Abstract
Omp21, a minor outer membrane protein of the soil bacterium Comamonas acidovorans, was purified from a spontaneous mutant lacking a surface layer and long-chain lipopolysaccharide. Omp21 synthesis is enhanced by oxygen depletion, and the protein has a variable electrophoretic mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis due to its heat-modifiable behavior. The structural gene omp21 encodes a precursor of 204 amino acids with a putative signal peptide of 21 amino acids. Mature Omp21 is a typical outer membrane protein with a high content of β structure as determined by infrared spectroscopy. Sequence comparisons show that it belongs to a new outer membrane protein family, characterized by eight amphipathic β strands, which includes virulence proteins, such as the neisserial opacity proteins, Salmonella typhimurium Rck, and Yersinia enterocolitica Ail, as well as the major outer membrane proteins OmpA from Escherichia coli and OprF from Pseudomonas aeruginosa.
Comamonas acidovorans is one of three species of the genus Comamonas, which belongs to the β subdivision of the class Proteobacteria (60). It is a soil bacterium but has also been observed in clinical isolates (6, 8). This organism possesses a typical gram-negative cell wall whose outer membrane contains two major protein components (21): a regular surface layer (S layer) protein, which covers the cell completely (22), and the constitutive porin Omp32 (29), which was shown to function as a strongly anion-selective pore protein (41), presumably as a result of adaptation to growth on organic acids, the preferred carbon source of C. acidovorans and related species (70). Among the less prominent components of the C. acidovorans outer membrane is a heat-modifiable protein of approximately 21 kDa, named Omp21 (21). Proteins of similar mass and characteristics have also been described for other gram-negative bacteria (33, 46), but their functional and structural properties have not been investigated in detail. In the course of a comprehensive characterization of the C. acidovorans outer membrane, we became interested in Omp21 and its unresolved importance for the cell.
In this study we describe the isolation and characterization of Omp21 from an S layer-deficient mutant. Sequence comparisons reveal that it belongs to a new family of outer membrane proteins containing eight transmembrane β strands.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The two strains of C. acidovorans used in this study are the type strain (DSM39, identical to ATCC 15688) and the mutant strain JL0 (see below). Cells were grown at 30°C either in complex medium containing 1% Bacto Tryptone, 0.5% yeast extract, 1% NaCl, and 0.1% glucose at pH 7.0 (9) or in a mineral medium containing (per liter) 2.28 g of K2HPO4 · 3H2O, 0.5 g of MgSO4 · 7H2O, 10 mg of CaCl2 · 2H2O, 15 ml of 20% NH4Cl, 9.4 g of malic acid, 5 ml of trace element solution SL6 (medium 27 [19]), and 10 ml of vitamin solution (medium 253 [19]). The pH was adjusted to 6.8 with solid NaOH. Growth experiments were performed in a 100-liter pilot fermentor (Bioengineering, Wald, Switzerland) containing 50 liters of mineral medium. The pH of 6.8 was maintained with a pH-stat with 4 M malic acid, which concurrently compensated for the consumption of the carbon source. Every 3 h samples were taken to determine the cell density (optical density at 600 nm), amount of protein, protein composition of the outer membrane, cell morphology (in the light microscope), and the concentration of malic acid in the supernatant. The pH and relative O2 concentration were continuously monitored. When necessary, samples were frozen at −20°C.
Isolation of the mutant strain JL0.
The strain JL0 of C. acidovorans was derived from the type strain as a spontaneous mutant as follows. Cells were grown in 50 ml of complex medium for 8 to 16 h, and 1 ml of cell suspension was used as an inoculum for the next passage. This procedure was repeated in order to enrich cells growing faster than the wild-type strain. After each three to five passages the culture was examined on agar plates (1.7% [wt/vol] Bacto Agar; 30°C) to search for a fast-growing phenotype, identified by particularly large colonies. The pattern of outer membrane proteins was compared to that of the type strain by means of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The procedure was continued until a mutant lacking the S layer protein was obtained (strain JL0).
Purification of Omp21.
Cell envelopes were isolated as described previously (9). Outer membranes were prepared by treatment with 1% (vol/vol) Triton X-100 and 0.02% (wt/vol) lysozyme in 10 mM sodium phosphate (pH 7.5) plus 3 mM NaN3 (buffer A), supplemented with 1 mM MgCl2, for 1 h at room temperature. This treatment solubilizes the cytoplasmic membrane but not the outer membrane (21). Membranes were collected at 40,000 × g for 15 min and washed twice with buffer A. The pellet was resuspended in buffer A to give a protein concentration of 5 mg/ml, and membrane proteins were solubilized with 2.5% (vol/vol; final concentration) n-octylpolyoxyethylene (C8En [n = 2 to 11]; Bachem) for 3 to 16 h at 30°C with gentle shaking. Insoluble material was removed by centrifugation at 40,000 × g for 15 min.
Chromatographic protein purification was performed with the GradiFrac system (Pharmacia) at a flow rate of 2 ml/min throughout. Extracted outer membrane proteins (200 mg to 2 g) were diluted to a protein concentration of 1 to 4 mg/ml and applied to a HiLoad 16/10 Q-Sepharose HP column (bed volume, 20 ml; Pharmacia) equilibrated with buffer A plus 0.5% C8En (buffer B). Bound proteins were eluted by applying a NaCl gradient (0 to 1 M) for 60 min. Omp21 appeared in the void volume. This solution, containing 70 to 75 mg of protein at a concentration of 0.2 to 0.3 mg/ml, was applied to a Bio-Gel HT hydroxyapatite column (bed volume, 50 ml; Bio-Rad) equilibrated with buffer B. Bound proteins were eluted with sodium phosphate (10 to 500 mM) for 80 min. Again, Omp21 appeared in the void volume.
In an alternative approach, 25 to 35 mg of extracted protein (2.5 to 3.5 mg/ml) was separated by gel filtration with Sepharose 300 HR (Pharmacia) (column length, 1 m; diameter, 5 cm) equilibrated with buffer B plus 150 mM NaCl. Fractions (8 ml) were collected and examined by SDS-PAGE. Fractions containing the Omp21 conformers at 21, 23, and 24 kDa (Omp21-I) and those containing the 21- and 24-kDa conformers (Omp21-II) were separately pooled and concentrated as described below. Protein samples from 21 runs were combined, resulting in 28.8 mg (1.8 mg/ml) of Omp21-I and 14.1 mg (0.47 mg/ml) of Omp21-II. These solutions were further purified by hydroxyapatite chromatography as described above.
Diluted samples were concentrated either by means of dry polyethylene glycol 40000 (Serva) or by dialysis against buffer A plus 10% polyethylene glycol 40000. To minimize the C8En content, concentrated samples were dialyzed against buffer A.
Isoelectric focusing.
The isoelectric point of Omp21 was determined by using the free-flow electrophoresis system OKTOPUS (Weber GmbH, Kirchheim, Germany [67]). Protein samples (0.3 mg/ml) were diluted by adding equal volumes of a solution containing 0.4% hydroxypropyl methylcellulose (Aldrich), 0.5% Resolyte (Merck), and 0.5% (vol/vol) C8En. The samples were applied to the system with a flow rate of 10.3 ml/h. The pH range was 4 to 8. Fractions (1 ml) were collected, the exact pH was measured with a pH electrode, and the protein composition was determined by SDS-PAGE.
