Abstract
Adult myoblasts retain plasticity in developmental potential and can be induced to undergo myogenic, adipogenic, or osteoblastogenic differentiation in vitro. In this report, we show that the balance between myogenic and adipogenic potential in myoblasts is controlled by Wnt signaling. Furthermore, this balance is altered during aging such that aspects of both differentiation programs are coexpressed in myoblasts due to decreased Wnt10b abundance. Mimicking Wnt signaling in aged myoblasts through inhibition of glycogen synthase kinase or through overexpression of Wnt10b resulted in inhibition of adipogenic gene expression and sustained or enhanced myogenic differentiation. On the other hand, myoblasts isolated from Wnt10b null mice showed increased adipogenic potential, likely contributing to excessive lipid accumulation in actively regenerating myofibers in vivo in Wnt10b-/- mice. Whereas Wnt10b deficiency contributed to increased adipogenic potential in myoblasts, the augmented myogenic differentiation potential observed is likely the result of a compensatory increase in Wnt7b during differentiation of Wnt10b-/- myoblasts. No such compensation was apparent in aged myoblasts and in fact, both Wnt5b and Wnt10b were down-regulated. Thus, alteration in Wnt signaling in myoblasts with age may contribute to impaired muscle regenerative capacity and to increased muscle adiposity, both characteristic of aged muscle.
INTRODUCTION
Adult skeletal muscle has remarkable regenerative capacity, largely mediated by satellite cells that reside between the sarcolemma and basal lamina of myofibers in postnatal muscle and remain throughout adult life (Bischoff, 1994). After muscle injury, satellite cells give rise to myoblasts that proliferate, migrate to the site of injury, and fuse into myofibers, thereby regenerating damaged or degenerating myofibers (Seale and Rudnicki, 2000). Deficits in the regenerative process occur as a function of age, some of which may be due to a decline in myoblast proliferative capacity (Schultz and Lipton, 1982; Dodson and Allen, 1987; Johnson and Allen, 1993; Renault et al., 2000) or to loss of factors required for satellite cell activity (Barton-Davis et al., 1998; Conboy et al., 2003). Although these deficits in myoblast activity may contribute to loss of muscle mass with age, they do not account for other features of aged muscle, in particular, increased lipid content (Goodpaster et al., 2001). We previously showed that compared with myoblasts isolated from adult mice (8 mo), those isolated from the muscle of aged mice (23 mo) showed increased expression of genes normally restricted to the adipocyte lineage (Taylor-Jones et al., 2002). Although genes involved in lipid trafficking and storage were overexpressed, a fully differentiated adipocyte phenotype is not achieved (Guan et al., 2002). Alteration of the expression and/or activity of peroxisome proliferator-activated receptor γ (PPARγ) and CAAT/enhancer binding protein α (C/EBPα), both activators of adipocyte-specific genes (Tontonoz et al., 1994; Mandrup and Lane, 1997; Hamm et al., 2001), was apparent as a function of age in myoblasts, potentially contributing to the increased adipogenic potential observed (Taylor-Jones et al., 2002). This is consistent with the observation that the muscle cell line C2C12 is converted to adipocytes by overexpression of PPARγ and C/EBPα (Hu et al., 1995; Rosen et al., 2000). Similarly, treatment with thiazolidinediones, potent activators of PPARγ, results in induction of genes involved in fatty acid uptake, storage, and metabolism (Teboul et al., 1995; Grimaldi et al., 1997).
The Wnt family of genes, encoding at least 19 lipid-modified signaling proteins (Willert et al., 2003), has been implicated in regulating both adipogenic and myogenic differentiation through the canonical β-catenin and noncanonical pathways (for review, see Huelsken and Behrens, 2002). In the absence of Wnt signaling, β-catenin is phosphorylated by glycogen synthase kinase (GSK), leading to rapid degradation (Kikuchi, 2000). Wnt ligands bind to transmembrane receptors of the Frizzled family, leading to inactivation of GSK within the complex, thereby stabilizing β-catenin, allowing translocation to the nucleus where it regulates T-cell factor (TCF)/leukocyte enhancing factor-dependent gene transcription. In the developing embryo, Wnt family members have been shown to be essential for differentiation of epaxial muscles (for review, see Buckingham et al., 2003). More recently, it has been shown that different Wnts have distinct effects during limb myogenic differentiation (Anakwe et al., 2003). Moreover, Wnt5a, 5b, 7a, and 7b promote myogenic commitment in adult muscle progenitor cells (Polesskaya et al., 2003). By contrast, Wnt signaling seems to inhibit adipogenic differentiation (Ross et al., 2000; Bennett et al., 2002; Moldes et al., 2003; Zhou et al., 2004). Blocking β-catenin activity in C2C12 cells through expression of dominant negative TCF resulted in inhibition of myogenic differentiation and activation of adipogenic differentiation (Ross et al., 2000). In particular, Wnt10b has been demonstrated to inhibit adipogenic differentiation both in C2C12 myoblasts and preadipocytes (Ross et al., 2000; Bennett et al., 2002). Because Wnt10b abundance decreases in muscle as a function of age (Taylor-Jones et al., 2002), the present study was designed to test the hypothesis that altered Wnt10b activity contributes to increased intramyocellular lipid accumulation that accompanies aging. Results suggest that Wnt10b specifically controls adipogenic potential in myoblasts and regenerating muscle, whereas other Wnt family members influence myogenic potential.
