Abstract
The Bacillus subtilis genome encodes at least 17 distinct sigma factors, including seven members of the extracytoplasmic function (ECF) subfamily. We have investigated the expression and regulation of the ECF ς factor encoded by the sigW gene. A ςW-dependent promoter (PW) precedes sigW, demonstrating that this transcription factor is positively autoregulated. Expression of sigW is regulated by both growth phase and medium composition. Maximal expression is attained in early-stationary-phase cells grown in rich medium. We previously reported that sigW mutants have elevated transcription of some ςX-controlled genes, and we now report that the converse is also true: in a sigX mutant, PW is derepressed during logarithmic growth. Thus, these two regulons are mutually antagonistic. Reconstituted ςW holoenzyme faithfully recognizes the PW preceding sigW but does not recognize the PX promoter preceding the sigX gene. Autoregulation of sigX is also highly specific: ςX holoenzyme initiates transcription from PX but recognizes PW poorly if at all. In contrast, several promoters that are at least partially under ςX control are active with both the ςX and ςW holoenzymes in vitro. This finding supports the suggestion that the ςW and ςX regulons overlap. Sequence comparisons suggest that promoters recognized by these two ς factors have similar −35 elements but are distinguished by different base preferences at two key positions within the −10 element.
The recognition of promoters by RNA polymerase (RNAP) requires an associated ς specificity factor (9, 13). In Bacillus subtilis, the principle ς subunit, ςA, can be substituted by any of a number of alternate ς factors (11). Alternate ς factors control the expression of stress responses (ςB), flagellar motility, chemotaxis, and cell wall-related functions (ςD), and the gene expression cascade leading to formation of a dormant endospore (ςH, ςE, ςF, ςG, and ςK). Alternate ς subunits may interact with a small fraction of the available core enzyme to activate transcription of a subset of genes or, as appears to happen during sporulation, become the predominant specificity factor present at a given time. The mechanisms acting to control ς factor activity are remarkably diverse and include the production and regulation of specific anti-ς factors, proteolytic processing of active ς factors from inactive precursors, and regulated turnover.
The sequencing of the B. subtilis genome has led to the identification of seven putative ς factors of the extracytoplasmic function (ECF) subfamily (18). ECF ς factors are found in a wide range of bacteria and control the uptake or secretion of specific molecules or ions and responses to a variety of extracellular stress signals (20). For example, ECF ς factors regulate ferric citrate uptake and heat shock responses in Escherichia coli (2, 7), alginate and exotoxin secretion in Pseudomonas aeruginosa (14, 21), antibiotic production in Streptomyces antibioticus (17), hairpin protein secretion in Erwinia amylovora (25), and carotenoid biogenesis in Myxococcus xanthus (8). The roles of the seven B. subtilis ECF ς factors, and the extent to which their functions may overlap, are largely unknown.
We have previously reported studies of one of the B. subtilis ECF ς factors, ςX. Mutants lacking this protein have an increased sensitivity to elevated temperatures (15). We have determined the consensus sequence for recognition by holoenzyme containing ςX (EςX) and, using this information, were able to identify several target genes recognized by EςX in vivo and in vitro (16). In the course of this work, we noticed a similar promoter element preceding the gene encoding another putative ECF ς factor designated ςW.
In this report, we demonstrate that the promoter element (PW) preceding the sigW-ybbM operon is transcribed by EςW both in vivo and in vitro. In addition, EςW recognizes a subset of promoters that are partially dependent on ςX for expression, consistent with previous in vivo data suggesting that these regulons overlap (16).
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are described in Table 1. For routine DNA manipulations, E. coli Jm2r− (4) was used as the host.
TABLE 1.
