Abstract
Hypoxia-inducible factor (HIF)-deficient placentas exhibit a number of defects, including changes in cell fate adoption, lack of fetal angiogenesis, hypocellularity, and poor invasion into maternal tissue. HIF is a heterodimeric transcription factor consisting of α and β aryl hydrocarbon receptor nuclear translocator or ARNT) subunits. We used undifferentiated trophoblast stem (TS) cells to characterize HIF-dependent adhesion, migration, and invasion. Arnt-/- and Hifα-/- TS cells exhibit reduced adhesion and migration toward vitronectin compared with wild-type cells. Furthermore, this defect is associated with decreased cell surface expression of integrin αvβ3 and significantly decreased expression of this integrin in focal adhesions. Because of the importance of adhesion and migration in tumor progression (in addition to placental development), we examined the affect of culturing B16F0 melanoma cells in 1.5% oxygen (O2). Culturing B16F0 melanoma cells at 1.5% O2 resulted in increased αvβ3 integrin surface expression and increased adhesion to and migration toward vitronectin. Together, these data suggest that HIF and O2 tension influence placental invasion and tumor migration by increasing cell surface expression of αvβ3 integrin.
INTRODUCTION
Oxygen (O2) deprivation or “hypoxia” acting through hypoxia-inducible factors (HIFs) influences cell adhesion and migration in a number of biological contexts. For example, HIF regulates progenitor cell adhesion and migration in ischemic tissue by inducing expression of SDF-1 and promotes tumor invasion through the tyrosine kinase receptor MET (Pennacchietti et al., 2003; Ceradini et al., 2004). Hif1α-/- macrophages exhibit poor migration and Matrigel invasion (Cramer et al., 2003), and directed migration of renal cell carcinoma cells is regulated by HIF activation of the chemokine receptor CXCR4 (Staller et al., 2003). HIF activity also is clearly important during placentation (Kozak et al., 1997; Adelman et al., 2000): placentas from embryos lacking HIF exhibit a variety of defects, including poor invasion of the maternal decidua (Adelman et al., 2000; Cowden Dahl, Mack, Compernolle, Adelman, Carmeliet, and Simon, unpublished data). Furthermore, tumor hypoxia is often clinically correlated with increased metastatic potential (Vaupel et al., 2001). The integrin adhesion receptors promote placental invasion, macrophage adhesion and migration, and metastatic progression of neoplasms (Zhou et al., 1997b; Ding et al., 1999; Jin and Varner, 2004), and hypoxia has been shown to increase mRNA levels of multiple integrins (β3, β2, and α5) (Walton et al., 2000; Iwaki et al., 2004; Koike et al., 2004; Kong et al., 2004). Therefore, HIF regulates cell motility in a variety of developmental and pathological situations.
The transcriptional response to hypoxia is mediated by the dimeric basic-helix-loop-helix-PAS (bHLH-PAS) transcription factor HIF. HIF is composed of an α subunit (HIF1α or HIF2α) and β subunit (aryl hydrocarbon receptor nuclear translocator; ARNT) and activates transcription by binding to hypoxic response elements (HREs) scattered throughout the genome (Gu et al., 2000). HIFα is labile under normoxic conditions, but stabilized by hypoxia, enabling dimerization with ARNT to induce genes such as angiogenic factors, glycolytic enzymes, glucose transporters, and erythropoietin (Wang and Semenza, 1993; Firth et al., 1994; Liu et al., 1995; Forsythe et al., 1996; Semenza et al., 1996; Ema et al., 1997; Okino et al., 1998). Arnt-/- mice die by embryonic day (E) 10.5 with defects in angiogenesis, cardiogenesis, hematopoiesis, and placentation (Kozak et al., 1997; Maltepe et al., 1997; Adelman et al., 1999, 2000; Abbott and Buckalew, 2000). Hif1α-/- embryos also die by E10.5, exhibiting angiogenic and cardiac defects (Iyer et al., 1998; Ryan et al., 1998; Compernolle et al., 2003). Mutations in Hif2α also result in embryonic lethality, with Hif2α-/- animals exhibiting defects in cardiac function, angiogenesis, and lung maturation (Tian et al., 1998; Peng et al., 2000; Compernolle et al., 2002). Therefore, HIF activity is essential for viability and cardiovascular development during embryogenesis.
Arnt-/- placentas are avascular, hypocellular, and exhibit aberrant cell fate adoption in addition to poor invasion. To study mechanisms underlying HIF regulation of multiple aspects of placentation, we used undifferentiated trophoblast stem (TS) cells from wild-type, Arnt-/-, Hif1α+/-Hif2α+/-, and Hif1α-/-Hif2α-/- embryos. TS cells are non-transformed, immortal precursor stem cells derived from blastocysts capable of differentiating into multiple placental lineages (e.g., trophoblast giant cells, spongiotrophoblasts, and syncytiotrophoblasts).
Because HIF-deficient placentas exhibit poor maternal invasion, we determined whether HIF regulates trophoblast invasion through integrin-mediated adhesion to the extracellular matrix (ECM). We show here that undifferentiated Arnt-/- and Hif1α-/-Hif2α-/- TS cells exhibit reduced αvβ3 integrin-mediated adhesion and migration due to decreased cell surface expression of this integrin. These studies explain in part the poorly invasive phenotype observed in Arnt-/- and Hif1α-/-Hif2α-/- placentas in vivo. Moreover, because HIF regulates αvβ3 integrin-mediated adhesion, we reasoned that hypoxia also may up-regulate αvβ3 integrin expression in tumors, leading to increased metastasis. Melanoma metastases contain hypoxic regions, and metastatic progression is associated with increased αvβ3 integrin expression (Gehlsen et al., 1992; Nip et al., 1992; Lartigau et al., 1997; Felding-Habermann et al., 2002). Poorly and highly metastatic melanoma cells (B16F0 and B16F10, respectively) were used to study hypoxic regulation of adhesion pathways that may be relevant to tumor metastasis. We show that hypoxia induces αvβ3 integrin cell surface expression in poorly metastatic B16F0 melanoma cells, resulting in increased adhesion and migration.
MATERIALS AND METHODS
Antibodies
The Novus HIF1α antibody (clone H1α67) was used for HIF1α electrophoretic mobility shift assay (EMSA) “supershift.” PE-RMV-7 from BD Biosciences PharMingen (San Diego, CA) was used for fluorescence-activated cell sorting (FACS) of αv integrin, and the Chemicon International (Temecula, CA) polyclonal αv integrin antibody was used for immunoprecipitation and Western blot assays. For β3 integrin, 2C9.G2 subclone from BD Biosciences PharMingen was used for blocking function, FACS, immunoprecipitation, immunoelectron microscopy (immuno-EM), and immunofluorescence. For β1 integrin, MAB1997 was used for immunoprecipitation. Additionally, the Chemicon International β3 integrin and β5 integrin antibodies were used in Western blot assays. For FACS analysis, BD Biosciences PharMingen's Ha2/5 was used for β1 integrin, the Santa Cruz Biotechnology (Santa Cruz, CA) (H-96) for β5 integrin, or BD Biosciences PharMingen's GoH3 for α6 integrin. For immunofluorescence, 58K (Abcam, Cambridge, MA), anti-calreticulin (StressGen Biotechnologies, San Diego, CA), phospho-Y397FAK (BD Transduction Laboratories, Lexington, KY), and anti-mouse-Alexa-fluor 488, anti-rabbit-Alexa-fluor 488, and streptavidin Alexa-fluor 568 from Molecular Probes (Eugene, OR) were used.
EMSAs
EMSAs were performed in binding buffer consisting of 10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 50 mM KCl, 1 mM MgCl2, 5 mM dithiothreitol, 1 mM EDTA, and 5% glycerol to which 0.1 mg/ml bovine serum albumin (BSA), 5 μg of nuclear extract, and 105 cpm of probe were added per 20 μl of reaction. The binding site sequence of the probe (E24F) is 5′-GCCCTACGTGCTGCCTCGCATGGC-3′. Cold cAMP response element-binding protein (CREB) competitor oligo (100× excess) containing the CREB binding site from the somatostatin promoter was added: 5′-GATCGCCTCCTTGGCTGACGTCAGAGAGCTAG-3′.
