Abstract
Purpose:
The HER2DX genomic test predicts pathological complete response (pCR) and survival outcome in early-stage HER2+ breast cancer. Here, we evaluated the association of HER2DX scores with 1) pCR according to hormone receptor status and various treatment regimens, and 2) survival outcome according to pCR status.
Methods:
Seven neoadjuvant cohorts with HER2DX and clinical individual patient data were evaluated (i.e., DAPHNe, GOM-HGUGM-2018-05, CALGB-40601, ISPY-2, BiOnHER, NEOHER and PAMELA). All patients were treated with neoadjuvant trastuzumab (n=765) in combination with pertuzumab (n=328), lapatinib (n=187) or without a second anti-HER2 drug (n=250). Event-free survival (EFS) and overall survival (OS) outcomes were available in a combined series of 268 patients (i.e., NEOHER and PAMELA) with a pCR (n=118) and without a pCR (n=150). Cox models were adjusted to evaluate whether HER2DX can identify patients with low- or high-risk beyond pCR status.
Results:
HER2DX pCR score was significantly associated with pCR in all patients (odds-ratio [OR] per 10-unit increase=1.59, 95% CI 1.43-1.77; AUC=0.75) with or without dual HER2 blockade. A statistically significant increase in pCR rate due to dual HER2 blockade over trastuzumab-only was observed in HER2DX pCR-high tumors treated with chemotherapy (OR=2.56, 1.29-5.20). A statistically significant increase in pCR rate due to multi-agent chemotherapy over a single taxane was observed in HER2DX pCR-medium tumors treated with dual HER2 blockade (OR=3.11, 1.54-6.49). The pCR rates in HER2DX pCR-low tumors were ≤30.0% regardless of treatment administered. After adjusting by pCR status, patients identified as HER2DX low-risk had better EFS (p<0.001) and OS (p=0.006) compared to patients with HER2DX high-risk.
Conclusion:
HER2DX pCR-score and risk-score might help identify ideal candidates to receive neoadjuvant dual HER2 blockade in combination with a single taxane in early-stage HER2+ breast cancer.
Keywords: HER2, breast cancer, HER2DX, pertuzumab, pathological complete response, neoadjuvant, genomics, biomarker
Neoadjuvant systemic therapy is standard for patients with clinical stage II-III HER2+ breast cancer1,2. The pathological complete response (pCR) rates are 29-46% following trastuzumab in combination with chemotherapy3-5. The addition of a second anti-HER2 agent, such as pertuzumab or lapatinib, to trastuzumab and chemotherapy increases pCR rates by 10-20%, albeit with modest improvements in long-term survival3,5-8. Nonetheless, patients with HER2+ disease who experience a pCR have better long-term survival outcomes than those without a pCR9,10. This observation seems valid irrespective of the type of systemic therapy received before surgery9,11-13. In patients who do not achieve a pCR, adjuvant T-DM1 improves invasive disease-free survival compared to trastuzumab14; thus, pCR is a highly clinically meaningful endpoint for multiple reasons.
Several clinical questions remain unanswered regarding the optimal neoadjuvant treatment approach in HER2+ breast cancer. For example, who benefits from pertuzumab when added to trastuzumab and chemotherapy is still unclear. In addition, it is unclear what the optimal chemotherapy backbone in combination with dual HER2 blockade is. The DAPHNe phase II trial treated 98 patients with stage II-III HER2+ disease with 3 months of paclitaxel, trastuzumab and pertuzumab (THP), with no further chemotherapy in 98% of patients who achieved a pCR15. The CompassHER2-pCR (NCT04266249) and the Decrescendo (NCT04675827) phase II clinical trials are currently evaluating survival outcomes following neoadjuvant THP and adjuvant HP only in the context of pCR across >3,000 patients. Thus, upfront identification of patients likely to benefit from a de-escalated chemotherapy treatment strategy such as THP might be clinically important.
The HER2DX genomic test16 is a single 27-gene expression and clinical feature-based classifier which provides two independent scores to predict both long-term prognosis and likelihood of pCR in patients with HER2+ early breast cancer. The assay integrates biological information tracking immune response, luminal differentiation, tumor cell proliferation and expression of the HER2 17q12-21 chromosomal amplicon, including the ERBB2 gene, with clinical information (i.e., tumor size and nodal status)16. The prognostic value of HER2DX was shown in 1,341 patients across 5 datasets, and the ability to predict pCR following trastuzumab-based therapy was demonstrated in 558 patients across 4 datasets, including 127 tumor samples from the ISPY-2 clinical trial, which evaluated HP in combination with anthracycline/taxane-based chemotherapy17, and 263 tumor samples from CALGB-40601, which evaluated paclitaxel with trastuzumab, lapatinib or the combination of both HER2 targeting drugs16. More recently, the HER2DX pCR score has been validated in 80 tumor samples from the DAPHNe neoadjuvant trial18, and a Spanish study of 155 patients treated with neoadjuvant docetaxel, carboplatin, trastuzumab with or without pertuzumab (i.e., GOM-HGUGM-2018-05 [GOM] cohort)19.
Here, we combined HER2DX and clinical data from ISPY-2, CALGB-40601, DAPHNe, GOM BiOnHER, NEOHER and PAMELA cohorts to test the ability of the HER2DX pCR score to predict pCR across different subgroups of patients. Specifically, we focused on two clinically relevant questions: who benefits from the addition of a second anti-HER2 agent, pertuzumab or lapatinib, as dual therapy with trastuzumab and chemotherapy, and who benefits from multi-agent chemotherapy over a single agent taxane when treated with dual HER2 blockade. Finally, we tested the ability of the HER2DX risk score to predict survival outcome according to pCR status in a combined dataset (i.e., NEOHER and PAMELA) with long-term follow-up.
Methods
ISPY-2 cohort
The ISPY-2 phase II trial17 adaptively randomized 128 patients with clinical stage II to III HER2+ breast cancer to 4 cycles of T-DM1 (3.6 mg/kg iv every 3 weeks) in combination with pertuzumab (n=52) or THP (n=45), or a common control arm of weekly paclitaxel (80 mg/m2) and trastuzumab for 12 weeks (n=31). All patients received 4 cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) intravenously, every 2–3 weeks, before surgery (Fig. S1). The results of the HER2DX assay in ISPY-2 have been previously reported in 127 patients (99.2%)16.
CALGB-40601 cohort
The CALGB-40601 study3,7 is a phase III clinical trial that randomized 305 women with untreated stage II and III HER2+ breast cancer to receive weekly paclitaxel (80 mg/m2) for 16 weeks combined with trastuzumab plus 1,000 mg/day of lapatinib (THL), trastuzumab (TH), or lapatinib (TL) (Fig. S1). The results of the HER2DX assay are available in 263 patients (86.2%).
DAPHNe cohort
DAPHNe is a prospective investigator-initiated, single-arm phase II study, where 98 patients were assigned to receive preoperative paclitaxel (80 mg/m2 weekly for 12 weeks) in combination with trastuzumab and pertuzumab (THP)15 (Fig. S1). The HER2DX results for 80 patients (81.6%) in DAPHNe are reported elsewhere18.
