Abstract
Besides its function as a cell cycle regulator, cyclin D1 interacts with transcription factors to regulate gene activation. In this study, we show that cyclin D1 is recruited to the p21waf1 promoter by a STAT3-NcoA complex. The association of cyclin D1 with DNA prevented the recruitment of the CBP histone acetylase and RNA polymerase II, leading to an inhibition of the p21waf1 gene. Confirming the transcriptional function of the protein, the expression of the p21waf1 gene was enhanced in cyclin D1–/– fibroblasts or upon siRNA-mediated down-regulation of the cyclin. Moreover, the STAT3-mediated activation of p21waf1 was also inhibited in breast cancer cells containing elevated levels of cyclin D1. Altogether, these results suggest that the transcriptional activities of cyclin D1 might play an important role in the regulation of cell-cycle regulatory genes and that these functions are probably involved in cell transformation.
INTRODUCTION
After mitogen stimulation of quiescent cells, genes encoding D-type cyclins get activated at the beginning of the G1 phase. Cyclin D then binds cdk4 or cdk6 to activate the kinase activity of these proteins, initiate Rb phosphorylation and induce cell cycle progression toward S phase. In light of these observation, it was predicted that the inactivation of the cyclin D1 gene would lead to important defects due to dysregulated cell proliferation. However, cyclin D1-deficient mice are viable but show developmental abnormalities to restricted tissues such as the retina, the nervous system, and the breast epithelium (Sicinski et al., 1995). In addition, inactivation of the cyclin D1 gene produced a small mouse phenotype (Fantl et al., 1995) and led to a disturbance of growth in Drosophila (Datar et al., 2000; Foley and Sprenger, 2000; Meyer et al., 2000) and Caenorhabditis elegans (Park and Krause, 1999). Overexpression of Arabidopsis cyclin D2 in transgenic tobacco leads to an increased rate of biomass accumulation, enhanced root growth, and a rapid attainment of flowering size (Cockcroft et al., 2000). In Arabidopsis, D-type cyclin expression is induced in the presence of sucrose, the major source of carbon that plays a central role in regulating cellular metabolism and physiology in plants. Therefore, cyclin D1 is involved in a large complexity of network interactions that regulate cell proliferation as well as cell growth.
Extending its cell cycle functions, cyclin D1 has been reported to have new functions that are independent of cyclin-dependent kinases. Cyclin D1 regulates the activity of various transcription factors such as MyoD, STAT3, Beta2/NeuroD, the estrogen, thyroid and androgen receptors or the v-myb, and DMP1 proteins (Bernards, 1999; Coqueret, 2002). Moreover, cyclin D1 interacts with several transcriptional cofactors such as the histone acetylase P/CAF (McMahon et al., 1999) and members of the p160 family of coactivators including NcoA/SRC1a or GRIP-1 (Zwijsen et al., 1998; Lazaro et al., 2002). A direct association with NcoA/SRC1a or GRIP-1 likely explains the effect of cyclin D1 on ER activation (Zwijsen et al., 1997, 1998) and on the activity of the MEF muscle factor (Lazaro et al., 2002). Cyclin D1 also regulates the activity of SP1 and Rb through its association with TAFII250, a component of TFIID (Adnane et al., 1999; Siegert et al., 2000). Additionally, it can also interact with the histone deacetylase HDAC3 (Lin et al., 2002), such that its inhibitory effects on the androgen and thyroid hormone receptors are lifted when cells are exposed to trichostatin A (TSA; Lin et al., 2002; Petre et al., 2002).
These studies suggest that cyclin D1 is contained within transcriptional regulatory complexes. However it remains to be determined if cyclin D1 is directly present on the DNA and if its effects are observed under physiological conditions or during carcinogenesis. We have previously shown that cyclin D1 interacts with STAT3 proteins to inhibit their transcriptional activity (Bienvenu et al., 2001). STAT3 transcription factors are cytoplasmic proteins that induce gene activation in response to cytokine stimulation. After tyrosine phosphorylation, STAT3 proteins dimerize, translocate into the nucleus, and activate the expression of cell cycle genes such as p21waf1, fos, cyclin D, myc, and pim-1 (Bromberg et al., 1999; Catlett-Falcone et al., 1999; Kiuchi et al., 1999; Shirogane et al., 1999; Ivanov et al., 2001). In the present study, using chromatin immunoprecipitation (ChIP) experiments, we show that cyclin D1 is recruited to the p21waf1 promoter by STAT3 proteins to down-regulate its activity. In the presence of cyclin D1, STAT3 and its transcriptional cofactor NcoA/SRC1a are normaly recruited to DNA, but the histone acetylase CBP and the RNA polymerase II do not associate with the promoter. Importantly, the cyclin D1-mediated inhibition of p21waf1 was also observed in breast cancer cells that contain elevated levels of cyclin D1. Altogether, these results indicate that cyclin D1 is part of a transcriptional complex that controls the activation of the p21waf1 gene, suggesting that this could play an important role during cell transformation.
MATERIALS AND METHODS
Cell Culture, Reagents, and Plasmid Constructs
Cyclin D1–/– fibroblasts were kindly provided by P. Sicinski and are derived from the same littermates as the parental fibroblasts used in this study. Polyclonal antibodies against STAT3 (C20), cyclin D1 (HD-11), p21waf1 (C19), NcoA/SRC1 (M-341), CBP (A-22), and RNA polymerase II (N-20) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phospho-STAT3-Tyr705 were from Cell Signaling Technology (Beverly, MA).
