Abstract
Context
Executive dysfunction is a well-recognized component of the cognitive phenotype of Klinefelter syndrome (KS), yet the neural basis of KS-associated cognitive weaknesses, and their association with testicular failure is unknown.
Objective
We investigated executive function, brain activation, and pubertal development in adolescents with and without KS.
Methods
Forty-three adolescents with KS (mean age 12.3 ± 2.3 years) and 41 typically developing boys (mean age 11.9 ± 1.8 years) underwent pubertal evaluation, behavioral assessment, and completed functional magnetic resonance imaging (fMRI) as they performed an executive function task, the go/no-go task. Group differences in activation were examined. Associations among activation, executive function, and pubertal development measures were tested in secondary analyses.
Results
Boys with KS exhibited reduced executive function, as well as lower activation in brain regions subserving executive function, including the inferior frontal gyrus, anterior insula, dorsal anterior cingulate cortex, and caudate nucleus. Secondary analyses indicated that the magnitude of activation differences in boys with KS was associated with severity of pubertal developmental delay, as indexed by lower testosterone (t(36) = 2.285; P = .028) and lower testes volume (t(36) = 2.238; P = .031). Greater parent-reported attention difficulties were additionally associated with lower testicular volume (t(36) = −2.028; P = .050).
Conclusion
These findings indicate a neural basis for executive dysfunction in KS and suggest alterations in pubertal development may contribute to increased severity of this cognitive weakness. Future studies that examine whether these patterns change with testosterone replacement therapy are warranted.
Keywords: executive function, brain, inhibition, Klinefelter syndrome, sex chromosome aneuploidy, fMRI
Klinefelter syndrome (KS) is a sex chromosome aneuploidy that occurs in roughly 1 in 500 males (1), in which boys are born with an extra X chromosome. In addition to physical features, such as hypogonadism, gynecomastia, and tall stature, KS is associated with a variety of behavioral alterations, including language and learning problems, increased anxiety, and depressed mood (2). One specific area of concern is executive function, a domain of cognition that encompasses a variety of higher-order processes such as working memory, inhibition, sustained attention, and cognitive flexibility. Growing evidence indicates that impairments in this cognitive domain likely emerge in childhood for boys with KS. Reductions in working memory, switching, and planning/problem-solving capabilities, for instance, have been noted in peripubertal boys with KS (3-9). Moreover, and consistent with the increased rates of attention-deficit/hyperactivity disorder that have been reported in KS (10), research in this area has noted that the greatest cognitive weakness resides in measures of attention (8, 9, 11, 12).
Despite these findings, and the clear negative influence of executive dysfunction on long-term academic, adaptive, and psychological functioning (13, 14), the neurobiological basis for executive function alterations in KS remain unknown. Further, it is unclear whether altered pubertal development and testosterone deficiency in this clinical population play a role in the pathogenesis of executive dysfunction and activation in associated brain regions. While pubertal development in boys with KS often begins at the same time as that of their typically developing (TD) peers, by mid- to late puberty, relative reductions in testosterone and stalled testicular growth become apparent (15-17). Testosterone supplementation represents the current standard of care to correct androgen deficiency in male patients with KS. Yet, the timing of when to initiate this treatment remains highly debated. As a result, pubertal deviations, when present, may play a substantial role in altered neurodevelopment in KS. Indeed, many brain regions that are important to executive function are densely population with androgen receptors (18-20) and undergo dynamic changes at puberty in typically developing males (21-28).
In the present study, we assessed neural activation in executive function networks in adolescent males, with and without KS. More specifically, using functional magnetic resonance imaging (fMRI), we examined brain function in 43 adolescents with KS (mean age 12.3 ± 2.3 years) and 41 TD control participants (mean age 11.9 ± 1.8 years) as they performed a well-validated executive function paradigm, the go/no-go task. We hypothesized that adolescents with KS would exhibit reduced performance on this task in comparison to their TD counterparts, and further, that these behavioral differences would be accompanied by anomalous activation of brain regions subserving sustained attention and inhibition, such as the inferior frontal gyrus, caudate, dorsal anterior cingulate cortex, and posterior parietal cortex. We additionally explored, in secondary analyses, whether the magnitude of KS-related alterations in activation were associated with severity of pubertal developmental delay as indexed by 2 measures: testosterone and testicular volume.
Materials and Methods
Participants
All study protocols were carried out in accordance with the latest version of the Declaration of Helsinki and were approved by the Nemours and Stanford University Institutional Review Boards. Prior to participation, informed consent was obtained from the parent/guardian and informed assent was obtained from each participant.
Data were collected as part of a longitudinal study examining cognition, mood, behavior, and neurodevelopment in school-aged boys with KS and their sex- and performance IQ-matched peers. Because lower verbal IQ is common in KS (29), groups were matched based on visual spatial subtests of the WISC-VSI (Visual Spatial Index) rather than overall IQ (Full Scale IQ). Participants with KS were recruited through university- and community-based pediatricians, pediatric endocrinologists, and medical geneticists as well as through advertisements in local and national chapters of organizations serving individuals with KS and their families (eg, the Association of X and Y Chromosome Variations/AXYS and the eXtraordinarY Kids Clinics at Nemours/Alfred I DuPont Hospital for Children and Stanford University). Within the KS group, 19 participants were diagnosed prenatally, 1 was diagnosed at birth, and 19 were diagnosed postnatally. For 3 participants in the KS group, the timing of diagnosis was unknown. The TD control group was recruited through advertisements in internet bulletin boards, schools, and parent organizations. For each participant in the KS group, diagnosis was confirmed by karyotype analysis demonstrating nonmosaic KS. Participants in both groups were excluded if they had history of traumatic brain injury, hypoxic-ischemic encephalopathy, uncontrolled seizure disorder, or psychosis. English was the primary language spoken for all participants.
