Abstract
Context
Liver fat content and visceral fat volume are associated with insulin resistance and cardiovascular disease and are higher in men than in women.
Objective
To determine the effect of estradiol and testosterone treatment on liver fat and visceral fat in transgender persons.
Design
Open-label intervention study (SHAMVA) with a 1-year follow-up.
Setting
Gender clinic in a hospital.
Patients
8 trans women and 18 trans men receiving hormone treatment.
Interventions
Trans women received an antiandrogen and after 6 weeks estradiol was added. Trans men were randomized to receive triptorelin, testosterone, and anastrozole for 12 weeks or triptorelin and testosterone for 12 weeks, followed by only testosterone until week 52.
Main outcome measures
Liver fat content, visceral and abdominal subcutaneous fat volume, measured by magnetic resonance spectrometry or imaging at baseline, 6, 8, 18, and 58 weeks in transwomen or at baseline; at 6 and 12 weeks in trans men with anastrozole; and at 52 weeks in trans men without anastrozole.
Results
In trans women, liver fat content decreased by 1.55% (−2.99 to −0.12) after 58 weeks, compared to week 6. Visceral fat did not change. In trans men with anastrozole, the liver fat content and visceral fat volume did not change. In trans men without anastrozole, after 52 weeks, liver fat content increased by 0.83% (0.14 to 1.52) and visceral fat volume increased by 34% (16 to 51).
Conclusions
Sex hormones regulate liver fat content and visceral fat in men and women.
Keywords: sex steroids, liver fat, visceral fat, transgender
It has been well established that the distribution of regional body fat is a more important risk factor for cardiovascular disease than the total amount of body fat (1-3). Specifically liver fat and visceral adipose tissue (VAT) are associated with insulin resistance and metabolic and cardiovascular disease risks (2, 4). Both liver fat and visceral fat show a clear sexual dimorphism. Men are more susceptible to store fat within the visceral compartment and liver whereas premenopausal women preferentially store fat in the subcutaneous compartment (5, 6). In both men and women, the amount of liver fat, VAT and the VAT/ subcutaneous adipose tissue (SAT) ratio (the amount of VAT relative to SAT) increase gradually with aging (6, 7). Around menopause, liver and visceral fat increase rapidly, resulting in a more masculine distribution of body fat in postmenopausal women (8, 9). These observations suggest a major role for sex steroids in the regulation of liver and visceral fat.
The role of estradiol in body fat distribution in women has been studied in postmenopausal women, where the decrease in estradiol is associated with an increase in liver fat and VAT. Increasing estradiol through hormone replacement treatment is associated with a decrease in liver fat and VAT (9, 10). High testosterone concentrations in women, on the other hand, are associated with more liver and visceral fat (11, 12). In men, the role of estradiol in body fat distribution is less clear. Circulating testosterone is converted to estradiol by the enzyme aromatase and is therefore difficult to study separately from estradiol. The importance of estradiol is emphasized by an animal model using male aromatase knockout mice. These mice display more liver and visceral fat than wild-type male mice, and these fat depots can be reduced by estradiol treatment (13). Furthermore, a case report on a man with congenital aromatase deficiency showed the same pattern of increased liver and abdominal fat, which could be reduced by estrogen treatment (14). Low testosterone concentrations in men are associated with increased liver and visceral fat (15-17).
As part of their transition, trans women (assigned male at birth, identify as female) and trans men (assigned female at birth, identify as male) can be treated with estradiol and antiandrogens or testosterone, respectively (18). This provides a unique clinical setting to determine whether the sex differences in body fat distribution and liver fat deposition are regulated by estradiol and/or testosterone. Previously, Elbers et al showed that VAT increased after administration of testosterone in trans men and also after administration of estradiol and antiandrogens in trans women. In the latter group, however, the VAT/SAT ratio decreased because of a concurrent increase in SAT (19, 20). The interpretation of the separate effects of testosterone and estradiol is hampered by the aromatization of testosterone to estradiol, which causes both sex steroids to affect body fat distribution at the same time. Therefore, in this study we (1) determine the effects of estradiol and testosterone on the amount of VAT in trans women and trans men and (2) for the first time determine the effects of estradiol and testosterone on liver fat content in trans women and trans men. This will provide unique mechanistic insights into the regulatory role of sex steroids in body fat distribution and improve transgender care.
Methods
Study Design
This study is part of the SHAMVA (the effects of Sex Hormone Administration on Marrow and Visceral Adiposity) study, a partly randomized open-label intervention study that is registered in the Dutch trial register Trial NL7513. This study was conducted in accordance with the declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013) and approved by the medical research ethics committee of the Amsterdam UMC. All participants were offered a minimum of 5 days to consider their decision before giving written informed consent. This study was conducted in The Center of Expertise on Gender Dysphoria of Amsterdam University Medical Centers (Amsterdam UMC), the largest gender clinic in the Netherlands. The screening and recruitment period ran from March 2019 until February 2021.
In this study trans women and trans men were included and liver fat content, and the amount of VAT and SAT was measured before and during their first year of hormone treatment. To separate the effects of estradiol and testosterone in trans women, they were first treated with triptorelin only [a gonadotrophin-releasing hormone (GnRH) analogue] for 6 weeks. To confirm testosterone suppression, trans women collected and sent us a saliva sample 4 weeks after their first triptorelin injection. The second step in trans women was increasing estradiol by simultaneous administration of triptorelin and estradiol for 52 weeks. SAT and VAT and liver fat content were quantified by magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) at baseline and after 6, 8, 18, and 58 weeks. Trans men were randomized to receive triptorelin and testosterone with anastrozole (an aromatase inhibitor, used to suppress the aromatization of testosterone into estradiol) or triptorelin and testosterone without anastrozole for 12 weeks, followed by only testosterone until week 52. VAT and liver fat content were determined at baseline and after 6, 12, and 52 weeks. For trans men with anastrozole, the study finished after 12 weeks, because of the negative effect of anastrozole on bone mineral density after 12 weeks (21).
In the initial protocol, trans men would start with gonadal suppression by triptorelin only. However, after 6 months of inclusion, only 1 trans man was willing to participate. This was due to the delay of the start of testosterone and possible negative effects of gonadal suppression in trans men (climacteric complaints). Therefore, the protocol was amended and approved to omit the 6 weeks of gonadal suppression and start with testosterone at baseline. We excluded the visits at 6 and 8 weeks for the first participant, who followed the initial protocol, because of the mismatch in hormone concentrations between this participant and the other participants in this group.
