Abstract
Context
Hip fracture is a serious injury that can lead to increased morbidity and mortality. Vitamin D binding protein (DBP) is a potential prognostic indicator of outcomes since it is important for actin scavenging and inflammation after tissue injury.
Objective
To determine whether circulating DBP is associated with mobility or mortality after hip fracture and its association with acute tissue injury markers.
Methods
Post hoc analysis of a multisite North American prospective study of 260 patients with hip fracture; mobility assessed at 30 and 60 days and mortality at 60 days after repair surgery. Biochemical markers were measured before, and 2 to 4 days after surgery. Tissue injury markers were measured in 100 randomly selected patients and controls. The primary outcome was mobility and mortality by DBP tertiles. Secondary outcomes were assessment of pre- and postoperative biomarkers.
Results
Among all patients (81 ± 9 years, BMI 25 ± 4 kg/m2; 72% female), the highest DBP tertile had greater mobility at 30 (OR: 2.66; 95% CI: 1.43, 4.92; P = .002) and 60 days (OR: 2.31; 95% CI: 1.17, 4.54; P = .014) and reduced mortality (OR: 0.18; 95% CI: 0.04, 0.86; P = .032) compared with the lowest DBP tertile (<28.0 mg/dL). Total 25-hydroxyvitamin-D did not differ between tertiles (22.0 ± 9.5 ng/mL). Circulating DBP and gelsolin were lower and interleukin-6, C-reactive protein, and F-actin were higher (P < .01) in patients vs controls, and worsened (P < .01) after surgery.
Conclusion
High circulating DBP concentrations are associated with better mobility and reduced mortality after hip fracture surgery. The role of DBP as an acute phase reactant to tissue injury and clinical outcomes should be addressed in future study.
Keywords: hip fracture, 25-hydroxyvitamin D, vitamin D binding protein, cytokines, mobility, mortality
Hip fracture is one of the most serious injuries which can lead to severe consequences, including morbidity and mortality. Older adults (65 years or older) account for the majority of hip fracture cases, and those who are unable to recover from fracture will suffer from a lower quality of life and increased risk of death. The ability to walk, unassisted or independently, is a major recovery goal after hip fracture surgery yet the patient recovery from hip fracture varies substantially. It is known that deficiency of total 25-hydroxyvitamin D (25OHD) (<12 ng/mL) is associated with the risk of hip fracture (1) and reduced mobility, but not necessarily mortality after hip fracture (2-5). Also, vitamin D supplementation alone does not consistently protect against falls and fractures (6-8). Thus, factors contributing to poor outcomes in patients after hip fracture remain a concern in the field.
One factor that may affect postoperative outcomes is the circulating Gc-globulin, also known as vitamin D binding protein (DBP). DBP is the major carrier protein of vitamin D, with an affinity for vitamin D metabolites that is approximately 1000 times greater than albumin, and it accounts for over 90% of circulating vitamin D. Less than 1% of vitamin D freely circulates in blood and for most tissues, only free vitamin D metabolites enters the cell to exert its biological effects. DBP is not only important for maintaining and distributing serum vitamin D metabolites (9-11), but also is responsible for clearing actin released from dead cells, known as the actin scavenging system that repairs tissue (12). In addition, DBP plays a major role in inflammation and immune cell modulation, and may influence circulating levels of cytokines, such as interleukin-6 (IL-6) and C-reactive protein (CRP). It has been found that a higher DBP concentration is associated with reduced organ dysfunction and a better survival rate in various clinical conditions (12-15), yet this has not been studied in patients with hip fracture. In this study, hip fracture is used as a prospective model to study DBP pre- and postsurgical responses and explore its role in acute tissue injury. The primary goal in this study is to determine whether DBP is a predictor of postsurgical outcomes in patients with hip fracture. In addition, this study includes an exploratory examination of the relationship between DBP and inflammatory cytokines and other mediators of acute tissue injury in patients with hip fracture.
Methods
Study Design and Participants
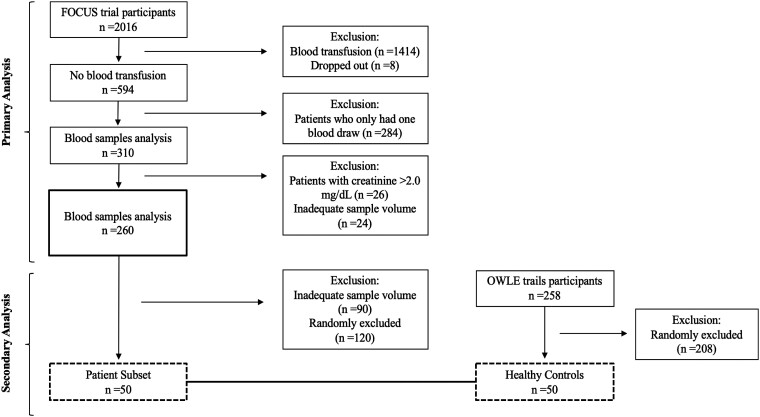
This study is a post hoc analysis of a subset of subjects enrolled in the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) Trial designed as a multisite prospective study to evaluate red blood cell transfusion threshold on mobility and mortality in patients who underwent hip fracture repair (ClinicalTrials.gov; NCT00071032) (16). The participants in FOCUS trial are patients who had cardiovascular disease (CVD; 63%) or cardiovascular risk factors (a history of or treatment for hypertension, diabetes, or hypercholesterolemia; high levels of cholesterol or LDL-cholesterol; cigarette smoking or tobacco use; or high creatinine). All races were included and self-reported. In this study, inclusion criteria were limited to those patients who did not receive blood transfusion, had a pre- and postoperative blood draw, had creatinine level ≤2.0 mg/dL, and with adequate sample volume. After these exclusions, a total of 260 patients were eligible for this study (Fig. 1).
