Abstract
Occludin is a tetraspan integral membrane protein in epithelial and endothelial tight junction (TJ) structures that is projected to have two extracellular loops. We have used peptides emulating central regions of human occludin's first and second loops, termed O-A:101–121 and O-B:210–228, respectively, to examine potential molecular interactions between these two regions of occludin and other TJ proteins. A superficial biophysical assessment of A:101–121 and O-B:210–228 showed them to have dissimilar solution conformation characteristics. Although O-A:101–121 failed to strongly interact with protein components of the human epithelial intestinal cell line T84, O-B:210–228 selectively associated with occludin, claudin-one and the junctional adhesion molecule (JAM)-A. Further, the presence of O-B:210–228, but not O-A:101–121, impeded the recovery of functional TJ structures. A scrambled peptide sequences of O-B:210–228 failed to influence TJ assembly. These studies demonstrate distinct properties for these two extracellular segments of the occludin protein and provide an improved understanding of how specific domains of occludin may interact with proteins present at TJ structures.
INTRODUCTION
Intercellular tight junction (TJ) structures established between adjacent epithelial cells provide a barrier to paracellular permeability and function to maintain cell polarity by fencing lipid bilayer components into segregated apical and basolateral domains (Sawada et al., 2003). Efforts to define mechanisms that establish these barrier and fence properties as well as regulate the passage of cells associated with innate immunity such as neutrophils (Huber et al., 2000; Liu et al., 2000) have helped identify TJ components that include several integral membrane and scaffolding proteins as well as a spectrum of regulatory proteins (Zahraoui et al., 2000; Matter and Balda, 2003). Scaffolding proteins, such as zonula occludens (ZO)-1 (Stevenson et al., 1986), that can bridge integral membrane proteins such as occludin to cytoskeletal elements such as actin (Fanning et al., 1998) form a link between TJ structures and an actomyosin contractile ring structure that is essential for the formation and stabilization of functional epithelial TJ structures. A number of factors, including growth factors and cytokines, can regulate TJ function through modulation of this actomyosin contractile ring as well as via direct modulation of TJ structural and regulatory components (Nusrat et al., 2000a, 2000b). Chronic dysregulation of TJ structures has been correlated with a spectrum of diseases (Sawada et al., 2003; Harhaj and Antonetti, 2004).
To date, several integral membrane proteins, including occludin, claudin(s) and the junctional adhesion molecule-A (JAM-A), have been localized to functional TJ structures. Human occludin has been predicted to have four transmembrane segments that position 46 amino acids (loop A) and 48 amino acids (loop B) at the exterior of the cell (Ando-Akatsuka et al., 1996). Claudins represent an extensive family of proteins with mixtures of these proteins establishing the paracellular and ionic perm-selective properties of different TJ structures (Colegio et al., 2002, 2003). Hydropathy plot analysis of claudin family members suggest two extracellular loops, a first loop of ∼50 amino acids and the second of 15 amino acids, that could be accessible for protein-protein contacts (Furuse et al., 1998). JAM-A is a type 1 transmembrane protein with one extracellular segment ∼230 amino acids that is segmented into two immunoglobulin (Ig)-like domains, termed D1 and D2. The technical difficulty of experimentally demonstrating molecular interactions involving integral membrane TJ proteins has been recognized (Itoh et al., 2001). One strategy to examine potential functions involving extracellular domains of integral TJ proteins has involved the use of peptides that might emulate these domains.
Initial studies using peptides emulating the complete extracellular loops of occludin demonstrated that addition of a chicken occludin loop B peptide could reversibly perturb the TJ permeability barrier of polarized Xenopus kidney (A6) epithelial cell monolayers, whereas a similar sized loop A peptide had no effect (Wong and Gumbiner, 1997). Although initial studies by Wong and others demonstrated decreased occludin levels in these A6 cells, subsequent studies examining the effect of this same peptide on polarized EpH4 mammary epithelial cells suggested that TJ perturbation might also occur through transcriptional regulation of β-catenin signal transduction pathway components (Vietor et al., 2001). Opposing data, however, has been obtained showing that a peptide with the entire human occludin loop B sequence had no effect on permeability properties of a human intestinal cell line, Caco-2, whereas a peptide emulating the entire loop A, or nested peptides of the proximal third of loop A, did affect TJ function (Tavelin et al., 2003). The findings of Tavelin et al. are consistent with earlier studies showing chicken occludin loop A decapeptides could impair A6 cell monolayer TJ resealing (Lacaz-Vieira et al., 1999).
We have used a novel bait peptide approach to identify potential molecular interactions of the central regions of loop A and loop B of human occludin and examined the role of these peptides in vitro using a polarized human intestinal epithelial cell line, T84. In this approach we prepared 20 amino acid peptides emulating the middle third regions of human occludin loops A and B and prepared one set of peptides that contained an N-terminal biotin moiety and a centrally located photoactive residue capable of covalent coupling after photo activation. This approach was similar to that used previously to identify binding partners for an intracellular occludin domain (Nusrat et al., 2000a). Using these peptides, we now demonstrate that the central region of occludin loop B can interact strongly with the plasma membrane of T84 cells, selectively associating with the TJ proteins occludin, claudin-1, and JAM-A, and impeding TJ reorganization. Studies with a similar peptide probe emulating the central region of human occludin loop A or a peptide with scrambled sequences of the loop B peptide failed to show any of these effects. Our data address potential protein-protein interactions involving specific domains of occludin's projected second extracellular loop and support a role for this region of occludin to influence TJ assembly.
MATERIALS AND METHODS
Peptide Synthesis
Peptides (see Table 1) were synthesized as previously described (Nusrat et al., 2000a). Briefly, an automated Pioneer Peptide Synthesizer (PE/ABI) with Fmoc-protected amino acids on Fmoc-PEG-PS-resin was used, denoted as Standard Peptide. The light-sensitive Fmoc-p-benzoylphenylalanine (Advanced ChemTech, Louisville, KY) amino acid was coupled into peptides using HBTU-HOBt/DIPEA, denoted as Bait Peptides. D-Biotin (Sigma, St. Louis, MO) was incorporated into peptides at the N terminus using HBTU-HOBt/DIPEA in Me2SO. Peptide resins were cleaved with a 1-h exposure of a 95% trifluoroacetic acid/2.5% triisopropylsilane/2.5% H2O solvent mixture. Released peptides were purified to >90% by preparative reversed-phase C18 high-performance liquid chromatography, characterized by electrospray ionization mass spectroscopy (Sciex API100), lyophilized to dryness, and stored at –20°C before use.
Table 1.