Plasmids and bacterial strains used in this study
| Plasmid or strain | Relevant characteristics | Reference or source |
|---|---|---|
| Plasmids | ||
| pDG646 | Antibiotic (erm) cassette plasmid (MLSr) | 10 |
| pJPM122 | Integrational vector for cat-lacZ operon fusion construction | 23 |
| pXH23 | PW PCR product cloned into pJPM122 | This study |
| pKF83 | pBKSII+ containing sigY (PCR product) | This study |
| pKF84 | pBKSII+ containing sigW (PCR product) | This study |
| pKF86 | pET16b containing sigW (NcoI-BamHI) | This study |
| pKF87 | pKF83 with MLSr cassette (pDG646) disrupting sigY | This study |
| pKF88 | pKF84 with MLSr cassette (pDG646) disrupting sigW | This study |
| B. subtilis strains | ||
| CU1065 | W168 trpC2 attSPβ | Lab stock |
| JH642 | trpC2 pheA1 | Lab stock |
| ZB307A | W168 SPβc2Δ2::Tn917::pSK10Δ6 (MLSr) | 26 |
| HB7007 | CU1065 sigX::spc (Spcr) [SigX−] | 15 |
| HB7013 | CU1065::pVA29 (Ermr) [RsiX−] | 15 |
| HB4245 | JH642 sigY::erm (MLSr) [SigY−] | This study |
| HB4246 | JH642 sigW::erm (MLSr) [SigW−] | This study |
| HB7063 | ZB307A SPβ7063 [PW-cat-lacZ] | This study |
| HB7070 | CU1065 SPβ7063 [PW-cat-lacZ] | This study |
| HB7077 | HB7007 SPβ7063 [PW-cat-lacZ; SigX−] | This study |
| HB7084 | HB4245 SPβ7063 [PW-cat-lacZ; SigY−] | This study |
| HB7091 | HB4246 SPβ7063 [PW-cat-lacZ; SigW−] | This study |
| HB7124 | HB7013 SPβ7063 [PW-cat-lacZ; RsiX−] | This study |
Growth conditions.
E. coli was grown in 2×YT (22) liquid medium or on LB plates containing antibiotics as indicated. B. subtilis was grown in liquid culture in either 4×SG medium, Difco sporulation medium (DSM [12]), LB, or morpholinepropanesulfonic acid (MOPS)-buffered minimal medium (6). Since ςW-dependent promoter activity is maximal in rich medium, we routinely used 4×SG medium (modified from 2×SG [12]), which contains 32 g of Difco nutrient broth, 2 g of KCl, and 2.4 g of MgSO4 · 7H2O per liter (adjusted to pH 7.0). After autoclaving, sterile solutions were used to add 2 ml of 1 M Ca(NO3)2, 2 ml of 0.1 M MnCl2, 2 ml of 1 mM FeSO4, and 4 ml of 50% (wt/vol) glucose per liter. Cultures were grown at 37°C with vigorous shaking. For growth on plates, DSM containing 2% (wt/vol) glucose was used.
In E. coli, ampicillin resistance was selected by using 100 μg of ampicillin per ml. For B. subtilis, antibiotics used for selection were erythromycin at 2 μg per ml, neomycin at 10 μg per ml, spectinomycin at 100 μg per ml, and macrolides-lincomycin-streptogramin B (MLS) with 2 μg of erythromycin and 10 μg of lincomycin per ml.
Construction of sigW and sigY mutants.
Primers 143 (5′-GGGGTACCATGGAAATGATGATTAAAAAA-3′) and 144 (5′-CGGGATCCTTAAAGATCCCTTAATTG-3′) were used to amplify sigW from chromosomal DNA. The PCR product was digested with KpnI and BamHI (underlined sites) for cloning into pBKSII+ (Stratagene) to generate plasmid pKF84. pKF84 was digested with HindIII, and the region between codons 43 and 125 was replaced with a 1.6-kb erm gene (MLSr) HindIII cassette isolated from plasmid pDG646 (10) to generate pKF88. In this plasmid, erm and sigW′ are oriented in the same direction. To construct a sigW::erm mutant strain, pKF88 was used to transform B. subtilis to MLSr to generate HB4246. The sigY mutant was constructed in a similar manner, using primers 141 (5′-GGGGTACCATGGATACACAAGAAGAACAG-3′) and 142 (5′-CGGGATCCTTATTCATCATCCCACTCCT-3′). The amplified product was digested with KpnI and BamHI (sites underlined) and cloned into pBKSII+ to generate plasmid pKF83, and then the erm gene was inserted as a HindIII fragment to replace sigY codons 39 through 63 to generate pKF87. Transformation of JH642 with pKF87 generated the sigY mutant strain, HB4245.
Construction of PW-cat-lacZ fusion.
The region containing the putative ςW-dependent promoter element was amplified by PCR with chromosomal DNA as the template. The forward primer, 180 (5′-ACGAATAAGCTTCTACACCCTGCCAAA-3′), and reverse primer, 181 (5′-AATGGATCCTGGTCGCCTTTTTTGA-3′), amplify a 161-bp region of B. subtilis DNA. The PCR product was digested with BamHI and HindIII (underlined sites) and cloned into pJPM122 (23) to generate a cat-lacZ operon fusion in plasmid pXH23. This plasmid was linearized with ScaI and integrated into strain ZB307A (26) to generate strain HB7063. For transduction into wild-type and mutant backgrounds (Table 1), SPβ specialized transducing phage carrying the PW-cat-lacZ operon fusion (SPβ7063) were recovered by heat induction and used to transduce recipient strains to neomycin resistance. Experiments were conducted in both CU1065 and JH642 wild-type backgrounds, and no differences were noted.