Participants and Sample Size
Participants were consecutively screened and recruited based on a list of trans persons who were starting hormone treatment. Eligible participants were trans women or trans men, diagnosed with gender dysphoria according to the revised fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (22) and planned to start with hormone treatment in our gender clinic in the Amsterdam UMC. Participants were between 18 and 50 years old, and trans men were premenopausal. Exclusion criteria were previous or current use of sex hormones or hormonal contraceptives or contraindications for MRI. Written informed consent was obtained from all participants.
Based on the data from a previous study by Elbers et al (19), we needed 6 subjects in each group to detect a 6 cm2 difference in VAT, with a power of 0.8 at a significance level of 0.05 (Software used: nQuery Advisor version 7.0). We measured VAT in volume instead of area; however, since our slices are 1 cm in thickness, volume is identical to area. To account for potential dropouts, we included 8 subjects in each group, resulting in 24 subjects in total. Due to the COVID pandemic, we missed multiple visits in the trans men without anastrozole group and therefore included 2 extra participants in that group to compensate. Trans men were randomized into 2 groups using nonstratified block randomization in the program R [R Core Team (2018)] and informed of the randomization allocation. Due to a subject withdrawal between randomization and the first study procedure, a new block randomization was performed for the last 3 participants.
Treatment Protocol
Trans women were treated with triptorelin, a GnRH analogue (3.75 mg every 4 weeks). After 6 weeks, transdermal estradiol (estradiol patch 100 mcg/24 hours) was added. After 18 weeks, at the discretion of the treating physician, 3 participants switched from transdermal to oral estradiol (4 mg once a day), while antiandrogen therapy was switched from triptorelin 3.75 mg every 4 weeks to triptorelin 1 125 mg every 3 months (n = 3) or cyproterone acetate 25 mg once a day (n = 3). Target values for serum estradiol concentrations were set at 200 to 600 pmol/L and at <2.5 nmol/L for testosterone, in accordance with our local protocol. Estradiol and antiandrogen dosages were adjusted if estradiol or testosterone concentrations were outside the target range.
Trans men were randomized into 2 groups. The first group, trans men with anastrozole (n = 8), was treated with triptorelin (3.75 mg every 4 weeks), testosterone gel (50 mg, once daily), and anastrozole, an aromatase inhibitor, (1 mg, once daily) for 12 weeks. The second group, trans men without anastrozole (n = 10), was treated with triptorelin (3.75 mg every 4 weeks) and testosterone gel (50 mg, once daily) for 12 weeks. At 12 weeks, trans men in this group continued for 40 weeks with only testosterone and could switch the testosterone gel to either testosterone undecanoate injections (1000 mg once every 12 weeks, n = 5) or testosterone esters (250 mg once every 3 weeks, n = 1). Serum testosterone target values were set at 10 to 30 nmol/L. Testosterone dosages were adjusted if testosterone concentrations were outside the target range.
Outcomes
Primary outcome measures were change in liver fat content, visceral fat volume (cm3), and subcutaneous fat volume (cm3).
Magnetic resonance acquisition
All participants were scanned on a 3.0 Tesla MRI scanner (Ingenia; Philips, Best, the Netherlands) using a 16-channel phased-array anterior coil and a 10-channelphase-arrayed posterior coil. All data were acquired in a single session of approximately 30 minutes. All scans were made by 2 observers who were blinded to the randomization group in the trans men and analyzed by 1 observer.
To estimate the liver fat content, 2 methods were used: MRS and MRI proton density fat fraction (PDFF). The MRS method is described in more detail in a previous paper (23). In short, data were acquired using a 1H-MRS multiecho stimulated-echo mode with a single voxel (20 × 20 × 20 mm3), placed in segment VII or VIII of the liver. Echo times (TEs) were 10, 15, 20, 25, and 30 ms with a repetition time of 3500 ms. The MRI method consisted of a two-dimensional multiecho gradient echo sequence with 6 TEs, according to our previously described protocol (24). To quantify the amount of VAT and SAT, a three-point Dixon method was used. This method was performed using a single slice two-dimensional multiecho gradient echo sequence with 3 TEs. The sequence acquired both magnitude and phase data. This sequence had a field of view of 420 × 300 mm2, a resolution of 2.4 × 2.4 mm2, and slice thickness of 10 mm. The same sequence was acquired at 2 different locations: the L2-L3 level and the umbilicus (L4 or L4-L5) level. The repetition time was 50 ms; the TEs were 3.1, 3.88, and 4.66 ms; the flip angle was 5°; the bandwidth was 436 Hz; and the acquisition duration was 19 seconds breath-hold per slice.
Magnetic resonance analysis
MRS data of the liver was analyzed according to a previous protocol (23). The AMARES algorithm (25) was used to fit spectral data in jMRUI version 4.0 (26). For each visit for each subject, PDFF MRS values were calculated, using the combined fat peak and T2-corrected water amplitudes, after correcting for the amplitudes of fat peaks overlapping the water peak (27). The MRI method of the liver was analyzed according to a previous protocol (24). One region of interest (ROI) was placed in the right hepatic lobe in each of the 3 slices, resulting in 3 ROIs. These ROIs were selected by avoiding liver edges, large vessels, and bile ducts. Mean signal intensity per TE was determined, and the PDFF was calculated in Matlab R2018a (Mathworks, Natick, MA, USA). The PDFFs of the 3 ROIs were averaged to calculate an average fat content of the liver. The three-point Dixon VAT and SAT image analysis was performed in Matlab, R2018a (Mathworks). Fat fraction images, scaled from 0% to 100% fat, were generated using a toolbox with multipoint fat-water separation using a hierarchical field map estimation (28) from the ISMRM Fat-Water Toolbox 2012. To calculate VAT volume, an ROI was selected including only VAT, excluding SAT and intermuscular fat. To calculate SAT volume, first a ROI was selected for the outer outline of the SAT and then a ROI for the inner outline of SAT was selected, excluding VAT and intermuscular fat. Both ROIs were used to perform a semiautomatic segmentation, identifying adipose tissue as all voxels with a fat fraction greater than the maximal fat fraction divided by 2 (29). The reproducibility of the VAT and SAT measurements was assessed in 10 volunteers (4 females and 6 males). Within-subject coefficient of variation values for the intraday measurements were 5.5% for VAT and 4.0% for SAT; within-subject coefficient of variation values for the interday measurements were 10.4% for VAT and 9.6% for SAT.
Laboratory Measurements
Fasting blood samples were collected at all visits. Baseline blood samples in trans men were not taken at a specific time point in the menstrual cycle. Serum measurements included estradiol, testosterone, aspartate transaminase, alanine transaminase, albumin, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, glucose, and creatinine.
