Abstract
Despite the demonstrated advantages of angiotensin receptor/neprilysin inhibitors in the management of heart failure, the pivotal Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure (PARADIGM-HF) trial, which explored this class of medications, did not include individuals from Saudi Arabia. Recognizing that different nations and ethnic groups may exhibit unique characteristics, this study aimed to compare the demographics and outcomes of patients in Saudi Arabia who received sacubitril/valsartan (Sac/Val) with those enrolled in the PARADIGM-HF trial. In this retrospective, multicenter cohort study, we included all adult patients diagnosed with heart failure with reduced ejection fraction (HFrEF) within a tertiary healthcare system in Saudi Arabia between January 2018 and December 2021 and were initiated on Sac/Val. The primary objective was to compare the patient characteristics of those initiating Sac/Val treatment with the participants in the PARADIGM-HF trial. The secondary endpoints included the initiation setting, dose initiation, and titration, as well as alterations in B-type natriuretic peptide and ejection fraction at the 6-month mark. Furthermore, we reported the hospitalization and mortality event rates at the 12-month time point. The study included 400 patients with HFrEF receiving Sac/Val. Compared with the PARADIGM-HF trial, the cohort had a younger mean age and a higher prevalence of diabetes mellitus. SAC/VAL was prescribed as the initial therapy for 34% of the patients, while the remaining participants were initially treated with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker before transitioning to Sac/Val. Approximately 75% of patients were initiated on 100 mg Sac/Val twice daily, and 90% initiated therapy in the inpatient setting. The mean ejection fraction significantly improved from 26.5 ± 8.4% to 30.5 ± 6.4% at 6 months (P < .001), while the median B-type natriuretic peptide level change was not significant (P = .39). Our study revealed notable disparities in the baseline characteristics of patients with HFrEF compared with those in the PARADIGM-HF trial. These findings offer valuable real-world insights into the prescription patterns and outcomes of Sac/Val in patients with HFrEF in Saudi Arabia, an aspect not previously represented in the PARADIGM-HF study.
Keywords: heart failure, sacubitril/valsartan, Saudi Arabia
1. Introduction
Heart failure (HF) represents a multifaceted array of clinical manifestations and symptoms that contribute significantly to elevated healthcare costs, morbidity, mortality, diminished quality of life, and reduced functional capacity.[1] Globally, this debilitating condition affects over 64 million individuals, underscoring the critical need for effective management and treatment strategies.[1] In a previously published study involving 2047 participants in Saudi Arabia, 0.6% had a documented history of HF.[2] The overall expenditure on HF in Saudi Arabia is predominantly influenced by factors such as hospitalization, medication, and diagnostic costs.[3]
Previous comparative analyses have revealed that patients diagnosed with HF in registries from Middle Eastern Arab nations tend to be younger than those from the United States and Europe. The early onset of HF in these regions can be ascribed to various contributing factors, such as the high prevalence of coronary artery disease (CAD), which is regarded as the principal cause of HF in these populations. Furthermore, the strikingly high occurrence of diabetes mellitus (DM) in these nations plays a substantial role in exacerbating CAD, and consequently, HF.[4,5]
In recent years, numerous novel pharmacological agents have emerged that demonstrate promising results in patients with HF. Consequently, it is recommended that they be integrated into clinical practice as the standard of care.[6] Among these agents, sacubitril/valsartan (Sac/Val), an angiotensin receptor-neprilysin inhibitor, has garnered significant attention. The United States Food and Drug Administration and the European Medicines Agency granted approval for Sac/Val in November 2015, following the positive outcomes observed in the Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure (PARADIGM-HF) trial.[6,7]
The American College of Cardiology includes Sac/Val in the 2022 update of the Guideline Directed Medical Therapy for the management of HF, with a class 1A recommendation to initiate Sac/Val in patients with HF with reduced ejection fraction (HFrEF) and New York Heart Association (NYHA) functional class II to III symptoms. In addition, among patients who tolerate angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ARBs), replacement with Sac/Val is currently recommended to reduce morbidity and mortality rates.[8] Moreover, the 2021 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic HF recommend replacing ACEi or ARB with Sac/Val in ambulatory patients with HFrEF who remain symptomatic despite optimal treatment.[9]
Despite the documented advantages of Sac/Val in managing HF, prior real-world evidence has shown low adoption rates.[10,11] The Get With The Guidelines-Heart Failure Registry, which included 21,078 patients hospitalized with HFrEF, revealed that only 2% of eligible patients received Sac/Val upon hospital discharge.[12] Furthermore, a recent examination of electronic medical records from Germany discovered that the recommended Sac/Val dosage of 200 mg twice daily was not prescribed to the majority of patients treated in real-world settings.[13] In a prospective small study conducted in Saudi Arabia, the initiation of Sac/Val was found to improve heart failure symptoms without any associated severe adverse events.[14]
Although the PARADIGM-HF trial was conducted across several countries in North America, Latin America, Europe, and Asia, Saudi Arabia was not a participating country in the study.[7] The characteristics and outcomes of patients with HF may vary between countries, and different nations and ethnic groups may have different patient characteristics.[15,16] Real-world data describing the contemporary uptake and outcomes of Sac/Val in Saudi Arabia are scarce. Therefore, this study aimed to describe the characteristics of Saudi Arabian patients versus those of patients enrolled in the PARADIGM-HF trial. In addition, our investigation included a description of the 6 to 12-month outcomes of patients who were administered Sac/Val therapy. This in-depth analysis aimed to evaluate the therapeutic efficacy, safety, and long-term implications of this treatment, thereby providing valuable insights into its optimal implementation in real-world clinical settings in Saudi Arabia.
