Abstract
The Rab GTPase Ypt1p and the large homodimer Uso1p are both required for tethering endoplasmic reticulum-derived vesicles to early Golgi compartments in yeast. Loss-of-function ypt1 and uso1 mutations are suppressed by SLY1-20, a dominant allele that encodes the Sed5p-associated protein, Sly1p. Here, we investigate the mechanism of SLY1-20 suppression. In wild-type strains, Ypt1p can be coimmunoprecipitated with Uso1p; however, in a ypt1Δ/SLY1-20 strain, which lacks this complex, membrane binding of Uso1p was reduced. In spite of Ypt1p depletion, Uso1p-dependent vesicle tethering was not bypassed under the ypt1Δ/SLY1-20 condition. Moreover, tethering and fusion assays with ypt1Δ/SLY1-20 membranes remained sensitive to Rab GDP dissociation inhibitor. These results indicate that an alternative Rab protein satisfies the Ypt1p requirement in Uso1p-dependent tethering when SLY1-20 is expressed. Further genetic and biochemical tests revealed that a related Rab protein, Ypt6, might substitute for Ypt1p in ypt1Δ/SLY1-20 cells. Additional experimentation to address the mechanism of SLY1-20 suppression in a cog2Δ [sec35Δ] strain indicated that the Cog2p subunit of the conserved oligomeric Golgi complex is either functionally redundant or is not directly required for anterograde transport to the Golgi complex.
INTRODUCTION
Protein transport through the eukaryotic secretory pathway is mediated by regulated mechanisms of vesicle budding, membrane tethering, and bilayer fusion. These mechanisms ensure that proper cargo is loaded into vesicles and that vesicles are targeted to and fuse with the appropriate acceptor membranes. Vesicle budding and cargo selection are dependent on sets of coat proteins (Schekman and Orci, 1996). Transport directionality and membrane fusion depend on the coordinated activities of Rab/Ypt GTPases and membrane-bound SNARE proteins (Rizo and Südhof, 2002). Although distinct Rabs and soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs) have been implicated in a multitude of intracellular membrane fusion reactions, it is less clear how Rabs, SNAREs, and their regulators cooperate to provide an essential degree of specificity. Therefore, it remains important to understand the mechanisms that couple proteins in membrane tethering and fusion.
Multisubunit tethering complexes have been proposed to contribute specificity to distinct intracellular fusion reactions. These complexes localize to various transport sites and share homology among some of their subunits, but thus far they seem to be unique enough in their composition to impart specificity (Pfeffer, 2001; Short and Barr, 2002; Whyte and Munro, 2002). The characterized complexes include the Exocyst at the plasma membrane (TerBush et al., 1996); the TRAPP I, TRAPP II complexes at the cis- and medial-Golgi compartments, respectively (Sacher et al., 2001); the conserved oligomeric Golgi (COG) complex involved in intra-Golgi targeting (Suvorova et al., 2002; Ungar et al., 2002); the GARP/VFT complex in endosome-to-late Golgi transport (Siniossoglou and Pelham, 2001; Conibear et al., 2003); and the HOPS/class C complex in homotypic vacuolar and Golgi-to-endosome fusion (Sato et al., 2000; Seals et al., 2000). Although these complexes share an apparent tethering role, it seems the molecular mechanisms for their function remain distinct.
Biochemical and genetic studies have shown that vesicle tethering to the early Golgi compartment in yeast requires the Rab GTPase Ypt1p, the large homodimer Uso1p, and the TRAPP I complex. TRAPP I is reported to provide the exchange activity for Ypt1p (Jones et al., 2000; Wang et al., 2000), and membrane extraction of Ypt1p with GDP dissociation inhibitor (GDI) reduces the level of membrane-bound Uso1p (Cao et al., 1998). Uso1p is homologous to the mammalian p115 protein that acts with the Rab1 GTPase in transport through the early secretory pathway (Allan et al., 2000). Uso1p also shares homology with the yeast Sys3p that interacts with Ypt6p in late Golgi compartments and possibly in Golgi-to-endoplasmic reticulum (ER) retrograde transport (Tsukada et al., 1999; Luo and Gallwitz, 2003).
To gain further insight into the mechanisms of vesicle tethering and fusion, we investigated ER/Golgi transport under conditions in which the YPT1 gene was deleted, but viability maintained through expression of the dominant SLY1-20 allele. This SLY1 allele was originally isolated as a suppressor of loss of Ypt1p (Dascher et al., 1991), but it also suppresses deletion of USO1 (Sapperstein et al., 1996). The Sly1p protein belongs to a conserved family of syntaxin-SNARE–associated proteins, termed the Sec1/Munc18 (SM) family, that govern SNARE protein activity in intracellular membrane fusion reactions (Rizo and Südhof, 2002). The collective genetic and biochemical evidence indicates that Sly1p operates downstream of Ypt1p and Uso1p and promotes ER/Golgi SNARE complex assembly (Sapperstein et al., 1996; Kosodo et al., 2002; Peng and Gallwitz, 2002; Williams et al., 2004). Therefore, it can be hypothesized that the SLY1-20 allele produces an activated form of Sly1p that somehow promotes SNARE-dependent membrane fusion and allows for a complete bypass of vesicle tethering. Alternatively, there could be redundant tethering mechanisms in transport to the Golgi complex that are permitted by SLY1-20. To test these possibilities, we examined ER/Golgi transport in ypt1Δ/SLY1-20 cells by using in vitro assays that monitor the subreactions of vesicle budding, vesicle tethering, and membrane fusion. Our data demonstrate that in the absence of Ypt1p, in vitro transport efficiency was decreased and membrane association of Uso1p was reduced. However, vesicle tethering and fusion remained dependent on Uso1p. Moreover, vesicle tethering and fusion in the ypt1Δ/SLY1-20 cells was sensitive to Rab GDI, indicative of a continued Rab protein requirement in the absence of Ypt1p. Our genetic and biochemical experiments suggest that Ypt6p may provide a substitute Rab protein activity in the absence of Ypt1p.
In addition to YPT1 and USO1, the SLY1-20 allele also suppresses null mutations in SEC35 and SEC34 (VanRheenen et al., 1998, 1999). Sec35p and Sec34p are subunits of a larger protein complex termed the COG complex and have been renamed Cog2p and Cog3p, respectively (Ungar et al., 2002). The COG complex is required for maintenance of Golgi structure/function and is proposed to act in retrograde targeting of intra-Golgi vesicles (Whyte and Munro 2001; Ram et al., 2002; Suvorova et al., 2002). When we examined the properties of a cog2Δ/SLY1-20 strain, no significant alterations in the distribution of known ER/Golgi transport factors were detected, and subreactions in transport from the ER to the Golgi complex were not compromised.
MATERIALS AND METHODS
General Materials and Techniques
Yeast strains used in this study are listed in Table 1 and were grown in rich medium (1% bacto-yeast extract, 2% Bacto-peptone, and 2% dextrose) or selective medium (0.67% nitrogen base without amino acids and 2% dextrose) with required supplements. Media containing 5-fluoro-orotic acid (5-FOA) was prepared as described previously (Guthrie and Fink, 1991). Bacterial strains DH5α and XL1-Blue were grown at 37°C (unless otherwise indicated) in LB medium (1% NaCl, 1% Bacto-tryptone, and 0.5% Bacto-yeast extract) containing 100 μg/ml ampicillin if required. For immunoblots, samples were resolved by SDS-PAGE (Laemmli, 1970), transferred to nitrocellulose (Towbin et al., 1979), and filter-bound secondary antibodies were detected by peroxidase-catalyzed chemiluminescence (enhanced chemiluminescence [ECL] method; Amersham Biosciences, Piscataway, NJ).