Transient Transfections, Preparation of Cell Extracts, and siRNA Experiments
Transfection experiments and cell extracts were done as described before (Giraud et al., 2002) and were repeated at least four times. The amount of transfected DNA was kept constant by addition of appropriate amounts of the parental expression vector. For siRNA experiments, cells, seeded into 10-cm cell culture dishes, were transfected with 5 μg/dish of short interfering RNAs (siRNAs) directed against SRC-1, cyclin D1, or lamin A/C using the lipofectamine 2000 reagent (Life Technologies, Rockville, MD)). Single-stranded RNAs were synthesized by Proligo (Boulder, CO) and correspond to the following sequences (Shang and Brown, 2002): for SRC-1: (sense strand: 5′-CCUCAGGGCAGAGAACCAUCUdTdT-3′; and antisense strand: 5′-AGAUGGUUCUCUGCCCUGAGGdTdT-3′), for lamin A/C:(sense strand: 5′CUGGACUUCCAGAAGAACAUCdTdT-3′; and antisense strand: 5′-GAUGUUCUUCUGGAAGUCCAGdTdT-3′). Cyclin D1 siRNAs were obtained from Santa Cruz Biotechnology.
Fusion Protein Purification, GST Pull-down Experiments, and In Vitro Transcription Translation
Bacterial pellets containing fusion proteins were lysed by sonication in phosphate-buffered saline (PBS) containing 1% Triton X-100, 1 mM EDTA. After centrifugation, fusion proteins were purified by affinity chromatography. Purified his-STAT3716–770, 50–100 ng, coupled to nickel-agarose resin was mixed with 50–100 ng GST-cyclin D1 and incubated for 30 min at 4°C in binding buffer (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM sodium vanadate, 100 mM sodium fluoride, 10 μg bovine serum albumin, and 1% Brij 96). Where indicated, his-STAT3716–770beads were preincubated for 30 min at 4°C with 200–300 ng GST or GSTSRC1a, extensively washed, and subsequently incubated with GST-cyclin D1. Beads were then washed three times with binding buffer, one time with Tris-50 mM HCl (pH 8), boiled for 5 min, and loaded on polyacrylamide gel for Western blot analysis. cDNAs were transcribed and translated in vitro in reticulocyte lysate in the presence of [35S]methionine, using the TNT kit (Promega, Madison, WI).
Chromatin Immunoprecipitation Assay
Cells grown to 60% confluence were serum-starved for 2 d. After stimulation (IL-6, 20 ng/ml), cells were washed and fixed with 1% formaldehyde at room temperature for 10 min. Cells were washed sequentially two times with 1 ml ice-cold PBS, centrifuged, and resuspended in 0.5 ml of lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin) and sonicated three times for 15 s each at the maximum setting. Supernatants were recovered by centrifugation at 12,000 rpm for 10 min at 4°C, diluted 2–4 times in dilution buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 1 mM PMSF, 2 mM sodium vanadate, 100 mM sodium fluoride, 20 mM Tris-HCl, pH 8.1) and subjected to one round of immuno-clearing for 2 h at 4°C with 2 μg sheared salmon sperm DNA, 2.5 μg preimmune serum and 20 μl of protein A sepharose (50% slurry). Immunoprecipitation was performed overnight with specific antibodies, then 2 μg sheared salmon sperm DNA and 20 μl of protein A-Sepharose (50% slurry) were further added for 1 h at 4°C. Note that the CBP and NcoA/SRC1a immunoprecipitations were performed in the presence of 1% Brij 97 or 1% NP40, respectively. Immunoprecipitates were washed sequentially for 10 min each in TSE I (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl), TSE II (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 500 mM NaCl) and buffer III (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH 8.1). Beads precipitates were then washed three times with TE buffer and eluted three times for 30 min at room temperature with 1% SDS, 0.1 M NaHCO3. Eluates were pooled and heated at 65°C overnight to reverse the formaldehyde cross-linking. For PCR, 10-μl from a 50 μl DNA preparation were used for 30 cycles of amplification. The following primers were used: for STAT3, CBP, cyclin D1, and NcoA/SRC1a binding: region –879/–593 of the p21 waf1 promoter 5′ TTCAGGAGACAGACAACTCACTCG 3′ (forward primer), 5′ GACACCCCAACAAAGCATCTTG 3′ (backward primer), region –105/+25 of the p21 waf1 promoter (RNA Pol II binding) 5′ GCGGCGCGGTGGGCCGAGCGCGGG 3′ (forward primer), 5′ GGCTCCACAAGGAACTGACT 3′ (backward primer), control region –2760/–2486 of the p21 waf1 promoter 5′-TTGTGCCACTGCTGACTTTGTC-3′ (forward primer), 5′ AGCCTGAAGAAGGAGGATGTGAGG-3′ (backward primer).