GOM cohort
GOM is an ongoing retrospective observational study since 2018 of consecutive patients with newly diagnosed stage I-III HER2+ breast cancer who were candidates for neoadjuvant therapy across 7 public hospitals in Spain. All patients received 6 cycles of docetaxel 75 mg/m2 iv every 3 weeks in combination with carboplatin AUC of 6 iv every 3 weeks and trastuzumab every 3 weeks (TCH). Once neoadjuvant pertuzumab was reimbursed in Spain, most patients received TCH in combination with pertuzumab 840 mg iv loading dose, followed by pertuzumab every 3 weeks (TCHP) depending on high-risk tumors at the clinician discretion and/or according to the hospital’s criteria for availability of the drug (Fig. S1). The results of the HER2DX assay are available in 155 patients and are reported elsewhere19.
BiOnHER cohort
BiOnHER is a single arm phase II trial performed at the Institute of Oncology (Barcelona, Spain), where 46 patients with clinical stage II to III HER2+ were treated with 1 cycle of trastuzumab and pertuzumab without chemotherapy, followed by weekly paclitaxel for 16 weeks in combination with tratuzumab and pertuzumab every 3 weeks (THP) (Fig. S1). The results of the HER2DX assay are available in all patients.
PAMELA cohort
SOLTI-1114 PAMELA was an open-label, single-group, phase II trial of 151 patients with HER2+ breast cancer, stage I–IIIA and a performance status of 0-120. Patients were given lapatinib (1,000 mg per day) and trastuzumab for 18 weeks; hormone receptor-positive patients were additionally given letrozole (2.5 mg per day) or tamoxifen (20 mg per day) according to menopausal status (Fig. S1). Treatment after surgery was left to treating physician discretion. The results of the HER2DX assay are available in 84 patients (55.6%) and are reported elsewhere16. For this analysis, the median follow-up was 6.4 years.
NEOHER cohort
NEOHER is based on two retrospective cohorts from Hospital Clinic of Barcelona and Padova University. Patients with early-stage HER2+ breast cancer and a performance status of 0-1 were treated, as per standard practice, with neoadjuvant trastuzumab-based chemotherapy for 3-6 months, followed by surgery (Fig. S1). Adjuvant treatment was completed with trastuzumab for up to 1 year, and a minimum of 5 years of hormonal therapy for patients with hormone receptor-positive tumors. Only 14 patients with residual disease at surgery received adjuvant T-DM1. Radiation therapy was administered according to local guidelines. The results of the HER2DX assay are available in 184 patients and are reported elsewhere16. For this analysis, the median follow-up was 5.9 years.
TCGA dataset
Clinical, genetic (i.e., somatic mutations), genomic (i.e., gene expression) and proteomic data from the breast cancer TCGA dataset was obtained from cbioportal21. HER2DX pCR score was applied onto RNA-seq data of 161 HER2+ tumors.
HER2DX assay
HER2DX was evaluated in tumor samples from pre-treatment baseline samples. In the GOM, BiOnHER, NEOHER, PAMELA and DAPHNe cohorts, the HER2DX standardized assay was performed using RNA extracted from FFPE tissue, as previously described16,18,19. In ISPY-2, CALGB-40601, HER2DX was applied onto publicly available microarray data (GSE181574) and mRNAseq data respectively (dbGaP website, under accession number phs001570.v3.p1), as previously described16. From FFPE RNA, the HER2DX standardized assay was performed on the nCounter platform (NanoString Technologies, Seattle, WA, USA). The HER2DX assay is based on 4 different gene signatures comprising 27 genes, including the 14-gene immunoglobulin (IGG) module (i.e., CD27, CD79A, HLA-C, IGJ, IGKC, IGL, IGLV3-25, IL2RG, CXCL8, LAX1, NTN3, PIM2, POU2AF1 and TNFRSF17). The other 3 genes signatures were: a 4-gene tumor cell proliferation signature (EXO1, ASPM, NEK2 and KIF23), a 5-gene luminal differentiation signature (BCL2, DNAJC12, AGR3, AFF3 and ESR1), and the 4-gene HER2 amplicon signature (ERBB2, GRB7, STARD3 and TCAP)16. Two scores were calculated for each patient i) HER2DX pCR score and ii) HER2DX risk score (both from 0 up to 100). Pre-established cut-offs were used to create HER2DX pCR groups [low [0, 33.3), medium [33.3-66.7) and high [66.7-100)], and also to create HER2DX risk groups [low [0, 50) and high [50,100)]16.
Statistical analyses
The first objective was to evaluate the association of the HER2DX pCR score with pCR status. Univariable and multivariable logistic regression models were used to investigate the association for each variable with pCR in terms of odds-ratios (ORs) with 95% confidence interval (95% CI). All variables evaluated in the univariable analysis were included in the multivariable model. The first multivariable analysis used multiple imputation of random missing values using the mice R package (Table S1). Additionally, two sensitivity analysis were performed i) without data imputation and ii) excluding data from CALGB-40601, ISPY-2, NEOHER and PAMELA cohorts because they were included in the original HER2DX validation. The association of the HER2DX pCR score with pCR was also evaluated in several clinically relevant subgroups of patients: 1) patients treated with chemotherapy and trastuzumab, 2) patients treated with chemotherapy and dual HER2 blockade, 3) patients treated only with dual HER2 blockade 4) patients with hormone receptor (HR)-positive disease and 5) patients with HR-negative disease. To summarize the overall effect, a patient-level analysis was performed adjusting by cohort. In all analyses, 57 patients who did not receive neoadjuvant trastuzumab (i.e., the TL arm from CALGB-40601) and 116 tumor samples from NEOHER which were used for building the HER2DX pCR score (i.e., training dataset)16 were excluded. Across the 7 cohorts, pCR was defined as ypT0/isN0.
The second objective was to evaluate the predictive ability of the HER2DX pCR score to identify patients who will achieve pCR to dual HER2 blockade when given with chemotherapy. The third objective was to assess the predictive capacity of HER2DX pCR score to identify patients who benefit from multi-agent chemotherapy in the context of taxane-based therapy and dual HER2 blockade. Interaction tests, adjusted by cohort, were used to evaluate the different effect of treatment according to HER2DX pCR groups. The fourth objective was to explore the biology of the HER2DX pCR groups using HER2+ tumor samples from TCGA breast cancer project21,22. To validate the performance of the HER2DX pCR score, the area under the ROC curve (AUC), the area under the precision-recall curve, and calibration plots were calculated23.
Finally, we evaluated the ability of the HER2DX risk-score to predict survival outcome according to pCR status. Event-free survival (EFS) and overall survival (OS) were available in 268 patients from the NEOHER and PAMELA cohorts. The Kaplan-Meier method was used to estimate survival outcomes at 6-years. Cox proportional-hazard models were used to obtain hazard ratios (HRs) in i) the overall population after adjusting by pCR status, ii) pCR only and iii) non-pCR only. The median follow-up was calculated using the reverse Kaplan-Meier method. For all statistical analyses, the significance level was set at two-sided alpha of 0.05 and all analyses were performed using R statistical software version 4.1.2.