2. Methods
2.1. Study design
This retrospective cohort study aimed to assess real-world utilization and outcomes of Sac/Val in adult patients with HFrEF in Saudi Arabia. The study included adult patients (aged ≥ 18 years) who were identified through a comprehensive review of electronic medical records from a multicenter healthcare system geographically distributed across Saudi Arabia. We included patients who received Sac/Val between January 2018 and December 2021, with January 2018 marking the date when Sac/Val was added to the formulary of the healthcare system. Patients prescribed Sac/Val in either inpatient or outpatient settings for a minimum of 12 months were included in the study. Follow-up assessments were performed on all patients to evaluate the study endpoints. A 1-year follow-up period from the date of data extraction was permitted. The Institutional Review Board of King Abdullah International Medical Research Center approved this study (reference no. NRC22R/088/02) and waived the need for informed consent from the participants due to the retrospective nature of the study.
2.2. Outcomes
The primary objective of this study was to compare the characteristics of patients initiated on Sac/Val with those of patients enrolled in the PARADIGM-HF trial. Secondary outcomes included examining the Sac/Val dose at initiation and titration patterns at 6 months, as well as determining the initiation setting (inpatient vs outpatient). Additionally, the study reported changes in B-type natriuretic peptide (BNP) levels and ejection fraction during the 6 months following initiation. Finally, the study reported the rate of deaths resulting from cardiovascular (CV) causes, non-CV causes, heart failure hospitalizations, and non-heart failure hospitalizations at 12 months.
2.3. Statistical analysis
Patient characteristics were presented using the same methodology as that employed in the PARADIGM-HF trial. Continuous variables are expressed as means with standard deviations or medians, accompanied by lower (Q1) and upper (Q3) quartiles. Categorical variables are reported as counts and percentages. Normality assumptions for all continuous variables were assessed using graphical histograms. To compare categorical variables, chi-squared or Fisher exact tests were employed, while Student t test was used to compare normally distributed continuous variables. To evaluate differences between baseline and follow-up outcomes, the chi-squared test was used for categorical variables, and the paired t test was used for continuous variables. Statistical significance was set at P < .05. All statistical analyses were performed using STATA/BE 17.0.
3. Results
3.1. Patient’s demographics
As shown in Table 1, 400 patients with HFrEF who received Sac/Val were included in the analysis. In this real-world evaluation, the mean age of patients who received Sac/Val was younger than that of the PARADIGM-HF population (58.6 ± 13.8 years vs 63.8 ± 11.5 years, respectively), and there was a higher percentage of female patients (30.8% vs 21%). Our cohort demonstrated a higher proportion of patients classified as NYHA class I (20.5% vs 4.3%) and IV (8.5% vs 0.8%), and a lower proportion of class II patients (48.75% vs 71.6%). The severity of heart failure was greater in our cohort, as evidenced by a median BNP level of 459 (range:139–600) compared with 255 (range:155–474) in the PARADIGM-HF trial. Additionally, our cohort exhibited a lower mean ejection fraction (EF) (26.5 ± 8.4% vs 29.6 ± 6.1%, respectively) at baseline. Moreover, the prevalence of DM was higher in our cohort (63.75% vs 34.7%), as was the incidence of stroke (11.5% vs 8.5%), whereas a lower history of atrial fibrillation was observed (20.8% vs 36.2%). The median estimated glomerular filtration rate (eGFR) of our cohort was 89 mL/minutes, with an interquartile range (IQR) of 66–100 mL/minutes. A significant proportion (90%) of patients who received Sac/Val exhibited an eGFR above 60 mL/minutes, while 7% presented with an eGFR between 60 and 30 mL/minutes, and a mere 3% had an eGFR within the 15 to 30 mL/minutes range. Notably, none of the patients displayed an eGFR < 15 mL/minutes, and no patients were undergoing dialysis treatment.