Figure 1.
Flow diagram of patients for the primary (n = 260) and secondary outcomes (n = 50 patients and 50 healthy controls). Abbreviations: FOCUS, Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair; OWLE, Osteoporosis, Weight Loss, and Endocrine database.
The mobility status of patients was assessed and documented at 30 and 60 days after surgery. Patients were defined as immobile if they were unable to walk 10 feet or across the room unassisted or independently. The mortality status at 30 and 60 days was determined by telephone call and confirmed according to the original protocol (16), and the 60-day mortality rate was analyzed in this study. Written informed consent was obtained from patients or their designated representatives for the FOCUS trial. In the current study, all patient information was deidentified and we analyzed stored samples from a repository. The study protocol was approved by the institutional review board at Rutgers University.
In those patients with adequate remaining sample volume, a subset of patients were randomly chosen (n = 50) to assess pre- and postoperative cytokines and markers (IL-6, CRP, tumor necrosis factor alpha [TNF-alpha], F-actin, and gelsolin). We also included a control group of 50 healthy individuals (Fig. 1), who were randomly selected from the Osteoporosis, Weight Loss, and Endocrine database (OWLE) and banked repository of baseline samples in our laboratory (ClinicalTrials.gov; NCT01631292, NCT00473031, NCT00472745).
Biochemical Analysis
Blood samples were collected before and 2 to 4 days after surgery and stored at −80 °C. Samples from the FOCUS trial were banked at the National Institutes of Health (NIH) repository and transferred to Rutgers University for analysis. The samples were generally drawn in the morning hours in a fasting state to avoid the influence of diurnal rhythms on biomarkers (17-19). Circulating DBP was measured by polyclonal antibody enzyme-linked immunosorbent assay (ELISA) (Immundiagnostik AG, K2314, RRID: AB_2943031) that avoids errors associated with different DBP genotypes when using the monoclonal method (20). The intra-assay and inter-assay coefficients of variation (CVs) are less than 5% and 10% for DBP, respectively. Serum 25OHD was measured by ELISA (Eagle Biosciences, VID31-K01, RRID: AB_2943034) and reference materials from the National Institute of Standards and Technology in each batch analysis; other external standards for total 25OHD were applied to improve the quality of measurements, as described previously (4). Serum intact parathyroid hormone (iPTH) and free 25OHD were measured by ELISA (ALPCO, 21-IPTHU-E01, RRID: AB_2943035; and DIAsource, KAPF1991, RRID: AB_2890998; respectively). The intra-assay and inter-assay CVs are less than 6.9% and 8.6% for total 25OHD; 5.5% and 6.3% for free 25OHD, and 3.4% and 7.7% for iPTH, respectively. Serum concentration of bioavailable 25OHD were calculated by using binding-affinity constant from Bikle et al (21) and equations adapted from Vermeulen et al (22). Circulating inflammatory cytokines, IL-6, CRP, and TNF-alpha were measured by ELISA (R&D Systems: D6050, RRID: AB_2928038; DCRP00, RRID: AB_2893119; and DTA00D, RRID: AB_2941365; respectively), and F-actin and gelsolin were also measured by ELISA (MyBioSource, MBS702018, RRID: AB_2943033; and LSBio, LS-F5675, RRID: AB_2943032; respectively) in a subset (n = 50). The intra-assay and inter-assay CVs are less than: 4.3% and 6.4% for IL-6; 4.4% and 6.0% for CRP; 3.0% and 8.4% for TNF-alpha; 4.1% and 6.2% for F-actin; and 10% and 12% for gelsolin, respectively. Due to limited sample volume, only one inflammatory cytokine (IL-6) was also measured in the entire population (n = 260). Samples from patients with hip fracture and control subjects were measured in the same analytic run to reduce inter-assay variability.
Circulating DBP concentrations were categorized into 3 groups by tertile: first tertile, <28.0 mg/dL (the lowest group); second tertile, 28.0 to 33.7 mg/dL (the middle group); and third tertile, >33.7 mg/dL (the highest group). Tertiles were also applied to other biomarkers, including total and free 25OHD, iPTH, albumin, and IL-6. The lowest tertile was used as a reference group.