MATERIALS AND METHODS
Animals
All studies were approved by the University Animal Care and Use Committee and overseen by the Division of Laboratory Animal Medicine (University Arkansas for Medical Sciences). For aging studies, DBA/2JNIA female mice were obtained from the NIA Aging Rodent Colony at Harlan (Indianapolis, IN) and were maintained on a normal chow diet until sacrifice. Generation and characterization of FVB Wnt10b-/- mice will be described in detail elsewhere (Lane and Leder, unpublished data). At 4 wk of age, female mice were randomly assigned to receive a low-fat diet (10% fat, D12450; Research Diets, New Brunswick, NJ) or a high-fat diet (45% fat, D12451; Research Diets). Wild-type and Wnt10b-/- mice were fed low- or high-fat diets until sacrifice.
Muscle Regeneration
To induce localized muscle injury, mice were anesthetized by isofluorane inhalation and an incision (∼5 mm) made in the skin of the lower hindlimb. The skin was opened with forceps, and the exposed tibialis anterior muscle was subjected to mild damage by application of a 2-mm, dry ice-tempered metal rod for 5 s. The incision was closed, and the mice killed at indicated times after injury. For widespread muscle degeneration, 10 μM cardiotoxin (snake venom from Naja mossambica; Sigma-Aldrich, St. Louis, MO) was injected directly into the tibialis anterior as described previously (Polesskaya et al., 2003).
Tissue Culture
Myoblasts were isolated from the tibialis anterior of aged (23 mo, n = 4) and adult (8 mo, n = 4) DBA/2JNIA, and 9-mo-old FVB/C57 Wnt10b+/+ (n = 4), and Wnt10b-/- (n = 4) mice as described previously (Rando and Blau, 1994). Pure populations (>98%) of each myoblast type were derived and maintained in growth medium (GM) containing 20% fetal calf serum (Taylor-Jones et al., 2002). For aging experiments, comparable results were obtained from myoblasts derived from individual mice, so results shown were obtained from myoblasts pooled from the four animals in each group. Individual myoblast cultures were established and analyzed from the four wild-type and 4 Wnt10b-/- mice. For differentiation experiments, myoblast cultures were switched to differentiation medium (DM) containing 2% horse serum for 72 h with daily refeeding. To promote adipogenic differentiation, myoblasts were incubated in GM supplemented with a cocktail of 3-isobutyl-1-methylxanthine (IBMX) (115 μg/ml; [Sigma-Aldrich]; 5 × 10-4 M dexamethasone (Sigma-Aldrich); and 0.25 U/ml insulin [Novolin, Clayton, NC]) as described previously (Taylor-Jones et al., 2002) for 3 d. In some experiments, cells were treated with IBMX + LiCl (L9650; Sigma-Aldrich) at a final concentration of 25 mM, and all treatments were repeated once a day for 3 d. Myoblasts were infected with either pLNCX (empty vector), pLCNWnt7b (Shimizu et al., 1997), or pLNCWnt10b as described previously (Ross et al., 2000). Cultures were assessed for adipogenic differentiation by Oil Red O staining as described below.
RNA Isolation and Gene Expression
RNA was isolated using the RNAqueous extraction kit (Ambion, Austin, TX). Purity and concentration were determined using the Spectramax 384 Plus (Molecular Devices, Sunnyvale, CA). Gene expression was assayed by semiquantitative, gel-based reverse transcriptase-polymerase chain reaction (RT-PCR) and by real-time RT-PCR. Semiquantitative RT-PCR was performed as described previously (Taylor-Jones et al., 2002). Briefly, Advantage RT-for-PCR and Advantage cDNA PCR kits (BD Biosciences Clontech, Palo Alto, CA) kits were used with glyceraldehyde-3-phosphate dehydrogenase as the positive control for sample normalization. Reactions were carried out using an Applied Biosystems (Foster City, CA) GeneAMP PCR System 2400. Cycle number and annealing temperatures were empirically determined for each set of primers to ensure that amplification reactions were specific and within the linear range. PCR products were resolved on 2% agarose gels by combining UltraPure agarose and FMC (Rockland, ME) NuSieve GTG agarose 1:1 and visualized with ethidium bromide on the ChemiImager Imaging System 5500 (Alpha Innotech, San Leandro, CA). The figure shown is representative, and reactions were repeated a minimum of three times.
