Abstract
One of the essential features of fungal morphogenesis is the polarized synthesis of cell wall components such as chitin. The actin cytoskeleton provides the structural basis for cell polarity in Aspergillus nidulans, as well as in most other eukaryotes. A class V chitin synthase, CsmA, which contains a myosin motor-like domain (MMD), is conserved among most filamentous fungi. The ΔcsmA null mutant showed remarkable abnormalities with respect to cell wall integrity and the establishment of polarity. In this study, we demonstrated that CsmA tagged with 9× HA epitopes localized near actin structures at the hyphal tips and septation sites and that its MMD was able to bind to actin. Characterization of mutants bearing a point mutation or deletion in the MMD suggests that the interaction between the MMD and actin is not only necessary for the proper localization of CsmA, but also for CsmA function. Thus, the finding of a direct interaction between the chitin synthase and the actin cytoskeleton provides new insight into the mechanisms of polarized cell wall synthesis and fungal morphogenesis.
INTRODUCTION
The filamentous fungus Aspergillus nidulans grows by generating ordered networks of filaments, or hyphae, which form a mycelium. Chitin, a β-1,4-linked homopolymer of N-acetylglucosamine (GlcNAc), is one of the major structural components of the fungal cell wall. The temporal and spatial regulation of its metabolism is very important for tip growth and the morphogenesis of a number of filamentous fungi (Bulawa, 1993; Cid et al., 1995). Actin is concentrated at the growing apices and sites of septum formation, where cell wall synthesis or septal synthesis is active (Harris et al., 1994; Momany and Hamer, 1997). Cytochalasin A, an inhibitor of actin polymerization, has been shown to induce the swelling of the hyphal tips and to block septum formation (Harris et al., 1994; Torralba et al., 1998). Thus, the actin cytoskeleton plays important roles in the determination of hyphal polarity (Torralba and Heath, 2001). Chitin synthases, membrane-bound proteins that catalyze the polymerization of GlcNAc from UDP-GlcNAc as a substrate, have been classified into at least six groups, classes I to VI, on the basis of the structures of their conserved region (Roncero, 2002). We have isolated five chitin synthase genes from A. nidulans and designated them as chsA, chsB, chsC, chsD, and csmA, and their gene products belong to classes II, III, I, IV, and V, respectively (Motoyama et al., 1994, 1996; Yanai et al., 1994; Fujiwara et al., 1997). The csmA (chitin synthase with a myosin motor-like domain) gene encodes a protein (1852 amino acids) consisting of an N-terminal myosin motor-like domain (MMD, ∼800 amino acids) and a C-terminal chitin synthase domain (CSD, ∼750 amino acids). Myosins are known as mechanoenzymes that convert chemical energy, liberated through ATP hydrolysis, into a mechanical force that is directed along actin filaments. The MMD of CsmA bears some consensus motifs of myosins, such as P-loop, Switch I, and Switch II, and belongs to class XVII of the myosin family (Hodge and Cope, 2000). The ΔcsmA null mutant showed remarkable abnormalities in both cell wall integrity and the establishment of polarity, including the presence of swollen tubes and newly formed intracellular hyphae referred to as “balloons” and “intrahyphal hyphae,” respectively. Abnormal conidiophore morphologies, such as short stalks and a small population of metulae on the vesicles, were occasionally observed. These phenotypes were suppressed to some extent by osmotic stabilizers. However, these phenotypes were not suppressed when only the CSD-coding region of csmA was expressed under the control of the alcA promoter of A. nidulans (Horiuchi et al., 1999). Over the past few years, the presence of genes encoding class V, CsmA-type chitin synthases has been reported in some filamentous fungi (Park et al., 1999; Zhang and Gurr, 2000; Chigira et al., 2002; Amnuaykanjanasin and Epstein, 2003; Madrid et al., 2003; Liu et al., 2004). CsmA-type chitin synthases have been shown to be required for the pathogenesis of Wangiella dermatidis, a human pathogen, and Fusarium oxysporum, a plant pathogen (Madrid et al., 2003; Liu et al., 2004). All filamentous fungi except for Ashbya gossypii, whose genome sequences are available in publicized databases, have the genes encoding CsmA-type chitin synthases. Moreover, ascomycete filamentous fungi, related to A. nidulans, possess two genes encoding chitin synthases with the MMD (Chigira et al., 2002). On the other hand, no orthologue exists in the genome of the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Thus, it is likely that CsmA links cell wall synthesis to actin cytoskeleton function; thus, CsmA appears to exert unique functions that are specific to filamentous polarized growth.
To date, no studies have been conducted in filamentous fungi to analyze the physiological functions of chitin synthases using biochemical or cell biological techniques. In our previous report, we constructed the CA2 strain that produced a functional CsmA tagged with nine copies of hemagglutinin A epitopes at its C-terminus (CsmA-HA) instead of CsmA (Takeshita et al., 2002). Furthermore, we showed that CsmA-HA with an approximate molecular mass of 210 kDa was produced during vegetative growth, and the amount of this CsmA-HA increased under low osmotic conditions. The purpose of the present study was to reveal the role played by the MMD in the localization of CsmA in the hyphae, with the aim of gaining a better understanding of the mechanism of polarized synthesis of the fungal cell wall and morphogenesis.
MATERIALS AND METHODS
Strains, Media, and Bacterial and Fungal Transformations
The A. nidulans strains used in this study are listed in Table 1. Complete medium, YG medium (0.5% yeast extract, 1% glucose, 0.1% trace elements), minimal medium (MMG), and complete medium or minimal medium containing 100 mM threonine instead of glucose (YT or MMT) for A. nidulans were used (Rowlands and Turner, 1973). YG and MMG plates consisted of YG and MMG containing 1.5% agar. MMG was supplemented with arginine at 0.2 mg/ml, biotin at 0.02 μg/ml, pyridoxine at 0.5 μg/ml, and uridine at 2.44 mg/ml, when necessary. All of the strains used in this study were grown at 37°C. Bacterial and fungal transformations were performed as described previously (Takeshita et al., 2002).
Table 1.