Northern Blot Analysis
Undifferentiated TS and embryonic stem (ES) cells were cultured for 16 h at 20% or 1.5% O2 and then washed with Dulbecco's phosphate-buffered saline (DPBS). RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to manufacturer's protocol. Fifteen micrograms of total RNA was electrophoresed in 1% agarose containing formaldehyde. The RNAs were transferred to Hybond N+ membranes (Amersham Biosciences, Piscataway, NJ). Full-length HIF1α and β-tubulin cDNAs were labeled as probes.
Cell Culture
Arnt+/+ and Arnt-/- TS were described previously (Adelman et al., 2000) and derived by standard protocols (Tanaka et al., 1998). Hif1α+/-Hif2α+/-, and Hif1α-/-Hif2α-/- TS cells are described by Cowden Dahl, Mack, Compernolle, Adelman, Carmeliet, and Simon (unpublished data). Briefly, TS cells were maintained in an undifferentiated state on mouse embryo fibroblasts (MEFs) in the presence of FGF4 and heparin. For all experiments, TS cells were cultured in the absence of MEFs but supplemented with MEF-conditioned media containing FGF4 and heparin. TS cells were plated on glass coverslips for immunofluorescence assays. B16F0 and B16F10 cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in DMEM supplemented with 10% serum, HEPES, penicillin, streptomycin, and l-glutamine. B16 melanoma cells were cultured either under standard culture conditions (20% O2) or at 1.5% O2 (in an IG750 3 gas incubator; Jouan, Winchester, VA) for 24 h. CO2 is maintained at 5% for all experiments.
Immunoprecipitation of Biotin-labeled Cell Surface Proteins
TS or melanoma cells were cultured to 75% confluence, placed on ice, and washed with ice cold DPBS with calcium and magnesium. Cells were then incubated in 1 mg/ml NHS-LC-Biotin (Pierce Chemical, Rockford, IL) for 90 min on ice and washed three times with 50 mM glycine in DPBS. Cell lysates were prepared in NP-40 buffer, pH 7.5 (0.1 M Tris-Base, 1% NP-40, 10 μg/ml Pefabloc, 1 mM E-64, 5 μg/ml leupeptin, 1 μg/ml pepstatin, 0.02 mg/ml aproptinin, and 0.5M EDTA). Debris was pelleted, and supernates were transferred to new tubes. Lysates were precleared overnight with protein A/G beads (Pierce Chemical). Bicinchoninic acid assays were performed on precleared lysates to determine protein concentration. Immunoprecipitations were performed on 250 μg of protein by using protein A/G beads and anti-αv integrin (Chemicon International), anti-β3 integrin (BD Biosciences PharMingen) or anti-β1 integrin (Chemicon International). After immunoprecipitation, beads were washed thrice with MDB buffer (50 mM Tris, pH 7.5, 0.1% SDS, 0.05% NP-40, and 0.3 M NaCl) and thrice with Tris-wash buffer (10 mM Tris, pH 7.5). Lysates were then resuspended in nonreducing sample buffer and run on an 8% SDS-polyacrylamide gel.
FACS Analysis
Cell surface expression of integrins was determined by FACS analysis. Cells were trypsinized and washed in DPBS and blocked with anti-CD16/32 for 15 min at room temperature. After washing in 1% BSA in DPBS, cells were incubated with primary antibody for 30 min on ice, washed with 1% BSA/DPBS, pelleted, and resuspended in isotonic buffer. Propidium iodide was used to exclude dead cells. FACS data were analyzed using CellQuest software, and error bars represent ±SE. Student's t tests were performed to determine statistical significance.
Adhesion, Migration, and Invasion Assays
Adhesion assays were performed on CytoMatrix cell adhesion strips containing fibronectin (FN), vitronectin (VN), collagen IV, and laminin (LN) according to manufacturer's instructions (Chemicon International). The concentration of ECM proteins and BSA for the adhesion and migration assays is not provided by Chemicon International. Briefly, strips were rehydrated with DPBS. Cells were prepared in a single cell suspension in serum-free media, and 1 × 105 cells were added to each well of the adhesion strips. Strips were incubated for 1 h at 37°C and washed twice with DPBS, stained with 0.2% crystal violet in 10% ethanol (EtOH), washed again, and solubilized in 50% 0.1 M NaH2PO4 in 50% EtOH. Absorbance was read at 560 nm on a microplate reader. Quantitative cell migration assays (QCM-FN and QCM-VN; Chemicon International) were performed on feeder-free TS cells according to manufacturer's instructions. Briefly, 2.5 × 105cells were placed in each well of FN-, VN-, or BSA-coated Boyden chambers. Cells were allowed to migrate for 16 h, and chambers were stained with crystal violet. Dye was then solubilized and read on a microplate reader. Absorbance was normalized to migration toward BSA. Invasion was measured on BD BioCoat Matrigel (BD Biosciences, San Jose, CA) invasion chambers according to manufacturer's instructions. Briefly, inserts were rehydrated with RPMI 1640 medium. Cells (4 × 105) were added to chambers and incubated for 22 h. Chambers were then cleaned and stained with crystal violet. Solubilized dye was measured on a microplate reader.
Western Blots
Cells were washed in DPBS and lysed in NP-40 buffer (described above). Lysates were cleared by centrifugation, and equal amounts of protein were separated on a 10% SDS-polyacrylamide gel. Proteins were transferred to nitrocellulose, and the membrane was blocked with 3% BSA in Tris-buffered saline/Tween 20 and incubated with antibodies before development with ECL.
Immunoelectron Microscopy
Briefly, samples were rinsed in buffer and fixed in 2% paraformaldehyde + 0. 2% glutaraldehyde in DPBS for 30 min and permeablized with 0.1% saponin. Fixative was removed, cells washed, and residual aldehyde was inactivated by treatment with 0.1% NaBH for 10 min. Cells were incubated with 1% ovalbumin + 5% normal horse or donkey serum and 0.2% cold water fish skin gelatin for 60 min at room temperature to prevent nonspecific binding. Primary antibody incubation was performed overnight at a dilution of 1:100 cells washed and treated with ultrasmall gold secondary overnight. After washing with DPBS cells were fixed in 1% glutaraldehyde in DPBS for 15 min. Cells were washed with DPBS and then with deionized water before enhancing with silver stain. After silver staining cells were osmicated with 1% osmium and dehydrated in graded alcohol and infiltration aided by propylene oxide and embedded in Epon. Ultrathin sections were mounted on Formvar-coated slotted copper grids and imaged in JEOL JEM 1010 aided by AMT HR-12 software and Hamamatsu Charge-coupled device camera.
Fluorescence Confocal Microscopy
TS cells were plated on uncoated coverslips and then fixed for 10 min at room temperature in –20°C acetone, washed in DPBS, permeabilized with DPBS/0.2%Triton X-100, and blocked in 0.2% BSA in DPBS-Tween 20. Cells were then incubated with primary and conjugated-secondary antibodies at room temperature for 1 h each. Coverslips were mounted on slides. Samples were viewed by confocal microscopy. Images were acquired for both red and green lasers and merged in Photoshop.
Statistical Analysis
Each experiment was performed at least three times. Error bars represent ± SE. Student's t tests were performed to determine statistical significance for the following assays: adhesion, migration, invasion, FACS, and immuno-EM quantitation.
