Abstract
Introduction
There is clinical equipoise as to whether hyperoxia is injurious to the myocardium, both in the setting of acute ischaemic insults and on the stable myocardium. This study examined the effect of extreme hyperoxia – in the form of hyperbaric oxygen treatment – on the myocardium through measurement of high-sensitivity cardiac troponin.
Methods
Forty-eight individuals were enrolled to undergo a series of 30 exposures to hyperbaric oxygen for treatment of non-cardiac pathologies. High-sensitivity troponin T was measured before and after each session.
Results
There was no clinically significant difference in troponin measurements following acute or recurrent sequential exposures to extreme hyperoxia, despite the studied patient population having a high rate of previous ischaemic heart disease or cardiovascular risk factors.
Conclusions
This study demonstrates that profound hyperoxaemia does not induce any measurable cardiac injury at a biochemical level. Neither is there a reduction in cardiac troponin to suggest a cardioprotective effect of hyperbaric hyperoxia. This provides some reassurance as to the cardiac safety of the routine use of hyperbaric oxygen treatment in management of non-cardiac pathology.
Keywords: Biomarkers, Cardiovascular, Health, Heart, Hyperbaric oxygen treatment
Introduction
Hyperbaric oxygen treatment (HBOT) is an intervention in which an individual intermittently breathes near 100% oxygen while inside a hyperbaric chamber pressurised to greater than 101.3 kPa (1 atmosphere absolute [atm abs]). At 203 kPa (2 atm abs) arterial oxygen tension is expected to be over 1,000 mmHg.[ 1]
The cardiac effects of HBOT have been studied in the context of acute cardiac insults, such as acute coronary syndromes, carbon monoxide poisoning, angioplasty and coronary artery bypass grafting.[ 1 - 3] These human and animal studies suggest HBOT has a protective effect on the myocardium as evidenced by reduced area of necrosis, lower levels of necrosis biomarkers, improved cardiac function, or improved clinical outcomes. A 2015 Cochrane review of effects of HBOT in acute coronary syndromes found some evidence from small trials to suggest that HBOT is associated with a reduction in the risk of death, the volume of damaged muscle, and the risk of major adverse cardiac events. However, insufficient evidence was found to support the routine use of HBOT in this setting.[ 1]
Conversely, there is evidence that hyperoxia may in fact be damaging to the myocardium in the setting of an acute ischaemic insult. Oxygen therapy (normobaric oxygen – NBO) in patients with ST-segment elevation myocardial infarction (STEMI) but without hypoxia may increase early myocardial injury and result in larger myocardial infarct size at six months.[ 4]
However, a subsequent randomised control trial comparing the effect of supplemental oxygen versus ambient air in patients with symptoms suggestive of acute myocardial infarction, but with oxygen saturations of 90% or higher, did not demonstrate any significant difference in peak troponin measurement or 1-year all-cause mortality.[ 5]
Thus, the effect of hyperoxia, whether that be normobaric or hyperbaric, on the acutely ischaemic myocardium remains unclear.
With respect to the stable myocardium, in the absence of an acute ischaemic insult, the effect of hyperoxia is also uncertain. A 2009 review found that hyperoxia resulting from NBO exposure resulted in increased coronary vascular resistance and reduced coronary blood flow.[ 6] These experimental studies produced modest hyperoxia in the range of 250 to 450 mmHg, markedly less than the arterial oxygen tensions achieved during HBO exposure. A small study in patients with stable multivessel coronary artery disease has indicated that inhalational hyperoxia in these patients may be associated with adverse effects of regional myocardial deoxygenation.[ 7]
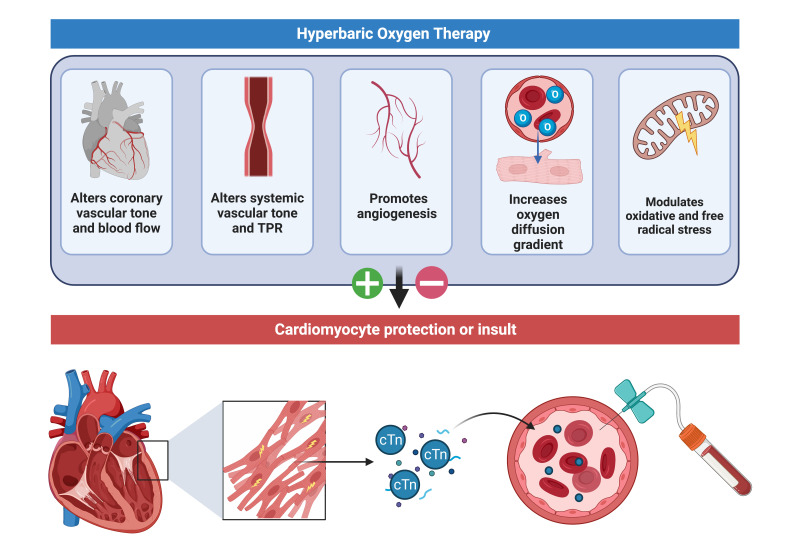
Hyperbaric oxygen is used globally in the treatment of a wide range of non-cardiac pathology. However, many of these patients have risk factors for coronary artery disease. Thus, there should be an index of concern for the effect of repeated HBOT exposures on the myocardium, even in the absence of an acute ischaemic insult. HBOT represents a possible double-edged sword with biological plausibility for cardio-protection or injury (Figure 1).