Overproduction and purification of ςW.
The sigW PCR amplification product (as described above) was digested with NcoI and BamHI and cloned into pET16b (Novagen) to generate pKF86. The sequence of the cloned sigW gene was confirmed by DNA sequencing of pKF86. Transformation of pKF86 into E. coli BL21/DE3 (24) generates strain HE7123. For purification of ςW, HE7123 was grown to mid-logarithmic phase at 37°C in 1 liter of 2×YT medium supplemented with 0.4% (wt/vol) glucose and 100 μg of ampicillin per ml. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to 0.4 mM, and cells were harvested after further incubation for 3 h. After centrifugation, the cell paste was suspended in 20 ml of disruption buffer (50 mM Tris-HCl [pH 8.0], 2 mM EDTA, 0.1 mM dithiothreitol [DTT], 1 mM β-mercaptoethanol, 233 mM NaCl, 10% glycerol) and lysed by sonication, and the inclusion bodies were recovered by centrifugation. The inclusion bodies were washed twice with 100 ml of TEDG buffer (10 mM Tris-HCl [pH 8.0], 0.1 mM EDTA, 1 mM DTT, 5% glycerol) containing 0.5% (vol/vol) Triton X-100 and 10 mM EDTA and then dissolved in 20 ml of TEDGX (TEDG containing 0.01% Triton X-100) containing 0.4% (wt/vol) Sarkosyl. The dissolved ςW was gradually diluted to 200 ml with TEDGX to allow renaturation of ςW, and the diluted ςW was dialyzed twice for 8 h against 10 vol of TEDGX at 4°C. The dialyzed material was centrifuged to remove any precipitate and loaded onto a 20-ml heparin-Sepharose CL-6B column equilibrated with TEDGX. After being washed with 200 ml of TEDGX and 0.2 M NaCl, ςW was eluted with TEDGX and 0.5 M NaCl. The peak fraction of ςW was further purified by chromatography on an FPLC (fast protein liquid chromatography) Superdex-75 column in TEDGX plus 0.2 M NaCl buffer, and the peak fractions were stored frozen at −80°C.
Runoff transcription assays.
Runoff transcription assays were performed with PCR-amplified promoter fragments from pJPM122 subclones that had been tested for in vivo expression activity and verified by DNA sequencing. Reactions (25 μl) were performed and analyzed as described previously for ςX-dependent transcription (15) except that the buffer contained 110 mM NaCl and lacked added potassium glutamate. Each reaction mixture contained 0.5 pmol of DNA template, 2.5 pmol of B. subtilis RNAP, and, where indicated, 40 pmol of the designated ς factor. Incubation was for 15 min at 37°C, and samples were analyzed by electrophoresis on an 8 M urea–6% polyacrylamide gels as described previously (15).
Primer extension assays.
RNA was prepared from late-logarithmic-phase cells (approximately T−1) as described elsewhere (16). One hundred micrograms of total RNA was precipitated with 2 pmol of end-labeled reverse primer 181. The pellet was resuspended in 40 μl of hybridization buffer (60 mM NaCl, 50 mM Tris-HCl [pH 8.0], 10 mM DTT), heated to 95°C for 3 min, and cooled slowly to 40°C over 30 min. Then 60 μl of extension solution (60 mM NaCl, 50 mM Tris-HCl [pH 8.0], 13 mM DTT, 10 mM MgCl2, 1 mM deoxynucleoside triphosphates, 20 U of avian myeloblastosis virus reverse transcriptase) was added, and the mixture was incubated at 37°C for 30 min. Nucleic acids were extracted with phenol-CHCl3 (1:1, vol/vol), precipitated with ethanol, resuspended in 10 μl of double-distilled H2O with 0.1 μg of DNase-free RNase per μl, and incubated at room temperature for 30 min; 10 μl of sequencing gel loading buffer was added, the reaction mixture was heated at 95°C for 3 min, and the nucleic acids were separated by electrophoresis on an 8 M urea–6% polyacrylamide gel and visualized by autoradiography. Double-stranded pXH23 DNA was sequenced by using phosphorylated primer 181, and the reaction products were electrophoresed adjacent to the primer extension products.
RESULTS
Identification of PW upstream of the sigW gene.
In a previous study, we searched the B. subtilis genome for sequences resembling the sigX autoregulatory site, PX (16). In the course of this analysis, we noticed an ECF-type promoter element upstream of the sigW gene (Fig. 1). Many ECF ς factors recognize promoters with similar consensus elements, particularly in the −35 element, and autoregulation is quite common (20). We therefore hypothesized that this sequence might be a ςW-dependent promoter, PW.
