Abstract
TrfA is the only plasmid-encoded protein required for initiation of replication of the broad-host-range plasmid RK2. Here we describe the isolation of four trfA mutants temperature sensitive for replication in Pseudomonas aeruginosa. One of the mutations led to substitution of arginine 247 with cysteine. This mutant has been previously described to be temperature sensitive for replication, but poorly functional, in Escherichia coli. The remaining three mutants were identical, and each of them carried two mutations, one leading to substitution of arginine 163 with cysteine (mutation 163C) and the other a codon-neutral mutation changing the codon for glycine 235 from GGC to GGU (mutation 235). Neither of the two mutations caused a temperature-sensitive phenotype alone in P. aeruginosa, and the effect of the neutral mutation was caused by its ability to strongly reduce the trfA expression level. The double mutant and mutant 163C could not be stably maintained in E. coli, but mutant 235 could be established and, surprisingly, displayed a temperature-sensitive phenotype in this host. Mutation 235 strongly reduced the trfA expression level also in E. coli. The glycine 85 codon in trfA mRNA is GGU, and a change of this to GGC did not significantly affect expression. In addition, we found that wild-type trfA was expressed at much lower levels in E. coli than in P. aeruginosa, indicating that this level is a key parameter in the determination of the temperature-sensitive phenotypes in different species. The E. coli lacZ gene was translationally fused at the 3′ end and internally in trfA, in both cases leading to elimination of the effect of mutation 235 on expression. We therefore propose that this mutation acts through an effect on mRNA structure or stability.
RK2 is a 60-kb self-transmissible plasmid capable of replicating in a large number of bacterial species (25). The plasmid is present in the cell at a copy number of four to seven per chromosome in Escherichia coli (9). Replication is known to be initiated at a single origin, oriV (26), and the only other plasmid locus essential for replication is the trfA gene (8). Minireplicons of RK2 containing only oriV and trfA were found to replicate in at least 12 gram-negative bacterial species (1, 12, 20).
The trfA gene of RK2 specifies two replication initiation proteins, a 44-kDa protein (TrfA-44) and a 33-kDa protein (TrfA-33), resulting from alternative translational starts within the same open reading frame (21, 24). In E. coli, either TrfA-44 or TrfA-33 alone is sufficient for replication, whereas in Pseudomonas aeruginosa, there is a specific requirement for TrfA-44 in the stable maintenance of the RK2 replicon (6). However, TrfA-33 can drive replication in P. aeruginosa if this protein is expressed at a sufficiently high level (22). The TrfA protein can be supplied in cis or in trans (with respect to oriV) to an RK2 origin plasmid, and it initiates replication by binding to the iterons at the origin (16–18).
A large number of mutations in the trfA gene have been shown to lead to enhanced copy numbers (4, 7, 11, 12), but the highest-copy-number mutants isolated in E. coli could not be established in other tested bacterial species. We later found that the reason for this is that each species has a specific upper maximum tolerable copy number tolerance, and none of the tested species tolerated as high copy numbers as E. coli (12). Similarly, we have found that trfA mutants temperature sensitive for replication do not display this phenotype in other species (11, 28). These observations have consequences for the construction of broad-host-range mini-RK2 cloning and expression vectors (1, 2) and are also important for the understanding of the nature of the broad-host-range properties of this replicon. In this report, we show that a codon-neutral trfA mutation can act in combination with a substitution mutation to generate a replication-temperature-sensitive phenotype in P. aeruginosa. The codon-neutral mutation acts by reducing the trfA expression level, and it therefore seems clear that this parameter is important for the determination of the species-specific phenotypes of replication-temperature-sensitive trfA mutants.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacteria and plasmids used in this study