Bait peptides emulating specific human protein extracellular sequences
| Peptide composition | Tight junction protein extracellular domain | Code name: Peptide no. | MW (Da) | |||
|---|---|---|---|---|---|---|
| Occludin Loop A | ||||||
| Biotin-SVGYPYGGSG (bpa) GSYGSGYGYG-NH2 | Bait peptide | O-A: 101-121a | 2263 | |||
| Ac-SVGYPYGGSG--F--GSYGSGYGYG-NH2 | Standard peptide | O-A: 101-121 | 2080 | |||
| Occludin Loop B | ||||||
| Biotin-SQIYALCNQ (bpa) YTPAATGLYVD-NH2 | Bait peptide | O-B: 210-228a | 2710 | |||
| Ac-SQIYALCNQ--F--YTPAATGLYVD-NH2 | Standard peptide | O-B: 210-228 | 2527 | |||
| Occludin Loop B | ||||||
| Ac-TSPYAQIYLANFQDTALGYCV-NH2 | Scrambled standard peptide | Scrambled | 2527 | |||
Peptides were acetylated (Ac) or biotinylated (biotin) at their N-terminus and amidated (NH2) at their C-terminus to improve stability.
Peptide Characterization
Homologous peptide association studies were performed for standard peptides and for bait peptides (those containing a photoactive residue and biotin) by incubating 20 μg peptide at room temperature for 30 min dissolved in Hanks' balanced salt solution (HBSS) with Ca2+ (HBSS+) that contained (in g/l) 0.185 CaCl2·2H2O, 0.1 MgSO4, 0.4 KCl, 0.06 KH2PO4, 8.0 NaCl, 0.04788 Na2HPO4 (anhydrous), and 1.0 d-glucose. Samples were then mixed 1:1 with a 2× sample loading buffer containing 20% glycerol, 3% SDS, and 750 mM Tris (pH 8.8) with or without 20 mM dithiothreitol. Samples were separated using a 16.5% Tris-tricine gel and electroblotted onto nitrocellulose. Biotinylated peptides were visualized using peroxidase-conjugated streptavidin. For circular dichroisms (CD) studies ∼0.2 mg peptide was accurately weighed on a Metler-Toledo microbalance and dissolved in distilled water or in 2,2,2-trifluoroethanol (Fluka, Buchs, Switzerland) to get an accurate concentration of ∼0.2 mg/ml. CD spectra were then acquired at 10 nm/min on the Jasco J720 spectropolarimeter in the near-UV (340–230 nm) and far UV (260–185 nm) regions with a 2-nm spectral bandwidth and a 4-s time constant using 10-mm and 0.2-mm path-length quartz cells, respectively. All spectra were corrected for solvent baseline. SDS spectra were obtained by adding (∼3 mg) solid SDS to water solutions and remeasured.
Cell Culture and Ca2+ Switch Protocol
T-84 cells (American Type Culture Collection, Manassas, VA, CCL-248) were grown in 5% CO2 in a 1:1 mixture of DMEM and Ham's F-12 medium with 6% NCS. Confluent T84 monolayers with transepithelial resistance (TER) values ∼2000 Ω · cm2 and apical-to-basolateral 3-kDa FITC-Dextran (FD-3) paracellular flux restricted to <100 ng/cm2/h were obtained 6 d after seeding on 0.33 cm2 (12 d for 5 cm2) collagen-coated permeable (0.4-μm pore size) polycarbonate Costar filter supports (Transwell Clears, Corning-Costar, Cambridge, MA). TER was measured using a volt-ohm-meter (World Precision Instruments, Sarasota, FL). The Ca2+ switch protocol involved subjecting confluent T84 monolayers to ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′-tetraacetic acid (EGTA; 2 mM, 20 min, 37°C) in Ca2+- and Mg2+-free HBSS with 10 mM HEPES (HBSS–) to chelate divalent cations and disrupt intercellular junctions (Parkos et al., 1995; Liu et al., 2000). After verification of TJ disruption, assessed by loss of TER, filters were transferred to complete cell culture media (containing Ca2+) to allow reconstitution of functional TJs.
Peptide Labeling and Cellular Distribution
At the time of Ca2+ repletion in the Ca2+ switch protocol, filters were exposed to various concentrations of peptides (Table 1) to final concentrations from 25 to 400 μM from initial stocks of 100 mM prepared in dimethyl sulfoxide. Filters were kept in low-level lighting to minimize premature photoactivation of bait peptides. At set periods of time, after unbound peptide was removed by washing with excess HBSS+, associated photoactive bait peptides were activated by a 15-min exposure to high-intensity UV light while on ice (Nusrat et al., 2000a). T84 cells labeled with bait peptides were scraped from filters with a plastic Teflon spatula directly into 4°C buffer R (100 mM KCl, 3 mM NaCl, 1 mM Na2ATP, 3.5 mM MgCl2, and 10 mM HEPES, pH 7.4) containing freshly prepared 5 mM diisopropyl fluorophosphate, 1.25 mM phenylmethylsulfonyl fluoride, and 10 μg/ml chymostatin (Sigma-Aldrich). Cells were disrupted at 4°C in a nitrogen cavitation bomb (Parr Instruments, Moline, IL) for 15 min using a N2 pressure of 200 psi and unbroken nuclei and cellular debris were removed from disrupted cells by a 1000 × g sedimentation at 4°C. Biotin-bound proteins were captured by passing the supernatant through a monomeric avidin-Sepharose column (Pierce, Rockford, IL). Samples were eluted according to manufacturer's instructions. The phosphorylation status of peptide-labeled occludin was determined by eluting biotin-peptide complexes from avidin beads as described above and capturing occludin-containing complexes by immuno-precipitation with antibodies to occludin. Immunoprecipitated occludin was subjected to SDS-PAGE and Western blotting with antibodies specific for occludin (Zymed Laboratories, South San Francisco, CA), phosphoserine (Zymed), phosphothreonine (Zymed), or phosphotyrosine (Upstate Biotechnology, Charlottesville, VA).
To determine peptide distribution in relation to TJ-associated proteins in T84 cells, filters were washed, fixed in absolute ethanol (20 min, –20°C) and blocked in 5% normal goat serum (1 h, room temperature [RT]) and incubated in a humidity chamber for 1 h with primary antibodies to either human occludin, claudin-1, ZO-1, (Zymed) or JAM-A (C. A. Parkos, Emory University, Atlanta, GA). Cell filters were then washed, probed with Alexa 488–conjugated goat anti-mouse/-rabbit IgG (1 h, RT; Molecular Probes, Eugene, OR), and mounted on phosphate-buffered saline/glycerol/p-phenylenediamine, 1:1:0.01 (vol/vol/v). For filamentous actin (F-actin) localization, cell filters were fixed in 3.7% paraformaldehyde (10 min, RT), permeabilized in 0.5% Triton X-100 (30 min, RT), incubated with Alexa 568–conjugated phalloidin (Molecular Probes; 1 h, RT), washed, and mounted as above. Bound biotinylated peptides were localized by labeling with FITC-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA). All fluorescent labels and cells were visualized on an LSM510 confocal microscope (Zeiss Microimaging, Thornwood, NY). Images shown are representative of at least six experiments, with multiple images taken per slide.