Real-time quantitative RT-PCR was performed using the protocols, chemistries, and the amplification and detection systems of Applied Biosystems. For each sample, cDNA was synthesized from 2 μg of total RNA by using components from the Taqman reverse transcription reagents (Applied Biosystems). The reaction volume of 100 μl also contained 1× reverse transcriptase buffer, 5.5 mM MgCl2, 0.5 mM dNTPs, 2.5 mM random hexamers, 40 U of RNase inhibitor, and 375 U of Multi-scribe reverse transcriptase. The primers were allowed to anneal for 10 min at 25°C before the reaction proceeded for 1 h at 37°C followed by 5 min at 95°C. Samples were aliquoted and stored at –80°C. Taqman primer/probes were selected using Applied Biosystems Assays on Demand: Mm00442104_m1, Mm00445880_m1, Mm00440387_m1, Mm00446194_m1, Mm00437337_m1, Mm00437347_m1, Mm00437357_m1, Hs99999901_s1, Mm00514283_s1, and Mm00440945_ m1. The latter primers to PPARγ amplified both PPARγ-1 and -2 mRNAs. PCR reactions were assembled so that only the addition of cDNA template and primers were required. Control reactions were run lacking template to check for reagent contamination. To optimize assay efficiency, PCR standard curves were produced using a pool containing each sample cDNA. Data points were generated using fourfold serial dilutions of cDNA. Eight microliters of the diluted cDNAs was added to 25 μl of final reaction volume. Gene expression was compared in individual samples by using 16 ng (1 ng for 18S) of RNA equivalents of cDNA and the standard curve method described in Applied Biosystems User Bulletin No. 2. Template amounts were adjusted for instances in which expression levels did not fall within the range of the standard curve. The reactions were performed in triplicate by using the ABI Prism 7700 sequence detection system and the instrument's universal cycling conditions: 95°C for 10 min, 40 cycles of 95°C for 15 s, and then 60°C for 1 min. RNA abundance for each gene of interest is expressed as a ratio normalized to RNA abundance of 18S in the same sample.
Western Analysis
Fractionation of cytosolic and membrane-bound proteins for β-catenin analysis was performed as described by Young et al. (1998). Samples (30–35 μg of protein) were resolved on 10% SDS-PAGE Criterion, precast gels (#345-0009; Bio-Rad, Hercules, CA) and blotted as described previously (Kiyokawa et al., 1994). Filters were stained with Ponceau S (P7170; Sigma-Aldrich) to verify equivalent loads. Primary antibody against β-catenin (610154; BD Transduction Laboratories, Lexington, KY) was diluted 1:1000. Total cellular extracts (50 μg of protein) were resolved for Western analysis with a PPARγ antibody (Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:167. A horseradish peroxidase-conjugated, goat anti-rabbit secondary antibody (Pierce Chemical, Rockford, IL) was added at a dilution of 1:100,000. Incubation times and washes were as described previously (Taylor-Jones et al., 2002). Protein bands were detected with the ChemiGlow West chemiluminescent substrate kit (CGW-8000; Alpha Innotech) per the user's manual and visualized on the ChemiImager 5500 Imaging System (Alpha Innotech).
Immunohistochemistry and Histochemistry
Tibialis anterior muscles were isolated from wild-type and Wnt10b-/- mice (n = 8 each) fed either a high-fat or low-fat diet (n = 4 each). From each animal, one tibialis anterior served as a control with the contralateral side subjected to freeze or cardiotoxin injury. Muscles were collected 7 or 28 d postinjury and flash frozen in liquid nitrogen. Serial cryostat sections (7 μm) were stained with hematoxylin and eosin or with Oil Red O as described previously (Taylor-Jones et al., 2002). For immunohistochemistry, sections were fixed in 2% paraformaldehyde for 30 min, blocked with 0.6% H2O2 for 30 min to block endogenous peroxide activity, and incubated for 1 h at room temperature with an antibody recognizing FABP4 (provided by D. A. Bernlohr, University of Minnesota, St. Paul, MN), myosin heavy chain (A4.1025, provided by H. M. Blau, Stanford University, Stanford, CA), or myogenin (Dupont-Versteegden et al., 1998). For myogenin detection, all incubations contained 0.1% Igepal (CA-630; Sigma-Aldrich) for permeabilization. Incubation with biotin-conjugated secondary antibodies (Zymed Laboratories, South San Francisco, CA) was followed by streptavidin peroxidase (Zymed Laboratories) and the DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA). Slides were rinsed in distilled water, dehydrated through alcohols/xylene, and coverslipped. For cells in culture, an alkaline phosphatase-conjugated secondary antibody was used (BD Biosciences PharMingen, San Diego, CA) and the alkaline phosphatase substrate kit (Vector Laboratories). Sections were visualized with a Nikon Eclipse E600 microscope by using Nikon Plan Fluor 20×/0.50 or 40×/0.75 objectives and photographed with a Photometrics CoolSnapES camera at room temperature.
Statistical Analysis
Experiments were conducted a minimum of three times on separate cell isolates with the different regimens. Western and PCR analysis were performed at least three times from each batch of treated cells, with individual samples run in either duplicate or triplicate. The variances for the groups were determined to be unequal using Bartlett's test. In light of this, a Welch's t test was used to compare the groups. The significance level was 0.05.