Study data were acquired from a total of 96 male participants, including 49 boys with KS and 47 TD boys. Research personnel reviewed MRI scan and behavioral data for quality. Six scans from the KS group and 6 scans from the TD group were excluded due to poor scan quality because of motion artifacts or because the participant was not performing the task as instructed. This resulted in final group sizes of 43 individuals with KS (mean age 12.3 ± 2.3 years) and 41 TD participants (mean age 11.9 ± 1.8 years). Two sibling pairs discordant for KS were included in the final study sample. All findings presented here remained unchanged when unaffected siblings from these 2 pairs were removed from the analyses.
Cognitive Testing
Cognitive and behavioral assessment was performed with the goal of completing these measures within 4 weeks of scanning. Parents completed the Behavior Assessment System for Children, Third Edition (BASC-3 (30)), and the Behavior Rating Inventory of Executive Function, Second Edition (BRIEF-2 (31)). Children were administered portions of the Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V (32)). Age-normed T-scores on BASC-3 and BRIEF subscales relevant to the construct of response inhibition (ie, Externalizing Problems, Hyperactivity, Attention Problems, and Behavioral Symptoms Index in BASC-3 and Inhibition Problems and Global Executive Composite in BRIEF), as well as T-scores on the Visual Spatial Index of the WISC-V, were used in analyses examining associations between cognitive ability and brain activation measures.
Pubertal Assessment
Pubertal development was assessed using physical examination of testes volume together with hormone levels extracted from blood serum. Testicular volume, a stable marker of testicular failure (33, 34), was determined by trained pediatric endocrinologists (T.A. and J.R.) using a Prader Orchidometer (Accurate Surgical and Scientific Instruments). Serum levels of total and free testosterone were assessed using liquid chromatography and tandem mass spectrometry, a highly reliable assay of gonadal steroids. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were assessed using immunoassay. Blood samples were collected in the early morning for all participants (between 8:20 and 9:30 Am).
Magnetic Resonance Imaging Acquisition
All participants were prepared for unsedated MRI scans through scan simulations performed by research staff. Participants were scanned at 1 of 2 imaging sites using identical GE 3T MR750 whole-body MR systems with matching 8-channel head coils and imaging protocols. T1-weighted structural images of the brain were acquired sagittally using a 3D fast spoiled gradient echo (FSPGR) echo sequence, with slice thickness = 1 mm, repetition time (TR) = 8.18 ms, echo time (TE) = 3.19 ms, inversion time (TI) = 450 ms, flip angle = 12°, 176 slices, matrix = 256 × 256, voxel size = 1 × 1 × 1 mm, scan duration: 4:54 minutes. Scans were repeated if needed, as per protocol, to increase the probability of obtaining a low-motion, usable scan. T2*-weighted functional images of the brain were acquired during the performance of each of 2 go/no-go task runs using an echo planar imaging sequence with TR = 2000 ms, TE = 30 ms, flip angle = 77°, field of view = 22 cm × 22 cm, 38 slices, matrix = 64 × 64, voxel size = 3.4375 × 3.4375 × 4 mm, scan duration: 8:26 minutes.
Go/No-Go Task Design
Stimuli were presented using E-Prime, version 2 (Psychology Software Tools), using a video projector that illuminated a rear projection screen at the end of the magnet. Participants viewed stimuli through an adjustable mirror attached to the head coil, and MRI acquisition was synchronized with the paradigm. Participants were presented with a fixed series of letters in 80-point font on the center of the screen and instructed to respond, using a response box held in their dominant hand, to every letter (“go” trial) with a key press, except to the letter “X” (“no-go” trial). Each letter trial was presented for 250 ms and was separated from the subsequent trial with a jittered intertrial interval that ranged from 750 ms to 8750 ms, during which participants passively viewed a fixation cross. The task was weighted toward go stimuli (N = 300 trials) to build up a prepotent tendency to respond, thereby increasing the inhibitory effort necessary to successfully withhold responding to no-go stimuli (N = 75 trials). The task was divided into 2 separate runs, each lasting 8.3 minutes. Accuracy of responses and response times (RTs) were recorded.
Behavioral Data Analyses
All statistical analyses were carried out using SPSS (version 26.0). Commission errors, omission errors, RT for correct “go” trials, and the signal detection measure, d-prime, were examined in separate linear regression models to test for the main effect of group. D-prime was computed by subtracting the z score of the false alarm rate (eg, commissions) from the z score of the correct response rate (eg, hits) and represents a preferred measure of task performance as it takes into consideration both the relative frequency of correct hits and correct rejections. A high d′ value indicates that the participant correctly responded to a high number of “go” trials and appropriately withheld a response to a high number of “no-go” trials. In contrast, a low d′ value signifies that the participant responded to fewer “go” trials (omission errors) and/or failed to withhold a response to a greater number of “no-go” trials. Age and site were included as covariates of noninterest in statistical analyses. Statistical significance was assessed using a 2-tailed α level of .05.