Statistical Analyses
To examine demographic characteristics before and after surgery, differences within groups were compared by paired t test. For subset analysis, patients were compared to a group of healthy controls, and continuous variables were compared by ANCOVA adjusted for age and body mass index (BMI). Categorical variables were compared by chi-square test. Pearson correlations were used to assess associations among circulating DBP, iPTH, vitamin D metabolites, and inflammatory cytokines. Logistic regression was used to determine association of DBP, total or free 25OHD, iPTH, albumin, or cytokine categories (by tertiles) with walking ability outcomes and mortality, and odds ratio (OR) and 95% CIs are reported. The logistic regression also performed in those patients without vitamin D deficiency (25OHD >12 ng/mL) to determine whether vitamin D deficiency is driving the association between biomarkers and outcomes. Multiple linear stepwise regression was used to evaluate the contribution of different independent variables (age, BMI, and the change of total 25OHD, albumin, iPTH, and IL-6) to variance explained in the change of DBP concentration. The subset of patients and healthy controls were randomly selected by using SAS PROC SURVEYSELECT program, and a sensitivity analysis was performed for the subset to compare it to the larger patient cohort. Statistical analyses were performed using SAS (SAS Inc, Cary, NC). A P value <.05 was considered significant.
Results
A total of 260 patients with hip fracture (72% women, aged 81 ± 9 years) were included in this study (Table 1). Fractures were at the intertrochanteric (50%), subtrochanteric regions (6%), and femoral neck (46%). The mean 25OHD level was 22.0 ± 9.5 ng/mL and 47% (n = 123) of patients had low 25OHD concentration (<20 ng/mL), and 11% (n = 29) had 25OHD deficiency (<12 ng/mL). Circulating mean DBP concentrations in the lowest, middle, and highest tertile groups were 24.0 ± 3.4, 30.6 ± 1.7, and 40.1 ± 6.3 mg/dL, respectively. Total 25OHD concentrations did not differ significantly between DBP tertile groups and averaged 21.3 ± 9.2, 21.1 ± 8.7, and 23.8 ± 10.4 ng/mL, respectively. Circulating total, free, and bioavailable 25OHD, albumin, and DBP significantly decreased after surgery (P < .001; Table 1). In contrast to other variables, circulating iPTH and IL-6 increased postoperatively (P < .001; Table 1). Also, as expected, there was a positive correlation between total and free 25OHD (r = 0.65, P < .001; Supplementary Table S1 (23)), but neither total or free 25OHD was associated with DBP, or iPTH, or IL-6. Circulating DBP and albumin were both inversely associated with IL-6 (r = −0.20, P = .002, and r = −0.23, P < .001, respectively; Supplementary Table S1 (23)).
Table 1.
Characteristics of patients at baseline and after surgery (n = 260)a
| Pre-op | Post-op | P valueb | |
|---|---|---|---|
| Age, years | 81 ± 9 (55-102) | ||
| Sex, female, % | 72% | ||
| Race, % | |||
| White | 92.7% | ||
| Black | 5% | ||
| Asian | 0.8% | ||
| Other | 1.5% | ||
| BMI, kg/m2 | 24.6 ± 4.4 | ||
| Albumin, g/dL | 3.7 ± 0.7 | 2.8 ± 0.6 | <.001 |
| DBP, mg/dL | 31.5 ± 7.9 | 27.9 ± 6.2 | <.001 |
| Total 25OHD, ng/mL | 22.0 ± 9.5 | 18.2 ± 7.7 | <.001 |
| Free 25OHD, pg/mL | 5.5 ± 2.1 | 5.1 ± 2.0 | <.001 |
| Bioavailable 25OHD, ng/mL | 1.7 ± 0.8 | 1.2 ± 0.6 | <.001 |
| iPTH, pg/mL | 50.2 ± 33.9 | 60.0 ± 33.7 | <.001 |
| IL-6, pg/mLc | 60.3 ± 66.1 | 157.8 ± 131.2 | <.001 |
Abbreviations: DBP, vitamin D binding protein; iPTH, intact parathyroid hormone; IL-6, interleukin-6; pre-op, preoperative; post-op, postoperative; 25OHD, 25-hydroxyvitamin D.
a Values are means ± SDs (range); n = 260.
b Paired t test for values pre- and 2 to 4 days postoperative.
c IL-6: normal values in healthy older adults (74 ± 4 years; median, interquartile range); 1.8 (1.3-2.8) pg/mL (50).
Clinical Outcomes
In 260 patients, 56% and 68% of the patients were mobile at 30 and 60 days, respectively and the 60-day mortality was 8%. In the preoperative patients without vitamin D deficiency (25OHD ≥12 ng/mL, n = 231), the frequency of mobility at the 30- and 60-day time points was 58% (n = 133) and 70% (n = 162), respectively, and there was 7% mortality (n = 16 deaths).