Table 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| ROH713-10A | Mata his3 leu2 ypt1Δ::HIS3 pYPT1-TM2 (LEU2 CEN YPT1-TM2) | Ossig et al. (1995) |
| CBY80 | Matα trp1Δ63 his3Δ200 ura3-52 leu2Δ1 lys2Δ202 | Winston et al. (1995) |
| CBY474 | MATα trp1-1 ade2-1 ura3-1 leu2-3,112 can1-100 ypt1-3 | Cao et al. (1998) |
| CBY528 | MATα trp1-1 ade2-1 ura3-1 leu2-3,112 can1-100 pUSO1-Myc (URA3 2μm USO1-Myc) | This study |
| CBY900 | MATa trp1Δ63 his3Δ200 ura3-52 leu2 | This study |
| CBY901 | MATa trp1Δ63 his3Δ200 ura3-52 leu2 pSK54 (URA3 2μm SLY1-20) | This study |
| CBY903 | MATa trp1Δ63 his3Δ200 ura3-52 leu2 ypt1Δ::HIS3 pSK54 (URA3 2μm SLY1-20) | This study |
| CBY1020 | MATα his3Δ ura3Δ leu2Δ pSK54 (URA3 2μm SLY1-20) | This study |
| CBY1021 | MATa his3Δ ura3Δ leu2Δ lys2Δ cog2Δ::KAN pSK54 (URA3 2μm SLY1-20) | This study |
| CBY1187 | MATα trp1Δ1 ade2-101 ura3-52 leu2Δ98 lys2-801 | Ross-Macdonald et al. (1999) |
| CBY1189 | MATα trp1Δ1 ade2-101 ura3-52 leu2Δ98 lys2-801 USO1-3HA | Ross-Macdonald et al. (1999) |
| CBY1297 | MATα his3Δ ura3Δ leu2Δ met15Δ lys2Δ uso1Δ::KAN pSK47 (URA3 2μm USO1) | This study |
| CBY1342 | Matα his3Δ ura3Δ leu2Δ lys2Δ ypt31Δ::KAN | Research Genetics |
| CBY1343 | Matα his3Δ ura3Δ leu2Δ lys2Δ ypt32Δ::KAN | Research Genetics |
| CBY1344 | Matα his3Δ ura3Δ leu2Δ lys2Δ ypt6Δ::KAN | Research Genetics |
| CBY1362 | MATα his3Δ ura3Δ leu2 ypt1Δ::HIS3 uso1Δ::KAN pSK47 (URA3 2μm USO1) pRS425-SLY1-20 (LEU2 2μm SLY1-20) | This study |
| CBY1381 | MATα his3Δ ura3Δ leu2Δ met15Δ lys2Δ uso1Δ::KAN pSK47 (URA3 2μm USO1) pRS425-SLY1-20 (LEU2 2μm SLY1-20) | This study |
| CBY1396 | MATa his3Δ ura3Δ leu2Δ ypt1Δ::HIS3 ypt6Δ::KAN pRB320 (URA3 2μm YPT1) pRS425-SLY1-20 (LEU2 2μm SLY1-20) | This study |
| CBY1399 | MATα his3Δ ura3Δ leu2Δ ypt1Δ::HIS3 ypt32Δ::KAN pRB320 (URA3 2μm YPT1) pRS425-SLY1-20 (LEU2 2μm SLY1-20) | This study |
| CBY1548 | MATα his3Δ ura3Δ leu2Δ ypt1Δ::HIS3 ypt31Δ::KAN pRB320 (URA3 2μm YPT1) pRS425-SLY1-20 (LEU2 2μm SLY1-20) | This study |
| CBY1550 | MATα trp1-1 ade2-1 ura3-1 leu2-3,112 can1-100 | This study |
| CBY1551 | MATa trp1Δ63 his3Δ200 ura3-52 leu2 ypt1Δ::HIS3 pRB320 (URA3 2μm YPT1) | This study |
Strain Construction
The CBY1187 and CBY1189 strains were provided by the Snyder laboratory (Ross-Macdonald et al., 1999). The Δypt1/SLY1-20 strain was generated from ROH713-10A (Ossig et al., 1995). This strain was crossed with wild-type CBY80. This YPT1/ypt1Δ::HIS3 diploid was transformed with pRS426-SLY1-20 to obtain CBY903. The isogenic wild-type strain CBY900 also was transformed with pRS426-SLY1-20, and this strain (CBY901) was grown on selective media to maintain plasmid selection. CBY1297 was generated from transformation of pRS426-USO1 into the Research Genetics diploid USO1/uso1Δ::KAN, followed by sporulation, asci dissection, and scoring to obtain the uso1Δ covered by the USO1 plasmid. To generate CBY1021, a cog2Δ[sec35Δ]::KAN strain from Research Genetics (Huntsville, AL) (Winzeler et al., 1999) was transformed with pRS426-SLY1-20. The isogenic wild-type from Research Genetics was transformed with pRS426-SLY1-20 rendering, CBY1020. The CBY1381 strain resulted from a transformation of pRS425-SLY1-20 into CBY1297. The CBY1362 strain was constructed from a cross of CBY1297 and CBY903. Strains CBY1396, 1399 and CBY1548 were constructed from crosses of CBY1551 with CBY1344, CBY1343, and CBY1342, respectively, and then transformed with pRS425-SLY1-20.
Protein Purification
Yeast GDI (Sec19p) was expressed in Escherichia coli and purified as described previously (Garrett et al., 1994). The peak fractions were dialyzed against buffer 88 (20 mM HEPES, pH 7.0, 150 mM KOAc, and 5 mM MgOAc) containing 0.5 mM dithiothreitol. Aliquots were frozen in liquid nitrogen and stored at –70°C. The COPII proteins (Barlowe et al., 1994) and Uso1p (Barlowe, 1997) were prepared as described previously. Purified LMA1 (Xu et al., 1997) was a generous gift from the Wickner laboratory (this department).
Antibody Production and Purification
To generate antigen for antibody production, the region encoding the N-terminal globular domain of Uso1p (amino acids 1–744) was subcloned from pRS426-USO1 as a BamHI/BglII fragment and ligated into pQE-70 (QIAGEN, Valencia, CA). The sequence encoding the C terminus of Uso1p (amino acids 1365–1812) was amplified as a BamHI/EcoRI fragment and ligated into pGEX-2T (Amersham Biosciences). The entire open reading frame of Bet3p was amplified from genomic DNA as a BamHI/EcoRI fragment and ligated into pGEX-2T (Amersham Biosciences). The His6-Cog2p [Sec35p] antigen was isolated after transformation of pSV20 (VanRheenen et al., 1998) into XL1-Blue. A GST-Cog3p [Sec34p] fusion protein was generated by amplifying a region of COG3 [SEC34] that encodes amino acids 8–293 as an EcoRI fragment and insertion into pGEX-3T (Amersham Biosciences). All fusion proteins were purified according to the manufacturer's specifications and used to immunize rabbits (Charles River PharmServices, Southbridge, MA). Antibodies directed against α-1,6-mannose linkages, Ypt1p, Sec61p, Sec22p, Sec23p, and Sly1p have been described previously (Cao and Barlowe, 2000). Antibodes against Erv41p (Otte et al., 2001) also were used. Antibodies against Ypt7p (Haas et al., 1995) were a generous gift from W. Wickner (this department). Antibodies against Ypt6p were a generous gift from D. Gallwitz (Max Planck Institute, Goettingen, Germany).
Membrane Binding Assays
Yeast semi-intact cells were prepared as described previously (Cao et al., 1998), except the final lysis washes were omitted to retain cytosol for subsequent binding experiments. For binding assays, 0.03 ml of unwashed semiintact cells (10 mg/ml) were diluted with buffer 88 and incubated with an ATP-regenerating system (Baker et al., 1988) and 0.1 mM GTP in a final volume of 0.14 ml at 29°C for 10 min. Reactions were stopped on ice, and total samples were collected. High-speed supernatant and membrane pellet fractions were isolated by centrifugation at 100,000 × g for 10 min in a TLA100.3 rotor (Beckman Coulter, Fullerton, CA). A sample of the supernatant was diluted 1/10 into SDS-PAGE sample buffer. After removal of the supernatant, the pellet membrane fraction was resuspended in 0.14 ml and diluted 1/10 in SDS-PAGE sample buffer. The levels of Ypt1p, Uso1p, Cog2p, Cog3p, Bet3p, Erv41p, Sec23p, and Sec22p were analyzed in each fraction by immunoblotting with polyclonal antibodies. Immunoblots were developed using the ECL method (Amersham Biosciences). The relative amounts of specific proteins were quantified by densitometric scanning of films and application of NIH Image 1.52 software.
Immunoprecipitations
Native immunoprecipitations of Uso1p-myc from detergent-solubilized lysates were performed as follows. Wild-type (CBY1550) and wild-type with pUSO1-myc (CBY528) cells were grown in selective media at 30°C. Semi-intact yeast cells were prepared as described previously (Cao et al., 1998) and washed with buffer 88, pH 8.0, to enrich for membrane-associated Uso1p-myc and Ypt1p. Approximately 3–4 mg of washed semi-intact cell protein in 0.4 ml was incubated in an ATP-regenerating system with 1 mM GTP at 20°C for 30 min. Purified GDI (3.8 μM final) was added at the start of these incubations where indicated. The semi-intact cell suspension was then solubilized with 1% digitonin at 25°C for 12 min. Solubilized supernatant fractions were isolated after a 2-min spin at 18,000 × g in a tabletop Eppendorf Microfuge and rotated with protein A beads at 4°C for 20 min. Precleared fractions were then incubated with ∼1.5 μg of anti-C-myc antibodies (Covance, Berkeley, CA) and protein A linked to Sepharose beads at 4°C for 1 h. Beads were washed five times with 0.1% digitonin in buffer 88, pH 8.0. Beads were resuspended in 0.04 ml of SDS-PAGE sample buffer, and samples were run on 6.5 and 12.5% SDS-PAGE gels. For Western analysis, proteins were probed with polyclonal antibodies. Native immunoprecipitations of Uso1p-hemagglutinin (HA) from digitonin solubilized lysates were performed as described above by using a strain with endogenously HA-tagged USO1. Immunoprecipitation was carried out using HA.11 antibodies (Covance, Berkeley, CA).
In Vitro Vesicle Budding, Tethering, and Transport Assays
Yeast semi-intact cells were prepared for in vitro tethering and transport assays as described previously (Barlowe, 1997). Transport assays were performed as described previously (Cao et al., 1998), except incubation time was extended to 80 min. The two-stage transport and tethering assays were performed as described previously (Cao and Barlowe, 2000) with vesicles isolated from wild-type CBY901 microsomes, and acceptor membranes were prepared from both CBY901 and CBY903 semi-intact cells. Transport and tethering assays shown were done in parallel with the same vesicle preparation. Two-stage transport and tethering assays were carried out at 23°C for 60 and 30 min, respectively. Data points represent the average of duplicate determinations, and error bars represent the range from this average.