| Bacterial strain or plasmid | Propertiesa | Source or reference(s) |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | endA1 hsdR17 supE44 thi-1 λ− recA1 gyrA96 relA1 ΔlacU169 (φ80d lacZΔM15) | Bethesda Research Laboratories |
| S17.1 | RP4 2-Tc::Mu-Km::Tn7 pro res mod+ | 23 |
| K37 | galK | 30 |
| K7487 | galK rho-1, rho-deficient derivative of K37 | 30 |
| P. aeruginosa PAO1161S | Spontaneous streptomycin-resistant derivative of PAO1161 | 12 |
| Plasmids | ||
| pRD110-34 | ColE1 replicon in which an EcoRI-PstI fragment of pBR322 was substituted with the trfA gene from plasmid RK2, Tcr, 4.8 kb | 7 |
| pPK34 | Derivative of pRD110-34 in which the NdeI site in the vector was removed and a 49-bp PflM1 fragment was deleted and the PflMI site was removed to simplify manipulations of trfA. These modifications do not affect TrfA expression. Designated pPK34 here. | 11 |
| pMC1871 | ColE1 replicon, Tcr, contains lacZ | Pharmacia |
| pFF1 | RK2 replicon with oriT | 7 |
| pFF1 163C,235 | P. aeruginosa ts mutant | This study |
| pFF1 163C | Derivative of pFF1 containing the trfA 163C mutation | This study |
| pFF1 235 | Derivative of pFF1 containing the trfA 235 mutation | This study |
| pFF1 247C | P. aeruginosa ts mutant | This study |
| E. coli ts mutant | 11, 28 | |
| pPK34 163C,235 | Derivative of pPK34, constructed by exchanging wild-type trfA (EcoRI/PstI fragment) with trfA from pFF1 163C,235 | This study |
| pPK34 163C | Derivative of pPK34 containing the trfA 163C mutation | This study |
| pPK34 235 | Derivative of pPK34 containing the trfA 235 mutation | This study |
| pPK34 85 | A codon-neutral mutant (GGU to GGC) at the position corresponding to glycine 85 of TrfA-44 | This study |
| pJB1002 | pRD110-34 derivative with trfA-lacZ translational fusion at codon 245 in trfA | This study |
| pJB1006 | pRD100-34 derivative with trfA-lacZ translational fusion at codon 379 in trfA | This study |
| pJB1004 | pPK34 derivative; equivalent to pJB1002 but with mutation 235 | This study |
| pJB1008 | pPK34 derivative; equivalent to pJB1006 but with mutation 235 | This study |
Apr, ampicillin resistant; Cmr, chloramphenicol resistant; Tcr, tetracycline resistant; ts, temperature sensitive.
Growth of bacteria, conjugative matings, electrotransformations, and standard molecular biology techniques.
E. coli and P. aeruginosa strains were grown in L broth or on L agar at 37°C unless otherwise stated (19). Antibiotics were used at the following concentrations: ampicillin, 200 μg/ml; carbenicillin, 400 μg/ml; and tetracycline, 15 μg/ml. Plasmids were transferred to P. aeruginosa either by electrotransformation (in all cases except for the construction of the pFF1 bank containing the mutagenized trfA genes and for the establishment of the trfA single mutants) as described for E. coli (10) or by conjugative matings from E. coli as described by Haugan et al. (12). Plasmids were transferred to E. coli by the method of Chung et al. (5).
Preparation of plasmid DNA, restriction endonuclease digestions, and agarose gel electrophoresis were performed according to standard protocols (19).
DNA sequencing was performed with the primers described by Haugan et al. (11) on an Applied Biosystems model 373A DNA sequencing system, using a TaqPRISM Ready Reaction Dye Deoxy Terminator Cycle Sequencing kit (Applied Biosystems). The conditions for the 30 thermal cycles were 30 s at 94°C, 15 s at 50°C, and 4 min at 60°C. Sequence assembly was performed with the Auto assembler software (Applied Biosystems).
Isolation of temperature-sensitive trfA mutants in P. aeruginosa.
The pFF1 bank containing the mutagenized trfA genes in E. coli S17.1 (12) was transferred into P. aeruginosa, and the resulting pool of about 120,000 transconjugants was used as a source for the isolation of temperature-sensitive mutants. The transconjugants were incubated on selective (carbenicillin) agar medium at 23°C until small colonies appeared, and the plates were then shifted to 42°C. Colonies that failed to increase in size at 42°C were characterized further. A total of about 100,000 colonies were inspected, leading to the identification of four plasmid mutants temperature sensitive for replication. The molecular analysis of the mutants was performed by localizing the mutations to a smaller fragment of the trfA gene as described by Haugan et al. (11), followed by DNA sequencing.
Site-directed mutagenesis and translational fusions.