A. nidulans strains used in this article
| Strain | Genotype | Source of reference |
|---|---|---|
| ABPU/A1 | biA1 pyrG89 argB2 pyroA4 wA3 [pSS1] | Fujiwara et al. (2000) |
| CA2 | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA:: csmA-9HA::pyrG | Takeshita et al. (2002) |
| CS4 | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-csmA | Horiuchi et al. (1999) |
| M2–6 | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB | Horiuchi et al. (1999) |
| CH5 | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-csmA(861–1852 aa) | Horiuchi et al. (1999) |
| CSMHA | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-csmA-9HA::pyrG | This study |
| CΔMHA | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-csmA(861–1852 aa)-9HA::pyrG | This study |
| AP | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-EGFP-csmA(G107A) | This study |
| AS | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-EGFP-csmA(S153A) | This study |
| APHA | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-EGFP-csmA(G107A)-9HA::pyrG | This study |
| ASHA | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-EGFP-csmA(S153A)-9HA::pyrG | This study |
| RA | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-csmA(R347A) | This study |
| D10 | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-csmA(Δ347–356) | This study |
| RAHA | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-csmA(R347A)-9HA::pyrG | This study |
| D10HA | biA1 pyrG89 argB2 pyroA4 wA3 ΔcsmA::argB::alcA(p)-csmA(Δ347–356)-9HA::pyrG | This study |
Plasmid Constructions
To create a strain in which the expression of CsmA would be under the control of the alcA promoter, we constructed the plasmid pM-ALC2 as follows. The 2.9-kb PstI-SalI fragment from pM-ALCΔP (Horiuchi et al., 1999) was ligated with PstI and SalI-digested pUC18, to yield pUCAM1. The 0.6-kb SpeI-HindIII fragment from pM-ALC-CHS5 (Horiuchi et al., 1999) was blunted and ligated with SmaI-digested pUCAM1, to yield pM-ALC2. The 3.4-kb EcoRI-PstI fragment of pM-ALC2 was used for transformation. To create strains that have a mutation in the P-loop (G107A) or Switch I (S153A) coding region of csmA (AP, APHA, AS, or ASHA), we introduced a mutation into the 0.8-kb KpnI-PstI fragment of pM-ALC2 using the Takara LA PCR in vitro Mutagenesis Kit (TakaRa, Ohtsu, Japan) and the following primers: 5′-AGTGGCGCCGCTAAAACGACTCTTC-3′ or 5′-CACCTACCGCGGCAAAAGCTGGATTG-3′ (the underlined letters represent nucleotide substitutions). The 0.8-kb KpnI-PstI fragments containing the site-directed mutations were substituted for the corresponding fragments of pM-ALC2, to yield pMAECP and pMAECS. The 4.1-kb EcoRI-PstI fragments of pMAECP and pMAECS were used for transformation. To create the strains (D10 or D10HA), we constructed the plasmid pAD10 as follows. The 2.2-kb PstI fragment from pMK10 (Fujiwara et al., 1997) was ligated with PstI-digested pM-ALC2, to yield pM-ALCP. A primer set of ad10 (5′-ACTGTCATGCTTGATCCCAAG-3′) and adre (5′-CATGAGCATGCCGCTTTTCCT-3′) was used to amplify a 0.8-kb fragment of pMK10 by KOD DNA polymerase (Toyobo, Osaka, Japan). The 0.8-kb fragment was phosphorylated and ligated with BstPI-digested and blunted pM-ALCP, to yield pAD10. The 4.0-kb Bpu1102I-KpnI fragment of pAD10 was used for transformation. To create the strains (RA or RAHA), we constructed the plasmid pARA as follows. A primer set of ara (5′-GCGACCAAGACCATCCATCGG-3′; the underlined letters represent nucleotide substitutions) and adre was used to amplify a 0.8-kb fragment of pMK10. The 0.8-kb fragment of pARA was phosphorylated and ligated with BstPI-digested and blunted pM-ALCP, to yield pARA. The 4.0-kb Bpu1102I-KpnI fragment was used for transformation. To produce the MMD (842 amino acid) fused to EGFP at its C-terminus (wtM) by an in vitro transcription and translation system, we used the plasmid pRSETMGS, which was constructed as follows. The 2.5-kb NcoI-SpeI fragment of pM5C, which contains csmA cDNA, was ligated with NcoI-digested pRSETB (Invitrogen, Carlsbad, CA), to yield pRSETM. The 0.7-kb BamHI-XbaI fragment from pEGFP (CLONTECH, Palo Alto, CA) was ligated with HindIII-digested pRSETM, to yield pR-SETMG. The 0.1-kb NdeI-BamHI fragment from pET-29a (Novagen, Madison, WI) was ligated with NdeI-digested pRSETMG, to yield pRSETMGS. To produce the mutated MMD fused to EGFP at its C-terminus (raM or d10M) by an in vitro transcription and translation system, we constructed pRSETMGSA and pRSETMGSD as follows. The 1.1-kb HpaI-SalI fragment from pARA and pAD10, respectively, was ligated with HpaI and SalI-digested pRSETMGS.
Indirect Immunofluorescence Microscopy
Cells were fixed and processed as described previously (Harris et al., 1994; Esnault et al., 1999). For cell wall digestion, the coverslips were overlaid for 1 h at room temperature with a 200-μl solution of phosphate-buffered saline (PBS; 13.7 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2HPO4) containing 6 mg Yatalase (TaKaRa), 0.6 mg LYSING ENZYMES L2265 (Sigma, St. Louis, MO), and 1% EGG WHITE (Sigma). The coverslips were washed in PBSP (PBS containing 0.1% Nonidet P-40) and immersed in ethanol at -20°C for 10 min. After being washed several times in PBSP, the coverslips were incubated for 1 h at room temperature with a mouse anti-HA primary antibody (BabCO, Richmond, CA) at a 1:200 dilution or a rabbit anti-actin primary antibody (Sigma) at a 1:500 dilution in PBSB (PBS containing 0.1% bovine serum albumin). After being washed several times in PBSP, the coverslips were incubated for 1 h at room temperature with FITC-conjugated anti-mouse IgG secondary antibody (Sigma) at a 1:100 dilution or Cy3-conjugated anti-rabbit IgG secondary antibody (Sigma) at a 1:500 dilution in the dark. The coverslips were washed several times in PBSP and stained with 0.01% Calcofluor white (fluorescent brightener 28, Sigma) for 5 min. Finally, the coverslips were washed several times in PBSP, mounted on glass slides in 10 μl glycerol-PBS (9:1 [vol/vol], 0.01% p-phenylenediamine), and observed using an Olympus BX52 microscope (Tokyo, Japan). Photographs were taken with ORCA-ER (Hamamatsu, Hamamatsu, Japan) and were developed with AQUACOSMOS (Hamamatsu). For the observation by confocal microscopy, photographs were taken with an Olympus FV500 and were developed with FLUOVIEW (Olympus).
Cytochalasin A Treatment
The CA2 strain was grown in YG medium on coverslips for 12 h at 37°C. The coverslips were overlaid with YG medium containing 0.02% dimethyl sulfoxide with or without 2 μg/ml cytochalasin A for 10, 30, or 60 min at 37°C. Then, the coverslips were washed and used for indirect immunofluorescence microscopy.
Coimmunoprecipitation and Western Blot Analysis
Total cellular extracts were prepared by grinding the mycelia (∼200 mg) with Metal-corn (Yasui kikai, Osaka, Japan) and then with glass beads using a Multi-beads shocker (Yasui kikai) in 200 μl extraction buffer (0.8 M sucrose, 10 mM Tris-HCl, pH 8.2) containing protease inhibitor cocktail (Sigma). The cellular extracts, to which 200 μl of extraction buffer was added, were centrifuged at 5000 × g for 10 min at 4°C. Then, 1.25 ml of ice-cold IP buffer (0.1% Triton X-100, 100 mM NaCl, 10 mM EDTA, 50 mM Tris-HCl) was added to 250 μl of the supernatants. These solutions were incubated with mouse anti-HA antibody (BabCO) with gentle agitation for 1 h at 4°C, and then the solutions were incubated with 10 μl of protein G-Sepharose (Amersham, Piscataway, NJ) for 1 h at 4°C. The reaction mixtures were centrifuged at 5000 × g for 10 min at 4°C to obtain the immunoprecipitated protein. Western blot analysis was performed as described previously (Takeshita et al., 2002). To detect actin, the samples were separated by electrophoresis on 10% polyacrylamide gel and electroblotted onto Hybond ECL nitrocellulose membranes. The membranes were incubated with anti-actin N.350 antibody (Amersham) at a 1:1000 dilution. To detect wtM, raM, and d10M, the samples were separated on 6% polyacrylamide gel, and rabbit polyclonal GFP antiserum (Invitrogen) was used at a 1:5000 dilution.