RESULTS
Ypt1p and Uso1p are both required for transport between the ER and Golgi in vivo and in vitro. Using in vitro assays that measure budding, tethering, and transport, we previously reported that Ypt1p and Uso1p are required for tethering of ER-derived vesicles to Golgi membranes. It also was shown that GDI (Sec19p) does not inhibit vesicle budding or fusion, but it specifically inhibits vesicle tethering (Cao et al., 1998). Physiological levels of GDI in cells are thought to participate in the general regulation of the Rab/Ypt GTPase cycle and are essential for cell viability, whereas high concentrations of recombinant GDI have been shown to extract the GDP-bound forms of Rab/Ypt proteins (Araki et al., 1990; Sasaki et al., 1990). In membrane binding assays, GDI extracts Ypt1p and prevents Uso1p binding (Cao et al., 1998). These data guided us to investigate the seemingly interactive roles of Ypt1p and Uso1p. Because GDI binds to all characterized Rab/Ypt proteins in yeast, we first wanted to ascertain that Ypt1p is the Rab specifically required for the membrane association of Uso1p.
Membrane Association of Uso1p Is Decreased in ypt1-3 and ypt1Δ
To determine the influence of Ypt1p mutants on Uso1p membrane association, we used a membrane binding assay as described in Materials and Methods. As shown in Figure 1A, the binding assay was performed at 29°C by using a ypt1-3 temperature-sensitive mutant strain. An immunoblot of soluble and membrane fractions prepared from ypt1-3 and wild-type strains revealed a decrease in membranebound Uso1p in the ypt1-3 mutant to levels below detection. The total amount of Ypt1p in the ypt1-3 mutant strain was reduced by 36% compared with a wild-type strain (Figure 1A), as observed previously (Sögaard et al., 1994). Therefore, the decrease in membrane associated Uso1p could be due to the point mutation in Ypt1p or to the fact that there is a lower amount of mutant Ypt1p in these cells or possibly both factors are involved. In any case, the ypt1-3 mutation has a specific effect on Uso1p and did not seem to significantly affect other Golgi- or vesicle-associated proteins such as Erv41p, the v-SNARE Sec22p, Cog2p, Cog3p, or the TRAPP subunit Bet3p.
Figure 1.
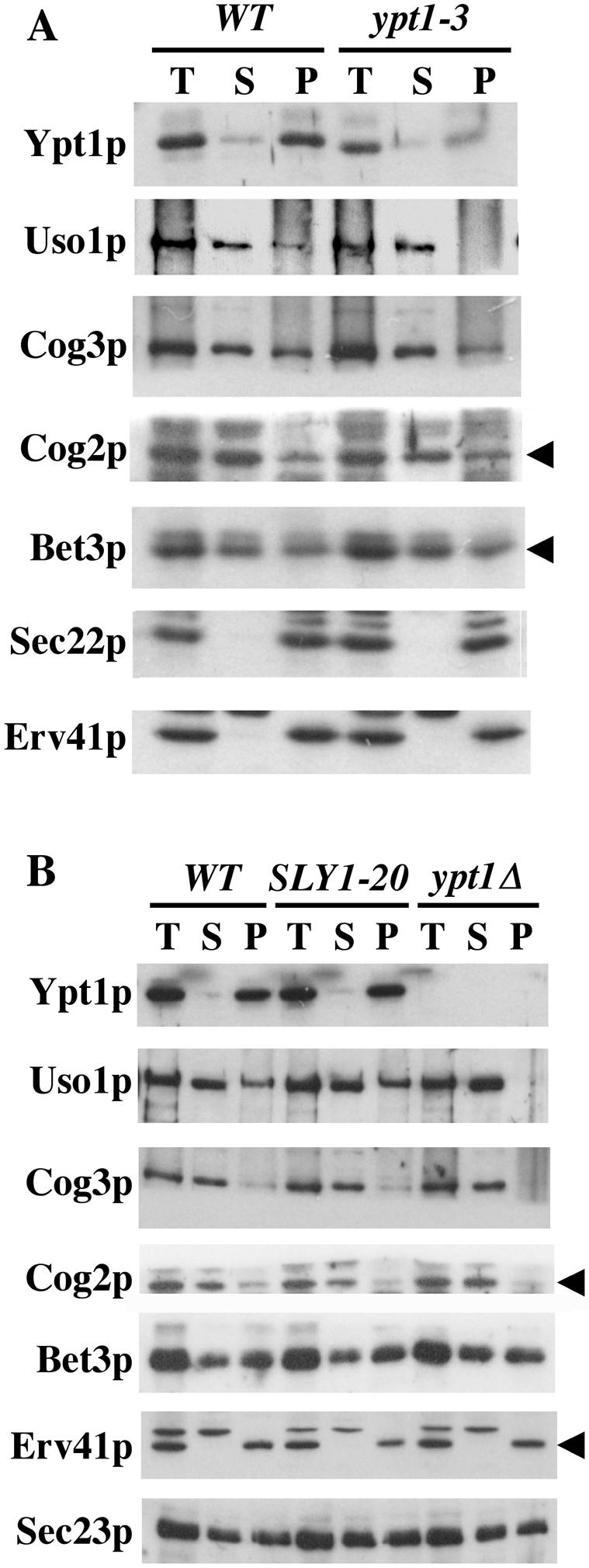
Ypt1p function influences Uso1p membrane association. (A) WT (CBY1550) and ypt1-3 (CBY474) semi-intact cells were incubated at 29°C with ATP/GTP in buffer for 10 min. High-speed supernatant and membrane pellet fractions were isolated by centrifugation at 100,000 × g. The total (T), supernatant (S), and resuspended pellet (P) fractions were diluted into SDS-PAGE sample buffer and analyzed by Western blot. (B) The experiment is similar to that in A, except it uses semi-intact cells from WT (CBY900), wild-type with pSLY1-20 (CBY901), and ypt1Δ/SLY1-20 (CBY903) strains.
In Figure 1B, we used the membrane binding assay to assess Uso1p and other proteins in a ypt1Δ strain expressing SLY1-20 (CBY903). Similar to the ypt1-3 strain, the ypt1Δ cells showed a striking decrease in the level of membrane-associated Uso1p. In wild-type cells, ∼35% of total Uso1p was associated with the pellet fraction, whereas in ypt1Δ/SLY1-20 cells the level of membrane-bound Uso1p ranged from 5% to undetectable. The membrane association of Cog2p and Cog3p also was decreased in the absence of Ypt1p. Cog2p and Cog3p are subunits of a larger COG complex, which has been reported to interact with Ypt1p-GTP (Suvorova et al., 2002) and therefore deletion of YPT1 could directly affect the membrane association of this complex. In replicate experiments, the level of Cog2p and Cog3p that cosedimented with the pellet fraction ranged from 30 to 50% of total in wild-type cells, whereas the level in ypt1Δ/SLY1-20 cells ranged from 10 to 25%. Expression of SLY1-20 alone in wild-type cells did not produce detectable changes in the distribution of proteins involved in ER/Golgi transport that we monitored (Figure 1B, middle). These results indicate that the marked decrease in membrane-bound Uso1p and modest decreases in Cog2p and Cog3p were due to the absence of Ypt1p and not caused by SLY1-20 expression.
Ypt1p Coimmunoprecipitates with Uso1p
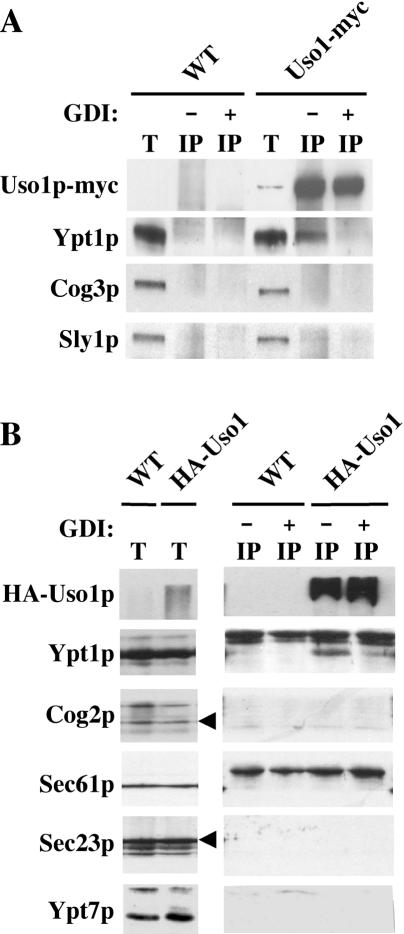
The results in Figure 1 indicated that Uso1p depends on Ypt1p for membrane association. Next, we tested whether Uso1p and Ypt1p were physically associated in solubilized membrane extracts. Native immunoprecipitation experiments were performed with Uso1p-myc expressed from pRS426 in a wild-type strain (CBY528). As shown in Figure 2A, a fraction of Ypt1p (0.2% of total) was recovered from a membrane-enriched digitonin-solubilized Uso1p-myc extract. Other peripherally associated Golgi proteins (i.e., Cog3p and Sly1p) were not detected in this Uso1p immunoprecipitation. Furthermore, the Uso1p–Ypt1p association was inhibited when semi-intact cells were first incubated with GDI. These results indicate that the small fraction of Ypt1p associated with Uso1p was specific. Because this association was detected in a strain with overexpressed Uso1p-myc, we tested whether the Ypt1p–Uso1p association was due to overexpression of Uso1p. Therefore, an endogenously HA-tagged version of Uso1p that was fully functional (Ross-Macdonald et al., 1999) was used in immunoprecipitation experiments as shown in Figure 2B. Precipitating HA-Uso1p under the same conditions as described above, a fraction of Ypt1p (0.6% of total) was again detected in association with Uso1p. This association was specific and also inhibited by GDI. We have attempted to detect a direct and specific interaction between purified Uso1p and Ypt1p. Although Uso1p does bind directly to GST-Ypt1p in a glutathione S-transferase pull-down assay, other Rab GTPases (e.g., Ypt7p) also bind to Uso1p under similar conditions (our unpublished data), suggesting a lack of specificity when purified proteins were used. Mammalian homologues of Uso1p and Ypt1p (i.e., p115 and Rab1) bind directly in vitro (Allan et al., 2000). We speculate that this is true for Uso1p and Ypt1p but that additional membrane factors are required for a specific association.
