Abstract
This article comments on:
Pavlovič A, Koller J, Vrobel O, Chamrád I, Lenobel R, and Tarkowski P. 2024. Is the co-option of jasmonate signalling for botanical carnivory a universal trait for all carnivorous plants? Journal of Experimental Botany 75, 334–349.
Keywords: Carnivorous plants, evolution, jasmonates, prey digestion
We don’t often think of plants as hunters. Yet, for a small but diverse group of flesh-eating plants, evolution has crafted them into skilled predators. Indeed, the leaves of plant carnivores have evolved snapping motions, hollow cage-like cavities, sticky secretions, and even suction power—all for the purpose of capturing small animals to provide nutrients not otherwise easily obtained from the nutrient-poor soils in which they grow. How these remarkable plants have evolved these killer abilities has long intrigued the scientific community and beyond. In this issue, Pavlovič et al. (2024) suggest that the evolutionary routes to carnivory may actually be broader than first thought.
Botanical carnivory has evolved more than once, with at least 11 different origins spread across 20 genera in six plant orders (Fleischmann et al., 2018; Lin et al., 2021). This polyphyletic origin makes the plants ideally suited for studies of convergent evolution: in how many ways can you evolve a killer plant? Traditionally, these studies were limited to descriptions of leaf trap morphologies and their mechanisms of catching prey. However, for a plant to be carnivorous, it not only must catch animal prey—typically insects or other small arthropods—but must also digest the prey and acquire the nutrients. Recent technological advances in molecular biology have facilitated studies that now probe these other aspects of the carnivorous syndrome and, in particular, prey digestion.
Sometimes, digestion in plant carnivores is achieved by other organisms that interact with the plant, such as microbes or mutualistic arthropods (Fleischmann et al., 2018). However, in many genera, digestion is achieved by the plant itself via the secretion of a mix of hydrolytic enzymes from specialized digestive glands (Schulze et al., 2012; Fukushima et al., 2017). These enzymes, which include chitinases, proteases, lipases, and phosphatases, are induced in non-carnivorous plants during defense responses; for example, to defend against fungal pathogens (Schlumbaum et al., 1986; Zhao and Chye, 1999). What then regulates the expression of these pathogenesis-related proteins in plant carnivores, and has this molecular pathway been selected for carnivory independently across diverse plant groups? This is the question addressed by Pavlovič et al. (2024).
Molecular phylogenetics has dated the evolution of the oldest known lineage of plant carnivores to ~95 million years ago, within the order Caryophyllales (Fleischmann et al., 2018). This lineage includes the Venus flytrap (Dionaea muscipula), with its bilobed, touch-sensitive snap-trap leaves, as well as sundews of the Drosera and pitcher plants of the Nepenthes genera, with adhesive and pitfall trap leaves, respectively. In these plants, the synthesis and secretion of digestive enzymes is regulated by prey capture (Bemm et al., 2016; Pavlovič and Mithöfer, 2019). Extensive studies over the last two decades have shown that the molecular link between prey recognition and enzyme secretion is the jasmonate (JA) signaling pathway (Pavlovič and Mithöfer, 2019). JAs are small, lipid-based phytohormones best known for their role in defense against herbivores and pathogens in non-carnivorous plants. Studies using non-carnivorous Arabidopsis thaliana (Arabidopsis) have revealed that electrical and calcium signals following herbivore attack lead to an increase in JA biosynthesis, both at the wounded site and in distal leaves (Mousavi et al., 2013; Toyota et al., 2018). Ultimately, JA signaling alters gene expression, including the induction of chitinases and many other pathogenesis-related genes (Zhao and Chye, 1999). A similar story unfolds in carnivorous Caryophyllales: electrical and calcium signals generated by prey are correlated with increases in JA biosynthesis and JA-dependent gene expression (Escalante-Pérez et al., 2011; Nakamura et al., 2013; Procko et al., 2022). Importantly, exogenous application of a JA analog can bypass prey feeding and recapitulate many aspects of the carnivorous syndrome, including digestive enzyme production (Escalante-Pérez et al., 2011).