RESULTS
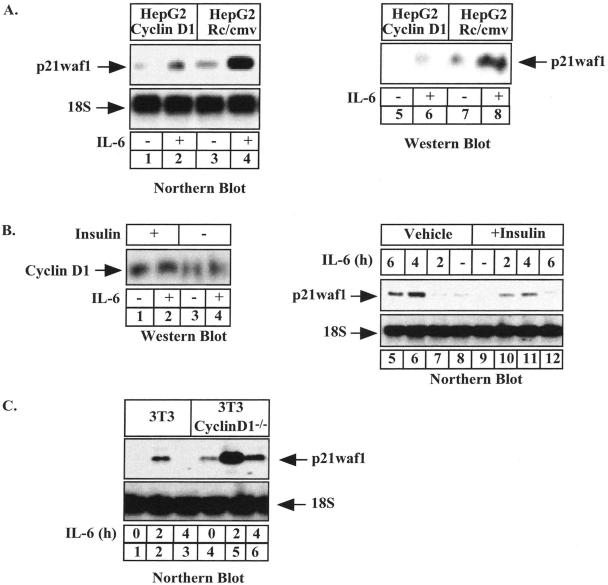
Cyclin D1 Prevents the Expression of the p21waf1 Gene
Because we have previously shown that cyclin D1 interacts with STAT3 to block its transcriptional activity (Bienvenu et al., 2001), we wanted to determine if this protein could prevent the IL-6–mediated activation of the p21waf1 gene. To this end, HepG2 cells stably transfected with cyclin D1 were serum-starved for 2 d and left untreated or were stimulated with IL-6 for 4 h. Under these conditions, Northern blot analysis showed that IL-6 induced the expression of the p21waf1 mRNA in control HepG2 cells, but that cyclin D1 reduced its expression (Figure 1A, compare lanes 1–4). Equal loading was verified using ribosomal 18S RNA staining. Western blot experiments confirmed that the expression of the p21waf1 protein was reduced in the presence of cyclin D1 (Figure 1A, lanes 5–8). Using a more physiological model to enhance the expression of cyclin D1, we then stimulated HepG2 cells with insulin, a well-known activator of the Akt kinase and cyclin D1 expression (Diehl et al., 1998). As expected, an enhanced expression of cyclin D1 was detected upon insulin stimulation (Figure 1B, lanes 1–4). Using Northern blot analysis, we also found that the IL-6–mediated activation of the p21waf1 mRNA was down-regulated when cells were pretreated with insulin (Figure 1B, lanes 5–12). To extend these results, we also evaluated if the ablation of cyclin D1 in mouse fibroblasts enhanced the cytokine-mediated activation of the p21waf1 gene. To this end, parental or cyclin D1–/– cells were serum-starved for 2 d and left untreated or were stimulated with IL-6 for 4 h. Under these conditions, Northern blot analysis effectively showed that IL-6 induced the expression of the p21waf1 mRNA to a higher extent in cyclin D1–/– cells than under control conditions (Figure 1C, compare lanes 1–3 and 4–6).
Figure 1.
Cyclin D1 inhibits the STAT3-mediated activation of the p21waf1 gene. (A) HepG2 cell lines expressing (lanes 1 and 2) or not cyclin D1 (lanes 3 and 4) were serum-starved for 2 d and stimulated for 4 h with IL-6 (10 ng/ml, lanes 2 and 4) or were left untreated (lanes 1 and 3). Total RNA was prepared and analyzed by Northern blot using a human cDNA probe. The expression of the p21waf1 protein was analyzed in parallel by Western blot after IL-6 stimulation (15 h, lanes 5–8). (B) HepG2 cells pretreated for 1 h with insulin (25 ng/ml) or its vehicle were stimulated with IL-6 as indicated. Nuclear extracts were prepared and the expression of cyclin D1 was analyzed by Western blot (lanes 1–4). The expression of the p21waf1 mRNA was analyzed after IL-6 stimulation of HepG2 cells in the presence (lanes 9–12) or absence (lanes 5–8) of insulin. (C) Wild-type and cyclin D1–/– 3T3 mouse fibroblasts derived from cyclin D1–/– transgenic mice were serum-starved for 2 d and stimulated with IL-6 (20 ng/ml) for 4 h. Total RNA was prepared and 20 μg of RNA was subjected to Northern blot analysis using a murine cDNA probe. The membrane was striped and reprobed with a 18S oligonucleotide (bottom panel).
Taken together, these results indicate that cyclin D1 inhibits the IL-6–mediated expression of the p21waf1 gene.
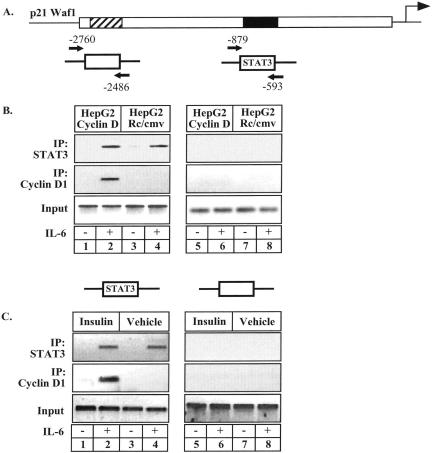
Cyclin D1 Is Associated with the p21waf1 Promoter
We then determined if cyclin D1 is recruited to the p21waf1 promoter to regulate its expression. To test this hypothesis, ChIP experiments were performed using two pairs of primers that cover either the STAT3 responsive region of the promoter or a control region 2 kb upstream of the STAT3 binding site (Figure 2A). The recruitment of cyclin D1 to the p21waf1 promoter was first examined in HepG2 cells that were serum-starved for 2 d and stimulated with IL-6 for 20 min. As expected, STAT3 was recruited to the p21waf1 promoter upon IL-6 stimulation (Figure 2B, top panel, lanes 1–4). Interestingly, cyclin D1 was also recruited to the p21waf1 promoter in cyclin D1-expressing cells (Figure 2B, bottom panel, lanes 1–4). Importantly, the presence of the protein on DNA did not affect the amount of STAT3 associated with its binding site. As a control of DNA sonication efficiency, PCR analysis did not detect any occupancy of the control region (Figure 2B, lanes 5–8). Given that insulin also induced a down-regulation of the p21waf1 mRNA, we then asked if this inhibitory effect was also associated with the recruitment of cyclin D1 to DNA. Using ChIP experiments, we effectively observed that insulin induced the association of cyclin D1 with the p21waf1 promoter but had no effect on the DNA binding activity of STAT3 (Figure 2C, lanes 1–4). No occupancy of a control region of the promoter was observed (Figure 2C, lanes 5–8).
Figure 2.
Cyclin D1 is associated with the p21waf1 promoter. (A) Schematic representation of the primers used in the ChIP experiments. (B) Soluble chromatin, prepared from HepG2 cells expressing or not cyclin D1 and treated with IL-6 or not for 20 min, was immunoprecipitated with the indicated antibodies. Final DNA extractions were amplified using a pair of primers that cover the STAT3 binding sites on the p21waf1 promoter (lanes 1–4) or a control, distal region (lanes 5–8). (C) ChIP analysis of the recruitment of STAT3 and cyclin D1 on the p21waf1 promoter in HepG2 cells in the presence of insulin (lanes 1 and 2, 5 and 6) or its vehicle (lanes 3 and 4, 7 and 8). Final DNA extractions were amplified as described in B.