RESULTS
TS Cells Exhibit High Levels of HIF Activity When Cultured in 20% O2
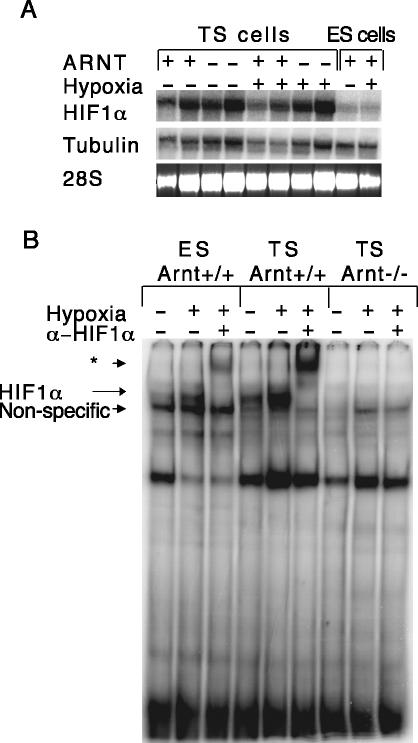
Arnt-/- TS cells exhibit a number of phenotypes reminiscent of defects observed in Arnt-/- placentas, including aberrant cell fate adoption upon differentiation and poor adhesion to ECM (see below). Because differentiated Arnt-/- TS cells exhibit defects in cell fate adoption, we chose to confine the experiments exclusively to undifferentiated TS cells. We determined that the degree of differentiation in both wild-type and Arnt-/- TS cells was very low based on trophoblast lineage-specific gene expression, morphology, and DNA content (our unpublished data). Interestingly, defects in Arnt-/- TS cells are detected under both normoxic and hypoxic conditions. To demonstrate that the Arnt+/+ and Arnt-/- TS cell phenotypes observed under normoxic conditions are mediated by the HIF pathway, we assessed the degree of HIF activity in normoxic and hypoxic TS cells. First, we examined mRNA expression of HIF1α and HIF2α in TS cells. For most experiments, we used two independent Arnt+/+ (0 and 2) and Arnt-/- (4 and 10) TS cell lines. In Figure 1A, both Arnt+/+ (0 and 2) and Arnt-/- (4 and 10) TS cell lines were examined for gene expression at 20% and 1.5% O2. Because tubulin expression is regulated by O2 tension, the 28S RNA band serves as an important second loading control. Even though the abundance of HIF1α mRNA somewhat varied between TS cell lines, HIF1α mRNA was present at two-to threefold higher levels under both normoxia (20% O2) and hypoxia (1.5% O2) in all TS cell lines compared with wild-type ES cells (Figure 1A). Although HIF2α is present in ES cells (our unpublished data), HIF2α RNA was not detected in undifferentiated TS cells, indicating that all TS HIF activity is therefore due to HIF1α/ARNT dimers. We then performed an EMSA to detect HIF DNA binding to an HRE residing in the erythropoietin (EPO) 3′ enhancer. Low levels of HIF binding activity were observed in normoxic ES cells, which increased upon exposure to 1.5% O2 (Figure 1B, lanes 1 and 2). Importantly, antibody against HIF1α completely shifted the hypoxic DNA binding complex, indicating that the inducible complex contains HIF (Figure 1B, lane 3). In comparison with ES cells, HIF DNA binding activity was detected in TS cells at relatively high levels at 20% O2; DNA binding activity was further enhanced by hypoxia and supershifted with α-HIF1α antibody (Figure 1B, lanes 4–6). These data suggested that HIF target genes might be regulated by HIF in TS cells even under normoxic conditions. As expected, no HIF DNA binding activity was present in Arnt-/- TS cells (Figure 1B, lanes 7–9). Furthermore, we observed decreased expression of the HIF target genes vascular endothelial growth factor (VEGF), ERO1-Lα, phosphofructose kinase (PFK), and Glut-1 (our unpublished data) in normoxic TS cells. Therefore, we concluded that HIF is partially active under normoxic conditions in Arnt+/+ TS cells and conducted all subsequent experiments at 20% O2 to study a homogenous stem cell population.
Figure 1.
TS cells exhibit high levels of HIF activity when cultured in 20% O2. (A) Northern blot analysis for HIF1α mRNA levels in two independent Arnt+/+ TS cell lines, two independent Arnt-/- TS cell lines, and wild-type ES cells. Tubulin and 28S RNA serve as loading controls. As expected, HIF1α mRNA levels are not increased by culture at 1.5% O2 in either TS or ES cells. Each of these experiments was performed three times. (B) EMSA analysis showing HIF DNA complex formation. Arnt+/+ ES cells demonstrate low levels of the HIF complex under normoxic conditions, but complex formation is enhanced under hypoxia. In addition to the hypoxia-inducible DNA complex, ES cells harbor a constitutive nonspecific complex as indicated. Arnt+/+ TS cells exhibit HIF complex formation under normoxia and hypoxia, but no complex forms in the absence of ARNT. HIF complexes are supershifted with an anti-HIF1α antibody, as indicated by the asterisk.
Arnt-/- TS Cells Exhibit Poor Migration and Adhesion
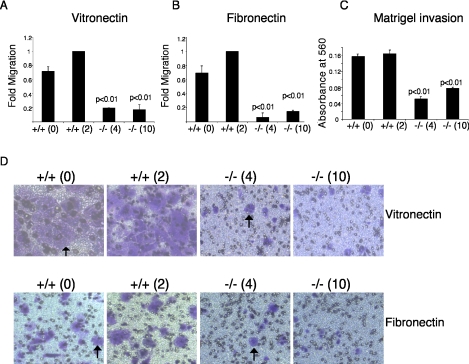
Because Arnt-/- trophoblasts exhibit poor invasion into maternal tissue, we investigated whether HIF activity plays a role in undifferentiated TS cell migration. Migration toward the ECM proteins vitronectin and fibronectin was analyzed using a Boyden chamber assay, and migration was normalized to the wild-type control +/+(2) TS cells for each experiment. Migration toward vitronectin was decreased by 5- and 5.9-fold, respectively, in two independent Arnt-/- TS cell lines compared with wild-type cells (p < 0.01 and p < 0.01) (Figure 2A). Additionally, migration toward fibronectin was decreased by 16.7- and 6.8-fold in Arnt-/- TS cell lines (Figure 2B). Photographs of the Boyden chambers showing decreased migration toward vitronectin (top) and fibronectin (bottom) are presented in Figure 2D. We also measured the ability of TS cells to invade basement membrane (Matrigel), which contains collagens, laminin, proteoglycans, matrix degrading enzymes and their inhibitors, and growth factors. Both Arnt+/+ cell lines exhibited roughly equivalent invasion. In contrast, Arnt-/- TS cell lines displayed decreased invasion (3.2- and 2.1-fold, respectively) into Matrigel compared with the control +/+(2) cells (p < 0.01) (Figure 2C). We concluded that trophoblasts lacking HIF activity exhibit poor migration and invasion.
Figure 2.
Arnt-/- TS cells exhibit impaired migration toward vitronectin and fibronectin and reduced invasion into Matrigel. (A) Boyden chamber assays for migration toward vitronectin. (B) Boyden chamber assays for migration toward fibronectin. (C) Invasion of Matrigel. (D) Photographs of migration toward vitronectin (top) and migration toward fibronectin (bottom). Original magnifications, 100×. Arrows indicate stained cells that have migrated through Boyden chamber pores. Each experiment was performed three times in duplicate. Error bars represent ± SEM. Student's t tests were used for statistical significance.
