Abstract
ADP-ribosylation factor 6 (Arf6) is a small-GTPase that regulates the membrane trafficking between the plasma membrane and endosome. It is also involved in the reorganization of the actin cytoskeleton. GTPase-activating protein (GAP) is a critical regulator of Arf function as it inactivates Arf. Here, we identified a novel species of GAP denoted as SMAP1 that preferentially acts on Arf6. Although overexpression of SMAP1 did not alter the subcellular distribution of the actin cytoskeleton, it did block the endocytosis of transferrin receptors. Knock down of endogenous SMAP1 also abolished transferrin internalization, which confirms that SMAP1 is needed for this endocytic process. SMAP1 overexpression had no effect on clathrin-independent endocytosis, however. Intriguingly, SMAP1 binds directly to the clathrin heavy chain via its clathrin-box and mutation studies revealed that its GAP domain and clathrin-box both contribute to the role SMAP1 plays in clathrin-dependent endocytosis. These observations suggest that SMAP1 may be an Arf6GAP that specifically regulates one of the multiple functions of Arf6, namely, clathrin-dependent endocytosis, and that it does so by binding directly to clathrin.
INTRODUCTION
In mammals, ADP-ribosylation factors (Arfs) constitute one family of the small molecular and GTP-binding protein superfamily (Cockcroft, 1996; Chavrier and Goud, 1999; Roth, 1999; Donaldson and Jackson, 2000; Wakeham et al., 2000; Turner et al., 2001). The Arf family includes six members of Arf1–6 and Arf-like proteins. In general, Arfs are involved in the regulation of vesicle budding and the reorganization of actin cytoskeleton. Arf1 and Arf6 are the best characterized of the Arfs. Arf1 is localized in the Golgi and the endosome and it regulates the membrane association of COPI, a coat protein used for intra-Golgi transport and retrograde transport from the cis-Golgi to the endoplasmic reticulum. It also regulates the membrane association of AP-1 and AP-3, which are adaptors for clathrin and are used for trans-Golgi network (TGN)-to-endosome transport and endosome-to-lysosome transport, respectively. In contrast, Arf6 localizes to both the plasma membrane and the recycling endosome and it regulates clathrin-dependent and -independent endocytosis, endosome recycling, and actin reorganization (D'Souza-Schorey et al., 1995; Radhakrishna et al., 1996; Radhakrishna and Donaldson, 1997; D'Souza-Schorey et al., 1998; Altschuler et al., 1999; Honda et al., 1999; Krauss et al., 2003; Naslavsky et al., 2003).
In clathrin-dependent endocytosis, AP-2 and clathrin molecules serve as coat components of the vesicles and Arf6 regulates this process (D'Souza-Schorey et al., 1995). For example, when Arf6Q67L, a GTP-bound and constitutively active form of Arf6, is expressed in Chinese hamster ovary (CHO) cells, it localizes on the plasma membrane and inhibits the internalization of transferrin receptors to the endosome. On the other hand, when Arf6T27N, a nucleotide-free and constitutively inactive form, is expressed, it localizes on the recycling endosomes and prevents the reappearance of endosomal transferrin receptors on the plasma membrane. One of the effector molecules that is targeted by the active form of Arf6 is phosphatidylinositol 4-phosphate 5-kinase (Honda et al., 1999) which generates phosphatidylinositol (4, 5)-bisphosphate (PIP2). The PIP2 that is thus generated attracts proteins that bear an affinity for it, such as AP-2, to the plasma membrane. This Arf6-induced process thereby promotes the nucleation of AP-2 and clathrin on coated pits that are formed on the plasma membrane (Krauss et al., 2003).
Like the other small GTPases, the activation and inactivation of Arf is evoked by guanine-nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), respectively. GEF replaces the Arf-bound GDP with GTP, whereas GAP activates the hydrolysis of ARF-bound GTP. GAP1 was the first molecule to be reported to act as a GAP for Arf and it was shown to exhibit substrate specificity for Arf1 (Cukierman et al., 1995). GAP1 contains, at its amino-terminus, a zinc finger motif that is responsible for its GAP activity. In agreement with its function as a GAP for Arf1, GAP1 localizes on the Golgi and interacts with COPI as well as KDEL receptors, which are cargo proteins that are exported to the endoplasmic reticulum (Aoe et al., 1997; Eugster et al., 2000).
There are many Arf GAP proteins in addition to GAP1, at least some of which function at various and distinct compartments within the cell (Brown et al., 1998; Premont et al., 1998; Andreev et al., 1999; Turner et al., 1999; Jackson et al., 2000; Vitale et al., 2000). ASAP1, GIT1, and AGAP1 are the examples of such Arf GAPs. ASAP1 has GAP as well as pleckstrin homology domains, and its GAP activity is necessary for the reorganization of the actin cytoskeleton (Brown et al., 1998). GIT1 was originally cloned as a protein that interacts with G protein–coupled receptor tyrosine kinases (Premont et al., 1998). Overexpression of GIT1 impairs the ligand-dependent internalization of G protein–coupled receptors and epidermal growth factor receptor (Claing et al., 2000). AGAP1 regulates AP-3–dependent endosome-to-lysosome trafficking (Nie et al., 2003). At least 26 genes have been found to be candidate human Arf GAPs (Bernards, 2003). Thus, an intriguing possibility is that each Arf GAP prefers a particular Arf and regulates a specific function of this Arf in intracellular trafficking and actin cytoskeleton reorganization.
Here, we report a novel GAP for Arf that is called SMAP1 and that prefers Arf6 to Arf1. SMAP1 is, however, not involved in the reorganization of actin cytoskeleton. Rather, it is involved in clathrin-dependent endocytosis such as the internalization of transferrin receptors. Furthermore, we show that SMAP1 can directly bind to the clathrin molecule. This novel interaction with clathrin in combination with GAP activity suggests a mechanism by which SMAP1 regulates clathrin-dependent endocytosis, which is discussed.
MATERIALS AND METHODS
Cells, Reagents, and Antibodies
HeLa and TRV-b1 cells were cultured in DMEM containing 10% (vol/vol) fetal calf serum (FCS). Brefeldin A was purchased from Sigma-Aldrich (St. Louis, MO). Rabbit antibody against the murine SMAP1 protein was raised by using the KLH-conjugated carboxy-terminal peptide QPPSTTAGWSGSSSG as an immunogen. The antibody was affinity-purified by passing the antiserum through a column in which the immunogen was coupled to HiTrap NHS-activated HP (Amersham Pharmacia Biotech, Piscataway, NJ). The following antibodies were purchased: the antitransferrin receptor mouse monoclonal antibody (mAb) B3/25 and the antiinfluenza hemagglutinin (HA) rat mAb 3F10 were from Roche Diagnostics (Indianapolis, IN), the mouse mAbs against FLAG (M2) and AP-1 (100/3) were from Sigma-Aldrich, the mouse mAbs against mannose 6-phosphate receptor (M6PR) (2G11), clathrin heavy chain (CHC) (X22) and AP-2 (AP.6), and the rabbit polyclonal antibody (pAb) against β-COP were from Affinity BioReagents (Golden, CO), the mouse mAb against AP-3 was from Transduction Laboratories (Lexington, KY), the anti-CD25 mouse mAb (7G7/B6) was from Upstate Biotechnology (Lake Placid, NY), the anti-6His mouse mAb was from Clontech Laboratories (Palo Alto, CA), the Cy3-conjugated goat anti-rat IgG was from Chemicon (Temecula, CA), and the Alexa488-conjugated goat anti-mouse and anti-rabbit IgGs, the Alexa488-conjugated human-transferrin and rhodamine-conjugated phalloidin were from Molecular Probes (Eugene, OR).