Paracellular Permeability Assays
Paracellular permeability to fluorescein-dextran (FD-3; MW 3000) was assessed at selected time points according to previously published methods (Sanders et al., 1995). Briefly, after TER was measured, monolayers were washed in HBSS+ and then equilibrated at 37°C for 10 min on an orbital shaker at 60 rpm. Monolayers were loaded apically with 1 mg/ml FD-3 (Molecular Probes) and basolateral samples were taken at t = 0, 30, 60, 90, and 120 min. HBSS+ replaced volume taken from basolateral samples. Fluorescence intensity was analyzed on a CytoFluor 2350 Fluorescence Measurement System (Millipore, Cambridge, MA) and the amount FD-3 transported, extrapolated from a standard curve, was expressed as ng FD-3 transported/cm2/h. Numerical values from individual experiments were pooled and expressed as mean ± SEM throughout. Control and test values at each time point were compared by two-tailed unpaired Student's t tests, with statistical significance assumed at p < 0.05.
RESULTS
Occludin Loop Peptides Can Self-Associate
Human occludin is composed of 522 amino acids (Figure 1A). Hydropathy plots have projected two extracellular loops for this protein; loop A from 90D to 135R and loop B from 196G to 243E (Ando-Akatsuka et al., 1996). Standard peptides (Table 1) were synthesized to emulate a central region of loop A and loop B of human occludin, termed O-A:101–121 and O-B:210–228, respectively (Figure 1A). Bait peptides were also prepared (identified with an asterisk in Table 1); these were similar to standard peptides but modified to contain a hydrophobic photoactive amino acid, benzoylphenylalanine (bpa), positioned to replace a centrally located hydrophobic amino acid of the native peptide sequence (these positions are highlighted in Table 1). A biotin moiety was positioned at the N-terminus of bait peptides.
Figure 1.
Properties of occludin synthetic peptides. (A) Cartoon of human occludin demonstrating sequence locations emulated by O-A:101–121 and O-B:210–228 peptides (detailed in Table 1). (B) Homologous peptide association studies performed with 20 μM bait peptide incubated in HBSS with Ca2+ and Mg2+ (HBSS+) in the presence or absence of 200 μM corresponding standard peptide. (C) Circular dichroism (CD) spectra of 0.2 mg standard peptide dissolved in H2O, sodium dodecylsulfate (SDS) micelles, or 92% trifluoroethanol (TFE).
Organizational aspects and biophysical properties of extracellular loop segments of occludin are unknown; thus it was important to obtain some preliminary characterization of O-A:101–121 and O-B:210–228. Homologous interactions of these peptides were monitored by determining the size of peptide aggregates formed by bpa activation (exposure to UV light) to induce covalent cross-linking in the presence and absence of excess standard (nonbait) peptide (Figure 1B). For the conditions used, which recreated studies performed with cells in culture, O-A:101–121* and O-B:210–228* demonstrated only low-molecular-weight associations. These results suggested that outcomes obtained in cell labeling studies are not secondary to formation of large self-associated peptide complexes. The presence of a 10-fold excess of standard peptides (O-A:101–121) produced complexes dominated by trimers and tetramers but not larger oligomers (Figure 1B). Oppositely, a 10-fold excess of O-B: 210–228 standard peptides did not significantly affect self-association properties of O-B:210–228*. Heterologous interactions of O-A:101–121* and O-B:210–228* were not examined since the peptides were not used in this type of format in the current studies.
Solvent-Dependent Conformations of Occludin Loop Peptides
The proposed extracellular loops of occludin could potentially have interactions not only within the hydrophilic environment at the surface of cells but also within the hydrophobic lipid bilayer of the plasma membrane. We examined the potential for O-A:101–121 and O-B:210–228 to be affected by different solvent conditions that might reflect differences between the aqueous extracellular and lipid bilayer environments. Circular dichroism (CD) spectra obtained for O-A: 101–121 in aqueous (H2O), interfacial (SDS micelles), and hydrophobic (92% 2,2,2-trifluoroethanol [TFE] in H2O) environments were very similar to one another, providing no evidence for solvent-dependent changes for this region of occludin (Figure 1C). CD spectra for O-B:210–228, however, were strongly dependent on solvent conditions and suggested a transition from very little structure in H2O to a highly structured organization in SDS micelles or in TFE (Figure 1C). These studies were only performed with standard peptides because of the potential for bait peptides to cross-link when exposed to UV light.
Peptide Interactions with TJ Proteins
Divalent cation chelation (the initial step of the Ca2+ switch protocol) induces disassembly of the apical junctional complex (AJC), composed of TJ and adherens junction (AJ) structures, in polarized T84 epithelial cell monolayers through an actin-mediated mechanism (Ivanov et al., 2004a, 2004b). Although AJ components become rapidly internalized by divalent cation depletion, TJ proteins become initially reorganized at the cell surface before internalization at later times (Ivanov et al., 2004a, 2004b). Thus, peptides used in Ca2+ switch protocol studies, added at the time of Ca2+ repletion, should have had at least some access to TJ protein components at even the earliest time points analyzed. Before cross-linking with UV light, unbound peptide was removed by washing. Covalent bait peptide-protein complexes were captured with streptavidin-Sepharose beads eluted, subjected to SDS-PAGE, and transferred onto nitrocellulose. Total biotin detection with avidin showed that O-A:101–121* did not efficiently label any T84 proteins during the Ca2+ switch protocol (Figure 2A). The same result was obtained when bait peptide was omitted from the protocol (–CTRL lane). Importantly, we could show that O-A:101–121* could label proteins in cell lysates demonstrating functional capacities of the incorporated bpa and biotin residues (unpublished data). Cells exposed to O-B:210–228*, however, showed strong labeling of five discrete protein bands when presented to T84 monolayers at the time of Ca2+ repletion (Figure 2A). The intensity of protein bands labeled with O-B:210–228* increased from 0 to 6 h after Ca2+ repletion; labeling intensity was comparable between samples obtained at 6 and 24 h.