RESULTS
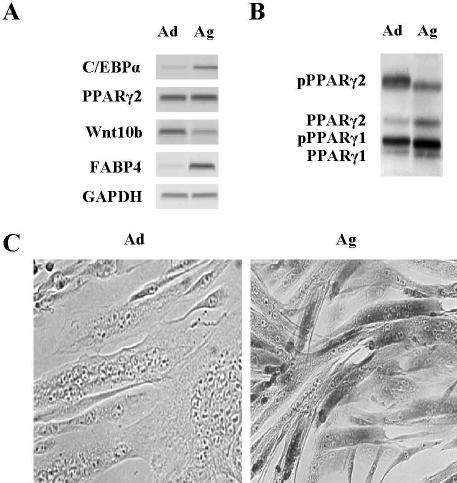
We reported previously that adipogenic potential increases as a function of age in isolated mouse myoblasts (Taylor-Jones et al., 2002). Analysis of gene expression in myoblasts from aged (24 mo) compared with adult (8 mo) mice demonstrated that genes normally associated with adipocyte differentiation are overexpressed, including C/EBPα and fatty acid binding protein 4 (FABP4, previously known as ap2) (Figure 1A). Although no difference in PPARγ2 mRNA abundance was apparent, PPARγ2 phosphorylation decreased with age (Figure 1B), suggesting increased activity. On the other hand, Wnt10b gene expression, which inhibits adipogenic differentiation (Ross et al., 2000; Bennett et al., 2002), was down-regulated with age (Figure 1A), potentially contributing to increased adipogenic potential. The relative abundance of myogenic and adipogenic gene products in aged myoblasts suggested that there was coexpression of these differentiation programs. Immunohistochemistry demonstrated that FABP4 was highly expressed in fully differentiated myotubes derived from aged mice, but not in myotubes from young adult mice (Figure 1C), confirming that both differentiation programs operate within individual cells with age. Furthermore, clonal analysis demonstrated that of the 26 clones analyzed, all underwent myogenic differentiation in low serum-containing myogenic DM and when exposed to a cocktail that promotes adipocyte differentiation in preadipocytes (IBMX), 25 of 26 were induced to express adipogenic markers, including C/EBPα, FABP4, and lipoprotein lipase as well as to accumulate lipid visualized by Oil Red O staining (our unpublished data).
Figure 1.
Myoblasts and differentiated myotubes from aged mice express adipocyte genes. (A) RT-PCR analysis of RNA from myoblasts maintained in GM from adult (Ad) and aged (Ag) mice shows differences in the expression of the indicated genes involved in adipocyte differentiation. (B) Western blot analysis of PPARγ shows age-related differences in protein abundance and phosphorylation. Filters were stained with Ponceau S to verify equivalent loads. (C) Immunocytochemical analysis of multinucleated myotubes after 72 h in DM shows FABP4 accumulates to high levels in aged (Ag) but not adult (Ad) differentiated myotubes.
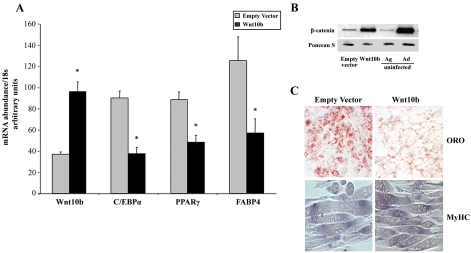
To determine whether Wnt10b controls gene expression in myoblasts with age, its expression was manipulated and the effect on differentiation potential was determined (Figure 2). Retroviral-mediated gene transfer of Wnt10b into aged myoblasts resulted in approximately threefold overexpression, quantified by real-time RT-PCR (Figure 2A). This increase was associated with increased abundance of cytosolic β-catenin to levels comparable with those observed in adult myoblasts (Figure 2B). Furthermore, Wnt10b overexpression led to inhibition of adipogenic gene expression, evidenced by decreased C/EBPα, PPARγ, and FABP4 mRNA abundance (Figure 2A), as well as decreased Oil Red O staining compared with empty vector-infected cells (Figure 2C). Myogenic differentiation was not significantly affected by Wnt10b overexpression, and fully differentiated myotubes expressing myosin heavy chain were apparent irrespective of Wnt10b abundance (Figure 2C). Treating aged myoblasts with LiCl, which inhibits activity of GSK, thereby stabilizing β-catenin, mimicked some aspects of Wnt10b overexpression. Western blot analysis demonstrated that daily treatment with LiCl for 4 h each day for 3 d (Figure 3A) resulted in increased cytosolic β-catenin, which was even more stabilized if cells were exposed to LiCl chronically for 3 d (Figure 3A, 24-h LiCl). Quantification of FABP4, C/EBPα, and PPARγ mRNA by real-time RT-PCR after chronic treatment with LiCl demonstrated that LiCl treatment decreased adipogenic gene expression (Figure 3B). LiCl treatment had the opposite effect on myogenic gene expression, augmenting expression of genes such as myogenin that promote myogenic differentiation (Figure 3B). Thus, although the canonical Wnt signaling pathway seems to promote myogenic differentiation and inhibit adipogenic differentiation in primary myoblasts, Wnt10b seems specific to the latter.
Figure 2.
Overexpression of Wnt10b results in decreased adipogenic gene expression in aged myoblasts with no effect on myogenic differentiation. (A) Real-time RT-PCR analysis of Wnt10b, C/EBPα, PPARγ, and FABP4 gene expression in aged myoblasts overexpressing Wnt10b by retroviral-mediated gene transfer compared with empty vector-infected cells after exposure to DM for 24 h. Data are expressed as mean ± SE. Asterisk (*) indicates significant difference from empty vector-infected cells, p ≤ 0.05. (B) Western blot analysis of cytosolic β-catenin in aged myoblasts overexpressing Wnt10b compared with empty vector-infected cells and to uninfected aged (Ag) and adult (Ad) myoblasts after exposure to DM for 24 h. A constitutively expressed protein visualized with Ponceau S is shown to verify equivalent loads. (C) Myoblasts overexpressing Wnt10b accumulate less lipid than empty vector-infected myoblasts, as demonstrated by Oil Red O (ORO) staining (top). Both cell populations differentiate efficiently to form myotubes that accumulate myosin heavy chain (MyHC; bottom) 72 h after exposure to DM.