Plasmid Construction
The murine SMAP1 cDNA has been described in Sato et al. (1998). Mutations were introduced into SMAP1 by using an in vitro site-directed mutagenesis kit for pBluescriptII (Takara, Kyoto, Japan). GAP1 cDNA was PCR-amplified using a rat liver cDNA library as a template. Murine Arf1 and Arf6 were obtained by PCR amplification using a murine brain-derived cDNA library as a template. cDNAs of human CHC and CD25 were provided by Drs. T. Nagase and H. Asao, respectively.
To express the cDNAs in mammalian cells, they were inserted into pCAGGS-Neo. SMAP1 and GAP1 were tagged at their amino-termini with HA, whereas Arf6 was tagged at its carboxy-terminus with FLAG.
To express the cDNAs in bacteria, they were inserted into pGEX5X-3 (Amersham Pharmacia). The sequences that were expressed were the amino-terminal 255 residues of SMAP1, the amino-terminal 246 residues of GAP1, the amino-terminal 390 residues of CHC, and the entire coding sequences of Arf1 and Arf6. All were fused at their amino-termini to glutathione S-transferase (GST) and tagged with hexahistidines at their carboxy-termini.
Preparation of Recombinant Proteins
The Escherichia coli strain BL21 was transformed with one of the expression plasmids and cultured in LB medium containing ampicillin. An overnight culture of 10 ml was inoculated into 250 ml of fresh LB medium and further incubated for 3 h. Isopropyl-β-d-thiogalactoside (IPTG; 0.4 mM) was then added to induce protein expression. The cells were harvested after 1 h by centrifugation at 5000 rpm for 15 min at 4°C. The GST-fusion proteins were then purified by using a B-PER GST-spin purification kit (Pierce Chemical, Rockford, IL). Briefly, the cells were suspended in B-PER supplemented with 5 mM MgCl2, 5 μg/ml DNase I, and 200 μg/ml lysozyme and gently shaken for 10 min at room temperature (RT). Debris and inclusion bodies were removed by centrifugation at 15000 rpm for 15 min at 4°C. One milliliter of immobilized glutathione was then added to the supernatant, and the mixture was shaken for 10 min at RT followed by centrifugation at 5000 rpm for 2 min. After several washes by centrifugation, the GST-fusion protein was eluted. The fusion protein was then digested with Factor Xa (Novagen, Madison, WI) and the GST-free protein was purified by using a 6× His spin purification kit (Pierce Chemical). The protein concentrations were assayed by a BCA protein assay kit (Pierce Chemical). The purity of the recovered proteins was ∼95% as judged by SDS-PAGE.
GAP Assay
GAP assays were performed as described in the literature (Randazzo et al., 1994; Randazzo, 1997). Briefly, recombinant nonmyristoylated Arf1 or Arf6 proteins were loaded with [α-32P] GTP in GTP loading buffer for 60 min at 30°C. GTP hydrolysis was initiated by the addition of GAP in GTP-hydrolysis buffer at 30°C. The reaction was terminated by the addition of washing buffer containing 20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2, and 2 mM dithiothreitol. Unbound nucleotides were removed by passing the reaction mixture through a 0.45-μm nitrocellulose filter (Millipore, Bedford, MA). GTP and GDP were eluted from the Arf protein by 2 M formic acid and separated by TLC, which was developed in 1 M LiCl/1 M formic acid. The plate was exposed to a phosphorimager screen, and radioactivity was detected by a Bio Imaging Analyzer (Fuji Photo Film Co., Ltd., Tokyo, Japan).
GST Pull-down and Immunoblot Analysis
HeLa cells were dissolved in lysis buffer containing 100 mM Mes, pH 6.8, 0.1% (vol/vol) Triton X-100, 1 mM EGTA, 0.5 mM MgCl2, and a complete mixture of protease inhibitors (Roche; Gaidarov et al., 2001), and the lysate was centrifuged at 12000 rpm for 5 min. The glutathione-bead-bound GST-fusion proteins were incubated with the cellular supernatant for 2 h with gentle agitation. The beads were then washed with lysis buffer three times and suspended in SDS sample buffer. Gel electrophoresis of the proteins, transfer of the proteins from the gel to a filter, and immunoreactions were performed as described previously (Chiba et al., 1997).
Immunofluorescence Microscopy
Cells on coverslips were transfected with plasmid DNA using Effectene (QIAGEN, Chatsworth, CA), fixed with 3.7% (wt/vol) paraformaldehyde and 0.1% (vol/vol) Triton X-100 in phosphate-buffered saline (PBS) for 15 min at RT, washed three times with PBS, and blocked with 1% (wt/vol) bovine serum albumin (BSA) and 0.1% Triton X-100 in PBS for 15 min at RT. In some cases, the transfected cells were treated with 50 μM AlCl3 and 30 mM NaF for 5 min before fixation. The fixed cells were incubated with appropriately diluted primary and then secondary antibodies for 45 min each at RT. After washing with PBS, the coverslips were mounted on slideglasses using Vectashield (Vector Laboratories, Burlingame, CA) and observed with a confocal microscope (LSM-401, Zeiss, Thornwood, NY).
Internalization of Transferrin and CD25 Antigen
To detect the internalization of transferrin, cells were rinsed with PBS and preincubated in 0.1% (wt/vol) BSA in DMEM for 30 min at 37°C. The cells were then incubated with 25 μg/ml Alexa488-conjugated human transferrin for 15 min at 37°C, rinsed with PBS, and fixed and processed for immunofluorescence as described above. Detection of CD25 internalization was performed as described previously (Radhakrishna and Donaldson, 1997). Thus, cells transfected with CD25 cDNA were chilled to 4°C, incubated with the anti-CD25 antibody for 30 min, and rinsed with ice-cold DMEM containing 10% (vol/vol) FCS. They were then incubated with prewarmed medium for 30 min at 37°C to permit the internalization of CD25. To remove excess antibodies that remained on the cell surface, the cells were chilled to 4°C again and rinsed with 0.5%(vol/vol) acetic acid, 0.5 M NaCl, pH 3.0. Thereafter, the cells were fixed and processed for immunofluorescence. To monitor the recycling of the CD25 antibody, the cells were warmed to 37°C for 30 min before fixation.
Small Interfering RNA
SMAP1 small interfering RNA (siRNA; 5′-CACUGAGCAAACUACAAAAUCAGAA-3′) and control firefly luciferase siRNA (5′-ACAUCACGUACGCGGAAUACUUCGA-3′) were provided by Hokkaido System Science (Sapporo, Japan). Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used to deliver the siRNAs into HeLa cells. Thus, cells in a six-well culture plate received 100 pmol of RNA duplex and were incubated for 72 h. They were then transfected with a second round of RNA and assayed after a 72-h incubation.
RESULTS
SMAP1 Is a Novel GAP for Arf6
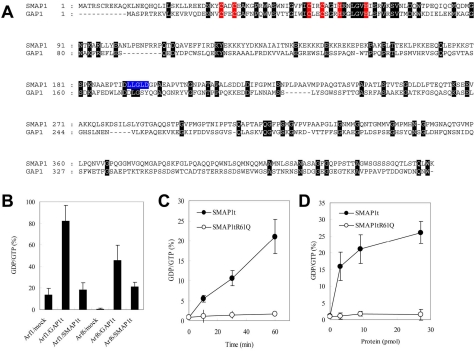
By searching databases and the literature, we found that the SMAP1 protein (Sato et al., 1998) contains a highly conserved Arf GAP domain. Figure 1A depicts the alignment of the amino acid sequence of SMAP1 with that of GAP1, the classical Arf1GAP. In the case of GAP1, the amino terminal 146 residues are required for its GAP catalytic activity; this region is therefore called a GAP domain (Cukierman et al., 1995; Goldberg, 1999). The amino-terminal 167 residues of SMAP1 exhibit 23% identity (highlighted by black boxes) and 43% similarity with this GAP domain of GAP1. The critical cysteine and histidine residues that form the C2C2H2 zinc finger motif found in various ArfGAPs (Zhang et al., 1998) are also conserved in the putative GAP domain of SMAP1 (highlighted by red boxes).