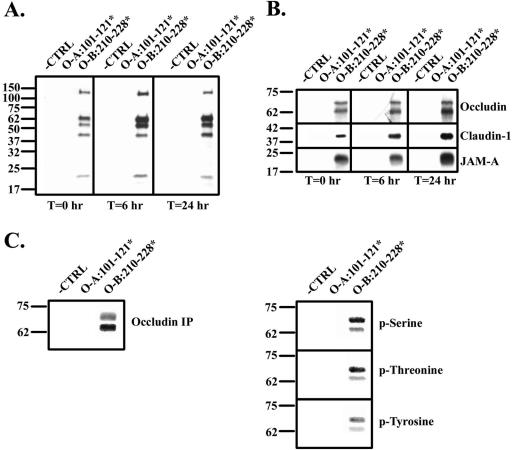
Figure 2.
Association of occludin bait peptides with cell proteins. Divalent cation depletion was used to dissemble tight junction (TJ) structures of polarized, confluent T84 cell monolayers. Bait peptide (200 μM O-A:101–121* or O-B:210–228*) was added at the time of Ca2+ repletion (T = 0) and monolayers were evaluated 0, 6, or 24 h later. After being washed free of unbound peptide monolayers were lysed. (A) After cell lysis and separation by SDS-PAGE, complete bait peptide labeling was determined by avidin labeling. (B) Monomeric avidin-Sepharose column-captured material was assessed by Western blot analysis. (C) Avidin-captured material was immunoprecipitated (IP) with an antibody recognizing human occludin, separated by SDS-PAGE, and Western-blotted an antibody to occludin. (D) Western blot analysis of IP occludin labeled by O-B:210–228* using antibodies that specifically recognize phospho-serine, phospho-threonine, and phosphotyrosine.
Western blot analysis demonstrated three of the five O-B: 210–228*-protein bands to be occludin, JAM-A, and claudin-1 (Figure 2B); identity of the other two bands is presently unknown. Previous studies have suggested that nonphosphorylated or poorly phosphorylated occludin is distributed along the basolateral membrane of epithelial cells, whereas hyperphosphosphorylated forms of the protein are selectively concentrated at the TJ and that these differences in occludin phosphorylation status provide a key step in TJ formation (Sakakibara et al., 1997). Although the resolving power of the SDS-PAGE system used for Figure 2A could not separate different phosphorylation forms of occludin, O-B:210–228*-labeled occludin bands separated in Figure 2B were consistent with molecular masses previously described for nonphosphorylated or poorly phosphorylated and phosphorylated occludin (Sakakibara et al., 1997). A more complete characterization of the phosphorylation status of O-B:210–228*-labeled occludin was performed. After separation by SDS-PAGE and Western blot analysis with an antioccludin antibody (Figure 2C), antibodies that specifically recognized occludin, phospho-serine, phospho-threonine, or phospho-tyrosine (Figure 2D) were used to confirmed that O-B:210–228* did indeed label phosphorylated occludin having phosphate modifications at serine, threonine, and tyrosine residues.
Effect of Peptides on Distribution of TJ Proteins
Addition of O-A:101–121* to T84 monolayers at the time of Ca2+ repletion did not affect the recovery of F-actin organization (demonstrated by Alexa568-phalloidin staining) at either 6 or 24 h compared with cells exposed to control media (Figure 3). Application of O-B:210–228*, however, showed that this peptide interacted strongly with T84 cells, forming aggregates of biotin label that increased over time and localized to sites where F-actin reorganization was retarded (Figure 3). The lack of O-A:101–121* association with T84 monolayers but the strong association of O-B:210–228* with these cells was consistent with results obtained in protein labeling studies (Figure 2).
Figure 3.
Localization of occludin peptides. Divalent cation depletion was used to dissemble tight junction (TJ) structures of polarized, confluent T84 cell monolayers. Bait peptide (200 μM O-A:101–121* or O-B: 210–228*) or media used for peptide addition (CTRL) was added at the time of Ca2+ repletion. After a 6- or 24-h incubation, monolayers were washed free of unbound peptide, fixed with 3.7% paraformaldehyde, permeabilized with 0.2% Triton X-100, and prepared for fluorescence microscopy. Distribution of bound bait peptides was determined by staining with FITC-conjugated streptavidin (green), whereas Alexa 568-phalloidin was used to highlight the F-actin architecture (red). Scale bar, 10 μm.
More extensive fluorescence microscopy studies were performed to determine the impact of 200 μM peptide treatment on the cellular distribution of known TJ proteins (Figure 4). Micrographs are presented where either control media or O-B:210–228* was added to cells at the time of Ca2+ repletion and incubated for either 0, 6, or 24 h. Data obtained with O-A:101–121* was identical to that obtained with control media-treated cells and was not included to minimize redundancy. Cells were kept in reduced light during incubation and washing steps before fixation to protect bait peptides from photoactivation.
Figure 4.
Effects of bait peptides on TJ architecture. Confluent T84 monolayers were subjected to a Ca2+ switch protocol with 200 μM O-A:101–121*, O-B:210–228*, or control media being added at the time of Ca2+ repletion. Immediately after (T = 0) or after 6- or 24-h incubation, monolayers were washed free of unbound peptide, fixed with ethanol or 3.7% paraformaldehyde, and prepared for fluorescence microscopy. Localization of claudin-1, occludin, JAM-A, and ZO-1 was determined by labeling with protein-specific antibodies that could be recognized by a fluorescently labeled secondary antibody (red). Distribution of bound bait peptides was determined by staining with FITC-conjugated streptavidin (green), Alexa 568-phalloidin as used to highlight the F-actin architecture (red). Scale bar, 10 μm.
Immediately after Ca2+ repletion (T = 0) all cells showed altered F-actin organization and internalized structures containing TJ proteins characteristic of divalent cation-depleted T84 cell monolayers (Ivanov et al., 2004a, 2004b). However, under conditions utilized in these studies we did observe a small fraction of TJ proteins in the plasma membrane. At this time a finely distributed arrangement of biotin (defining the location of O-B:210–228*) was detectable. Addition of control media (or O-A:101–121*; unpublished data) allowed intracellular actin distribution to return to pretreated conditions by 6 h after Ca2+ repletion; this organization of F-actin characteristic of polarized, confluent epithelial cells was maintained at 24 h (Figure 4). Cellular distribution of claudin-1, occludin, JAM-A, and ZO-1 at 6 and 24 h were reorganized to the cell membrane in a manner similar to that observed in cells before divalent cation-depletion conditions (Figure 4).