Primary Analyses: Group Differences in Activation
Preprocessing of fMRI data was conducted in FSL (FMRIB Software Library, version 5.0.10), using FEAT (FMRI Expert Analysis Tool, version 6.0.0). The first 3 volumes of each scan were discarded to allow for the stabilization of longitudinal magnetization. The remaining images were preprocessed using a series of steps. First, nonbrain material was removed from both the anatomical and functional images using the Brain Extraction Tool (35). Preprocessing included motion correction to the mean image (36), spatial smoothing using a Gaussian smoothing kernel of 6-mm FWHM, and high-pass temporal filtering (37). Linear registration was performed using FMRIB's Linear Image Registration Tool to linearly align each individual's functional data to his high-resolution anatomical image (38). Linear registration was used to align each individual's anatomical image to Montreal Neurological Institute (MNI) standard space. The linear and transformations were combined to register each individual's functional data to template space.
Because 2 separate task runs were conducted for each participant, time-series statistical analyses were carried out at a single-run intraindividual level using a generalized linear model that modeled each condition and accuracy type (“go” correct, “go” incorrect, “no-go” correct, “no-go” incorrect) using a synthetic hemodynamic response function and its first derivative, as well as motion correction parameters and time points that exceeded a motion threshold (75th percentile plus 1.5 times the interquartile range) defined by FSL's motion outliers tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers). Both runs were combined in a fixed-effects analysis for each participant to provide individual-specific summaries of activation.
To test for voxel-based group differences in activation, individual-specific activation summary maps for “no-go” correct minus “go” correct (“no-go” > go”) contrast were computed and carried to higher-level voxel-based analyses that controlled for site and age. Corrected significance maps of the interaction of group by condition on activation were computed using FSL's randomize permutation tool (39); this approach uses a threshold-free cluster enhancement procedure, and a correction for family-wise error (P < .05) with 5000 iterations.
Secondary Analyses: Associations Between Activation and Clinical and Behavioral Measures
Associations between activation and behavioral, cognitive, and clinical measures were examined within the KS group in secondary analyses. Activation values were extracted for each significant cluster resulting from primary analyses, then fed into separate linear regressions that modeled activation as the dependent variable, and behavioral or pubertal measures (testes volume, total testosterone) as covariates of interest. Duration of time between neural and pubertal assessments was included as a covariate of noninterest, as was age.
Results
Participants
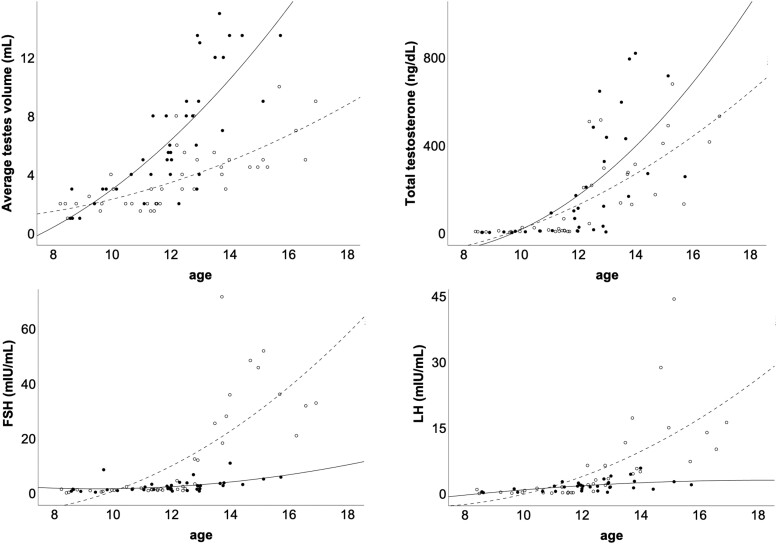
Participant characteristics are presented in Table 1. The KS and TD groups did not differ with respect to age (t(82) = −0.929; P = .356) or visuospatial IQ (t(75) = 0.985; P = .328). Of the 43 participants with KS, 5 were receiving testosterone replacement therapy (TRT) at the time of study. As we note later, to assess whether TRT confounded our findings, all models were rerun after excluding these 5 participants. Because of the positive skew to testosterone, LH, FSH, and testicular volume, a log transformation was performed on these values prior to statistical analysis. Increased FSH (t(75) = 2.367; P = .021) and reduced testicular volume (t(75) = −5.413; P < .001) was observed in the KS relative to the TD group (see Table 1 and Fig. 1). The main effect of group was not statistically significant for testosterone (t(71) = −0.856; P = .395) or LH (t(75) = 0.152; P = .880). To test whether pubertal measures varied between groups as a function of age, the interaction of group by age was entered as a predictor. A statistically significant group by age interaction was observed for testes volume (t(72) = −2.937; P = .004), LH (t(76) = 3.097; P = .003), and FSH (t(76) = 4.102; P < .001); testes volume was decreased and LH and FSH were increased in the KS relative to the TD group at older ages. No interaction of group by age was observed for testosterone (t(68) = −1.661; P = .101). With respect to behavior, parents in the KS group reported greater difficulties in attention (attention subscale of the BASC-PRS; t(68) = 4.961; P < .001), impulsivity (inhibition subscale of the BRIEF; t(68) = 4.349; P < .001), and executive function (global executive composite of the BRIEF; t(68) = 6.113; P < .001). All findings remained unchanged when boys with KS who were currently receiving TRT (N = 5) were excluded from analyses. Moreover, exploratory analyses comparing the aforementioned measures between boys who were diagnosed prenatally vs postnatally revealed no statistically significant differences (Ps > .085).
Table 1.