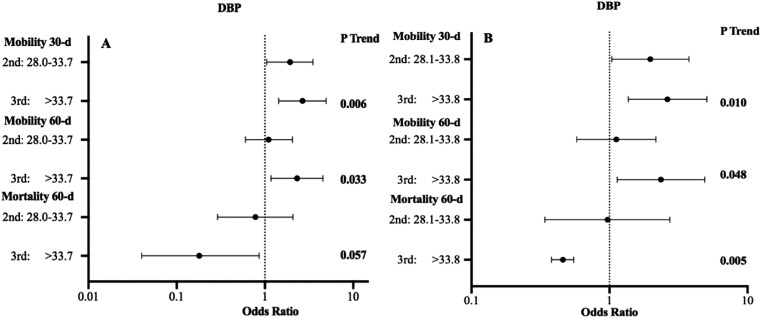
Higher circulating preoperative DBP levels were associated with improved mobility at both 30 and 60 days, and a lower rate of mortality at 60 days after hip fracture surgery. The lowest DBP tertile was used as reference group. Compared with the DBP <28.0 mg/dL, patients in the middle DBP tertile (OR: 1.91; 95% CI: 1.05, 3.50; P = .034) and the highest DBP tertile (OR: 2.66; 95% CI: 1.43, 4.92; P = .002) had greater mobility at 30 days (Fig. 2A). At 60 days, patients within the highest DBP tertile group had greater mobility (OR: 2.31; 95% CI: 1.17, 4.54; P = .014) and reduced mortality (OR: 0.18; 95% CI: 0.04, 0.86; P = .032) compared with the lowest group (Fig. 2A). In those patients without vitamin D deficiency (25OHD >12 ng/mL), it was still found that the highest DBP tertile were associated with better mobility at both 30 days (OR: 2.63; 95% CI: 1.37, 5.07; P = .004) and 60 days (OR: 2.36; 95% CI: 1.14, 4.90; P = .020), and reduced risk of death (OR: 0.46; 95% CI: 0.38, 0.55; P = .007) (Fig. 2B). There was no association between postoperative DBP categories with mobility at any time point or survival rate.
Figure 2.
Odds ratio of outcomes after hip fracture surgery assessed by preoperative DBP tertiles in (A) whole patient population (n = 260) and (B) patient without vitamin D deficiency (25OHD ≥12 ng/mL, n = 231) for mobility and mortality compared to the lowest tertile (reference group). P trends show the differences across all tertile groups.
In addition, there were no significant associations between total or free 25OHD with postoperative outcomes (Supplementary Fig. S1A and S1B (23)). Circulating IL-6 was also measured in the full population, and indicated that patients with higher values were less likely to walk at 60 days after surgery (P < .001), and there was a trend of lower survival rate (P = .063; Supplementary Fig. S1C (23)). Multiple regression analysis indicated that the change in IL-6, albumin, and 25OHD predicted the change in DBP concentration and explained 17% of its variation (Table 2).
Table 2.
Multiple linear stepwise regression model analyzing independent predictors of the change in DBP concentration (n = 260)a
| Variable | β coefficient | P value |
|---|---|---|
| Albumin | 0.21 | <.001 |
| IL-6 | −0.19 | <.001 |
| Total 25OHD | 0.18 | .004 |
| Model R2 = 0.17 |
Abbreviation: DBP, vitamin D binding protein; IL-6, interleukin-6; 25OHD, 25-hydroxyvitamin D.
a Model includes independent predictors (age, BMI, and the change in total 25OHD, albumin, iPTH, and IL-6). Only significant predictors are included in the table.
Subset Analysis
To determine whether DBP plays a role in the actin scavenging system and inflammation, 50 patients were compared to 50 controls (Table 3). Compared to the patients, the healthy controls had higher values of BMI, albumin, DBP, total and bioavailable 25OHD, and gelsolin, but lower cytokines and nearly undetectable F-actin (P < .05). The elevated preoperative concentrations of IL-6 and CRP after hip fracture increased further after surgery (P < .001; Table 3). Circulating IL-6 and CRP were both inversely associated with DBP (r = −0.34, P = .017, and r = −0.31, P = .035, respectively; Supplementary Table S2 (23)). Unlike controls, the majority of patients (90%) had detectable preoperative F-actin, and while it declined 2 to 4 days after surgery, it remained high compared to healthy controls (P < .001). In addition, the low circulating gelsolin levels further reduced postoperatively (P < .001; Table 3) and was positively associated with DBP (r = 0.35, P = .017). In contrast to DBP, vitamin D metabolites did not correlate with the cytokines or gelsolin (Supplementary Table S2 (23)).
Table 3.