Figure 2.
Ypt1p coimmunoprecipitates with Uso1p. (A) Semi-intact cells from WT (CBY1550) and WT with 2 μm pUso1p-myc (CBY528) were washed in buffer to remove cytosol. Washed membranes were incubated with or without ATP/GTP and GDI at 20°C for 30 min, followed by solubilization in 1% digitonin at 25°C for 12 min. Solubilized membranes were immunoprecipitated with anti-myc monoclonal antibodies and protein A beads at 4°C for 1 h. Washed beads were resuspended in SDS-PAGE sample buffer and analyzed by Western blot. In this experiment, the total lanes (T) represent 0.6% of the total immunoprecipitated (IP) input. (B) The experiment is similar to that in A, except immunoprecipitation was carried out using semi-intact cells from a strain with endogenously HA-tagged USO1 (CBY1189) and an isogenic wild-type (CBY1187). Anti-HA precipitation was performed using HA monoclonal antibodies. The T lanes represent 2.0% of the total IP input.
Budding, Tethering, and Fusion in ypt1Δ/pSLY1-20
The above-mentioned findings in addition to other lines of evidence indicate that Uso1p and Ypt1p work in concert to facilitate tethering. We next investigated how tethering may be linked to fusion. Tethering is thought to occur before fusion because downstream fusion factors can suppress upstream tethering factors. However, a direct protein-to-protein mechanism between these two steps remains unclear. We postulated that if the gain-of-function SLY1-20 allele obviates the need for Uso1p and Ypt1p function by mass action, then the act of tethering should be bypassed in the presence of the Sly1-20p protein. Therefore, we investigated how cells with SLY1-20 sustain transport to the Golgi in the absence of Ypt1p. Specifically, are the requirements for in vitro budding, vesicle tethering, and fusion the same for ypt1Δ/SLY1-20 as in wild type?
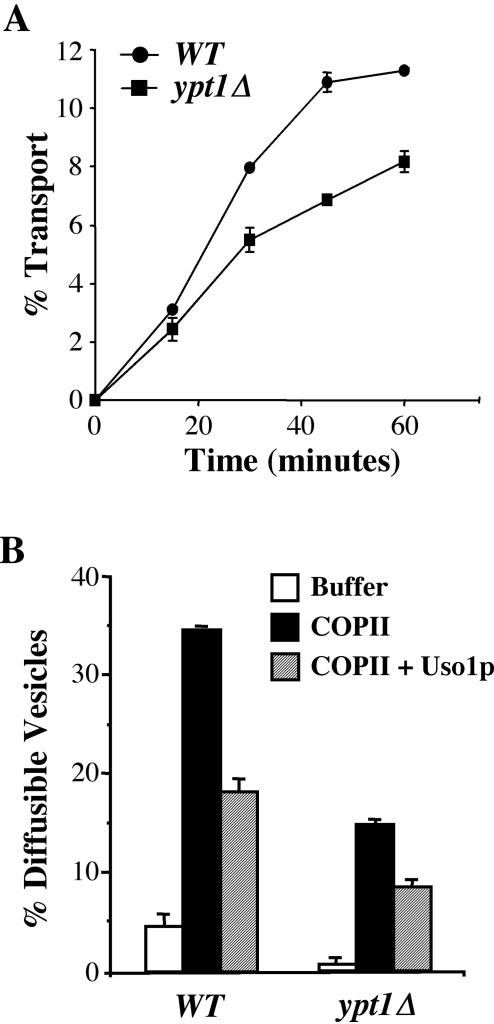
To assay ER/Golgi transport in vitro, semi-intact cells were made from both the WT/SLY1-20 (CBY901) and ypt1Δ/SLY1-20 (CBY903) strains. As shown in Figure 3A, in vitro transport was measured at 15, 30, 45, and 60 min. The results show that purified reconstitution proteins (COPII, Uso1p, and LMA1) support transport in the ypt1Δ strain but at a reduced rate compared with wild type. In vitro vesicle budding and tethering were measured in both of these strains (Figure 3B). Purified COPII proteins (Sar1p, Sec23p/Sec24p, and Sec13p/Sec31p) stimulated vesicle budding from ypt1Δ membranes but at a lower efficiency than wild type. Surprisingly, addition of Uso1p produced comparable tethering in both strains. It should be noted that the amount of translocated [35S]gp-α-factor was 53% lower in the ypt1Δ strain compared with the wild type (our unpublished data), suggesting the ER membranes were compromised and that this may also influence the efficiency of vesicle budding. However, it seems clear that this ypt1Δ strain can operate by using a COPII- and Uso1p-dependent transport process.
Figure 3.
In vitro transport, budding, and tethering in the absence of Ypt1p. (A) Transport kinetics was measured in WT (CBY901) and ypt1Δ/SLY1-20 (CBY903) semi-intact cells in the presence of purified transport factors (COPII, Uso1p, and LMA1) at 0, 15, 30, 45, and 60 min. The percentage of transport represents the amount of [35S]gp-α-factor that has been modified by the addition of Golgi-specific α1,6-mannose residues. (B) Budding and tethering in WT (CBY901) and ypt1Δ/SLY1-20 (CBY903) semi-intact cells was assayed with COPII and Uso1p proteins at 23°C for 30 min. The percentage of diffusible vesicles represents the amount of [35S]gp-α-factor released into a medium-speed supernatant fraction divided by the total amount of [35S]gp-α-factor contained in the reaction.
GDI Inhibits Overall Transport in ypt1Δ
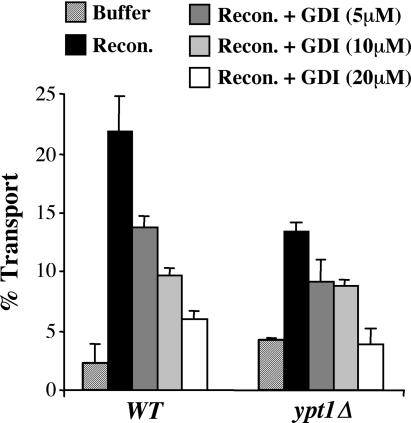
In a previous study, Uso1p-dependent vesicle tethering was inhibited by GDI (Cao et al., 1998). Given our results in Figure 3 indicating that the ypt1Δ/SLY1-20 strain maintained an Uso1p-dependent tethering step, we next explored whether this tethering was sensitive to GDI. As shown in Figure 4, in vitro transport was measured in both strains with increasing amounts of GDI. Transport in the ypt1Δ/SLY1-20 strain was again clearly stimulated by adding COPII, Uso1p, and LMA1. Surprisingly, GDI inhibited the ypt1Δ strain with similar efficacy as in the wild type. The results from these experiments raised two questions: How does Uso1p contribute to transport stimulation in the ypt1Δ strain, and how does GDI inhibit transport in the absence of the only known Rab protein to function in yeast ER/Golgi transport?
Figure 4.
GDI inhibits transport in the absence of Ypt1p. Transport in WT (CBY901) and ypt1Δ/SLY1-20 (CBY903) semi-intact cells in the presence of purified transport factors (COPII, Uso1p, and LMA1) and increasing amounts of GDI (micromolar) at 23°C for 70 min. The percentage of transport represents the amount of [35S]gp-α-factor that has been modified by the addition of Golgi-specific α-1,6-mannose residues.