Serum estradiol concentrations were measured in serum using an in-house developed Liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (30). The LC-MS/MS method has a lower limit of quantification of 10 pmol/L for estradiol, the intra-assay variation was <5% between 20 and 1700 pmol/L, and the inter-assay variation was 9% at 21 pmol/L and < 7% at 179 and 760 pmol/L. Serum testosterone was also measured using LC-MS/MS with a limit of quantification of 0.1 nmol/L, a mean inter-assay variation of 2.1%, and a mean intra-assay variation of 2.4% Total cholesterol, HDL, and triglyceride levels were measured using an enzymatic method (Roche Cobas 8000 module c502, Roche Diagnostics, Mannheim, Germany), with an inter-assay coefficient of variation of 1.6% and 1.9%, respectively. LDL values were derived using the Friedewald formula.
Statistical Analysis
Baseline characteristics are presented as mean ± SD or as median [interquartile range (IQR)] when not normally distributed. Outcome measures are presented as mean (95% confidence interval). Liver fat content is presented in percentage; the change in liver fat content is expressed in absolute percentage change. VAT and SAT volumes were calculated by taking the mean of the volume in slice L2-L3 and slice umbilicus. If the volume was only measured in 1 of these slices, the only available slice was used in the analyses (4 visits in trans women, 6 in trans men). Sensitivity analyses showed no differences when we excluded measurements with 1 slice. The change in VAT and SAT volumes is presented as percentage change. The change in VAT/SAT ratio is presented as absolute change in ratio.
Linear mixed models were used to analyze the association between the treatment protocol and change in liver fat content, percentage change in VAT, percentage change in SAT, and VAT/SAT ratio. The visits were chosen as fixed effect, repeated measures were nested within the participants, and a random intercept was included in the model. For each study group, a log likelihood ratio test was performed to test if adding a random slope improved the model. The normality of the residuals was checked by histograms. Linear mixed models were used to handle missing data (31). These analyses were done separately for trans women, trans men with anastrozole, and trans men without anastrozole. In trans women, visit 2 (start of estradiol) was entered as baseline to test for possible differences in effects between only a GnRH analogue and a GnRH analogue and estradiol. The changes in metabolic features were analysed by paired t-tests for each study group. In trans women and trans men without anastrozole, changes between baseline and 1 year were analyzed, and in trans men without anastrozole, changes between baseline and 3 months (end of study for this group) were analyzed.
STATA Statistical Software, version 15.1 (Statacorp, College Station, TX, USA) was used to perform analyses and GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, CA, USA) was used to create graphs.
Results
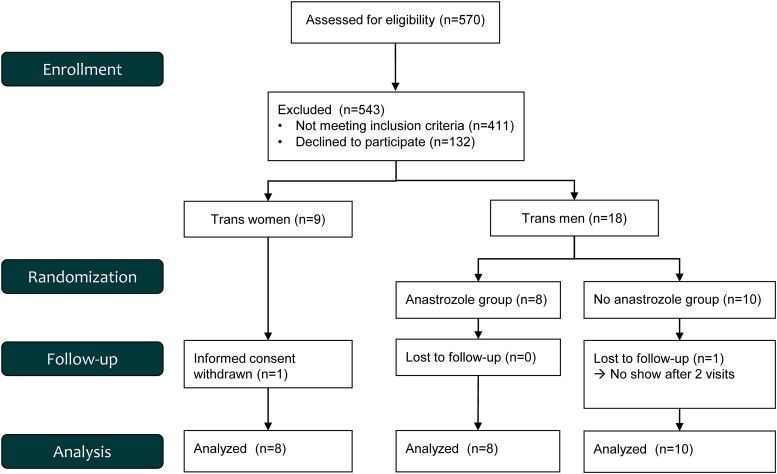
The flowchart of the study is depicted in Fig. 1. The baseline characteristics for the 8 trans women and 18 trans men who completed the study protocol are shown in Table 1.
Figure 1.
Flowchart study.
Table 1.
Baseline characteristics
| Trans women (n = 8) | Trans men | ||
|---|---|---|---|
| With anastrozole (n = 8) | Without anastrozole (n = 10) | ||
| Age (years) | 26 (20-29) | 23 (20-26) | 22 (19-25) |
| BMI (kg/m2) | 22 ± 4 | 28 ± 8 | 25 ± 4 |
| MRS liver fat content (%) | 2.33 (1.07-2.92) | 1.41 (0.45-4.81) | 1.13 (0.60-1.68) |
| VAT (cm3) | 104 ± 74 | 86 ± 63 | 56 ± 36 |
| SAT (cm3) | 121 ± 73 | 289 ± 192 | 201 ± 94 |
| VAT/SAT ratio | 0.91 ± 0.06 | 0.31 ± 0.03 | 0.28 ± 0.04 |
| Estradiol (pmol/L) | 106 (93-149) | 133 (119-438) | 172 (109-446) |
| Testosterone (nmol/L) | 19.9 ± 3.4 | 1.3 ± 0.3 | 1.7 ± 0.6 |
| AST (U/L) | 26.0 ± 7.7 | 22.3 ± 6.7 | 20.6 ± 4.7 |
| ALT (U/L) | 25.1 ± 13.3 | 21.8 ± 14.5 | 21.2 ± 9.1 |
| Albumin (g/L) | 44.9 ± 2.5 | 43.1 ± 3.3 | 43.9 ± 4.3 |
| Cholesterol (mmol/L) | 4.4 ± 0.85 | 4.3 ± 0.4 | 4.5 ± 0.4 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.2 | 1.3 ± 0.3 | 1.4 ± 0.3 |
| LDL cholesterol (mmol/L) | 2.7 ± 0.7 | 2.5 ± 0.4 | 2.7 ± 0.5 |
| Triglycerides (mmol/L) | 1.3 ± 0.5 | 1.2 ± 0.5 | 0.8 ± 0.2 |
| Glucose (mmol/L) | 4.0 ± 0.8 | 4.4 ± 0.7 | 3.9 ± 0.7 |
| Creatinine (µmol/L) | 78.6 ± 10.3 | 64.9 ± 8.5 | 62.0 ± 5.3 |
Baseline characteristics, displayed per study group.
Abbreviations: AST, aspartate transaminase; ALT, alanine transaminase; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MRS, magnetic resonance spectroscopy; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
VAT and SAT are presented in mean volumes of the umbilicus and L2-3 slice (both 1 cm slice thickness).