Characteristics of subset of patients and healthy controls (n = 50/group)a
| Pre-opb | Post-opb | P valuec | Control (CTL) | Adjusted P value (pre-op vs CTL)d | |
|---|---|---|---|---|---|
| Age, years | 79 ± 8 (59-98) | 66 ± 3 (60-75) | <.001 | ||
| BMI, kg/m2 | 24.8 ± 4.9 | 27.7 ± 2.4 | <.001 | ||
| Albumin, g/dL | 3.6 ± 0.6 | 2.9 ± 0.5 | <.001 | 4.5 ± 0.3 | <.001 |
| DBP, mg/dL | 32.1 ± 7.0 | 29.3 ± 5.6 | .003 | 39.4 ± 5.1 | <.001 |
| Total 25OHD, ng/mL | 21.8 ± 10.7 | 18.8 ± 8.6 | <.001 | 29.7 ± 7.8 | <.001 |
| Free 25OHD, pg/mL | 5.5 ± 2.1 | 5.2 ± 2.2 | .011 | 5.9 ± 1.7 | .637 |
| Bioavailable 25OHD, ng/mL | 1.5 ± 0.9 | 1.2 ± 0.6 | <.001 | 2.0 ± 0.4 | .035 |
| iPTH, pg/mL | 52.3 ± 32.4 | 61.9 ± 39.5 | .043 | 38.9 ± 23.9 | .013 |
| IL-6, pg/mL | 58.7 ± 52.4 | 154.9 ± 136.4 | <.001 | 2.1 ± 2.0 | <.001 |
| CRP, mg/L | 11.5 ± 12.7 | 20.2 ± 8.3 | <.001 | 2.6 ± 2.3 | <.001 |
| TNF-alpha, pg/mL | 22.2 ± 35.5 | 25.7 ± 31.8 | .060 | 12.1 ± 13.4 | .631 |
| F-actin, ng/mL | 6.5 ± 4.8 | 4.9 ± 4.5 | <.001 | 0.7 ± 0.9 | <.001 |
| Gelsolin, mg/L | 119.3 ± 51.7 | 80.1 ± 41.1 | <.001 | 175.0 ± 40.3 | <.001 |
Values of DBP, free and total 25OHD, and albumin in the subset did not differ significantly (P > .10) compared to the larger patient population (n = 260; Table 1).
Abbreviations: CTL, control; CRP, C-reactive protein; DBP, vitamin D binding protein; iPTH, intact parathyroid hormone; IL-6, interleukin-6; pre-op, preoperative; post-op, postoperative; TNF-alpha, tumor necrosis factor alpha; 25OHD, 25-hydroxyvitamin D.
a Values are means ± SDs (range); n = 50.
b Blood samples were taken before hip fracture surgery (Pre-op) and collected again 2 to 4 days after surgery (Post-op).
c Paired t test for values pre- and postoperative.
d ANCOVA, analysis adjusted for age and BMI.
Discussion
The majority of hip fracture cases occur in older adults and are serious injuries that may result in long recovery periods or mortality. One of the main metrics of recovery after hip fracture surgery is the ability to walk independently. However, recovery and survival rates have not significantly improved over recent decades (24), highlighting the importance of understanding the risk factors that contribute to postoperative mobility and mortality. Previous prospective studies, including one from our lab, have shown that a deficient level of total 25OHD (<12 ng/mL) is associated with immobility after hip fracture (4, 5, 25). However, many patients without vitamin D deficiency are immobile or do not survive after surgery (2-5). Vitamin D binding protein is not only an important carrier of serum vitamin D metabolites, but also plays a major role in the actin scavenging system, immune cells modulation, and inflammatory response; furthermore, higher DBP concentration is found to associate with a better survival rate in other conditions (13-15, 26). Therefore, we hypothesized that low circulating DBP would be associated with poor outcomes. To our knowledge, no prior prospective study has assessed the association between circulating DBP with mobility and mortality after hip fracture. In the current study, we found that higher preoperative circulating DBP was associated with better mobility at both 30 and 60 days and lower risk of mortality at 60 days in both the whole cohort and in those with vitamin D ≥ 12 ng/mL. In addition, there was a positive relationship between circulating DBP and gelsolin, and a negative one with IL-6 and CRP. These findings have important clinical implications indicating that the circulating DBP is a predictor of prognostic outcomes after hip fracture, regardless of vitamin D status, and there is a strong association with markers of tissue injury and inflammation.
Circulating DBP decreases in clinical conditions associated with cell lysis, such as during infection, sepsis, acute liver failure, surgery, and trauma (27, 28). Cellular death causes large amounts of intracellular structural proteins, including actin (which polymerizes to F-actin), to be released into the bloodstream, leading to intravascular coagulation and organ injuries if not cleared from circulation (29-31). DBP and gelsolin are major proteins in the extracellular actin scavenger system. Low circulating gelsolin and high F-actin are observed in patients after injury and trauma associated with worse clinical outcomes (13, 32, 33). As expected, in the current study, F-actin level is increased after hip fracture, and while it decreases a few days after surgery, it remains elevated. Circulating DBP and gelsolin levels were lower in the patients than in healthy controls, and both proteins decreased further after surgery. This may be partially explained by acute utilization of DBP and gelsolin to clear excessive F-actin in an effort to support tissue repair and improve postsurgical recovery. Also, the DBP-actin complex works as an essential cofactor for immune regulation by triggering neutrophil recruitment to sites of inflammation (34, 35). In the current study, circulating DBP is inversely associated with inflammatory markers (eg, IL-6 and CRP). Together, these findings support a role for DBP in the acute phase response to tissue injury.