Pull-down Assay
wtM, raM, and d10M were expressed with a TnT T7 Quick Coupled Transcription/Translation System (Promega, Madison, WI) using pRSETMGS, pRSETMGSA, and pRSETMGSD, respectively, according to the manufacturer's instructions, and the samples were centrifuged for 1 h at 100,000 × g at 4°C. The supernatants were used for pull-down assays. To prepare the F-actin, we polymerized G-actin using an Actin Filament Biochem Kit (Cytoskeleton, Denver, CO) according to the manufacturer's instructions. Two microliters of the supernatant containing wtM, raM, or d10M were incubated with 100 μl of solution containing F-actin or G-actin at 24°C for 1 h. The samples were then centrifuged for 1 h at 100,000 × g at 24°C. The supernatants and pellets were analyzed by Western blotting.
RESULTS
Localization of CsmA-HA
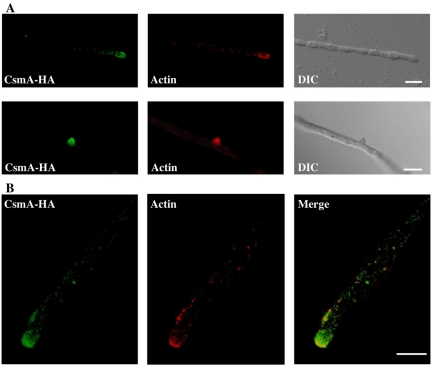
The CA2 strain that produced CsmA-HA instead of CsmA showed wild-type growth and morphology, suggesting that CsmA-HA fully complements the function of CsmA (Takeshita et al., 2002). We stained the hyphae of the CA2 strain with anti-HA antibodies and fluorescent secondary antibodies and showed that CsmA-HA localized to the hyphal tips and sites of septum formation (Figures 1 and 2). No signals were detected in the hyphae of the wild-type strain stained with anti-HA antibodies. To elucidate the relationship to actin distribution, we stained the hyphae of the CA2 strain with anti-HA and anti-actin antibodies. Actin was observed as fine spots and was found to be concentrated at the hyphal tips; these results are in good agreement with those of previous studies using filamentous fungi (Harris et al., 1994; Degousee et al., 2000). The fluorescence of CsmA-HA was observed at sites similar to those at which the actin spots were observed, and the fluorescence was also concentrated at the hyphal tips; however, the fluorescence of CsmA-HA was more diffuse than the spots representing actin (Figure 1A). When imaged by confocal microscopy, the actin spots localized primarily along the apical membrane and were concentrated at the hyphal tips. CsmA-HA localized primarily in and around these actin spots (Figure 1B). CsmA-HA and actin were also concentrated at the tips of the hyphal branches (Figure 1A).
Figure 1.
Localization of CsmA-HA and actin at the hyphal tips. (A) Hyphae of the CA2 strain grown in YG medium overnight were fixed, stained with anti-HA and anti-actin antibodies, and visualized by indirect immunofluorescence. The images obtained from a hyphal tip (top panels) and a branched tip (bottom panels) are shown. (B) The images of a hyphal tip are shown in a single section obtained by confocal microscopy. Scale bar, 5 μm.
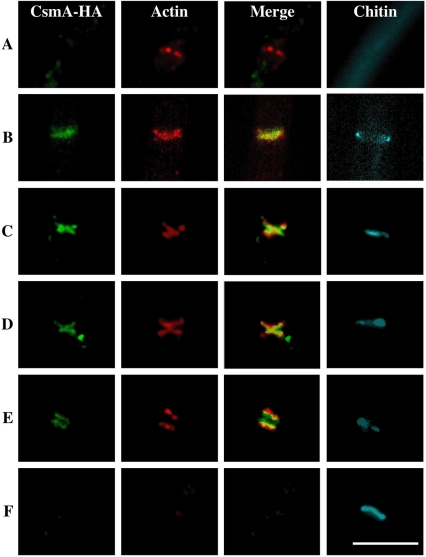
Figure 2.
Changes in CsmA-HA, actin, and chitin localization at septation sites. Hyphae of the CA2 strain grown in YG medium overnight were fixed, stained with anti-HA and anti-actin antibodies, and visualized by indirect immunofluorescence. The images of CsmA-HA and actin were merged. These hyphae were stained simultaneously with Calcofluor white. Each image was arranged in the order of the deduced time course of septum development (A–F). Scale bar, 5 μm.
Septum formation in A. nidulans has been suggested to proceed according to the following series of steps: contraction of the actin ring, actin-mediated invagination of the plasma membrane, and deposition of the chitinous primary septum (Momany and Hamer, 1997). The images sets for CsmA-HA, actin, and chitin that were visualized with Calcofluor white were aligned in the order of events occurring during septum development, which was deduced from the distribution patterns of actin and chitin (Figure 2, A–F). The characteristics of each set are as follows: (A) Actin appeared as a single band and was most likely forming a ring. No CsmA-HA or Calcofluor white fluorescence was detected. (B) CsmA-HA appeared as a single band at almost the same position of the actin band. Calcofluor white fluorescence appeared as a ring at the rim of the same septation site. (C and D) The actin was shaped like the letter X. In fact, this should have been an hourglass-like structure. CsmA-HA formed a structure very similar to actin in its proximity. (E) Both X-shaped actin and CsmA-HA were split into two bands. Both of them were presumably assuming a ring or disk-like form, and the two CsmA-HA bands appeared between the two actin bands. CsmA-HA was only partially colocalized with actin. At this stage, the actin ring was in the cytoplasm, whereas the CsmA-HA was probably anchoring to the membranous structures such as the plasma membrane. Calcofluor white fluorescence localized between the two CsmA-HA bands. (F) Most of the septa were stained with Calcofluor white, and neither actin nor CsmA-HA localized in the vicinity of the septa. These results suggest that CsmA-HA localizes temporarily at septation sites during septum formation, and then CsmA-HA disappear at those sites after the maturation of the septa.