A codon-neutral mutation at glycine 85 of the trfA gene (from GGT to GGC) (mutation 85) was made by modified restriction site PCR (13). The unique restriction sites EcoRI and SfiI at the 5′ end of the trfA gene were used for this purpose. The four primers used in the experiment were 5′-GCAGGGGATCAAGATCGACG-3′, 5′-ATCCGGGTAATTCCGGGGCA-3′, 5′-TGATCTGCTGCTTCGTGTGT-3′, and 5′-CTTCGCCAAGCCTGCCGCCT-3′. The trfA-lacZ translational fusions were made by first PCR amplifying lacZ from plasmid pMC1871. Two different fragments were made, one containing a BsmI and another containing a SexAI restriction endonuclease site at the 5′ end of lacZ. The primers used to generate these 5′ ends were 5′-GCCGATGAATGCCCCGGGGATCCCGTC-3′ (BsmI site) and 5′-GCCGATACCTGGTTCCCGGGGATCCCGTC-3′ (SexAI site). For both fragments, the same 3′ primer (5′-GAATGAAGCCATACCAAACG-3′) was used. This primer corresponds to sequences downstream of the PstI site 3′ of lacZ. The BsmI and SexAI sites are naturally present in trfA, allowing in-frame translational fusions at positions corresponding to codons 245 and 379, respectively. There is also a PstI site downstream of trfA in pRD110-34 and pPK34, simplifying insertions of the PCR fragments in the appropriate positions.
Western blotting and quantification of β-galactosidase activity and total protein.
Samples for Western analysis were prepared by boiling the cells for 2 to 5 min in the loading buffer (18a). The samples were subjected to electrophoresis in 10% sodium dodecyl sulfate-polyacrylamide gels according to the procedure of Laemmli (14). The proteins were transferred from the gel to Immobilon-P transfer membranes (Millipore) as described by Towbin et al. (27). The Immobilon sheets were incubated with a polyclonal anti-TrfA antibody diluted 1:1,000 in Tris-buffered saline (20 mM Tris chloride [pH 7.5], 150 mM NaCl, 3% bovine serum albumin, 0.2% Triton X-100) for 2 h at 37°C, followed by incubation with peroxidase-conjugated goat anti-rabbit immunoglobulin diluted 1:5,000 in Tris-buffered saline for 1 h at room temperature (25°C). The blots were then processed for detection of the TrfA antibody complexes by using the Pierce SuperSignal Western blotting kit, which uses a chemiluminescent reaction for visualization.
β-Galactosidase activities were measured as described by Sambrook et al. (19).
For quantification of protein, the soluble fractions obtained by centrifugation of sonicated cells were analyzed by the Bio-Rad Coomassie brilliant blue-based protein assay (as described in the Bio-Rad manual).
RESULTS
Isolation and characterization of mutants temperature sensitive for replication in P. aeruginosa.
E. coli S17.1 cells containing plasmid pFF1 with mutagenized trfA genes were conjugated into P. aeruginosa, and the transconjugants were pooled. The use of strain S17.1 prevents loss of plasmids nonfunctional in E. coli because S17.1 expresses wild-type TrfA from the chromosome. Screening of the transconjugants indicated that the frequency of temperature-sensitive mutants was about 4 × 10−5, and four such mutants were identified and characterized further (Table 2). The mutants fell into two phenotype classes. One mutant (mutant 1) allowed cell growth in the presence of carbenicillin up to 37°C, while the remaining three strains grew at 23°C but not at any of the higher tested temperatures.
TABLE 2.
Temperature dependence of growth for P. aeruginosa cells containing pFF1 plasmid mutantsa
| pFF1 construct | Growth at:
|
|||
|---|---|---|---|---|
| 23°C | 30°C | 37°C | 42°C | |
| Wild type | + | + | + | + |
| Mutant 1 | + | + | + | − |
| Mutant 2 | + | − | − | − |
| Mutant 3 | + | − | − | − |
| Mutant 4 | + | − | − | − |
P. aeruginosa PAO1161S cells containing the pFF1 plasmids were grown at 23°C in the presence of carbenicillin to stationary phase, diluted, and plated (about 300 cells/plate) on agar medium containing carbenicillin. The plates were incubated overnight (2 days for incubations at 23°C) at the temperatures indicated and then inspected for visible growth.
All four mutations were found to map between the SfiI and NdeI restriction endonuclease sites in trfA, and the corresponding DNA fragments were therefore sequenced in all four mutants (Fig. 1). The results showed that the mutation in mutant 1 leads to replacement of arginine 247 with cysteine. This mutation has previously been described for E. coli, and its phenotype was characterized in this host and to some extent in P. aeruginosa (11, 28). In E. coli the mutant was found to display a temperature-sensitive phenotype, and functional replication at the permissive temperature was found to require high TrfA expression levels. The phenotype of the mutant was also tested in P. aeruginosa, and it was found to be able to replicate at 42°C at low but not at high concentrations of carbenicillin (100 and 800 μg/ml, respectively). In the present work 400 μg/ml was used, and based on these data this mutant was not studied further.