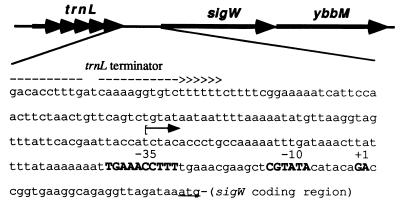
FIG. 1.
Diagram of the sigW-ybbM region (16.6°; 195 kb) of the B. subtilis chromosome. The sequence of the region between trnL and sigW is shown. The inverted repeat predicted to terminate transcription of the trnL operon is illustrated. The bracket and arrow indicate the upstream boundary of the PW-containing fragment used in construction of the PW-cat-lacZ reporter. The −35 and −10 regions of PW, and the two observed transcription start points, are shown in boldface.
To determine if the putative PW element was active, we generated a cat-lacZ operon fusion integrated at SPβ. Specialized transducing phage were used to transduce the PW-cat-lacZ reporter fusion into wild-type and sigW mutant strains, and expression was assessed on DSM plates containing 2% glucose and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). While the wild-type strain is light blue on this medium, there was no detectable expression from the PW-cat-lacZ reporter fusion in the sigW mutant strain. We also tested the effects of mutations in two other genes encoding ECF ς factors, sigX and sigY, on expression of the PW-cat-lacZ reporter fusion. Mutation of sigY had no effect on expression, while mutation of sigX led to increased expression.
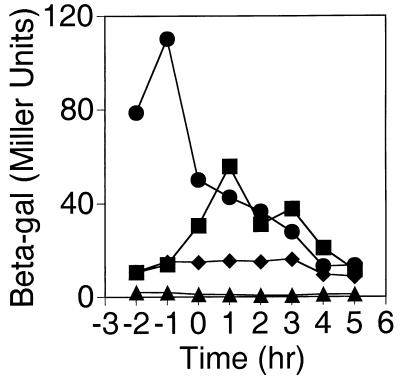
These results were quantified by β-galactosidase assay of cells grown in liquid media. In rich (4×SG) medium, optimal expression of the PW-cat-lacZ fusion occurs between 1 and 3 h after the end of logarithmic growth phase (T1 to T3), and as on plates, this expression is completely dependent on ςW (Fig. 2). We conclude that ςW contributes to its own expression and PW is not recognized to a significant extent by other ς factors, including other ECF ς factors, active in the cell under these conditions. Activity of the PW-cat-lacZ fusion is also affected by the composition of the growth medium. Expression is highest in 4×SG and is reduced between two- and fivefold when cells are grown in either DSM (with or without 2% glucose), LB, or MOPS-buffered minimal medium.
FIG. 2.
Expression of PW-cat-lacZ. Expression of β-galactosidase (Miller Units) is plotted as a function of growth phase for cells grown in 4×SG. Time zero is the end of logarithmic-phase growth. The strains used contain SPβ7063 (PW-cat-lacZ) reporter phage in either wild-type (■), sigW::erm (▴), sigX::spc (•), or rsiX::pVA29 (⧫) mutant background.
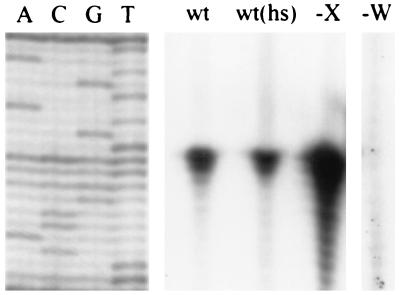
We used primer extension start site mapping to determine if the putative PW element (Fig. 1) was responsible for transcription in these strains. In the wild-type strain, transcription initiates with G and A residues located 7 and 8 nucleotides downstream from the CGTATA −10 element (Fig. 3). These transcripts are more abundant in the sigX mutant, consistent with the derepression observed on plates, and are completely absent from a sigW mutant. There was no induction of the sigW transcript when cells were heat shocked by transfer to 50°C for 15 min prior to RNA isolation. We did not detect any longer transcripts in this assay under conditions that would detect transcripts initiating anywhere within 300 bases upstream of PW. Thus, expression of ςW appears to be completely dependent on ςW, at least under these growth conditions. We cannot exclude the possibility that some basal level of expression is contributed by readthrough of the terminator for the upstream trnL operon (Fig. 1).
FIG. 3.
Primer extension analysis of PW activity. RNA samples were prepared from late-logarithmic-phase cells of the wild-type strain CU1065 grown at 37°C (wt) or 15 min after a shift to 50°C [wt(hs)] and analyzed by reverse transcription. Samples were also prepared from the sigX::spc (−X) and sigW::erm (−W) mutants. The sequencing ladder on the left was generated by using the same reverse primer (primer 181) and double-stranded pXH23 DNA. The two start sites are A and G residues as shown in Fig. 1.