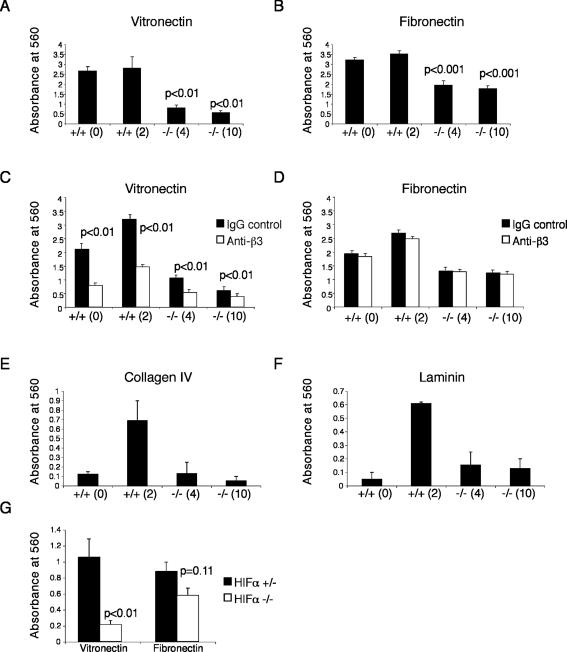
ECM is composed of a number of proteins that serve as ligands for integrins, including fibrous proteins (e.g., collagen), linker proteins (e.g., fibronectin, laminin, and vitronectin), and glycosaminoglycans. We reasoned that the decreased migration exhibited by Arnt-/- TS cells might be due to a defect in ECM adhesion. TS cells were plated on vitronectin-, fibronectin-, collagen IV-, or laminin-coated plates for 1 h to measure binding to different substrates. Binding to vitronectin was decreased by approximately fivefold in both Arnt-/- TS cell lines (p < 0.01) (Figure 3A), whereas adhesion to fibronectin was decreased by ∼1.8-fold (p < 0.001) (Figure 3B). Because integrin αvβ3 is the prototypic receptor for vitronectin and has affinity for fibronectin, we determined whether the ECM adhesion defects were due to a decrease in αvβ3 activity. We treated Arnt+/+ and Arnt-/- TS cells with a β3 integrin-blocking antibody and then performed adhesion assays. Adhesion to vitronectin was greatly decreased in Arnt+/+ and Arnt-/- TS cells by blocking β3 integrin activity (p < 0.01); however, adhesion to fibronectin was not (Figure 3, C and D). This suggested that inhibition of β3 activity is sufficient to decrease adhesion to vitronectin, but blocking β3 activity alone does not inhibit adhesion to fibronectin. Adhesion to collagen IV and laminin was equivalent between Arnt-/- cell lines and one of the wild-type cell lines +/+(0) (Figure 3, E and F). The second wild-type cell line [+/+(2)] exhibited more adhesion to laminin and collagen IV than the other lines, indicating that this effect was not genotype specific. To demonstrate that the decrease in adhesion to vitronectin and fibronectin was due to inactivation of the HIF pathway, ECM adhesion for TS cells lacking both Hif1α and Hif2α (designated Hifα-/-) was examined. Hifα-/- TS cells exhibited a fivefold decrease in adhesion to vitronectin compared with Hif1a+/- Hif2a+/- (Hifα+/-) TS cells (p < 0.01) (Figure 3G). Adhesion to fibronectin was also slightly decreased in Hifα-/- TS cells (1.5-fold), but this change was not significant. Importantly, adhesion of Hifα+/- TS cells to laminin was equivalent to Hifα-/- TS cells (our unpublished data). We concluded that Arnt-/- and Hifα-/- TS cells exhibit striking defects in adhesion to vitronectin and fibronectin.
Figure 3.
Arnt-/- TS cells adhere poorly to both vitronectin and fibronectin. Arnt+/+ and Arnt-/- TS cells were plated on strips coated with vitronectin (A) or fibronectin (B) and stained with crystal violet. Solubilized crystal violet incorporated by adherent cells was measured on a plate reader at 560 nm. Two independent wild-type and mutant lines were used. Treatment of TS cells with a blocking antibody for β3 integrin reduces vitronectin (C) but not fibronectin (D). Arnt-/- TS cells adhere to collagen IV (E) and laminin (F) as well as Arnt+/+ TS line 0. Hifα-/- TS cells demonstrate a substantial reduction in adhesion to vitronectin (p < 0.01) and are somewhat reduced in adhesion to fibronectin coated-strips compared with Hifα+/- TS cells (G). Each experiment was performed three times in triplicate. Error bars represent ± SEM. Student's t tests were used for statistical significance.
In the Absence of ARNT, αvβ3 Integrin Does Not Localize to the Cell Surface
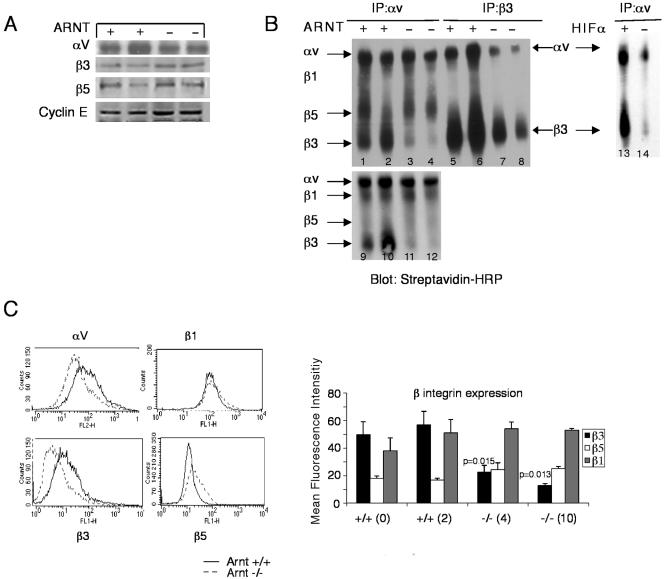
Because the adhesion and migration defects were specific for the ECM proteins vitronectin and fibronectin, we evaluated a role for HIF in the regulation of adhesion receptors. As stated above, integrin αvβ3 is a receptor for both vitronectin and fibronectin. Furthermore, αvβ3 integrin activity has been shown to be important in cytotrophoblast invasion in the human placenta (Zhou et al., 1997a). First, we determined whether HIF regulates expression of αvβ3 integrin. We performed Western blots on TS cell extracts to examine protein levels for αv, β3, and β5 integrins. We found comparable levels of each of these integrins in whole cell lysates prepared from Arnt+/+ and Arnt-/- TS cells (Figure 4A). Cyclin E was used as a loading control. These data suggest that the αv and β3 integrins are not regulated at the level of transcription or translation and therefore are not likely to be direct HIF target genes in TS cells.
Figure 4.
TS cells lacking Arnt exhibit reduced cell surface αvβ3 integrin. (A) Immunoblots showed that integrins αv, β3, and β5 are expressed equivalently in wild-type and mutant TS whole cell lysates. Cyclin E is used as a loading control. (B) TS cells were surface labeled with biotin, and lysates were immunoprecipitated with antibodies for αv integrin or β3 integrin and separated by nonreducing SDS-PAGE, which was subsequently blotted with streptavidin-HRP. Positions of the individual integrins are indicated. Lanes 1, 2, 5, 6, 9, and 10 are Arnt+/+ cell lysates; lanes 3, 4, 7, 8, 11, and 12 are Arnt-/- cell lysates; lane 13 is Hifα+/- cell lysate, whereas lane 14 is Hifα-/- cell lysate. (C) Arnt+/+ and Arnt-/- cells were analyzed by FACS for surface expression of integrins αv, β1, β3, and β5. Representative histograms are shown as well as bar graphs for the mean fluorescence intensity of at least three independent experiments for each integrin. Error bars represent ± SEM. Student's t tests were performed to determine statistical significance. Of note, only one Arnt-/- TS line exhibited reduced surface integrin αv expression.
Next, we examined the cell surface expression of αvβ3 integrin on Arnt+/+, Arnt-/-, Hifα+/-, and Hifα-/- TS cells. TS cell surfaces were biotinylated, and whole cell lysates were immunoprecipitated with antibodies to either αv integrin or β3 integrin. Immunoprecipitations (IPs) were fractionated by nonreducing SDS-PAGE, blotted, and probed with streptavidin-horseradish peroxidase (HRP). The top left panel of Figure 4B presents an experiment where IPs were washed under high salt conditions (0.6 M NaCl), causing β1 integrin to dissociate from αv integrin. The bottom panel represents IPs washed less stringently (0.3 M NaCl), and the position of β1 integrin is indicated. Arnt+/+ TS cells lysates immunoprecipitated with antibody to αv integrin exhibited expression of αvβ1, αvβ5, and αvβ3 integrin dimers on their cell surface (Figure 4B, lanes 1, 2, 9, and 10). Additionally, when Arnt+/+ extracts were immunoprecipitated with β3 integrin antibody, substantial αvβ3 integrin was detected on the cell surface (Figure 4B, lanes 5 and 6). In contrast, Arnt-/- TS cells showed dramatically decreased αvβ3 integrin on the cell surface when lysates were immunoprecipitated with antibody against αv integrin (lanes 3, 4, 11, and 12) or β3 integrin (lanes 7 and 8). The top right panel of Figure 4B depicts Hifα+/- and Hifα-/- TS cells that have been biotinylated and immunoprecipitated with integrin αv. Hifα-/- TS cells exhibit reduced surface αvβ3 integrin compared with Hifα+/- TS cells (Figure 4B, lanes 13 and 14). Importantly, Arnt-/- TS cells expressed comparable levels of αvβ1 integrin and αvβ5 integrin on their cell surface compared with Arnt+/+ TS cells. We also used FACS analysis to determine the extent of cell surface expression for integrins αv, β1, β3, and β5. Only one of the Arnt-/- TS cell lines expressed less αv integrin on the cell surface than wild-type cells, so this is not a genotype-specific phenomenon. Instead, we found that β3 integrin cell surface expression was decreased in both Arnt-/- TS cell lines (Figure 4C) whereas integrins β1 and β5 were expressed at wild-type levels. In conclusion, HIF regulation of integrin αvβ3 cell surface expression seems to mediate adhesion to vitronectin and fibronectin.