SDS-PAGE and staining procedures.
SDS-PAGE was performed with gradient gels (10 to 15%) as described by Laemmli (39). Samples containing 2 to 4 μg of protein were precipitated according to the method of Wessel and Flügge (69). The dried pellets were resuspended in 10 μl of sample buffer, heated for 5 min at 100°C, and loaded onto the gel. Protein was stained with AgNO3 as described by Morrissey (45), and lipopolysaccharide (LPS) was stained by the procedure of Tsai and Frasch (61).
FTIR spectroscopy.
Aqueous Omp21 solutions containing 10 to 100 μg of protein were dried on Ge crystals. Spectra were recorded by the method of attenuated total reflection Fourier transform infrared (FTIR) spectroscopy in a Nicolet FTIR 740. The protein secondary structure composition was determined by means of Fourier self-deconvolution (59) and band form analysis as described by Kleffel et al. (36). The relative absorption at 1,750 to 1,700 cm−1 was used to judge the LPS content.
MALDI mass spectrometry.
For matrix-assisted laser desorption ionization (MALDI) mass spectrometry samples containing 1 mg of Omp21/ml were dialyzed against 10 mM sodium phosphate buffer (pH 7.5)–3 mM NaN3. The target was prepared with a saturated solution of α-cyanohydroxycinnamic acid in 2/3 acetonitrile and 1/3 0.1% trifluoroacetic acid in water as the matrix. As the second layer, the dialyzed sample was mixed with this matrix to a final protein concentration of 20 pmol/μl. MALDI mass spectra were obtained with a Reflex II time-of-flight mass spectrometer (Bruker, Bremen, Germany) equipped with a 337-nm-wavelength nitrogen laser and a Scout sample stage source.
Amino acid sequence analysis.
Samples (20 μg) of purified Omp21 were run on linear SDS-PAGE gradient gels (7 to 15% acrylamide), stained with Serva Blue R, and destained by the usual procedures. Gel regions containing the protein to be analyzed were cut out, and the protein was directly digested with endopeptidase LysC according to the method of Eckerskorn and Grimm (20). The resulting peptides were separated in a linear gradient of 0 to 60% acetonitrile at a flow rate of 0.3 ml/min and analyzed by automated sequencing in a gas-phase sequenator (model 470 A; Applied Biosystems) according to the instructions of the manufacturer.
Isolation of DNA and nucleotide sequence analysis.
To isolate genomic DNA of C. acidovorans, cells were grown to an optical density at 600 mm of 5 and embedded in low-melting-point agarose formed into plugs, where cell lysis and proteolysis took place as described by Applegate et al. (2). Intact genomic DNA was obtained by melting the agarose plugs at 70°C for 15 min and 25-fold dilution with sterile MilliQ water.
Direct PCR was performed with 300 ng of genomic DNA and degenerate oligonucleotides, derived from the sequences of Omp21 peptide fragments. Each reaction mixture contained 6% (vol/vol) formamide. A 125-bp fragment obtained by this method was used to synthesize complementary oligonucleotides in opposite directions. For inverse PCR 2 μg of DNA from one agarose plug was digested with 100 U of restriction enzyme NcoI (New England Biolabs) in a 250-μl sample volume. After incubation at 37°C overnight, the digested DNA was purified by means of the gel extraction kit from Bio-Rad and religated with 400 U of T4 ligase (Stratagene) in a 100-μl volume. These DNA fragments, together with complementary primers derived from the 125-bp fragment, were used for inverse PCR. The resulting 1,055-bp fragment was sequenced with the PRISM Ready Reaction Dye Deoxy Terminator cycle sequencing kit (Applied Biosystems, Inc.) and the sequencing system model 373 DNA Stretch XL (Applied Biosystems, Inc.).
Northern blot.
RNA from mutant cells was prepared following the RNeasy miniprotocol for the isolation of total RNA from bacteria as described by Qiagen. After elution with RNase-free MilliQ water, the absorption at 260 nm was measured and the RNA concentration was calculated. Total RNA of C. acidovorans mutant cells was separated by formaldehyde-agarose gel electrophoresis and transferred to nylon membranes (Amersham). The required single-stranded gene probe containing 90% of the structural gene omp21 was labeled with digoxigenin (DIG)-11-dUTP (Boehringer, Mannheim, Germany) via direct PCR with 1 nmol per nucleotide, and 2 nmol of DIG-11-dUTP per 100 μl of PCR assay mixture. Hybridization and detection followed the procedure described by Boehringer for the DIG luminescent detection kit.
Sequence comparisons and secondary-structure prediction.
The Omp21 sequence was compared to protein sequences in the National Center for Biotechnology Information nonredundant database (http://ncbi.nlm.nih.gov) by using BLAST version 2.0.4 software (1), and related sequences were detected at probabilities of chance occurrence between 2 × 10−38 (for Y4mB; Rhizobium sp.) and 2 × 10−9 (for DoxH; Pseudomonas sp.). These sequences form group I (see the alignment in Fig. 7). In a second step we searched for more distant Omp21 relatives by using PSI-BLAST software (1) with a cutoff probability of 10−4 (PSI-BLAST is a position-specific, iterative procedure representing a profile search with evolving profiles). The first sequence not represented in group I, OprF (Pseudomonas aeruginosa), was included in the significant set at the third iteration. After the seventh iteration, the significant set contained more than 100 database entries, including all the proteins shown in Table 1. The detected region of similarity focused mainly on the second half of the proteins, in the region marked as β strands 5 to 8 (see Fig. 7). All the proteins represented in the significant set after the seventh iteration were extracted from the database, purged of sequences more than 95% identical to each other, and subdivided into five groups by their relative similarity, yielding 8 sequences in group I, 29 sequences in group IIa, 19 sequences in group IIb, 16 sequences in group IIIa, and 6 sequences in group IIIb. Further PSI-BLAST searches were made with members of each of these five groups at the same significance cutoff, in order to confirm that the same set of proteins was detected in the database independently of the starting sequence. In no case was a protein detected that did not belong to one of the five groups. The searches were ended when at least one member of each of the five groups appeared in the significant set.