This co-option of the JA pathway towards the regulation of prey digestion in Caryophyllales has in part led to the hypothesis that botanical carnivory evolved from an insect and pathogen defense pathway (Pavlovič and Mithöfer, 2019). This is perhaps not so surprising; for example, secretory glands—including glandular hairs, or trichomes—are common in the non-carnivorous sister clade to carnivorous Caryophyllales and are very similar to digestive glands (Heubl et al., 2006; Fleischmann et al., 2018). Such glandular hairs are well known to provide chemical and structural defense to insect predation, synthesizing poisons and secreting sticky exudates to deter and impede small herbivores. Indeed, it has been hypothesized that the ancestral pre-adapted population that gave rise to modern-day Caryophyllales carnivores had these sticky, glandular hairs, which would facilitate both digestion and prey capture via adhesion (Heubl et al., 2006). However, any link between glandular hair stimulation—whether mechanical or chemical—and JA biosynthesis has not been well explored outside of carnivorous plants. Despite this, in light of these morphological, physiological, and molecular similarities between plant carnivory and defense, we can forgive the 18th century poet-naturalist Erasmus Darwin for erroneously writing in his work The Botanic Garden that the sticky secretions from hairs of the sundew plant were not for attack but rather to protect the leaves from insect herbivores. It was his grandson, Charles Darwin, who supposed that glandular hairs could also be a first rung on the evolutionary ladder towards botanical carnivory (Darwin, 1875).
This hypothesis of repurposing the JA defense pathway towards carnivory is certainly an attractive one. However, while likely to be true for carnivorous Caryophyllales, is this a common route through which carnivory has evolved in other plant lineages? Pavlovič et al. now suggest that the answer to this question is an emphatic ‘no’.
To address this question, the authors looked at two different types of pitcher plants: the purple trumpet pitcher plant Sarracenia purpurea, from the order Ericales, and the independently evolved Australian pitcher plant Cephalotus follicularis (Cephalotus), of the order Oxalidales. Both species use ‘pitfall’ pitcher-shaped leaves for catching prey and exhibit digestive enzyme activity that bears a close resemblance to that seen in the carnivorous Caryophyllales (Fukushima et al., 2017). Unlike the touch-sensitive Venus flytrap and sundews, Pavlovič and his colleagues found that purple and Cephalotus pitcher plants do not display obvious electrical signaling in response to prey capture. However, as the authors write, this in itself does not exclude the possibility of JA involvement for carnivory; for example, other genera within the Caryophyllales—specifically Nepenthes and Drosophyllum—also appear to lack robust and/or detectable electrical impulses in response to prey perception but do employ JAs for regulating digestive enzyme production (Yilamujiang et al., 2016; Pavlovič et al., 2024). In these plants, a requirement for electrical impulses may have been lost over time. More strikingly, the purple and Cephalotus pitcher plants fail to produce a detectable increase in JAs following prey capture. In contrast, mechanical wounding caused measureable changes in JA levels, similar to non-carnivorous plants.
While this work is understandably not an exhaustive look at all carnivorous plant genera, it is striking that to date the involvement of the JA pathway in regulating digestive enzymes in response to prey has only been observed in the Caryophyllales (Fig. 1). Is, therefore, digestive enzyme secretion in other plant carnivores no longer dependent on prey perception? While Pavlovič et al. suggest that this may be true for Cephalotus, it is likely that prey perception does regulate some enzymatic activity in the purple pitcher plant. Here, the pitcher is open to rainfall that may flush away valuable proteins inside, and which may thus necessitate controlled release of digestive enzymes only as needed. Furthermore, previous work from these same authors, and on which they build their current study (Kocáb et al., 2020), showed that the carnivorous genus Pinguicula of the order Lamiales has strongly inducible secretion of pathogenesis-related enzymes which are also independent of JAs. Thus, it seems that these plants probably employ a signal transduction pathway linking prey perception to enzyme biosynthesis and secretion that is not JA based. But if not JAs, then what is this signaling intermediate? To date, the involvement of other phytohormones in carnivorous responses has not been well established, including for other defense-related hormones such as salicylic acid. Why (or how) these other phytohormones would independently regulate genes typically within the domain of JA defense responses is unclear. The answer then to this question remains unresolved.
Fig. 1.