Results
Clinical-pathological features
Seven hundred sixty-five patients with available pre-treatment baseline HER2DX and clinical data were available across 7 cohorts (Fig. S2). Mean age was 51.6 years (range, 22-86), clinical T1 disease represented 21.2%, clinical node-positive disease (cN1-3) represented 46.5%, and 63.7% of tumors were HR-positive (Table 1). Patients were treated with neoadjuvant trastuzumab in combination with multi-agent chemotherapy (n=337), a single taxane (n=344), no chemotherapy (n=84), pertuzumab (n=328), lapatinib (n=187) or without a second anti-HER2 drug (n=250). The overall pCR rate was 49.9% (95% CI 46.3-53.5): 40.7% (36.4-45.3) in patients with HR-positive disease, 66.1% (60.1-71.6) in patients with HR-negative disease, 46.0% (39.7-52.4) in patients treated with chemotherapy and trastuzumab, 29.8% (20.5-40.9) in patients treated with dual HER2 blockade in the absence of chemotherapy and 56.1% (51.3-60.9) in patients treated with chemotherapy and dual HER2 blockade. Among patients with chemotherapy and dual HER2 blockade (n=431), the pCR rate with pertuzumab or lapatinib as a second anti-HER2 agent was 56.7% (51.1-62.1) and 54.4% (44.3-64.1), respectively.
Table 1:
Clinical-pathological characteristics of the seven neoadjuvant cohorts evaluated.
| Overall (n=765) |
CALGB- 40601 (n=206) |
ISPY-2 (n=127) |
DAPHNe (n=80) |
GOM (n=155) |
BIOnHER (n=46) |
NEOHER (n=67) |
PAMELA (n=84) |
||
|---|---|---|---|---|---|---|---|---|---|
| Age (mean and range) | 52 (22-86) | 49 (24-75) | NA | 50 (26-78) | 50 (22-74) | 60 (35-83) | 54 (34-81) | 56 (30-86) | |
| Clinical tumor stage, n (%) | T1 | 132 (21.2) | 16 (8.4) | NA | 15 (18.8) | 35 (22.6) | 14 (30.4) | 13 (19.4) | 39 (46.4) |
| T2-T3-T4 | 491 (78.8) | 175 (91.6) | NA | 65 (81.2) | 120 (77.4) | 32 (69.6) | 54 (80.6) | 45 (53.6) | |
| Clinical nodal stage, n (%) | N0 | 231 (53.5) | NA | NA | 52 (65.0) | 56 (36.1) | 27 (58.7) | 42 (62.7) | 54 (64.3) |
| N1-N2-N3 | 201 (46.5) | NA | NA | 28 (35.0) | 99 (63.9) | 19 (41.3) | 25 (37.3) | 30 (35.7) | |
| Hormone receptor, n (%) | Negative | 277 (36.3) | 84 (41.0) | 44 (34.6) | 23 (29.1) | 50 (32.3) | 15 (32.6) | 18 (26.9) | 43 (51.2) |
| Positive | 486 (63.7) | 121 (59.0) | 83 (65.4) | 56 (70.9) | 105 (67.7) | 31 (67.4) | 49 (73.1) | 41 (48.8) | |
| PAM50, n (%) | Basal-like | 82 (10.8) | 32 (15.5) | 27 (21.3) | 5 (6.2) | 8 (5.2) | 1 (2.4) | 2 (3.0) | 7 (8.3) |
| HER2-E | 316 (41.6) | 46 (22.3) | 28 (22.0) | 46 (57.5) | 80 (51.6) | 26 (63.4) | 33 (49.3) | 57 (67.9) | |
| Luminal A | 153 (20.1) | 38 (18.4) | 31 (24.4) | 14 (17.5) | 38 (24.5) | 6 (14.6) | 12 (17.9) | 14 (16.7) | |
| Luminal B | 131 (17.2) | 45 (21.8) | 25 (19.7) | 10 (12.5) | 26 (16.8) | 8 (19.5) | 12 (17.9) | 5 (6.0) | |
| Normal-like | 78 (10.3) | 45 (21.8) | 16 (12.6) | 5 (6.2) | 3 (1.9) | 0 (0) | 8 (11.9) | 1 (1.2) | |
| Systemic therapy, n (%) | TH | 115 (15.0) | 103 (50.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 12 (17.9) | 0 (0) |
| AC–TDM1–P | 52 (6.8) | 0 (0) | 52 (40.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| AC–TH | 66 (8.6) | 0 (0) | 31 (24.4) | 0 (0) | 0 (0) | 0 (0) | 35 (52.2) | 0 (0) | |
| AC–THP | 61 (8.0) | 0 (0) | 44 (34.6) | 0 (0) | 0 (0) | 0 (0) | 17 (25.4) | 0 (0) | |
| HL | 84 (11.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 84 (100) | |
| TCH | 69 (9.0) | 0 (0) | 0 (0) | 0 (0) | 67 (43.2) | 0 (0) | 2 (3.0) | 0 (0) | |
| TCHP | 89 (11.6) | 0 (0) | 0 (0) | 0 (0) | 88 (56.8) | 0 (0) | 1 (1.5) | 0 (0) | |
| THL | 103 (13.5) | 103 (50.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| THP | 126 (16.5) | 0 (0) | 0 (0) | 80 (100) | 0 (0) | 46 (100) | 0 (0) | 0 (0) | |
| Cytotoxic therapy and HER2 blockade, n (%) | Single CT and trastuzumab | 115 (15.0) | 103 (50.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 12 (17.9) | 0 (0) |
| Single CT and dual blockade | 229 (29.9) | 103 (50.0) | 0 (0) | 80 (100) | 0 (0) | 46 (100) | 0 (0) | 0 (0) | |
| Multi-agent CT and trastuzumab | 135 (17.6) | 0 (0) | 31 (24.4) | 0 (0) | 67 (43.2) | 0 (0) | 37 (55.2) | 0 (0) | |
| Multi-agent CT and dual blockade | 202 (26.4) | 0 (0) | 96 (75.6) | 0 (0) | 88 (56.8) | 0 (0) | 18 (26.9) | 0 (0) | |
| Dual blockade alone | 84 (11.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 84 (100) | |
| HER2DX pCR score groups, n (%) | Low | 257 (33.6) | 72 (35.0) | 42 (33.1) | 31 (38.8) | 53 (34.2) | 12 (26.1) | 29 (43.3) | 18 (21.4) |
| Med | 246 (32.2) | 66 (32.0) | 42 (33.1) | 22 (27.5) | 54 (34.8) | 20 (43.5) | 17 (25.4) | 25 (29.8) | |