Clinical characteristics of study participants
| Characteristic | KS | TD | P |
|---|---|---|---|
| General information | |||
| No. | 43 | 41 | — |
| Age, y | 12.3 ± 2.3 | 11.9 ± 1.8 | .356 |
| Diagnosis prenatal/postnatal/unknown, No. | 19/19/3 | — | — |
| Go/no-go task performance | |||
| Commission errors | 24.1 ± 13.9 | 32.3 ± 18.6 | .023 |
| Omission errors | 88.0 ± 57.7 | 47.1 ± 48.0 | <.001 |
| RT to correct “go” trials | 489.6 ± 67.8 | 463.1 ± 70.4 | .080 |
| d-prime | 1.24 ± 0.74 | 1.46 ± 0.88 | .054 |
| Behavioral measures (T scores) | |||
| BASC-3 PRS attention problems | 56.9 ± 8.2 | 46.6 ± 8.6 | <.001 |
| BRIEF-2 inhibition problems | 57.3 ± 10.8 | 46.4 ± 9.6 | <.001 |
| BRIEF global executive composite | 64.7 ± 10.9 | 48.9 ± 9.6 | <.001 |
| WISC-V processing speed index Composite | 101.6 ± 14.1 | 104.5 ± 12.4 | .328 |
| Clinical measures | |||
| Total testosterone, ng/dL | 158.0 ± 195.4 | 188.9 ± 251.2 | .395a |
| FSH, mIU/mL | 13.0 ± 18.4 | 2.6 ± 2.2 | .021a |
| LH, mIU/mL | 6.1 ± 9.3 | 1.7 ± 1.3 | .880a |
| Testicular volume, mL | 3.8 ± 2.2 | 6.4 ± 4.1 | <.001a |
Values are presented as mean ± SD.
Abbreviations: BASC-3, Behavior Assessment System for Children, Third Edition; BRIEF-2, Behavior Rating Inventory of Executive Function, Second Edition; FSH, follicle-stimulating hormone; KS, Klinefelter syndrome; LH, luteinizing hormone; RT, response time; TD, typically developing; WISC-V, Wechsler Intelligence Scale for Children, Fifth Edition.
a Significance values resulting from comparison of log-transformed values, controlling for age.
Figure 1.
Pubertal measures in each group. The dashed line and unfilled markers represent the Klinefelter syndrome group. The solid line and filled markers represent the typically developing group. Raw values are plotted. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Go/No-Go Task Performance
Commission errors, omission errors, RT for correct “go” trials, and the signal detection measure, d-prime are presented in Table 1. Analyses of behavioral metrics, controlling for age and site, showed that group was a marginally significant predictor of d-prime (t(83) = −1.953; P = .054) and a significant predictor of omission error rate (t(83) = 4.240; P < .001); boys in the KS group exhibited lower d-prime values and higher rate of omission errors relative to the control group. No statistically significant difference was observed between the 2 groups for RT for correct “go” trials (t(83) = 1.848; P = .080). Boys in the TD group showed a higher rate of commission errors relative to the KS group (t(83) = −2.313; P = .023). These patterns remained unchanged when boys treated with TRT were removed from the analyses. Comparison of these measures between prenatally diagnosed vs postnatally diagnosed boys with KS yielded no statistically significant differences (Ps > .161).
Primary Analyses: Group Differences in Activation
Analyses of activation occurring during “no-go” vs “go” trials between KS and TD groups that controlled for site and age indicated that the main effect of site, and the interaction of group by site, was not statistically significant. Site was therefore removed from the model. Moreover, because task performance showed trend-level differences between groups, d-prime was added as a covariate of noninterest to permit the identification of activation differences between KS and TD participants that were not confounded by behavior. The final voxel-wise model included group as a factor, and age and d-prime as covariates of noninterest.
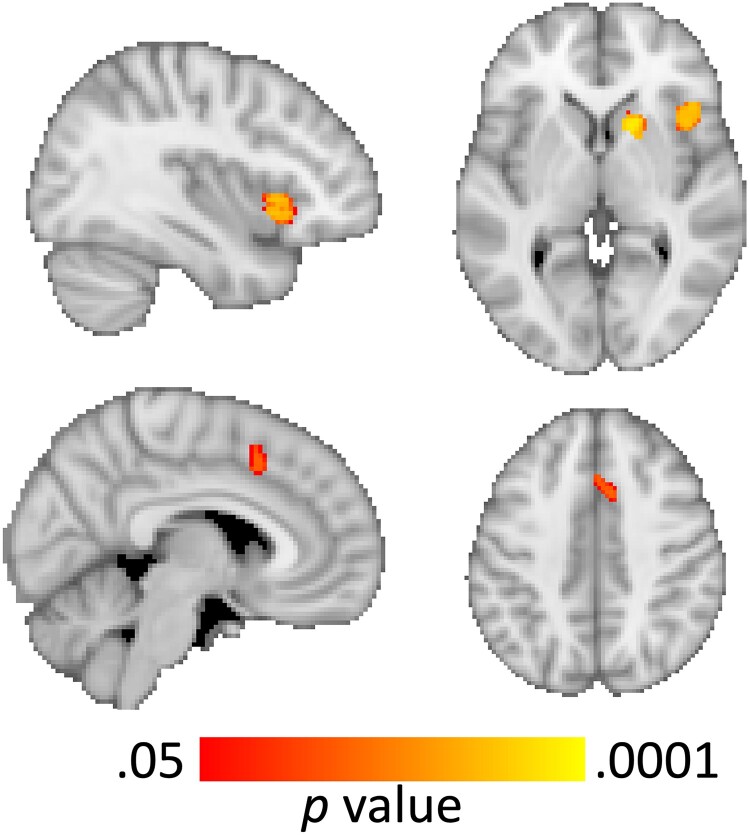
Results from primary analyses indicated a statistically significant effect of group for the contrast of “no-go” > “go” in 2 clusters: a region that was centered in the right anterior insula and included parts of the caudate nucleus and inferior frontal gyrus (x/y/z peak MNI coordinates = 36, 68, 35, k = 711 voxels; P < .001), and another that was centered in the paracingulate gyrus and included parts of the anterior cingulate gyrus (x/y/z peak MNI coordinate = 41, 70, 60, k = 111 voxels; P < .001; Fig. 2). Decomposition of multifactor effects indicated greater activation in both regions in the control group relative to the KS group during “no-go” trials (ts[1, 83] < −3.298; Ps < .001), but no difference between the 2 groups during “go” trials (ts[1, 83] < 1.042; Ps > .299). These findings remained generally unchanged when the 5 participants from the KS group who were receiving TRT at the time of study were excluded. Moreover, comparison of activation in these clusters did not vary significantly as a function of prenatal vs postnatal diagnosis within the KS group (Ps > .082).