Figure 1.
SMAP1 is an ArfGAP. (A) Alignment of the amino acid sequences of SMAP1 (Sato et al., 1998) and GAP1 (Cukierman et al., 1995). The C2C2H2 zinc finger motif that is conserved among various ArfGAPs is indicated in red. The amino acid residues that are identical in SMAP1 and GAP1 are indicated by dark highlighting. A putative clathrin box in SMAP1 is indicated in blue. (B) Substrate specificity of ArfGAPs. One microgram of GTP-loaded Arf1 or Arf6 was incubated for 60 min with 9 pmol of GAP1t or SMAP1t in the indicated combinations. Mock means no addition of GAP1t or SMAP1t. GTP and GDP bound to Arfs were extracted and separated from each other by TLC. Phosphor-images of chromatograms were scanned by a densitometer, and the percentages of the GTP that had been hydrolyzed to GDP were calculated as the GDP/GTP ratios. The averages and SDs from two independent experiments are presented for each Arf and ArfGAP combination. (C) Time course of GTP-hydrolysis by SMAP1t. One microgram of GTP-loaded Arf6 and 9 pmol of SMAP1t or SMAP1t R61Q were incubated for the indicated time. The reaction mixture was processed as in B, and the percentages of GTP-hydrolysis were calculated. The averages and SDs from two independent experiments are presented for each point. Closed and open circles represent SMAP1t and SMAP1t R61Q, respectively. (D) Dependence of GTP-hydrolysis on the concentration of SMAP1t. One microgram of GTP-loaded Arf6 was incubated for 60 min with the indicated amount of SMAP1t or SMAP1t R61Q. The reaction mixture was proceeded as in B, and the percentages of GTP-hydrolysis were calculated. The averages and SDs from two independent experiments are presented for each point. Closed and open circles represent SMAP1t and SMAP1t R61Q, respectively.
We next assayed whether recombinant SMAP1 protein can catalyze the hydrolysis of GTP that is bound to recombinant nonmyristoylated Arf1 and Arf6. We found that the expression of the full-length SMAP1 protein was toxic to the bacterial host cells because it inhibited their growth. Consequently, a truncated form of SMAP1 consisting of its amino-terminal 255 residues (designated as SMAP1t) was used in this study. As shown in Figure 1B, SMAP1t did not catalyze hydrolysis when Arf1-loaded GTP was used as a substrate (compare 13% of mock with 18% of SMAP1t) but catalyzed significantly when Arf6-GTP was used (compare 1% of mock with 20% of SMAP1t). As a control, when a truncated form of GAP1 consisting of its amino-terminal 246 residues GAP1t was used, its catalytic activity on Arf1-bound GTP (82%) was higher compared with that on Arf6-bound GTP (45%). This is as expected for GAP1 as Arf1-GAP. Because the hydrolysis of Arf6-bound GTP by SMAP1t was as moderate as 20%, we examined its activity in more detail. As seen in panels C and D, the degree to which Arf6-bound GTP was hydrolyzed by SMAP1t was incubation time-dependent as well as protein concentration–dependent, increasing the likelihood of SMAP1 to be Arf6-GAP. The 50th arginine residue is known to be vital for the GAP activity of GAP1 (Szafer et al., 2000) and is also conserved as the 61th residue in SMAP1 (see Figure 1A). We replaced this 61R with a glutamine residue (designated as SMAP1t R61Q) and assayed its activity. As seen in panels C and D, SMAP1t R61Q did not show any catalytic activity on Arf6-bound GTP. Thus, the amino-terminal region of SMAP1 indeed functions as a GAP domain for Arf6, at least in vitro.
SMAP1 Is Not Involved in Actin Reorganization
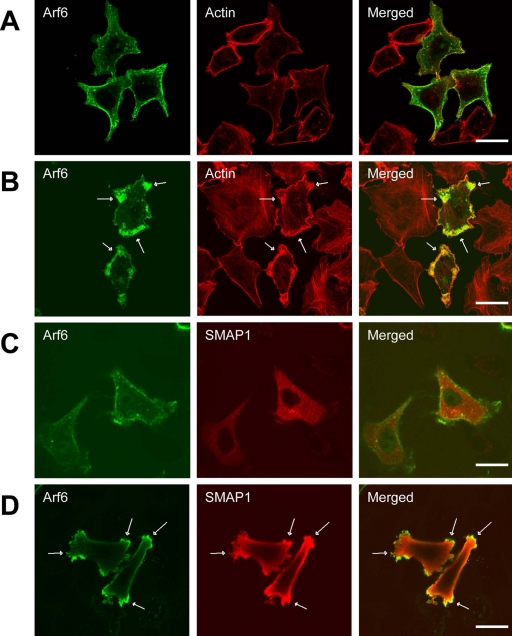
Arf6 is known to be involved in actin reorganization (Radhakrishna et al., 1996; Honda et al., 1999). This was confirmed when HeLa cells were transfected with FLAG-tagged Arf6 and the cells were stained with anti-FLAG antibody together with rhodamine-conjugated phalloidin (Figure 2, A and B). Arf6 and F-actin were mainly detected beneath the plasma membrane (panel A). When the cells were treated with AlF, the cells formed membrane protrusions into which the activated Arf6 and subcortical F-actin had accumulated and colocalized (indicated by the arrows in panel B).
Figure 2.
Effect of overexpressing SMAP1 protein on the Arf6-induced protrusion of the plasma membrane. HeLa cells were transfected with Arf6-FLAG on its own (A and B) or along with HA-SMAP1 (C and D). In B and D, the cells were treated with 50 μM AlCl3 and 30 mM NaF for 5 min before fixation. The fixed cells were subjected to immunofluorescent staining using anti-FLAG and anti-HA antibodies to detect Arf6 and SMAP1, respectively. F-actin was stained by rhodamine-conjugated phalloidin. The arrows indicate the membrane protrusions that were induced by the activated Arf6 protein. Bar, 20 μm.
Because SMAP1 is a putative Arf6GAP, we checked whether it is also involved in the membrane protrusion. Thus, the amino-terminus of SMAP1 was tagged with HA and transfected into cells together with Arf6-FLAG (Figure 2, C and D). It must be noted that, in this and following transfection experiments into HeLa cells, the intact/full length SMAP1 protein was used. Immunofluorescent staining using an anti-HA antibody revealed that SMAP1 is diffused throughout the cytoplasm and on the plasma membrane, whereas Arf6 was localized beneath the plasma membrane (panel C). Treatment of SMAP1-transfected cells with AlF still induced the membrane protrusions (indicated by the arrows in panel D), which can be recognized as sites of Arf6 (and F-actin) accumulation. Thus, overexpression of SMAP1 does not appear to perturb the Arf6-induced reorganization of actin that results in membrane protrusions.