Hormone Concentrations in Trans Women
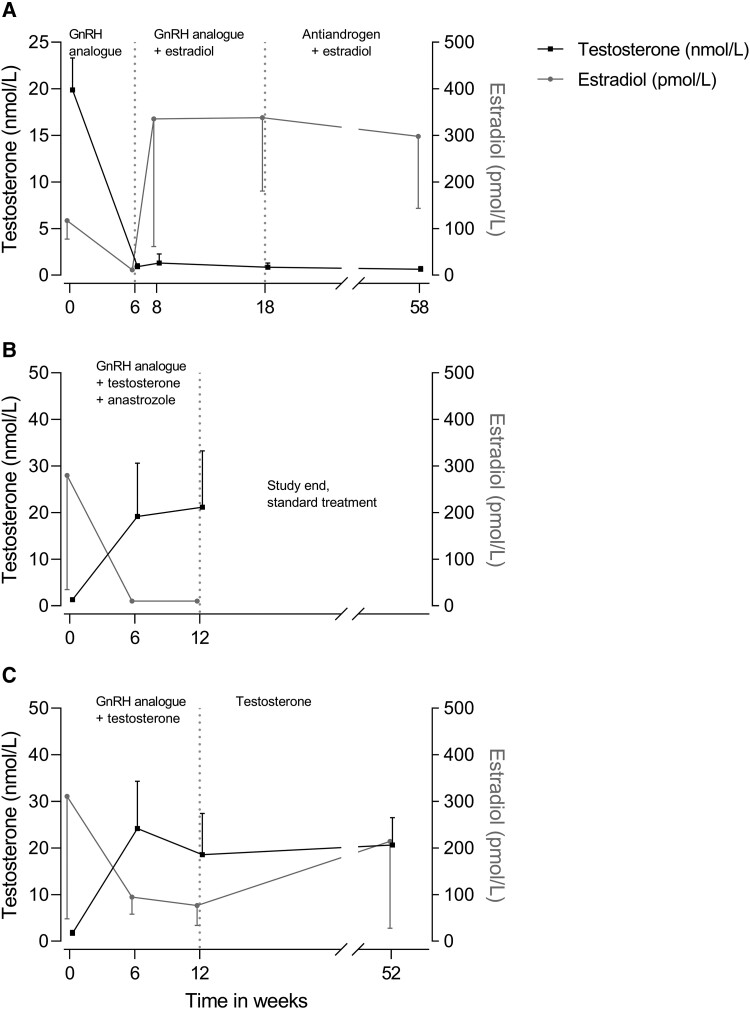
Serum hormone concentrations of trans women during the study are presented in Fig. 2. The testosterone concentration at baseline was 19.8 nmol/L (17.7-21.9), decreased to 0.9 nmol/L (0.7 to 1.2) after 6 weeks of triptorelin, and remained stably suppressed throughout the study. The estradiol concentration was 117 pmol/L (91-143) at baseline and decreased below the detection limit in all trans women after 6 weeks of triptorelin. The estradiol concentration increased to 336 pmol/L (106 to 565) after 2 weeks of estradiol (week 8) and remained stable throughout the study.
Figure 2.
Serum estradiol and testosterone concentrations in (A) trans women, (B) trans men without anastrozole, and (C) trans men with anastrozole. Data presented as mean ± SD.
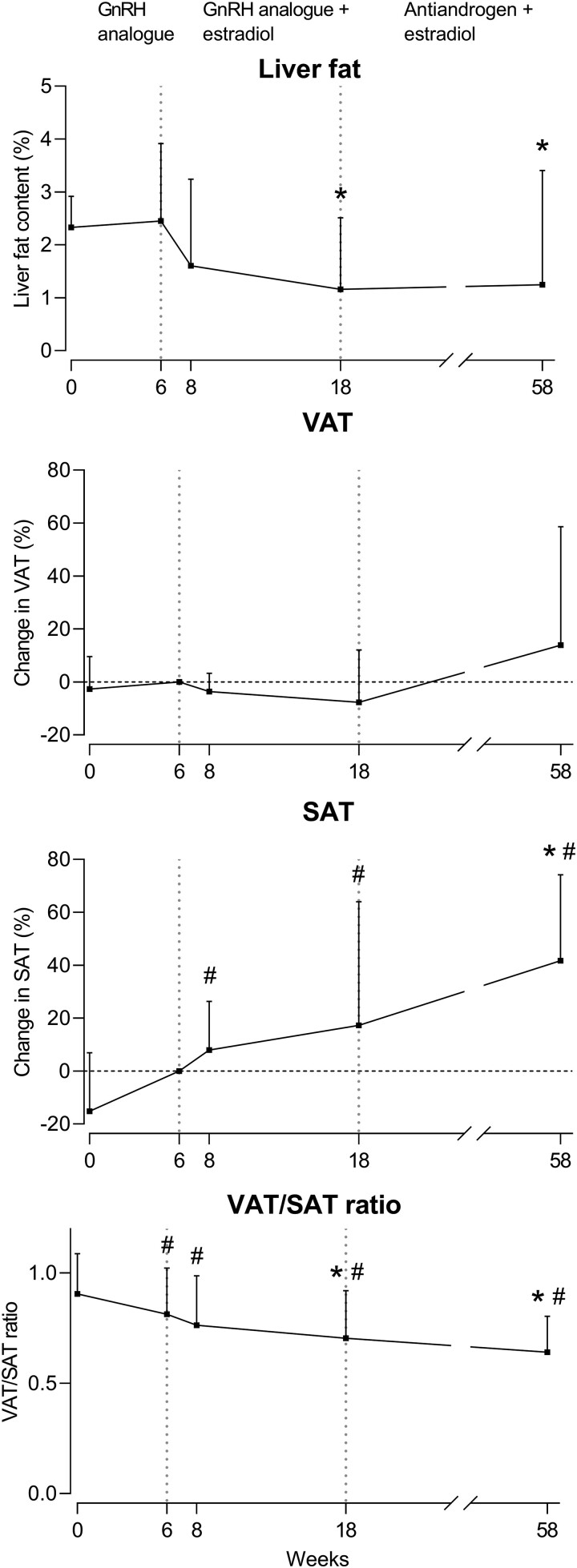
Liver, Visceral and Subcutaneous fat in Trans Women
The liver fat content (measured by MRS), percentage change in VAT volume and SAT volume, and VAT/SAT ratio in trans women are shown in Fig. 3. The liver fat content was 2.33% (IQR 1.07-2.92) at baseline and did not change after 6 and 8 weeks but decreased (absolute change) by 1.93% (−3.43 to −0.44) after 18 weeks and by 1.55% (−2.99 to −0.12) after 58 weeks, compared to 6 weeks. VAT volume was 123 cm3 (41-204) at baseline and did not change after 6, 8, 18, or 58 weeks. SAT volume was 130 cm3 (44-216) at baseline and did not change after 6, 8, or 18 weeks but increased by 42.08% (14.19 to 69.98) after 58 weeks, compared to 6 weeks. The VAT/SAT ratio at baseline was 0.91 (0.75-1.06), decreased by 0.10 (−0.17 to −0.03) at 6 weeks compared to baseline, and decreased further by 0.12 (−0.21 to −0.03) after 18 weeks and 0.19 (−0.28 to −0.10) after 58 weeks, compared to 6 weeks. The changes in liver fat content measured by MRI were comparable to MRS and are shown in supplemental Supplementary Fig. S1 (32).