Figure 2.
Voxel-based activation differences illustrating areas of decreased activation in the Klinefelter syndrome relative to the typically developing group in the anterior insula, inferior frontal gyrus, and caudate nucleus (top row), and the paracingulate and dorsal anterior cingulate cortices (bottom row).
Secondary Analyses: Associations Between Activation, Behavior and Puberty
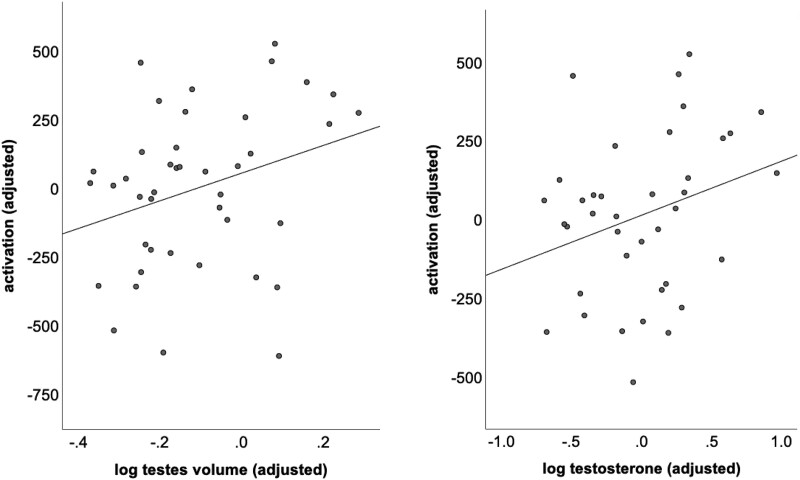
Because of the positive skew to testosterone, LH, FSH, and testicular volume, a log transformation was performed on these values prior to statistical analysis. Within the KS group, decreased activation in the paracingulate cluster was associated with lower total testosterone (t(36) = 2.285; P = .028) and lower testes volume (t(36) = 2.238; P = .031; Fig. 3). No associations were observed between pubertal and activation metrics in the cluster centered in the anterior insula (ts[36] < 0.679; Ps > .501), nor were there statistically significant associations between activation in either cluster and parent-reported measures of executive function (ts[36] > 1.238; Ps > .223).
Figure 3.
Scatterplots demonstrating associations within the Klinefelter syndrome group between activation in the paracingulate/dorsal anterior cingulate cluster and testicular volume and testosterone.
With respect to behavior, a statistically significant association was observed within the KS group between parent-reported executive function and testicular volume. Specifically, greater attention difficulties, as indexed by higher scores on the attention subscale of the BASC-PRS, were associated with lower testes volume (t(36) = −2.028; P = .050). No associations were observed between testosterone or testes volume and other parent-reported measures of behavior (ts[36] > −0.258; Ps > .797), nor were there associations between pubertal measures and go/no-go task performance (ts[72] < 0.581; Ps > .563). Again, these findings remained generally unchanged regardless of whether the 5 participants from the KS group who were receiving TRT at the time of study were excluded from analysis.
Discussion
In this study, we assessed activation patterns in adolescent males with KS as compared to an age-matched TD control group as they performed the go/no-go task, a well-validated executive function paradigm. Results of our analysis indicated that, relative to their TD peers, adolescents with KS demonstrated reduced task accuracy, as well as decreased activation in brain regions subserving executive function, including the right inferior frontal gyrus, anterior insula, striatum, and dorsal anterior cingulate gyrus. Statistically significant associations were found between activation patterns and metrics of pubertal development in secondary analyses within the KS group. Specifically, lower activation of the paracingulate and dorsal anterior cingulate cortex in the KS group was associated with lower serum total testosterone and with lower testes volume. Further, greater parent-reported executive dysfunction was associated with lower testes volume. Taken together, these findings indicate a neural basis for executive dysfunction in KS and offer preliminary evidence in support of a role for altered pubertal development and testosterone deficiency in the magnitude of these differences.
The present findings add to the existing literature documenting alterations in brain areas subserving social cognition (40, 41) and verbal fluency (42)—behavioral features that are notably affected in males with KS. Our study is the first, to our knowledge, to investigate brain function during an executive function task in boys with KS. While the precise mechanisms underlying functional alterations of executive control regions are unclear, our findings indicate that KS-associated deviations in pubertal development may play a role. Puberty represents a dynamic period of neurodevelopment, during which subregions of the executive control network undergo considerable structural changes in response pubertal fluctuations in testosterone (21-27). Although many boys with KS may initiate puberty at the same time as their TD peers, with an initial enlargement of the testes, and a rise in gonadotropins and testosterone to a pubertal range (43-47), by mid-puberty, LH and FSH levels increase to above-normal levels and testes volume is observed to be lower relative to age-matched, TD controls. This is likely the result of a depletion of germ cells, hyalinization of seminiferous tubules, and the degeneration of the Sertoli cells (48, 49). By late puberty, testosterone levels show significant declines, when Leydig cells fail to produce testosterone, despite increased levels of LH (15-17). In the present study, although there was a trend for reduced testosterone in the KS group at older ages, no difference was observed between the 2 groups with respect to testosterone overall. The exact reason for this is unclear but is likely the result of the relatively younger age of our sample (mean age = 12.3 years). In addition, there are wide variations in testosterone in adolescence. The combination of lower testes volume and higher FSH levels nevertheless likely reflects some degree of testicular failure. Future studies that examine physical and hormonal aspects of puberty from larger samples spanning the entire peripubertal developmental period, including additional participants sampled at older ages, would be helpful in confirming this possibility.