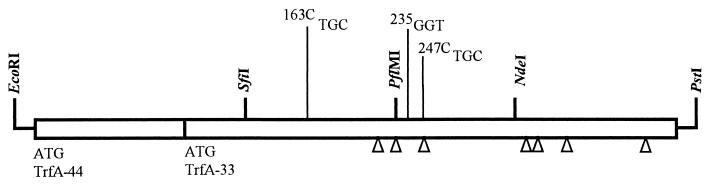
FIG. 1.
Map of trfA mutations leading to a replication-temperature-sensitive phenotype. Mutant number designations correspond to amino acid residues affected by the mutations (the first methionine from the amino-terminal end in the 44-kDa protein represents residue 1). The three-letter designations following the numbers indicate the new DNA sequence at the corresponding codon. Arrowheads indicates the locations of the mutations previously reported to lead to a replication-temperature-sensitive phenotype in E. coli (11).
The sequence data of the remaining three mutants (mutants 2, 3, and 4) showed that they were identical, consistent with their indistinguishable phenotypes (Fig. 1). These mutants contained two mutations, one of which involved substitution of arginine 163 with cysteine (mutation 163C). This mutation has to our knowledge not been previously reported. The second mutation involves a base substitution (C to T) at the position corresponding to glycine 235 (mutation 235). Surprisingly, this mutation does not lead to an amino acid substitution.
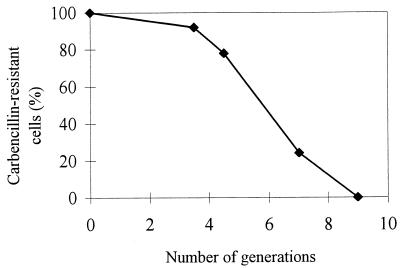
The phenotype of double mutant 163C,235 in P. aeruginosa is consistent with the assumption that replication is temperature sensitive; to confirm that this is the case, we carried out a stability test in which plasmid loss at a nonpermissive temperature was monitored (Fig. 2). The data show that a switch from growth at 23°C to growth at 37°C leads to a rapid loss of the plasmids, consistent with a replication deficiency.
FIG. 2.
Plasmid loss kinetics of P. aeruginosa cells containing the mutant plasmid pFF1 163C,235. Cells were grown exponentially at 23°C in the presence of carbenicillin, diluted 104-fold (0 generations), and then incubated further at 37°C in the absence of carbenicillin. At the times indicated, the cells were diluted and plated on agar medium without antibiotics. After incubation at 23°C, individual colonies were picked and tested for carbenicillin resistance at 23°C. Wild-type pFF1 is not lost at significant frequencies over such short incubation periods.
Separation of the mutations in the double mutant and characterization of phenotypes in P. aeruginosa and E. coli.
The two mutations in the double mutant could easily be separated by the unique PflMI site localized between the mutations. The single mutations were later moved to pFF1 as EcoRI-PstI fragments and were established in E. coli S17.1. The phenotypes of the corresponding mutants (163C and 235, respectively) were then analyzed in P. aeruginosa (Table 3). Surprisingly, none of the two mutants displayed a temperature-sensitive phenotype, and it could therefore be concluded that the codon-neutral mutation 235, together with mutation 163C, is somehow involved in determining the temperature-sensitive phenotype.
TABLE 3.
Temperature dependence of growth for P. aeruginosa cells containing trfA single mutants (in pFF1)a
| trfA | Growth at:
|
|||
|---|---|---|---|---|
| 23°C | 30°C | 37°C | 42°C | |
| Wild type | + | + | + | + |
| Mutant | ||||
| 163C,235 | + | − | − | − |
| 163C | + | + | + | + |
| 235 | + | + | + | + |
The experiment was performed as described in the footnote to Table 2.