Expression of PW in a sigX mutant.
We noticed that PW-cat-lacZ expression is derepressed in the sigX mutant strain as observed in plate assays, in the primer extension experiment (Fig. 3), and during growth in liquid culture (Fig. 2). This derepression occurs during late logarithmic phase, when ςX activity is normally the highest (15, 16). This finding suggests that ςX antagonizes the activity of ςW. However, since sigX is cotranscribed with a negative regulatory gene, rsiX (3, 15), it is also possible that the antagonism is due to RsiX and the observed derepression is due to polarity of the sigX::spc insertion. Since RsiX appears to function as an anti-ς factor (3), it could inhibit ςW activity directly. To test this idea, we compared expression of PW-cat-lacZ in the wild type and an rsiX mutant (Fig. 2). In the rsiX mutant, expression of PW-cat-lacZ no longer increases upon entry into stationary phase, suggesting that the increased activity of ςX in the absence of RsiX further antagonizes ςW. The opposite result would have been expected if RsiX was able to inhibit ςW directly.
Reconstitution and activity of ςW holoenzyme.
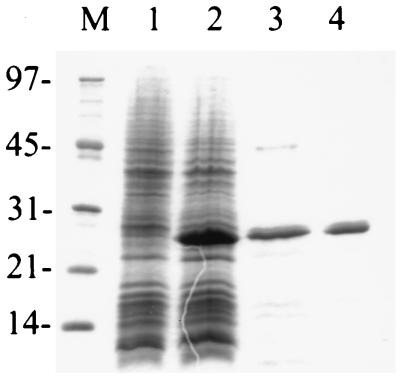
We overproduced and purified ςW by using a T7 RNAP-based overexpression system. As found previously for several other B. subtilis ς factors, ςW accumulates in inclusion bodies upon overproduction. Since ςW precipitated during renaturation from inclusion bodies dissolved in 6 M guanidine hydrochloride, we used Sarkosyl as a denaturant as originally described for E. coli ς32 (5). ςW was about 90% pure after heparin-Sepharose chromatography and >95% pure after Superdex-75 chromatography (Fig. 4). ςW elutes during gel exclusion chromatography with an apparent molecular mass of 31.0 kDa, which is somewhat greater than the calculated monomer mass of 21.6 kDa. This is typical of ς factors and has been interpreted as an indication of an asymmetric shape.
FIG. 4.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of fractions from the ςW purification. Lane M, molecular weight markers (sizes in kilodaltons are indicated on the left); lane 1, 100 μg of whole-cell lysate of HE7123 prior to induction with IPTG; lane 2, 100 μg of whole-cell lysate after 3 h of induction with IPTG; lane 3, 10 μg of peak fractions after heparin-Sepharose CL-6B chromatography; lane 4, 10 μg of peak fractions after FPLC Superdex-75 chromatography.
Addition of ςW to purified core RNAP specifically activates transcription of the PW element to give runoff products of the expected size (Fig. 5). In contrast, a specific transcript is not observed in reactions with the core RNAP alone, and there is only a very slight stimulation in reactions in which ςX is added in place of ςW. Similarly, only ςX is active in stimulating transcription from the sigX-dependent promoter preceding the sigX gene (Fig. 5). There is a low level of transcription with the core fraction, suggesting that this core preparation is contaminated with ςX (as seen previously [15]), but there is no further stimulation of transcription upon addition of ςW. This in vitro transcription analysis is consistent with the in vivo observations that PW is completely dependent on sigW (Fig. 2) and PX is dependent on sigX (15).
FIG. 5.
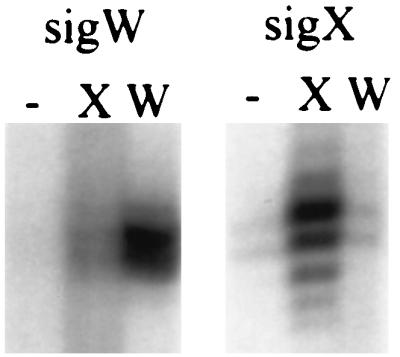
In vitro runoff transcription of PW and PX. PCR products containing either PW or PX were used as templates for runoff transcription reactions with core RNAP either alone (−) or after supplementation with either ςX (X) or ςW (W). Note that the core RNAP preparation has a low level of activity in the absence of added ς, suggesting that this preparation has some contaminating ςX as noted previously (15).
Overlapping promoter selectivity of ςW and ςX.