Figure 3.
Mimicking Wnt signaling with LiCl results in decreased adipogenic and increased myogenic potential. (A) Western blot analysis of cytosolic β-catenin in aged control myoblasts (C), NaCl-treated 4 h a day for 3 d, LiCl-treated 1 h a day for 3 d, LiCl-treated 4 h a day for 3 d, or LiCl-treated 24 h a day for 3 d. PC is the β-catenin–positive control included with the antibody. All cells were cultured in GM + IBMX. A constitutively expressed protein visualized with Ponceau S is shown to verify equivalent loads. (B) Realtime RT-PCR quantification of myogenin (MyoG), FABP4, C/EBPα, and PPARγ mRNAs in untreated cells compared with cells treated chronically with LiCl (24 h LiCl from A). Data are expressed as mean ± SE. Asterisk (*) indicates significant difference from untreated, p ≤ 0.05.
Because Wnt10b clearly inhibits adipogenic gene expression in myoblasts, we hypothesized that knocking out its expression in primary myoblasts would cause them to express an “aged” phenotype, with increased expression of markers of adipogenic differentiation. To test this hypothesis, myoblasts were isolated from mice at 9 mo of age with a deletion of the Wnt10b open reading frame (Lane and Leder, unpublished data). The most striking feature of the Wnt10b-/- myoblasts was that myogenic differentiation was significantly accelerated compared with myoblasts obtained from wild-type mice (Figure 4). Within 24 h of exposure to DM massive fusion of myoblasts into large myotubes expressing myosin heavy chain was observed from knockout mice (Figure 4A), whereas wild-type myoblasts were just initiating myogenic differentiation (Figure 4A). Increased fusion in Wnt10b-/- myoblasts was apparently not due to increased follistatin production (Iezzi et al., 2004), because follistatin mRNA was expressed at slightly higher levels in wild-type compared with Wnt10b-/- myoblasts (our unpublished data). The MyoD gene was overexpressed in Wnt10b-/- myoblasts 24 h after induction of differentiation (Figure 4B), which may account for the increased fusion potential (Brennan et al., 1990). Transcripts encoding the adipogenic transcription factors C/EBPα and PPARγ also were overexpressed in null compared with wild-type myoblasts at this early stage of differentiation. By 72 h in DM, no difference in transcription factor gene expression was apparent between Wnt10b-/- and wild-type myotubes (our unpublished data), however, at this stage, down-stream genes, such as FABP4, were differentially expressed (Figure 4C).
Figure 4.
Wnt10b-/- myoblasts demonstrate accelerated myogenic differentiation and augmented adipogenic gene expression. (A) Myoblasts isolated from 9 mo Wnt10b-/- or wild-type (Wnt10b+/+) mice cultured 24 h in DM were incubated with an antibody to myosin heavy chain. Wnt10b-/- myoblasts form massive, fully differentiated myotubes by this stage. (B) Real-time RT-PCR analysis of MyoD, FABP4, C/EBPα, and PPARγ gene expression after 24 h in DM. Data are expressed as mean ± SE. Asterisks (*) indicates significant difference from wild type, p ≤ 0.05. (C) Real-time RT-PCR analysis of FABP4 gene expression after 72 h in DM. Data are expressed as mean ± SE. Asterisks (*) indicates significant difference from wild type, p ≤ 0.05.
The distinct in vitro differentiation potential of myoblasts from Wnt10b-/- mice suggested that the muscle phenotype in vivo also would be altered. Analysis of cryostat sections demonstrated no difference in muscle size or morphology between wild-type Wnt10b+/+ (Figure 5A) and Wnt10b-/- null mice (Figure 5C). To examine muscle regenerative capacity, both freeze injury and cardiotoxin injection were used to induce either localized (freeze) or widespread (cardiotoxin) muscle fiber degeneration and satellite cell activation in the tibialis anterior. Muscle regeneration in wild-type muscle was typical (Figure 5B), characterized by satellite cell proliferation and fusion to regenerate muscle fibers, identified by their small size and central nuclei. Muscle regeneration also proceeded efficiently in Wnt10b-/- mice (Figure 5D); however, areas of regeneration accumulated large amounts of lipid as detected by Oil Red O staining, whereas adjacent nonregenerating areas showed no increased lipid content. Higher magnification analysis of Wnt10b-/- mice (Figure 6) showed that lipid accumulation in regenerating muscle was heterogeneous with some regenerating fibers demonstrating no accumulation, some showing small lipid inclusions (Figure 6A), and others becoming completely filled with lipid (Figure 6, A and B). Although lipid clearly accumulated in regenerating muscle fibers, small cells surrounding the fibers also were Oil Red O positive (Figure 6A). The location and frequency of these cells suggested they were activated satellite cells or myoblasts, verified by nuclear accumulation of myogenin (Dupont-Versteegden et al., 1999) (Figure 7A). Myogenin was overexpressed in regenerating muscle from Wnt10b-/- mice (Figure 7A), compared with wild type (Figure 7E), confirming in vitro findings that myogenic potential was increased in the absence of Wnt10b. Myogenic cells in Wnt10b-/- mice (Figure 7B) but not Wnt10b+/+ mice (Figure 7F) coexpressed FABP4. The nearby lipid-filled fibers in Wnt10b-/- mice (Figure 7C) did not survive fixation with alcohol and manifested as void volumes upon hematoxylin and eosin staining (Figure 7D), and immunohistochemical analysis (Figure 7, A and B), whereas muscle integrity was maintained through processing in Wnt10b+/+ mice (Figure 7, E–H). Thus, myogenic and adipogenic gene programs are simultaneously expressed in regenerating muscle from Wnt10b-/- mice. This may have functional and metabolic consequences as the adipocity in muscle is apparent even 1 mo after injury in Wnt10b-/- mice (Figure 8). It should be noted that the increased adipogenic potential of regenerating muscle in vivo is only observed in Wnt10-/- mice fed a high-fat diet. Muscle regeneration in Wnt10b-/- mice maintained on a low-fat diet (our unpublished data) was indistinguishable from that observed in wild-type mice (Figure 5B), suggesting that a stimulus to accumulate lipid also is required.