The statistically significant associations that we observed between low testosterone and testes volume and decreased neural and executive function point to a role for altered pubertal development at the onset and maintenance of executive dysfunction in KS. Future studies that test whether neural and behavioral manifestations of executive dysfunction partially reverse following treatment with TRT, as has been suggested in the small extant structural neuroimaging literature (50, 51), remains an important area of further research. Studies examining whether timing of the initiation of treatment with TRT moderates clinical and behavioral outcomes in KS are also critical, since the question of whether to initiate TRT when FSH and LH levels begin to rise vs waiting for testosterone to fall below the normative range is widely debated (52, 53).
Our findings of reduced executive functioning in young male patients with KS, as demonstrated by impaired performance on the go/no-go task, as well as higher scores on the attention problems of the BASC-3 PRS, and Inhibition Problems and Global Executive Composite subscales of the BRIEF, are consistent with the small number of studies in this area. Lee and colleagues (9), for example, reported reduced performance on tests of visual attention, task switching, spatial working memory, and planning in a sample of 27 participants with KS ranging in age from 9 to 25 years. In a sample of 23 boys who were between ages 9 and 18 years, van Rijn and Swaab (12) reported KS-associated cognitive weaknesses in inhibition and sustained attention, as well as reduced mental flexibility and visual working memory. Most recently, Janusz and colleagues (11) reported weaknesses in executive function in a sample of 77 boys with KS who ranged in age from 8 to 18 years; the most pronounced weaknesses were that of attention, whereas moderate weaknesses were observed in working memory, switching, and planning/problem-solving. Our study extends these prior findings by demonstrating that weaknesses in attention are the most pronounced among boys with greater pubertal impairment, as indicated by reduced testicular volume.
Some limitations of this investigation should be noted. First, while we have interpreted activation associations with testosterone and testes volume to be indicative of pubertal effects on the brain, we cannot exclude the involvement of genetic factors related to a supernumerary X chromosome. Research into specific genes that underlie brain and behavioral alterations in KS remains a pressing area of study. Second, because exploratory analyses of associations among brain activation, behavior, and testosterone and testes volume were not corrected for multiple comparisons, we cannot exclude the possibility of type II error. Third, while we have interpreted our findings of brain function in boys with KS as underlying behavioral symptoms of executive dysfunction, we cannot conclude a causal association between these measures. Fourth, the small number of participants in our study may have resulted in reduced power to detect group differences in brain function and/or correlations with behavior. Fifth, 5 of the 43 boys in our KS cohort were receiving TRT at the time of study, and this treatment may have had a confounding effect on our findings. However, our results remained unchanged when these 5 participants were removed from our analyses. Therefore, we consider this possibility unlikely. Lastly, although previous studies have found better outcomes for prenatally diagnosed children (54-56), exploratory analyses did not indicate an effect of prenatal vs postnatal diagnosis on neural or behavioral function. However, due to our small sample sizes, additional studies using large samples are needed to address this question.
In summary, we present findings demonstrating reduced activation of brain regions subserving executive functions in young adolescent males with KS. The magnitude of these alterations was significantly correlated with reduced serum testosterone levels and testicular volume, suggesting a putative role of altered gonadal hormones in the development of executive dysfunction. If replicated, the present study results demonstrating dysfunction in executive control regions could be used as end points in syndrome-specific intervention trials. Our findings bolster KS as a model for studying the role of the X chromosome, and androgens, in the functional development of brain areas subserving executive function. Whether alterations in brain activation normalize in response to testosterone supplementation represents an area of future research that is of critical importance both to the neuroscientific and clinical communities.
Acknowledgments
The authors thank the participants and their families and the clinical and imaging staff at each study site. We also gratefully acknowledge AXYS for its assistance and support with participant recruitment.
Abbreviations
- BASC-3
Behavior Assessment System for Children, Third Edition
- BRIEF-2
Behavior Rating Inventory of Executive Function, Second Edition
- fMRI
functional magnetic resonance imaging
- FSH
follicle-stimulating hormone
- KS
Klinefelter syndrome
- LH
luteinizing hormone
- MNI
Montreal Neurological Institute
- RT
response time
- TD
typically developing
- TRT
testosterone replacement therapy
- WISC-V
Wechsler Intelligence Scale for Children, Fifth Edition
Contributor Information
Lara C Foland-Ross, Center for Interdisciplinary Brain Sciences Research, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA.
Elnaz Ghasemi, Center for Interdisciplinary Brain Sciences Research, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA.
Vanessa Lozano Wun, Department of Psychology, University of Minnesota, Minneapolis, MN 55455, USA.
Tandy Aye, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA 93405, USA.