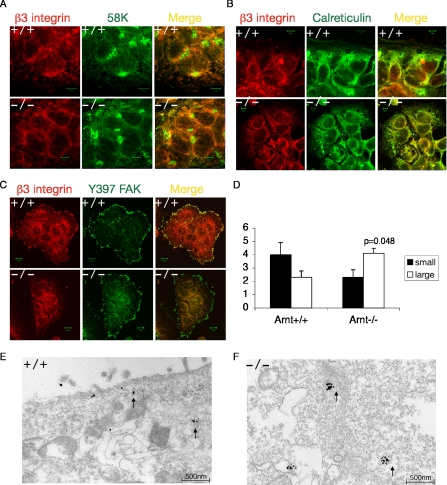
Because β3 integrin seemed to be translated normally in Arnt-/- TS cells, we examined cellular localization of β3 integrin by performing immunofluorescence confocal microscopy analysis. TS cells grow as epithelial sheets, so the micrographs shown consist of undifferentiated cells within a sheet and not individual cells. We first determined whether β3 integrin localized to the Golgi apparatus by analyzing colocalization between β3 integrin and 58K, a microtubule-binding Golgi protein (Bashour and Bloom, 1998). Arnt+/+ and Arnt-/- TS cells were initially stained for β3 integrin (in red) and 58K (in green) by using fluorescently conjugated antibodies (Figure 5A). The yellow color present in the merged image of both Arnt+/+ and Arnt-/- TS cells indicates colocalization between β3 integrin and 58K, suggesting that β3 integrin is present in the Golgi (Figure 5A). We subsequently assessed colocalization between β3 integrin (red) and the ER protein calreticulin (green). β3 integrin was detected in the ER in both Arnt+/+ and Arnt-/- TS cells (Figure 5B). When integrins are activated, they cluster at focal adhesions to mediate attachment to the ECM (Giancotti and Ruoslahti, 1999). Focal adhesions are composed of structural proteins, cytoskeletal proteins, and signaling molecules. On integrin clustering, focal adhesion kinase (FAK) becomes phosphorylated at tyrosine 397 (Rodriguez-Fernandez, 1999). To determine whether β3 was present in TS cell focal adhesions, colocalization between β3 integrin and phosphorylated FAK was examined. Although integrin β3 was localized with phosphorylated FAK in wild-type cells, very little β3 integrin was associated with focal adhesions in Arnt-/- TS cells (Figure 5C). In agreement with the decreased β3 integrin observed by FACS and immunoprecipitation assays, β3 integrin transits through the Golgi and ER in Arnt-/- TS cells but is not transported to focal adhesions at the cell surface.
Figure 5.
Subcellular localization of integrin β3 in TS cells. Confocal immunofluorescence microscopy was performed on Arnt+/+ and Arnt-/- TS cells showing colocalization of β3 integrin with the Golgi marker 58K (A), calreticulin in the ER (B), and phosphorylated FAK Y397 (C). Integrin β3 is in red and the other markers are in green. The yellow image is a red/green merge to show colocalization. Intracellular small and large gold particle clusters for β3 integrin in wild-type and Arnt-/- TS cells from immunoelectron micrographs was quantitated (D). Immunoelectron micrographs for β3 integrin in Arnt+/+ (E) and Arnt-/- TS cells (F). Bar, 500 nm. Error bars represent ± SEM. Student's t tests were performed to determine statistical significance.
To further analyze β3 subcellular localization, we performed immuno-EM for β3 integrin. Immuno-EM is an unbiased approach used to examine the association of a particular protein with ultrastructural features or subcellular compartments. In Arnt+/+ TS cells, β3 integrin-specific gold particles were localized near the cell surface and in small intracellular clusters (Figure 5E). In direct contrast, β3 integrin was never present on the cell surface in Arnt-/- cells. Instead, there were large clusters of intracellular β3-specific gold particles, indicating that β3 integrin was localized in the cell interior (Figure 5F). These gold particle aggregates seemed to be in membrane-bound compartments, which may be some type of vesicle. To quantitate these findings, we counted small gold particle clusters (1 gold particle) or large gold particle clusters (≥3 gold particles) for at least nine electron micrographs for Arnt+/+ and Arnt-/- TS cells. Arnt-/- TS cells had significantly more large clusters of gold particles (p = 0.048) and fewer small clusters of gold particles (Figure 5D) than wild-type cells. Therefore, β3 integrin seems to be accumulating in a post-Golgi, membrane-bound compartment in Arnt-/- TS cells. Accumulation in this compartment either prevents transport of β3 integrin to the cell surface or β3 integrin accrues in this location because it is unable to be transported properly to the surface. These results provide further evidence that β3 integrin is intracellular but does not reach the cell surface in TS cells deficient for HIF.
Hypoxia Regulates αvβ3 Integrin-mediated Adhesion and Migration in Melanoma Cells
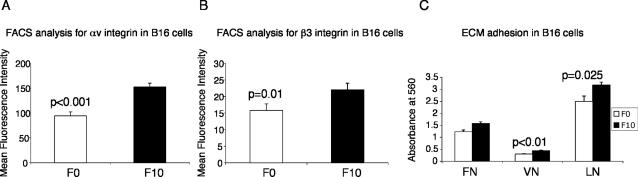
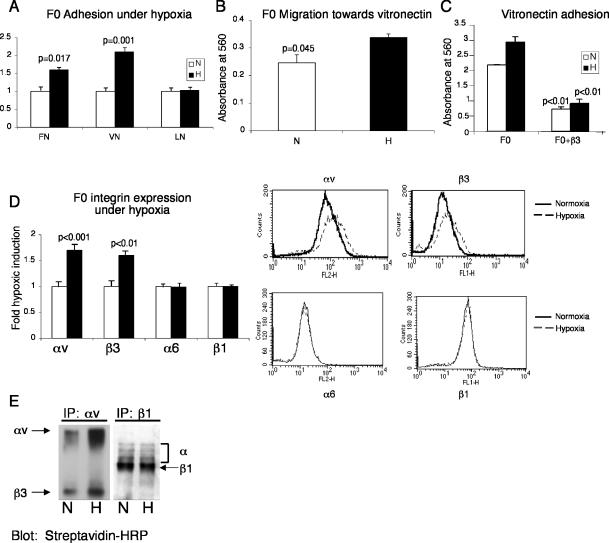
Integrin expression and activation is important in both developmental processes and pathological conditions. It has been reported that hypoxia is associated with radiation resistance in tumors and predicts poor patient outcome (Vaupel et al., 2001). Additionally, αvβ3 integrin expression correlates with metastasis or an increased invasive phenotype in a number of tumor cell types such as melanoma, ovarian carcinoma, hepatocellular carcinoma, and breast cancer (Carreiras et al., 1996; Wong et al., 1998; Nejjari et al., 2002; Jin and Varner, 2004). We examined the possible regulation of αvβ3 integrin by hypoxia in melanoma cells. We assayed both highly metastatic B16F10 murine melanoma cells and poorly metastatic B16F0 cells for cell surface expression of αvβ3 integrin. Based on FACS analysis, B16F10 cells exhibited 50% more αv integrin (p < 0.001) and 25% more β3 integrin (p = 0.01) on the cell surface than B16F0 cells (Figure 6, A and B). Unlike the TS cells, culturing cells at 1.5% O2 for 24 h induced αvβ3 integrin protein expression in B16F0 and F16F10 cells approximately twofold (our unpublished data). Of note, the highly metastatic B16F10 cells were more adherent to vitronectin (p < 0.01) and laminin (p = 0.025) than the poorly metastatic B16F0 cells (Figure 6C). To further investigate the role of hypoxia in tumor progression, we examined changes in adhesion properties of melanoma cells in response to hypoxic culture conditions. We subjected B16F0 cells to 24 h of 1.5% O2 and measured adhesion to fibronectin, vitronectin, and laminin. Hypoxia increased B16F0 adhesion to fibronectin and vitronectin by 1.6-fold (p = 0.017) and 1.7-fold (p = 0.001), respectively, compared with culture in 20% O2 (Figure 7A). In contrast, adhesion to laminin was unaltered by hypoxic exposure (Figure 7A).