Overexpression of SMAP1 Inhibits Clathrin-dependent Endocytosis
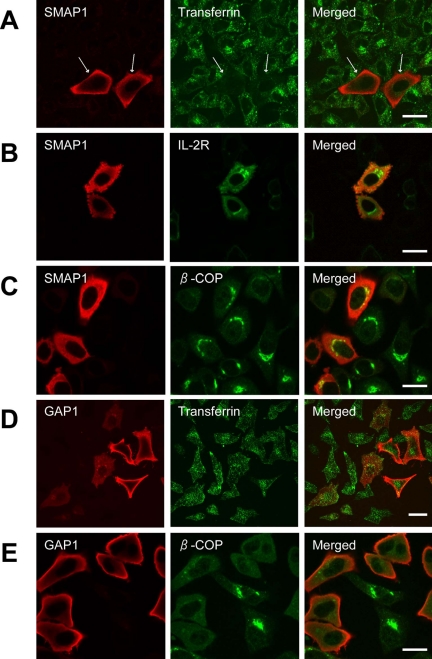
Because Arf6 is known to be involved in clathrin-dependent endocytosis (D'Souza-Schorey et al., 1995; Palacios et al., 2002; Krauss et al., 2003), we examined the effect of overexpressing SMAP1 on this process. HeLa cells express receptors for transferrin and constitutively endocytose them. Thus, HeLa cells were transfected with SMAP1, incubated with Alexa488-labeled transferrin, and then observed microscopically (Figure 3A). The cells that were not transfected by SMAP1 internalized transferrin efficiently but the cells that overexpressed SMAP1 failed to incorporate these molecules (indicated by the arrows). When the transferrin receptors themselves were stained by a specific antibody, this revealed that in SMAP1-untransfected cells, the transferrin receptors were internalized into the cytoplasm and detected around the perinuclear region (unpublished data). In contrast, in SMAP1-transfected cells, the transferrin receptors remained on the plasma membrane (unpublished data). A similar result was obtained when a CHO-derived and human transferrin-transduced cell line, TRVb-1 (D'Souza-Schorey et al., 1995), was transfected with SMAP1 and tested for the incorporation of transferrin (unpublished data).
Figure 3.
Effect of overexpressing SMAP1 protein on clathrin-dependent endocytosis. HeLa cells were transfected with the expression plasmids encoding HA-SMAP1 (A–C) or HA-GAP1 (D and E). In A and D, the cells were incubated with Alexa488-transferrin before fixation to assay transferrin internalization. In B, the cells were cotransfected with the CD25 (IL-2R) expression plasmid to assay IL-2R internalization. The cells were fixed and stained with anti-HA antibody on its own (A and D) or together with antibodies specific for β-COP (C and E) or CD25 (B). Bar, 20 μm.
It must be noted that Arf6 is also involved in a clathrin-independent pathway of endocytosis (Radhakrishna and Donaldson, 1997). CD25 antigen, which is the α subunit of interleukin-2 receptor (IL-2R), does not contain any tyrosines or the dileucine motif in its cytoplasmic tail and hence cannot utilize the pathway that is facilitated by clathrin. However, Figure 3B shows that the anti-CD25 antibody is internalized normally in SMAP1-transfected cells. Moreover, the internalized anti-CD25 antibody was recycled normally to the cell surface in these cells (unpublished data). Thus, overexpression of SMAP1 only impairs the Arf6-regulated clathrin-dependent pathway of endocytosis.
GAP1 is an Arf1GAP and is involved in the membrane traffic between the Golgi and endoplasmic reticulum. Overexpression of intact/full length GAP1 in HeLa cells disrupted the Golgi structure as shown by the disappearance of β-COP staining (Figure 3E) but did not affect transferrin internalization (Figure 3D). In contrast, overexpression of SMAP1 did not affect the distribution of β-COP (Figure 3C). Thus, SMAP1 is involved in Arf6-regulated pathways and not in those that are regulated by Arf1.
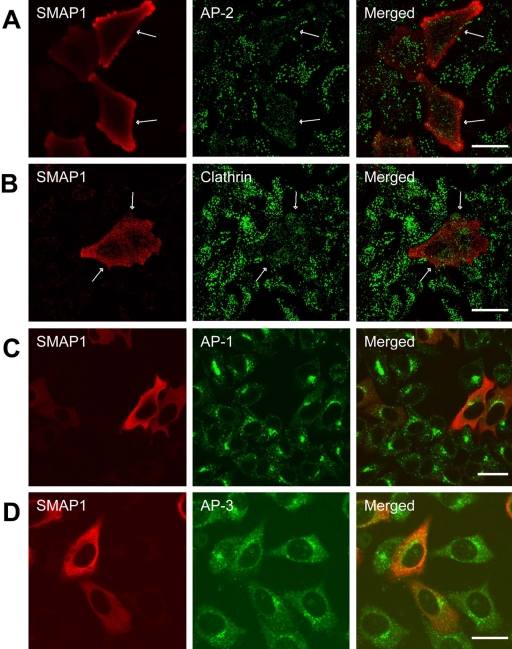
Overexpression of SMAP1 Impairs the Recruitment of AP-2 and Clathrin to the Plasma Membrane
Clathrin-dependent endocytosis is initiated by the interaction of cargo proteins with AP-2 followed by the recruitment of clathrin to the plasma membrane. Therefore, we examined the effect of overexpressing SMAP1 on the subcellular distribution of AP-2 and CHC proteins by double immunofluorescence (Figure 4). In untransfected cells, both AP-2 and CHC appeared as multiple and punctate stains, which probably correspond to coated pits that have formed on the plasma membrane (panels A and B). In contrast, such discrete staining patterns could not be detected for AP-2 or CHC in the SMAP1-expressing cells (see the cells indicated by arrows). These results indicate that the impairment of transferrin internalization seen in SMAP1-overexpressing cells is probably due to the perturbation in recruiting AP-2 and clathrin to coated pits. It is probable that overexpression of SMAP1 induces Arf6 to adopt its inactive GDP-bound form, which is not favorable for recruiting AP-2 to the membrane.
Figure 4.
Effect of overexpressing SMAP1 protein on the subcellular distribution of adaptor proteins and clathrin. HeLa cells were transfected with the HA-SMAP1 expression plasmid, fixed, and stained with anti-HA antibody together with antibodies specific for AP-2 (A), CHC (B), AP-1 (C), or AP-3 (D). Bar, 20 μm.
AP-1 and AP-3 are also involved in clathrin-dependent vesicle transport but in the TGN-to-endosome and endosome-to-lysosome pathways, respectively. Overexpression of SMAP1 did not alter the distribution of either AP-1 or AP-3 (Figure 4, C and D), which suggests that SMAP1 is not involved in AP-1- or AP-3-regulated vesicle transportation.
Biological Activity of a SMAP1 Mutant Protein that Lacks GAP Activity
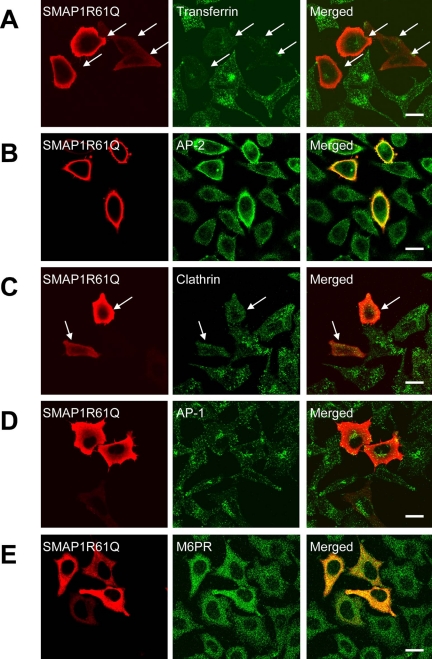
We have shown that the SMAP1 protein, when overexpressed, inhibits transferrin internalization. We then wondered whether the GAP activity of SMAP1 is necessary for this effect. To address this question, we introduced SMAP1 R61Q, a GAP-negative mutant as shown in Figure 1, into HeLa cells. As shown in Figure 5A, SMAP1 R61Q displayed a distribution pattern to the cytosol and the plasma membrane that is similar to that in the case of the wild-type SMAP1. Notably, the expression of SMAP1 R61Q still inhibited the internalization of transferrin (see the cells indicated by the arrows in Figure 5A) and the transferrin receptors remained on the plasma membrane of transfected cells (unpublished data). Although this was unexpected, it does not mean that the GAP activity of SMAP1 is not needed for its inhibition of transferrin internalization, because we found that the mutant SMAP1 inhibits transferrin internalization in a manner different from that of the wild-type SMAP1. In SMAP1 R61Q-overexpressing cells, AP-2 staining appeared to be located on the plasma membrane (Figure 5B), and it was more intense compared than that of the untransfected cells, which suggests that the recruitment of AP-2 to coated pits is in fact more efficient in mutant SMAP1-expressing cells. A GAP-negative mutant of SMAP1 is expected to promote the activation of Arf6 into a GTP-bound form, which favors the recruitment of AP-2 to the membrane. We then examined the distribution of CHC (Figure 5C). Surprisingly, CHC was not detected on coated pits on the plasma membrane (see the cells indicated by the arrows). Therefore, the inability of SMAP1 R61Q-transfected cells to incorporate transferrin appears to be due to the defect of CHC recruitment in these cells.