Figure 5.
In vivo analysis of Wnt10b-/- and wild-type (Wnt10b+/+) muscle from 9-mo-old mice fed a high-fat diet. Lipid accumulation visualized by Oil Red O staining on 7-μm cryostat sections was assessed in Wnt10b+/+ (A and B) and Wnt10b-/- (C and D) tibialis anterior muscle. No obvious difference in muscle was apparent in uninjured muscle (A and C). One week after freeze injury, lipid accumulation was apparent in Wnt10b-/- muscle (D) compared with wild type (B), restricted to regenerating areas. No significant lipid accumulation was apparent in regenerating muscle from Wnt10b-/- mice fed a low-fat diet (our unpublished data). One small area of lipid accumulation in wild-type muscle is indicated by the arrow in B. Bar, 100 μm.
Figure 6.
Lipid accumulation in regenerating Wnt10b-/- muscle is heterogeneous. (A) Higher magnification analysis of sections shown in Figure 5 shows large Oil Red O-positive lipid droplets in regenerating fibers (arrows with tails). Small cells surrounding the fibers also accumulate lipid (arrows without tails). (B) In some cases, regenerating fibers, identified by central nucleation (arrows), become completely filled with lipid. Bars, 50 μm (A) and 100 μm (B).
Figure 7.
Myogenic and adipogenic programs are coexpressed in regenerating Wnt10b-/- muscle. Serial cryostat sections of muscle from Wnt10b-/- (A–D) or wild-type (Wnt10b+/+; E–H) mice 1 wk after freeze injury were processed for myogenin immunohistochemistry (MyoG; A and E), FABP4 immunohistochemistry (B and F), Oil Red O staining (ORO; C and G), or hematoxylin and eosin staining (H&E; D and H). Bar, 100 μm.
Figure 8.
Lipid accumulation persists in Wnt10b-/- muscle 1 mo after injury. Oil Red O staining of Wnt10b-/- and Wnt10b+/+ muscle 1 mo after cardiotoxin injection to induce widespread degeneration indicates that lipid accumulation persists specifically within muscle fibers from Wnt10b-/- mice. Bar, 100 μm.
The observations that Wnt10b-/- muscle and isolated myoblasts undergo myogenic differentiation more efficiently than those from wild-type animals, even in the face of increased adipogenic gene expression, suggest two possibilities: 1) Wnt10b normally dampens myogenic differentiation, or 2) other Wnt family member(s) that are more myogenic may be compensating for Wnt10b deficiency. That overexpression of Wnt10b in myoblasts inhibits adipogenic potential with no obvious affect on myogenic differentiation (Figure 2) argues against the first possibility. Real-time RT-PCR analysis demonstrated that the Wnt7b gene is overexpressed in Wnt10b-/- compared with wild-type myoblasts during the first 24 h of differentiation (Table 1). This suggests that the myogenic potential of these cells is increased, because Wnt7b gene expression has been shown to increase rapidly in regenerating muscle and to induce myogenic commitment in resident muscle stem cells (Polesskaya et al., 2003). No such compensation is apparent in aged myoblasts, and in fact, both Wnt5b and Wnt10b are down-regulated as a function of age (Table 1), resulting in decreased cytosolic β-catenin with age (Figure 2B).
Table 1.
Wnt mRNA expression in wild-type, Wnt10b–/–, and aged myoblasts
| Wnt 5a | Wnt 5b | Wnt 7b | Wnt 10b | FABP4 | |
|---|---|---|---|---|---|
| 24 h in DM | |||||
| Wild type | 5.1 | 5.3 | 9.0 | nd | 7.4 |
| Wnt 10b–/– | 4.2 | 2.9 | 21.9a | nd | 8.4 |
| 72 h in DM | |||||
| Wild type | 6.0 | 14.9 | 14.0 | nd | 9.3 |
| Wnt 10b–/– | 6.4 | 12.8 | 17.0 | nd | 31.9b |
| Adult | 19.2 | 23.0 | 14.0 | 19.5 | 2.2 |
| Aged | 16.7 | 8.4c | 9.9 | 6.5d | 19.2e |
nd, not determined (Wnts 1, 3a, and 7a were below the level of detection).