Introduction of O-B:210–228* at the time of Ca2+ repletion did not dramatically influence the recovery of polarized F-actin organization at 6 or 24 h (Figure 4); however, minor differences in actin organization were observed, in agreement of previous studies (Figure 3). In general, TJ proteins in O-B:210–228*-treated T84 monolayers reorganized to a plasma membrane by 6 h post-Ca2+ repletion except at locations where small aggregates of O-B:210–228* could be detected (Figure 4). At 24 h post-Ca2+ repletion O-B:210–228* was present in larger aggregates, with each aggregate being associated with a loss of normal organization for the TJ proteins claudin-1, occludin, JAM-A, and ZO-1 (Figure 4). Reorganization of E-cadherin to the plasma membrane at sites of cell-cell contact was not affected by incubation with O-A:101–121* or O-B:210–228*, being identical to control monolayers, over this same time course (unpublished data). A control peptide, having scrambled amino acid sequences of the O-B:210–228 peptide (Table 1), failed to affect the reorganization of TJ structures when added at the time of Ca2+ repletion (unpublished data). Structural prediction (http://www.cmpharm.ucsf.edu/~nomi/nnpredict.html) of O-B:210–228 suggested no predictable structure outside of an extended conformation for residues YAL near the N-terminus of the SQIYALCNQFYTPAATGLYVD peptide. The control peptide TSPYAQIYLANFQDTALGYCV was prepared to have a similar predicted extended conformation of AQIYL in a centrally located region of the peptide. The two peptides, O-B:210–228 and the scrambled control peptide, had identical predicted overall hydrophobic and iso-electric properties.
Overall, these results demonstrate that O-A:101–121* did not affect the recovery of TJ structures after Ca2+ repletion and that this peptide did not interact strongly with T84 cells. Oppositely, O-B:210–228* strongly associated with T84 cells and was observed to form complexes at the surface of T84 cells that increased in size over time (comparing the 0-, 6-, and 24-h time points). Micrographs (Figures 3 and 4) obtained at focal planes of the apical neck region of T84 cell monolayers showed that TJ proteins claudin-1, occludin, JAM-A, and ZO-1 failed to organize to TJ sites at the lateral membrane where complexes of O-B:210–228* were observed. Although claudin-1, occludin, and JAM-A were directly excluded from the lateral membrane of these cells wherever O-B:210–228* was distributed, apparent interruptions in ZO-1 may have been due to indirect actions resulting from a loss of organized integral TJ proteins from the lateral membrane. Indeed, lower-level focal plane images demonstrated ZO-1 present at the lateral membrane at locations inferior to O-B:210–228* distribution (unpublished data).
O-B:210–228 Influences TJ Function
Previous studies examining the actions of O-A:101–121 and O-B:210–228 were performed at 200 μM. To verify that this represented an appropriate peptide concentration, we performed a dose-response study. O-B:210–228 concentrations < 200 μM were found to be insufficient, by the criteria of transepithelial electrical resistance (TER), to block recovery of organized TJ structures in the T84 epithelial cell Ca2+ switch model system used in these studies (Figure 5A). O-A:101–121 failed to affect TER recovery compared with control media addition over the same time frame and same concentrations tested for O-B:210–228 (unpublished data). These results support the use of 200 μM concentrations of O-A:101–121 and O-B:210–228 in the present studies.
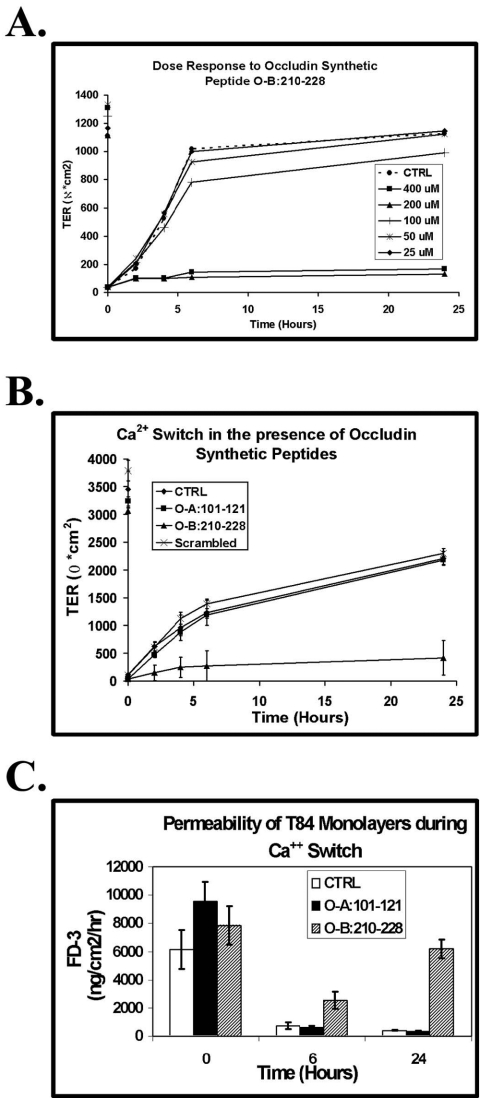
Figure 5.
Effects of peptides on TJ function. Divalent cation depletion was used to dissemble tight junction (TJ) structures of polarized, confluent T84 cell monolayers. (A) O-B:210–228 was added to final concentrations from 25 to 400 μM at the time of Ca2+ repletion. Addition of media used for peptide additions was used as a control (CTRL). Transepithelial electrical resistance (TER) measurements were performed over the next 24 h using “chopstick” electrodes. (B) After divalent cation depletion, 200 μM O-A:101–121, O-B:210–228, control (scrambled O-B:210–228 sequence) peptides, or control media was added to T84 monolayers at the time of Ca2+ repletion. TER measurements were made over the next 24 h using chopstick electrodes. (C) After addition of 200 μM O-A:101–121, O-B:210–228, or control media at the time of Ca2+ repletion, paracellular permeability was determined as a rate of 3-kDa fluorescent dextran transport at T = 0, 6, and 24 h.
Direct comparison of recovery rates of TJ function after Ca2+ repletion were performed using 200 μM O-A:101–121 or O-B:210–228 by monitoring TER values (Figure 5B). Addition of either O-A:101–121 or control peptides for O-B: 210–228 (scrambled peptide) resulted in comparable TER recovery rates to that observed for T84 monolayers treated with control media (Figure 5B). Incubation with 200 μM O-B:210–228, however, inhibited reestablishment of TER (Figure 5B). Additional studies demonstrated that the effects of 200 μM O-B:210–228 on TER continued for up to at least 48 h after Ca2+ repletion (unpublished data). The above results were observed with both the bait and standard version of the O-B:210–228 peptide.