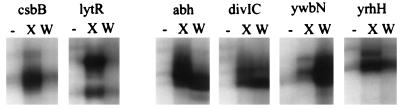
In a previous study, we noted that a subset of putative ςX-dependent promoters were active in a sigX mutant but were, in some cases, inactive in a sigX sigW double mutant (16). We therefore suggested that these ς factors might have overlapping promoter selectivity. To further define the extent to which the ςX and ςW regulons might overlap, we used in vitro runoff transcription assays to test the ability of EςW to recognize promoters identified originally as part of the EςX regulon. In these experiments, for the csbB and lytR promoters, EςW failed to stimulate transcription above the level detected in reactions with core RNAP alone. In contrast, both EςX and EςW were quite active with abh, divIC, ywbN, and yrhH templates (Fig. 6). Thus, both holoenzymes can recognize these promoter elements in vitro.
FIG. 6.
In vitro runoff transcription of EςX and EςW on six ςX-dependent promoters. PCR products containing the indicated promoter regions were amplified from the corresponding pJPM122 derivatives (16) and used as templates for runoff transcription reactions with core RNAP either alone (−) or after supplementation with either ςX (X) or ςW (W). Promoter sequences are shown in Table 2 together with the deduced transcription start sites. All runoff transcripts are between 110 and 180 nucleotides in length, allowing start sites to be assigned with an accuracy of ±2 nucleotides. The start sites for the in vitro EςX transcripts have been previously determined at nucleotide resolution by primer extension mapping (16). Some of the transcript heterogeneity (most notably in the lytR reaction) is apparently due to different 3′ ends resulting from variability in the PCR products since a single well-defined start point is observed in primer extension mapping experiments (data not shown and reference 16).
DISCUSSION
The B. subtilis genome encodes multiple ECF ς factors of largely unknown function (18). As one approach to defining the physiological role of these proteins, we have sought to identify sets of target genes dependent on one or more ECF ς factors for expression. Previously, we identified an autoregulatory ςX-dependent promoter and used saturation mutagenesis to define the bases relevant for ςX recognition in vivo. We then used the resulting consensus sequence to identify a set of eight promoters that were either partially or completely dependent on ςX for expression (16). The genes of the ςX regulon include lytR, a regulator of autolysins (19), and csbB, encoding a putative membrane-bound glucosyltransferase that is also part of the ςB regulon (1). Thus, ςX is postulated to control genes expressed in late logarithmic phase that act to modify the cell surface.
To define the physiological role of ςW, it will be necessary to identify both the target operons and the regulatory signals leading to their expression. As a first step toward defining ςW promoter selectivity, we have characterized an autoregulatory promoter (PW) preceding the sigW-ybbM operon. PW is the prototype for a large family of highly conserved ςW-dependent promoters: at least 12 additional promoters, similar in both the −35 and −10 elements to PW, control the expression of more than two dozen, mostly membrane-localized proteins (our unpublished data). Our ongoing identification of target operons will facilitate physiological studies of the sigW mutant strain which is apparently unaffected in sporulation, competence, and most other post-exponential-phase functions.
PW is completely dependent on ςW in vivo (Fig. 2) and is actively transcribed by EςW in vitro (Fig. 5). By primer extension analysis, we do not detect any additional promoters in the trnL-sigW intergenic region, suggesting that this promoter is primarily, if not exclusively, responsible for sigW transcription. The previously described sigX autoregulatory promoter is also transcribed with high specificity: PX is dependent on ςX in vivo and is recognized in vitro by EςX but not by EςW (Fig. 5). Whereas expression of sigX (15) and ςX-controlled genes (16) is maximal in late-logarithmic-phase cells (T−2 to T0), expression of sigW is highest in early stationary phase (approximately T1 to T3). Thus, the ςX and ςW regulons appear to be quite distinct both in the time of their optimal expression and in their constituent operons.
In contrast with the high selectivity of autoregulation, both lacZ reporter and start site mapping data indicate that some ςX-dependent promoters are still active in a sigX mutant and are therefore recognized by at least one additional ς factor in vivo (16). To better define the sequence features that distinguish ςX- from ςW-dependent promoters, we have tested several ςX-dependent promoters for the ability to be recognized in vitro by EςW. Reconstituted EςW fails to stimulate transcription from the sigX, csbB, or lytR promoter in vitro (Fig. 5 and 6), consistent with the in vivo observation that transcripts from these sites are greatly reduced or eliminated in a sigX mutant strain (16). Thus, these promoters seem to be ςX specific. In contrast, both EςX and EςW recognize promoters for abh, divIC, yrhH, ywbN (Fig. 6), and rapD (data not shown). Therefore, these promoters have properties that allow them to be recognized by either holoenzyme, at least in vitro.