Figure 5.
Biological activity of the GAP-negative mutant of SMAP1. HeLa cells were transfected with the expression plasmid encoding HA-SMAP1 R61Q. In A, the cells were incubated with Alexa488-transferrin before fixation to assay transferrin internalization. The cells were fixed and stained with anti-HA antibody on its own (A) or together with antibodies specific for AP-2 (B), CHC (C), AP-1 (D), or M6PR (E). Bar, 20 μm.
These observations show that although the recruitment of clathrin but not AP-2 is impaired in GAP-negative SMAP1-expressing cells, the recruitment of both AP-2 and clathrin is impaired in wild-type SMAP1-expressing cells. Thus, the GAP activity of SMAP1 is necessary for the impairment of AP-2 recruitment by wild-type SMAP1. Both the wild-type and mutant SMAP1 proteins resulted in the same phenotype, however, because the lack of membrane association of either AP-2 and/or clathrin blocked the ability of the cells to form endocytic vesicles.
AP-1 is a TGN-located clathrin adaptor protein and is regulated by Arf1. The staining pattern of AP-1 was not altered by the transfection of SMAP1 R61Q (Figure 5D). M6PR is a cargo protein that is transported from the TGN to the endosome in an AP-1– and clathrin-dependent manner (Le Borgne et al., 1996). M6PR was detected in the endosomes and had not accumulated at the TGN of SMAP1 R61Q-transfected cells (Figure 5E). This suggests that the clathrin molecules in the SMAP1 R61Q-transfected cells are functioning properly in the AP-1–dependent TGN-to-endosome pathway.
SMAP1 Interacts Directly with the CHC
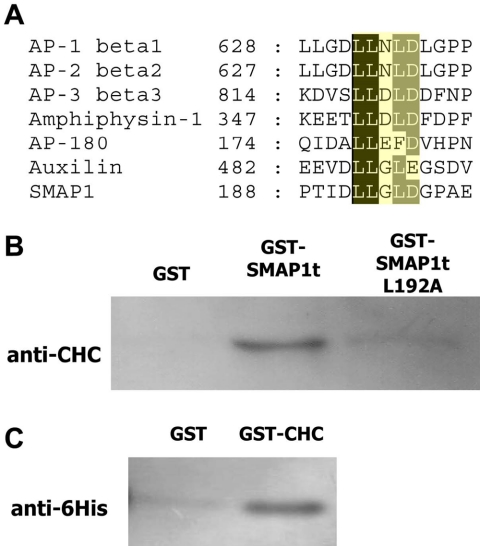
Recruitment of clathrin molecules to coated pits was impaired in the GAP-negative SMAP1-transfected cells. Overexpression of AP-180, a clathrin assembly protein, similarly impairs the recruitment of clathrin to the plasma membrane and this effect is mediated by a carboxy-terminal region of AP-180 to which clathrin molecules bind (Ford et al., 2001). One possibility would be that clathrin molecules bind to the GAP-negative SMAP1 protein and cannot be incorporated into coated pits. Therefore, we examined a possibility that SMAP1 is somehow involved in vesicle formation by interacting with clathrin. The interaction between the clathrin molecule and proteins, including adaptor proteins, requires that the latter proteins carry a conserved penta-peptide called a “clathrin-box.” Indeed, a clathrin-box-like sequence was detected in the SMAP1 protein at amino acids 192–196 (see the LLGLD sequence in blue in Figure 1A). The alignment of various clathrin-boxes and the putative box found in SMAP1 is shown in Figure 6A.
Figure 6.
Binding of SMAP1 protein to the CHC molecule. (A) Alignment of clathrin-box sequences. Typical clathrin-boxes are found in proteins that are components of clathrin-mediated membrane traffic (ter Haar et al., 2000), including β-adaptin (Shih et al., 1995; Dell'Angelica et al., 1998), β-arrestin (Krupnick et al., 1997), AP-180 (Morris et al., 1993), amphiphysin (Ramjaun and McPherson, 1998), and epsin 1 (Rosenthal et al., 1999). A clathrin box is a pentapeptide and it binds to clathrin by means of a “peptide-in-groove” interaction (ter Haar et al., 2000). The third residue in the clathrin-box is usually of a polar nature. However, a DnaJ homologue, auxilin, can also bind to clathrin through its LLGLE candidate clathrin-box (Smith et al., 2004). The putative clathrin box in SMAP1 has the LLGLD sequence. (B) Pull down assays using purified recombinant GST, GST-SMAP1t, and GST-fused SMAP1t L192A. In the SMAP1t L192A molecule, the five residues of the putative clathrin box of SMAP1t were replaced with alanine. Each protein was incubated with the lysate of untransfected HeLa cells. The proteins bound to glutathione beads were recovered, separated by SDS-PAGE, and subjected to immunoblot analysis using the anti-CHC antibody. (C) GST and GST-CHC proteins were incubated with histidine-tagged SMAP1t protein. The material bound to glutathione beads was recovered and detected using the anti-6His antibody.
To determine whether SMAP1 interacts directly with clathrin, we performed a GST-pull-down experiment in which a recombinant GST-SMAP1t protein was incubated with the lysate of untransfected HeLa cells. The SMAP1t proteins we used consisted of the amino-terminal 255 residues of SMAP1 as well as its mutated version. In the mutant SMAP1t L192A, all of the residues in the putative clathrin-box were replaced by alanine. The pulled down material was subjected to immunoblot analysis using the anti-CHC antibody (Figure 6B). The wild-type SMAP1t pulled down CHC from the cell lysate but GST alone did not. In the case of the SMAP1t L192A, the amount of CHC that was coprecipitated was greatly reduced compared with the case of the wild-type SMAP1t. Thus, the putative clathrin-box is necessary for the association between SMAP1 and CHC.
We then sought to determine whether the clathrin-box of SMAP1 interacts directly or indirectly with clathrin. A recombinant GST-CHC protein representing an amino-terminal 390 residues of CHC was incubated with a recombinant SMAP1t protein tagged by hexa-histidines. The pulled down material was subjected to immunoblot analysis using the anti-6His antibody. Figure 6C shows that the CHC and SMAP1 molecules can bind to each other in the absence of any other protein.