TJ structures limit paracellular movement of molecules and establish a TER. Using truncated forms of occludin, previous studies have demonstrated a functional dissociation of these two TJ properties (Balda et al., 1996). Thus, it was important to also assess the effects of 200 μM O-B:210–228 on paracellular permeability. This assessment was performed by monitoring the transport of 3-kDa dextran labeled with fluorescein (FD-3) across T84 monolayers (Figure 5C). After divalent cation depletion a maximal rate of FD-3 transport is measured and this rate returns to a restricted level once functional TJ structures reorganize after Ca2+ repletion. The recovery profile of restricted FD-3 flux for T84 cell monolayers dosed with 200 μM O-A:101–121 mirrored that of cells receiving control media at the time of Ca2+ repletion (Figure 5B). Incubation with 200 μM O-B:210–228, however, inhibited reestablishment of TER (Figure 5B). Interestingly, the presence of 200 μM O-B:210–228 allowed for a partial recovery of FD-3 flux restriction at 6 h but the continued presence of this peptide resulted in a complete loss of FD-3 restricted flux by 24 h (Figure 5C). Additional studies demonstrated that the enhanced paracellular permeability of T84 monolayers resulting from treatment with 200 μM O-B:210–228 continued for up to at least 48 h after Ca2+ repletion (unpublished data).
DISCUSSION
Epithelial TJs are composed of several classes of integral membrane proteins that include occludin, claudins, and JAMs. TJs are dynamic structures that allow for the migration of white cells, events associated with wound repair and the regulation of paracellular solute movement. An improved understanding of molecular interactions involving the extracellular domains of these integral membrane proteins may provide new methods of regulating critical functions performed by TJs. Crystallography studies have revealed the large extracellular structure of JAM-A to have two concatenated extracellular Ig-like domains that dimerize through extensive ionic and hydrophobic interactions (Prota et al., 2003). Claudins and occludin, however, are extremely hydrophobic molecules with much smaller proposed extracellular components that make it technically difficult to determine their organization using conventional biochemical methods including x-ray crystallography. We have used a bait peptide approach to identify potential binding partners and functions for central regions of the two putative extracellular loop domains of human occludin. Our studies suggest that these two regions differ dramatically in their ability to interact with epithelial cell proteins and affect TJ functional properties, supporting the concept that occludin contains multiple, distinct, functional domains.
Our results suggest that the central region of the presumed first extracellular loop of occludin (O-A:101–121) can self-associate in solution to a limited extent (possibly forming tetramers). These data are interesting because O-A:101–121, and indeed the majority of the first loop of occludin, has a very high content of tyrosine and glycine residues similar to proteins known for their capacity to self-associate: some hair proteins (Aoki et al., 1997) and silk fibroin (Mita et al., 1994). Others have suggested that peptide segments with high glycine content can permit backbone-to-backbone Cα-H···O = C formation through short proximity interactions (Senes et al., 2001), providing one potential mechanism of O-A:101–121 self-association. CD spectra obtained for O-A:101–121 in H2O, SDS micelles or in a hydrophobic solvent (92% TFE) were not significantly different and showed strong absorbance bands at ∼200 nm and ∼230 nm that were consistent with a stable β-hairpin peptide conformation. Indeed, CD spectra obtained for a peptide known to adopt a two-stranded β-hairpin conformation with four residues per strand and a two residue type I' β-turn were striking similar to those obtained for O-A:101–121 (Pastor et al., 2002). Internal interactions suggested to occur within a two-stranded β-hairpin conformation could explain why O-A:101–121 failed to change conformation in the different solvents examined. The inability of O-A:101–121 to interact with membrane surface components of intact T84 cells could reflect either an inability of this peptide to access specific binding partners or its exclusion from organized structures at the cell surface of T84 cells, possibly due to a propensity to establish homologous interactions that might limit interactions with cell proteins.
O-A:101–121 represents a central block of 21 amino acids of the projected first extracellular loop of human occludin, a segment proposed to contain 46 amino acids (Ando-Akatsuka et al., 1996). Peptides emulating the entire first extracellular occludin loop (sequences from several species) have provided conflicting results: either having no effect on epithelial cell TJ function (Wong and Gumbiner, 1997; Vietor et al., 2001), inhibiting cell adhesion induced by occludin expression (Van Itallie and Anderson, 1997) or significantly reducing the paracellular permeability barrier to mannitol flux (Tavelin et al., 2003). Examination of a variety of shorter peptide sequences emulating various regions of occludin's first extracellular loop demonstrated its actions on TJ structure/function to be focused in the proximal third of the sequence (Lacaz-Vieira et al., 1999; Tavelin et al., 2003). These peptides affected TJ structure/function when added to the basolateral surface of confluent monolayers or as part of a Ca2+ switch study, similar to the studies presently reported. It is possible that the striking differences observed in these various studies were due, at least in part, to the cell systems and peptide preparations used and their manner of application.
O-B:210–228 emulates a central domain of the proposed second extracellular loop of human occludin. Unlike O-A: 101–121, CD spectra obtained for O-B:210–228 were strikingly different in H2O compared with SDS micelles or 92% TFE. O-B:210–228 appears to take on an extended left-handed helical, or PII, conformation in H2O (Park et al., 1997). Unlike α-helices that twist to the right at a rate of 3.6 residues per turn, a PII conformation has three residues per turn to position every third side-chain in a line, resulting in three parallel columns spaced uniformly about the long helical axis (Shi et al., 2002). O-B:210–228 showed clear evidence of α-helix and some β-sheet structure (evidenced by negative troughs at 208 and 222 nm and the positive band at 195 nm) in SDS micelles and TFE. A similar conformational transition has been observed for other peptides as they move from a hydrophilic to hydrophobic environment (Park et al., 1997). We speculate that the ability of O-B:210–228 to interact strongly with T84 cells may reflect its ability to undergo solvent-dependent conformation changes associated with lipid bilayer interaction.
Studies performed using peptides to emulate structural and functional domains of integral membrane proteins with the potential to form complex associations within the peculiar environment of the TJ present a unique set of concerns. First of all, these peptide domains have the potential to self-associate or to interact with some other region of occludin or possibly another TJ protein. Second, interactions observed with such peptides may represent cis-membrane (same cell) or transmembrane (between opposing cells) contacts. Third, these peptides may, as a critical aspect of their structure and function, interact with the plasma membrane. Although the peptides used in our studies were from projected extracellular regions of occludin, the extremely close proximity of adjacent cell plasma membranes at the TJ suggests that these loop regions have the potential to come in close contact with membrane surfaces. This unique combination of potential peptide-protein and peptide-membrane contacts makes identifying appropriate control peptides very challenging. Because it is unclear how any scramble might affect essential aspects of the parent peptide that could dictate interactions with membrane or protein components (e.g., hydrophobicity, charge distribution, or conformation), any results obtained with scrambled peptide in our studies cannot be used to identify functional aspects of O-B:210–228. For the studies described presently, a scrambled peptide for the amino acid sequence of O-B:210–228 did have, based upon prediction algorithms, comparable and similar structural content although displaced at different regions of the sequence. It should also be noted that in the present studies, particularly because of their clearly different properties, O-A:101–121 and O-B:210–228 peptides are not intended to act as controls for each other.