Table 1.
Patients characteristics who were initiated on Sac/Val in comparison to the patients enrolled in the PARADIGM-HF trial.
| Variable | Real-World N = 400 | PARADIGM-HF N = 4187 | P value |
|---|---|---|---|
| Age – yr (mean ± SD) | 58.6 ± 13.8 | 63.8 ± 11.5 | <.0001 |
| Female sex – no. (%) | 123 (30.8) | 879 (21.0) | <.0001 |
| Body-mass index (mean ± SD) | 29.5 ± 6.8 | 28.1 ± 5.5 | <.0001 |
| NYHA functional classification – no. (%) | |||
| Class I | 82 (20.50) | 180 (4.3) | <.0001 |
| Class II | 195 (48.75) | 2998 (71.6) | <.0001 |
| Class III | 89 (22.25) | 969 (23.1) | .6867 |
| Class IV | 34 (8.50) | 33 (0.8) | <.0001 |
| Median B-type natriuretic peptide, median (IQR) – pg/mL | 459 (139–600) | 255 (155–474) | <.0001 |
| Left ventricular ejection fraction – % (mean ± SD) | 26.5 ± 8.4 | 29.6 ± 6.1 | <.0001 |
| Systolic blood pressure – mm Hg (mean ± SD) | 121 ± 17 | 122 ± 15 | .2525 |
| Heart rate – beats/min (mean ± SD) | 73 ± 10 | 72 ± 12 | .3452 |
| Medical history – no. (%) | |||
| Hypertension | 270 (67.5) | 2969 (70.9) | .1344 |
| Myocardial infarction | 193 (48.3) | 1818 (43.4) | .0503 |
| Stroke | 46 (11.5) | 355 (8.5) | .0314 |
| Hospitalization for heart failure | NA | 2607 (62.3) | NA |
| Atrial fibrillation | 83 (20.8) | 1517 (36.2) | <.0001 |
| Diabetes mellitus | 255 (63.75) | 1451 (34.7) | <.0001 |
| Previous use of ACEi | 138 (34.50) | 3266 (78.0) | <.0001 |
| Previous use of ARB | 127 (31.8) | 929 (22.2) | <.0001 |
| Serum creatinine – mg/dL (mean ± SD) | 1.07 ± 0.31 | 1.13 ± 0.3 | .0001 |
| Device therapy – no. (%) | |||
| Implantable cardioverter–defibrillator | 56 (14) | 623 (14.9) | .6132 |
| Cardiac resynchronization therapy | 45 (11.3) | 292 (7.0) | .0009 |
| Heart failure medication – no. (%) | |||
| Diuretic | 328 (82) | 3363 (80.3) | .3926 |
| Beta-blocker | 364 (91) | 3899 (93.1) | .1169 |
| Mineralocorticoid receptor antagonist | 252 (63) | 2271 (54.2) | .0004 |
| Sodium-glucose Cotransporter-2 inhibitors | 177 (44.3) | NA | NA |
| Digoxin | 40 (10) | 1223 (29.2) | <.0001 |
ACE = angiotensin-converting-enzyme, ARB = angiotensin receptor blocker, IQR = interquartile range, NYHA = New York Heart Association, Sac/val = sacubitril/valsartan, SD = standard deviation.
Overall, our cohort demonstrated similar utilization of diuretic and beta-blocker therapies to those enrolled in the PARADIGM-HF trial. However, a higher percentage of patients in our cohort received mineralocorticoid receptor antagonists (MRAs) and sodium-glucose cotransporter-2 inhibitors (SGLT-2i) than those in the PARADIGM-HF trial. It is important to note that the PARADIGM-HF trial did not include SGLT-2i, as their positive CV outcomes were not established at that time. Among 255 patients with DM, the mean Hemoglobin A1c (HbA1c) level was 7.7%, with a standard deviation of ± 2.1%. The primary antidiabetic medications prescribed were metformin (55%), insulin (41%), sulfonylureas (13%), and glucagon-like peptide-1 agonists (6%). Of 177 patients treated with SGLT-2 inhibitors, 160 (90%) were diagnosed with DM.