Karen Kowal, Department of Pediatrics, Nemours Children's Hospital Delaware, Wilmington, DE 19803, USA; Department of Pediatrics, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Judith Ross, Department of Pediatrics, Nemours Children's Hospital Delaware, Wilmington, DE 19803, USA; Department of Pediatrics, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Allan L Reiss, Center for Interdisciplinary Brain Sciences Research, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA; Department of Pediatrics, Stanford University School of Medicine, Stanford, CA 93405, USA; Department of Radiology, Stanford University School of Medicine, Stanford, CA 94304, USA.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD092847).
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Data Availability
The data sets generated and analyzed for this study are not publicly available. Availability will be considered on reasonable request.
References
- 1. Smyth CM, Bremner WJ. Klinefelter syndrome. Arch Intern Med. 1998;158(12):1309‐1314. [DOI] [PubMed] [Google Scholar]
- 2. Herlihy AS, McLachlan RI, Gillam L, Cock ML, Collins V, Halliday JL. The psychosocial impact of Klinefelter syndrome and factors influencing quality of life. Genet Med. 2011;13(7):632‐642. [DOI] [PubMed] [Google Scholar]
- 3. DeLisi LE, Maurizio AM, Svetina C, et al. . Klinefelter's syndrome (XXY) as a genetic model for psychotic disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;135(1):15‐23. [DOI] [PubMed] [Google Scholar]
- 4. Fales CL, Knowlton BJ, Holyoak KJ, Geschwind DH, Swerdloff RS, Gonzalo IG. Working memory and relational reasoning in Klinefelter syndrome. J Int Neuropsychol Soc. 2003;9(6):839‐846. [DOI] [PubMed] [Google Scholar]
- 5. Boone KB, Swerdloff RS, Miller BL, et al. . Neuropsychological profiles of adults with Klinefelter syndrome. J Int Neuropsychol Soc. 2001;7(4):446‐456. [DOI] [PubMed] [Google Scholar]
- 6. Kompus K, Westerhausen R, Nilsson LG, et al. . Deficits in inhibitory executive functions in Klinefelter (47, XXY) syndrome. Psychiatry Res. 2011;189(1):135‐140. [DOI] [PubMed] [Google Scholar]
- 7. Ross JL, Roeltgen DP, Stefanatos G, et al. . Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A. 2008;146(6):708‐719. [DOI] [PubMed] [Google Scholar]
- 8. Ross JL, Zeger MPD, Kushner H, Zinn AR, Roeltgen DP. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47, XYY syndrome or 47, XXY Klinefelter syndrome. Dev Disabil Res Rev. 2009;15(4):309‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee NR, Wallace GL, Clasen LS, et al. . Executive function in young males with Klinefelter (XXY) syndrome with and without comorbid attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2011;17(3):522‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruining H, Swaab H, Kas M, van Engeland H. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics. 2009;123(5):e865‐e870. [DOI] [PubMed] [Google Scholar]
- 11. Janusz J, Harrison C, Boada C, et al. . Executive function in XXY: comparison of performance-based measures and rating scales. Am J Med Genet C Semin Med Genet. 2020;184(2):469‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Rijn S, Swaab H. Executive dysfunction and the relation with behavioral problems in children with 47, XXY and 47, XXX. Genes Brain Behav. 2015;14(2):200‐208. [DOI] [PubMed] [Google Scholar]
- 13. Boada R, Janusz J, Hutaff-Lee C, Tartaglia N. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Dev Disabil Res Rev. 2009;15(4):284‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jahromi LB, Stifter CA. Individual differences in preschoolers’ self-regulation and theory of mind. Merrill-Palmer Q. 2008;54(1):125‐150. [Google Scholar]
- 15. Aksglæde L, Skakkebæk NE, Almstrup K, Juul A. Clinical and biological parameters in 166 boys, adolescents and adults with nonmosaic Klinefelter syndrome: a Copenhagen experience. Acta Paediatr. 2011;100(6):793‐806. [DOI] [PubMed] [Google Scholar]
- 16. Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999;80(2):192‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rohayem J, Nieschlag E, Zitzmann M, Kliesch S. Testicular function during puberty and young adulthood in patients with Klinefelter's syndrome with and without spermatozoa in seminal fluid. Andrology. 2016;4(6):1178‐1186. [DOI] [PubMed] [Google Scholar]
- 18. Sato T, Matsumoto T, Kawano H, et al. . Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101(6):1673‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simerly R, Swanson L, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76‐95. [DOI] [PubMed] [Google Scholar]
- 20. Nuñez JL, Huppenbauer CB, McAbee MD, Juraska JM, DonCarlos LL. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. J Neurobiol. 2003;56(3):293‐302. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen TV, McCracken J, Ducharme S, et al. . Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013;23(6):1424‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bramen JE, Hranilovich JA, Dahl RE, et al. . Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21(3):636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koolschijn PCMP, Peper JS, Crone EA. The influence of sex steroids on structural brain maturation in adolescence. PLoS One. 2014;9(1):e83929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neufang S, Specht K, Hausmann M, et al. . Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19(2):464‐473. [DOI] [PubMed] [Google Scholar]
- 25. Paus T, Nawaz-Khan I, Leonard G, et al. . Sexual dimorphism in the adolescent brain: role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm Behav. 2010;57(1):63‐75. [DOI] [PubMed] [Google Scholar]