To study this further, we also attempted to transfer the mutants to E. coli DH5α, which does not express wild-type TrfA. The double mutant could not be established at any temperature, and the same was true for mutant 163C (Table 4). However, mutant 235 could be established at 23°C but not at higher temperatures. Thus, both single mutations display a strong negative effect on replication in E. coli. We have previously observed that overexpression of the trfA gene may lead to a reduction of temperature sensitivity for replication, and we therefore also analyzed the phenotypes of the mutants in a two-plasmid system which is known to lead to an approximately 10-times-higher trfA expression level (7, 28). In this system, trfA is expressed from the ColE1 replicon pPK34 (a derivative of plasmid pBR322), while oriV is on a replicon (pSV16) lacking the trfA gene. The results showed that the double mutant could still not be established in E. coli, while cells containing mutant 235 could now grow also at 30°C in the presence of pSV16 selection (Table 4). We were also able to get some transformants at 23°C with mutant 163C, but the plasmids in these cells could not be stably maintained. Thus, overexpression of trfA reduces but does not eliminate the temperature sensitivity.
TABLE 4.
Temperature dependence of growth for E. coli DH5α cells containing trfA single mutants
| Plasmid, trfA form | Growth at:
|
|||
|---|---|---|---|---|
| 23°C | 30°C | 37°C | 42°C | |
| pFF1, wild type | + | + | + | + |
| pPK34, wild type | + | + | + | + |
| pFF1, 163C,235 | − | − | − | − |
| pPK34, 163C,235 | − | − | − | − |
| pFF1, 163C | − | − | − | − |
| pPK34, 163C | (+) | − | − | − |
| pFF1, 235 | + | − | − | − |
| pPK34, 235 | + | + | − | − |
The experiment was performed as described in the footnote to Table 2 except that the agar medium contained ampicillin instead of carbenicillin. Cells containing pPK34 or its mutant derivatives also contained pSV16. (+) indicates poor growth.
Mutation 235 acts by reducing the trfA expression level.
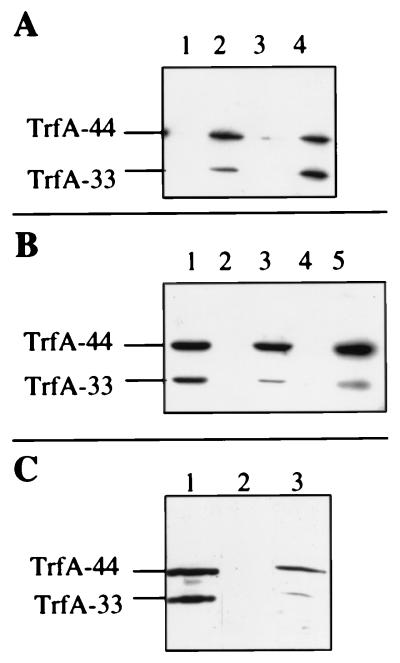
One possible way of explaining the puzzling effect of mutation 235 is to assume that it somehow acts by reducing the trfA expression level. To study this, we used Western blotting and a TrfA polyclonal antibody (Fig. 3). The data showed that trfA in mutant 163C is expressed at levels similar to wild-type levels in P. aeruginosa, while the expression levels in the double mutant and in mutant 235 were severely reduced (Fig. 3A). This finding strongly supports the above hypothesis concerning the effect of mutation 235, and it therefore also follows that single mutant 163C displays a temperature-sensitive phenotype at low but not high trfA expression levels in P. aeruginosa.
FIG. 3.
Western blot analysis of TrfA protein produced by different plasmid mutants. (A) Derivatives of pFF1 in P. aeruginosa. Lanes: 1, 235; 2, 163C; 3, 163C,235; 4, wild type. (B) Derivatives of pPK34 in E. coli DH5α. Lanes: 1, 85; 2, 235; 3, 163C; 4, 163C,235; 5, wild type. (C) Comparison of trfA expression in E. coli and P. aeruginosa. Lanes: 1, pFF1 in P. aeruginosa; 2, pFF1 in E. coli DH5α; 3, pPK34 in E. coli DH5α. The amounts of lysed cells loaded on the gel correspond to the following quantities of soluble protein: 6 μg (A), 20 μg (B), and 7.5 μg (C). Note that signal intensities cannot be compared between panels, as film exposure times varied.
We were interested in studying the effect of mutation 235 also in E. coli; by expressing trfA from plasmid pPK34, the expression is decoupled from replication. The results indicate that the relative expression levels are similar to those in P. aeruginosa. Mutation 235 leads to a severe reduction of expression relative to wild-type trfA, while this is not the case for mutation 163C (Fig. 3B). Another very interesting observation is that wild-type trfA is expressed from pFF1 at much lower levels in E. coli than in P. aeruginosa (Fig. 3C). Furthermore, relative to total soluble protein, the expression level from pPK34 in E. coli was also lower than that from pFF1 in P. aeruginosa.