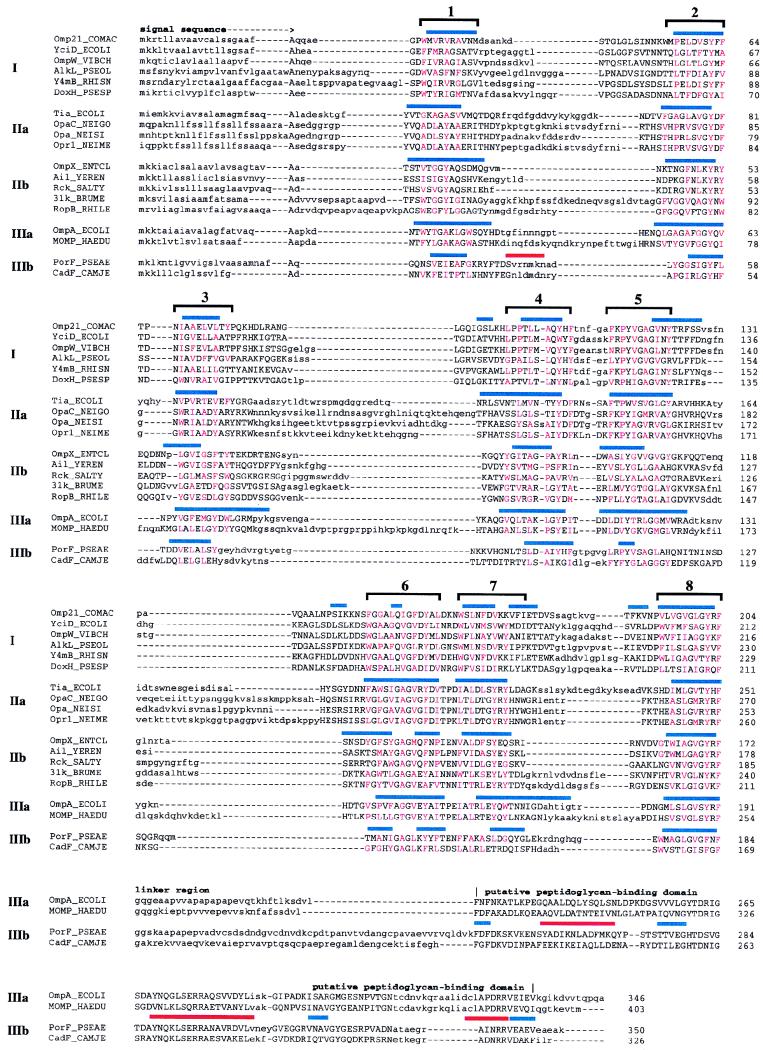
FIG. 7.
Alignment and secondary-structure prediction for Omp21 and homologous proteins. Representative proteins from each group in Table 1 are shown. For sequences in group III, the start of the linker region leading to the putative peptidoglycan-binding domain is denoted. Sequence regions that could not be aligned within any of the five groups are shown in lowercase letters. Red capitals mark the hydrophobic amino acids located in the conserved amphipathic sequence stretches. Bars above each aligned group indicate β strands (blue) and α helices (red) predicted by the PHD program (groups I, IIb, and IIIb) or by the method of Gromiha and Ponnuswamy (groups IIa and IIIa). Brackets above complete alignments emphasize the predicted eight-stranded β sheet of this family of outer membrane proteins.
TABLE 1.
Homology groups of Omp21-related proteins
| Group | Protein | Organism | Mass (kDa)a | Locationb | Heat modi- fiablec | Functiond | References |
|---|---|---|---|---|---|---|---|
| I | Omp21 | Comamonas acidovorans | 22.0 | OM | + | − | This paper |
| YciD | Escherichia coli | 22.9 | − | − | − | 55 | |
| OmpW | Vibrio cholerae | 21.5 | OM | − | − | 34 | |
| AlkL | Pseudomonas oleovorans | 24.9 | OM | + | Part of alkane metabolic pathway | 62 | |
| Y4mB | Rhizobium sp. | 24.6 | − | − | − | 28 | |
| DoxH | Pseudomonas sp. | 22.9 | − | − | Part of dibenzothiophene metabolic pathway | 18 | |
| IIa | Opa66 | Haemophilus influenzae | 13.2 | − | − | LPS biosynthesis? | 26, 48 |
| OpaD | Haemophilus influenzae | 19.5 | − | − | − | 26 | |
| Tia | Escherichia coli | 27.9 | OM | − | − | 25 | |
| OpaC | Neisseria gonorrhoeae | 30.3 | OM | + | Adhesion | 63 | |
| OpaA | Neisseria gonorrhoeae | 26.7 | OM | + | Adhesion | 5 | |
| Opa | Neisseria sicca | 28.2 | OM | + | − | 71 | |
| Opa | Neisseria flava | 29.1 | OM | + | − | 71 | |
| Opr1 | Neisseria meningitidis | 28.9 | OM | + | − | 54 | |
| IIb | OmpX | Enterobacter cloacae | 18.6 | OM | − | − | 56 |
| OmpK17 | Klebsiella pneumoniae | 18.4 | OM | − | − | 11 | |
| Ail | Yersinia enterocolitica | 19.6 | OM-associated | − | Adhesion | 43 | |
| Rck | Salmonella typhimurium | 19.7 | OM | − | Serum resistance, cell invasion | 10 | |
| PagC | Salmonella typhimurium | 20.6 | OM | − | − | 49 | |
| Att | Salmonella typhimurium | 17.4 | − | − | − | 16 | |
| VLom | Phage λ | 21.9 | OM (E. coli) | − | − | 4 | |
| Omp31 | Brucella melitensis | 25.3 | OM | − | − | 64 | |
| Omp25 | Brucella melitensis | 23.2 | OM | − | − | 12 | |
| RopB | Rhizobium leguminosarum | 22.5 | OM | − | − | 50 | |
| Pap31 | Bartonella henselae | 29.9 | − | − | − | 7 | |
| IIIa | OmpA | Escherichia coli | 37.2 | OM | + | Receptor diffusion channel stabilization of mating aggregates | 44, 53, 58 |
| MOMP | Haemophilus ducreyi | 44.2 | OM | + | Stabilization of cell shape | 37 | |
| OmpA1 | Aeromonas salmonicida | 35.8 | OM | − | − | 13 | |
| Omp | Vibrio cholerae | 34.3 | OM | − | − | ||
| IIIb | OprFe | Pseudomonas aeruginosa | 37.6 | OM | + | Uptake of di- and tetrasaccharides, stabilization of cell shape | 24 |
| OmpCD | Branhamella catarrhalis | 48.3 | OM | + | − | 46 | |
| CadF | Campylobacter jejuni | 36.7 | OM | + | − | 38 |
Calculated from sequence data, including putative signal sequence.
OM, outer membrane; −, unknown.
+, heat modifiable; −, unknown.
−, unknown.
PorF_PSEAE in National Center for Biotechnology Information nonredundant database.
Each group of sequences was aligned in MACAW by using the BLOSUM62 matrix (40), and the regions aligned significantly within a group are shown in capital letters (see Fig. 7). The alignments were converted to MSF format and submitted to the PredictProtein server at EMBL (http://www.embl-heidelberg.de/predictprotein/phd_pred.html) for consensus secondary-structure prediction by the PHD method (51). In a final step, representative members of the five groups were aligned in MACAW with the amphipathic residue pattern, the predicted secondary structure, and the location of conserved sequences as guidelines. The resulting alignment (see Fig. 7) contained eight predicted β-strand regions. The significance of the alignments in these regions was 5 × 10−5 for β2 and <10−20 for β5, β6, β7, and β8. The alignment was not considered significant in the regions of β1, β3, and β4.
Nucleotide sequence accession number.