Overlapping holoenzyme selectivity is consistent with previous in vivo primer extension data demonstrating the persistence of transcripts from these sites even in a sigX mutant (16). Most surprising, ywbN and yrhH transcripts were not detected in wild-type cells, were easily detected in sigX mutant cells, but were not detected in sigX sigW double-mutant cells. It now seems likely that transcripts were detected in the sigX mutant because of increased ςW activity during late logarithmic phase, as noted above for PW (Fig. 2). Thus, ywbN and yrhH are members of the ςW regulon. As noted previously, however, analyses of lacZ reporter fusions suggest that these genes are also part of the ςX regulon (16). Transcription from the ςX-like promoter upstream of divIC is reduced less than twofold in a sigX mutant and is still detectable even in a sigX sigW double-mutant strain. We suspect that there is at least one additional holoenzyme that can recognize this site. Thus, while divIC is an active template for both EςX and EςW in vitro (Fig. 6), it is difficult to assess the relative contribution of these ς factors to in vivo expression until the remaining holoenzyme forms allowing initiation at this site are identified. In summary, our in vitro transcription results (Fig. 6), together with previous in vivo data (16), suggest that the ςX and ςW regulons overlap, with the relative contributions of these two ς factors depending on both growth phase and nutritional factors.
Expression of PX, and some ςX-dependent genes, is elevated in a sigW mutant (16). Similarly, we now find that expression of sigW is derepressed in late logarithmic phase in a sigX mutant and repressed in an rsiX mutant (Fig. 2). The basis of this mutually antagonistic behavior is not clear. It is possible that when the ςX holoenzyme is active, it binds and recognizes PW but fails to initiate transcription efficiently, thereby preventing autoactivation of this site during late logarithmic phase. Then, when ςW become active in early stationary phase, it may bind and competitively inhibit expression from those promoter sites exclusively dependent on ςX (e.g., preceding csbB and lytR) while extending expression from those sites able to be recognized by either holoenzyme. In fact, the csbB and lytR promoters, which apparently cannot be recognized by ςW, are the most dramatically induced in a sigW mutant as assessed on X-Gal plates (16). Alternatively, the ςX and ςW regulons may be partially redundant in function and respond to an overlapping set of signals. By this model, expression of one regulon may decrease the need to express the other.
A comparison of promoter sites recognized by either ςX or ςW, or both, suggests that two positions in the −10 region may be key to understanding holoenzyme selectivity (Table 2). Mutational analysis of the sigX promoter identified a −10 consensus of CGwC (where w represents A or T). In contrast, promoters exclusively dependent on ςW (PW and other characterized ςW-dependent promoters [our unpublished data]) contain a −10 region of CGTA. The ability of ςW to transcribe some ςX-dependent promoters (Table 2) demonstrates that this holoenzyme can recognize CGTC in addition to CGTA. Thus, the sequence specificity for ςW seems to be CGT(A or C), although this has not been directly tested by mutagenesis. This suggests a simple model for the partially overlapping recognition observed both in vitro (Fig. 6) and in vivo (16): those promoters that have a −10 element of CGAC are dependent on ςX, those containing CGTC can, at least in principle, be recognized by either ςX or ςW, and those containing CGTA are exclusively dependent on ςW (Table 2).
TABLE 2.
Comparison of promoter sequences recognized in vitro by EςX, EςX and EςW, or EςW alone
| Sequence recognized by: | Gene | Promoter sequence | In vitro activity
|
|
|---|---|---|---|---|
| ςX | ςW | |||
| “−35” −10 +1 | ||||
| EςX | sigX | aaTGTAACTTttcaagctattcataCGACAAaaaagtGaacg | + | − |
| csbB | atTGTAACAAaaaacaggtttaaaCGACTTtaaaaaAAggaa | + | − | |
| lytR | aaTGAAACTTttttttataaaaaaCGACTAttttagGatttc | + | − | |
| EςX and EςW | abh | cgGGAAACTTtttcaaagtttcattCGTCTAcgataTAttGa | + | + |
| divIC | ttTGAAACTTcttcctgtgaaaatgCGTCTAactttTAgacg | + | + | |
| ywbN | taCAAAACAAatgatcagtcctataCGTCTTatgatAaatta | + | + | |
| yrhH | atTGAAACATttttcaatacattgcCGTCTAgttggTacctt | + | + | |
| EςW | sigW | atTGAAACCTtttgaaacgaagctCGTATAcatacaGAccgg | − | + |
It is likely that other features of the promoter, such as preferred spacer length and the −35 region, may also contribute to selectivity. However, deletion of one base from the PX spacer region reduced but did not eliminate activity, and two ςX-dependent promoters, csbB and lytR, have a shorter spacer region (16). EςW also tolerates at least a 1-bp variation in spacer length. Both PX and PW contain a highly conserved AAC motif in the −35 region, similar to promoters recognized by diverse ECF ς factors, and there is no apparent correlation between sequence in this region and holoenzyme selectivity.