The mutant 235-mediated effect on the trfA expression level is site specific and is probably the result of an effect on mRNA structure.
The glycine codon GGU in mutant 235 has been reported to be used about three times less frequently in P. aeruginosa than the original GGC codon (29). In E. coli, the GGU codon is used at a frequency similar to that of GGC (29). We also recently showed that the celB gene (encoding phosphoglucomutase) from the bacterium Acetobacter xylinum could be expressed at very high levels in E. coli (1, 2), and inspection of the celB sequence showed that it contains six GGU codons (3). This made us suspect that the effect of the GGU codon in 235 is due to a more specific effect than, for instance, insufficient supplies of the relevant tRNA. To test this further, we inspected the trfA gene sequence and found that glycine 85 is encoded by GGU, and the possible effect of changing this codon to GGC could thus be tested. The mutant was made by PCR and the mutation did not significantly affect the trfA expression level (Fig. 3B). We also constructed a double mutant of 85 and 235, and trfA expression was then similar to that of mutant 235 (results not shown). Thus, mutation 235 appeared to act by some site-specific mechanism.
To further analyze the mechanism by which mutation 235 reduces trfA expression we constructed two pairs of in-frame translational lacZ fusions with trfA. In the first of these pairs, lacZ was fused at the position corresponding to codon 245 in the wild type and mutant 235, generating plasmids pJB1002 and pJB1004, respectively. In the second pair, the fusions were near the 3′ terminus of trfA, such that only four of the carboxy-terminal amino acids in the TrfA protein are missing (constructs pJB1006 and pJB1008). The constructs were used for Western blotting with the TrfA antibody, and the results confirmed that the fusion proteins were being produced, as the molecular masses had increased as expected (Fig. 4A). Surprisingly, there was no significant difference between the expression levels of any of the constructs. Thus, both fusions eliminate the effect of mutation 235. To confirm these data by an independent method, we also analyzed the β-galactosidase activities in extracts prepared from the same strains. All activities were similar (980, 1,060, 1,020, and 1,010 U for pJB1002, pJB1004, pJB1006, and pJB1008, respectively), confirming the Western blot data.
FIG. 4.
Western blot analysis of expression of TrfA-LacZ fusion proteins in E. coli DH5α (A) and of TrfA in an E. coli rho-deficient host mutant (B). (A) Lanes: 1, pJB1002; 2, pJB1004; 3, pJB1006; 4, pJB1008. (B) Lanes: 1, K37(pRD110-34); 2, K7487(pRD110-34); 3, K37(pPK34 235); 4, K7487(pPK34 235). In each lane, lysed cells corresponding to 25 μg of soluble protein were loaded. The numbers represent deduced molecular masses in kilodaltons. The intensities of the bands from the pPK34 extracts were found to be similar to those from the trfA-lacZ fusions (not shown).
One possible way by which mutation 235 might act is by creating a site for rho-dependent transcriptional termination. This could be tested by comparing expression in the rho-deficient E. coli mutant K7487 and its parent strain K37 (Fig. 4B). The results showed that significantly more TrfA was produced in K7487 than in K37, but this difference was not affected by mutation 235. We noted that strain K37 grew much faster than strain K7487, which is probably the reason for the mutation 235-independent differences in the expression levels. Thus, mutation 235 probably does not act by generating a rho-dependent transcription termination site.
DISCUSSION
The trfA mutant bank used to isolate the temperature-sensitive mutants from P. aeruginosa originated from a previously constructed E. coli mutant bank estimated to contain about 60,000 original clones (12). The frequency of temperature-sensitive mutants in P. aeruginosa was found to be as low as about 1 in 25,000, which probably explains the repeated (three times) isolation of the double mutant. By using a more complex mutant bank, it might therefore have been possible to identify other temperature-sensitive mutants in P. aeruginosa.