Effect of Cytochalasin A on the Localization of CsmA
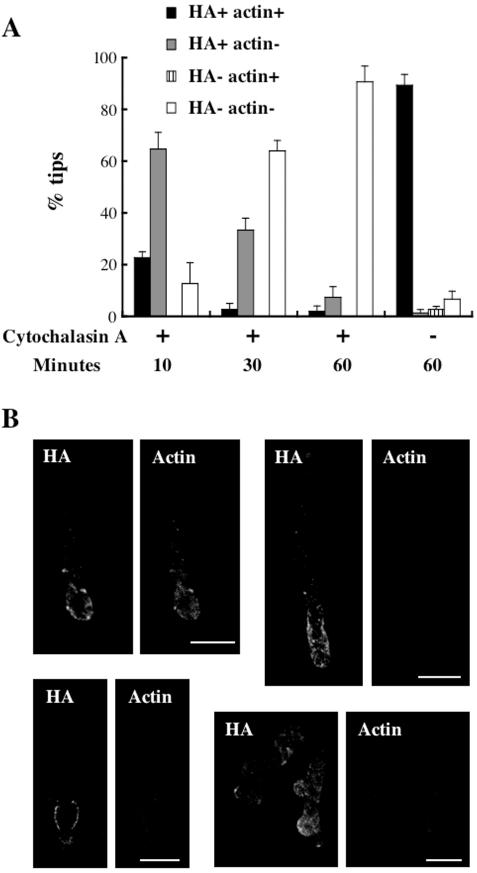
To investigate the role of filamentous actin in the localization of CsmA, we examined effect of cytochalasin A, an inhibitor of actin polymerization, on the localization of CsmA in the CA2 strain (see Materials and Methods). After a 10-min treatment with cytochalasin A, weak fluorescence of filamentous actin was only observed at ∼20% of hyphal tips. CsmA-HA fluorescence signals were observed at roughly 90% of hyphal tips and often diffused to subapical regions (Figure 3, A and B, top panels). After 30-min, morphologically abnormal hyphal tips were frequently observed. Actin structures became hardly detectable at the hyphal tips and the number of hyphal tips with the CsmA-HA fluorescence decreased to 40% of total. The detectable CsmA-HA often localized at the swollen hyphae nearby the tips (Figure 3B, bottom panels). After 60-min, CsmA-HA was detected at ∼10% of hyphal tips (Figure 3A). Without cytochalasin A treatment, most hyphae appeared morphologically normal and both actin and CsmA-HA were detected at all the hyphal tips so far examined (Figure 3A). These results suggest that inhibition of actin filament formation impairs the localization of CsmA-HA at the hyphal tips. Because actin and CsmA-HA localize at only a limited number of septa, even under conditions lacking cytochalasin A treatment, we were unable to determine the frequency of septa at which the actin and CsmA-HA disappeared after treatment with cytochalasin A; however, we did observe septa at which CsmA-HA but not actin localized (data not shown). Such septa tended to be rarer after longer cytochalasin A treatment.
Figure 3.
Effect of cytochalasin A treatment on the localization of CsmA. (A) The CA2 strain, grown in YG medium for 12 h, was treated with or without 2 μg/ml cytochalasin A for 10, 30, or 60 min. Thereafter, hyphae of the CA2 strain were fixed, stained with anti-HA and anti-actin antibodies, and visualized by indirect immunofluorescence. The presence of actin and/or CsmA-HA was scored in 50 tips. The percentage of the tips stained with both anti-HA and anti-actin antibodies (HA+ actin+), only anti-HA antibody (HA+ actin-), only anti-actin antibody (HA-actin+), and neither anti-HA nor anti-actin antibody (HA-actin-) is indicated. The data are expressed as the mean ± SD (N = 3). (B) The images of hyphal tips treated with cytochalasin A for 10 min (top panels: left, HA+ actin+; right, HA+ actin-) or 30 min (bottom panels, HA+ actin-) are shown in a single section obtained by confocal microscopy. Scale bar, 10 μm.
Role of MMD in the Localization of CsmA
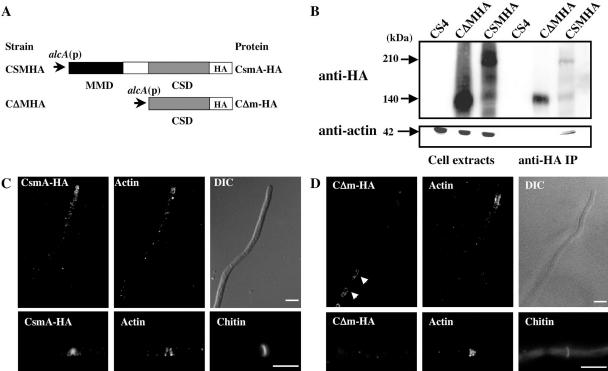
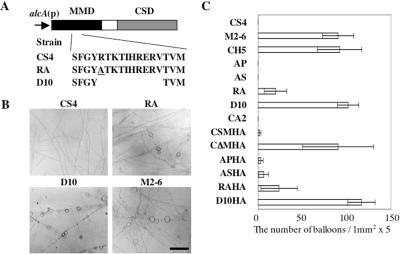
To investigate the role of the MMD in the localization of CsmA, we constructed a CΔMHA strain that expressed the CSD with an HA-tag at its C-terminus (CΔm-HA) under the control of the alcA promoter. We also constructed a CSMHA strain that expressed CsmA-HA under the same promoter as CΔm-HA (Figure 4A). The wild-type csmA was deleted in both strains. Although the CSMHA strain exhibited almost the same phenotype as the ABPU/A1 (wild-type) strain on MMT plates (the alcA promoter-inducing condition) and although very low-frequency, meandering hyphae on agar surface and balloons were observed. These features are morphological characteristics of the M2–6 (ΔcsmA null mutant). This result may have been caused by the additive effect of the addition of the HA-tag to CsmA and the overproduction of CsmA, because neither the CS4 strain expressing csmA under the control of the same alcA promoter, nor the CA2 strain showed such mutant morphologies on the MMT plates (Horiuchi et al., 1999; Takeshita et al., 2002). Successive balloons and intrahyphal hyphae were frequently observed in the CΔMHA strain on the MMT plates, as was observed in the case of the CH5 strain that expressed the CSD lacking the HA-tag under the control of the alcA promoter (Horiuchi et al., 1999). The frequency of balloon formation of the CΔMHA strain was similar to that of the CH5 strain (data are summarized in Figure 6C). It has been suggested that the CsmA-type chitin synthases contain four putative transmembrane regions in the CSD and between the MMD and the CSD (Choquer et al., 2004). It is likely that CΔm-HA has the same membrane topology as CsmA, because CΔm-HA contains all of the putative transmembrane regions.
Figure 4.
Role played by the MMD in the localization of CsmA. (A) Cartoon depicting CsmA-HA and CΔm-HA. The MMD and the CSD are indicated by a black box and a gray box, respectively. (B) Cell extracts of CS4, CΔMHA, and CSMHA strains grown in YT medium, were immunoprecipitated with anti-HA antibody. Immunoprecipitation input and the immunoprecipitated pellets were analyzed by Western blotting using anti-HA or anti-actin antibody. (C and D) Localization of overproduced CsmA-HA (C) or CΔm-HA (D) and actin at the hyphal tips and septa. Hyphae of the CSMHA (C) and the CΔMHA (D) strain, grown in MMT medium overnight, were fixed, stained with anti-HA and anti-actin antibodies, and visualized by indirect immunofluorescence. These hyphae were stained simultaneously with Calcofluor white. Scale bar, 5 μm.
Figure 6.
The effect of mutations of the deduced actin-binding region in the MMD on the function of CsmA. (A) Cartoon depicting ra-CsmA and d10-CsmA. (B) Hyphal morphology of CS4, RA, D10, and M2–6 strains grown on MMT plates for 3 d. Scale bar, 100 μm. (C) The total number of balloons of each strain grown on MMT plates for 3 d. The total number was calculated in five randomly selected 1-mm2 areas of an MMT plate for each strain. The data are expressed as the mean ± SD (N = 3).