ACKNOWLEDGMENTS
We thank A. Gaballa for helpful comments on the manuscript.
This research was supported by National Institutes of Health grant GM47446.
REFERENCES
- 1.Akbar S, Price C W. Isolation and characterization of csbB, a gene controlled by Bacillus subtilis general stress transcription factor ςB. Gene. 1996;177:123–128. doi: 10.1016/0378-1119(96)00287-9. [DOI] [PubMed] [Google Scholar]
- 2.Braun V. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch Microbiol. 1997;167:325–331. doi: 10.1007/s002030050451. [DOI] [PubMed] [Google Scholar]
- 3.Brutsche S, Braun V. SigX of Bacillus subtilis replaces the ECF sigma factor FecI of Escherichia coli and is inhibited by RsiX. Mol Gen Genet. 1997;256:416–425. doi: 10.1007/s004380050585. [DOI] [PubMed] [Google Scholar]
- 4.Bsat N, Chen L, Helmann J D. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J Bacteriol. 1996;178:6579–6586. doi: 10.1128/jb.178.22.6579-6586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess R R. Purification of overproduced Escherichia coli RNA polymerase ς factors by solubilizing inclusion bodies and refolding from Sarkosyl. Methods Enzymol. 1996;273:145–149. doi: 10.1016/s0076-6879(96)73014-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, James L P, Helmann J D. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially regulated by metal ions. J Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Las Penas A, Connolly L, Gross C A. The sigma-E-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigma-E. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 8.Gorham H C, McGowan S J, Robson P R H, Hodgson D A. Light-induced carotenogenesis in Myxococcus xanthus: light-dependent membrane sequestration of ECF sigma factor CarQ by anti-sigma factor CarR. Mol Microbiol. 1996;19:171–186. doi: 10.1046/j.1365-2958.1996.360888.x. [DOI] [PubMed] [Google Scholar]
- 9.Gross C A, Lonetto M, Losick R. Bacterial sigma factors. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1992. pp. 129–176. [Google Scholar]
- 10.Guérout-Fleury A-M, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 11.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley and Sons, Ltd.; 1990. [Google Scholar]
- 13.Helmann J D. Bacterial sigma factors. In: Conaway R C, Conaway J, editors. Transcription: mechanisms and regulation. New York, N.Y: Raven Press; 1994. pp. 1–17. [Google Scholar]
- 14.Hershberger C D, Ye R W, Parsek M R, Xie Z D, Chakrabarty A M. The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative sigma factor (sigma-E) Proc Natl Acad Sci USA. 1995;92:7941–7945. doi: 10.1073/pnas.92.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Decatur A, Sorokin A, Helmann J D. The Bacillus subtilis ςX protein is an extracytoplasmic function sigma factor contributing to the survival of high-temperature stress. J Bacteriol. 1997;179:2915–2921. doi: 10.1128/jb.179.9.2915-2921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Helmann J D. Identification of target promoters for the Bacillus subtilis ςX factor using a consensus-directed search. J Mol Biol. 1998;279:165–173. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- 17.Jones G H, Paget M S B, Chamberlin L, Buttner M J. Sigma-E is required for the production of the antibiotic actinomycin in Streptomyces antibioticus. Mol Microbiol. 1997;23:169–178. doi: 10.1046/j.1365-2958.1997.2001566.x. [DOI] [PubMed] [Google Scholar]
- 18.Kunst F, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 19.Lazarevic V, Margot P, Soldo B, Karamata D. Sequencing and analysis of the Bacillus subtilis lytRABC divergon: a regulatory unit encompassing the structural genes of the N-acetylmuramoyl-l-alanine amidase and its modifier. J Gen Microbiol. 1992;138:1949–1961. doi: 10.1099/00221287-138-9-1949. [DOI] [PubMed] [Google Scholar]
- 20.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochsner U A, Johnson Z, Lamont I L, Cunliffe H E, Vasil M L. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol Microbiol. 1997;21:1019–1028. doi: 10.1046/j.1365-2958.1996.481425.x. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1990. [Google Scholar]
- 23.Slack F J, Mueller J P, Sonenshein A L. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993;175:4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 25.Wei Z M, Beer S V. HrpL activates Erwinia amylovora hrp gene transcription and is a member of the ECF subfamily of sigma factors. J Bacteriol. 1995;177:6201–6210. doi: 10.1128/jb.177.21.6201-6210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuber P, Losick R. Role of AbrB and Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]