3.2. Initiation settings and titration patterns
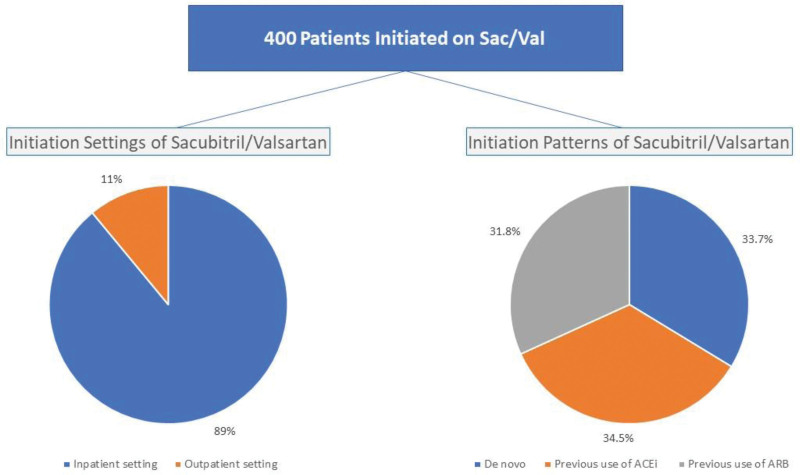
The evaluation of initiation settings for Sac/Val revealed that a majority of patients (n = 356; 89%) began treatment in the inpatient setting, while the remaining 44 (11%) initiated therapy in the outpatient setting. Notably, 135 (34%) patients commenced Sac/Val therapy de novo, while the rest were previously on either an ACEi or ARB before transitioning to Sac/Val, as illustrated in Figure 1.
Figure 1.
initiation settings and patterns of sacubitril/valsartan.
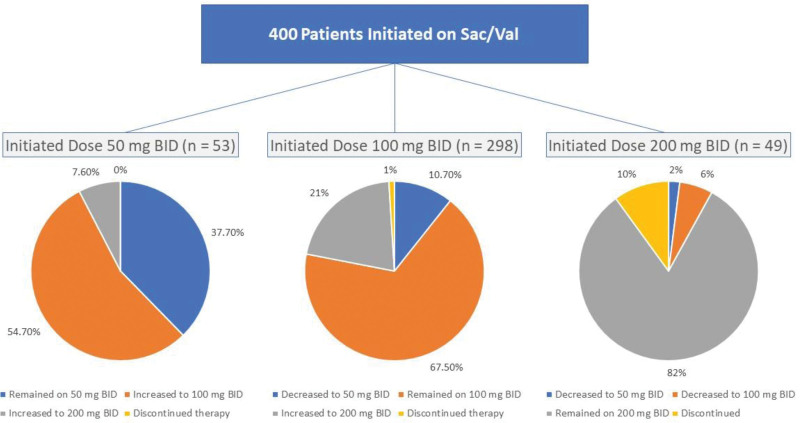
As shown in Figure 2, analysis of the initiation pattern indicated that 53 (13.25%), 298 (74.50%), and 49 (12.25%) patients started Sac/Val with 50, 100, and 200 mg bid doses, respectively. Upon examining the titration pattern at the 6-month mark, 7.6% of patients who initiated treatment with a 50 mg bid dose reached the target dose of 200 mg bid. Moreover, 21.8% of the patients who began with a 100 mg bid dose achieved the target dose of 200 mg bid. At the 6-month follow-up, 8 (2%) patients discontinued Sac/Val treatment. Among these patients, 3 stopped owing to hypotension, 3 based on the physician’s discretion, and 2 declined to continue taking Sac/Val after the index date (initiation date).
Figure 2.
initiation doses and titration patterns of sacubitril/valsartan at six months.
3.3. BNP and LVEF within 6 months following Sac/Val therapy initiation
Of the 400 patients enrolled in the study, baseline and 6-month follow-up data on EF were available for 355. The mean EF demonstrated a significant improvement, increasing from 26.5 ± 8.4% at baseline to 30.5 ± 6.4% at the 6-month follow-up (P = .02). Furthermore, baseline and 6-month follow-up data on BNP levels were available for 351 of the 400 patients included in the study. During the 6-month period, the median BNP level increased slightly from 459 (range:139–600) to 501 (range:120–633); however, this change was not statistically significant (P = .39).
3.4. Hospitalizations and mortality events within 12 months post-initiation of Sac/Val therapy
In this study, data were available for 390 of the 400 patients who were administered Sac/Val for a minimum of 12 months. During the first year of treatment, 65 patients (16.7%) experienced at least 1 HF-related hospitalization, whereas 59 patients (15.1%) had a minimum of 1 hospitalization due to other CV-related causes. At the 12-month period, 11 patients (2.8%) died, of which 7 were attributed to CV causes and 4 were attributed to non-CV causes.
4. Discussion
In this retrospective study conducted in Saudi Arabia, we described the baseline characteristics and outcomes of patients with HFrEF receiving Sac/Val, and compared them to those enrolled in the PARADIGM-HF trial. The findings revealed notable disparities between the baseline characteristics of patients with HFrEF in the Saudi Arabian cohort and those in the PARADIGM-HF trial, emphasizing the importance of examining real-world data from diverse populations.