Figure 1.
Possible mechanisms by which hyperbaric hyperoxia may have a protective or injurious effect on human myocardium; cTN – cardiac troponin; TPR – total peripheral resistance
In this study we sought to assess any injurious effect of hyperbaric hyperoxia on the myocardium in patients undergoing a course of HBOT for non-cardiac pathologies, through measurement of the cardiac-restricted biomarker of necrosis, troponin T.
Methods
The study was approved by South Eastern Sydney Local Health District human research ethics committee (HREC: 2021/ETH00415).
Cardiac troponin T (hs-cTnT) was measured using a high-sensitivity assay (Roche Elecsys),[ 8] in consecutive patients planned to undergo greater than twenty HBOT exposures for treatment of non-cardiac pathology. Each treatment consisted of 90 min exposure to > 203 kPa (2 atm abs) pressure whilst intermittently breathing 100% oxygen, and the planned treatment course consisted of five exposures a week for 4–6 weeks.
Individuals were not enrolled if they had suffered an acute coronary syndrome or undergone cardiac intervention in the last six months, or if they had known chronic kidney disease stage four or greater.
Forty-eight individuals were prospectively enrolled (mean age 69 years, 65% male). Two participants withdrew from the study before the collection of any paired samples and were not included in data analysis; one due to patient preference, and one due to reporting chest pain before commencing treatment. Participants represented a high-risk demographic for cardiac disease: 25% had a known history of established ischaemic heart disease (IHD), 13% had previous coronary stents or bypass grafting, 13% were diabetic, and 52% were on at least one antihypertensive or cholesterol lowering agent. Of measured baseline troponins, 36% had serum hs-cTnT greater than the upper reference limit of 14 ng·L-1.
Serum hs-cTnT was measured before and after HBOT sessions 1, 10, 20, and 30 (a total of four paired hs-cTnT measurements). Logistical issues affecting HBOT treatments and phlebotomy during the COVID-19 pandemic meant that a complete set of paired samples was not achieved for all participants. Nonetheless, all participants contributed at least one paired set of data to the analysis, and 83% of individuals contributed 2, 3, or 4 paired sets of data. No participant experienced cardiac symptoms during acute exposure to HBO.
Where available, paired samples were compared before and after individual treatments to assess for acute changes in hs-cTnT following exposure to HBOT. Comparison was also made between the 1st and 30th treatment, to assess for chronic changes to hs-cTnT during a course of repeated HBOT exposure.
Results
No statistically significant difference in hs-cTnT was detected either as the result of acute or chronic repeated exposures to HBOT (using paired t-test) (Table 1). Repeated measures ANOVA also failed to demonstrate significant changes in hs-cTnT when applied to the 24 subjects who had a complete set of hs-cTnT measurements before their 1st, 10th, 20th, and 30th treatments (Greenhouse-Geisser correction P = 0.767).
Table 1. Statistical analysis of paired troponin levels measured in ng·L-1; HBOT – hyperbaric oxygen treatment; hs-cTnT – cardiac troponin; SEM – standard error of mean .