Uso1p Is Required in the Absence of Ypt1p
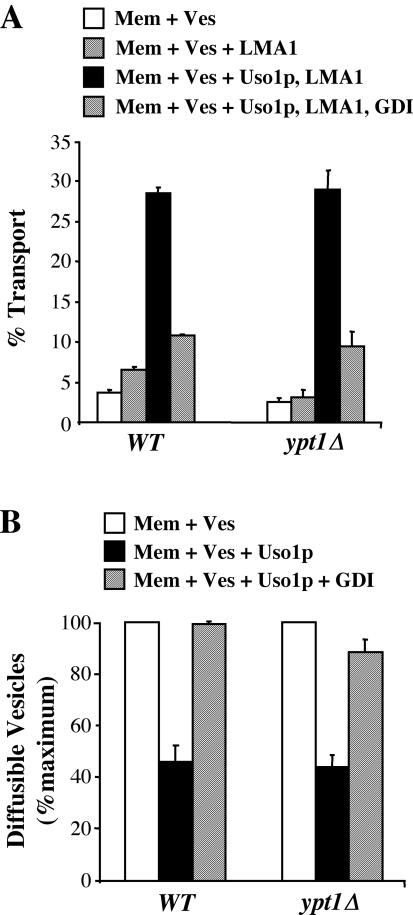
To address these questions regarding transport in ypt1Δ/SLY1-20 cells, we carried out in vitro assays by using a two-stage reaction. Thus, we could assay the integrity of the ypt1Δ membrane as an acceptor by using wild-type vesicles. Figure 5A shows the results from in vitro transport by using a two-stage reaction. Wild-type COPII vesicles were isolated from microsomes and added to a second stage fusion reaction containing wild-type/SLY1-20 membranes or ypt1Δ/SLY1-20 membranes under various conditions. With no addition, a very low level of background transport was observed for both strains. With addition of the fusion factor LMA1, transport was only slightly above background. On addition of Uso1p and LMA1, transport was significantly stimulated in both the wild-type and ypt1Δ strains. Again, transport remained sensitive to GDI in both strains. In Figure 5B, in vitro tethering also was measured in a two-stage reaction similar to that in Figure 5A. Wild-type vesicles were isolated and incubated in a tethering reaction with wild-type or ypt1Δ membranes. As in the transport experiments, vesicles tethered to both membranes upon addition of Uso1p. These results together confirm that ypt1Δ/SLY1-20 cells maintain a requirement for Uso1p to tether vesicles, and this Uso1p-dependent tethering is sensitive to GDI.
Figure 5.
Transport and tethering in the absence of Ypt1p requires Uso1p and is sensitive to GDI. (A) COPII vesicles were isolated in vitro from wild-type membranes at 20°C and incubated in a second transport stage with WT (CBY901) or ypt1Δ/SLY1-20 (CBY903) acceptor membranes with buffer alone, indicated transport factors and transport factors plus GDI at 23°C for 60 min. The percentage of transport represents the amount of [35S]gp-α-factor that has been modified by the addition of Golgi-specific α-1,6-mannose residues. (B) COPII vesicles were isolated as described in A and were incubated in a second stage tethering reaction with WT (CBY901) or ypt1Δ/SLY1-20 (CBY903) acceptor membranes in the presence of buffer, Uso1p, or Uso1p plus GDI. The percentage of diffusible vesicles represents the amount of [35S]gp-α-factor released into a medium-speed supernatant fraction divided by the total amount of [35S]gp-α-factor contained in the reaction.
SLY1-20 Does Not Suppress ypt1Δ uso1Δ
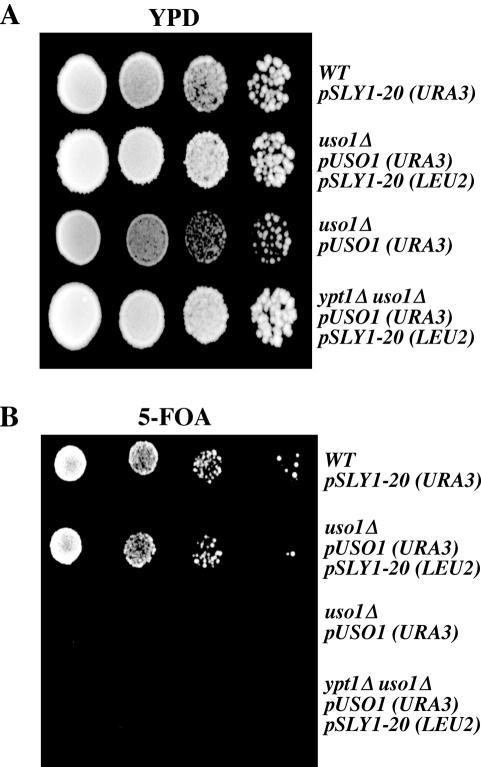
A tethering requirement for Uso1p in the ypt1Δ/SLY1-20 cells in vitro, indicates that tethering is not entirely by-passed in the absence of YPT1. Previous reports demonstrated that the SLY1-20 allele suppresses either a ypt1Δ or an uso1Δ mutation. However, if Uso1p-dependent tethering occurs in the ypt1Δ/SLY1-20 strain, then one would predict that SLY1-20 could not suppress the loss of both activities in a ypt1Δ uso1Δ double mutant. To test this, we constructed a haploid yeast strain containing SLY1-20 and the ypt1Δ uso1Δ deletions in which viability is maintained by expression of USO1 on a URA3-linked plasmid. In this strain, loss of the USO1-URA3 plasmid, indicative of Uso1p and Ypt1p bypass, can be scored by growth on media containing 5-FOA. As seen in Figure 6, no growth of the SLY1-20 ypt1Δ uso1Δ strain was observed on 5-FOA plates. As controls, a SLY1-20 uso1Δ strain can lose the USO1-URA3 plasmid and grow on 5-FOA plates, whereas the uso1Δ strain in the absence of the SLY1-20 allele cannot grow under this condition. This genetic test supports the idea that the function of both Uso1p and Ypt1p cannot be bypassed by SLY1-20.
Figure 6.
SLY1-20 does not suppress a double ypt1Δ uso1Δ strain. Serial dilutions of WT/pSLY1-20 (CBY901), uso1Δ/pUSO1 pSLY1-20 (CBY1381), uso1Δ/pUSO1 (CBY1297), and ypt1Δ uso1Δ/pUSO1 pSLY1-20 (CBY1362) strains were spotted on YPD (A) or minimal 5-FOA (B) plates and incubated at 24°C.
SLY1-20 Suppresses ypt1Δypt31Δ, ypt1Δypt32Δ, but Not ypt1Δypt6Δ
Our data showing GDI sensitivity in the absence of Ypt1p led us to explore the possibility that another Rab fulfills the GTPase function of Ypt1p in the ypt1Δ strain. Although no other Rab is known to function in anterograde transport to the cis-Golgi, we postulated that a nearby Rab could substitute. Potential candidates are the Rab GTPases Ypt31p/32p, which function in intra-Golgi transport and are required for exit from the trans-Golgi (Benli et al., 1996; Jedd et al., 1997). Individually, Ypt31p and Ypt32p are nonessential; however, yeast strains lacking both proteins are inviable. TRAPP I has been reported to catalyze nucleotide exchange activity for both Ypt1p and Ypt31p, and the Ypt32p exchange factor is a putative effector of Ypt1p (Jones et al., 2000; Wang et al., 2000; Wang and Ferro-Novick, 2002). An additional candidate is Ypt6p, a nonessential Rab that acts in endosome-to-Golgi, retrograde intra-Golgi and possibly Golgi-to-ER transport (Luo and Gallwitz, 2003). Furthermore, ypt6Δ is suppressed by both SLY1-20 and YPT1. Given the reported relationships of these Rabs with Ypt1p, we set up a genetic test to determine whether YPT31, YPT32, or YPT6 may substitute for YPT1.
We hypothesized that deletion of the putative substitute Rab in a ypt1Δ/SLY1-20 strain would be a lethal event. Thus, we made double deletions of YPT1 and each of the individual Rabs in a SLY1-20 strain that also contained the YPT1 gene on a URA3-linked plasmid. On 5-FOA–containing media, these strains cannot depend on the YPT1-URA3 plasmid for growth, and therefore viability on this media would indicate that the double deletion is suppressed by SLY1-20. Conversely, inviability on 5-FOA would indicate that SLY1-20 cannot suppress the double deletion and suggest a redundant relationship.
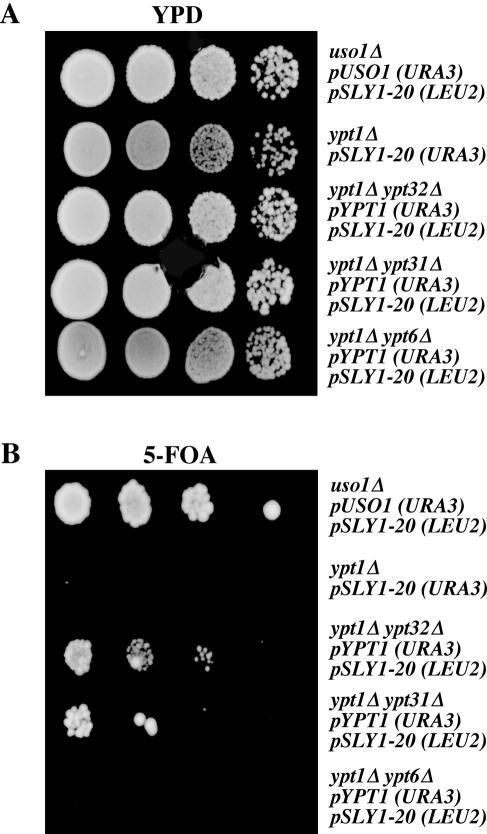
We constructed the necessary strains by using the viable single deletions in ypt31Δ, ypt32Δ, and ypt6Δ (Winzeler et al., 1999). As shown in Figure 7, A and B, each of these ypt1Δ double deletions and controls were plated in parallel on YPD and 5-FOA plates and grown at 24°C. This test revealed that although SLY1-20 can suppress ypt1Δ, it cannot suppress the ypt1Δypt6Δ mutation. However, SLY1-20 can suppress ypt1Δypt31Δ and ypt1Δypt32Δ. These results indicate that in the absence of YPT1, YPT6 becomes essential and suggests Ypt6p may substitute for Ypt1p in its absence.
Figure 7.