In comparison to those enrolled in the PARADIGM-HF trial, our cohort was notably younger, with an average age of 58.6 ± 13.8 years versus 63.8 ± 11.5 years. The prevalence of comorbidities in our patient population was largely comparable to that in the PARADIGM-HF trial, with the notable exception of DM, which was significantly more prevalent in our study (63.75% vs 34.7%, respectively). The observed percentages of young age and high rates of DM align with previous registries, indicating that patients with HF in Middle Eastern Arab countries tend to be younger and have a higher prevalence of DM than those in the United States and Europe.[5] The earlier onset of HF in these regions can be attributed to multiple factors, such as the high prevalence of CAD in Middle Eastern Arab nations, which is recognized as the principal cause of HF among these populations. Additionally, the remarkably elevated incidence of DM in these countries is a critical factor contributing to CAD and, subsequently, HF.[5] This phenomenon can be attributed to the increasing prevalence of obesity and significant socioeconomic transformations that have resulted in the uptake of detrimental behaviors, including the consumption of high-calorie foods, tobacco use, and insufficient physical activity.[17]
A systematic review of real-world observational studies of Sac/Val in patients with HFrEF also reported variations in the baseline characteristics between the studies compared with PARADIGM-HF.[18] Similar to our study, patients included in the systematic review had a higher incidence of DM and more patients were on MRA than in the PARADIGM-HF study.[18] Another systematic review and meta-analysis of 16,952 patients from 21 studies highlighted the discrepancy between patients with HFrEF who received Sac/Val and those enrolled in the PARADIGM-HF trial.[19] The differences were mostly reported in age and the prevalence of DM and hypertension, which were lower than those in patients enrolled in the PARADIGM-HF study.[20]
Additionally, our study had a considerably higher percentage of HF patients with NYHA functional class I and IV and the mean LFEF in our cohort was significantly lower than that in the PARADIGM-HF study (26.5 ± 8.4 vs 29.6 ± 6.1). Our research also revealed a higher proportion of patients with NYHA class IV and elevated BNP levels, suggesting that our study included patients with more severe HFrEF than the PARADIGM-HF trial.
Our study identified a significantly higher percentage of patients with HFrEF receiving MRAs (63% vs 54.2%) and a significantly lower percentage of patients administered digoxin compared to the PARADIGM-HF trial. Moreover, 44.3% of the patients in our study were prescribed SGLT-2i, which was not a recommended treatment option in the PARADIGM-HF trial. This variation in prescription practices may be attributed to differences in contemporary guidelines, HF severity, and the presence of comorbidities within our cohort, thereby influencing therapeutic choices for these individuals.
In contrast to the PARADIGM-HF study, our investigation revealed that 34% of the patients with HFrEF were initiated on de novo Sac/Val therapy. The majority (74.5%) of these patients received an initial dose of 100 mg twice daily. Despite the The American College of Cardiology guidelines recommending gradual titration of Sac/Val to achieve a target dose of 200 mg twice daily within 3 to 6 months, only 26.5% of our patients reached the target dose. This proportion is significantly lower than that reported in previous studies, which indicated that more than 50% of heart failure patients achieved the target dose.[20,21] The delay in titrating the Sac/Val to the recommended target dose may be attributed to the various patient characteristics. Notably, 67.5% of our study population had hypertension, while concomitant use of medications with hypotensive effects was prevalent: beta-blockers (91%), MRA (63%), and SGLT-2i (44%). These factors could potentially exacerbate hypotension, volume depletion, and renal insufficiency, thereby impeding timely up-titration of Sac/Val.
The findings from the prospective study of biomarkers, symptom improvement, and ventricular remodeling during sacubitril/valsartan therapy for heart failure trial demonstrated a substantial improvement in LVEF at both 6 and twelve-month intervals.[22] This is consistent with the results of our study, in which we observed a significant improvement in the mean EF 6 months after Sac/Val initiation (26.5 ± 8.4% to 30.5 ± 6.4%, P < .001). Furthermore, the study’s overall mortality rate at 12 months (2.8%) was considerably lower than that reported in the PARADIGM-HF trial (13.3%). Additionally, it should be noted that the follow-up durations of the 2 studies (the present study vs PARADIGM-HF) were different, and the 12-month mortality rate was assessed in the present study, whereas PARADIGM-HF reported outcomes at a median follow-up of 27 months.