Based on results previously reported, it appears that temperature-sensitive trfA mutants isolated from E. coli in most cases display a wild-type phenotype in P. aeruginosa. Furthermore, mutants that show some degree of reduced functionality or are temperature sensitive tend to be those that are particularly poorly functional in E. coli (11, 28). The temperature-sensitive mutants isolated in P. aeruginosa are poorly (247C) or not (163C,235) functional in E. coli. Another way of stating this is to propose that poorly functional TrfA proteins generally works better in supporting the replication of RK2 minireplicons in P. aeruginosa than in E. coli. The observation that trfA is expressed (from Pneo) at much higher levels in P. aeruginosa than in E. coli may partly explain why such a pattern appears to exist. The reason is simply that functionality can be improved by expressing more protein. This is also consistent with our previous observation that increased expression levels lead to a less temperature-sensitive phenotype in E. coli (11). Even more striking is the observation that mutation 235 can make the substitution mutant 163C temperature sensitive by leading to reduced levels of trfA expression. Despite all this evidence, we believe that there most probably also exist other factors that influence the phenotypes. The results presented in Fig. 3 indicate that the expression level of mutant 163C,235 in P. aeruginosa is much lower than that of 163C in E. coli. The same protein is presumably produced in both cases, but functionality is still observed only in P. aeruginosa.
Another interesting problem raised by the results reported here is how mutation 235 acts to reduce the trfA expression level. It seems clear that it is not the codon as such that leads to reduced expression but rather the location of it. Early transcriptional rho-dependent termination also appears unlikely since the relative difference between the trfA expression levels in a rho-deficient strain relative to its parent wild-type strain was not selectively affected by mutation 235. In contrast, the effect of codon 235 was eliminated by two different trfA-lacZ fusions. The most likely explanation for this sensitivity to lacZ fusions appears to be that the mutation 235 effect depends on the generation of some particular mRNA structure. Such a new structure could in principle lead to reduced mRNA stability or translatability (15). We have tried to model the folding patterns by a computer program (the Zuker-Turner RNA folding package; Washington University Medical School, St. Louis, Mo.). The program predicted a significant difference in the folding of wild-type and mutant 235 mRNAs. However, such effects were also observed by some randomly tested single base changes in the sequence, and we therefore feel that the biological significance of such analyses is doubtful. To fully understand the effect of mutation 235 it will therefore be necessary to study this system in much more detail.
We also found it surprising that the codon-neutral mutation alone displayed a temperature-sensitive phenotype in E. coli. We do not know the reason for this, but it could be that the expression level becomes reduced beyond a subcritical level at elevated temperatures, possibly due to an effect of temperature on the mechanism by which mutation 235 acts. It could also be that TrfA is intrinsically slightly temperature sensitive, such that more protein is needed at higher temperatures or that expression is somewhat reduced at elevated temperatures for reasons unrelated to mutation 235.
ACKNOWLEDGMENTS
This work was supported by grants from The Norwegian Research Council and by the Norwegian University of Science and Technology.
We thank D. R. Helinski for his generous gift of the TrfA antibody.
REFERENCES
- 1.Blatny J M, Brautaset T, Winther-Larsen H C, Haugan K, Valla S. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl Environ Microbiol. 1997;63:370–379. doi: 10.1128/aem.63.2.370-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blatny J M, Brautaset T, Winther-Larsen H C, Karunakaran P, Valla S. Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in Gram-negative bacteria. Plasmid. 1997;38:35–51. doi: 10.1006/plas.1997.1294. [DOI] [PubMed] [Google Scholar]
- 3.Brautaset T, Standal R, Fjærvik E, Valla S. Nucleotide sequence and expression analysis of the Acetobacter xylinum phosphoglucomutase gene. Microbiology. 1994;140:1183–1188. doi: 10.1099/13500872-140-5-1183. [DOI] [PubMed] [Google Scholar]
- 4.Cereghino J L, Helinski D R, Toukdarian A E. Isolation and characterization of DNA-binding mutants of a plasmid replication initiation protein utilizing an in vivo binding assay. Plasmid. 1994;31:89–99. doi: 10.1006/plas.1994.1009. [DOI] [PubMed] [Google Scholar]