When applied to T84 cells at a time of TJ reorganization, O-B:210–228 was found to interact with claudin-1, occludin, JAM-A, and two other, as yet undefined, proteins. Association with one or all of these protein-interaction partners is likely critical to the actions observed for O-B:210–228 to disrupt TJ reorganization events. We hypothesize that it is through these specific interactions with this small cadre of proteins that O-B:210–228 exerts its ability to affect TER and increase paracellular permeability over a time frame that coincides with increased cell association of the peptide. Given the limited peptide supply and low abundance of many TJ proteins, we resorted to using the avidin pull-down and Western blotting approach rather than more conventional silver-stained gels to identify components of the protein complex that associate with bound peptides. However, using our approach it is rather remarkable that only a limited number of proteins were identified in spite of the several hundred proteins that would theoretically be accessible to this peptide on the cell surface.
We believe the effects of O-B:210–228 are stoichiometric in nature because a threshold dose of the peptide (200 μM) was required for effects to be observed and sites of TJ disruption correlated with focal aggregates of the peptide. Although focal sites of O-B:210–228 aggregation revealed local loss of occludin, claudin-1, and JAM-A at the lateral membrane, lesser effects were observed for the TJ cytoplasmic plaque protein, ZO-1 and no effect on the adherens junction (AJ) protein E-cadherin was observed. This observation is consistent with previous findings that demonstrated distinct translocation routes for AJ and TJ proteins in the Ca2+ switch model using T84 cell monolayers (Ivanov et al., 2004a). Thus, local disruption of TJ adhesion function, possibly through interruption of protein-protein contacts by O-B:210–228, does not necessarily require disturbance of TJ plaque protein organization or AJ structures.
Previous studies using peptides emulating the entire proposed second extracellular loop of occludin have shown it to perturb TJ permeability and down-regulate occludin (Wong and Gumbiner, 1997) either directly or through a functional cross-talk between AJ and TJ elements that involves accumulation of nuclear β-catenin (Vietor et al., 2001). It is important to note, however, that others have not observed permeability effects after treatment with a peptide emulating the entire 48 amino acids making up this projected second extracellular loop (Tavelin et al., 2003). Our findings with O-B:210–228 instead showed that a centrally located 21 amino acid segment of the second loop can profoundly affect TJ permeability and TER. It is unclear why such variability is observed between various studies using peptides that emulate the extracellular loops of occludin although most of these studies have used different cell models and occludin sequences from a variety of animal species. It is important to note that in the studies we now report there was a completely consistent set of observations that supported the ability of O-B:210–228 (and lack of ability of O-A:101–121) to interact with proteins at the membrane surface during reorganization of TJ structures and the capacity to affect TER and paracellular permeability.
We believe our studies are the first to use a strategy to provide simultaneous assessments of protein-peptide interactions and physiological outcomes for extracellular loop domains of occludin. Such a strategy must still be viewed cautiously because the photoactive or biotin moieties of the bait peptides used in these studies could affect properties or actions of the peptide. To rule out such concerns we have used standard peptides (emulating the exact peptide sequence of these regions and lacking photoactive and biotin moieties) wherever possible to verify outcomes observed with bait peptides and to demonstrate specificity of bait peptides interactions. Additionally, concerns exist over extrapolating conformational or protein-association data using peptides that may represent domains of a protein that might be physically constrained or restricted in ways that could not be represented accurately by this peptide approach. Despite these issues, and in light of the highly selective nature of the interactions observed, we feel this bait peptide approach has provided novel information related to potential protein binding partners and actions by discrete extracellular domains of the integral TJ protein occludin.
Occludin has been suggested to interact in a homotypic manner through extracellular loop interactions (Tsukita et al., 2001). Our findings support that concept and also suggest heterotypic contacts between the proposed second extracellular occludin loop and TJ adhesion proteins claudin-1 and JAM-A. To our knowledge these finding are the first example of such heterotypic interactions and provide additional information concerning possible protein-protein contacts comprising the epithelial TJ. Our recent studies examining potential protein interactions involving the latter half of the putative first extracellular loop of claudin-1 have shown this domain to interact specifically with occludin (unpublished results). Thus, it is possible that this domain of claudin-1 may contact the central domain of occludin's loop B. Because of demonstrated strong membrane affinities for peptides used to emulate these occludin and claudin-1 domains, it is possible that these occludin-claudin-1 interactions occur in conjunction with membrane bilayers and thus the mechanism of interaction may be complicated significantly by this issue of lipid involvement. Indeed, interactions between integral membrane TJ protein must be considered in the context of the two-dimensional environment experienced within plasma membrane as well as within a third dimension where extracellular loop domains could interact with components from the same cells (cis contacts) or with those of an adjacent epithelial cell (trans contacts).
In summary, we have described preliminary structural information as well as an initial assessment on protein-protein contacts involving a central domain of the second extracellular loop (B) of occludin and demonstrated a capacity of that segment to regulate the organization of epithelial TJ structures. Together, these results provide the first data addressing potential structure/function relationships of this extracellular segment of human occludin. With this perspective, it is interesting to note that claudin proteins appear to establish the backbone of TJ membrane-associated fibrils and functionally competent barrier structures (Hoevel et al., 2002), whereas occludin lacks the ability to form extended lateral membrane polymers (Medina et al., 2000) but may play a role in the dynamic regulation of TJ function (Matter and Balda, 2003).
Acknowledgments
We thank L. M. Winfree and Susan Voss for their technical assistance. This work was supported by funding from the National Institutes of Health (DK 59888, DK 64399).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–06–0465) on January 19, 2005.
Abbreviations used: TJ, tight junction; JAM, junctional adhesion molecule; TER, trans-epithelial electric resistance; CD, circular dichroism.