Altogether, these results indicate that cyclin D1 is recruited to the p21waf1 promoter upon STAT3 activation.
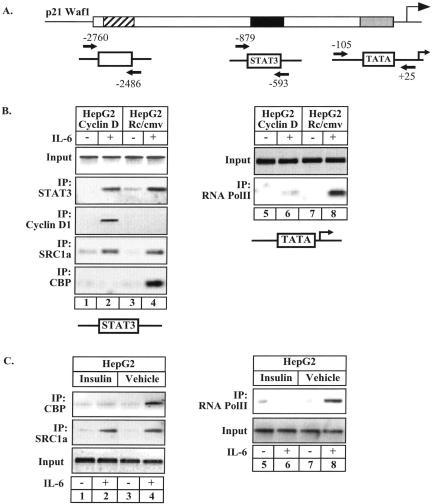
Cyclin D1 Inhibits the Occupancy of the p21waf1 Promoter by the CBP Coactivator and RNA Polymerase II
We have recently shown that the transcriptional activity of STAT3 is dependent on the recruitment of the NcoA/SRC1a and CBP coactivators (Giraud et al., 2002). To determine if cyclin D1 might modify the association of these proteins with the p21waf1 promoter, their recruitment was therefore analyzed by ChIP in HepG2 cells. Owing to their interaction with STAT3, the recruitment of NcoA/SRC1a and CBP was analyzed on the STAT3-responsive region of the promoter, whereas the recruitment of the RNA polymerase II was evaluated on its binding site, the TATA box (Figure 3A). In control HepG2 cells, ChIP experiments showed that the association of STAT3 and NcoA/SRC1a with the p21waf1 promoter was associated with the recruitment of CBP and the RNA pol II (Figure 3B, lanes 3–4 and 7–8). Whereas cyclin D1 was not significantly detected in control cells, it was found associated with the STAT3-responsive region in cyclin D1-expressing cells (Figure 3B, lanes 1–2, middle panel). In the presence of cyclin D1, STAT3 effectively induced the recruitement of NcoA/SRC1a, but failed to induce any CBP or RNA pol II association (Figure 3B, lanes 1–2 and 5–6). No occupancy of a control region of the promoter was observed (data not shown). Because insulin also induced the recruitment of cyclin D1 to the p21waf1 promoter, we also investigated its effects on the association of CBP and RNA pol II with DNA. Confirming the above results, ChIP experiments indicated that insulin prevented the STAT3-mediated recruitement of CBP and RNA pol II to the p21waf1 promoter (Figure 3C, lanes 1–2 and 5–6). In contrast, the association of NcoA/SRC1a and STAT3 with DNA was not modified (Figure 3C, lanes 1–4 and data not shown).
Figure 3.
ChIP analysis of the recruitment of STAT3, NcoA/SRC1a, CBP, and RNA Pol II in cyclin D1–expressing cells. (A) Schematic representation of the primers used in the ChIP experiments. (B) Stable HepG2 cell lines expressing cyclin D1 or a control plasmid as indicated were left untreated or stimulated with IL-6 for 20 min. Soluble chromatin was prepared and immunoprecipitated with the indicated antibodies. The final DNA extractions were amplified using two pairs of primers that cover either the STAT3 binding site for STAT3, cyclin D1, SRC1a, and CBP (lanes 1–4), or the RNA Pol II binding site (lanes 5–8). (C) ChIP analysis of the recruitment of SRC1a, CBP (lanes 1–4) and RNA Pol II (lanes 5–8) on the p21waf1 promoter of HepG2 cells in the presence or absence of insulin.
Altogether, these results suggest that cyclin D1 prevents the recruitment of CBP and of the polymerase to inhibit the activation of the p21waf1 promoter.
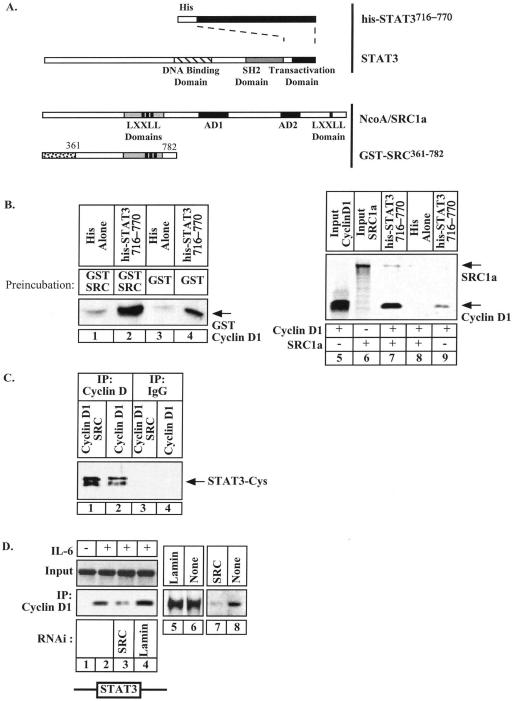
NcoA/SRC1a Enhances the Interaction between STAT3 and Cyclin D1
Because STAT3, cyclin D1, and NcoA/SRC1a are present on the p21waf1 promoter, we then examined if a ternary complex could be formed between these proteins. To determine the effect of NcoA/SRC1a on the STAT3-cyclin D1 interaction, his-tagged STAT3716–770 beads were preincubated for 30 min with GST alone or with GST-SRC361–782 (see Figure 4A). This preincubation allowed the association of the two proteins, as we have previously shown that the activation domain of STAT3 interacts with the residues 361–567 of NcoA/SRC1a (Giraud et al., 2002). After extensive washings, GST-cyclin D1 proteins were then added to the bound complexes. Under these conditions, results indicated that the binding of the cyclin was significantly enhanced when his-tagged STAT3716–770 was preincubated with GST-SRC361–782 as compared with GST alone (Figure 4B, compare lanes 2 and 4). Because we were concerned that the GST proteins could dimerize, these binding experiments were repeated using bacterially produced his-STAT3 proteins and 35S-methionine–labeled cyclin D1. Where indicated, in vitro–translated NcoA/SRC1a proteins were added to the binding reaction to determine if it could potentiate the STAT3-cyclin D1 interaction. As expected, in vitro translated cyclin D1 was retained by his-tagged STAT3716–770 immobilized on beads but not by histidine alone (Figure 4B, lanes 8–9). Moreover, the binding of the cyclin was significantly enhanced when his-tagged STAT3716–770 was preincubated with NcoA/SRC1a (Figure 4B, compare lanes 7 and 9).