SLY1-20 does not suppress ypt1Δ ypt6Δ. Serial dilutions of uso1Δ/pUSO1 pSLY1-20 (CBY1381), ypt1Δ/pSLY1-20 (CBY903), ypt1Δ ypt31Δ/pYPT1 pSLY1-20 (CBY1548), ypt1Δ ypt32Δ/pYPT1 pSLY1-20 (CBY1399), and ypt1Δ ypt6Δ/pYPT1 pSLY1-20 (CBY1396) were spotted on YPD (A) or minimal 5-FOA (B) plates and incubated at 24°C.
YPT6 Is Overexpressed in ypt1Δ/pSLY1-20 Cells
If Ypt6p is fulfilling the Rab function in the absence of Ypt1p, we hypothesized that this could have an affect on its level of expression. A Western blot of total cell extracts from wild-type, wild-type/SLY1-20, ypt1Δ/SLY1-20, ypt32Δ, and ypt6Δ strains is shown in Figure 8. In these strains, the expression levels Ypt7p (a vacuolar rab protein) and Erv41p were not detectably different. In addition, no changes were observed in the levels of Ypt1p expression in this set of strains, with the exception of the ypt1Δ cells, which lack the encoding gene. Interestingly, the level of Ypt6p in ypt1Δ cells was twofold higher than in wild-type cells or in the wild type expressing SLY1-20. The increase in Ypt6p could facilitate the substitution of Ypt6p in the absence of Ypt1p. We also observed a 0.4-fold reduction in the level of Ypt6p in ypt32Δ cells. This result suggests that Ypt32p influences Ypt6p, although further experimentation will be required to explore this relationship. The protein species migrating below Ypt6p also was recognized by the anti-Ypt6p antibody and was not present in the ypt6Δ strain. We speculate that this form of Ypt6p was either an unprenlyated species or a degradation product derived from the mature protein. Regardless, increased expression of Ypt6p in the ypt1Δ/SLY1-20 cells supports the hypothesis that Ypt6p can substitute for Ypt1p during SLY1-20 suppression.
Figure 8.
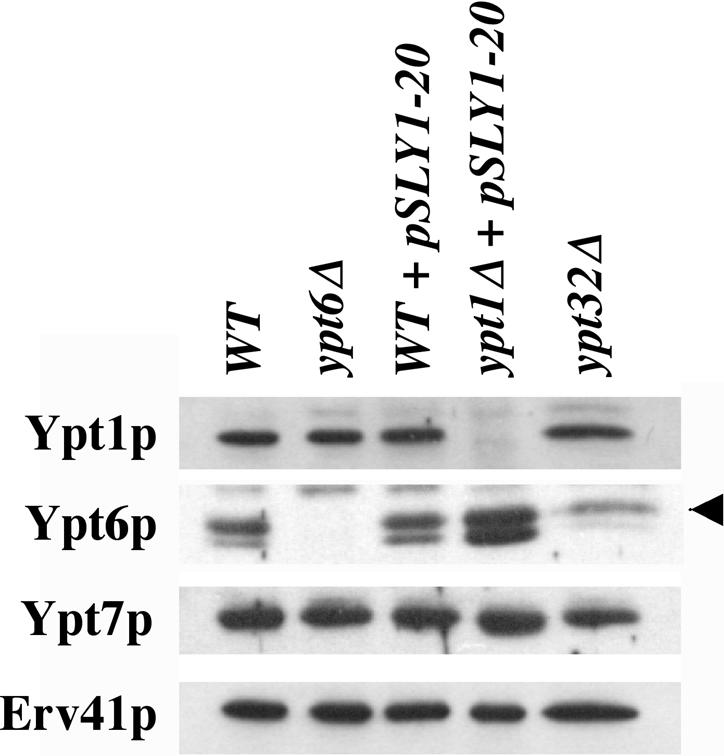
Ypt6p is up-regulated in ypt1Δ/SLY1-20 cells. Equal protein amounts of semi-intact cells from WT (CBY740), ypt6Δ (CBY1344), WT/SLY1-20 (CBY901), ypt1Δ/SLY1-20 (CBY903), and ypt32Δ (CBY1343) strains were washed once with buffer 88 and resuspended in equal amounts of buffer 88 and SDS PAGE sample buffer. For Western analysis, protein samples were separated on 12.5% gels and blotted with anti-Ypt1p, anti-Ypt6p, anti-Erv41p, and anti-Ypt7p polyclonal antibodies.
Characterization of the cog2Δ/SLY1-20 Strain
Cog2p and Cog3p are subunits of the COG complex and have been shown to interact with Ypt1p in vitro (Suvorova et al., 2002; Ungar et al., 2002). Because mutations in COG2 [SEC35] and COG3 [SEC34] are suppressed by SLY1-20 (VanRheenen et al., 1998), this provided an opportunity to investigate the function of the COG complex in ER/Golgi transport. First, the influence of cog2Δ/pSLY1-20 on the distribution of ER/Golgi transport factors was assayed. We used a membrane fractionation procedure as in Figure 1. The assay in Figure 9 was performed using isogenic wild-type (CBY1020) and cog2Δ/SLY1-20 (CBY1021) semi-intact cells. Immunoblots of the fractionation show that loss of Cog2p does not affect the distribution of any of the known tethering factors (Ypt1p, Uso1p, or the TRAPP subunit Bet3p) or several other proteins involved in ER/Golgi transport (Erv41p, Sec23p, and ε-COPI). However, the expression level of Cog3p in the cog2Δ/SLY1-20 cells was reduced by 27% compared with wild-type cells, and the amount of membrane associated Cog3p was reduced by 40%.
Figure 9.
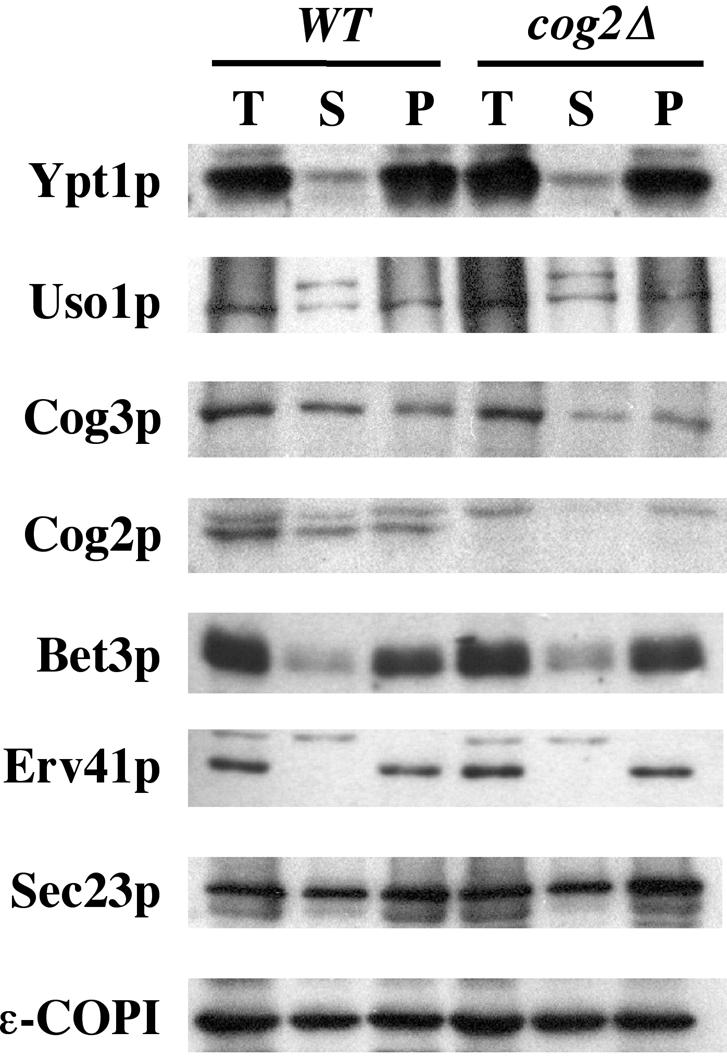
Loss of Cog2p does not influence the association of known Golgi-tethering factors. WT/SLY1-20 (CBY1020) and cog2Δ/SLY1-20 (CBY1021) semi-intact cells were incubated at 29°C with ATP/GTP in buffer 88 for 10 min. High-speed supernatant and -membrane pellet fractions were isolated by centrifugation at 100,000 × g. The total, supernatant, and resuspended pellet fractions were diluted in SDS-PAGE sample buffer and analyzed by Western blot.