The cytokine release due to inflammation after tissue injury is important for tissue healing (36, 37). However, persistent high IL-6 not only has a detrimental effect on soft tissues, but also contributes to bone loss (38, 39), a particular concern after hip fracture surgery. In addition, it has been suggested that IL-6 contributes to the inhibition of proteins in the liver, such as albumin (40) and possibly DBP. In the current study, circulating IL-6 and CRP are both higher in the patients with hip fracture than in healthy older controls, and levels increase after surgery by 2.6- and 1.8-fold, respectively, whereas TNF-alpha shows no further increase postoperatively. This marked increase in IL-6 following major tissue injury is consistent with previous studies (41-43), supporting its role as one of the earliest and most important mediators of the short-term response to tissue injury. However, excessive production and prolonged elevation of IL-6 can be associated with increased injury severity (43), surgical complications (42), morbidity, and mortality (44, 45). Because of the importance of IL-6 in surgical patients, we measured its concentrations in the full cohort, yet found it was not associated with mobility or mortality.
There was no significant association found between preoperative total or free 25OHD and mobility or mortality in this population with a large age range (55-102 years). We previously examined only older adults (>65 years) after hip fracture and found an association with total 25OHD (<12 ng/mL) and immobility (4). Overall, there are mixed results in the literature, indicating that low circulating total and free 25OHD in a wide range of patients does not always explain the variability in prognostic outcomes in patients with hip fracture (2, 4, 15, 46). This may be due to the high variability of total and free 25OHD concentrations that are influenced by the rapid change in DBP after trauma and surgery. In addition, the vitamin D metabolites do not correlate with gelsolin and cytokines, unlike DBP which is highly correlated. Overall, our findings in the current study support the hypothesis that higher DBP concentration is important for acute phase response after tissue injury and may explain its association with better postsurgical outcomes.
This study has a few strengths and limitations. One strength is that the large sample size from multiple centers, and patients in this cohort had a wide age range (55-102 years) and had CVD or at least one CVD risk factor. Since the majority of the general and hip fracture populations have one or more CVD risk factors and more than 90% of hip fractures occur in people over 50, this increases the generalizability of the data (47-49). In addition, no previous study to our knowledge has examined a combination of circulating vitamin D metabolites and their binding proteins (DBP and albumin), and IL-6 at multiple time points using a prospective design to determine their association with postsurgical outcomes, which provides important clinical relevance. In addition, assessment of acute phase reactants, induced by tissue injury, allowed for a better understanding of why DBP may be a better predictor of postsurgical outcomes. However, a limitation is that these findings cannot prove a causal relationship; instead, they can provide the basis for future studies to examine mechanisms of DBP function and its role after injury or inflammation. In addition, this study has limited racial diversity, although patients were examined in 47 sites in the United States and Canada. Furthermore, a limitation is the use of short-term design to examine clinical outcomes and the limited number of deaths, although the mortality rate is proportional to that in the larger FOCUS population (n = 2016 patients) (16).
The results from this multicenter study provide new evidence that higher DBP concentrations are associated with greater mobility and reduced mortality after hip fracture surgery. An improved understanding of DBP as an acute phase reactant in the inflammatory response after tissue injury and its association with clinical outcomes should be addressed in future studies.
Acknowledgments
We thank all teams of the study group for their contribution and the study participants who contributed their information.
Contributor Information
Lingqiong Meng, Department of Nutritional Sciences, and the Institute of Food, Nutrition & Health, Rutgers University, New Brunswick, NJ 08901, USA.
Xiangbing Wang, Department of Medicine, Rutgers–Robert Wood Johnson Medical School, New Brunswick, NJ 08901, USA.
Jeffrey L Carson, Department of Medicine, Rutgers–Robert Wood Johnson Medical School, New Brunswick, NJ 08901, USA.
Yvette Schlussel, Department of Nutritional Sciences, and the Institute of Food, Nutrition & Health, Rutgers University, New Brunswick, NJ 08901, USA.
Sue A Shapses, Department of Nutritional Sciences, and the Institute of Food, Nutrition & Health, Rutgers University, New Brunswick, NJ 08901, USA; Department of Medicine, Rutgers–Robert Wood Johnson Medical School, New Brunswick, NJ 08901, USA.
Funding
The original FOCUS trial was supported by grants from the National Heart, Lung, and Blood Institute (U01 HL073958 and U01 HL 074815). This study was supported by NJ-Institute of Food, Nutrition & Health, USDA-NIFA (0153866) and the National Institute of Aging (AG-12161).