| High | 262 (34.2) | 68 (33.0) | 43 (33.9) | 27 (33.8) | 48 (31.0) | 14 (30.4) | 21 (31.3) | 41 (48.8) | |
TH: Paclitaxel and trastuzumab; AC-TDM1-P: Doxorubicin, cyclophosphamide, ado-trastuzumab emtansine and pertuzumab; AC-TH: Doxorubicin, cyclophosphamide, paclitaxel and trastuzumab; AC-THP: Doxorubicin, cyclophosphamide, paclitaxel, trastuzumab and pertuzumab; TCH: Docetaxel, carboplatin and trastuzumab; TCHP: Docetaxel, carboplatin, trastuzumab and pertuzumab; THL: Paclitaxel, trastuzumab and lapatinib; THP: Paclitaxel, trastuzumab and pertuzumab
HER2DX pCR score versus pCR
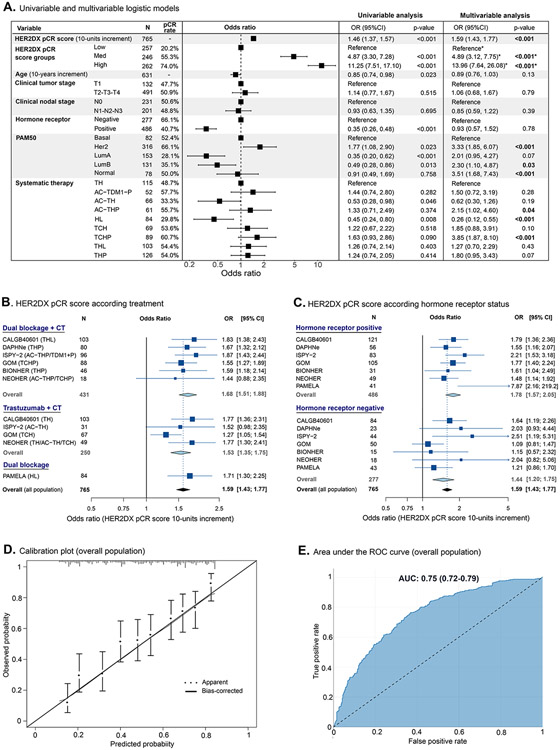
In the combined cohort, HER2DX pCR score (as a continuous variable from 0 to 100) was significantly associated with pCR (odds-ratio [OR] per 10-unit increase=1.59, 95% CI 1.43-1.77, p<0.001) after adjusting for treatment and clinicopathological factors (Fig. 1A). Similar results were obtained in the sensitivity analysis (Table S2). The ability of HER2DX pCR score to predict pCR was confirmed in patients treated with dual HER2 blockade and chemotherapy or trastuzumab and chemotherapy, and within HR-positive and HR-negative disease (Fig. 1B-C). Calibration plots comparing predicted and observed probabilities showed a correct calibration performance (Fig. 1D). The AUC for HER2DX pCR score was 0.75 (95% CI 0.72-0.79) (Fig. 1E, all populations), 0.78 (chemotherapy and dual HER2 blockade), 0.70 (chemotherapy and trastuzumab), 0.75 (HR-positive) and 0.70 (HR-negative) (Fig. S3). The area under the precision-recall curves was 0.73 for all populations (Fig. S4).
Figure 1. Association of HER2DX pCR score with pCR in the combined neoadjuvant cohort of 765 patients.
(A) Univariable and multivariable logistic models to predict pCR (n=765). (B) Pooled results in patients treated with chemotherapy and dual HER2 blockade (n=431), with chemotherapy and trastuzumab (n=250) or dual HER2 blockade alone (n=84). (C) Pooled results in patients with HR-positive (n=486) or HR-negative (n=277) disease. (D) Calibration plots for the pCR endpoint. X-axis shows average predicted probability values for each decile, and y-axis shows corresponding observed probability in each decile. Error bars represent 95% confidence intervals of mean predicted probabilities. The diagonal line represents the perfect calibration, the dotted curve represents the estimated calibration, and the solid curve the corrected estimation after correction for overfitting (bootstrap validation with resampling of 1000 interactions) (E) Area under the ROC curve with 95% confidence interval of HER2DX pCR score to predict pCR in all patients (n=765). OR: odds ratio; 95% CI: 95% confidence interval; pCR: pathological complete response; AUC: area under the ROC curve; TH: Paclitaxel and trastuzumab; AC-TDM1-P: Doxorubicin, cyclophosphamide, ado-trastuzumab emtansine and pertuzumab; AC-TH: Doxorubicin, cyclophosphamide, paclitaxel and trastuzumab; AC-THP: Doxorubicin, cyclophosphamide, paclitaxel, trastuzumab and pertuzumab; HL: Trastuzumab and lapatinib; TCH: Docetaxel, carboplatin and trastuzumab; TCHP: Docetaxel, carboplatin, trastuzumab and pertuzumab; THL: Paclitaxel, trastuzumab and lapatinib; THP: Paclitaxel, trastuzumab and pertuzumab. *A separate multivariable model has been performed using HER2DX pCR groups instead of HER2DX pCR score. To avoid multicollinearity, HER2DX pCR groups and HER2DX pCR score cannot be included in the same model.
To better stratify patients in clinical practice, the predefined cut-offs were used to classify patients in HER2DX pCR groups. The proportion of tumors in HER2DX low-, medium- and high-pCR groups was 33.6%, 32.2% and 34.2% in the overall population, 49.8%, 35.4%, 14.8% in the HR-positive population, and 5.1%, 26.4%, 68.6% in the HR-negative population, respectively. The pCR rates in HER2DX pCR-high, pCR-medium and pCR-low groups were 74.0%, 55.3% and 20.2% (high vs. low: OR=15.14, 95% CI 9.83-23.79, p<0.001).
HER2DX pCR score and dual HER2 blockade response
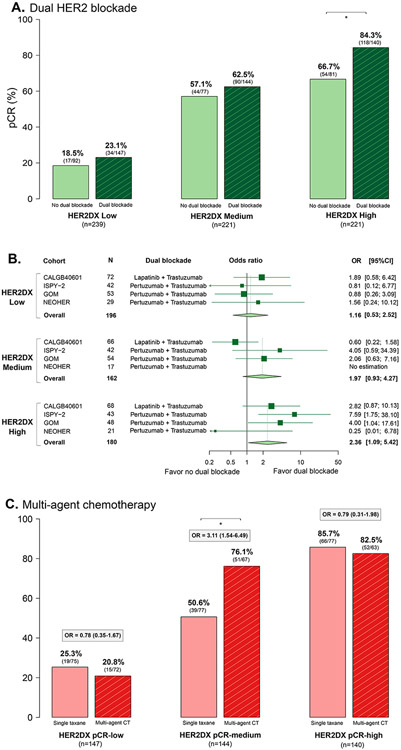
Among patients who received chemotherapy (n=681), 431 patients (63.3%) received dual HER2 blockade and 250 (36.7%) received trastuzumab alone. The overall pCR rate in patients treated with and without dual HER2 blockade was 56.1% and 46.0% (i.e., delta of 10.1%, OR=1.50, 95% CI 1.10-2.06, p=0.01). This difference in pCR rates is consistent with the known effect of adding lapatinib or pertuzumab to trastuzumab and chemotherapy in other randomized trials such as NeoSphere5, NSABP-B4124 and NeoALTTO25.