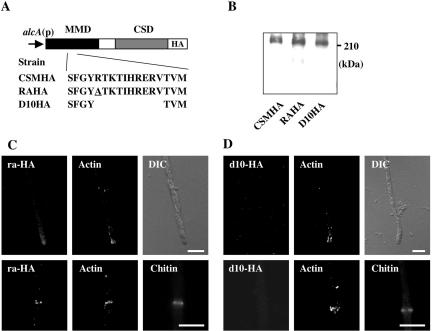
Western blot analysis by anti-HA antibody showed signals of ∼210 and 140 kDa in the CSMHA cell extract and a signal of ∼140 kDa in the CΔMHA cell extract (Figure 4B). The protein corresponding to the 140-kDa band in the CSMHA cell extract is likely to have been a degradation product of CsmA-HA without the MMD (Takeshita et al., 2002). The CSMHA and CΔMHA strains were grown under the alcA promoter-inducing condition (MMT medium), and they were stained with anti-HA and anti-actin antibodies, and with Calcofluor white. In the CSMHA strain, overproduced CsmA-HA was concentrated at both the hyphal tips and the septation sites (Figure 4C), although the fluorescence signals at the hyphal tips were more diffuse than those that appeared in the CA2 strain (Figure 1). In the CΔMHA strain, actin was concentrated at both the hyphal tips and the septation sites, although CΔm-HA was not concentrated at these sites; however, CΔm-HA was localized in large organelles or tubular structures (Figure 4D, arrow), such as vacuoles and/or prevacuolar compartments. These results suggest that the MMD is necessary for the proper localization of CsmA-HA at the hyphal tips and septation sites.
The Characteristics of the MMD
It is possible that the motor activity of the MMD is necessary for the localization of CsmA at hyphal tips and septation sites. To examine this possibility, we constructed four ΔcsmA strains that produced CsmA or CsmA-HA variants under the control of the alcA promoter, and each of the CsmA and CsmA-HA variants contained an amino acid substitution in the P-loop (G107A) or Switch I (S153A) of the MMD (AP, AS, APHA, and ASHA strains; Table 1). This type of substitution in the P-loop or Switch I abolished the GTPase activity of Ras2 of S. cerevisiae or the ATPase activity of Myosin II of Dictyostelium discoideum (Denmat and Jacquet, 1997; Shimada et al., 1997). The ATPase activities of the myosins are essential for their motor activity. The AP and AS strains on the MMT plates (i.e., the alcA promoter-inducing condition) revealed the same hyphal morphology as the CS4 and wild-type strains. Western blot analysis of the extracts of CSMHA, APHA, and ASHA strains with anti-HA antibody showed bands of nearly the same intensity at the expected molecular masses. The APHA and ASHA strains grown on MMT plates exhibited phenotypes similar to that of the CSMHA strain, suggesting that the addition of the HA tag had almost the same effect on the function of the CsmA variants as it did on the wild-type CsmA. We stained the hyphae of the APHA and ASHA strains grown in MMT medium with anti-HA and anti-actin antibodies and Calcofluor white. The CsmA-HA variants in the APHA and ASHA strains localized at both the hyphal tips and the septation sites; moreover, the localization of these variants was identical to that of CsmA-HA in the CSMHA strain (data not shown). These results suggest the possibility that the ATPase activity of the MMD is dispensable for the proper localization and function of CsmA.
Furthermore, we investigated whether or not the MMD is able to bind to actin. CsmA-HA and CΔm-HA were immunoprecipitated with anti-HA antibody from the respective cell extracts of CSMHA and CΔMHA strains, and the immunoprecipitates were analyzed by Western blotting using anti-actin antibody. Actin was coprecipitated with CsmA-HA, but not with CΔm-HA (Figure 4B), suggesting that CsmA interacts with actin through the MMD in vivo. A direct interaction between the MMD and actin filaments was demonstrated by in vitro pull-down assay. The MMD (842 amino acids), which was fused with EGFP at its C-terminus (wtM), was produced by in vitro transcription and translation (Figure 5A) and was incubated with F-actin or G-actin. The wtM was coprecipitated with F-actin, but not with G-actin (Figure 5B). These results indicate that the MMD can directly bind to actin filaments.
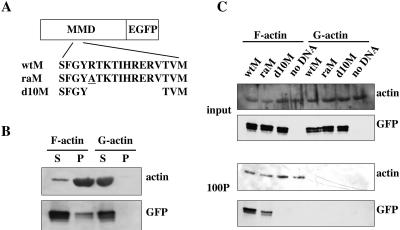
Figure 5.
Interaction between the MMD and actin. (A) Cartoon depicting the wild-type or mutated MMD fused to EGFP at its C-terminus. The amino acid changed in raM is underlined. (B) The MMD-EGFP fusion protein (wtM) was produced by in vitro transcription and translation and was centrifuged at 100,000 × g for 1 h at 4°C. The supernatants were incubated with F- or G-actin for 1 h at 24°C. Then, the samples were centrifuged at 100,000 × g for 1 h at 24°C. The supernatants and pellets were analyzed by Western blotting using anti-actin or anti-GFP antibody. (C) wtM, raM, or d10M was produced in vitro and centrifuged at 100,000 × g for 1 h at 4°C. The supernatants were incubated with F- or G-actin for1hat 24°C. Lysates of an in vitro transcription and translation system without DNA were also incubated as a control. After incubation with F- or G-actin, the samples were centrifuged at 100,000 × g for 1 h at 24°C. The samples were precentrifuged, and the pellets were analyzed by Western blotting using anti-actin or anti-GFP antibody.
The Function and Localization of CsmA Depends on the Interaction between the MMD and the Actin Cytoskeleton
To clarify which region of the MMD is essential for binding to F-actin, we constructed MMD mutants containing either the substitution of an amino acid or a deletion in the deduced actin-binding region of the MMD. One of the putative actin-binding sites of Dictyostelium myosin II is the myopathy loop (Ile398-Leu-Ala-Gly-Arg-Asp-Leu-Val405, the amino acid residues are numbered from the N-terminus), which is located at the distal end of the upper 50-kDa subdomain and is adjacent to the conserved arginine (Arg397). The deletion of the myopathy loop from myosin II abolished its interaction with actin, and the substitution of Gln for Arg397 (R397Q) in myosin II lowered its affinity for actin (Fujita et al., 1997; Sasaki et al., 1999). On the basis of the sequence similarity of the conserved region between myosin II and the MMD, we designed two mutant MMDs, raM and d10M, and produced them by in vitro transcription and translation. In raM, Arg347 (numbered from the N-terminus of CsmA) was substituted with Ala, and in d10M, 10 amino acids (Arg347-Val356) corresponding to the putative myopathy loop were deleted. Both were fused to EGFP at their C-termini (Figure 5A). Their interaction with actin was analyzed by in vitro pull-down assay. The raM coprecipitated with F-actin; however, this proceeded less effectively than in the case of wtM (wild-type MMD), and d10M was not coprecipitated with F-actin at all (Figure 5C). These results indicate that the substitution of Arg347 with Ala (R347A) lowered the affinity of the MMD for actin and that the deletion of 10 amino acids abolished interactions between the MMD and F-actin.