Figure 3.
Percentage change in liver fat content, VAT, SAT, and VAT/SAT ratio over time in trans women. Liver fat is presented as median with IQR. Change in VAT, SAT, and VAT/SAT ratio are presented as mean ± SD., # P < 0.05 compared to week 0, * P < 0.05 compared to week 6.
Abbreviations: IQR, interquartile range; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Hormone Concentrations in Trans men
The serum hormone concentrations in trans men during this study are presented in Fig. 2. In trans men with anastrozole, the mean testosterone concentration at baseline was 1.3 nmol/L (1.1-1.6), which increased to 19.2 nmol/L (7.2 to 31.2) at 6 weeks and remained stable at 12 weeks. The mean estradiol concentration at baseline was 280 pmol/L (110-450) and decreased below the detection limit in all trans men at both 6 and 12 weeks. In trans men without anastrozole, the mean testosterone concentration at baseline was 1.7 nmol/L (1.3-2.1), increased to 24.2 nmol/L (15.7 to 32.6) after 6 weeks, and remained stable at 12 and 52 weeks. The mean estradiol concentration was 311 pmol/L (148-474) at baseline, decreased to 95 (67 to 123) after 6 weeks, and remained stable at 12 weeks. At 52 weeks, the mean estradiol concentration increased to 218 pmol/L (83 to 352).
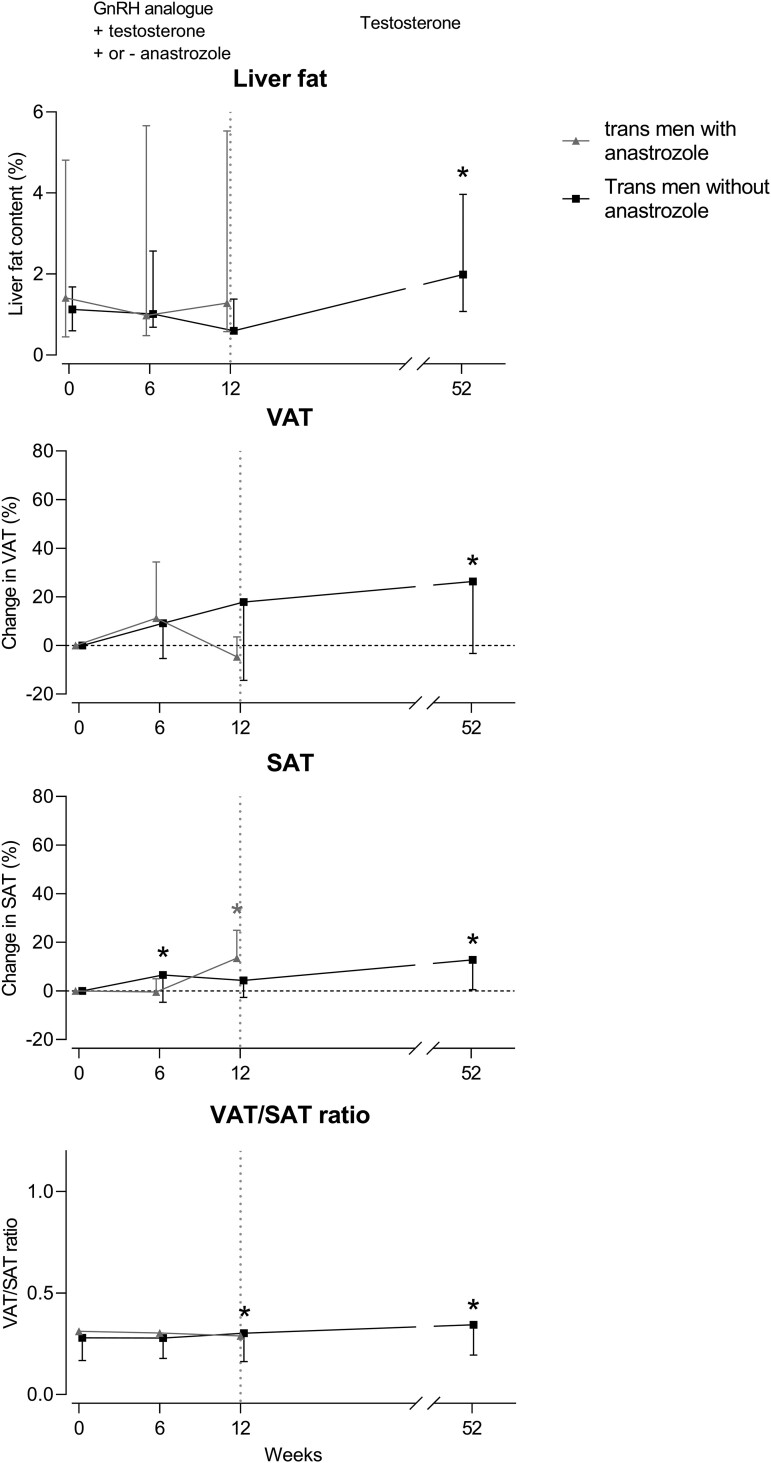
Liver, Visceral and Subcutaneous fat in Trans men
The changes in liver fat content (measured by MRS), VAT volume, SAT volume, and VAT/SAT ratio in trans men are shown in Fig. 4. In trans men with anastrozole, the liver fat content at baseline was 1.41% (IQR 0.45-4.81), which did not change after 6 and 12 weeks. VAT volume was 76 cm3 (19-133) at baseline and did not change after 6 and 12 weeks. SAT volume was 247 cm3 (81-414) at baseline and did not change after 6 weeks but increased by 13.16% (6.45 to 19.87) after 12 weeks. The VAT/SAT ratio was 0.31 (0.24-0.39) at baseline and did not change significantly after 6 and 12 weeks.
Figure 4.
Percentage change in liver fat content, VAT, SAT, and VAT/SAT ratio over time in trans men with anastrozole (grey) and without anastrozole (black). Liver fat is presented as median with IQR. Change in VAT, SAT, and VAT/SAT ratio are presented as mean ± SD. * = P < 0.05 compared to baseline.