References
- Ando-Akatsuka, Y., Saitou, M., Hirase, T., Kishi, M., Sakakibara, A., Itoh, M., Yonemura, S., Furuse, M., and Tsukita, S. (1996). Interspecies diversity of the occludin sequence: cDNA of human, mouse, dog, and rat-kangaroo homologues. J. Cell Biol. 133, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, N., Ito, K., and Ito, M. (1997). Isolation and characterization of mouse high-glycine/tyrosine proteins. J. Biol. Chem. 272, 30512–30518. [DOI] [PubMed] [Google Scholar]
- Balda, M. S., Whitney, J. A., Flores, C., Gonzalez, S., Cereijido, M., and Matter, K. (1996). Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134, 1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio, O. R., Van Itallie, C., Rahner, C., and Anderson, J. M. (2003). Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 284, C1346–C1354. [DOI] [PubMed] [Google Scholar]
- Colegio, O. R., Van Itallie, C. M., McCrea, H. J., Rahner, C., and Anderson, J. M. (2002). Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 283, C142–C147. [DOI] [PubMed] [Google Scholar]
- Fanning, A. S., Jameson, B. J., Jesaitis, L. A., and Anderson, J. M. (1998). The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 273, 29745–29753. [DOI] [PubMed] [Google Scholar]
- Furuse, M., Fujita, K., Hiiragi, T., Fujimoto, K., and Tsukita, S. (1998). Claudin-1 and -2, novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141, 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj, N. S., and Antonetti, D. A. (2004). Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 36, 1206–1237. [DOI] [PubMed] [Google Scholar]
- Hoevel, T., Macek, R., Mundigl, O., Swisshelm, K., and Kubbies, M. (2002). Expression and targeting of the tight junction protein CLDN1 in CLDN1-negative human breast tumor cells. J. Cell. Physiol. 191, 60–68. [DOI] [PubMed] [Google Scholar]
- Huber, D., Balda, M. S., and Matter, K. (2000). Occludin modulates transepithelial migration of neutrophils. J. Biol. Chem. 275, 5773–5778. [DOI] [PubMed] [Google Scholar]
- Itoh, M., Sasaki, H., Furuse, M., Ozaki, H., Kita, T., and Tsukita, S. (2001). Junctional adhesion molecule (JAM) binds to PAR-3, a possible mechanism for the recruitment of PAR-3 to tight junctions. J. Cell Biol. 154, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, A. I., McCall, I. C., Parkos, C. A., and Nusrat, A. (2004a). Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol. Biol. Cell 15, 2639–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, A. I., Nusrat, A., and Parkos, C. A. (2004b). Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 15, 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaz-Vieira, F., Jaeger, M. M., Farshori, P., and Kachar, B. (1999). Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J. Membr. Biol. 168, 289–297. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Nusrat, A., Schnell, F. J., Reaves, T. A., Walsh, S., Pochet, M., and Parkos, C. A. (2000). Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113(Pt 13), 2363–2374. [DOI] [PubMed] [Google Scholar]
- Matter, K., and Balda, M. S. (2003). Signalling to and from tight junctions. Nat. Rev. Mol. Cell. Biol. 4, 225–236. [DOI] [PubMed] [Google Scholar]
- Medina, R., Rahner, C., Mitic, L. L., Anderson, J. M., and Van Itallie, C. M. (2000). Occludin localization at the tight junction requires the second extracellular loop. J. Membr. Biol. 178, 235–247. [DOI] [PubMed] [Google Scholar]
- Mita, K., Ichimura, S., and James, T. C. (1994). Highly repetitive structure and its organization of the silk fibroin gene. J. Mol. Evol. 38, 583–592. [DOI] [PubMed] [Google Scholar]
- Nusrat, A., Chen, J. A., Foley, C. S., Liang, T. W., Tom, J., Cromwell, M., Quan, C., and Mrsny, R. J. (2000a). The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J. Biol. Chem. 275, 29816–29822. [DOI] [PubMed] [Google Scholar]
- Nusrat, A., Turner, J. R., and Madara, J. L. (2000b). Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am. J. Physiol. Gastrointest Liver Physiol. 279, G851–G857. [DOI] [PubMed] [Google Scholar]
- Park, S. H., Shalongo, W., and Stellwagen, E. (1997). The role of PII conformations in the calculation of peptide fractional helix content. Protein Sci. 6, 1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos, C. A., Colgan, S. P., Bacarra, A. E., Nusrat, A., Delp-Archer, C., Carlson, S., Su, D. H., and Madara, J. L. (1995). Intestinal epithelia (T84) possess basolateral ligands for CD11b/CD18-mediated neutrophil adherence. Am. J. Physiol. 268, C472–C479. [DOI] [PubMed] [Google Scholar]
- Pastor, M. T., Lopez de la Paz, M., Lacroix, E., Serrano, L., and Perez-Paya, E. (2002). Combinatorial approaches: a new tool to search for highly structured beta-hairpin peptides. Proc. Natl. Acad. Sci. USA 99, 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prota, A.E., Campbell, J. A., Schelling, P., Forrest, J. C., Watson, M. J., Peters, T. R., Aurrand-Lions, M., Imhof, B. A., Dermody, T. S., and Stehle, T. (2003). Crystal structure of human junctional adhesion molecule 1, implications for reovirus binding. Proc. Natl. Acad. Sci. USA 100, 5366–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara, A., Furuse, M., Saitou, M., Ando-Akatsuka, Y., and Tsukita, S. (1997). Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 137, 1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, S. E., Madara, J. L., McGuirk, D. K., Gelman, D. S., and Colgan, S. P. (1995). Assessment of inflammatory events in epithelial permeability: a rapid screening method using fluorescein dextrans. Epithelial Cell Biol. 4, 25–34. [PubMed] [Google Scholar]
- Sawada, N., Murata, M., Kikuchi, K., Osanai, M., Tobioka, H., Kojima, T., and Chiba, H. (2003). Tight junctions and human diseases. Med. Electron Microsc. 36, 147–156. [DOI] [PubMed] [Google Scholar]
- Senes, A., Ubarretxena-Belandia, I., and Engelman, D. M. (2001). The Calpha—HO hydrogen bond: a determinant of stability and specificity in transmembrane helix interactions. Proc. Natl. Acad. Sci. USA 98, 9056–9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z., Olson, C. A., Rose, G. D., Baldwin, R. L., and Kallenbach, N. R. (2002). Polyproline II structure in a sequence of seven alanine residues. Proc. Natl. Acad. Sci. USA 99, 9190–9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, B. R., Siliciano, M. S., Mooseker, M. S., and Goodenough, D. A. (1986). Identification of ZO-1, a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 103, 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavelin, S., Hashimoto, K., Malkinson, J., Lazorova, L., Toth, I., and Artursson, P. (2003). A new principle for tight junction modulation based on occludin peptides. Mol. Pharmacol. 64, 1530–1540. [DOI] [PubMed] [Google Scholar]
- Tsukita, S., Furuse, M., and Itoh, M. (2001). Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell. Biol. 2, 285–293. [DOI] [PubMed] [Google Scholar]
- Van Itallie, C. M., and Anderson, J. M. (1997). Occludin confers adhesiveness when expressed in fibroblasts. J. Cell Sci. 110, 1113–1121. [DOI] [PubMed] [Google Scholar]
- Vietor, I., Bader, T., Paiha, K., and Huber, L. A. (2001). Perturbation of the tight junction permeability barrier by occludin loop peptides activates beta-catenin/TCF/LEF-mediated transcription. EMBO Rep. 2, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, V., and Gumbiner, B. M. (1997). A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol. 136, 339–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui, A., Louvard, D., and Galli, T. (2000). Tight junction, a platform for trafficking and signaling protein complexes. J. Cell Biol. 151, F31–F36. [DOI] [PMC free article] [PubMed] [Google Scholar]