Author Contributions
L.M. and S.S. contributed to the conceptualization and study design. J.C. contributed to the original study design. L.M. and S.S. contributed to the data collection. L.M. performed the statistical analysis and wrote the first draft of the manuscript. X.W., J.C., Y.S., and S.S. contributed to the review and editing of the manuscript. All authors reviewed and approved the final version of the manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
Data Availability
The datasets generated during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author upon reasonable request.
Clinical Trial Information
Clinicaltrials.gov registration no. NCT00071032.
References
- 1. Holvik K, Ahmed LA, Forsmo S, et al. Low serum levels of 25-hydroxyvitamin D predict hip fracture in the elderly: a NOREPOS study. J Clin Endocrinol Metab. 2013;98(8):3341‐3350. [DOI] [PubMed] [Google Scholar]
- 2. Dauny V, Thietart S, Cohen-Bittan J, et al. Association between vitamin D deficiency and prognosis after hip fracture surgery in older patients in a dedicated orthogeriatric care pathway. J Nutr Health Aging. 2022;26(4):324‐331. [DOI] [PubMed] [Google Scholar]
- 3. Ingstad F, Solberg LB, Nordsletten L, Thorsby PM, Hestnes I, Frihagen F. Vitamin D status and complications, readmissions, and mortality after hip fracture. Osteoporos Int. 2021;32(5):873‐881. [DOI] [PubMed] [Google Scholar]
- 4. Hao L, Carson JL, Schlussel Y, Noveck H, Shapses SA. Vitamin D deficiency is associated with reduced mobility after hip fracture surgery: a prospective study. Am J Clin Nutr. 2020;112(3):613‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mak JC, Mason RS, Klein L, Cameron ID. An initial loading-dose vitamin D versus placebo after hip fracture surgery: randomized trial. BMC Musculoskelet Disord. 2016;17(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chakhtoura M, Bacha DS, Gharios C, et al. Vitamin D supplementation and fractures in adults: a systematic umbrella review of meta-analyses of controlled trials. J Clin Endocrinol Metab. 2022;107(3):882‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LeBoff MS, Murata EM, Cook NR, et al. Vitamin D and OmegA-3 TRIAL (VITAL): effects of vitamin D supplements on risk of falls in the US population. J Clin Endocrinol Metab. 2020;105(9):2929‐2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847‐858. [DOI] [PubMed] [Google Scholar]
- 9. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144Pt A:132‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507‐515. [DOI] [PubMed] [Google Scholar]
- 11. Henderson CM, Fink SL, Bassyouni H, et al. Vitamin D-binding protein deficiency and homozygous deletion of the GC gene. N Engl J Med. 2019;380(12):1150‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiødt FV, Ott P, Bondesen S, Tygstrup N. Reduced serum Gc-globulin concentrations in patients with fulminant hepatic failure: association with multiple organ failure. Crit Care Med. 1997;25(8):1366‐1370. [DOI] [PubMed] [Google Scholar]
- 13. Luebbering N, Abdullah S, Lounder D, et al. Endothelial injury, F-actin and vitamin-D binding protein after hematopoietic stem cell transplant and association with clinical outcomes. Haematologica. 2021;106(5):1321‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dahl B, Schiødt FV, Kiaer T, Ott P, Bondesen S, Tygstrup N. Serum Gc-globulin in the early course of multiple trauma. Crit Care Med. 1998;26(2):285‐289. [DOI] [PubMed] [Google Scholar]
- 15. Suberviola B, Lavin BA, Jimenez AF, Perez-San Martin S, Garcia-Unzueta M, Santibañez M. Vitamin D binding protein, but not vitamin D or vitamin D-related peptides, is associated with septic shock mortality. Enferm Infecc Microbiol Clin (Engl Ed). 2019;37(4):239‐243. [DOI] [PubMed] [Google Scholar]
- 16. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rejnmark L, Lauridsen AL, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Diurnal rhythm of plasma 1,25-dihydroxyvitamin D and vitamin D-binding protein in postmenopausal women: relationship to plasma parathyroid hormone and calcium and phosphate metabolism. Eur J Endocrinol. 2002;146(5):635‐642. [DOI] [PubMed] [Google Scholar]
- 18. Jones KS, Redmond J, Fulford AJ, et al. Diurnal rhythms of vitamin D binding protein and total and free vitamin D metabolites. J Steroid Biochem Mol Biol. 2017;172:130‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10(4):307‐312. [DOI] [PubMed] [Google Scholar]
- 20. Denburg MR, Hoofnagle AN, Sayed S, et al. Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res. 2016;31(6):1128‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954‐959. [DOI] [PubMed] [Google Scholar]
- 22. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666‐3672. [DOI] [PubMed] [Google Scholar]
- 23. Meng L, Wang X, Carson JL, Schlussel Y, Shapses SA. Data from: A prospective study of Vitamin D binding protein and post-surgical outcomes after hip fracture: evidence for its role as a tissue injury marker. figshare. Deposited August 23, 2023. 10.6084/m9.figshare.24021027.v2 [DOI] [PMC free article] [PubMed]