In line with our findings, a separate prospective observational study involving patients with HFrEF treated with Sac/Val also noted a significant decrease in rehospitalization rates due to worsening heart failure and all-cause mortality.[23] A prior investigation demonstrated that in patients with HFrEF receiving Sac/Val therapy, there was considerable improvement in left ventricular global longitudinal strain and left atrial reservoir strain, typically within a 6-month treatment period.[24] These sensitive biomarkers may serve as valuable indicators of therapeutic responses and prognostic outcomes. Our current study revealed enhancements in other LVEF parameters. An additional real-world study conducted in Korea demonstrated similar results, including cardiac reverse remodeling, left ventricular size reduction, and LFEF improvement following the initiation of Sac/Val therapy.[25]
In the current study, there was a modest increase in BNP levels over the course of 6 months of treatment, rising from 459 (IQR 139–600) pg/mL to 501 (IQR 120–633) pg/mL. Importantly, it should be noted that the baseline BNP levels in our cohort were higher than those reported in the PARADIGM-HF trial. In the aforementioned trial, the median BNP concentration prior to treatment was 202 pg/mL (IQR 126–335), which later increased to 235 ng/L (IQR 128–422) after 8 to 10 weeks of treatment. When interpreting BNP levels among patients receiving Sac/Val, it is crucial to recognize several confounding factors that may complicate the assessment. Specifically, neprilysin inhibition by sacubitril leads to increased circulating BNP levels due to decreased degradation, which makes it challenging to distinguish between elevated BNP levels attributable to heart failure and the effect of medication. Furthermore, individual patient responses to Sac/Val may vary, resulting in disparate degrees of BNP elevation. This heterogeneity renders it difficult to establish a standardized reference range for BNP levels within the patient’s demographic. Clinicians should consider these factors when evaluating BNP levels in such patients and may consider employing alternative biomarkers such as N-terminal pro-brain natriuretic peptide (NT-proBNP). Given that neprilysin does not degrade NT-proBNP, it serves as a more dependable biomarker for patients receiving Sac/Val therapy.[19,26,27] One of the major limitations of our study is that we were unable to acquire NT-proBNP measurements because of the unavailability of this particular biomarker at the participating research centers.
Among the total study population, there were 11 (2.8%) mortality cases, with 7 (1.8% of the total study population) CV mortality cases during the 12-month period. The lower mortality rate in the present study might be attributable to the younger study population or the higher usage of SGLT-2 inhibitors compared with other cohorts, which will be investigated in a separate study.
To the best of our knowledge, this study is the first and most extensive real-world investigation of the characteristics and outcomes of HFrEF patients receiving Sac/Val therapy in Saudi Arabia. Prior randomized controlled trials and observational studies have not included this specific population. Although our real-world study reflects patients from clinical practice, thereby enhancing the external validity of our findings, the retrospective design may limit the generalizability of our results. In contrast to the PARADIGM-HF trial, our study included both inpatients and outpatients with HF. This inclusion may account for some discrepancies in the baseline patient characteristics. Additionally, our study’s follow-up duration was shorter than that of the PARADIGM-HF trial, which could result in an underestimation of the Sac/Val benefits. Moreover, our study had limitations in examining and reporting the history of hospitalization for HF in our cohort. This issue primarily stems from inadequate documentation in the electronic medical records used in our research, potentially affecting the internal validity of our findings. It is crucial to acknowledge that our study incorporated an additional class of disease-modifying medications, SGLT-2i. This inclusion may have influenced the outcomes, rendering the comparison with the PARADIGM-HF trial less straightforward.
5. Conclusion
This study revealed significant discrepancies in the baseline characteristics of patients with HFrEF who received Sac/Val compared with those enrolled in the PARADIGM-HF trial. These observations provide crucial real-world evidence regarding the utilization and outcomes of Sac/Val therapy for patients with HFrEF in Saudi Arabia, an aspect that has not been comprehensively addressed within the scope of the PARADIGM-HF investigation. This comprehensive analysis of prescription patterns and outcomes for Sac/Val in patients with HFrEF broadens the existing knowledge base and underscores the importance of considering regional differences when evaluating the effectiveness of novel therapies.
Acknowledgments
We would like to thank Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R78), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia. We also thank the investigators who participated in this project from the Saudi critical care pharmacy research (SCAPE) platform.
Author contributions
Conceptualization: Hisham A. Badreldin, Mousa H. Aljohani, Omar A. Alshaya, Khalid Al Sulaiman, Lolwa Alabdelmuhsin, Faisal Alanazi, Ghada M. Alqannam, Ohoud Almadani, Ohoud Aljuhani, Awatif Hafiz, Ghalyah Aljowaie, Ehssan Basha.
Data curation: Mousa H. Aljohani, Lolwa Alabdelmuhsin, Dahlia M. Almutairi, Ohoud Aljuhani, Ehssan Basha.
Funding acquisition: Ghazwa B. Korayem.
Investigation: Basel A. Alenazy, Dahlia M. Almutairi.
Methodology: Ghazwa B. Korayem, Basel A. Alenazy, Dahlia M. Almutairi.
Project administration: Ghada M. Alqannam.
Resources: Mousa H. Aljohani, Khalid Al Sulaiman, Huda Alenazi, Ghada M. Alqannam, Awatif Hafiz.