The pCR rates with and without dual HER2 blockade differed according to HER2DX pCR score (Fig. 2A). In patients with HER2DX pCR-high, -medium, and -low disease, the difference in pCR rates with dual blockade versus single anti-HER2 were 17.6%, 5.4% and 4.6% in favor of dual HER2 blockade, respectively. A significant increase in pCR rate due to dual HER2 blockade was only found in HER2DX pCR-high tumors (OR=2.36, 95% CI 1.09-5.42, p=0.03) but not in HER2DX pCR-medium or low tumors (Fig. 2B). However, the interaction tests after adjusting by cohort type did not reach statistically significance (HER2DX pCR-high versus others, p=0.130; HER2DX pCR-high versus pCR-low, p=0.070).
Figure 2. Association of HER2DX pCR groups with response to dual HER2 blockade and with response to multi-agent chemotherapy in the combined neoadjuvant cohort.
(A) Bar plots showing the pCR rates across the HER2DX pCR groups based on single versus dual HER2 blockade. (B) Forest plots evaluating the association of HER2DX pCR groups with pCR according to dual HER2 blockade administration in cohorts that compared dual blockade vs. single anti-HER2 (DAPHNe, BiOnHER and PAMELA cohorts were not included). (C) Bar plots showing the pCR rates across the HER2DX pCR groups based on single taxane versus multi-agent chemotherapy in the cohort of 367 patients treated with dual HER2 blockade. OR: odds ratio; 95% CI: 95% confidence interval; pCR: pathological complete response.
HER2DX pCR score and multi-agent chemotherapy response
Among the 431 patients receiving dual HER2 blockade and chemotherapy, 229 (53.1%) received a single taxane and 202 (46.9%) received multi-agent chemotherapy. The overall pCR rate in patients treated with dual HER2 blockade with and without multi-agent chemotherapy was 58.4% and 54.1% (i.e., delta of 4.3%, OR=1.19, 95% CI 0.81-1.74, p=0.37). The pCR rates with and without multi-agent chemotherapy differed according to HER2DX pCR score. In patients with HER2DX pCR-high, -medium, and -low disease, the difference in pCR rates (with multi-agent chemotherapy versus a single taxane) were −4.5%, 25.5% and −3.2%, respectively. A significant increase in pCR rate due to multi-agent chemotherapy was only found in HER2DX pCR-medium tumors (OR=3.11, 95% CI 1.54-6.49, p=0.002) but not in HER2DX pCR-high or low tumors (Fig. 2C). A statistically significant interaction was observed between HER2DX pCR-medium group and the other groups after adjusting by cohort type (p=0.001).
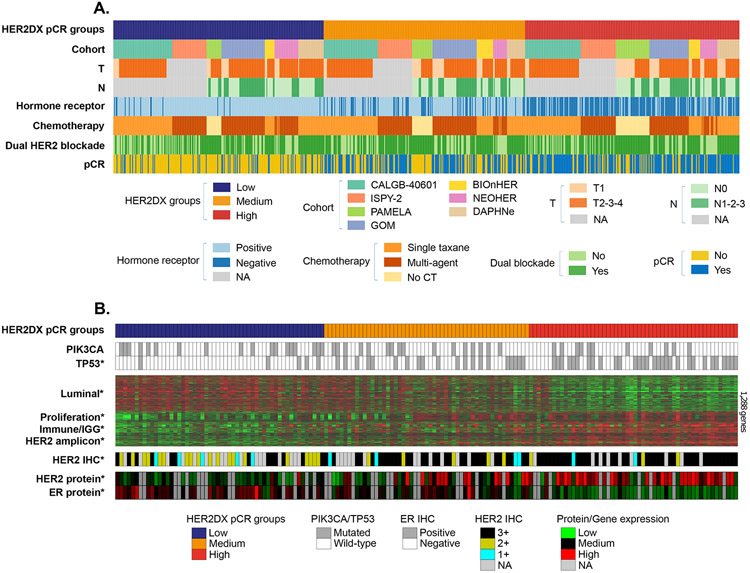
Overall, the value of HER2DX pCR groups to identify patients who benefit from multi-agent chemotherapy and dual HER2 blockade was independent of clinicopathological characteristics (Fig. 3A, Fig. 1A).
Figure 3: HER2DX pCR groups association with clinical-pathological variables and with genomic and proteomic data from The Cancer Genome Atlas (TCGA) breast cancer project.
(A) HER2DX pCR groups ranking and association with clinical-pathological variables, type of treatment and therapy response in the combined cohort (n=765). Each column represents the information for a patient. (B) HER2DX pCR score was evaluated in 161 HER2+ tumor samples from TCGA breast cancer dataset using the cbioportal21 data portal. Tumor samples were rank ordered based on their HER2DX pCR score (from low [left] to high [right]). Below the tumor samples with HER2DX pCR score data, DNA somatic mutation status in TP53 and PIK3CA, gene expression patterns of 1,283 genes, and the expression of HER2 and ER proteins (from reverse-phase protein array data) are shown. The heatmap reveals the expression patterns of the top 1,283 genes whose expression was found differentially expressed across the HER2DX pCR groups (false discovery rate<1%). T: Clinical tumor stage; N: Clinical nodal stage, pCR: pathological complete response. ICH: Immunohistochemistry, IGG: Immunoglobulin G signature, ER: Estrogen receptor.
Biology associated with HER2DX pCR score
To explore the biological differences among HER2DX pCR groups, we interrogated the HER2DX test as well as genomic and proteomic data from 161 HER2+ tumors of the TCGA breast cancer dataset21,22 (Fig. 3B). At the DNA level, TP53 somatic mutations were found in 56.0%, 38.0% and 9.3% of HER2DX pCR-high, -medium and -low tumors (p<0.001). No statistically significant differences across the HER2DX pCR groups were observed regarding PIK3CA mutations. At the RNA level, 3,033 of 12,369 (24.5%) genes were found differentially expressed across the HER2DX pCR groups (false discovery rate <1%). The significant genes generally tracked the 4 biological processes identified by the HER2DX assay (i.e., luminal differentiation, proliferation, HER2 amplicon and immune). As expected, HER2DX pCR-high tumors showed the highest expression of the HER2 amplicon-related genes, immune genes, and proliferation-related genes, and the lowest expression of luminal genes. Finally, we evaluated the protein expression of HER2 and estrogen receptor by reverse-phase protein arrays across the HER2DX pCR groups. Concordant with the gene expression results, HER2DX pCR-high tumors showed the highest and lowest expression of HER2 and ER, respectively (Fig. 3B).