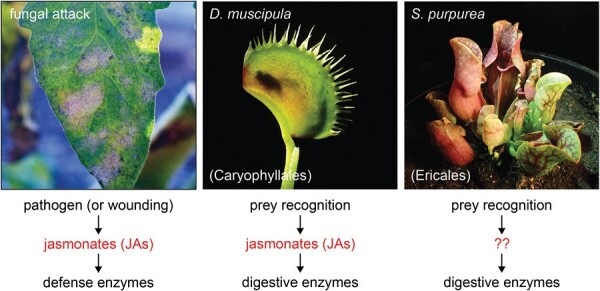
Co-option of defense-associated genes for prey digestion in carnivorous plants. Powdery mildew fungal infection of a non-carnivorous tomato plant leaf (Solanum lycopersicum; left), the snap-trap leaves of the Venus flytrap (Dionaea muscipula, order Caryophyllales; middle), and pitfall trap leaves of the purple pitcher plant (Sarracenia purpurea, order Ericales; right). Both carnivorous plant species have co-opted one aspect of plant defense during their independent evolution towards carnivory: the expression of pathogenesis-related hydrolytic enzymes. During defense in non-carnivorous plants, these enzymes help protect against fungal pathogens and insect herbivores, and are typically regulated by jasmonate (JA) hormones (left). In botanical carnivory, these enzymes function in digestion of animal prey. The expression of these enzymes is regulated by prey perception in both carnivorous species; however, only the Venus flytrap and other carnivores of the order Caryophyllales regulate enzyme expression by also co-opting the JA signaling pathway.
Not only does it seem that JAs are not required for the induction of digestive enzymes outside the Caryophyllales, but Pavlovič et al. also demonstrate that the expression of these genes in non-Caryophyllales pitcher plants is unaffected by exogenous treatment with a JA analog. This is despite the ancestral role of these genes in defense processes. Could there be a benefit to this decoupling of JA signaling and pathogenesis-related hydrolytic enzyme expression in plant carnivores? It is possible to imagine a scenario where carnivory could be in conflict with JA-mediated defenses, which must respond to and protect the plant from environmental stressors independently of whether a trap has caught an animal meal or not. In this scenario, it is beneficial for the plant to use different regulatory pathways: one for defense and the other for prey digestion. Likewise, in Arabidopsis plants, the electrical signals that induce JA biosynthesis in response to herbivory are systemic, initiating defense responses in leaves distal to the site of attack (Mousavi et al., 2013). While systemic electrical and JA signaling probably confers an advantage when protecting oneself from herbivores or pathogens that can easily move between leaves, it does not benefit a plant carnivore, which requires only a local response at the site of prey capture. Indeed, it is noteworthy that electrical signals generated by prey in touch-sensitive Venus flytrap and sundews do not advance beyond the stimulated leaf (Williams and Pickard, 1972). It is conceivable that in these carnivorous Caryophyllales, the JA-regulated hydrolytic enzymes play a dual role, both in defense against pathogens and for prey digestion. However, in non-Caryophyllales, it would seem that the purpose of many is restricted to JA-independent carnivorous functions.
Undoubtedly, massively parallel sequencing technologies—which have revealed insights into carnivorous plant genomes and in particular trap transcriptomes and their regulation (Bemm et al., 2016; Fukushima et al., 2017)—as well as recent advances in carnivorous plant transformation and targeted genetic approaches (Procko et al., 2023)—will continue to be instrumental in resolving these open questions. However, for now it seems that the field must rethink how it extrapolates lessons learned about botanical carnivory from the well-studied Caryophyllales to other carnivorous plant groups. Indeed, as Pavlovič et al. well conclude, it seems that co-option of JA defense signaling to carnivory in the Caryophyllales is perhaps the anomaly and not the norm; rather, there are more ways than one to evolve a killer plant.
Acknowledgements
The authors thank S. Morrison for providing the image of fungal infection of plant leaves.
Contributor Information
Carl Procko, Plant Biology Laboratory, Salk Institute for Biological Studies, 10010 N. Torrey Pines Rd, La Jolla, CA 92037, USA.
Joanne Chory, Plant Biology Laboratory, Salk Institute for Biological Studies, 10010 N. Torrey Pines Rd, La Jolla, CA 92037, USA; Howard Hughes Medical Institute, Chevy Chase, MD, USA.