Figure 4.
NcoA/SRC1a enhances the interaction between STAT3 and cyclin D1. (A) Representation of the fusion proteins used in these experiments. (B) Purified histidine or his-STAT3716–770 coupled to nickel-agarose resin were preincubated with 200–300 ng purified GST (lanes 3 and 4) or GST-SRC361–782 fusion proteins (lanes 1 and 2) for 30 min. After extensive washings, the complexes bound to the resin were further incubated with purified GST-cyclin D1 proteins (50–100 ng). Samples were then washed four times and cyclin D1 binding on preformed complexes was detected by Western blot. In parallel, purified histidine or his-STAT3716–770 coupled to beads were tested for binding to in vitro 35S-labeled full-length cyclin D1 proteins, in the presence (lane 7) or absence (lane 9) of 35S-labeled full-length NcoA/SRC1. Bound proteins were analyzed by SDS-PAGE and autoradiography. (C) COS cells were cotransfected with vectors expressing cyclin D1 and the activated form of STAT3, STAT3-Cys, in the presence (lanes 1 and 3) or absence (lanes 2 and 4) of NcoA/SRC1a. After 2 d, nuclear extracts were immunoprecipitated with polyclonal antibodies directed against cyclin D1 proteins (lanes 3–4) or a control serum (lanes 1 and 2) and analyzed by Western blot analysis with antibodies directed against STAT3 proteins. (D) HepG2 cells expressing cyclin D1 were transfected with siRNAs directed against either NcoA/SRC1a (lanes 3 and 7) or lamin A/C (lanes 4 and 5). ChIP analysis was conducted as described in Figure 1 to detect the association of cyclin D1 with the p21waf1 promoter (lanes 1–4). Western blot analysis was performed in parallel to analyze the expression of NcoA/SRC1a (lanes 5–8).
To extend these results, COS cells were transfected with vectors expressing cyclin D1, the activated form of STAT3, STAT3-Cys (Bromberg et al., 1999), in the presence or absence of a vector encoding for NcoA/SRC1a. Under these conditions, STAT3-Cys was effectively found to coimmunoprecipitate with cyclin D1 but this association was significantly enhanced in the presence of NcoA/SRC1a (Figure 4C, compare lanes 1 and 2). Because these results suggested that the interaction between STAT3 and cyclin D1 is mediated by NcoA/SRC1a, we then used siRNA to down-regulate the expression of the cofactor and determine if this could affect the interaction between the two proteins. Interestingly, ChIP experiments indicated that the siRNA-mediated down-regulation of NcoA/SRC1a prevented the recruitment of cyclin D1 to the p21waf1 promoter (Figure 4D, lanes 1–4). As a control, lamin siRNA did not affect the recruitment of cyclin D1 to DNA. The expression of NcoA/SRC1a was verified by Western blot analysis in each condition (Figure 4D, lanes 5–8).
Altogether, these results suggest that the interaction between STAT3 and cyclin D1 is indirect and mediated by NcoA/SRC1a. This also suggests that the STAT3-SRC-cyclin D1 complex might not bind to CBP as opposed to STAT3-SRC only.
Regulation of the p21waf1 Promoter by Cyclin D1 in Breast Cancer Cells
As a first attempt to determine if the transcriptional effects of cyclin D1 could be observed in tumor cells, we then used the MCF7 breast cancer cell line, because these cells have been reported to express high levels of cyclin D1 (Russell et al., 1999). MCF7 cells were serum-starved for 2 d and stimulated with IL-6, and Northern blot and ChIP experiments were then performed to analyze the regulation of the p21waf1 gene. Surprisingly, IL-6 stimulation of MCF7 cells did not lead to a detectable p21waf1 mRNA expression (Figure 5A, lanes 1–3), whereas serum stimulation activated the p21waf1 gene as previously reported (Coqueret et al., 1998). Interestingly, we found using ChIP experiments that cyclin D1 was recruited to the p21waf1 promoter upon cytokine stimulation (Figure 5A, lanes 4–5). To determine if cyclin D1 is associated with the down-regulation of the p21waf1 gene, we used the LY294002 pharmalogical inhibitor of the PI3K/Akt pathway to accelerate the proteolytic degradation of cyclin D1 (Diehl et al., 1998). As expected, Western blot analysis showed that LY294002 led to a down-regulation of cyclin D1 expression without affecting control tubulin expression (Figure 5B, compare lanes 1–2 and 3–4). Interestingly, we observed a shift in cyclin D1 mobility that is probably be due to an enhanced phosphorylation of the cyclin by the glycogen synthase kinase-3beta as previously reported (Diehl et al., 1998). Consequently, ChIP experiments indicated that the association of cyclin D1 with the p21waf1 promoter was also inhibited (Figure 5B, lanes 5–8). Northern blot experiments were then performed to determine if the down-regulation of cyclin D1 induced an enhanced expression of the cell cycle inhibitor. Interestingly, results showed that the LY294002 treatment effectively restored the expression of the p21waf1 mRNA upon stimulation of MCF7 cells (Figure 5C, lanes 1–4).
Figure 5.