Wnt10b–/– significantly different from wild type, p < 0.05
Aged significantly different from adult, p < 0.05
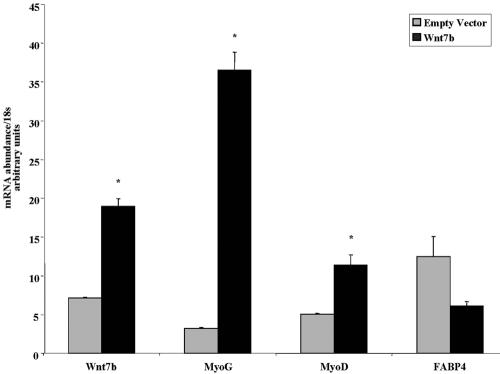
To test the hypothesis that Wnt7b contributes to increased myogenic potential, Wnt7b was overexpressed in aged myoblasts by retroviral-mediated gene transfer (Figure 9). Whereas Wnt7b expression had no significant affect on the adipogenic genes FABP4 (Figure 9), C/EBPα, or PPARγ (our unpublished data), myogenic gene expression was dramatically increased within 24 h of exposure to DM. Thus, the compensatory increase in Wnt7b in Wnt10b-/- myoblasts leads to more robust coexpression of myogenic and adipogenic gene programs than in aged myoblasts, where low levels of Wnt10b may be permissive for expression of adipogenic genes with no augmentation of myogenic gene expression.
Figure 9.
Overexpression of Wnt7b results in increased myogenic differentiation with no significant effect on adipogenic differentiation. (A) Real-time RT-PCR analysis of Wnt7b, myogenin (MyoG), MyoD, and FABP4 gene expression in aged myoblasts overexpressing Wnt7b by retroviral-mediated gene transfer compared with empty vector-infected cells after exposure to DM for 24 h. Data are expressed as mean ± SE. Asterisk (*) indicates significant difference from empty vector-infected cells, p ≤ 0.05.
DISCUSSION
The loss in muscle and bone mass with age is accompanied by an increase in fat mass (Pahor and Kritchevsky, 1998). Thus, aging in muscle is characterized not only by a decrease in the total number of muscle fibers and a reduced fiber cross-sectional area but also by decreased muscle density associated with increased intra- and intermuscular fat (Porter et al., 1995; Goodpaster et al., 2001). These changes in body composition and tissue remodeling contribute significantly to the decline in muscle strength and function. In fact, the increase in lipid content within muscle fibers that accompanies aging may account for the observation that older subjects are weaker than one would predict based on muscle mass alone (Kallman et al., 1990). Although mechanisms underlying increased muscle adiposity during aging are relatively undefined, a substantial literature exists examining lipid content within skeletal muscle and its link to insulin resistance and type 2 diabetes (for review, see Kelley and Goodpaster, 2001; McGarry, 2002; Unger, 2003). Defects in the pathways for fatty acid oxidation leading to diminished use of fatty acids and increased esterification and storage of lipid within skeletal muscle have been strongly implicated in the pathogenesis of insulin resistance (Bays et al., 2004). Changes in fatty acid oxidation with age have been less well studied; however, availability of free fatty acids increases with age, likely contributing to increased lipid content of muscle through increased storage. Our results demonstrate that adipocyte-specific regulatory genes as well as genes involved in fatty acid trafficking are overexpressed in myoblasts from aged mice, potentially exacerbating muscle lipid accumulation with age. Other muscle degenerative processes such as Duchenne muscular dystrophy (Cullen and Mastaglia, 1980) and denervation (Bacou et al., 1982; Dulor et al., 1998) also involve increased intramyocellular lipid accumulation. Thus, we hypothesize that changes in gene expression due to altered Wnt signaling in myogenic progenitors may contribute to muscle adiposity during aging and in other pathological conditions.
Wnts have been shown previously to initiate myogenesis during development (Munsterberg et al., 1995; Stern et al., 1995; Hoppler et al., 1996; Ikeya and Takada, 1998; Tajbakhsh et al., 1998; Borello et al., 1999; Cossu and Borello, 1999; Ridgeway et al., 2000) and to inhibit adipogenic differentiation (Ross et al., 2000; Bennett et al., 2002). Our results demonstrate that Wnt signaling promotes myogenic and inhibits adipogenic differentiation simultaneously within primary adult myoblasts. Analysis of Wnt10b null mice suggests that Wnt10b normally represses the aberrant expression of genes involved in lipid storage in adult muscle, but this function is only apparent during muscle regeneration. Thus, upon satellite cell activation leading to expression of the myogenic differentiation program, adipogenic genes were activated in Wnt10b-/- myoblasts, leading to excessive lipid accumulation within regenerating myofibers. However, lipid in regenerating fibers was diffuse and thus may not be properly associated with lipid droplet-associated proteins for efficient utilization. In aged muscle, satellite cell activation and regeneration are ongoing due to increased susceptibility to damage and denervation (Brooks and Faulkner, 1994), suggesting that loss of Wnt10b that normally occurs during aging may contribute to increased muscle adipocity with age. This increase in adipogenic potential is likely mediated by C/EBPα and PPARγ.