- 24. Mundi S, Pindiprolu B, Simunovic N, Bhandari M. Similar mortality rates in hip fracture patients over the past 31 years. Acta Orthop. 2014;85(1):54‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pioli G, Lauretani F, Pellicciotti F, et al. Modifiable and non-modifiable risk factors affecting walking recovery after hip fracture. Osteoporos Int. 2016;27(6):2009‐2016. [DOI] [PubMed] [Google Scholar]
- 26. Yuan C, Song M, Zhang Y, et al. Prediagnostic circulating concentrations of vitamin D binding protein and survival among patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2020;29(11):2323‐2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schoenmakers I, Fraser WD, Forbes A. Vitamin D and acute and severe illness—a mechanistic and pharmacokinetic perspective. Nutr Res Rev. 2023;36(1):23‐38. [DOI] [PubMed] [Google Scholar]
- 28. Binkley N, Coursin D, Krueger D, et al. Surgery alters parameters of vitamin D status and other laboratory results. Osteoporos Int. 2017;28(3):1013‐1020. [DOI] [PubMed] [Google Scholar]
- 29. Dahl B, Schiødt FV, Ott P, et al. Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Crit Care Med. 2003;31(1):152‐156. [DOI] [PubMed] [Google Scholar]
- 30. Antoniades CG, Berry PA, Bruce M, et al. Actin-free Gc globulin: a rapidly assessed biomarker of organ dysfunction in acute liver failure and cirrhosis. Liver Transpl. 2007;13(9):1254‐1261. [DOI] [PubMed] [Google Scholar]
- 31. Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57(4):897‐909. [DOI] [PubMed] [Google Scholar]
- 32. Wang H, Cheng B, Chen Q, et al. Time course of plasma gelsolin concentrations during severe sepsis in critically ill surgical patients. Crit Care. 2008;12(4):R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee PS, Drager LR, Stossel TP, Moore FD, Rogers SO. Relationship of plasma gelsolin levels to outcomes in critically ill surgical patients. Ann Surg. 2006;243(3):399‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trujillo G, Habiel DM, Ge L, Ramadass M, Cooke NE, Kew RR. Neutrophil recruitment to the lung in both C5a- and CXCL1-induced alveolitis is impaired in vitamin D-binding protein-deficient mice. J Immunol. 2013;191(2):848‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kew RR, Tabrizian T, Vosswinkel JA, Davis JE, Jawa RS. Vitamin D-binding protein deficiency in mice decreases systemic and select tissue levels of inflammatory cytokines in a murine model of acute muscle injury. J Trauma Acute Care Surg. 2018;84(6):847‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saribal D, Hocaoglu-Emre FS, Erdogan S, Bahtiyar N, Caglar Okur S, Mert M. Inflammatory cytokines IL-6 and TNF-α in patients with hip fracture. Osteoporos Int. 2019;30(5):1025‐1031. [DOI] [PubMed] [Google Scholar]
- 37. Bermejo-Bescós P, Martín-Aragón S, Cruz-Jentoft AJ, Merello de Miguel A, Vaquero-Pinto MN, Sánchez-Castellano C. Peripheral IL-6 levels but not sarcopenia are predictive of 1-year mortality after hip fracture in older patients. J Gerontol A Biol Sci Med Sci. 2020;75(10):e130‐e137. [DOI] [PubMed] [Google Scholar]
- 38. Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13(5):1189‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kotake S, Sato K, Kim KJ, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11(1):88‐95. [DOI] [PubMed] [Google Scholar]
- 40. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond). 1990;79(2):161‐165. [DOI] [PubMed] [Google Scholar]
- 42. Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79(8):757‐760. [DOI] [PubMed] [Google Scholar]
- 43. Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Brückner UB. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135(3):291‐295. [DOI] [PubMed] [Google Scholar]
- 44. Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011;26(2):73‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stensballe J, Christiansen M, Tønnesen E, Espersen K, Lippert FK, Rasmussen LS. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol Scand. 2009;53(4):515‐521. [DOI] [PubMed] [Google Scholar]
- 46. Rosendahl-Riise H, Spielau U, Ranhoff AH, Gudbrandsen OA, Dierkes J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: a systematic review and meta-analysis. J Hum Nutr Diet. 2017;30(1):3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwartz AV, Kelsey JL, Maggi S, et al. International variation in the incidence of hip fractures: cross-national project on osteoporosis for the World Health Organization Program for Research on Aging. Osteoporos Int. 1999;9(3):242‐253. [DOI] [PubMed] [Google Scholar]
- 48. Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480‐486. [DOI] [PubMed] [Google Scholar]
- 49. Dominic E, Brozek W, Peter RS, et al. Metabolic factors and hip fracture risk in a large Austrian cohort study. Bone Rep. 2020;12:100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54(5):566‐572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author upon reasonable request.