Cyclin D1 is associated with the p21waf1 promoter to prevent its expression in breast cancer cells (A) MCF-7 cells were serum-starved for 2 d and stimulated for 4 h with IL-6 (20 ng/ml) or 10% serum as indicated. Total RNA was prepared and 20 μg of RNA was subjected to Northern blot analysis using a human cDNA probe (lanes 1–3). The membrane was striped and reprobed with a 18S oligonucleotide (bottom panel). The recruitment of cyclin D1 to the p21waf1 promoter was analyzed in parallel by ChIP after IL-6 stimulation (lanes 4–5). (B) Serum-starved MCF-7 cells were pretreated for 1 h with LY294002 (50 μM, lanes 3 and 4, 7 and 8) or with dimethyl sulfoxide (DMSO; vehicle, lanes 1 and 2, 5 and 6), and then left untreated or stimulated with IL-6 for 20 min. Cyclin D1 expression was analyzed by Western blot (lanes 1–4). ChIP analysis were performed to analyze the recruitment of cyclin D1 on the p21waf1 promoter after LY294002 pretreatment (lanes 5–8). Cells were treated as explained in A and stimulated for 20 min, and ChIP experiments were performed as described Figure 2. (C) Northern blot analysis of the expression of the p21waf1 mRNA in the presence or absence of LY294002. (D) Serum-starved MCF-7 cells were pretreated for 1 h with LY294002 (+) or with DMSO (–) and were then stimulated with IL-6 for 20 min. The association of STAT3 and CBP with the p21waf1 promoter was analyzed by ChIP using one pair of primers that cover the STAT3 binding site (lanes 1–4). The association of the RNA pol II with the p21waf1 promoter was analyzed in parallel with one pair of primers that cover the TATA box binding site (lanes 5–8).
Finally, ChIP experiments were conducted in the presence or absence of LY294002 to analyze the association of the CBP and RNA pol II complexes with DNA. On IL-6 stimulation of MCF7 cells, we found that STAT3 and NcoA/SRC1a were recruited to the p21waf1 promoter (Figure 5D, lanes 1–4 and 5–8). As previously reported, low but detectable levels of NcoA/SRC1a were recovered in MCF-7 cells (data not shown and Shang and Brown, 2002). However, STAT3 activation failed to induce any CBP or RNA polymerase II association with the p21waf1 promoter in control conditions (Figure 5D, lanes 1–2 and 5–6). Because LY294002 restored the expression of p21waf1, we reasoned that it should also reinduce the association of the cofactors with the promoter. As expected, ChIP analysis indicated that LY294002 allowed the association of CBP with the p21waf1 promoter (Figure 5D, lanes 3–4). Consequently, the RNA pol II was also found associated with DNA after LY294002 treatment and cyclin D1 degradation (Figure 5D, lanes 7–8). LY294002 did not affect STAT3 DNA binding and no association of these complexes was observed with a control DNA region (data not shown)
Taken together, these results suggest that cyclin D1 is associated with the p21waf1 promoter in breast cancer cells to prevent the activation of the gene.
The Expression of p21waf1 Is Enhanced upon Cyclin D1 Down-regulation
To verify that endogeneous cyclin D1 functions in the regulation of p21waf1 expression, MCF7 or HepG2 cells were transiently transfected with siRNA targeting cyclin D1 before stimulation. As controls, siRNA targeting lamin were also included. As shown in Figure 6A, siRNA transfection resulted in a significant reduction in cyclin D1 levels in MCF7 cells. Interestingly, whereas transfection of cells with lamin-specific siRNA had no effect on the expression of the cell cycle inhibitor, reduction of cyclin D1 levels by siRNA significantly restored the IL-6–mediated activation of p21waf1 (Figure 6A, lanes 3–6). To confirm these results, siRNA against cyclin D1 were then introduced into HepG2 cells before treatment with insulin and IL-6. Similar to the case of MCF7 cells, siRNA significantly knocked down cyclin D1 expression (Figure 6B, lanes 1–2). Confirming the results of Figure 1, insulin-inhibited p21waf1 expression in the presence of lamin-specific siRNA (Figure 6B, lanes 3–6). By contrast, cyclin D1 disruption eliminated the insulin-mediated repression of the p21waf1 gene (Figure 6B, compare lanes 6 and 10).
Figure 6.
Downregulating cyclin D1 enhanced p21waf1 expression. (A) MCF7 cells were transiently transfected with siRNAs against cyclin D1 (+) or lamin (–) used as a negative control. After 2 d, total protein extracts were prepared and analyzed by Western blotting using a polyclonal anticyclin D1 antibody (lanes 1 and 2). In parallel, cells were serum-starved for 2 d and stimulated or not with IL-6 as indicated. mRNA levels were then analyzed by RT-PCR with primers specific for the p21waf1 or GAPDH mRNAs (lanes 3–6). (B) The expression of cyclin D1 was analyzed by Western blot (lanes 1 and 2) in HepG2 cells transiently transfected with siRNAs against cyclin D1 (+) or lamin (–). Two days after transfection, cells were pretreated for 1 h with insulin or its vehicle and further stimulated with IL-6 as indicated. mRNA levels were then analyzed by RT-PCR with primers specific for the p21waf1 or GAPDH mRNAs (lanes 3–10).
Together, these data confirm that the endogeneous cyclin D1 can repress the IL-6–dependent activation of p21waf1 in breast cancer cells or upon insulin stimulation.
DISCUSSION
Besides its cell cycle role, cyclin D1 fullfills additional functions during transcriptional regulation. In this study, we have shown that cyclin D1 is recruited by a STAT3-SRC complex to the p21waf1 promoter. As a consequence, the CBP histone acetylase and the RNA polymerase II do not associate with the promoter and the p21waf1 mRNA is down-regulated.