Abbreviations: IQR, interquartile range; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
In trans men without anastrozole, the liver fat content at baseline was 1.13% (IQR 0.60-1.68) and did not change significantly after 6 and 12 but increased (absolute change) by 0.83% (0.14 to 1.52) after 52 weeks, compared to baseline. VAT volume was 56 cm3 (29-84) at baseline and did not change after 6 or 12 weeks but increased by 26.8% (9.34 to 44.4) after 52 weeks, compared to baseline. SAT volume was 200 cm3 (128-274) at baseline and increased by 6.5% (0.93 to 12.11) after 6 weeks, did not change after 12 weeks, and increased by 12.9% (3.84 to 21.9) after 52 weeks, compared to baseline. The VAT/SAT ratio was 0.28 (0.19-0.37) at baseline and did not change after 6 weeks but increased by 0.04 (0.01 to 0.07) after 12 weeks and by 0.06 (CI 0.02 to 0.11) after 52 weeks, compared to baseline. The changes in liver fat content measured by MRI were comparable to MRS and are shown in Supplementary Fig. S1 (32).
Changes in Metabolic Features in Trans Women and Trans men
The mean serum aspartate transaminase concentration decreased in trans women by 4.57 U/L (95% CI 6.83 to −2.32, P = .003) after 1 year and did not change in trans men with or without anastrozole. Alanine transaminase and albumin did not change in both trans women and trans men. Total cholesterol increased in trans men with anastrozole by 0.50 mmol/L (95% CI 0.10-0.90, P = .02) after 3 months and did not change in trans women and trans men without anastrozole. Serum concentrations of HDL, LDL, triglycerides, and glucose did not change in either trans women and trans men. Mean serum creatinine concentration decreased in trans women by 9.38 µmol/L (95% CI −16.78 to −1.99, P = .02) after 1 year, increased in trans men with anastrozole by 6.86 µmol/L (95% CI 3.77-9.94, P = .002) after 3 months, and increased in trans men without anastrozole by 8.62 µmol/L (95% CI 1.85-15.40, P = .02) after 1 year.
Discussion
In this study, we researched the effects of testosterone and estradiol treatment on liver fat content and visceral and subcutaneous adipose tissue volume in trans women and trans men. We show that trans women transition into a more feminine body fat distribution, with lower liver fat content and more subcutaneous adipose tissue. Conversely, trans men without anastrozole, transition into a more masculine body fat distribution with higher liver fat content and more visceral adipose tissue.
As far as we know, this is the first longitudinal study describing the effect of hormone treatment on liver fat content in transgender persons. The decrease in liver fat content in trans women and the increase in liver fat content in trans men is in line with epidemiological studies on the prevalence of nonalcoholic fatty liver disease (NAFLD). Several studies have shown that men and postmenopausal women have an increased risk of NAFLD compared to premenopausal women (33, 34). Furthermore, in postmenopausal women with NAFLD, the duration of estrogen deficiency is inversely associated with the risk of fibrosis (35). In addition, postmenopausal women using hormone replacement are less likely to have NAFLD compared to women without hormone replacement (34). Finally, the use of selective estrogen receptor modulators in breast cancer patients is associated with an increase in fat content in the liver and an increased risk of NAFLD (36, 37). The findings of the current study are also in line with a cross-sectional study by Ciardullo et al showing that a higher android/gynoid ratio is associated with a higher prevalence of NAFLD in both women and men (38). Furthermore, in women, a higher android/gynoid ratio was associated with a higher prevalence of fibrosis. Future research should determine whether this also applies to trans men.
We observed no change in liver fat content in trans men using anastrozole, which seems to contradict previous research. Multiple studies have shown the importance of estradiol in the accumulation of liver fat. This is illustrated by men with aromatase deficiency, who display severe hepatic steatosis that could be reduced by administration of estradiol (14, 39). This discrepancy may be explained by the relatively short duration of anastrozole use in our study. Our results indicate that aromatization of testosterone to estradiol does not play a major role in trans men in the first 3 months of hormone treatment. The increase in liver fat content in trans men without anastrozole after 12 and 52 weeks is in line with previous research in women with polycystic ovary syndrome, who have an increased risk of NAFLD (40).
The increase in SAT and decrease in VAT/SAT ratio in trans women and the increase in VAT and increase in VAT/SAT ratio in trans men, after 1 year of hormone treatment, are in line with the known sex differences in body fat distribution [reviewed in (41)]. We observed no change in VAT in trans women. The association between hormone treatment and VAT in trans women is less consistent than that of SAT as previous studies have shown different results. Elbers et al found a small increase in VAT measured by MRI in 20 trans women after 1 year of hormone treatment (19). In contrast, Klaver et al found no change in VAT measured by DXA in 179 trans women after 1 year of hormone treatment (42). The latter study observed a large individual range (−57-52%) in changes in VAT. The difference in imaging method may have contributed to this difference. A previous study in patients with coronary artery disease showed that DXA underestimates the longitudinal changes in VAT compared to MRI (43). However, in the present study, VAT was measured by MRI and we observed similar results as Klaver et al. Another difference between our study and the study of Elbers et al is the use of 17β-estradiol in our study vs the use of ethinyl estradiol in the study by Elbers et al. Ethinyl estradiol is a highly potent synthetic analogue of estradiol but is not measurable in the serum, making it difficult to compare the estrogen exposure between Elbers et al and our study. This could be of relevance, since Wiepjes et al observed a threshold of 200 pmol/L estradiol in serum, above which bone mineral density increased and below which bone mineral density decreased (44). Both Elbers et al and Wiepjes et al did not stratify for estrogen exposure. Additional studies with larger groups of trans women are needed to assess a possible estradiol threshold for changes in VAT.
In trans men using anastrozole, testosterone administration was not associated with changes in VAT, liver fat content, or VAT/SAT ratio after 6 and 12 weeks. We observed a small but significant increase of SAT after 12 weeks. In trans men not using anastrozole, testosterone administration increased liver fat content, VAT, SAT, and VAT/SAT ratio after 52 weeks. The effects on SAT are not in accordance with the study by Finkelstein et al, showing an increase in SAT in men receiving a GnRH analogue, testosterone, and anastrozole but no increase in men receiving only a GnRH analogue and testosterone (45). Our results on VAT are not in accordance with the study by Finkelstein et al, who showed that the intra-abdominal fat area increased more in men using anastrozole than in men not using anastrozole. This discrepancy with our study might be explained by the different study duration (16 weeks vs 12 weeks) or the larger sample size (198 men vs 18 trans men). The increase in VAT in trans men not using anastrozole is in line with the study by Elbers et al, who showed an increase in VAT after 12 months of testosterone administration in trans men (19). This is not in accordance with the study by Klaver et al, which found no change in VAT in trans men after 1 year of hormone treatment (42). Again, this study used DXA to estimate VAT, which might explain this difference. Together these data suggest that 12 months of testosterone administration increases VAT in trans men.