The nucleotide sequence of omp21 was deposited in the EMBL nucleotide sequence database with accession no. AJ001918.
RESULTS
Induction of Omp21 expression.
The synthesis of the outer membrane protein Omp21 from C. acidovorans is regulated, because Omp21 was expressed in cells grown in complex medium but not in mineral medium. We supplemented mineral medium with various constituents of yeast extract and peptone. Neither inorganic salts nor organic acids, amino acids, or other carbon sources had any specific effect upon Omp21 expression, but the oxygen pressure in the cell suspension did: Omp21 was expressed independently of the growth medium in poorly aerated cultures, whereas cells grown in identical, but sufficiently aerated, cultures contained Omp21 only at low concentrations (Fig. 1). In order to exclude possible influences of pH or nutrient starvation and to determine the apparent oxygen concentration at which Omp21 is induced, we performed long-term growth experiments in a fermentor with mineral medium and an approximately constant concentration of malate. At growth-limiting O2 concentrations Omp21 was detected after 3 h in cells of the logarithmic growth phase (μ = 0.4 h−1, where μ is the growth rate). At high aeration, Omp21 appeared after 10 h (μ = 0.53 h−1 [Fig. 2]), when the O2 content in solution decreased to about 20% of the initial level (∼0.05 mmol/liter). By using O2-limiting growth conditions, the yield of Omp21 could be increased at least 10-fold.
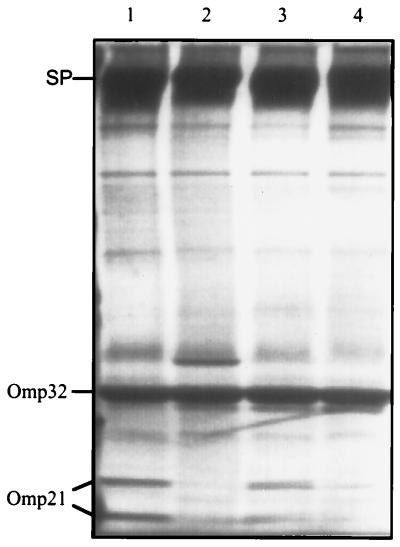
FIG. 1.
SDS-PAGE of the outer membrane proteins from C. acidovorans grown in batch cultures under different aeration conditions. The gel shows the protein patterns of cells grown in complex medium at low (lane 1) and high (lane 2) aeration and cells grown in mineral medium at low (lane 3) and high (lane 4) aeration. Omp21, running at 21 and 24 kDa, is expressed in cultures with limited oxygen. SP: regular surface protein; Omp32: porin.
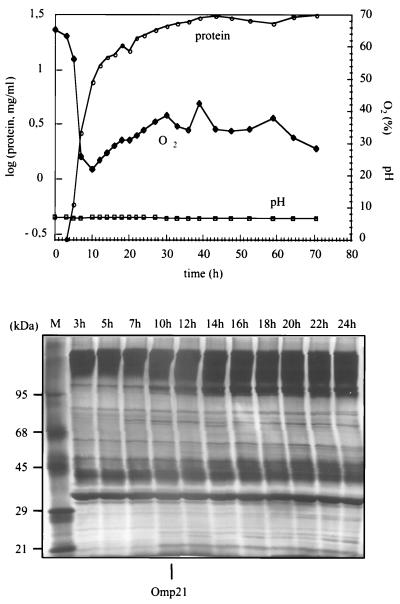
FIG. 2.
Induction of Omp21 expression in cells grown in mineral medium at high aeration in the fermentor under controlled pH, malic acid, and temperature conditions. Top: total cell protein (μ = 0.53 h−1) and relative oxygen concentration (100% denotes 8.5 mg of O2/liter). Bottom: outer membrane protein patterns in SDS-PAGE from samples taken at different times of growth. Omp21 appears after 10 h at a relative O2 concentration of about 20% and is present in all samples beyond 24 h.
Isolation of surface protein-deficient mutant cells.
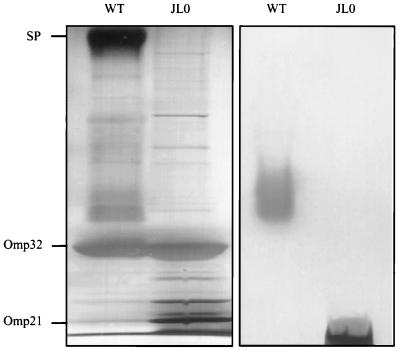
Attempts to purify Omp21 were hampered by the fact that the S layer protein (Fig. 3) is closely associated with the outer membrane (22) and thus was usually coextracted and copurified. It is known that bacteria occasionally lose their S layers in culture, when mutant cells outgrow the wild-type. To exploit this effect, C. acidovorans cells were grown continuously by repeated passages from log-phase cultures to fresh complex medium. After 52 changes, quickly growing colonies with rough surfaces were detected on agar plates among the smaller, smooth, wild-type colonies. Outer membranes isolated from the mutant colonies showed no S layer protein in SDS-PAGE and, unexpectedly, contained only short-chain LPS (Fig. 3).
FIG. 3.
SDS-PAGE of outer membrane preparations from C. acidovorans type strain (WT) and mutant strain JL0. The gels were stained for protein (left panel) and for LPS (right panel). Mutant cells (JL0) are deficient in the surface layer protein (SP) and exhibit short-chain LPS only.
Conformers of Omp21 with different mobilities.
After extraction of the outer membrane proteins Omp21 could be purified by means of gel filtration or ion-exchange chromatography, followed by hydroxyapatite chromatography in either case (Fig. 4). Omp21 from wild-type samples migrates at molecular masses of 21 and 24 kDa in SDS-PAGE, depending upon the solubilization temperature and the compositions of the buffer solutions (21) (Fig. 1). Omp21 from mutant outer membranes exhibited up to three protein bands at 21, 23, and 24 kDa. In order to clarify whether these proteins were derived from the same polypeptide, they were extracted from gels and digested with endopeptidase LysC. The resulting peptides were separated chromatographically as outlined in Materials and Methods. All the elution profiles were identical, and peptides from selected but corresponding peaks had the same amino acid sequence (i.e., J1, J2, and J3 and W1 and W2 in Fig. 5).
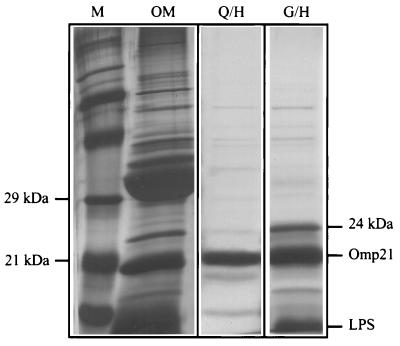
FIG. 4.
SDS-PAGE of Omp21 preparations from mutant strain JL0. Left panel: marker proteins (M) and C8En-extracted outer membrane proteins (OM). Middle panel: Omp21 purified by ionic exchange (Q) and hydroxyapatite chromatography (H). This preparation contains a particularly low level of LPS and almost no conformers at 23 and 24 kDa. Right panel: Omp21 purified by gel filtration (G) and hydroxyapatite chromatography (H). Note the additional conformer at 24 kDa. The protein was silver stained.