HER2DX risk score beyond pCR status
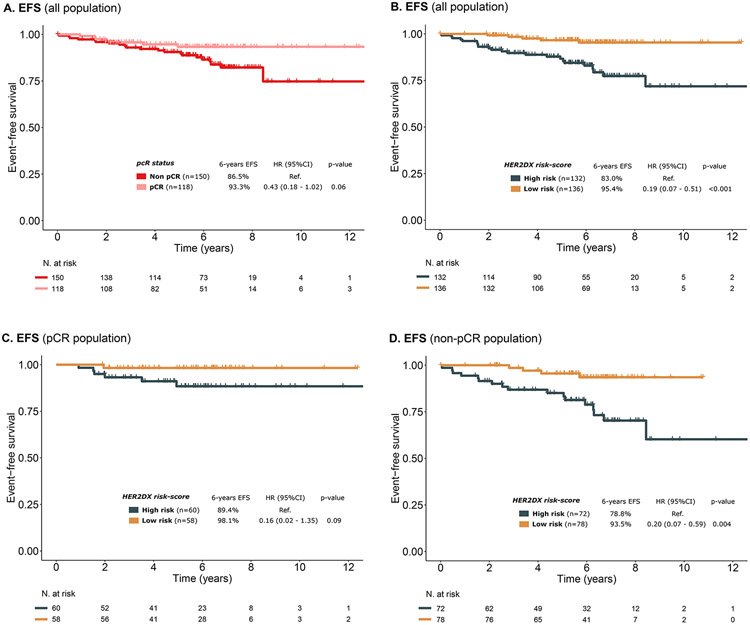
To evaluate the ability of HER2DX risk score to identify patients with lower risk of recurrence irrespective of pCR status, survival outcomes were evaluated in 268 patients treated with (neo)adjuvant trastuzumab-based therapy with long-term follow-up (NEOHER and PAMELA cohorts, median follow-up of 6.2 years). In this cohort, pCR status showed a tendency for association with better EFS (HR=0.43, 95% CI 0.18-1.02, p=0.06) (Fig. 4). The HR estimate of 0.43 in our study is consistent with previous studies9,10. Of note, only 14 (9.3%) patients with residual disease at surgery received adjuvant T-DM1.
Figure 4. Event-free survival (EFS) by pCR status and HER2DX risk group in the NEOHER and PAMELA combined cohorts (n=268).
(A) EFS in the overall population by pCR status (n=268), (B) EFS in the overall population by HER2DX risk group (n=268), (C) EFS in the pCR population by HER2DX risk group (n=118), (D) EFS in the non-pCR population by HER2DX risk group (n=150). EFS: event-free survival; HR: hazard ratio; 95% CI: 95% confidence interval.
To evaluate the clinical utility of the HER2DX risk score, the predefined risk cut-off was used to classify patients in HER2DX low-risk vs high-risk. Among patients who achieved a pCR (n=118), the 6-years EFS for patients with HER2DX low- and high-risk disease was 98.1% and 89.4%, respectively. Among patients who did not achieve a pCR (n=150), EFS outcomes were also better for HER2DX low-risk patients compared to HER2DX high-risk patients (EFS at 6 years of 93.5% vs 78.8%) (Fig. 4). In the multivariable analysis, HER2DX risk group was statistically associated with EFS (low vs high risk, HR= 0.19, 95% CI 0.07-0.49, p<0.001) and OS (low vs high risk, HR= 0.13, 95% CI 0.03-0.56, p=0.006) after adjusting by pCR (Table S3; Fig. S5).
Discussion
We present the largest study to date of the HER2DX as a predictor of pCR following neoadjuvant trastuzumab-based chemotherapy. Specifically, we confirm that the HER2DX pCR score is significantly associated with pCR independent of the type of chemotherapy and anti-HER2 therapy, and HR status. Importantly, we confirm that pCR rates of 80-90% can be achieved in patients with HER2DX pCR-high disease following a single taxane and dual HER2 blockade. In addition, multi-agent chemotherapy does not seem to increase the pCR rate in HER2DX pCR-low or high tumors but does in pCR-medium tumors.
The underlying biological explanation of our observations is that HER2DX pCR-high tumors are the most HER2 addicted, the most proliferative, the most immune infiltrated and the ones with the lowest expression of luminal features. These biological features have been previously linked to response to HER2-directed therapy and chemotherapy sensitivity, even within HER2+/HR-positive disease26-29. On the other extreme, the pCR rates in HER2DX pCR-low disease are ≤30% whether dual HER2 blockade and/or multi-agent chemotherapy are administered. The underlying biological explanation is that this group of tumors is the least HER2 addicted, the least proliferative and the least immune infiltrated, while it has the highest expression of luminal features16. These biological features are linked to resistance to anti-HER2 therapy and chemotherapy but linked to sensitivity to endocrine therapy26-29. Finally, the HER2DX pCR-medium group has an intermediate biological state, and multi-agent chemotherapy is particularly active in this group of tumors and increases the pCR rate over a single taxane. Of note, each HER2DX pCR group represents approximately one third of patients with early-stage HER2+ breast cancer.
Upfront identification of patients with early-stage HER2+ breast cancer who benefit the most from neoadjuvant dual HER2 blockade is needed. Despite FDA and EMA approval of (neo)adjuvant pertuzumab in clinically high-risk HER2+ breast cancer, the absolute increase in pCR rates in unselected patients with stage II-III disease is ≤20%5. Similar results are observed in randomized trials with chemotherapy and trastuzumab with or without lapatinib3,24,25. In addition, the absolute increase in invasive disease-free survival when 1-year of adjuvant pertuzumab or lapatinib is added to trastuzumab-based chemotherapy is small6,8,30, except for pertuzumab in node-positive disease in the APHINITY trial (delta of 4.9% at 8-years)6,30. Moreover, no overall survival benefit has been observed in any subgroup in APHINITY30.
In the context of early-stage HER2+ breast cancer, an important clinical consideration is determining which patients may be eligible for neoadjuvant therapy and can safely transition from multi-agent chemotherapy to single-agent taxane-based therapy. Neoadjuvant THP is currently not recommended by clinical guidelines, and two ongoing phase II clinical trials are evaluating this approach. The CompassHER2-pCR study (NCT04266249) led by ECOG-ACRIN will treat 2,156 patients with stage II-III HER2+ breast cancer (both HR-positive and HR-negative) with 3 months of a single taxane with trastuzumab and pertuzumab. If pCR is achieved, patients do not receive additional chemotherapy and continue with HP to complete 1 year. The Decrescendo trial (NCT04675827) led by BIG will treat 1,065 patients with stage II-III HER2+/HR-negative breast cancer with 3 months of a single taxane, trastuzumab and pertuzumab. If a pCR is achieved, patients will not receive additional chemotherapy and will continue with HP to complete 1 year. The primary endpoint of both trials is 3-year recurrence-free survival in patients who achieve a pCR. In this context, HER2DX pCR score would allow an upfront identification of patients most likely to benefit from this treatment approach (i.e., those with HER2DX pCR-high disease), and could help avoid the need for treatment escalation post-operatively by identifying those patients that may need more than just a single taxane in the preoperative setting.
The de-escalation of systemic therapy may require additional prognostic information beyond pCR status. Our study revealed that the HER2DX risk-score provides independent prognostic information that goes beyond pCR status. Consequently, HER2DX risk-score may assist in identifying patients with low-risk disease, irrespective of their pCR status or whether they receive adjuvant T-DM1 therapy. These findings are consistent with a recent combined patient-level analysis of five neoadjuvant trials31, which found that baseline tumor size and nodal status were associated with survival outcomes in patients with a pCR across subtypes, including HER2+ breast cancer. Additionally, the current results are consistent with our previous findings from the CALGB-40601 phase III trial, in which the HER2DX risk-score was assessed in-silico using genomic signatures only, without considering tumor size or nodal status. In that study, the HER2DX risk-score was significantly associated with EFS and OS, independent of pCR status16.