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by National Institutes of Health (NIH) award 5R35GM122604 (to JC) and the Howard Hughes Medical Institute (to JC).
References
- Bemm F, Becker D, Larisch C, et al. 2016. Venus flytrap carnivorous lifestyle builds on herbivore defense strategies. Genome Research 26, 812–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin CR. 1875. Insectivorous plants. London: John Murray. [Google Scholar]
- Escalante-Pérez M, Krol E, Stange A, Geiger D, Al-Rasheid KAS, Hause B, Neher E, Hedrich R.. 2011. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proceedings of the National Academy of Sciences, USA 108, 15492–15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A, Schlauer J, Smith SA, Givnish TJ.. 2018. Evolution of carnivory in angiosperms. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology, and evolution. Oxford: Oxford University Press, 22–41. [Google Scholar]
- Fukushima K, Fang X, Alvarez-Ponce D, et al. 2017. Genome of the pitcher plant Cephalotus reveals genetic changes associated with carnivory. Nature Ecology & Evolution 1, 59. [DOI] [PubMed] [Google Scholar]
- Heubl G, Bringmann G, Meimberg H.. 2006. Molecular phylogeny and character evolution of carnivorous plant families in Caryophyllales—revisited. Plant Biology 8, 821–830. [DOI] [PubMed] [Google Scholar]
- Kocáb O, Jakšová J, Novák O, Petřík I, Lenobel R, Chamrád I, Pavlovič A.. 2020. Jasmonate-independent regulation of digestive enzyme activity in the carnivorous butterwort Pinguicula × Tina. Journal of Experimental Botany 71, 3749–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Ané C, Givnish TJ, Graham SW.. 2021. A new carnivorous plant lineage (Triantha) with a unique sticky-inflorescence trap. Proceedings of the National Academy of Sciences, USA 118, e2022724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE.. 2013. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Reichelt M, Mayer VE, Mithöfer A.. 2013. Jasmonates trigger prey-induced formation of ‘outer stomach’ in carnivorous sundew plants. Proceedings of the Royal Society B: Biological Sciences 280, 20130228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Koller J, Vrobel O, Chamrád I, Lenobel R, Tarkowski P.. 2024. Is the co-option of jasmonate signalling for botanical carnivory universal trait for all carnivorous plants? Journal of Experimental Botany 75, 334–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Mithöfer A.. 2019. Jasmonate signalling in carnivorous plants: copycat of plant defence mechanisms. Journal of Experimental Botany 70, 3379–3389. [DOI] [PubMed] [Google Scholar]
- Procko C, Radin I, Hou C, Richardson RA, Haswell ES, Chory J.. 2022. Dynamic calcium signals mediate the feeding response of the carnivorous sundew plant. Proceedings of the National Academy of Sciences, USA 119, e2206433119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko C, Wong WM, Patel J, Mousavi SAR, Dabi T, Duque M, Baird L, Chalasani SH, Chory J.. 2023. Mutational analysis of mechanosensitive ion channels in the carnivorous Venus flytrap plant. Current Biology 33, 3257–3264.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumbaum A, Mauch F, Vögeli U, Boller T.. 1986. Plant chitinases are potent inhibitors of fungal growth. Nature 324, 365–367. [Google Scholar]
- Schulze WX, Sanggaard KW, Kreuzer I, et al. 2012. The protein composition of the digestive fluid from the Venus flytrap sheds light on prey digestion mechanisms. Molecular & Cellular Proteomics 11, 1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S.. 2018. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361, 1112–1115. [DOI] [PubMed] [Google Scholar]
- Williams SE, Pickard BG.. 1972. Properties of action potentials in Drosera tentacles. Planta 103, 222–240. [DOI] [PubMed] [Google Scholar]
- Yilamujiang A, Reichelt M, Mithöfer A.. 2016. Slow food: insect prey and chitin induce phytohormone accumulation and gene expression in carnivorous Nepenthes plants. Annals of Botany 118, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K-J, Chye M-L.. 1999. Methyl jasmonate induces expression of a novel Brassica juncea chitinase with two chitin-binding domains. Plant Molecular Biology 40, 1009–1018. [DOI] [PubMed] [Google Scholar]