FIG. 5.
Nucleotide sequence and derived amino acid sequence of Omp21 from C. acidovorans. The putative ribosome-binding site (AAGA) is printed in boldface. The characteristic rho-independent terminator region is indicated by dotted lines. Translation stop codons are marked by asterisks. Amino acid residues +1 to +21 represent a typical bacterial signal sequence. Underlined amino acid sequences, corresponding to 51% of the mature protein, were determined by amino acid sequence analysis (J1 to J3, W1, and W2 indicate the identical peptides from the various protein bands separated in SDS-PAGE [see the text]). Dashed lines with a plus every 10 nucleotides are provided for orientation.
Determinations of the isoelectric point were only possible in a free-flow electrophoresis system because massive protein precipitation prevented experiments using the usual approach. The pI was >9, and a separation of different Omp21 forms was not observed. MALDI mass spectrometry data confirmed that all Omp21 variants were identical and exhibited a single clear signal at 20,000 Da, in agreement with the apparent molecular mass of 21 kDa. From this point on, all Omp21 preparations were made from outer membranes of C. acidovorans JL0.
Nucleotide sequence of omp21.
The sequences of peptides obtained from limited proteolysis of Omp21 were used to design primer oligonucleotides for PCR experiments. The first attempts to synthesize DNA fragments from the omp21 gene via direct PCR with genomic DNA and degenerate primers failed, possibly because of the high G+C content of the C. acidovorans DNA (67%). Only when DNA was prepared in agarose plugs and 6% formamide was added to PCR samples could a 125-bp DNA fragment be produced. Based on the sequence of this fragment, homologous primers in opposite directions were constructed for inverse PCR. As many as 25 different restriction enzymes were tested in single- and double-digestion probes, of which only NcoI produced useful DNA fragments. Inverse PCR resulted in one product of 1,055 bp whose nucleotide sequence is shown in Fig. 5. It contained an open reading frame of 612 bp coding for 204 amino acids. Within this gene, all the peptide sequences obtained from Omp21 by means of amino acid sequence analysis could be located, showing that we had identified the omp21 structural gene.
The G+C content of the omp21 gene amounts to 61%. A possible Shine-Dalgarno sequence is located between nucleotides 221 and 224, which differs from the typical AGGNNNNATG (N stands for nucleotide), but is consistent with the general features of ribosome-binding sites (57). Between positions 915 and 966 a strong palindrome, typical of factor-independent transcription termination signals, was found (14). In order to evaluate whether omp21 is part of an operon, the RNA of C. acidovorans was isolated. Northern hybridization experiments with an omp21-specific gene probe yielded one transcript of 857 nucleotides, suggesting that omp21 is transcribed as a monocistronic mRNA and is not part of an operon. Due to the position of the terminator sequence, we conclude that the entire structural gene and its promoter are located on the sequenced 1,055-bp NcoI fragment, but a putative promoter sequence resembling the Escherichia coli consensus was not found.
The first 21 amino acid residues of the translated gene product have the typical features of a signal sequence (66). The putative cleavage site lies at the C-terminal end of Ala19-Phe-Ala, which corresponds to the frequently occurring cleavage site motif Ala-X-Ala (47). The first amino acid of the mature Omp21 protein is presumably Gln22, suggesting that the previously observed resistance of Omp21 to Edman degradation is due to the formation of an N-terminal pyroglutamate, as has been shown for the porin Omp32 of C. acidovorans (30). Correspondingly, mature Omp21 consists of 183 amino acids with a calculated mass of 19,989 Da, which is supported by the value determined by MALDI. The pI of the mature protein (9.78; calculated by GENETYX) is also in agreement with the experimental value.
Secondary-structure determination and sequence comparisons.
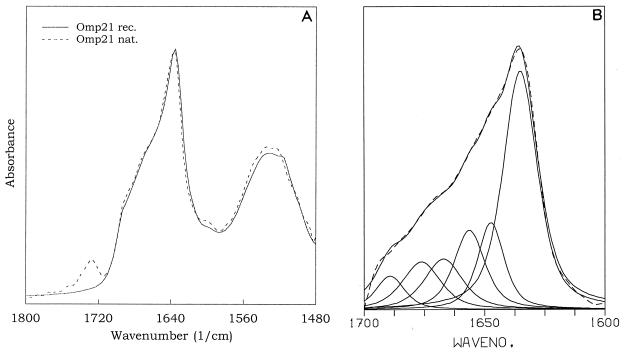
Omp21 shows amino acid sequence patterns typical of amphipathic β strands, and in accordance with that, FTIR spectroscopy revealed a high β-structure content (Fig. 6). This applies to preparations still containing residual LPS as well as to recombinant and LPS-free Omp21 obtained recently (3). The distinct peak at 1,635 cm−1 in the amide I band, in combination with the shoulder at 1,693 cm−1, is indicative of antiparallel β sheets. Quantitative analysis showed that at least 50% of Omp21 consists of β strands (Fig. 6B).
FIG. 6.
Attenuated total reflection FTIR spectra of Omp21. (A) Spectra of recombinant Omp21 (rec.) and native Omp21 (nat.) in association with LPS. The spectra are displayed in the amide I and amide II regions centered around 1,640 and 1,530 cm−1. The absorption at 1,730 cm−1 originates from carbonyl-ester vibrations of fatty acids bound in LPS molecules that do not contribute to the amide I band absorptions used for secondary-structure quantification. (B) Band form analysis between 1,700 and 1,600 cm−1 reveals a number of vibrations (solid lines) whose superposition defines the spectrum (dotted line). The strong absorption at 1,635 cm−1 and the band at 1,693 cm−1 are indicative of antiparallel β-sheet structure.
In order to assign these in the Omp21 sequence, we used a combined approach of consensus secondary-structure prediction and conservation analysis (23, 32), which has yielded reliable results for 16- and 18-stranded porins. As a first step, we identified related sequences in the current nonredundant database by using the programs BLAST and PSI-BLAST. These searches yielded a surprisingly large number of Omp21 homologs (a representative subset is shown in Table 1 and Fig. 7), among them YciD from E. coli (55) and OmpW from Vibrio cholerae (34), the Opa proteins from organisms of the family Neisseriaceae, virulence proteins of enteric bacteria (Ail from Yersinia enterocolitica and Rck from Salmonella typhimurium), OmpA (E. coli), and OmpA-related molecules (OprF from P. aeruginosa). We divided these proteins into three main groups, containing sequences most similar to Omp21 (group I) and more distant homologs without (group II) and with (group III) a peptidoglycan-binding domain. Groups II and III were again subdivided according to the relative similarity of the sequences they comprised. Each of the five data sets was aligned, and consensus secondary-structure predictions were obtained with the PHD program (51). For groups IIa and IIIa, secondary-structure predictions obtained by a similar method had already been published by Gromiha and Ponnuswamy (32) which agreed very well with the ones obtained by us (Fig. 7). Consensus secondary-structure predictions and the location of conserved sequence blocks corresponded closely among the five alignments, revealing the location of eight amphipathic β strands representing putative transmembrane segments. As in 16- and 18-stranded porins, the central β strands are arranged in pairs connected by short sequences (turns), and the pairs are connected to each other and to the two terminal β strands by more extended loops. By analogy, we propose a folding model for Omp21 in which eight membrane-spanning β strands are connected by four loops at the exoplasmic side and three turns at the periplasmic side of the outer membrane and where the two termini are located in the periplasm.