Regarding de-escalation of trastuzumab, several non-inferiority randomized studies with over 10,000 patients32-36 have shown a small absolute reduction in risk of recurrence and a small absolute increase in risk of cardiac toxicity with 12 months of therapy compared with shorter durations (e.g., 6 months). Although decreasing the duration of adjuvant trastuzumab has not been endorsed by clinical guidelines, HER2DX could help identify patients with very low risk of recurrence and low probability of pCR, who would be ideal candidates for a shorter duration of anti-HER2 therapy. In METABRIC16 and SCAN-B37 datasets, HER2DX risk-score has shown prognostic value beyond the use of trastuzumab. Thus, further studies could also determine the value of HER2DX to identify patients who might be cured with locoregional therapy without the need of any systemic therapy, including trastuzumab.
Our study has several limitations worth noting. First, the retrospective nature of this study. Second, the lack of a study which randomized patients to a single taxane versus multi-agent chemotherapy. Third, the lack of long-term survival outcome for most of the cohorts. Fourth, the fact that HER2DX was evaluated in-silico in the ISPY-2 and CALGB-40601 cohorts.
To conclude, HER2DX test results are associated with likelihood of pCR following neoadjuvant trastuzumab-based chemotherapy and might help identify patients with stage II-III disease who are candidates for neoadjuvant HP in combination with a single taxane over multi-agent chemotherapy. Independent external validation of HER2DX in CompassHER2-pCR trial is planned.
Supplementary Material
Acknowledgements:
We would like to thank the patients and family members who participated in all the cohorts analyzed in this study.
Funding/support
This study was funded by Hospital General Universitario Gregorio Marañón (Madrid, Spain) and Reveal Genomics (Barcelona, Spain). M.M. received funding by CIBER and Center of Biomedical Research on Cancer (CIBERONC, group CB16/12/00471). F. B-M received funding from Fundación Científica Asociación Española Contra el Cáncer (Ayudas Investigador AECC 2021- INVES21943BRAS). EAM acknowledges support as the Rob and Karen Hale Distinguished Chair in Surgical Oncology. OMS is a SEOM Visiting Fellow 2022.
Footnotes
Conflicts of Interest Disclosure
G.V has received a speaker’s fee from MSD, Pfizer, GSK and Pierre Fabrer, has held an advisory role with AstraZeneca and received consultant fees from Reveal Genomics. C. B-M received honoraria as speakers’ honoraria from Roche, Novartis, Lilly, M.S.D and AstraZeneca and G.S.K and Travel Grant from Roche, Novartis and Pfizer. I.E. reports Speaker fees from Roche, Teva, Novartis, Pfizer, Lily and advisory role from Lilly and Astrazeneca. S.L-T reports consulting or advisory role fees from AstraZeneca, Novartis, Roche, Pfizer, Pierre Fabre, Lilly, Seagen, Daichii-Sankyo Europe GmbH, Gilead Sciences, MDS, GlaxoSmithKline, Veracyte and speakers Bureau fees from Lilly. T.M. reports consulting or advisory boards fees from AstraZeneca, Novartis, Roche, GSK and travel grants from Novartis and Astra Zeneca. Y.J. reports consultant or advisory role fees from Novartis, Pfizer, Roche, and AstraZeneca, she received speaker honoraria from Roche, Novartis, Lilly and AstraZeneca, travel and trainnig grants from Roche, Novartis, Pfizer, and Lilly. JA.G-S reports consulting and advisory services fees for Seagen, Gilead, Sanofi. Consultancy/speaker fees from Novartis, Celgene, Eli Lilly, EISAI, AstraZeneca, Daiichi Sankyo, MSD, Pierre Fabre. Institution research funding from AstraZeneca and travel support from Novartis, Roche, Pfizer. F.M. reports consulting or advisory role fees from Roche/Genentech, Novartis, Pfizer, AstraZeneca, MSD, Daiichi Sankyo/Astrazeneca and speakers' bureau fees from Pfizer. Travel, Accommodations, Expenses from Roche/Genentech, Daiichi Sankyo/Astra Zeneca. A.R-L reports consultant Fees from Roche, AstraZeneca, Pfizer, Seagen, Novartis, Lilly, AstraZeneca and Pierre-Fabré and Speaker Bureau fees from Daiichi-Sankyo, Msd and Gilead. AI.B. reports consulting or advisory fees from Pfizer, Novartis, Seagen, Roche, Pierre Fabre. Travel and accommodations fees from Pfizer. A.P. reports advisory and consulting fees from Roche, Pfizer, Novartis, Amgen, BMS, Puma, Oncolytics Biotech, MSD, Guardant Health, Peptomyc and Lilly, lecture fees from Roche, Pfizer, Novartis, Amgen, BMS, Nanostring Technologies and Daiichi Sankyo, institutional financial interests from Boehringer, Novartis, Roche, Nanostring, Sysmex Europa GmbH, Medica Scientia Innovation Research, SL, Celgene, Astellas and Pfizer; stockholder and consultant of Reveal Genomics, SL; A.P. is also listed as an inventor on patent applications for the HER2DX assay. C.M.P is an equity stockholder and consultant of BioClassifier LLC, and for Reveal Genomics. C.M.P is also listed as an inventor on patent applications for the Breast PAM50 assay. J.S.P is an equity stockholder and consultant for Reveal Genomics and is also listed as an inventor on patent applications for the Breast PAM50 assay. F.B-M. has a patent application EP21383165. L.P is listed as an inventor on patent PCT/EP2021/070788. F.S. has no competing financial and non-financial interests. EAM reports compensated service on scientific advisory boards for AstraZeneca, Exact sciences (formerly Genomic Health), Merck, Roche/Genentech; uncompensated service on steering committees for Bristol Myers Squibb, Lilly, and Roche/Genentech; and institutional research support from Roche/Genentech (via SU2C grant) and Gilead. She receives research funding from Susan Komen for the Cure for which she serves as a Scientific Advisor. She also reports uncompensated participation as a member of the American Society of Clinical Oncology Board of Directors. SMT has served as a consultant for: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, Odonate Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Gilead, Mersana, Certara, Chugai Pharma, Ellipses Pharma, Infinity, 4D Pharma, OncoSec Medical Inc., BeyondSpring Pharmaceuticals, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics; SMT has received institutional research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, NanoString Technologies, Cyclacel, Nektar, Gilead, Odonate Therapeutics, Sanofi, Seattle Genetics. M.M. reports Research grants from PUMA; consulting/advisory fees from Roche, Novartis, AstraZeneca, Daiichi-Sankyo, Seagen, Lilly, Sanofi; speakers’ honoraria from Seagen, Lilly, AstraZeneca, Pfizer, Daiichi-Sankyo, Roche. Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: Chairman, GEICAM Member of Board of Directors, TRIO. The rest of authors declare no conflict of interest. AGW receives institutional research funding from Genentech/Roche, Macrogenics, and Merck.