To investigate whether or not the binding of the MMD to actin is a prerequisite for the proper localization and function of CsmA, we constructed RA and D10 strains that produced ra-CsmA and d10-CsmA, respectively, instead of CsmA; in these strains, ra-CsmA and d10-CsmA were produced under the control of the alcA promoter. In this series, ra-CsmA contained an R347A substitution, and d10-CsmA contained a deletion of 10 amino acids (Arg347-Val356) in the MMD of CsmA (Figure 6A). The growth rates of the RA and D10 strains on the MMT plates (the alcA promoter-inducing condition) were similar to that of the CS4 strain. Balloons were occasionally observed in the RA strain on the MMT plates; however, the frequency of their appearance was lower than that of balloons of the M2–6 strain (ΔcsmA; Horiuchi et al., 1999), thus suggesting that ra-CsmA functions only weakly (Figure 6, B and C). On the other hand, successive balloons and intrahyphal hyphae were observed in the D10 strain on the MMT plates, and this phenotype of D10 was very similar to that of the CH5 strain, which produced only the CSD under the control of the alcA promoter (Horiuchi et al., 1999). Hence, it is suggested that d10-CsmA is not functional. Next, we constructed two strains, RAHA and D10HA, which produced ra-CsmA and d10-CsmA, respectively, with the HA-tag at their C-termini instead of CsmA; in both of these strains, the CsmA-HA variants were produced under the control of the alcA promoter. These tagged proteins were referred to as ra-HA and d10-HA, respectively (Figure 7A). Western blot analysis of the extracts of these strains using anti-HA antibody showed bands of almost the same intensity at the expected molecular mass of 210 kDa (Figure 7B), suggesting that ra-HA and d10-HA are as stable as CsmA-HA. The RAHA and D10HA strains grown on MMT plates exhibited nearly the same phenotype as those of the RA and D10 strains, respectively (Figure 6C). We stained the hyphae of the RAHA and D10HA strains grown in MMT medium with anti-HA and anti-actin antibodies, and with Calcofluor white. In the RAHA strain, ra-HA was concentrated at both the hyphal tips and septation sites (Figure 7C). This pattern of fluorescent was similar to that of CsmA-HA in the CSMHA strain. In the D10HA strain, actin was concentrated at both the hyphal tips and septation sites, whereas d10-HA was not concentrated at these sites (Figure 7D). Thus, the results presented here suggest that the binding of the MMD to actin is essential for CsmA localization and that its proper localization is necessary for the function of CsmA.
Figure 7.
The effect of mutations of the deduced actin-binding region in the MMD on the localization of CsmA. (A) Cartoon depicting ra-HA and d10-HA. (B) Cell extracts of CSMHA, RAHA, and D10HA strains grown in YT medium were analyzed by Western blotting using anti-HA antibody. (C and D) Localization of overproduced ra-HA (C) or d10-HA (D) and actin in a hyphal tip and a septum. Hyphae of the RAHA (C) or the D10HA (D) strain, grown in MMT medium overnight, were fixed, stained with anti-HA and anti-actin antibodies, and visualized by indirect immunofluorescence. These hyphae were stained simultaneously with Calcofluor white. Scale bar, 5 μm.
DISCUSSION
Here, we demonstrated that CsmA-HA was concentrated at the hyphal tips and septation sites near actin structures. This localization was dependent on the presence of the MMD, particularly on its actin-binding ability, because mutant CsmA-HA lacking the MMD and mutant CsmA-HA with a small deletion that covered the putative actin-binding motif in the MMD were not concentrated at these sites. The actin-binding ability of MMD-EGFP was shown in vitro by its cosedimentation with filamentous actin and the failure of cosedimentation of a mutant MMD-EGFP that had undergone the small deletion mentioned above. In addition to these observations, A. nidulans strains that expressed only mutant CsmA defective in the actin-binding motif, exhibited the same aberrant phenotype as that of the mutant that produced CsmA without the MMD. These findings suggest that the actin binding ability of the MMD is not only important for the intracellular localization of CsmA, but is also essential for the function of CsmA.
The mutant CsmA-HA that had undergone a substitution mutation at the conserved amino acid residue in the P-loop or the Switch I region of the MMD resided at the hyphal tips and septation sites. Furthermore, the mutant CsmA that bearing the above mutation reversed the defective phenotype of the ΔcsmA null mutant. Thus, the motor activity of the MMD does not appear to be relevant to the localization and function of CsmA. Although we cannot eliminate the possibility that the mutant CsmA possesses some residual ATPase activity and might suppress defects due to its overproduction, this possibility seems unlikely, because all of the defects observed in the ΔcsmA null mutant were completely reversed, i.e., the wild-type phenotype was restored by the expression of the mutant CsmA. Although myoA encoding a class I myosin, MYOA, is essential for the growth of A. nidulans, the motor activity of MYOA is thought not to be (Liu et al., 2001). Thus, it is possible that both the class I myosins and the MMD of CsmA-type chitin synthases exert certain crucial functions that are not dependent on motor activity for the growth of filamentous fungi.
One possible function of the MMD is to serve as an anchor of CsmA at the hyphal tips and septation sites where F-actins are concentrated and linked with the plasma membrane, but the MMD does not serve as a motor for transportation to the plasma membrane. In the hyphal tips, CsmA-HA localized on and inside the apical membrane (Figure 1C). The fluorescence observed inside the apical membrane might have originated from CsmA-HA on the trafficking vesicles. In filamentous fungi, as well as in most other eukaryotes, both kinesins and cytoplasmic dyneins are presumed to play important roles in the intracellular trafficking of various proteins, vesicles, and organelles (Xiang and Plamann, 2003; Steinberg and Fuchs, 2004). In the polarized cells of filamentous fungi, it has been suggested that microtubule-based motors are directly involved in long-range vesicle transport toward the Spitzenkörper (Wu et al., 1998; Seiler et al., 1999; Zhang et al., 2003). The Spitzenkörper contains a cluster of vesicles in the growing hyphal tips of some filamentous fungi and is thought to function as a vesicle supply center (Bartnicki-Garcia et al., 1989; Reynaga-Pena et al., 1997). One of the proteins transported by kinesin might be CsmA or other chitin synthases, because the deletion of a gene encoding conventional kinesin impaired chitin deposition in Neurospora crassa (Seiler et al., 1997). CsmA might be transported near the hyphal tips or septation sites by microtubule-based motors, and thereafter it might be transferred to the actin cytoskeleton by binding to actin through the MMD, leading to CsmA anchoring at those sites. If this is indeed the case, then it is conceivable that mutated CsmA, which lost its interaction with actin, is transported to the hyphal tips or septation sites, but it is unable to anchor at either of those sites. Some of the mutant CsmA may be retrieved into intracellular organelles such as vacuoles and may be degraded there. Another possibility is that the binding of the MMD to actin is required for the transport of CsmA by microtubule-based motors. Recently, the interaction of actin- and microtubule-based motors in the intracellular trafficking of various vesicles and organelles has been revealed (Gross et al., 2002; Manneville et al., 2003). The binding of the MMD to actin might result in the effective transport of CsmA to the hyphal tips or septation sites by microtubule-based motors.