DISCUSSION
Omp21 is a characteristic constituent of the outer membrane of C. acidovorans. It is heat modifiable, i.e., it shows differences in electrophoretic mobility upon heating (21). This property has also been observed in several Omp21 homologs (Table 1) and may be attributed to incomplete unfolding or tight association with peptidoglycan fragments and/or LPS (12, 64). We identified up to three Omp21 conformers by SDS-PAGE. None of the variants appeared to be glycosylated, but FTIR analyses usually revealed a significant content of LPS in these samples even after hydroxyapatite chromatography, indicating a strong interaction between LPS and the protein. The results of amino acid sequence determinations and MALDI showed that all Omp21 conformers corresponded to the same polypeptide.
Omp21 is a member of a large family of known and putative outer membrane proteins characterized by eight transmembrane β strands. It is an open question whether these form a β barrel with a central pore. Liposome-swelling tests indicated that OmpA could form unspecific diffusion channels (58), but electrophysiological measurements with planar membranes showed no pore-forming activity of Omp21 (42). A possible structural model for such a barrel is provided by the lipocalins (27). Several members of this family, which are typically of sizes similar to that of Omp21, have a known three-dimensional structure, consisting of an eight-stranded barrel formed by amphipathic β strands. The main difference between the lipocalin barrel and the one proposed for Omp21 lies in the fact that the hydrophobic residues of the β strands point towards the central cavity in lipocalins but out towards the membrane in Omp21, making one essentially an inside-out version of the other. The lipocalin barrel is also closed at one end by a mobile lid, a feature which we do not envisage for the Omp21 family.
Proteins of the Omp21 family resemble 16- and 18-stranded porins in many respects: they contain a signal sequence, have a high content of β structure, are made up of amphipathic β strands flanked by aromatic amino acids, expose short turns to the periplasmic side and long loops to the exoplasmic side of the outer membrane (52, 68), and generally end with a phenylalanine, which has been shown to be required for the correct assembly of porins in the outer membrane (15). Despite these similarities (which may well be the result of convergent evolution), neither 16- nor 18-stranded porins were identified as related to Omp21 in database searches, indicating that the family of eight-stranded outer membrane proteins represents a homologous group of sequences. This family has several unique features, providing a further indication of homology. The turn connecting β strands 6 and 7 is of invariant length. Glycine occurs frequently in the hydrophilic positions of the amphipathic β strands (including an invariant glycine in β strand 6) but not in the membrane-oriented positions or in the connecting sequences. Finally, the hydrophilic positions of the transmembrane β strands are the most highly conserved (including a near-invariant tyrosine in β strand 5), suggesting similar properties for the putative pore region of the proposed β barrel in all members.
Our proposed folding model for Omp21 is similar to models suggested for the Omp21-related proteins OmpX (56), OmpK17 (11), Rck (10), RopB (50), OmpA (44), and OmpA1 (13), for some of which the surface-exposed position of the long loops was proven (10, 44). The model may thus be taken as representative of the family of eight-stranded β-sheet proteins.
Interestingly, OmpA and related proteins are also members of this family, supporting previous proposals that they exhibit eight transmembrane β strands (44). However, unlike other proteins in this family, they possess an additional domain connected to the C-terminal β strand via a highly variable, Pro-Ala-rich linker region. This domain is located in the periplasmic space (44) and is thought to bind to the peptidoglycan (17). Alternative models suggesting a 16-stranded structure (53), which place eight transmembrane β strands in the putative peptidoglycan-binding domain, are not supported by our analysis of proteins in this family (Fig. 7).
At present, it is not known whether Omp21 forms oligomers in vivo. There are indications from experiments with nondenaturating gel electrophoresis that the protein assembles into complexes, possibly tetramers (3). If so, the association would be much weaker than that between the subunits of trimeric porins.
The function(s) of Omp21 and of its most closely related proteins (group I in Table 1) are still unknown. DoxH (18) and AlkL (62) are encoded in operons involved in naphthalene and alkane catabolic pathways, respectively, but the deletion of AlkL had no detectable phenotype (62). Although omp21 does not appear to be organized in a multicistronic operon like the DoxH and AlkL genes, this does not exclude the possibility that omp21 is regulated together with other genes. The regulatory signal is very likely coupled to oxygen or to the redox state of cells. The nucleotide sequence upstream of omp21 did not reveal any sequence similarities to motifs known to bind the redox-regulated transcription factors Fnr or Arc of E. coli (31, 35), but this is not surprising, as these factors regulate the expression of enzymes of the fermentative metabolism and C. acidovorans is known to be nonfermentative (70). Nevertheless, C. acidovorans can grow in oxygen-depleted environments and was shown to excrete incompletely oxidized metabolites when the oxygen level became extremely low (65). Omp21 may have a functional role under such conditions.
Finally, it is noteworthy that Omp21 is homologous to virulence proteins, among them Ail, Rck, PagC, and Lom. These are involved in adhesion to and invasion of epithelial cells (4, 10, 43, 49). Since C. acidovorans can cause secondary infections (6, 8), Omp21 may also contribute to pathogenicity.
ACKNOWLEDGMENTS
We thank J. Kellermann for the amino acid sequence analyses, C. Eckerskorn for MALDI mass spectrometry, and G. Weber for measuring the isoelectric point and putting his equipment at our disposal. We also thank Marius Boicu for DNA sequencing, Uta Schimanko for synthesizing oligonucleotides, Anton Mathes for conductivity measurements, and M. Kania for critically reading the manuscript.
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (SFB 266/D4).
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Applegate M, Juhn G, Liechty M, Moore M, Hozier J. Use of DNA purified in situ from cells embedded in agarose plugs for the molecular analysis of tk−/− mutants recovered in the L5178Y tk+/− 3.7.2C mutagen assay system. Mutat Res. 1990;245:55–59. doi: 10.1016/0165-7992(90)90026-g. [DOI] [PubMed] [Google Scholar]
- 3.Baldermann, C., and H. Engelhardt. Unpublished data.