Data sharing statement
Deidentified participant data and trial protocol will be made available upon a reasonable request to the corresponding author. Proposals for any purpose will be considered.
References
- 1.Korde LA, Somerfield MR, Carey LA, et al. : Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. Journal of Clinical Oncology 39:1485–1505, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso F, Kyriakides S, Ohno S, et al. : Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology 30:1194–1220, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Carey LA, Berry DA, Cirrincione CT, et al. : Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib. Journal of Clinical Oncology 34:542–549, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gianni L, Eiermann W, Semiglazov V, et al. : Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. The Lancet 375:377–384, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Gianni L, Pienkowski T, Im Y-H, et al. : Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. The Lancet Oncology 13:25–32, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Piccart M, Procter M, Fumagalli D, et al. : Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years' Follow-Up. Journal of Clinical Oncology 39:1448–1457, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Martinez A, Krop IE, Hillman DW, et al. : Survival, Pathologic Response, and Genomics in CALGB 40601 (Alliance), a Neoadjuvant Phase III Trial of Paclitaxel-Trastuzumab With or Without Lapatinib in HER2-Positive Breast Cancer. Journal of Clinical Oncology 38:4184–4193, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccart-Gebhart M, Holmes E, Baselga J, et al. : Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. Journal of Clinical Oncology 34:1034–1042, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortazar P, Zhang L, Untch M, et al. : Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 384:164–172, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Squifflet P, Saad ED, Loibl S, et al. : Re-Evaluation of Pathologic Complete Response as a Surrogate for Event-Free and Overall Survival in Human Epidermal Growth Factor Receptor 2–Positive, Early Breast Cancer Treated With Neoadjuvant Therapy Including Anti–Human Epidermal Growth Factor Receptor 2 Therapy. Journal of Clinical Oncology 0:JCO.22.02363. [DOI] [PubMed] [Google Scholar]
- 11.Nitz U, Gluz O, Graeser M, et al. : De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR−): survival outcomes from a multicentre, open-label, randomised, phase 2 trial. The Lancet Oncology 23:625–635, 2022 [DOI] [PubMed] [Google Scholar]
- 12.Hurvitz SA, Martin M, Jung KH, et al. : Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. Journal of Clinical Oncology 37:2206–2216, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbeck N, Nitz U, Christgen M, et al. : LBA14 De-escalated neoadjuvant T-DM1 with or without endocrine therapy (ET) vs trastuzumab+ET in early HR+/HER2+ breast cancer (BC): ADAPT-TP survival results. Annals of Oncology 31:S1146, 2020 [Google Scholar]
- 14.von Minckwitz G, Huang C-S, Mano MS, et al. : Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. New England Journal of Medicine 380:617–628, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Waks AG, Desai NV, Li T, et al. : A prospective trial of treatment de-escalation following neoadjuvant paclitaxel/trastuzumab/pertuzumab in HER2-positive breast cancer. npj Breast Cancer 8:63, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prat A, Guarneri V, Pascual T, et al. : Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. eBioMedicine 75, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark AS, Yau C, Wolf DM, et al. : Neoadjuvant T-DM1/pertuzumab and paclitaxel/trastuzumab/pertuzumab for HER2+ breast cancer in the adaptively randomized I-SPY2 trial. Nature Communications 12:6428, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waks AG, Ogayo ER, Paré L, et al. : Independent validation of the HER2DX assay in HER2-positive breast cancer patients treated with neoadjuvant paclitaxel, trastuzumab and pertuzumab on the DAPHNe trial. submitted, JAMA Oncol 2023. In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bueno-Muiño C, Echavarría I, López-Tarruella S, et al. : HER2DX in HER2-positive breast cancer following neoadjuvant chemotherapy and trastuzumab with or without pertuzumab. SABCS, JAMA Oncol 2023. In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llombart-Cussac A, Cortés J, Paré L, et al. : HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. The Lancet Oncology 18:545–554, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Cerami E, Gao J, Dogrusoz U, et al. : The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discovery 2:401–404, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koboldt DC, Fulton RS, McLellan MD, et al. : Comprehensive molecular portraits of human breast tumours. Nature 490:61–70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SH, Han K: Methodologic Guide for Evaluating Clinical Performance and Effect of Artificial Intelligence Technology for Medical Diagnosis and Prediction. Radiology 286:800–809, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Robidoux A, Tang G, Rastogi P, et al. : Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. The Lancet Oncology 14:1183–1192, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Bradbury I, Eidtmann H, et al. : Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. The Lancet 379:633–640, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual T, Fernandez-Martinez A, Tanioka M, et al. : Independent Validation of the PAM50-Based Chemo-Endocrine Score (CES) in Hormone Receptor–Positive HER2-Positive Breast Cancer Treated with Neoadjuvant Anti–HER2-Based Therapy. Clinical Cancer Research 27:3116–3125, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianchini G, Kiermaier A, Bianchi GV, et al. : Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Research 19:16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianchini G, Pusztai L, Pienkowski T, et al. : Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Annals of Oncology 26:2429–2436, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Schettini F, Pascual T, Conte B, et al. : HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: A systematic review and meta-analysis. Cancer Treatment Reviews 84:101965, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loibl S, Jassem J, Sonnenblick A, et al. : VP6-2022: Adjuvant pertuzumab and trastuzumab in patients with early HER-2 positive breast cancer in APHINITY: 8.4 years' follow-up. Annals of Oncology, 2022 [Google Scholar]
- 31.Huober J, van Mackelenbergh M, Schneeweiss A, et al. : Identifying breast cancer patients at risk of relapse despite pathological complete response after neoadjuvant therapy. npj Breast Cancer 9:23, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pivot X, Romieu G, Debled M, et al. : 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. The Lancet 393:2591–2598, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Joensuu H, Fraser J, Wildiers H, et al. : Effect of Adjuvant Trastuzumab for a Duration of 9 Weeks vs 1 Year With Concomitant Chemotherapy for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: The SOLD Randomized Clinical Trial. JAMA Oncology 4:1199–1206, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conte P, Bisagni G, Frassoldati A, et al. : Final analysis of the phase III multicentric Italian study Short-HER: 9 weeks vs 1 year adjuvant trastuzumab for HER2+ early breast cancer. Annals of Oncology 29:2328–2333, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Earl HM, Hiller L, Vallier A-L, et al. : 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. The Lancet 393:2599–2612, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mavroudis D, Saloustros E, Malamos N, et al. : Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Annals of Oncology 26:1333–1340, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Pascual T, Villacampa G, Brasó-Maristany F, et al. : HER2DX risk-score in early-stage HER2-positive (HER2+) breast cancer: a correlative analysis of 757 patients from the Sweden Cancerome Analysis Network - Breast (SCAN-B) dataset. Annals of Oncology (2023) 8 (1suppl_4): 101219–101219. 10.1016/esmoop/esmoop101219.2023 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data and trial protocol will be made available upon a reasonable request to the corresponding author. Proposals for any purpose will be considered.