Figure 6.
Poorly metastatic B16F0 melanoma cells express less cell surface αvβ3 integrin than highly metastatic B16F10 cells. (A) Mean fluorescence intensity for αv integrin on B16F0 and B16F10 cells based on FACS analysis. (B) Mean fluorescence intensity for β3 integrin on B16F0 and B16F10 cells. (C) B16F0 and B16F10 cells were plated on strips coated with FN, VN, or LN and stained with crystal violet. Solubilized crystal violet incorporated by adherent cells was measured on a plate reader at 560 nm. Error bars represent ± SEM. Student's t tests were performed to determine statistical significance.
Figure 7.
Adhesion to vitronectin and migration toward vitronectin is increased in B16F0 melanoma cells cultured in 1.5% O2 versus 20% O2, via increased cell surface expression of αvβ3. (A) An increase in adhesion to FN and VN is observed in hypoxic B16F0 cells. Adhesion to LN is not changed by hypoxia. (B) Migration toward vitronectin is also enhanced by hypoxia in F0 cells. (C) Adhesion to vitronectin under normoxia and hypoxia is mediated by the β3 integrin because a blocking antibody for β3 integrin diminishes adhesion to vitronectin. (D) B16F0 cells exhibit increased αvβ3 integrin surface expression when treated with 1.5%O2 for 24 h. Surface levels of α6 and β1 integrins are unaltered by hypoxia. FACS analysis for integrins αv, β3, α6, and β1 was performed on cells cultured in either 20% O2 (solid line) or 1.5%O2 (dotted line). (E) B16F0 cells were surface labeled with biotin and immunoprecipitated for αv integrin or β1 integrin. IPs were separated by nonreducing SDS-PAGE and blotted with streptavidin-HRP. Positions of αv integrin, β3 integrin, and β1 integrin are indicated. Error bars represent ± SEM. Student's t tests were performed to determine statistical significance.
We also measured B16F0 cell migration toward vitronectin. Treatment of B16F0 cells with 1.5% O2 resulted in increased vitronectin-associated migration (p = 0.045) (Figure 7B). Assays for invasion of B16F0 and B16F10 cells into Matrigel were performed; unfortunately, both cell lines failed to perform in these experiments When a blocking antibody for β3 integrin was added to B16F0 cells, adhesion to vitronectin under both normoxia and hypoxia was decreased (p < 0.01) (Figure 7C), suggesting that adhesion to vitronectin is mediated by β3 integrin. In contrast to B16F0 cells, exposure of B16F10 cells to 1.5% O2 for 24 h did not significantly increase adhesion to vitronectin (our unpublished data). We then determined the effect of hypoxia on αvβ3 integrin cell surface expression. B16F0 cells were cultured at 20% O2 and 1.5% O2 for 24 h, and cells were harvested, stained with specific antibodies, and analyzed by FACS. B16F0 cells exhibited increased αv integrin (p < 0.001) and β3 integrin (p < 0.01) cell surface expression under hypoxia (Figure 7D). However, cell surface expression of α6 and β1 integrins did not increase after hypoxic exposure (Figure 7D).
B16F0 cell surfaces also were labeled with biotin to detect membrane-bound proteins. Immunoprecipitation with antibodies to αv integrin confirmed that B16F0 cells exhibited increased αvβ3 integrin cell surface expression under hypoxia, whereas immunoprecipitation with an antibody for β1 integrin showed no change in the abundance of integrin β1 on the cell surface after hypoxic treatment (Figure 7E). In conclusion, hypoxia specifically induces αvβ3 integrin expression in the poorly metastatic B16F0 melanoma cells, resulting in increased adhesion and migration. However, the mechanism of hypoxic induction is slightly different from that of TS cells because hypoxia increases αvβ3 total cellular protein in B16F0 melanoma cells.
DISCUSSION
Data presented here demonstrate that HIF signaling is important for both adhesion of undifferentiated TS cells to vitronectin and migration properties of these cells, via control of subcellular localization of αvβ3 integrin. We also show that hypoxia promotes tumor cell migration through regulation of αvβ3 integrin-mediated adhesion. Therefore, the HIF signaling pathway and O2 availability are important in cell motility and adhesion in a number of biological contexts.
HIF Regulates Adhesion and Migration
Placental development initially occurs in a natural O2 gradient, and O2 sensing via HIF activity in the placenta is critical for trophoblast cell fate adoption and trophoblast invasion (Adelman et al., 2000; Cowden Dahl, Mack, Compernolle, Adelman, Carmeliet, and Simon, unpublished data). Trophoblast adhesion and invasion are critical for embryo implantation into the maternal tissue and subsequent placentation. Therefore, we examined the ability of HIF to regulate trophoblast adhesion and migration. Because embryos lacking ARNT or the HIFα subunits exhibit shallow placental invasion, we used TS cells derived from Arnt-/- blastocysts to assess whether the invasion defect in vivo is autonomous to trophoblasts. Normoxic undifferentiated TS cells were analyzed because they represent the most homogenous cell population. TS cells exhibit unusually high levels of HIF activity at 20% O2 conditions, thereby allowing us to study HIF-dependent processes under normal O2 tensions. TS cells deficient in the ARNT subunit of HIF migrated and adhered poorly to vitronectin and fibronectin, suggesting HIF regulates these events. The specificity of the adhesion defect suggests that HIF specifically mediates adhesion through the αvβ3 integrin pair and not through other integrins.
Other groups have examined hypoxic regulation of αvβ3 integrin in endothelial cells. Hypoxia transcriptionally stimulates integrin αv and β3 mRNA levels in human umbilical vein endothelial cells (HUVECs) (Walton et al., 2000) and integrin β2 in human monocytes (Kong et al., 2004). However, we did not observe changes in integrin αvβ3 protein levels in hypoxic TS cell or HUVECs (Figure 4C; out unpublished data). Treatment of bovine retinal endothelial cells with exogenous VEGF induces αv and β3 integrin expression; however, VEGF treatment does not alter expression of either αv integrin or β3 integrin in TS cells (Suzuma et al., 1998; our unpublished data). In Arnt-/- TS cells, β3 integrin is transcribed, translated, and transits through the Golgi and ER, but it fails to cluster at focal adhesions on the cell surface. Therefore, the regulation of αvβ3 integrin in TS cells seems to be distinct from that in endothelial cells.
As the trophectoderm of the blastocyst differentiates into trophoblasts, cell polarity, motility, and adhesion are altered (Sutherland, 2003). Integrins α5, α6, αv, αIIb, β1, and β3 are expressed on the surface of blastocysts (Sutherland et al., 1993; Schultz et al., 1997). Antibodies against integrins α5, αv, β1, and β3 block adhesion of blastocysts to fibronectin, an early event in trophoblast differentiation; therefore, αvβ3 integrin-mediated adhesion may be important in trophoblast differentiation and function (Schultz and Armant, 1995). After the embryo implants into the uterus, integrin localization changes. Contact with uterine ECM induces protein trafficking of the laminin receptors α5β1 integrin and α7β1 integrin from the basal surface to the apical surface of the trophectoderm (Schultz et al., 1997; Klaffky et al., 2001; Sutherland, 2003). Trafficking of integrins in trophoblasts seems to be an important regulatory step in trophoblast differentiation.