- 26. Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28‐37. [DOI] [PubMed] [Google Scholar]
- 27. Raznahan A, Lee Y, Stidd R, et al. . Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A. 2010;107(39):16988‐16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goddings AL, Beltz A, Peper JS, Crone EA, Braams BR. Understanding the role of puberty in structural and functional development of the adolescent brain. J Res Adolesc. 2019;29(1):32‐53. [DOI] [PubMed] [Google Scholar]
- 29. Graham JM Jr, Bashir AS, Stark RE, Silbert A, Walzer S. Oral and written language abilities of XXY boys: implications for anticipatory guidance. Pediatrics. 1988;81(6):795‐806. [PubMed] [Google Scholar]
- 30. Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. 3rd ed. NCS Pearson Inc; 2015. [Google Scholar]
- 31. Gioia NK, Isquith PK, Kenworthy L. Behavior Rating Inventory of Executive Function. 2nd ed. (BRIEF2). PAR Inc; 2015. [Google Scholar]
- 32. Wechsler D. Wechsler Intelligence Scale for Children. 5th ed. PCS Pearson; 2014. [Google Scholar]
- 33. Bahk JY, Jung JH, Jin LM, Min SK. Cut-off value of testes volume in young adults and correlation among testes volume, body mass index, hormonal level, and seminal profiles. Urology. 2010;75(6):1318‐1323. [DOI] [PubMed] [Google Scholar]
- 34. Ruiz-Olvera S, Rajmil O, Sanchez-Curbelo JR, Vinay J, Rodriguez-Espinosa J, Ruiz-Castañé E. Association of serum testosterone levels and testicular volume in adult patients. Andrologia. 2018;50(3):e12933. [DOI] [PubMed] [Google Scholar]
- 35. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825‐841. [DOI] [PubMed] [Google Scholar]
- 37. Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370‐1386. [DOI] [PubMed] [Google Scholar]
- 38. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143‐156. [DOI] [PubMed] [Google Scholar]
- 39. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92(100):381‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Rijn S, Swaab H, Baas D, de Haan E, Kahn RS, Aleman A. Neural systems for social cognition in Klinefelter syndrome (47, XXY): evidence from fMRI. Soc Cogn Affect Neurosci. 2012;7(6):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whitman ET, Liu S, Torres E, et al. . Resting-state functional connectivity and psychopathology in Klinefelter syndrome (47, XXY). Cereb Cortex. 2021;31(9):4180‐4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Rijn S, Aleman A, Swaab H, Vink M, Sommer I, Kahn RS. Effects of an extra X chromosome on language lateralization: an fMRI study with Klinefelter men (47, XXY). Schizophr Res. 2008;101(1-3):17‐25. [DOI] [PubMed] [Google Scholar]
- 43. Pacenza N, Pasqualini T, Gottlieb S, et al. . Clinical presentation of Klinefelter's syndrome: differences according to age. Int J Endocrinol. 2012;2012:324835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ratcliffe SG. The sexual development of boys with the chromosome constitution 47, XXY (Klinefelter's syndrome). Clin Endocrinol Metab. 1982;11(3):703‐716. [DOI] [PubMed] [Google Scholar]
- 45. Bastida MG, Rey RA, Bergadá I, et al. . Establishment of testicular endocrine function impairment during childhood and puberty in boys with Klinefelter syndrome. Clin Endocrinol (Oxf). 2007;67(6):863‐870. [DOI] [PubMed] [Google Scholar]
- 46. Topper E, Dickerman Z, Prager-Lewin R, Kaufman H, Maimon Z, Laron Z. Puberty in 24 patients with Klinefelter syndrome. Eur J Pediatr. 1982;139(1):8‐12. [DOI] [PubMed] [Google Scholar]
- 47. Salbenblatt JA, Bender BG, Puck MH, Robinson A, Faiman C, Winter JSD. Pituitary-gonadal function in Klinefelter syndrome before and during puberty. Pediatr Res. 1985;19(1):82‐86. [DOI] [PubMed] [Google Scholar]
- 48. Wikström AM, Raivio T, Hadziselimovic F, Wikström S, Tuuri T, Dunkel L. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab. 2004;89(5):2263‐2270. [DOI] [PubMed] [Google Scholar]
- 49. Aksglaede L, Wikström AM, Rajpert-De Meyts E, Dunkel L, Skakkebæk NE, Juul A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update. 2006;12(1):39‐48. [DOI] [PubMed] [Google Scholar]
- 50. Foland-Ross LC, Ross JL, Reiss AL. Androgen treatment effects on hippocampus structure in boys with Klinefelter syndrome. Psychoneuroendocrinology. 2019;100:223‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patwardhan AJ, Eliez S, Bender B, Linden MG, Reiss AL. Brain morphology in Klinefelter syndrome: extra X chromosome and testosterone supplementation. Neurology. 2000;54(12):2218‐2223. [DOI] [PubMed] [Google Scholar]
- 52. Chang S, Skakkebæk A, Davis SM, Gravholt CH. Morbidity in Klinefelter syndrome and the effect of testosterone treatment. Am J Med Genet C Semin Med Genet. 2020;184(2):344‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vogiatzi M, Tursi JP, Jaffe JS, Hobson S, Rogol AD. Testosterone use in adolescent males: current practice and unmet needs. J Endocr Soc. 2021;5(1):bvaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robinson A, Bender BG, Linden MG. Prognosis of prenatally diagnosed children with sex chromosome aneuploidy. Am J Med Genet. 1992;44(3):365‐368. [DOI] [PubMed] [Google Scholar]
- 55. Ross JL, Roeltgen DP, Kushner H, et al. . Behavioral and social phenotypes in boys with 47, XYY syndrome or 47, XXY Klinefelter syndrome. Pediatrics. 2012;129(4):769‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tartaglia NR, Ayari N, Hutaff-Lee C, Boada R. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. J Dev Behav Pediatr. 2012;33(4):309‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed for this study are not publicly available. Availability will be considered on reasonable request.