The results shown in Figure 2 suggest that the localization of CsmA at the septation sites is well correlated with that of the actin cytoskeleton during septum formation. It was shown that actin, the formin SepA, and the septin AspB appeared at septation sites before observable chitin staining (Momany and Hamer, 1997, Sharpless and Harris, 2002, Westfall and Momany, 2002). CsmA-HA was not observed at the septation sites that were not yet stained with Calcofluor white, suggesting that CsmA localizes to the septation sites after actin, SepA, and AspB deposit there. It appears likely that CsmA and actin disappear simultaneously from the septa, because without cytochalasin A treatment, we scarcely observed septation sites where only CsmA-HA localized. It is also possible that the time lag of their disappearance is too short to detect any difference. The MMD might enable CsmA to synthesize septum chitin regionally and temporally by binding to the localized actin cytoskeleton, which plays important roles in septum formation. In the ΔcsmA null mutant, intrahyphal hyphae were occasionally observed, although a large number of septa were formed normally. It was previously suggested that intrahyphal hyphae originate from the “normal” septa, as observed by Calcofluor staining (Horiuchi et al., 1999). In N. crassa, a new hyphal tip was produced on the septum adjacent to a septal pore and grew in the hyphae like a intrahyphal hyphae when a Woronin body, a unique organelle in filamentous fungi, plugged the septal pore (Jedd and Chua, 2000). Thus, it is possible that intrahyphal hyphae are generated by the closing of septal pores in the ΔcsmA null mutant. The deletion of csmA may disturb the strictly regulated chitin synthesis at septation sites and occasionally lead to the formation of abnormal septa such as septa without septal pores.
Based on our results, it appears that CsmA plays an important role in hyphal tip growth, as CsmA-HA is concentrated at the hyphal tips, and because the MMD is able to interact with the actin cytoskeleton, which is involved in the determination of hyphal polarity. However, the ΔcsmA null mutant rarely exhibited defects in hyphal tip growth and only produced frequently meandering hyphae on the agar surface. The genome sequence of A. nidulans indicates that there is another gene encoding this type of chitin synthase, although its MMD is shorter (∼450 amino acids) and does not possess a P-loop, nor does it have either a Switch I or a Switch II motif. We designated this gene as csmB. The function of CsmB and its functional relationship with CsmA are currently being investigated; thus far, the csmA csmB double mutant has exhibited severe defects in hyphal tip growth (unpublished results). Thus, CsmA and CsmB perform some overlapping functions in terms of hyphal tip growth. CsmA in the hyphal tip might also be involved in maintaining cell wall integrity, because lysis in the subapical region and the formation of balloons, both of which were suppressed to some extent with osmotic stabilizers, were observed in the ΔcsmA null mutants; moreover, the amount of csmA mRNA became abundant under low osmotic conditions (Takeshita et al., 2002). It has also been suggested that CsmA functions in the maintenance of the cell wall in the chsA chsC double mutant (Yamada et al., 2005).
In S. cerevisiae, the distribution of the actin cytoskeleton is regulated in response to several types of signals, which are required for morphogenesis and tolerance to stressors. These processes may be basically conserved in fungi that show polarized growth. The present results suggest that changes in actin distribution lead to changes in CsmA distribution. In other words, CsmA transmits cell polarity to the localized synthesis of the cell wall at the hyphal tips and septation sites via its association with the actin cytoskeleton. The chitin content of the cell wall in most of filamentous fungi is much higher than that in the cell wall of S. cerevisiae (Siestma and Wessels, 1981). Filamentous fungi form complex hyphal networks and differentiate into asexual reproductive structures and spores, where chitin synthesis must be regulated temporally and spatially in an elaborate manner. The association of CsmA with the actin cytoskeleton could be a key process in this complex mechanism.
Acknowledgments
We thank Dr. Hiroyuki Adachi for valuable discussion. N. T. was financially supported by research fellowships from the Japan Society for the Promotion of Science for Young Scientists. This work was performed using the facilities of the Biotechnology Research Center of the University of Tokyo and was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-09-0761) on February 9, 2005.
References
- Amnuaykanjanasin, A., and Epstein, L. (2003). A class V chitin synthase gene, chsA is essential for conidial and hyphal wall strength in the fungus Colletotrichum graminicola (Glomerella graminicola). Fungal. Genet. Biol. 38, 272-285. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia, S., Bartnicki, D., and Gierz, G. (1989). Computer simulation of fungal morphogenesis and the mathematical basis for hyphal (tip) growth. Protoplasma 153, 46-57. [Google Scholar]
- Bulawa, C. E. (1993). Genetics and molecular biology of chitin synthesis in fungi. Annu. Rev. Microbiol. 47, 505-534. [DOI] [PubMed] [Google Scholar]
- Chigira, Y., Abe, K., Gomi, K., and Nakajima, T. (2002). chsZ, a gene for a novel class of chitin synthase from Aspergillus oryzae. Curr. Genet. 41, 261-267. [DOI] [PubMed] [Google Scholar]
- Choquer, M., Boccara, M., Goncalves, I. R., Soulie, M., and Vidal-Cros, A. (2004). Survey of the Botrytis cinerea chitin synthase multigenic family through the analysis of six euascomycetes genomes. Eur. J. Biochem. 271, 2153-2164. [DOI] [PubMed] [Google Scholar]
- Cid, V. J., Duran, A., del Rey, F., Snyder, M. P., Nombela, C., and Sanchez, M. (1995). Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol. Rev. 59, 345-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degousee, N., Gupta, G. D., Lew, R. R., and Heath, I. B. (2000). A putative spectrin-containing membrane skeleton in hyphal tips of Neurospora crassa. Fungal Genet. Biol. 30, 33-44. [DOI] [PubMed] [Google Scholar]
- Denmat, H. S., and Jacquet, M. (1997). Yeast RAS2 mutations modulating the ras-guanine exchange factor interaction. FEBS Lett. 403, 95-99. [DOI] [PubMed] [Google Scholar]
- Esnault, K., el Moudni, B., Bouchara, J. P., Chabasse, D., and Tronchin, G. (1999). Association of a myosin immunoanalogue with cell envelopes of Aspergillus fumigatus conidia and its participation in swelling and germination. Infect. Immun. 67, 1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, H., Sugiura, S., Momomura, S., Omata, M., Sugi, H., and Sutoh, K. (1997). Characterization of mutant myosins of Dictyostelium discoideum equivalent to human familial hypertrophic cardiomyopathy mutants. Molecular force level of mutant myosins may have a prognostic implication. J. Clin. Invest. 99, 1010-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, M., Horiuchi, H., Ohta, A., and Takagi, M. (1997). A novel fungal gene encoding chitin synthase with a myosin motor-like domain. Biochem. Biophys. Res. Commun. 236, 75-78. [DOI] [PubMed] [Google Scholar]
- Fujiwara, M., Ichinomiya, M., Motoyama, T., Horiuchi, H. Ohta, A., and Takagi, M. (2000). Evidence that the Aspergillus nidulans class I and class II chitin synthase genes, chsC and chsA, share critical roles in hyphal wall integrity and conidiophore development. J. Biochem. 127, 359-366. [DOI] [PubMed] [Google Scholar]