Placental development is coordinated with invasion into maternal decidua. In preeclampsia, cytotrophoblast invasion is greatly compromised. Preeclampsia is a pregnancy-associated disease that affects 4–5% of all pregnancies and can be fatal (VanWijk et al., 2000). Hypoxia may affect placental invasion in vivo because preeclamptic placentas are hypoxic and exhibit reduced invasion into maternal deciduae (VanWijk et al., 2000; Goldman-Wohl and Yagel, 2002). Preeclamptic placentas express low levels of β3 integrins throughout the placenta compared with normal placentas (Zhou et al., 1997a), suggesting that this integrin may be important in proper placental progression. Furthermore, αvβ3 integrin and αIIbβ3 integrin regulate migration and trophoblast outgrowth on fibronectin (Rout et al., 2004). Interestingly, β3 integrin is expressed throughout the murine placenta. However, the subcellular localization of β3 integrin is different for distinct trophoblast subtypes (Bowen and Hunt, 1999). β3 integrin is localized to the membrane in labyrinthine trophoblasts, whereas it is diffuse and cytoplasmic in spongiotrophoblasts (Bowen and Hunt, 1999), suggesting that β3 integrin localization and expression may be a developmentally regulated event. β3 integrin is not essential for placentation because integrin β3-/- mice are viable; however, β3-/- mice exhibit reduced fecundity. β3 integrin function is likely important for normal pregnancy, because the viability of integrin β3-/- embryos is reduced due to placental defects (Hodivala-Dilke et al., 1999). Of note, 80% of αv integrin-null embryos die at midgestation with placental defects such as a reduced labyrinthine layer, fewer intermingled fetal and maternal blood vessels, and aberrant spongiotrophoblast and giant cell layers (Bader et al., 1998). We have shown that HIF activity is critical to proper establishment of each placental layer (Cowden Dahl, Mack, Compernolle, Adelman, Carmeliet, and Simon, unpublished data). HIF may regulate trophoblast differentiation and invasion, in part, through regulation of αvβ3 integrin. Importantly, TS cells differentiated for 4 d also fail to express high levels of cell surface αvβ3 integrin (our unpublished data), suggesting that αvβ3 integrin is not regulated properly during differentiation either. These results strongly suggest that misexpression of αvβ3 may occur in HIF-deficient placentas. Integrin-mediated defects are not as severe as those noted for HIF-deficient placentas, likely due to misexpression of additional target genes in HIF mutants. In summary, HIF regulation of genes critical to placentation in addition to the timing of αvβ3 integrin surface expression contributes to placental invasion of maternal tissue.
HIF is critical to trophoblast adhesion and migration and is also important in these processes in a variety of disease states. For example, hypoxia and oxidative stress are major factors in inflammation, cardiovascular disease, and cancer (Paul et al., 2004; Peyssonaux and Johnson, 2004). HIF is critical to inflammatory responses because myeloid cells lacking HIF1α are defective in migration and invasion (Cramer et al., 2003). Similar to our findings in TS cells, αvβ3 integrin may be important in the normal oxygen-regulated inflammatory responses of macrophages. For example, macrophage differentiation is marked by increased expression of αvβ3 integrin (Lafrenie et al., 2002).
Hypoxia Promotes Tumor Cell Motility
As tumors expand, they can outgrow their vascular supply, resulting in regions of the tumor deficient in O2 and nutrients. Induction of genes in this hypoxic environment is important for further tumor progression. For example, VEGF induction in melanomas enhances both tumor angiogenesis and metastasis (Rofstad and Danielsen, 1999). In a variety of human cancer, tumor hypoxia is associated with increased metastatic potential. Although many mechanisms are involved in this process, changes in integrin activity may contribute to metastatic progression. We propose that one way tumor cells become more motile is by hypoxic exposure, because hypoxic exposure enhances αvβ3 integrin surface expression and adhesion to vitronectin in poorly metastatic B16F0 cells. Additionally, modulation of αvβ3 integrin expression could have consequences for tumor angiogenesis, which is linked to αvβ3 integrin. To this end, integrin inhibitors are currently in clinical trials as cancer therapeutics.
In summary, we demonstrate that HIF activity is a critical regulator of undifferentiated TS cell adhesion and migration. Functional inactivation of HIF results in decreased αvβ3 integrin cell surface expression. αvβ3 integrin is not appropriately transported to the cell surface of Arnt-/- TS cells, which adhere poorly to αvβ3 integrin ligands such as vitronectin and fibronectin. Furthermore, hypoxia regulates adhesion and migration of mouse melanoma cells through the regulation of αvβ3 integrin expression at the plasma membrane. These data illustrate that HIF activity controls adhesion, migration, and invasion through regulation of a key integrin in both development and cancer.
Acknowledgments
We thank Q. C. Yu and Neelima Shah for work on the immuno-EM experiments. This work is supported by National Research Service Award predoctoral fellowship award F31 HL10378 (to K.D.C.D.), National Institutes of Health grant R01 66331, and the Abramson Family Cancer Research Institute (to K.D.C.D. and M.C.S.). M.C.S. is an investigator for the Howard Hughes Medical Institute.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-12-1082) on February 2, 2005.
References
- Abbott, B. D., and Buckalew, A. R. (2000). Placental defects in ARNT-knock-out conceptus correlate with localized decreases in VEGF-R2, Ang-1, and Tie-2. Dev. Dyn. 219, 526-538. [DOI] [PubMed] [Google Scholar]
- Adelman, D. M., Gertsenstein, M., Nagy, A., Simon, M. C., and Maltepe, E. (2000). Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 14, 3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman, D. M., Maltepe, E., and Simon, M. C. (1999). Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 13, 2478-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader, B. L., Rayburn, H., Crowley, D., and Hynes, R. O. (1998). Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all α v integrins. Cell 95, 507-519. [DOI] [PubMed] [Google Scholar]
- Bashour, A. M., and Bloom, G. S. (1998). 58K, a microtubule-binding Golgi protein, is a formiminotransferase cyclodeaminase. J. Biol. Chem. 273, 19612-19617. [DOI] [PubMed] [Google Scholar]
- Bowen, J. A., and Hunt, J. S. (1999). Expression of cell adhesion molecules in murine placentas and a placental cell line. Biol. Reprod. 60, 428-434. [DOI] [PubMed] [Google Scholar]
- Carreiras, F., Denoux, Y., Staedel, C., Lehmann, M., Sichel, F., and Gauduchon, P. (1996). Expression and localization of αv integrins and their ligand vitronectin in normal ovarian epithelium and in ovarian carcinoma. Gynecol. Oncol. 62, 260-267. [DOI] [PubMed] [Google Scholar]
- Ceradini, D. J., Kulkarni, A. R., Callaghan, M. J., Tepper, O. M., Bastidas, N., Kleinman, M. E., Capla, J. M., Galiano, R. D., Levine, J. P., and Gurtner, G. C. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 10, 858-864. [DOI] [PubMed] [Google Scholar]
- Compernolle, V., et al. (2002). Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat. Med. 8, 702-710. [DOI] [PubMed] [Google Scholar]
- Compernolle, V., Brusselmans, K., Franco, D., Moorman, A., Dewerchin, M., Collen, D., and Carmeliet, P. (2003). Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxiainducible factor-1α. Cardiovasc. Res. 60, 569-579. [DOI] [PubMed] [Google Scholar]
- Cramer, T., Yamanishi, Y., Clausen, B. E., Forster, I., Pawlinski, R., Mackman, N., Haase, V. H., Jaenisch, R., Corr, M., Nizet, V., et al. (2003). HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Z. M., et al. (1999). Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 163, 5029-5038. [PubMed] [Google Scholar]
- Ema, M., Taya, S., Yokotani, N., Sogawa, K., Matsuda, Y., and Fujii-Kuriyama, Y. (1997). A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl. Acad. Sci. USA 94, 4273-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann, B., Fransvea, E., O'Toole, T. E., Manzuk, L., Faha, B., and Hensler, M. (2002). Involvement of tumor cell integrin αvβ3 in hematogenous metastasis of human melanoma cells. Clin. Exp. Metastasis. 19, 427-436. [DOI] [PubMed] [Google Scholar]