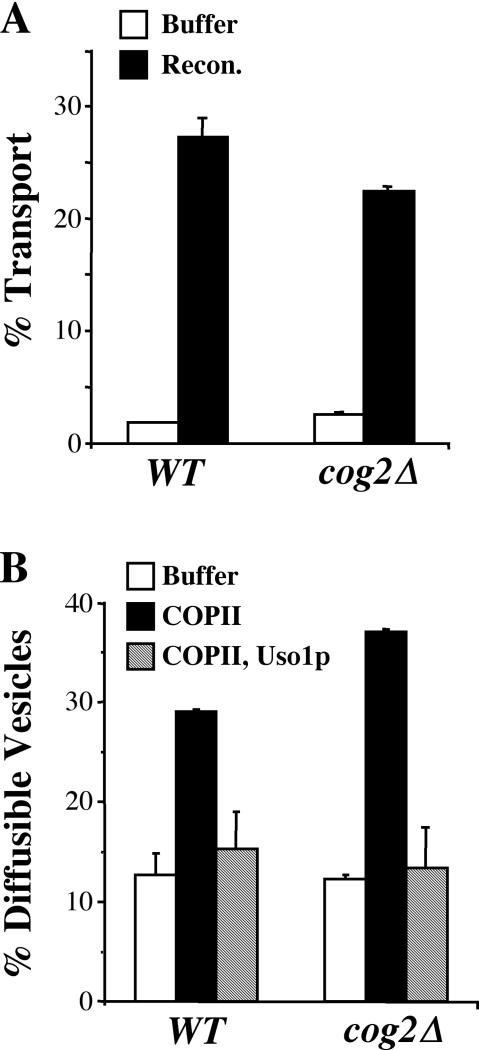
Next, we performed in vitro transport and budding assays comparing the wild-type and cog2Δ/SLY1-20 semi-intact cells. Surprisingly, purified reconstitution proteins stimulated transport in the cog2Δ strain to a level comparable with that of wild-type (Figure 10A). Furthermore, addition of COPII stimulated budding and addition of Uso1p tethered vesicles at wild-type levels (Figure 10B). Together with the fractionation data, we note that cog2Δ/SLY1-20 cells display properties that were quite similar to wild-type strains when the ER/Golgi transport pathway was monitored.
Figure 10.
Transport, budding, and tethering in the absence of Cog2p (A) Transport in semi-intact cells from WT/SLY1-20 (CBY1020) and cog2Δ/SLY1-20 (CBY1021) was assayed with buffer 88 alone or with purified reconstitution factors (COPII, Uso1p, and LMA1) at 23°C for 70 min. The percentage of transport represents the amount of [35S]gp-α-factor that has been modified by the addition of Golgi-specific α1,6-mannose residues. (B) Budding and tethering in the same semi-intact cells was assayed with COPII and Uso1p proteins at 23°C for 30 min. The percentage of diffusible vesicles represents the amount of [35S]gp-α-factor released into a medium-speed supernatant fraction divided by the total amount of [35S]gp-α-factor contained in the reaction.
DISCUSSION
SLY1-20 suppression of ypt1Δ and of uso1Δ mutants had suggested this gain-of-function allele produces an activated conformation of Sly1p that promotes Sed5p-dependent membrane fusion (Dascher et al., 1991; Pfeffer, 1996; Sapperstein et al., 1996). Experiments showing Uso1p and Ypt1p function in vesicle tethering upstream of Sly1p and Sed5p led to the proposal that tethering activates Sly1p and that Sly1-20p may mimic the activated state to bypass tethering (Sapperstein et al., 1996; Cao et al., 1998). Recent structural studies have revealed that the Sly1-20p point mutation resides in a surface helix distant from the Sed5p interaction domain (Bracher and Weissenhorn, 2002), consistent with a potential regulatory influence. These authors reason that this surface helix may act as a Rab-regulated lid to control Sly1p activity and that Sly1-20p may have a permanently opened lid. In the current report, we tested the hypothesis that SLY1-20 bypasses the Uso1p/Ypt1p-dependent tethering step in anterograde transport from the ER to the Golgi complex.
First, we demonstrated that membrane association of Uso1p was dependent upon Ypt1p and that this association was reduced in ypt1Δ cells suppressed by SLY1-20. However, addition of purified Uso1p continued to tether ER-derived vesicles and stimulate transport in cell free assays with ypt1Δ/SLY1-20 membranes. Furthermore, we observed that the Uso1p-dependent tethering step remained sensitive to GDI, indicating that a Rab requirement was maintained during SLY1-20 suppression. The inability of SLY1-20 to suppress the double uso1Δ ypt1Δ mutant (Figure 6) provides further evidence that tethering cannot be entirely bypassed. Instead, these results imply that SLY1-20 permits substitution by a related rab protein.
The notion of a substitute Rab or Rab cross-talk is not unfounded. Ypt31p and Ypt32p can substitute for each other (Benli et al., 1996), and although they function together in intra-Golgi transport, some of their interactions are distinct. The TRAPP I/II complex is the guanine nucleotide exchange factor (GEF) for Ypt31p and Ypt1p (Jones et al., 2000; Wang et al., 2000), but TRAPP I/II is not the exchange factor for Ypt32p. In fact, the exchange factor for Ypt32p is reported to be an effector of Ypt1p (Wang and Ferro-Novick, 2002). This type of Rab “cascade” also has been reported between Ypt32p and Sec4p (Ortiz et al., 2002) where Ypt32p aids in the recruitment of the GEF, Sec2p, for its substrate Sec4p. These established examples compelled us to genetically test both YPT31 and YPT32 for a role in the ypt1Δ mutant. We also included YPT6 in our test, because this Rab has been assigned additional roles in retrograde transport, including endosome to Golgi, intra-Golgi, and Golgi to ER (Luo and Gallwitz, 2003). Interestingly, ypt6 mutants are suppressed by SLY1-20 or by overexpression of YPT1 (Li and Warner, 1998; Luo and Gallwitz, 2003). Furthermore, YPT6 overexpression studies suggest that Ypt6p interacts with shared Ypt1p-interacting proteins (Li and Warner, 1998).
We show that a ypt6Δypt1Δ strain cannot be suppressed by SLY1-20, whereas ypt31Δypt1Δ and ypt32Δypt1Δ are both suppressed. This finding suggests that YPT6 provides a functionally redundant activity with YPT1. If Ypt6p does fulfill the role of Ypt1p in ypt1Δ/SLY1-20 cells, the presumed interactions between Ypt6p and Uso1p may be weaker than between Uso1p and Ypt1p. This may explain why the level of membrane-associated Uso1p is decreased in ypt1Δ/SLY1-20 cells (Figure 1). Furthermore, a weakened set of interactions may explain the slower growth rate, the temperature sensitivity, and the lowered transport efficiency in ypt1Δ/SLY1-20 strains. Nonetheless, when this weaker substitute is coupled with SLY1-20, the level of tethering and fusion seems to be sufficient.
Based on the collective evidence, we envisage that SLY1-20 renders the Sly1–20p/Sed5p complex in an active and unregulated state. We speculate that proper Ypt1p/Uso1p-mediated tethering in wild-type cells relays a signal to Sly1p/Sed5p, causing a conformational change that promotes trans-SNARE pairing and membrane fusion. For anterograde transport to the Golgi in wild-type membranes, Ypt1p is apparently the exclusive Rab that can confer this signal to Sly1p/Sed5p. However under the SLY1-20 condition, a substitute Rab can interact with Uso1p to tether vesicles, but no signal need be transmitted to the continually active Sly1-20p. Although this mechanism remains speculative, our results do show that Rab protein activity can be substituted but not circumvented in the presence of Sly1-20p and highlight the importance of Rab proteins and tethering in membrane fusion.
Further genetic analyses have indicated that SLY1-20 more generally suppresses mutations that act upstream of SED5 or UFE1 in other membrane fusion reactions (Li and Warner, 1998; Reilly et al., 2001; Luo and Gallwitz, 2003). Among this group are the cog2 [sec35] and cog3 [sec34] mutants, which are suppressed by SLY1-20 (VanRheenen et al., 1998, 1999) and were originally identified as sec mutants that accumulated organelles and vesicles in the early secretory pathway (Wuestehube et al., 1996). Our in vitro experiments suggested that Cog2p and Cog3p were required for anterograde transport to the Golgi (VanRheenen et al., 1998, 1999). More recent studies show that Cog2p and Cog3p are subunits of an octameric COG complex, which is thought to participate in retrograde and anterograde Golgi transport (Whyte and Munro, 2001; Ram et al., 2002; Suvorova et al., 2002; Ungar et al., 2002). When we examined a cog2Δ/SLY1-20 strain to determine the consequences of cog2Δ on ER/Golgi transport, no changes were detected compared with wild type. These findings suggest that a functionally related tethering complex may substitute for the COG complex in the presence of SLY1-20. The TRAPP I/II complexes are candidates for providing such an activity. TRAPP I acts in anterograde transport to the Golgi complex, whereas TRAPP II is thought to function in intra-Golgi stages (Sacher et al., 2001). Both TRAPP I and TRAPP II have been shown to stimulate guanine nucleotide exchange on Ypt1p (Jones et al., 2000; Wang et al., 2000). The GARP/VFT tethering complex, thought to act at later Golgi compartments (Siniossoglou and Pelham, 2001; Conibear et al., 2003), also could substitute for the COG complex in the presence of SLY1-20. However it also is possible that the COG complex does not act directly in anterograde transport to the Golgi. In this scenario, the cog2 and cog3 mutations may disrupt retrograde-Golgi transport and have indirect consequences on early Golgi compartments. The SLY1-20 allele may then act to restore retrograde pathways and alleviate transport defects to early Golgi compartments.
Our findings concerning yeast Sly1p may have broader implications for the related SM family of proteins. Although the manner in which SM proteins bind to and regulate their syntaxin-like SNARE partners differs (Gallwitz and Jahn, 2003), several lines of evidence suggest these proteins serve a central role in the coordinating Rab and SNARE protein activities (Rizo and Südhof, 2002). In this regard, the study of yeast Sly1p suggests this role is as a regulatory checkpoint that signals to the SNARE machinery when a correct tethering event has occurred. For Sly1-20p, incorrect tethering is permitted apparently without a detrimental result. However, other SM-imposed checkpoints may be critical to maintain cellular organization. Further experimentation to determine the mechanism by which Sly1p coordinates Rab and SNARE protein activity during intracellular membrane fusion remains critical.