The mechanisms underlying the sex difference in liver fat content and body fat distribution remain poorly understood. The major estrogen receptor (ER) that regulates VAT, SAT, and liver fat in both men and women is ERα (46, 47). Heine et al was the first to show that ERα whole body knockout mice, both female and male, were more prone to obesity (47). They further demonstrated that the disruption of ERα, but not ERβ, signaling is associated with an increase in VAT. One of the mechanisms by which estrogen signaling protects against hepatic steatosis is reduction of de novo lipogenesis (9). Adipose tissue further expresses the androgen receptor gene in both men and women and shows no gender dimorphism in androgen binding (48). The androgen receptor gene is expressed more in VAT than in SAT. Therefore, testosterone effects would be more pronounced in VAT than in SAT. Furthermore, testosterone seems to upregulate the density of the androgen receptor, while estradiol downregulates this density (49).
This is the first study to examine the longitudinal effect of testosterone and estradiol on the liver fat content in trans gender persons. The liver fat percentages of both trans women and trans men remained largely within the normal range [< 5.56% (50)] throughout the study, except for 2 trans men who met the criterion for hepatic steatosis (liver fat content > 5%). This could have clinical implications and physicians should be aware of this. A limitation of this study is the short follow-up time in the anastrozole group. Therefore, we cannot draw conclusions about the long-term effects of aromatization on VAT, SAT, and liver fat. Another limitation is the lack of control groups in both trans women and trans men. We cannot rule out that VAT naturally changes during the timespan of our study. Likewise, we cannot exclude that changes in physical activity and diet have contributed to the observed changes but deem this unlikely based on the study by Jones et al, who showed that both transgender women and transgender men engage in significantly less exercise than cis women and cis men (51). Furthermore, they found that transgender persons using gender-affirming hormone treatment engaged in more exercise than transgender persons who did not. When compared to age matched cisgender persons, trans women exercised as much as cis women; however, trans men exercised less than cis men. As far as we know, there are no prospective studies on the effect of hormone treatment on nutritional habits in transgender persons. Three trans women switched from triptorelin to cyproterone acetate after the fourth visit; therefore cyproterone acetate could have confounded the results at the fifth visit. The effects of cyproterone acetate on fat depots are not precisely known. However, we observed no change in trend on the effect on the different fat depots within these trans women. Based on the results of our study, we cannot compare between different routes of estradiol administration, ie, oral or transdermal, since all women were started on transdermal administration and only 3 switched to oral before the last visit.
In conclusion, we showed that sex steroids play a major role in the regulation of body fat distribution in both men and women. In trans women, combined estradiol and antiandrogen treatment decreases liver fat content and increases subcutaneous adipose tissue. Testosterone treatment increases liver fat content and visceral adipose tissue in trans men. Differences in sex hormones between men and women may explain the sexual dimorphism in liver fat content. We observed no relevant differences between trans men with and without anastrozole after 6 and 12 weeks, indicating that aromatization has no major role in the regulation of fat depots in trans men within a time frame of 3 months.
Contributor Information
Marieke Tebbens, Department of Endocrinology, Amsterdam UMC Vrije Universiteit Amsterdam, Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; Amsterdam Movement Sciences, Amsterdam, The Netherlands.
Moya Schutte, Department of Endocrinology, Amsterdam UMC Vrije Universiteit Amsterdam, Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; Amsterdam Movement Sciences, Amsterdam, The Netherlands.
Marian A Troelstra, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Amsterdam, The Netherlands.
Eveline Bruinstroop, Department of Endocrinology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Movement Sciences, Amsterdam, The Netherlands.
Renée de Mutsert, Department of Clinical Epidemiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands.
Aart J Nederveen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Amsterdam, The Netherlands.
Martin den Heijer, Department of Endocrinology, Amsterdam UMC Vrije Universiteit Amsterdam, Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; Amsterdam Movement Sciences, Amsterdam, The Netherlands.
Peter H Bisschop, Department of Endocrinology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Movement Sciences, Amsterdam, The Netherlands.
Funding
This work was supported by Amsterdam Movement Sciences, the Netherlands [VUmc PhD call 2016].
Author Contributions
M.T. Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing—Original Draft, Project administration. M.S. Validation, Investigation, Data Curation, Writing—Review & Editing, Project administration. M.T. Validation, Resources, Writing—Review & Editing. E.B. Writing—Review & Editing. R.de M. Conceptualization, Writing—Review & Editing. A.J.N. Resources, Writing—Review & Editing. M.d.H. Conceptualization, Methodology, Writing—Review & Editing, Supervision. P.H.B. Conceptualization, Methodology, Writing—Review & Editing, Supervision
Disclosures
No conflicts of interest have been reported.
Data Availability
All datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
Registration in a public trials registry: the Dutch trial register Trial NL7513.