- 5.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherichia coli: Transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durland R H, Helinski D R. The sequence encoding the 43-kilodalton trfA protein is required for efficient replication or maintenance of minimal RK2 replicons in Pseudomonas aeruginosa. Plasmid. 1987;18:164–169. doi: 10.1016/0147-619x(87)90044-8. [DOI] [PubMed] [Google Scholar]
- 7.Durland R H, Toukdarian A, Fang F, Helinski D R. Mutations in the trfA replication gene of the broad-host-range plasmid RK2 result in elevated plasmid copy numbers. J Bacteriol. 1990;172:3859–3867. doi: 10.1128/jb.172.7.3859-3867.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figurski D H, Meyer R J, Helinski D R. Suppression of ColE1 replication properties by the IncP-1 plasmid RK2 in hybrid plasmids constructed in vitro. J Mol Biol. 1979;133:295–318. doi: 10.1016/0022-2836(79)90395-4. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991;204:63–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 11.Haugan K, Karunakaran P, Blatny J M, Valla S. The phenotypes of temperature-sensitive mini-RK2 replicons carrying mutations in the replication control gene trfA are suppressed nonspecifically by intragenic cop mutations. J Bacteriol. 1992;174:7026–7032. doi: 10.1128/jb.174.21.7026-7032.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haugan K, Karunakaran P, Tøndervik A, Valla S. The host range of RK2 minimal replicon copy-up mutants is limited by species-specific differences in the maximum tolerable copy number. Plasmid. 1995;33:27–39. doi: 10.1006/plas.1995.1004. [DOI] [PubMed] [Google Scholar]
- 13.Ito W, Ishiguro H, Kurosawa Y. A general method for introducing a series of mutations into cloned DNA using the polymerase chain reaction. Gene. 1991;102:67–70. doi: 10.1016/0378-1119(91)90539-n. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Makrides S C. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev. 1996;60:512–538. doi: 10.1128/mr.60.3.512-538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perri S, Helinski D R, Toukdarian A. Interactions of plasmid-encoded replication initiation proteins with the origin of DNA replication in the broad host range plasmid RK2. J Biol Chem. 1991;266:12536–12543. [PubMed] [Google Scholar]
- 17.Perri S, Helinski D R. DNA sequence requirements for interactions of the RK2 replication initiation protein with plasmid origin repeats. J Biol Chem. 1993;268:3662–3669. [PubMed] [Google Scholar]
- 18.Pinkney M, Diaz R, Lanka E, Thomas C M. Replication of mini RK2 plasmid in extracts of Escherichia coli requires plasmid-encoded protein TrfA and host-encoded proteins DnaA, B, G, DNA gyrase and DNA polymerase III. J Mol Biol. 1988;203:927–938. doi: 10.1016/0022-2836(88)90118-0. [DOI] [PubMed] [Google Scholar]
- 18a.Promega Corp. Protocols and applications guide. 2nd ed. Madison, Wis: Promega Corp.; 1991. [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Schmidhauser T J, Helinski D R. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985;164:446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shingler V, Thomas C M. Analysis of the trfA region of broad-host-range plasmid RK2 by transposon mutagenesis and identification of polypeptide products. J Mol Biol. 1984;175:229–249. doi: 10.1016/0022-2836(84)90346-2. [DOI] [PubMed] [Google Scholar]
- 22.Shingler V, Thomas C M. Analysis of nonpolar insertion mutations in the trfA gene of IncP plasmid RK2 which affect its broad host range property. Biochim Biophys Acta. 1989;1007:301–308. doi: 10.1016/0167-4781(89)90152-8. [DOI] [PubMed] [Google Scholar]
- 23.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 24.Smith C A, Thomas C M. Nucleotide sequence of the trfA gene of broad host range plasmid RK2. J Mol Biol. 1984;175:251–262. doi: 10.1016/0022-2836(84)90347-4. [DOI] [PubMed] [Google Scholar]
- 25.Thomas C M, Helinski D R. Vegetative replication and stable inheritance of IncP plasmids. In: Thomas C M, editor. Promiscuous plasmids of Gram-negative bacteria. London, England: Academic Press; 1989. pp. 1–25. [Google Scholar]
- 26.Thomas C M, Stalker D M, Helinski D R. Replication and incompatibility properties of segments of the origin region of replication of the broad host range plasmid RK2. Mol Gen Genet. 1981;181:1–7. doi: 10.1007/BF00338996. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valla S, Haugan K, Durland R H, Helinski D R. Isolation and properties of temperature-sensitive mutants of the trfA gene of the broad host range plasmid RK2. Plasmid. 1991;25:131–136. doi: 10.1016/0147-619x(91)90025-r. [DOI] [PubMed] [Google Scholar]
- 29.Wada K, Wada Y, Ishibashi F, Gojobori T, Ikemura T. Codon usage tabulated from the GeneBank genetic sequence data. Nucleic Acids Res. 1992;20:2111–2118. doi: 10.1093/nar/20.suppl.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng C, Friedman D I. Reduced Rho-dependent transcription termination permits NusA-independent growth of Escherichia coli. Proc Natl Acad Sci USA. 1994;91:7543–7547. doi: 10.1073/pnas.91.16.7543. [DOI] [PMC free article] [PubMed] [Google Scholar]