SMAP1 Needs its Clathrin-box to Inhibit Endocytosis
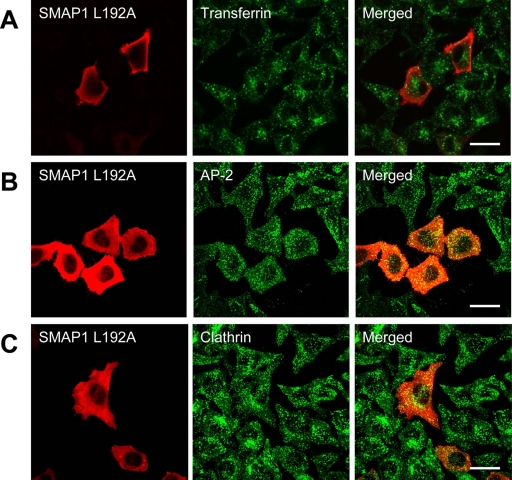
The significance of the clathrin-binding ability of SMAP1 with regard to its role in transferrin internalization was then examined. Thus, HeLa cells were transfected with the clathrin-box-mutated SMAP1 and subjected to immunofluorescent staining (Figure 7). Transferrin was internalized into these cells to a similar extent as in the untransfected cells (panel A), and AP-2 (panel B) and clathrin (panel C) were recruited normally to the plasma membrane. Therefore, SMAP1 needs its clathrin-box to inhibit clathrin-dependent endocytosis.
Figure 7.
Biological activity of clathrin-box–mutated SMAP1. HeLa cells were transfected with the plasmid expressing the mutated HA-SMAP1 in which amino acid residues 192–196 were replaced with alanine. In A, the cells were incubated with Alexa488-transferrin before fixation to assay transferrin internalization. The cells were fixed and stained with anti-HA antibody alone (A) or together with antibodies specific for AP-2 (B) or CHC (C). Bar, 20 μm.
Clathrin-dependent Endocytosis Does Not Occur in the Absence of SMAP1
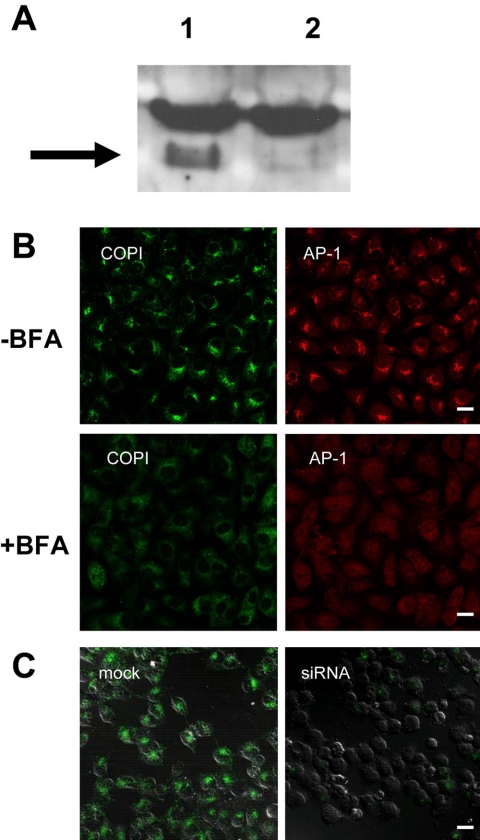
So far, we have examined the effect of overexpressing SMAP1 or its mutants on transferrin internalization. We then checked whether reducing endogenous SMAP1 expression in the cells would affect their clathrin-dependent endocytosis. We used an siRNA method to reduce SMAP1 expression (Figure 8A) and showed by immunoblot analysis with an anti-SMAP1 peptide antibody that HeLa cells treated with siRNA (lane 2) but not with control RNA (lane 1) lacked the 62-kDa band of SMAP1. The siRNA-treated HeLa cells showed intact patterns of β-COP and AP-1 staining, which represent the Golgi and TGN structures, respectively (Figure 8B, top panels). Treatment of siRNA-cells with brefeldin A, a Golgi-targeting compound, dissociated β-COP and AP-1 from the membranes of the Golgi apparatus (Figure 8B, bottom panels). Therefore, the reduction of endogenous SMAP1 does not appear to alter the morphology or drug-responsiveness of the Golgi apparatus. In contrast, the cells with reduced expression of SMAP1 failed to internalize transferrin, unlike the control cells (Figure 8C). These results are consistent with the notion that endogenous SMAP1 is necessary for clathrin-dependent endocytosis.
Figure 8.
Effect of reducing endogenous SMAP1 protein levels on clathrin-dependent endocytosis. (A) Immunoblot analysis of endogenous SMAP1 protein levels in HeLa cells transfected with the control RNA (lane 1) or with the siRNA against SMAP1 (lane 2). After incubating the cells with the RNAs, their lysates were subjected to immunoblot analysis using the anti-SMAP1 peptide antibody. The 62-kDa band indicated by the arrow represents the SMAP1 protein. The 62-kDa mass is much larger than the calculated 48-kDa mass of SMAP1, probably because SMAP1 is relatively basic and migrates slowly. (B) The subcellular distribution of β-COP and AP-1 in siRNA-treated HeLa cells. The cells were transfected with the siRNA against SMAP1 and incubated with (bottom panel) or without (top panel) 5 μg/ml brefeldin A for 2 min before fixation. The fixed cells were stained with anti-β-COP and anti-AP-1 antibodies. (C) Effect of siRNA treatment on transferrin internalization. HeLa cells were transfected with control RNA or siRNA and incubated with Alexa488-transferrin before fixation. DIC images are included to visualize the cells. Bar, 20 μm.
DISCUSSION
In this study, we have shown that SMAP1 is a novel Arf6GAP. We also revealed that it is not involved in actin reorganization; rather, it regulates clathrin-dependent endocytosis. Furthermore, we showed that SMAP1 associates directly with clathrin through its clathrin box. To our knowledge, the association of an ArfGAP with clathrin has not been described previously.
An in vitro GAP assay revealed that SMAP1 prefers Arf6 to Arf1 as a substrate. Supporting this is that the overexpression of SMAP1 had no effect on the distribution of the Golgi, whose membrane traffic is dependent on the function of Arf1. Arf6, on the other hand, regulates actin reorganization. This was confirmed when we transfected HeLa cells with Arf6 and treated them with AlF, because these cells reorganized their peripheral actin and induced membrane protrusions. Molecules such as ACAP1, ACAP2, and GIT2 are all considered to possess Arf6GAP activity, and cotransfection of one of these inhibits the protrusion of the actin (Jackson et al., 2000; Mazaki et al., 2001). Unlike these molecules, however, coexpression of SMAP1 did not perturb the formation of the Arf6-induced membrane protrusion (Figure 2). Thus, SMAP1 appears to be distinct from the other Arf6GAP members that have been identified to date.
Arf6 also regulates the endocytosis of both transferrin and IL-2R, which are transported via clathrin-dependent and -independent pathways, respectively. It has been shown that overexpression of Arf6GAP such as GIT1 does not affect the transferrin internalization. On the other hand, when one kind of Arf6 mutant is introduced into cells, it affects only the actin reorganization but not the vesicle trafficking (Al-Awar et al., 2000). Overexpression of SMAP1 impaired clathrin-dependent endocytosis but did not perturb clathrin-independent endocytosis or actin reorganization. Therefore, it is plausible to hypothesize that Arf6 separately regulates actin rearrangement and the clathrin-dependent and -independent pathways of vesicle trafficking by interacting with different sets of Arf6GAPs. This notion is supported by observations that suggest that the Arf6GEFs EFA6 and ARNO are separately involved in the distinct functions exerted by Arf6. For instance, overexpression of EFA6 inhibits transferrin endocytosis and simultaneously induces membrane ruffles (Franco et al., 1999), whereas overexpression of ARNO enhances the endocytosis of the beta2-adrenergic receptor (Claing et al., 2001).