- 4.Barondess J J, Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature. 1990;346:871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- 5.Bhat K S, Gibbs C P, Barrera O, Morrison S G, Jahnig F, Stern A, Kupsch E M, Meyer T F, Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 6.Bofill L, Wessolossky M, Vicent E, Salas M, Besso J, Merentes A, Istúriz R, Guzmán M, Murillo J. Septic shock due to Comamonas acidovorans: a most unusual association. Infect Dis Clin Pract. 1996;5:73–74. [Google Scholar]
- 7.Bowers T J, Sweger D, Anderson B E. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Cloning, sequencing, and expression of a Bartonella henselae bacteriophage 31kDa protein, abstr. C-119; p. 141. [Google Scholar]
- 8.Castagnola E, Tasso L, Conte M, Nantron M, Barretta A, Giacchino R. Central venous catheter-related infection due to Comamonas acidovorans in a child with non-Hodgkin’s lymphoma. Clin Infect Dis. 1994;19:559–560. doi: 10.1093/clinids/19.3.559-a. [DOI] [PubMed] [Google Scholar]
- 9.Chalcroft J P, Engelhardt H, Baumeister W. Three-dimensional structure of a regular surface layer from Pseudomonas acidovorans. Arch Microbiol. 1986;144:196–200. [Google Scholar]
- 10.Cirillo D M, Heffernan E J, Wu L, Harwood J, Fierer J, Guiney D G. Identification of a domain in Rck, a product of the Salmonella typhimurium virulence plasmid, required for both serum resistance and cell invasion. Infect Immun. 1996;64:2019–2023. doi: 10.1128/iai.64.6.2019-2023.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Climent N, Ferrer S, Rubires X, Merino S, Tomas J M, Regue M. Molecular characterization of a 17-kDa outer-membrane protein from Klebsiella pneumoniae. Res Microbiol. 1997;148:133–143. doi: 10.1016/S0923-2508(97)87644-9. [DOI] [PubMed] [Google Scholar]
- 12.Cloeckaert A, Verger J-M, Grayon M, Zygmunt M S, Grépinet O. Nucleotide sequence and expression of the gene encoding the major 25-kilodalton outer membrane protein of Brucella ovis: evidence for antigenic shift, compared with other Brucella species, due to a deletion in the gene. Infect Immun. 1996;64:2047–2055. doi: 10.1128/iai.64.6.2047-2055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello G M, Vipond R, MacIntyre S. Aeromonas salmonicida possesses two genes encoding homologs of the major outer membrane protein, OmpA. J Bacteriol. 1996;178:1623–1630. doi: 10.1128/jb.178.6.1623-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d’Aubenton Carafa Y, Brody E, Thermes C. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol. 1990;216:835–858. doi: 10.1016/s0022-2836(99)80005-9. [DOI] [PubMed] [Google Scholar]
- 15.de Cock H, Struyve M, Kleerebezem M, van der Krift T, Tommassen J. Role of the carboxy-terminal phenylalanine in the biogenesis of outer membrane protein PhoE of Escherichia coli K-12. J Mol Biol. 1997;269:473–478. doi: 10.1006/jmbi.1997.1069. [DOI] [PubMed] [Google Scholar]
- 16.De Groote M A, Ochsner U A, Shiloh M, Nathan C, McCord J, Dinauer M C, Fang F C. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. The role of Cu,Zn-superoxide dismutase (SodC) in Salmonella typhimurium pathogenesis, abstr. B-279; p. 77. [Google Scholar]
- 17.De Mot R, Vanderleyden J. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both gram-positive and gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol Microbiol. 1994;12:333–334. doi: 10.1111/j.1365-2958.1994.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 18.Denome S A, Stanley D C, Olson E S, Young K D. Metabolism of dibenzothiophene and naphthalene in Pseudomonas strains: complete DNA sequence of an upper naphthalene catabolic pathway. J Bacteriol. 1993;175:6890–6901. doi: 10.1128/jb.175.21.6890-6901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deutsche Sammlung von Mikroorganismen. Catalogue of strains. Göttingen, Germany: Gesellschaft für biotechnologische Forschung mbH; 1983. [Google Scholar]
- 20.Eckerskorn C, Grimm R. Enhanced in situ gel digestion of electrophoretically separated proteins with automated peptide elution onto mini reversed-phase columns. Electrophoresis. 1996;17:899–906. doi: 10.1002/elps.1150170511. [DOI] [PubMed] [Google Scholar]
- 21.Engelhardt H, Gerbl Rieger S, Krezmar D, Schneider-Voss S, Engel A, Baumeister W. Structural properties of the outer membrane and the regular surface protein of Comamonas acidovorans. J Struct Biol. 1990;105:92–102. [Google Scholar]
- 22.Engelhardt H, Gerbl Rieger S, Santarius U, Baumeister W. The three-dimensional structure of the regular surface protein of Comamonas acidovorans derived from native outer membranes and reconstituted two-dimensional crystals. Mol Microbiol. 1991;5:1695–1702. doi: 10.1111/j.1365-2958.1991.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferenci T. From sequence alignment to structure prediction: the case of the OmpF porin family. Mol Microbiol. 1994;14:188–189. doi: 10.1111/j.1365-2958.1994.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 24.Finnen R L, Martin N L, Siehnel R J, Woodruff W A, Rosok M, Hancock R E. Analysis of the Pseudomonas aeruginosa major outer membrane protein OprF by use of truncated OprF derivatives and monoclonal antibodies. J Bacteriol. 1992;174:4977–4985. doi: 10.1128/jb.174.15.4977-4985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleckenstein J M, Kopecko D J, Warren R L, Elsinghorst E A. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect Immun. 1996;64:2256–2265. doi: 10.1128/iai.64.6.2256-2265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedlom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 27.Flower D R, North A C T, Attwood T K. Structure and sequence relationships in the lipocalins and related proteins. Protein Sci. 1993;2:753–761. doi: 10.1002/pro.5560020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freiberg C, Perret X, Broughton W J, Rosenthal A. Sequencing the 500-kb GC-rich symbiotic replicon of Rhizobium sp. NGR234 using dye terminators and a thermostable “sequenase”: a beginning. Genome Res. 1996;6:590–600. doi: 10.1101/gr.6.7.590. [DOI] [PubMed] [Google Scholar]
- 29.Gerbl-Rieger S, Engelhardt H, Peters J, Kehl M, Lottspeich F, Baumeister W. Topology of the anion-selective porin Omp32 from Comamonas acidovorans. J Struct Biol. 1992;108:14–24. doi: 10.1016/1047-8477(92)90003-s. [DOI] [PubMed] [Google Scholar]
- 30.Gerbl-Rieger S, Peters J, Kellermann J, Lottspeich F, Baumeister W. Nucleotide and derived amino acid sequences of the major porin of Comamonas acidovorans and comparison of porin primary structures. J Bacteriol. 1991;173:2196–2205. doi: 10.1128/jb.173.7.2196-2205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green J, Irvine A S, Meng W, Guest J R. FNR-DNA interactions at natural and semi-synthetic promoters. Mol Microbiol. 1996;19:125–137. doi: 10.1046/j.1365-2958.1996.353884.x. [DOI] [PubMed] [Google Scholar]
- 32.Gromiha M M, Ponnuswamy P K. Prediction of transmembrane beta-strands from hydrophobic characteristics of proteins. Int J Pept Protein Res. 1993;42:420–431. doi: 10.1111/j.1399-3011.1993.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 33.Hancock R E W, Carey A M. Outer membrane of Pseudomonas aeruginosa: heat- and 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979;140:902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jalajakumari M B, Manning P A. Nucleotide sequence of the gene, ompW, encoding a 22kDa immunogenic outer membrane protein of Vibrio cholerae. Nucleic Acids Res. 1990;18:25. doi: 10.1093/nar/18.8.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser M, Sawers G. Overlapping promoters modulate Fnr- and ArcA-dependent anaerobic transcriptional activation of the focApfl operon in Escherichia coli. Microbiology. 1997;143:775–783. doi: 10.1099/00221287-143-3-775. [DOI] [PubMed] [Google Scholar]
- 36.Kleffel B, Garavito R M, Baumeister W, Rosenbusch J P. Secondary structure of a channel-forming protein: porin from E. coli outer membranes. EMBO J. 1985;4:1589–1592. doi: 10.1002/j.1460-2075.1985.tb03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klesney-Tait J, Hiltke T J, Maciver I, Spinola S M, Radolf J D, Hansen E J. The major outer membrane protein of Haemophilus ducreyi consists of two OmpA homologs. J Bacteriol. 1997;179:1764–1773. doi: 10.1128/jb.179.5.1764-1773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konkel M E, Garvis S G, Tipton S L, Anderson D E, Jr, Cieplak W., Jr Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol Microbiol. 1997;24:953–963. doi: 10.1046/j.1365-2958.1997.4031771.x. [DOI] [PubMed] [Google Scholar]
- 39.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence C E, Altschul S F, Boguski M S, Liu J S, Neuwald A F, Wootton J C. Detecting subtle sequence signals: a Gibbs sampling strategy for multiple alignment. Science. 1993;262:208–214. doi: 10.1126/science.8211139. [DOI] [PubMed] [Google Scholar]
- 41.Mathes, A., and H. Engelhardt. Nonlinear and asymmetric open channel characteristics of an ion-selective porin in planar membranes. Biophys. J., in press. [DOI] [PMC free article] [PubMed]
- 42.Mathes, A., C. Baldermann, and H. Engelhardt. Unpublished data.