- Gross, S. P., Tuma, M. C., Deacon, S. W., Serpinskaya, A. S., Reilein, A. R., and Gelfand, V. I. (2002). Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 156, 855-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. D., Morrell, J. L., and Hamer, J. E. (1994). Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136, 517-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge, T., and Cope, M. J. (2000). A myosin family tree. J. Cell Sci. 113, 3353-3354. [DOI] [PubMed] [Google Scholar]
- Horiuchi, H., Fujiwara, M., Yamashita, S., Ohta, A., and Takagi, M. (1999). Proliferation of intrahyphal hyphae caused by disruption of csmA, which encodes a class V chitin synthase with a myosin motor-like domain in Aspergillus nidulans. J. Bacteriol. 181, 3721-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd, G., and Chua, N. H. (2000). A new self-assembled peroxisomal vesicle required for efficient resealing of the plasma membrane. Nat. Cell Biol. 2, 226-231. [DOI] [PubMed] [Google Scholar]
- Liu, X., Osherov, N., Yamashita, R., Brzeska, H., Korn, E. D., and May, G. S. (2001). Myosin I mutants with only 1% of wild-type actin activated MgATPase activity retain essential in vivo function(s). Proc. Natl. Acad. Sci. USA 98, 9122-9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Kauffman, S., Becker, J. M., and Szaniszlo, P.J. (2004). Wangiella (Exophiala) dermatitidis WdChs5p, a class V chitin synthase, is essential for sustained cell growth at temperature of infection. Eukaryot. Cell 3, 40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid, M. P., Di Pietro, A., and Roncero, M. I. (2003). Class V chitin synthase determines pathogenesis in the vascular wilt fungus Fusarium oxysporum and mediates resistance to plant defence compounds. Mol. Microbiol. 47, 257-266. [DOI] [PubMed] [Google Scholar]
- Manneville, J. B., Etienne-Manneville, S., Skehel, P., Carter, T., Ogden, D., and Ferenczi, M. (2003). Interaction of the actin cytoskeleton with microtubules regulates secretory organelle movement near the plasma membrane in human endothelial cells. J. Cell Sci. 116, 3927-3938. [DOI] [PubMed] [Google Scholar]
- Momany, M., and Hamer, J. E. (1997). Relationship of actin, microtubules, and crosswall synthesis during septation in Aspergillus nidulans. Cell Motil. Cytoskeleton 38, 373-384. [DOI] [PubMed] [Google Scholar]
- Motoyama, T., Kojima, N., Horiuchi, H., Ohta, A., and Takagi, M. (1994). Isolation of a chitin synthase gene (chsC) of Aspergillus nidulans. Biosci. Biotechnol. Biochem. 58, 2254-2257. [DOI] [PubMed] [Google Scholar]
- Motoyama, T., Fujiwara, M., Kojima, N., Horiuchi, H., Ohta, A., and Takagi, M. (1996). The Aspergillus nidulans genes chsA and chsD encode chitin synthases which have redundant functions in conidia formation. Mol Gen Genet 251, 442-450. (corrected and republished article originally appeared in 1997. Mol. Gen. Genet. 253, 520-528) [DOI] [PubMed] [Google Scholar]
- Park, I. C., Horiuchi, H., Hwang, C. W., Yeh, W. H., Ohta, A., Ryu, J. C., and Takagi, M. (1999). Isolation of csm1 encoding a class V chitin synthase with a myosin motor-like domain from the rice blast fungus, Pyricularia oryzae. FEMS Microbiol. Lett. 170, 131-139. [DOI] [PubMed] [Google Scholar]
- Reynaga-Pena, C. G., Gierz, G., and Bartnicki-Garcia, S. (1997). Analysis of the role of the Spitzenkorper in fungal morphogenesis by computer simulation of apical branching in Aspergillus niger. Proc. Natl. Acad. Sci. USA 94, 9096-9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero, C. (2002). The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41, 367-378. [DOI] [PubMed] [Google Scholar]
- Rowlands, R. T., and Turner, G. (1973). Nuclear and extranuclear inheritance of oligomycin resistance in Aspergillus nidulans. Mol. Gen. Genet. 126, 201-216. [DOI] [PubMed] [Google Scholar]
- Sasaki, N., Asukagawa, H., Yasuda, R., Hiratsuka, T., and Sutoh, K. (1999). Deletion of the myopathy loop of Dictyostelium myosin II and its impact on motor functions. J. Biol. Chem. 274, 37840-37844. [DOI] [PubMed] [Google Scholar]
- Seiler, S., Nargang, F. E., Steinberg, G., and Schliwa, M. (1997). Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa. EMBO J. 16, 3025-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler, S., Plamann, M., and Schliwa, M. (1999). Kinesin and dynein mutants provide novel insights into the roles of vesicle traffic during cell morphogenesis in Neurospora. Curr. Biol. 9, 779-785. [DOI] [PubMed] [Google Scholar]
- Sharpless, K. E., and Harris, S. D. (2002). Functional characterization and localization of the Aspergillus nidulans formin SEPA. Mol. Biol. Cell 13, 469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siestma, J. H. and Wessels, J. G. (1981). Solubility of (1–3)-β-D/(1–6)-β-D-glucan in fungal walls: Importance of presumed linkages between glucan and chitin. J. Gen. Microbiol. 125, 209-212. [DOI] [PubMed] [Google Scholar]
- Shimada, T., Sasaki, N., Ohkura, R., and Sutoh, K. (1997). Alanine scanning mutagenesis of the switch I region in the ATPase site of Dictyostelium discoideum myosin II. Biochemistry 36, 14037-14043. [DOI] [PubMed] [Google Scholar]
- Steinberg, G., and Fuchs, U. (2004). The role of microtubules in cellular organization and endocytosis in the plant pathogen Ustilago maydis. J. Microsc. 214, 114-123. [DOI] [PubMed] [Google Scholar]
- Takeshita, N., Ohta, A., and Horiuchi, H. (2002). csmA, a gene encoding a class V chitin synthase with a myosin motor-like domain of Aspergillus nidulans, is translated as a single polypeptide and regulated in response to osmotic conditions. Biochem. Biophys. Res. Commun. 298, 103-109. [DOI] [PubMed] [Google Scholar]
- Torralba, S., Raudaskoski, M., Pedregosa, A. M., and Laborda, F. (1998). Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology 144, 45-53. [DOI] [PubMed] [Google Scholar]
- Torralba, S., and Heath, I. B. (2001). Cytoskeletal and Ca2+ regulation of hyphal tip growth and initiation. Curr. Top. Dev. Biol. 51, 135-187. [DOI] [PubMed] [Google Scholar]
- Westfall, P. J., and Momany, M. (2002). Aspergillus nidulans septin AspB plays pre- and postmitotic roles in septum, branch, and conidiophore development. Mol. Biol. Cell 13, 110-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Q., Sandrock, T. M., Turgeon, B. G., Yoder, O. C., Wirsel, S. G., and Aist, J. R. (1998). A fungal kinesin required for organelle motility, hyphal growth, and morphogenesis. Mol. Biol. Cell 9, 89-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, X., and Plamann, M. (2003). Cytoskeleton and motor proteins in filamentous fungi. Curr. Opin. Microbiol. 6, 628-633. [DOI] [PubMed] [Google Scholar]
- Yanai, K., Kojima, N., Takaya, N., Horiuchi, H., Ohta, A., and Takagi, M. (1994). Isolation and characterization of two chitin synthase genes from Aspergillus nidulans. Biosci. Biotechnol. Biochem. 58, 1828-1835. [DOI] [PubMed] [Google Scholar]
- Yamada, E., Ichinomiya, M., Ohta, A., and Horiuchi, H. (2005). Class V chitin synthase gene csmA is crucial for the growth of chsA chsC double mutant in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 69, 87-97. [DOI] [PubMed] [Google Scholar]
- Zhang, J., Li, S., Fischer, R., and Xiang, X. (2003). Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent. Mol. Biol. Cell 14, 1479-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., and Gurr, S. J. (2000). Walking into the unknown: a `step down' PCR-based technique leading to the direct sequence analysis of flanking genomic DNA. Gene 253, 145-150. [DOI] [PubMed] [Google Scholar]