The recruitment of AP-2 to the plasma membrane was impaired in SMAP1-transfected cells, whereas it was rather enhanced in the cells transfected by the GAP-negative mutant of SMAP1. It is thus evident that the GAP activity of SMAP1 is one factor that determines the subcellular localization of AP-2. A GAP-negative mutant of SMAP1 probably leads Arf6 to its active, GTP-bound form. In accordance, we have observed previously that the transfection of HeLa cells with the constitutively active Arf6Q67L protein does not impair the membrane recruitment of AP-2 (unpublished data). Curiously, however, transfection of Arf6Q67L does block transferrin internalization (unpublished data). These apparent discrepancies may be due to the possibility that Arf6Q67L cannot be involved in the triggering of vesicle formation because it cannot cycle between the GTP/active and GDP/inactive forms. Alternatively, Arf6Q67L may affect the actin reorganization that is associated with and is necessary for vesicle formation. In contrast, SMAP1 (and its mutant) may regulate the activity of Arf6 molecules, but only those Arf6 molecules that are engaged in the clathrin-dependent endocytic pathway.
SMAP1 harbors a clathrin-box in its central region. This motif is found in many proteins that are known to be essential components of clathrin-mediated membrane traffic (Figure 6A; ter Haar et al., 2000), including β-adaptin (Shih et al., 1995; Dell'Angelica et al., 1998), β-arrestin (Krupnick et al., 1997), AP-180 (Morris et al., 1993), amphiphysin (Ramjaun and McPherson, 1998), epsin 1 (Rosenthal et al., 1999), auxilin (Smith et al., 2004), and phosphatidylinositol 3-kinase (Gaidarov et al., 2001). These proteins function in the assembly/disassembly of clathrin and the membrane-association of clathrin. The AP-180 molecule, which facilitates clathrin assembly, is particularly notable because its overexpression inhibits the internalization of transferrin, whereas its mutant that lacks a clathrin-box does not have this inhibitory effect on endocytosis (Ford et al., 2001). We observed that SMAP1 also needs its clathrin-box to inhibit transferrin internalization. This suggests that SMAP1, probably as in the case of AP-180, regulates endocytosis by interacting with the clathrin molecule.
At the Golgi membrane, Arf1 recruits both coat proteins (COPI) and Arf1GAP (GAP1), and these three components together form a ternary complex. Indeed, GAP1 is able to directly interact with β-COP and γ-COP, which are components of COPI-coated vesicles (Eugster et al., 2000). This interaction with COPI further potentiates the ability of GAP1 to activate the Arf1-GTPase (Goldberg, 1999). Furthermore, cargo proteins, if present, suppress the GAP1- and COPI-guided hydrolysis of Arf1-bound GTP, thereby facilitating vesicle formation. On the other hand, noncargo proteins have no effect on GTPase activity (Lanoix et al., 1999; Goldberg, 2000). Thus, at the Golgi, GAP1 plays a critical role in decoding the signals that allow cargo and noncargo proteins to be sorted from each other. Similarly, another member of Arf1GAP, AGAP1, can bind to the AP-3 molecule and regulate AP-3–dependent vesicle transport from the endosome to the lysosome (Nie et al., 2003). A somewhat analogous but distinct system may be functioning in the constitutive and clathrin-dependent endocytosis at the plasma membrane. We showed that SMAP1 is an Arf6GAP and can directly interact with the clathrin molecule. Although the interaction between Arf6 and coat proteins has not been reported, one of the effector molecules of Arf6 is PIP2. The enhanced production of PIP2 probably favors the recruitment of AP-2 to the membrane (Krauss et al., 2003). Therefore, it is possible that Arf6, Arf6GAP (SMAP1), AP-2, and clathrin form a quaternary complex and that clathrin and/or its adaptor proteins modulate the activity of SMAP1. SMAP1 is the first example of a GAP that is capable of interacting with clathrin. As such, it may be a valuable tool in elucidating the mechanism underlying Arf6- and clathrin-dependent endocytosis.
Acknowledgments
We are grateful to the following scientists for providing us with valuable experimental tools: N. Yanai and M. Obinata for the murine SMAP1 cDNA, T. Nagase for the human CHC cDNA, H. Asao for the human CD25 cDNA, H. Kawabata and T. E. McGraw for the TRVb-1 cells, and Hokkaido System Bioscience, Co. Ltd for the siRNAs. We also thank K. Nakayama for critically reading the manuscript and M. Kuji for secretarial work. This work was supported in part by Grants in Aid from the Ministry of Education, Science, Sports, Culture, and Technology, Japan. M.S. is a member in the 21st century COE program “Center for Innovative Therapeutic Development Toward the Conquest of Signal Transduction Diseases,” which is headed by K. Sugamura at Tohoku University.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–08–0683) on January 19, 2005.
Abbreviations used: Arf, ADP-ribosylation factor; CHC, clathrin heavy chain; GAP, GTPase-activating protein; GEF, guanine-nucleotide exchange factor; GST, glutathione S-transferase; HA, influenza hemagglutinin; IL-2R, interleukin-2 receptor; M6PR, mannose 6-phosphate receptor; PIP2, phosphatidylinositol (4, 5)-bisphosphate; siRNA, small interfering RNA; TGN, trans-Golgi network.
References
- Al-Awar, O., Radhakrishna, H., Powell, N. N., and Donaldson, J. G. (2000). Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant. Mol. Cell. Biol. 20, 5998–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler, Y., Liu, S., Katz, L., Tang, K., Hardy, S., Brodsky, F., Apodaca, G., and Mostov, K. (1999). ADP-ribosylation factor 6 and endocytosis at the apical surface of Madin-Darby canine kidney cells. J. Cell Biol. 147, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev, J., Simon, J. P., Sabatini, D. D., Kam, J., Plowman, G., Randazzo, P. A., and Schlessinger, J. (1999). Identification of a new Pyk2 target protein with Arf-GAP activity. Mol. Cell. Biol. 19, 2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoe, T., Cukierman, E., Lee, A., Cassel, D., Peters, P. J., and Hsu, V. W. (1997). The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 16, 7305–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards, A. (2003). GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta 1603, 47–82. [DOI] [PubMed] [Google Scholar]
- Brown, M. T., Andrade, J., Radhakrishna, H., Donaldson, J. G., Cooper, J. A., and Randazzo, P. A. (1998). ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol. Cell. Biol. 18, 7038–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier, P., and Goud, B. (1999). The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 11, 466–475. [DOI] [PubMed] [Google Scholar]
- Chiba, N., Watanabe, T., Nomura, S., Tanaka, Y., Minowa, M., Niki, M., Kanamaru, R., and Satake, M. (1997). Differentiation dependent expression and distinct subcellular localization of the protooncogene product, PEBP2beta/CBFbeta, in muscle development. Oncogene 14, 2543–2552. [DOI] [PubMed] [Google Scholar]
- Claing, A., Chen, W., Miller, W.E., Vitale, N., Moss, J., Premont, R. T., and Lefkowitz, R. J. (2001). Beta arrestin-mediated ARF6 activation and beta2-adrenergic receptor endocytosis. J. Biol. Chem. 276, 42509–42513. [DOI] [PubMed] [Google Scholar]
- Claing, A., Perry, S. J., Achiriloaie, M., Walker, J. K., Albanesi, J. P., Lefkowitz, R. J., and Premont, R. T. (2000). Multiple endocytic pathways of G protein-coupled receptors delineated by GIT1 sensitivity. Proc. Natl. Acad. Sci. USA 97, 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft, S. (1996). ARF-regulated phospholipase D: a potential role in membrane traffic. Chem. Phys. Lipids 80, 59–80. [DOI] [PubMed] [Google Scholar]
- Cukierman, E., Huber, I., Rotman, M., and Cassel, D. (1995). The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science 270, 1999–2002. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica, E. C., Klumperman, J., Stoorvogel, W., and Bonifacino, J. S. (1998). Association of the AP-3 adaptor complex with clathrin. Science 280, 431–434. [DOI] [PubMed] [Google Scholar]