- Firth, J. D., Ebert, B. L., Pugh, C. W., and Ratcliffe, P. J. (1994). Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc. Natl. Acad. Sci. USA 91, 6496-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe, J. A., Jiang, B. H., Iyer, N. V., Agani, F., Leung, S. W., Koos, R. D., and Semenza, G. L. (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen, K. R., Davis, G. E., and Sriramarao, P. (1992). Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin. Exp. Metastasis 10, 111-120. [DOI] [PubMed] [Google Scholar]
- Giancotti, F. G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028-1032. [DOI] [PubMed] [Google Scholar]
- Goldman-Wohl, D., and Yagel, S. (2002). Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol. Cell. Endocrinol. 187, 233-238. [DOI] [PubMed] [Google Scholar]
- Gu, Y. Z., Hogenesch, J. B., and Bradfield, C. A. (2000). The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 40, 519-561. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke, K. M., McHugh, K. P., Tsakiris, D. A., Rayburn, H., Crowley, D., Ullman-Cullere, M., Ross, F. P., Coller, B. S., Teitelbaum, S., and Hynes, R. O. (1999). β3-Integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Investig. 103, 229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki, T., Yamamoto, K., Matsuura, T., Sugimura, M., Kobayashi, T., and Kanayama, N. (2004). Alteration of integrins under hypoxic stress in early placenta and choriocarcinoma cell line BeWo. Gynecol. Obstet. Investig. 57, 196-203. [DOI] [PubMed] [Google Scholar]
- Iyer, N. V., et al. (1998). Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H., and Varner, J. (2004). Integrins: roles in cancer development and as treatment targets. Br. J. Cancer. 90, 561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaffky, E., Williams, R., Yao, C. C., Ziober, B., Kramer, R., and Sutherland, A. (2001). Trophoblast-specific expression and function of the integrin α7 subunit in the peri-implantation mouse embryo. Dev. Biol. 239, 161-175. [DOI] [PubMed] [Google Scholar]
- Koike, T., et al. (2004). Hypoxia induces adhesion molecules on cancer cells: a missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc. Natl. Acad. Sci. USA 101, 8132-8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, T., Eltzschig, H. K., Karhausen, J., Colgan, S. P., and Shelley, C. S. (2004). Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of β2 integrin gene expression. Proc. Natl. Acad. Sci. USA 101, 10440-10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak, K. R., Abbott, B., and Hankinson, O. (1997). ARNT-deficient mice and placental differentiation. Dev. Biol. 191, 297-305. [DOI] [PubMed] [Google Scholar]
- Lartigau, E., Randrianarivelo, H., Avril, M. F., Margulis, A., Spatz, A., Eschwege, F., and Guichard, M. (1997). Intratumoral oxygen tension in metastatic melanoma. Melanoma Res. 7, 400-406. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Cox, S. R., Morita, T., and Kourembanas, S. (1995). Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 77, 638-643. [DOI] [PubMed] [Google Scholar]
- Maltepe, E., Schmidt, J. V., Baunoch, D., Bradfield, C. A., and Simon, M. C. (1997). Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386, 403-407. [DOI] [PubMed] [Google Scholar]
- Nejjari, M., et al. (2002). Expression, regulation, and function of αV integrins in hepatocellular carcinoma: an in vivo and in vitro study. Hepatology 36, 418-426. [DOI] [PubMed] [Google Scholar]
- Nip, J., Shibata, H., Loskutoff, D. J., Cheresh, D. A., and Brodt, P. (1992). Human melanoma cells derived from lymphatic metastases use integrin αvβ3 to adhere to lymph node vitronectin. J. Clin. Investig. 90, 1406-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okino, S. T., Chichester, C. H., and Whitlock, J. P., Jr. (1998). Hypoxiainducible mammalian gene expression analyzed in vivo at a TATA-driven promoter and at an initiator-driven promoter. J. Biol. Chem. 273, 23837-23843. [DOI] [PubMed] [Google Scholar]
- Paul, S. A., Simons, J. W., and Mabjeesh, N. J. (2004). HIF at the crossroads between ischemia and carcinogenesis. J. Cell. Physiol. 200, 20-30. [DOI] [PubMed] [Google Scholar]
- Peng, J., Zhang, L., Drysdale, L., and Fong, G. H. (2000). The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. Proc. Natl. Acad. Sci. USA 97, 8386-8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti, S., Michieli, P., Galluzzo, M., Mazzone, M., Giordano, S., and Comoglio, P. M. (2003). Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 3, 347-361. [DOI] [PubMed] [Google Scholar]
- Peyssonaux, C., and Johnson, R. S. (2004). An unexpected role for hypoxic response: oxygenation and inflammation. Cell Cycle 3, 168-171. [PubMed] [Google Scholar]
- Rodriguez-Fernandez, J. L. (1999). Why do so many stimuli induce tyrosine phosphorylation of FAK? Bioessays 21, 1069-1075. [DOI] [PubMed] [Google Scholar]
- Rofstad, E. K., and Danielsen, T. (1999). Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br. J. Cancer 80, 1697-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout, U. K., Wang, J., Paria, B. C., and Armant, D. R. (2004). α5β1, αVβ3 and the platelet-associated integrin αIIbβ3 coordinately regulate adhesion and migration of differentiating mouse trophoblast cells. Dev. Biol. 268, 135-151. [DOI] [PubMed] [Google Scholar]
- Ryan, H. E., Lo, J., and Johnson, R. S. (1998). HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 17, 3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, J. F., and Armant, D. R. (1995). β1- and β3-Class integrins mediate fibronectin binding activity at the surface of developing mouse peri-implantation blastocysts. Regulation by ligand-induced mobilization of stored receptor. J. Biol. Chem. 270, 11522-11531. [DOI] [PubMed] [Google Scholar]
- Schultz, J. F., Mayernik, L., Rout, U. K., and Armant, D. R. (1997). Integrin trafficking regulates adhesion to fibronectin during differentiation of mouse peri-implantation blastocysts. Dev. Genet. 21, 31-43. [DOI] [PubMed] [Google Scholar]
- Semenza, G. L., Jiang, B. H., Leung, S. W., Passantino, R., Concordet, J. P., Maire, P., and Giallongo, A. (1996). Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 271, 32529-32537. [DOI] [PubMed] [Google Scholar]
- Staller, P., Sulitkova, J., Lisztwan, J., Moch, H., Oakeley, E. J., and Krek, W. (2003). Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 425, 307-311. [DOI] [PubMed] [Google Scholar]
- Sutherland, A. (2003). Mechanisms of implantation in the mouse: differentiation and functional importance of trophoblast giant cell behavior. Dev. Biol. 258, 241-251. [DOI] [PubMed] [Google Scholar]
- Sutherland, A. E., Calarco, P. G., and Damsky, C. H. (1993). Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development 119, 1175-1186. [DOI] [PubMed] [Google Scholar]
- Suzuma, K., Takagi, H., Otani, A., and Honda, Y. (1998). Hypoxia and vascular endothelial growth factor stimulate angiogenic integrin expression in bovine retinal microvascular endothelial cells. Investig. Ophthal. Vis. Sci. 39, 1028-1035. [PubMed] [Google Scholar]
- Tanaka, S., Kunath, T., Hadjantonakis, A. K., Nagy, A., and Rossant, J. (1998). Promotion of trophoblast stem cell proliferation by FGF4. Science 282, 2072-2075. [DOI] [PubMed] [Google Scholar]
- Tian, H., Hammer, R. E., Matsumoto, A. M., Russell, D. W., and McKnight, S. L. (1998). The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 12, 3320-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWijk, M. J., Kublickiene, K., Boer, K., and VanBavel, E. (2000). Vascular function in preeclampsia. Cardiovasc. Res. 47, 38-48. [DOI] [PubMed] [Google Scholar]
- Vaupel, P., Kelleher, D. K., and Hockel, M. (2001). Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin. Oncol. 28, 29-35. [DOI] [PubMed] [Google Scholar]
- Walton, H. L., Corjay, M. H., Mohamed, S. N., Mousa, S. A., Santomenna, L. D., and Reilly, T. M. (2000). Hypoxia induces differential expression of the integrin receptors α(vβ3) and α(vβ5) in cultured human endothelial cells. J. Cell. Biochem. 78, 674-680. [DOI] [PubMed] [Google Scholar]
- Wang, G. L., and Semenza, G. L. (1993). Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513-21518. [PubMed] [Google Scholar]
- Wong, N. C., Mueller, B. M., Barbas, C. F., Ruminski, P., Quaranta, V., Lin, E. C., and Smith, J. W. (1998). αv integrins mediate adhesion and migration of breast carcinoma cell lines. Clin. Exp. Metastasis 16, 50-61. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., Damsky, C. H., and Fisher, S. J. (1997a). Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J. Clin. Investig. 99, 2152-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., Fisher, S. J., Janatpour, M., Genbacev, O., Dejana, E., Wheelock, M., and Damsky, C. H. (1997b). Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Investig. 99, 2139-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]