| Paired hs-cTnT measurements before and after 90 min exposure to HBOT | ||||
| Treatment number | Number of paired samples | Mean (SEM) pre-treatment | Mean (SEM) post-treatment | P-value |
| 1 | 35 | 18.56 (6.03) | 18.76 (7.37) | 0.91 |
| 10 | 30 | 18.83 (9.29) | 18.27 (8.43) | 0.62 |
| 20 | 33 | 20.60 (7.94) | 19.79 (7.76) | 0.22 |
| 30 | 28 | 20.23 (9.05) | 19.28 (8.28) | 0.24 |
| Paired hs-cTnT measurements before and after a course of 30 HBOT exposures | ||||
| Treatment number | Number of paired samples | Mean (SEM) treatment 1 | Mean (SEM) treatment 30 | P-value |
| 1 vs 30 | 35 | 18.07 (6.06) | 18.77 (7.25) | 0.64 |
Discussion
To our knowledge this is the first study to rigorously assess the effect of hyperbaric hyperoxia on baseline myocardial health through measurement of hs-cTnT. This was a cohort of patients with a high prevalence of established IHD, or risk factors for such. This is reflected in the fact that the mean hs-cTnT levels were consistently above the 99th percentile upper reference limit of the Roche Elecsys assay.[ 9]
In this population, our study demonstrated that exposure to profound hyperoxaemia did not induce any measurable cardiac injury at a biochemical level. Equally, there was no reduction in circulating serum hs-cTnT to suggest a cardioprotective effect of hyperbaric hyperoxia. This provides some degree of reassurance as to the cardiac safety of HBOT for patient undergoing routine hyperbaric treatment for non-cardiac pathologies.
Footnotes
Acknowledgements
Professor Michael Bennett contributed substantially to this study but died before its final publication; the research team would like to recognise the unique contribution of Professor Bennett to the field of Hyperbaric Medicine over the course of his distinguished career. Thanks to M Turley, M Grealish, H Sangha and P Shively for their invaluable contribution to data collection. Thanks to Roche for provision of discounted assay reagents, and the Department of Chemical Pathology, NSW Health Pathology, Randwick, for the diligent analysis of samples. Figures created with BioRender.com.
Conflict of interest and funding:
Roche provided discounted assay reagents but had no role in study design or implementation, or in analysis of results.
Contributor Information
Jack Marjot, Department of Diving and Hyperbaric Medicine, Prince of Wales Hospital, Sydney.
John Mackenzie, Department of Diving and Hyperbaric Medicine, Prince of Wales Hospital, Sydney.
Nigel Jepson, Department of Cardiology, Prince of Wales Hospital, Sydney.
Ewan Reeves, Department of Diving and Hyperbaric Medicine, Prince of Wales Hospital, Sydney.
Michael Bennett, Department of Diving and Hyperbaric Medicine, Prince of Wales Hospital, Sydney.
References
- Bennett MH, Lehm JP. Hyperbaric oxygen therapy for acute coronary syndrome. Cochrane Database Syst Rev. 2015;2015(7):CD004818. doi: 10.1002/14651858.CD004818.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos L, Serra AJ, Antônio EL, Hull EL, Tucci PJ. Hyperbaric oxygenation applied immediately after coronary occlusion reduces myocardial necrosis and acute mortality in rats. Clin Exp Pharmacol Physiol. 2009;36(5-6):594–8. doi: 10.1111/j.1440-1681.2008.05118.x. [DOI] [PubMed] [Google Scholar]
- Dekleva M, Neskovic A, Putnikovic B, Beleslin B, Ostojic M. Adjunctive effect of hyperbaric oxygen treatment after thrombolysis on left ventricular function in patients with acute myocardial infarction. Am Heart J. 2004;148(4):E14. doi: 10.1016/j.ahj.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, et al. AVOID Investigators. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131:2143–50. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N, et al. DETO2X–SWEDEHEART Investigators. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377:1240–9. doi: 10.1056/NEJMoa1706222. [DOI] [PubMed] [Google Scholar]
- Farquhar H, Weatherall M, Wijesinghe M, Perrin K, Ranchord A, Simmonds M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158:371–7. doi: 10.1016/j.ahj.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Guensch DP, Fischer K, Yamaji K, Luescher S, Ueki Y, Jung B, et al. Effect of hyperoxia on myocardial oxygenation and function in patients with stable multivessel coronary artery disease. J Am Heart Assoc. 2020;9(5):e014739. doi: 10.1161/JAHA.119.014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerbin G, Tate JR, Hickman PE. Analytical characteristics of the Roche highly sensitive troponin T assay and its application to a cardio-healthy population. Ann Clin Biochem. 2010;47(Pt 6):524–8. doi: 10.1258/acb.2010.010033. [DOI] [PubMed] [Google Scholar]
- Eggers KM, Al-Shakarchi J, Berglund L, Lindahl B, Siegbahn A, Wallentin L, et al. High-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. Am Heart J. 2013;166:541–8. doi: 10.1016/j.ahj.2013.07.004. [DOI] [PubMed] [Google Scholar]