- Donaldson, J. G., and Jackson, C. L. (2000). Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 12, 475–482. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey, C., Li, G., Colombo, M. I., and Stahl, P. D. (1995). A regulatory role for ARF6 in receptor-mediated endocytosis. Science 267, 1175–1178. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey, C., van Donselaar, E., Hsu, V.W., Yang, C., Stahl, P. D., and Peters, P. J. (1998). ARF6 targets recycling vesicles to the plasma membrane: insights from an ultrastructural investigation. J. Cell Biol. 140, 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster, A., Frigerio, G., Dale, M., and Duden, R. (2000). COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 19, 3905–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, M. G., Pearse, B. M., Higgins, M. K., Vallis, Y., Owen, D. J., Gibson, A., Hopkins, C. R., Evans, P. R., and McMahon, H. T. (2001). Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051–1055. [DOI] [PubMed] [Google Scholar]
- Franco, M., Peters, P. J., Boretto, J., van Donselaar, E., Neri, A., D'Souza-Schorey, C., and Chavrier, P. (1999). EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 18, 1480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov, I., Smith, M. E., Domin, J., and Keen, J. H. (2001). The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol. Cell 7, 443–449. [DOI] [PubMed] [Google Scholar]
- Goldberg, J. (1999). Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell 96, 893–902. [DOI] [PubMed] [Google Scholar]
- Goldberg, J. (2000). Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell 100, 671–679. [DOI] [PubMed] [Google Scholar]
- Honda, A. et al. (1999). Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521–532. [DOI] [PubMed] [Google Scholar]
- Jackson, T. R., Brown, F. D., Nie, Z., Miura, K., Foroni, L., Sun, J., Hsu, V. W., Donaldson, J. G., and Randazzo, P. A. (2000). ACAPs are arf6 GTPase-activating proteins that function in the cell periphery. J. Cell Biol. 151, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss, M., Kinuta, M., Wenk, M. R., De Camilli, P., Takei, K., and Haucke, V. (2003). ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type I{gamma}. J. Cell Biol. 162, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick, J. G., Goodman, O. B., Jr., Keen, J. H., and Benovic, J. L. (1997). Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J. Biol. Chem. 272, 15011–15016. [DOI] [PubMed] [Google Scholar]
- Lanoix, J., Ouwendijk, J., Lin, C. C., Stark, A., Love, H. D., Ostermann, J., and Nilsson, T. (1999). GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J. 18, 4935–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne, R., Griffiths, G., and Hoflack, B. (1996). Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J. Biol. Chem. 271, 2162–2170. [DOI] [PubMed] [Google Scholar]
- Mazaki, Y. et al. (2001). An adp-ribosylation factor gtpase-activating protein git2-short/kiaa0148 is involved in subcellular localization of paxillin and actin cytoskeletal organization. Mol. Biol. Cell 12, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, S. A., Schroder, S., Plessmann, U., Weber, K., and Ungewickell, E. (1993). Clathrin assembly protein AP180, primary structure, domain organization and identification of a clathrin binding site. EMBO J. 12, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky, N., Weigert, R., and Donaldson, J. G. (2003). Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol. Biol. Cell 14, 417–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, Z., Boehm, M., Boja, E. S., Vass, W. C., Bonifacino, J. S., Fales, H. M., and Randazzo, P. A. (2003). Specific regulation of the adaptor protein complex AP-3 by the Arf GAP AGAP1. Dev. Cell 5, 513–521. [DOI] [PubMed] [Google Scholar]
- Palacios, F., Schweitzer, J. K., Boshans, R. L., and D'Souza-Schorey, C. (2002). ARF6-GTP recruits Nm23–H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 4, 919–936. [DOI] [PubMed] [Google Scholar]
- Premont, R. T., Claing, A., Vitale, N., Freeman, J. L., Pitcher, J. A., Patton, W. A., Moss, J., Vaughan, M., and Lefkowitz, R. J. (1998). beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA 95, 14082–14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna, H., and Donaldson, J. G. (1997). ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna, H., Klausner, R. D., and Donaldson, J. G. (1996). Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J. Cell Biol. 134, 935–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjaun, A. R., and McPherson, P. S. (1998). Multiple amphiphysin II splice variants display differential clathrin binding: identification of two distinct clathrin-binding sites. J. Neurochem. 70, 2369–2376. [DOI] [PubMed] [Google Scholar]
- Randazzo, P. A. (1997). Functional interaction of ADP-ribosylation factor 1 with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 272, 7688–7692. [PubMed] [Google Scholar]
- Randazzo, P. A., Terui, T., Sturch, S., and Kahn, R. A. (1994). The amino terminus of ADP-ribosylation factor (ARF) 1 is essential for interaction with Gs and ARF GTPase-activating protein. J. Biol. Chem. 269, 29490–29494. [PubMed] [Google Scholar]
- Rosenthal, J. A., Chen, H., Slepnev, V. I., Pellegrini, L., Salcini, A. E., Di Fiore, P. P., and De Camilli, P. (1999). The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J. Biol. Chem. 274, 33959–33965. [DOI] [PubMed] [Google Scholar]
- Roth, M. G. (1999). Snapshots of ARF1, implications for mechanisms of activation and inactivation. Cell 97, 149–152. [DOI] [PubMed] [Google Scholar]
- Sato, Y., Hong, H. N., Yanai, N., and Obinata, M. (1998). Involvement of stromal membrane-associated protein (SMAP-1) in erythropoietic microenvironment. J. Biochem. (Tokyo) 124, 209–216. [DOI] [PubMed] [Google Scholar]
- Shih, W., Gallusser, A., and Kirchhausen, T. (1995). A clathrin-binding site in the hinge of the beta 2 chain of mammalian AP-2 complexes. J. Biol. Chem. 270, 31083–31090. [DOI] [PubMed] [Google Scholar]
- Smith, C. J., Dafforn, T. R., Kent, H., Sims, C.A., Khubchandani-Aswani, K., Zhang, L., Saibil, H. R., and Pearse, B. M. (2004). Location of auxilin within a clathrin cage. J. Mol. Biol. 336, 461–471. [DOI] [PubMed] [Google Scholar]
- Szafer, E., Pick, E., Rotman, M., Zuck, S., Huber, I., and Cassel, D. (2000). Role of coatomer and phospholipids in GTPase-activating protein-dependent hydrolysis of GTP by ADP-ribosylation factor-1. J. Biol. Chem. 275, 23615–23619. [DOI] [PubMed] [Google Scholar]
- ter Haar, E., Harrison, S. C., and Kirchhausen, T. (2000). Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc. Natl. Acad. Sci. USA 97, 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C. E., Brown, M. C., Perrotta, J. A., Riedy, M. C., Nikolopoulos, S. N., McDonald, A. R., Bagrodia, S., Thomas, S., and Leventhal, P. S. (1999). Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 145, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C. E., West, K. A., and Brown, M. C. (2001). Paxillin-ARF GAP signaling and the cytoskeleton. Curr. Opin. Cell Biol. 13, 593–599. [DOI] [PubMed] [Google Scholar]
- Vitale, N., Patton, W. A., Moss, J., Vaughan, M., Lefkowitz, R. J., and Premont, R.T. (2000). GIT proteins, a novel family of phosphatidylinositol 3,4,5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J. Biol. Chem. 275, 13901–13906. [DOI] [PubMed] [Google Scholar]
- Wakeham, D. E., Ybe, J. A., Brodsky, F. M., and Hwang, P. K. (2000). Molecular structures of proteins involved in vesicle coat formation. Traffic 1, 393–398. [DOI] [PubMed] [Google Scholar]
- Zhang, C. J., Cavenagh, M. M., and Kahn, R. A. (1998). A family of Arf effectors defined as suppressors of the loss of Arf function in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 273, 19792–19796. [DOI] [PubMed] [Google Scholar]