- 43.Miller V L, Bliska J B, Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990;172:1062–1069. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morona R, Klose M, Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984;159:570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrissey J H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 46.Murphy T F, Kirkham C, Lesse A J. The major heat modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol Microbiol. 1993;10:87–97. doi: 10.1111/j.1365-2958.1993.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 47.Perlman D, Halvorson H O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983;167:391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- 48.Preston A, Maskell D, Johnson A, Moxon E R. Altered lipopolysaccharide characteristic of the I69 phenotype in Haemophilus influenzae results from mutations in a novel gene, isn. J Bacteriol. 1996;178:396–402. doi: 10.1128/jb.178.2.396-402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulkkinen W S, Miller S I. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J Bacteriol. 1991;173:86–93. doi: 10.1128/jb.173.1.86-93.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roest H P, Mulders I H, Wijffelman C A, Lugtenberg B J. Isolation of ropB, a gene encoding a 22-kDa Rhizobium leguminosarum outer membrane protein. Mol Plant-Microbe Interact. 1995;8:576–583. [PubMed] [Google Scholar]
- 51.Rost B, Sander C. Improved prediction of protein secondary structure by use of sequence profiles and neural networks. Proc Natl Acad Sci USA. 1993;90:7558–7562. doi: 10.1073/pnas.90.16.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schirmer T, Keller T A, Wang Y F, Rosenbusch J P. Structural basis for sugar translocation through maltoporin channels at 3.1 Å resolution. Science. 1995;267:512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- 53.Stathopoulos C. An alternative topological model for Escherichia coli OmpA. Protein Sci. 1996;5:170–173. doi: 10.1002/pro.5560050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stern A, Meyer T F. Common mechanism controlling phase and antigenic variation in pathogenic Neisseriae. Mol Microbiol. 1987;1:5–12. doi: 10.1111/j.1365-2958.1987.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 55.Stoltzfus A, Leslie J F, Milkman R. Molecular evolution of the Escherichia coli chromosome. I. Analysis of structure and natural variation in a previously uncharacterized region between trp and tonB. Genetics. 1988;120:345–358. doi: 10.1093/genetics/120.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoorvogel J, van Bussel M J A W M, Tommassen J, van de Klundert J A M. Molecular characterization of an Enterobacter cloacae outer membrane protein (OmpX) J Bacteriol. 1991;173:156–160. doi: 10.1128/jb.173.1.156-160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stormo G D, Schneider T D, Gold L M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982;10:2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugawara E, Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992;267:2507–2511. [PubMed] [Google Scholar]
- 59.Susi H, Byler D M. Resolution-enhanced Fourier transform infrared spectroscopy of enzymes. Methods Enzymol. 1986;130:290–311. doi: 10.1016/0076-6879(86)30015-6. [DOI] [PubMed] [Google Scholar]
- 60.Tamaoka J, Ha D-M, Komagata K. Reclassification of Pseudomonas acidovorans den Dooren de Jong 1926 and Pseudomonas testosteroni Marcus and Talalay 1956 as Comamonas acidovorans comb. nov. and Comamonas testosteroni comb. nov., with an emended description of the genus Comamonas. Int J Syst Bacteriol. 1987;37:52–59. [Google Scholar]
- 61.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 62.van Beilen J B, Eggink G, Enequist H, Bos R, Witholt B. DNA sequence determination and functional characterization of the OCT-plasmid-encoded alkJKL genes of Pseudomonas oleovorans. Mol Microbiol. 1992;6:3121–3136. doi: 10.1111/j.1365-2958.1992.tb01769.x. [DOI] [PubMed] [Google Scholar]
- 63.van der Ley P. Three copies of a single protein II-encoding sequence in the genome of Neisseria gonorrhoeae JS3: evidence for gene conversion and gene duplication. Mol Microbiol. 1988;2:797–806. doi: 10.1111/j.1365-2958.1988.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 64.Vizcaíno N, Cloeckaert A, Zygmunt M S, Dubray G. Cloning, nucleotide sequence, and expression of the Brucella melitensis omp31 gene coding for an immunogenic major outer membrane protein. Infect Immun. 1996;64:3744–3751. doi: 10.1128/iai.64.9.3744-3751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vollbrecht D, El Nawawy M A. Restricted oxygen supply and excretion of metabolites. Eur J Appl Microbiol. 1980;9:1–8. [Google Scholar]
- 66.Von Heijne G. Signal sequences: the limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 67.Weber G, Bocek P. Optimized continuous flow electrophoresis. Electrophoresis. 1996;17:1906–1910. doi: 10.1002/elps.1150171216. [DOI] [PubMed] [Google Scholar]
- 68.Weiss M S, Abele U, Weckesser J, Welte W, Schiltz E, Schulz G E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991;254:1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- 69.Wessel D, Flügge U I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 70.Willems A. The genus Comamonas. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. III. New York, N.Y: Springer-Verlag; 1992. [Google Scholar]
- 71.Wolff K, Stern A. Identification and characterization of specific sequences encoding pathogenicity associated proteins in the genome of commensal Neisseria species. FEMS Microbiol Lett. 1995;125:255–263. doi: 10.1111/j.1574-6968.1995.tb07366.x. [DOI] [PubMed] [Google Scholar]