Software: Mousa H. Aljohani, Khalid Al Sulaiman, Huda Alenazi, Ghada M. Alqannam, Ohoud Almadani, Awatif Hafiz.
Supervision: Hisham A. Badreldin, Khalid Al Sulaiman, Ohoud Almadani, Awatif Hafiz, Mosaad Alhussein.
Validation: Basel A. Alenazy, Ohoud Almadani.
Visualization: Huda Alenazi, Faisal Alanazi, Seba K. Alobathani.
Writing – original draft: Hisham A. Badreldin, Ghazwa B. Korayem, Basel A. Alenazy, Mousa H. Aljohani, Omar A. Alshaya, Khalid Al Sulaiman, Lolwa Alabdelmuhsin, Huda Alenazi, Faisal Alanazi, Seba K. Alobathani, Ohoud Aljuhani, Ghalyah Aljowaie, Ehssan Basha, Tariq Alqahtani.
Writing – review & editing: Hisham A. Badreldin, Ghazwa B. Korayem, Basel A. Alenazy, Mousa H. Aljohani, Omar A. Alshaya, Khalid Al Sulaiman, Lolwa Alabdelmuhsin, Huda Alenazi, Seba K. Alobathani, Ohoud Aljuhani, Ghalyah Aljowaie, Tariq Alqahtani.
Abbreviations:
- ARB
- angiotensin receptor blocker
- BNP
- B-type natriuretic peptide
- CAD
- coronary artery disease
- CV
- cardiovascular
- DM
- diabetes mellitus
- EF
- ejection fraction
- eGFR
- estimated glomerular filtration rate
- HF
- heart failure
- HFrEF
- heart failure with reduced ejection fraction
- IQR
- interquartile range
- MRA
- mineralocorticoid receptor antagonists
- NT-proBNP
- N-terminal pro-brain natriuretic peptide
- NYHA
- New York Heart Association
- PARADIGM-HF
- Angiotensin–Neprilysin inhibition versus enalapril in heart failure trial
- Sac/Val
- sacubitril/valsartan
- SGLT-2i
- sodium-glucose cotransporter-2 inhibitors
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
This work was supported by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R78), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
The authors have no conflicts of interest to disclose.
How to cite this article: Badreldin HA, Korayem GB, Alenazy BA, Aljohani MH, Alshaya OA, Al Sulaiman K, Alabdelmuhsin L, Alenazi H, Almutairi DM, Alanazi F, Alobathani SK, Alqannam GM, Almadani O, Aljuhani O, Hafiz A, Aljowaie G, Basha E, Alqahtani T, Alhussein M. Real-world analysis of integration of sacubitril/valsartan into clinical practice in Saudi Arabia. Medicine 2023;102:51(e36699).
Contributor Information
Ghazwa B. Korayem, Email: ghazwa.krayem@gmail.com.
Basel A. Alenazy, Email: basil.alsalem501@gmail.com.
Mousa H. Aljohani, Email: m.h.aljohani@hotmail.com.
Omar A. Alshaya, Email: omaraalshaya@gmail.com.
Khalid Al Sulaiman, Email: alsulaimankh@hotmail.com.
Lolwa Alabdelmuhsin, Email: Alabdelmuhsinlo@ngha.med.sa.
Huda Alenazi, Email: ALENAZIHU@ngha.med.sa.
Dahlia M. Almutairi, Email: bellusdahlia@gmail.com.
Faisal Alanazi, Email: alanazi.faisal.h@gmail.com.
Seba K. Alobathani, Email: Alobathaniseba@gmail.com.
Ghada M. Alqannam, Email: Ghada.alqannam@gmail.com.
Ohoud Almadani, Email: oaljuhani@kau.edu.sa.
Ohoud Aljuhani, Email: oaljuhani@kau.edu.sa.
Awatif Hafiz, Email: awatifhafiz@yahoo.com.
Ghalyah Aljowaie, Email: Aljowaie053@ksau-hs.edu.sa.
Ehssan Basha, Email: Basha271@ksau-hs.edu.sa.
Tariq Alqahtani, Email: Qahtanita@ksau-hs.edu.sa.
Mosaad Alhussein, Email: HusseinM@ngha.med.sa.