In light of these results, we propose that cyclin D1 prevents the expression of p21waf1 through CBP inhibition. Gene activation involves the recruitment of coactivator complexes that modify or remodel chromatin at target promoters through histone acetylation or phosphorylation. A transcriptionally active chromatin structure is then obtained, allowing the association of the initiation complex with DNA (Kingston and Narlikar, 1999; Strahl and Allis, 2000). CBP contains an intrinsic acetyltransferase activity, which can modify histones as well as several transcription factors. Thus, our first hypothesis is that cyclin D1 affects the CBP-mediated acetylation of the p21waf1 promoter. We have recently shown that the transcriptional activity of STAT3 is mediated by an increase in histone H3 acetylation and by the recruitment of BRG1, the ATPase subunit of the Swi/Snf complex (Giraud et al., 2004). These recruitments are followed by an increased accessibility of the proximal promoter, which facilitates the binding of the RNA polymerase. Thus, if cyclin D1 prevents the access of the histone acetylase CBP, this could inhibit histone H3 acetylation and indirectly affects nucleosome positioning on DNA. By preventing histone modification, cyclin D1 would inhibit chromatin remodeling on the proximal p21waf1 promoter and mask the binding sequences involved in the loading of the initiation complex. However, because other acetylases are probably also present on the promoter, it will be important to determine if these proteins are also affected by cyclin D1. In addition, determining if additional histone modifications are necessary for p21waf1 to be activated is also an important issue to adress.
We also speculate that cyclin D1 might affect a CBP activity not directed against histones. Because CBP proteins serve as scaffolds for the association of multiprotein complexes, it is tempting to speculate that cyclin D1 disrupts contacts between STAT3 and essential coactivators that would be bridged by CBP. Although this remains controversial (Petre et al., 2002), it has been previously suggested that cyclin D1 might inhibit the transcriptional activity of the androgen receptor through the dissociation of a CBP-P/CAF complex (Reutens et al., 2001). Interestingly, PCAF has been reported to be associated with the TBP-associated factors (TAFs; Ogryzko et al., 1998). These subunits of the TFIID complex play an important role in the recruitment of the preinitiation complex. In addition, it has been reported that the RNA helicase A (RHA) functions as a bridging factor connecting CBP and RNA polymerase II (Nakajima et al., 1997). Thus, if the contacts between STAT3 and the polymerase are effectively mediated by either a CBP-RHA or CBP-P/CAF complex, cyclin D1 could either inhibit the direct recruitment of the RNA pol II or prevent the access of RHA and P/CAF activities to the p21waf1 promoter. In this case, the cyclin D1 effects would not be related to histone acetylation but more likely to steric hindrance.
Altogether, these results lead to the hypothesis that the cyclin D1 oncogene, or an oncogenic pathway that relies on cyclin D1, might prevent the activation of the p21waf1 promoter to enhance cell transformation. Cyclin D1 would thereby guide the transcriptional activity of STAT3 toward the activation of growth promoting genes at the expense of cell cycle inhibitory proteins. This could be a necessary step in the activation of the STAT3 and cyclin D1 oncogenic potentials, particularly in breast cancer cells were both oncogenes play important roles and might therefore cooperate (Dechow et al., 2004). Further experiments are now needed to determine if these observations can be extended to other genes or are only specific to the p21waf1 promoter.
Acknowledgments
We thank Dr. P. Sicinski for the gift of cyclin D1–/– cells; Drs. A. Nepveu, R. Roth, and D. Leprince for the gift of various expression vectors; and Drs. Lundquist and Sicinski for a critical reading of the manuscript. This work was supported by a fellowhip (F.B) from the Ligue Nationale contre le Cancer, fellowships (S.G. and B.B.) from the Ministere dela Recherche et dela Technologie and by a grant from the Ligue contre le Cancer, Comite Departemental deMaine et Loire.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–08–0654) on January 19, 2005.
References
- Adnane, J., Shao, Z., and Robbins, P. D. (1999). Cyclin D1 associates with the TBP-associated factor TAF(II)250 to regulate Sp1-mediated transcription. Oncogene 18, 239–247. [DOI] [PubMed] [Google Scholar]
- Bernards, R. (1999). CDK-independent activities of D type cyclins. Biochim. Biophys. Acta 1424, M17–22. [DOI] [PubMed] [Google Scholar]
- Bienvenu, F., Gascan, H., and Coqueret, O. (2001). Cyclin D1 represses STAT3 activation through a Cdk4-independent mechanism. J. Biol. Chem. 276, 16840–16847. [DOI] [PubMed] [Google Scholar]
- Bromberg, J., Wrzeszczynska, M., Devgan, G., Zhao, Y., Pestell, R., Albanese, C., and Darnell, J. (1999). Stat3 as an oncogene. Cell 98, 295–303. [DOI] [PubMed] [Google Scholar]
- Catlett-Falcone, R. et al. (1999). Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10, 105–115. [DOI] [PubMed] [Google Scholar]
- Cockcroft, C. E., den Boer, B. G., Healy, J. M., and Murray, J. A. (2000). Cyclin D control of growth rate in plants. Nature 405, 575–579. [DOI] [PubMed] [Google Scholar]
- Coqueret, O. (2002). Linking cyclins to transcriptional control. Gene 299, 35–55. [DOI] [PubMed] [Google Scholar]