Acknowledgments
We thank Dieter Gallwitz for providing anti-Ypt6p antibodies and Christine Bentivoglio for help with this study. This work was supported by the Nation Institutes of Health.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–08–0725) on February 2, 2005.
References
- Allan, B. B., Moyer, B. D., and Balch, W. E. (2000). Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444–448. [DOI] [PubMed] [Google Scholar]
- Araki, S., Kikuchi, A., Hata, Y., Isomura, M., and Takai, Y. (1990). Regulation of reversible binding of smg p25A, a ras p21-like GTP binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J. Biol. Chem. 265, 13007–13015. [PubMed] [Google Scholar]
- Baker, D., Hicke, L., Rexach, M., Schleyer, M., and Schekman, R. (1988). Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell 54, 335–344. [DOI] [PubMed] [Google Scholar]
- Barlowe, C. (1997). Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol. 139, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, D., Hamamoto, S., Salama, N., Rexach, M., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the ER. Cell 77, 895–907. [DOI] [PubMed] [Google Scholar]
- Benli, M., Döring, F., Robinson, D. G., Yang, X., and Gallwitz, D. (1996). Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 15, 6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Bracher, A., and Weissenhorn, W. (2002). Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J. 21, 6114–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., Ballew, N., and Barlowe, C. (1998). Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 17, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., and Barlowe, C. (2000). Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J. Cell Biol. 149, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear, E., Cleck, J. N., and Stevens, T. H. (2003). Vps51p mediates the association of the GARP (Vps52/53/54) complex with the late Golgi t-SNARE Tlg1p. Mol. Biol. Cell 14, 1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher, C., Ossig, R., Gallwitz, D., and Schmitt, H. D. (1991). Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS Superfamily. Mol. Cell. Biol. 11, 872–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz, D., and Jahn, R. (2003). The riddle of the Sec1/Munc-18 proteins - new twists added to their interactions with SNAREs. Trends Biochem. Sci. 28, 113–116. [DOI] [PubMed] [Google Scholar]
- Garrett, M. D., Zahner, J. E., Cheney, C. M., and Novick, P. J. (1994). GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 13, 1718–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G. R. (1991). Guide to yeast genetics and molecular biology. Methods Enzymol. 194, 309–311. [PubMed] [Google Scholar]
- Haas, A., Scheglmann, D., Lazar, T., Gallwitz, D., and Wickner, W. (1995). The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 14, 5258–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd, G., Mulholland, J., and Segev, N. (1997). Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 137, 563–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S., Newman, C., Liu, F., and Segev, N. (2000). The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol. Biol. Cell 11, 4403–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo, Y., Noda, Y., Adachi, H., and Yoda, K. (2002). Binding of Sly1 to Sed5 enhances formation of the yeast early Golgi SNARE complex. J. Cell Sci. 115, 3683–3691. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Li, B., and Warner, J. R. (1998). Genetic interaction between YPT6 and YPT1 in Saccharomyces cerevisiae. Yeast 14, 915–922. [DOI] [PubMed] [Google Scholar]
- Luo, Z., and Gallwitz, D. (2003). Biochemical and genetic evidence for the involvement of yeast Ypt6-GTPase in protein retrieval to different Golgi compartments. J. Biol. Chem. 278, 791–799. [DOI] [PubMed] [Google Scholar]
- Ortiz, D., Medkova, M., Walch-Solimena, C., and Novick, P. (2002). Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossig, R., Laufer, W., Schmitt, H. D., and Gallwitz, D. (1995). Functionality and specific membrane localization of transport GTPases carrying C-terminal membrane anchors of synaptobrevin-like proteins. EMBO J. 15, 3645–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte, S., Belden, W. J., Heidtman, M., Liu, J., Jensen, O. N., and Barlowe, C. (2001). Erv41p and Erv46p: new components of COPII vesicles involved in transport between the ER and Golgi complex. J. Cell Biol. 152, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, R., and Gallwitz, D. (2002). Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J. Cell Biol. 157, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S. R. (2001). Vesicle tethering factors united. Mol. Cell 8, 729–730. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. R. (1996). Transport vesicle docking: SNAREs and Associates. Annu. Rev. Cell Dev. Biol. 12, 441–461. [DOI] [PubMed] [Google Scholar]
- Ram, R. J., Li, B., and Kaiser, C. A. (2002). Identification of Sec36p, Sec37p, and Sec38p: components of yeast complex that contains Sec34p and Sec35p. Mol. Biol. Cell 13, 1484–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly, B. A., Kraynack, B. A., VanRheenen, S. M., and Waters, M. G. (2001). Golgi-to-endoplasmic reticulum (ER) retrograde traffic in yeast requires Dsl1p, a component of the ER target site that interacts with a COPI coat subunit. Mol. Biol. Cell 12, 3783–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo, J., and Südhof, T. C. (2002). Snares and Munc18 in synaptic vesicle fusion. Nat. Rev. Neurosci. 8, 641–653. [DOI] [PubMed] [Google Scholar]
- Ross-Macdonald, P., et al. (1999). Large-scale analysis of the yeast genome by transposon tagging and gene disruption. Nature 402, 413–418. [DOI] [PubMed] [Google Scholar]
- Sacher, M., Barrowman, J., Wang, W., Horecka, J., Zhang, Y., Pypaert, M., and Ferro-Novick, S. (2001). TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol. Cell 7, 433–442. [DOI] [PubMed] [Google Scholar]
- Sapperstein, S. K. Lupashin, V. V., Schmitt, H. D., and Water, M. G. (1996). Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell Biol. 132, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, T., Kikuchi, A., Araki, S., Hata, Y., Isomura, M., Kuroda, S., and Takai, Y. (1990). Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J. Biol. Chem. 265, 2333–2337. [PubMed] [Google Scholar]
- Sato, T. K., Rehling, P., Peterson, M. R., and Emr, S. D. (2000). Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell 6, 661–671. [DOI] [PubMed] [Google Scholar]
- Schekman, R., and Orci, L. (1996). Coat proteins and vesicle budding. Science 271, 1526–1533. [DOI] [PubMed] [Google Scholar]
- Seals, D., Eitzen, G., Margolis, N., Wickner, W., and Price, A. (2000). A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short, B., and Barr, F. (2002). Membrane traffic: exocyst III–makes a family. Curr. Biol. 12, R18–R20. [DOI] [PubMed] [Google Scholar]
- Siniossoglou, S., and Pelham, H. R. (2001). An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 20, 5991–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sögaard, M., Tani, K., Ye, R. R., Geromanos, S., Tempst, P., Kirchhausen, T., Rothman, J. E., and Sollner, T. (1994). A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell 78, 927–948. [DOI] [PubMed] [Google Scholar]
- Suvorova, E. S., Duden, R., and Lupashin, V. V. (2002). The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J. Cell Biol. 157, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush, D. R., Maurice, T., Roth, D., and Novick, P. (1996). The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Towbin, H., Straehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada, M., Will, E., and Gallwitz, D. (1999). Structural and functional analysis of a novel coiled-coil protein involved in Ypt6 GTPase-regulated protein transport in yeast. Mol. Biol. Cell 10, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar, D., Toshihiko, O., Brittle, E. E., Vasile, E. Lupashin, V. V., Chatterton, J. E., Heuser, J. E., Krieger, M., and Waters, M. G. (2002). Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function. J. Cell Biol. 157, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen, S. M., Cao, X., Lupashin, V. V., Barlowe, C., and Waters, M. G. (1998). Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking. J. Cell Biol. 141, 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen, S. M., Cao, X., Sapperstein, S. K., Chiang, E. C., Lupashin, V. V., Barlowe, C., and Waters, M. G. (1999). Sec34p, a protein required for vesicle tethering to the Yeast Golgi Apparatus, is in a complex with Sec35p. J. Cell Biol. 147, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., and Ferro-Novick, S. (2002). A Ypt32p exchange factor is a putative effector of Ypt1p. Mol. Biol. Cell 13, 3336–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Sacher, M., and Ferro-Novick, S. (2000). TRAPP stimulates Guanine Nucleotide exchange on Ypt1p. J. Cell Biol. 151, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte, J.R.C., and Munro, S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 113, 3473–3484. [DOI] [PubMed] [Google Scholar]
- Whyte, J.R.C., and Munro, S. (2001). The Sec34/35 Golgi transport complex is related to the Exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell 1, 527–537. [DOI] [PubMed] [Google Scholar]
- Williams, A. L., Ehm, S., Jacobson, N. C. Xu, D., and Hay, J. C. (2004). rsly1 binding to syntaxin 5 is required for endoplasmic reticulum-to-Golgi transport but does not promote SNARE motif accessibility. Mol. Biol. Cell 15, 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler, E. A., et al. (1999). Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906. [DOI] [PubMed] [Google Scholar]
- Wuestehube, L. J., Duden, R., Eun, A., Hamamoto, S., Korn, P., Ram, R., and Schekman, R. (1996). New mutants of Saccharomyces cerevisiae affected in the transport of proteins from the endoplasmic reticulum to the Golgi complex. Genetics 142, 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, A., Mayer, A., Muller, E., and Wickner, W. (1997). A heterodimer of thioredoxin and IB2 acts with Sec18p (NSF) to promote yeast vacuole inheritance. J. Cell Biol. 136, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]