References
- [1].Savarese G, Becher PM, Lund LH, et al. Coats AJS Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118:3272–87. [DOI] [PubMed] [Google Scholar]
- [2].Alhabib KF, Batais MA, Almigbal TH, et al. Demographic, behavioral, and cardiovascular disease risk factors in the Saudi population: results from the Prospective Urban Rural Epidemiology study (PURE-Saudi). BMC Public Health. 2020;20:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alghamdi A, Algarni E, Balkhi B, et al. Healthcare expenditures associated with heart failure in saudi arabia: a cost of illness study. Healthcare (Basel). 2021;9:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alhabeeb W, Elasfar A, AlBackr H, et al. Clinical characteristics, management and outcomes of patients with chronic heart failure: results from the heart function assessment registry trial in Saudi Arabia (HEARTS-chronic). Int J Cardiol. 2017;235:94–9. [DOI] [PubMed] [Google Scholar]
- [5].Sulaiman K, Panduranga P, Al-Zakwani I, et al. Clinical characteristics, management, and outcomes of acute heart failure patients: observations from the Gulf acute heart failure registry (Gulf CARE). Eur J Heart Fail. 2015;17:374–84. [DOI] [PubMed] [Google Scholar]
- [6].Badreldin HA, Aldosari N, Alnashwan L, et al. What the near future holds for sacubitril/valsartan: a summary of major ongoing studies. J Cardiovasc Dev Dis. 2022;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- [8].Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- [9].McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- [10].DeVore AD, Hill CL, Thomas L, et al. Patient, provider, and practice characteristics associated with sacubitril/valsartan use in the United States. Circ Heart Fail. 2018;11:e005400. [DOI] [PubMed] [Google Scholar]
- [11].Kim BJ, Huang CW, Chung J, et al. Real-world use patterns of angiotensin receptor-neprilysin inhibitor (sacubitril/valsartan) among patients with heart failure within a large integrated health system. J Manag Care Spec Pharm. 2022;28:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Luo N, Fonarow GC, Lippmann SJ, et al. Early adoption of sacubitril/valsartan for patients with heart failure with reduced ejection fraction: insights from get with the guidelines-heart failure (GWTG-HF). JACC Heart Fail. 2017;5:305–9. [DOI] [PubMed] [Google Scholar]
- [13].Wachter R, Fonseca AF, Balas B, et al. Real-world treatment patterns of sacubitril/valsartan: a longitudinal cohort study in Germany. Eur J Heart Fail. 2019;21:588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alhabeeb W, Al Ayoubi F, Hayajneh A, et al. Efficacy and Safety of sacubitril/valsartan in patients with heart failure. IntJ Pharm Res Allied Sci. 2020;9(4):11–8. [Google Scholar]
- [15].Dewan P, Jhund PS, Shen L, et al. Heart failure with reduced ejection fraction: comparison of patient characteristics and clinical outcomes within Asia and between Asia, Europe and the Americas. Eur J Heart Fail. 2019;21:577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Badreldin HA, Alghnam S. Cardiovascular diseases guideline-directed medical therapy in low- and middle-income countries: a call for action. Saudi J Clin Pharm. 2022;1:67–8. [Google Scholar]
- [17].Alharbi M, Alharbi F, AlTuwayjiri A, et al. Assessment of health-related quality of life in patients with heart failure: a cross-sectional study in Saudi Arabia. Health Qual Life Outcomes. 2022;20:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Proudfoot C, Studer R, Rajput T, et al. Real-world effectiveness and safety of sacubitril/valsartan in heart failure: a systematic review. Int J Cardiol. 2021;331:164–71. [DOI] [PubMed] [Google Scholar]
- [19].Vodovar N, Séronde MF, Laribi S, et al. Elevated plasma B-type natriuretic peptide concentrations directly inhibit circulating neprilysin activity in heart failure. JACC Heart Fail. 2015;3:629–36. [DOI] [PubMed] [Google Scholar]
- [20].Giovinazzo S, Carmisciano L, Toma M, et al. Sacubitril/valsartan in real-life European patients with heart failure and reduced ejection fraction: a systematic review and meta-analysis. ESC Heart Fail. 2021;8:3547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ganesananthan S, Shah N, Shah P, et al. Real-world treatment switching to sacubitril/valsartan in patients with heart failure with reduced ejection fraction: a cohort study. Open Heart. 2020;7:e001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Januzzi JL, Jr., Prescott MF, Butler J, et al. Association of change in N-terminal pro-b-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Polito MV, Silverio A, Rispoli A, et al. Clinical and echocardiographic benefit of sacubitril/valsartan in a real-world population with HF with reduced ejection fraction. Sci Rep. 2020;10:6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moon MG, Hwang IC, Lee HJ, et al. Reverse remodeling assessed by left atrial and ventricular strain reflects treatment response to sacubitril/valsartan. JACC Cardiovasc Imaging. 2022;15:1525–41. [DOI] [PubMed] [Google Scholar]
- [25].Park JJ, Lee SE, Cho HJ, et al. Real-World usage of sacubitril/valsartan in Korea: a multi-center, retrospective study. Int J Heart Fail. 2022;4:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zile MR, Claggett BL, Prescott MF, et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68:2425–36. [DOI] [PubMed] [Google Scholar]
- [27].Myhre PL, Vaduganathan M, Claggett B, et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019;73:1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]