References
- 1. Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72(3):1150‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kammerlander AA, Lyass A, Mahoney TF, et al. . Sex differences in the associations of visceral adipose tissue and cardiometabolic and cardiovascular disease risk: the Framingham Heart Study. J Am Heart Assoc. 2021;10(11):e019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simon TG, Roelstraete B, Hagström H, Sundström J, Ludvigsson JF. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut. 2022;71(9):1867‐1875. [DOI] [PubMed] [Google Scholar]
- 4. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55(10):2622‐2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ulbrich EJ, Fischer MA, Manoliu A, et al. . Age- and gender dependent liver fat content in a healthy normal BMI population as quantified by fat-water separating DIXON MR imaging. PLoS One 2015;10(11):e0141691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunter GR, Gower BA, Kane BL. Age related shift in visceral fat. Int J Body Compos. Res. 2010;8(3):103‐108. [PMC free article] [PubMed] [Google Scholar]
- 8. Samargandy S, Matthews KA, Brooks MM, et al. . Abdominal visceral adipose tissue over the menopause transition and carotid atherosclerosis: the SWAN heart study. Menopause. 2021;28(6):626‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Della Torre S. Beyond the X factor: relevance of sex hormones in NAFLD pathophysiology. Cells. 2021;10(9):2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papadakis GE, Hans D, Rodriguez EG, et al. . Menopausal hormone therapy is associated with reduced total and visceral adiposity: the OsteoLaus cohort. J Clin Endocrinol Metab. 2018;103(5):1948‐1957. [DOI] [PubMed] [Google Scholar]
- 11. Sarkar MA, Suzuki A, Abdelmalek MF, et al. . Testosterone is associated with nonalcoholic steatohepatitis and fibrosis in premenopausal women with NAFLD. Clin Gastroenterol Hepatol. 2021;19(6):1267‐1274.e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the study of Women's Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring, Md). 2010;18(3):604‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hewitt KN, Pratis K, Jones MEE, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145(4):1842‐1848. [DOI] [PubMed] [Google Scholar]
- 14. Maffei L, Murata Y, Rochira V, et al. . Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89(1):61‐70. [DOI] [PubMed] [Google Scholar]
- 15. Völzke H, Aumann N, Krebs A, et al. . Hepatic steatosis is associated with low serum testosterone and high serum DHEAS levels in men. Int J Androl. 2010;33(1):45‐53. [DOI] [PubMed] [Google Scholar]
- 16. Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 2000;24(4):485‐491. [DOI] [PubMed] [Google Scholar]
- 17. Yassin AA, Alwani M, Talib R, et al. . Long-term testosterone therapy improves liver parameters and steatosis in hypogonadal men: a prospective controlled registry study. Aging Male. 2020;23(5):1553‐1563. [DOI] [PubMed] [Google Scholar]
- 18. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. . Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869‐3903. [DOI] [PubMed] [Google Scholar]
- 19. Elbers JM, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276(2):E317‐E325. [DOI] [PubMed] [Google Scholar]
- 20. Elbers JM, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82(7):2044‐2047. [DOI] [PubMed] [Google Scholar]
- 21. Finkelstein JS, Lee H, Leder BZ, et al. . Gonadal steroid–dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126(3):1114‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013. [Google Scholar]
- 23. Runge JH, Smits LP, Verheij J, et al. . MR Spectroscopy-derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis. Radiology. 2018;286(2):547‐556. [DOI] [PubMed] [Google Scholar]
- 24. Troelstra MA, Witjes JJ, van Dijk AM, et al. . Assessment of imaging modalities against liver biopsy in nonalcoholic fatty liver disease: the Amsterdam NAFLD-NASH cohort. J Magn Reson Imaging. 2021;54(6):1937‐1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 26. Stefan D, Cesare F, Andrasescu A, et al. . Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol. 2009;20(10) 104035. [Google Scholar]
- 27. Hamilton G, Yokoo T, Bydder M, et al. . In vivo characterization of the liver fat ¹H MR spectrum. NMR Biomed. 2011;24(7):784‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsao J, Jiang Y. Hierarchical IDEAL: fast, robust, and multiresolution separation of multiple chemical species from multiple echo times. Magn Reson Med. 2013;70(1):155‐159. [DOI] [PubMed] [Google Scholar]
- 29. Poonawalla AH, Sjoberg BP, Rehm JL, et al. . Adipose tissue MRI for quantitative measurement of central obesity. J Magn Reson Imaging. 2013;37(3):707‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verdonk SJE, Vesper HW, Martens F, Sluss PM, Hillebrand JJ, Heijboer AC. Estradiol reference intervals in women during the menstrual cycle, postmenopausal women and men using an LC-MS/MS method. Clin Chim Acta. 2019;495:198‐204. [DOI] [PubMed] [Google Scholar]
- 31. Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol. 2013;66(9):1022‐1028. [DOI] [PubMed] [Google Scholar]
- 32. Tebbens M, Schutte M, Troelstra MA, et al. . Supplemental data for: Sex steroids regulate liver fat content and body fat distribution in both men and women: a study in transgender persons. Uploaded March, 2023. 10.21942/uva.22439203 [DOI]
- 33. Yang JD, Abdelmalek MF, Pang H, et al. . Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(4):1406‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649‐1657. [DOI] [PubMed] [Google Scholar]
- 35. Klair JS, Yang JD, Abdelmalek MF, et al. . A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016;64(1):85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nishino M, Hayakawa K, Nakamura Y, Morimoto T, Mukaihara S. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. AJR Am J Roentgenol. 2003;180(1):129‐134. [DOI] [PubMed] [Google Scholar]
- 37. Pan HJ, Chang HT, Lee CH. Association between tamoxifen treatment and the development of different stages of nonalcoholic fatty liver disease among breast cancer patients. J Formos Med Assoc. 2016;115(6):411‐417. [DOI] [PubMed] [Google Scholar]
- 38. Ciardullo S, Oltolini A, Cannistraci R, Muraca E, Perseghin G. Sex-related association of nonalcoholic fatty liver disease and liver fibrosis with body fat distribution in the general US population. Am J Clin Nutr. 2022;115(6):1528‐1534. [DOI] [PubMed] [Google Scholar]
- 39. Chen Z, Wang O, Nie M, et al. . Aromatase deficiency in a Chinese adult man caused by novel compound heterozygous CYP19A1 mutations: effects of estrogen replacement therapy on the bone, lipid, liver and glucose metabolism. Mol Cell Endocrinol. 2015;399:32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelley CE, Brown AJ, Diehl AM, Setji TL. Review of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. World J Gastroenterol. 2014; 20(39):14172‐14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karastergiou K, Fried SK. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ. 2012;3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klaver M, van Velzen D, de Blok C, et al. . Change in visceral fat and total body fat and the effect on cardiometabolic risk factors during transgender hormone therapy. J Clin Endocrinol Metab. 2022;107(1):e153‐e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taylor JL, Holland DJ, Coombes JS, Keating SE. Accuracy of dual-energy x-ray absorptiometry for assessing longitudinal change in visceral adipose tissue in patients with coronary artery disease. Int J Obes. 2021;45(8):1740‐1750. [DOI] [PubMed] [Google Scholar]
- 44. Wiepjes CM, de Jongh RT, de Blok CJ, et al. . Bone safety during the first ten years of gender-affirming hormonal treatment in transwomen and transmen. J Bone Miner Res. 2019;34(3):447‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Finkelstein JS, Lee H, Burnett-Bowie SA, et al. . Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qiu S, Vazquez JT, Boulger E, et al. . Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci Rep. 2017;7(1):1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729‐12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joyner J, Hutley L, Cameron D. Intrinsic regional differences in androgen receptors and dihydrotestosterone metabolism in human preadipocytes. Horm Metab Res. 2002;34(5):223‐228. [DOI] [PubMed] [Google Scholar]
- 49. Björntorp P. Hormonal control of regional fat distribution. Hum Reprod. 1997;12(Suppl 1):21‐25. [DOI] [PubMed] [Google Scholar]
- 50. Szczepaniak LS, Nurenberg P, Leonard D, et al. . Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462-–E4468.. [DOI] [PubMed] [Google Scholar]
- 51. Jones BA, Haycraft E, Bouman WP, Arcelus J. The levels and predictors of physical activity engagement within the treatment-seeking transgender population: A matched control study. J Phys Act Health. 2018;15(2):99‐107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.