- Coqueret, O., Berube, G., and Nepveu, A. (1998). The mammalian Cut homeodomain protein functions as a cell-cycle dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J. 17, 4680–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datar, S. A., Jacobs, H. W., de la Cruz, A. F., Lehner, C. F., and Edgar, B. A. (2000). The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J. 19, 4543–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechow, T. N., Pedranzini, L., Leitch, A., Leslie, K., Gerald, W. L., Linkov, I., and Bromberg, J. F. (2004). Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc. Natl. Acad. Sci. USA 101, 10602–10607. Epub 12004 Jul 10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl, J. A., Cheng, M., Roussel, M. F., and Sherr, C. J. (1998). Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12, 3499–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl, V., Stamp, G., Andrews, A., Rosewell, I., and Dickson, C. (1995). Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9, 2364–2372. [DOI] [PubMed] [Google Scholar]
- Foley, E., and Sprenger, F. (2000). Cyclins: growing pains for Drosophila. Curr. Biol. 10, R665–667. [DOI] [PubMed] [Google Scholar]
- Giraud, S., Bienvenu, F., Avril, S., Gascan, H., Heery, D. M., and Coqueret, O. (2002). Functional interaction of STAT3 transcription factor with the coactivator NcoA/SRC1a. J. Biol. Chem. 277, 8004–8011. [DOI] [PubMed] [Google Scholar]
- Giraud, S., Hurlstone, A., Avril, S., and Coqueret, O. (2004). Implication of BRG1 and cdk9 in the STAT3-mediated activation of the p21waf1 gene. Oncogene 23, 7391–7398. [DOI] [PubMed] [Google Scholar]
- Ivanov, V. N., Bhoumik, A., Krasilnikov, M., Raz, R., Owen-Schaub, L. B., Levy, D., Horvath, C. M., and Ronai, Z. (2001). Cooperation between STAT3 and c-jun suppresses Fas transcription. Mol. Cell 7, 517–528. [DOI] [PubMed] [Google Scholar]
- Kingston, R. E., and Narlikar, G. J. (1999). ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 13, 2339–2352. [DOI] [PubMed] [Google Scholar]
- Kiuchi, N., Nakajima, K., Ichiba, M., Fukada, T., Narimatsu, M., Mizuno, K., Hibi, M., and Hirano, T. (1999). STAT3 is required for the gp130-mediated full activation of the c-myc gene. J. Exp. Med. 189, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro, J. B., Bailey, P. J., and Lassar, A. B. (2002). Cyclin D-cdk4 activity modulates the subnuclear localization and interaction of MEF2 with SRC-family coactivators during skeletal muscle differentiation. Genes Dev. 16, 1792–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H. M., Zhao, L., and Cheng, S. Y. (2002). Cyclin D1 is a ligand-independent co-repressor for thyroid hormone receptors. J. Biol. Chem. 277, 28733–28741. [DOI] [PubMed] [Google Scholar]
- McMahon, C., Suthiphongchai, T., DiRenzo, J., and Ewen, M. E. (1999). P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc. Natl. Acad. Sci. USA. 96, 5382–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, C. A., Jacobs, H. W., Datar, S. A., Du, W., Edgar, B. A., and Lehner, C. F. (2000). Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. EMBO J. 19, 4533–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, T., Uchida, C., Anderson, S. F., Lee, C. G., Hurwitz, J., Parvin, J. D., and Montminy, M. (1997). RNA helicase A mediates association of CBP with RNA polymerase II. Cell 90, 1107–1112. [DOI] [PubMed] [Google Scholar]
- Ogryzko, V. V., Kotani, T., Zhang, X., Schiltz, R. L., Howard, T., Yang, X. J., Howard, B. H., Qin, J., and Nakatani, Y. (1998). Histone-like TAFs within the PCAF histone acetylase complex. Cell 94, 35–44. [DOI] [PubMed] [Google Scholar]
- Park, M., and Krause, M. W. (1999). Regulation of postembryonic G(1) cell cycle progression in Caenorhabditis elegans by a cyclin D/CDK-like complex. Development 126, 4849–4860. [DOI] [PubMed] [Google Scholar]
- Petre, C. E., Wetherill, Y. B., Danielsen, M., and Knudsen, K. E. (2002). Cyclin D1, mechanism and consequence of androgen receptor co-repressor activity. J. Biol. Chem. 277, 2207–2215. [DOI] [PubMed] [Google Scholar]
- Reutens, A. T., Fu, M., Wang, C., Albanese, C., McPhaul, M. J., Sun, Z., Balk, S. P., Janne, O. A., Palvimo, J. J., and Pestell, R. G. (2001). Cyclin D1 binds the androgen receptor and regulates hormone-dependent signaling in a p300/CBP-associated factor (P/CAF)-dependent manner. Mol. Endocrinol. 15, 797–811. [DOI] [PubMed] [Google Scholar]
- Russell, A., Thompson, M. A., Hendley, J., Trute, L., Armes, J., and Germain, D. (1999). Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene 18, 1983–1991. [DOI] [PubMed] [Google Scholar]
- Shang, Y., and Brown, M. (2002). Molecular determinants for the tissue specificity of SERMs. Science 295, 2465–2468. [DOI] [PubMed] [Google Scholar]
- Shirogane, T., Fukada, T., Muller, J. M., Shima, D. T., Hibi, M., and Hirano, T. (1999). Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity 11, 709–719. [DOI] [PubMed] [Google Scholar]
- Sicinski, P., Donaher, J. L., Parker, S. B., Li, T., Fazeli, A., Gardner, H., Haslam, S. Z., Bronson, R. T., Elledge, S. J., and Weinberg, R. A. (1995). Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82, 621–630. [DOI] [PubMed] [Google Scholar]
- Siegert, J. L., Rushton, J. J., Sellers, W. R., Kaelin, W. G., Jr., and Robbins, P. D. (2000). Cyclin D1 suppresses retinoblastoma protein-mediated inhibition of TAFII250 kinase activity. Oncogene 19, 5703–5711. [DOI] [PubMed] [Google Scholar]
- Strahl, B. D., and Allis, C. D. (2000). The language of covalent histone modifications. Nature 403, 41–45. [DOI] [PubMed] [Google Scholar]
- Zwijsen, R. M., Buckle, R. S., Hijmans, E. M., Loomans, C. J., and Bernards, R. (1998). Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 12, 3488–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwijsen, R. M., Wientjens, E., Klompmaker, R., van der Sman, J., Bernards, R., and Michalides, R. J. (1997). CDK-independent activation of estrogen receptor by cyclin D1. Cell 88, 405–415. [DOI] [PubMed] [Google Scholar]