Other Wnt family members, in particular Wnt7b, seem to contribute to enhanced myogenic potential in Wnt10b-/- myoblasts and when overexpressed in aged myoblasts. Using gain- and loss-of-function studies, it was recently shown that different Wnt family members have distinct effects on chick limb muscle development, controlling the number of terminally differentiated cells and fiber type distribution (Anakwe et al., 2003). Because both Wnt10b and Wnt7b seem to increase cytosolic β-catenin, the mechanism underlying the specificity in response is currently unknown, but it is consistent with the observation that stabilization of β-catenin promoted myogenic differentiation and inhibited adipogenic potential in aged myoblasts. Clearly, the regulation of differentiation potential in myoblasts by β-catenin is complex (Goichberg et al., 2001; Martin et al., 2002), and we cannot rule out the possibility that Wnt10b and Wnt7b also differentially affect noncanonical signaling pathways (Nelson and Nusse, 2004). This is supported by the observation that the abundance of cytosolic β-catenin was not consistently different between myoblasts from wild-type and null mice (our unpublished data). The compensatory increase in other Wnt family members in response to decreased Wnt10b did not occur in aged myoblasts, suggesting that alterations in Wnt signaling may contribute to both decreased regenerative potential and increased adiposity in muscle as a function of age.
That myoblasts can be induced to undergo adipogenic differentiation has been demonstrated in several different experimental paradigms in vitro. Enhancing PPARγ activity by treatment with thiazolidinediones, synthetic ligands for PPARγ, has been reported to convert C2C12 cells to adipocytes (Teboul et al., 1995; Grimaldi et al., 1997). Thiazolidinediones alone did not enhance adipogenic potential in primary mouse myoblasts (Taylor-Jones et al., 2002), suggesting that additional events may be required, such as inhibition of the p38 pathway (Yeow et al., 2001), or decrease in Wnt10b abundance that occurs in myoblasts with age. Thus, in aged mice and high-fat–fed Wnt null mice, abundant free fatty acid derivatives may act as natural ligands for PPARγ enhancing the adipogenic potential of myoblasts in vivo. Moreover, aged muscle is a more oxidized environment with ongoing denervation, both of which have been shown to augment adipogenesis in isolated myoblasts and muscle (Dulor et al., 1998; Csete et al., 2001). Together, these results suggest that during aging, altered Wnt signaling may combine with the effects of elevated circulating oxidized free fatty acids and ongoing denervation/regeneration to lead to the expression of genes that promote lipid storage in muscle.
Acknowledgments
We thank P. Leder for considerable contribution to creation of Wnt10b-/- mice, J. Kitajewski for retroviral vectors, D. A. Bernlohr and M. D. Lane for antibodies to FABP4, C. M. Gurley and R. A. Dennis for technical assistance, J. Mays for help with manuscript preparation, and M. MacNicol for thoughtful reading of the manuscript. This work was supported by grants to C.A.P. from the National Institutes of Health (AG20941) and the Central Arkansas Veterans Health Care System, and by the University of Arkansas for Medical Sciences Microarray Facility through Act 1, The Arkansas Tobacco Settlement Proceeds Act of 2000, and by National Institutes of Health grant #P20 RR-16460 from the BRIN Program of the National Center for Research Resources. This work also was supported by grants from the National Institutes of Health to O.A.M. (DK-51563 and DK-62876).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-08-0720) on January 26, 2005.
Abbreviations used: C/EBPα, CAAT/enhancer binding protein α; DM, differentiation medium; FABP4, fatty acid binding protein 4; GM, growth medium; GSK, glycogen synthase kinase; IBMX, 3-isobutyl-1-methylxanthine; PPARγ, peroxisome proliferator-activated receptor γ; RT-PCR, reverse transcriptase-polymerase chain reaction; TCF, T cell factor.
References
- Anakwe, K., et al. (2003). Wnt signalling regulates myogenic differentiation in the developing avian wing. Development 130, 3503-3514. [DOI] [PubMed] [Google Scholar]
- Bacou, F., Vigneron, P., and Massoulie, J. (1982). Acetylcholinesterase forms in fast and slow rabbit muscle. Nature 296, 661-664. [DOI] [PubMed] [Google Scholar]
- Barton-Davis, E. R., Shoturma, D. I., Musaro, A., Rosenthal, N., and Sweeney, H. L. (1998). Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. USA 95, 15603-15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays, H., Mandarino, L., and DeFronzo, R. A. (2004). Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 89, 463-478. [DOI] [PubMed] [Google Scholar]
- Bennett, C. N., Ross, S. E., Longo, K. A., Bajnok, L., Hemati, N., Johnson, K. W., Harrison, S. D., and MacDougald, O. A. (2002). Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 277, 30998-31004. [DOI] [PubMed] [Google Scholar]
- Bischoff, R. (1994). The satellite cell and muscle regeneration. In: Myology, ed. A. G. Engel and C. Franzini-Armstrong, New York: McGraw Hill, 97-118.
- Borello, U., Coletta, M., Tajbakhsh, S., Leyns, L., De Robertis, E. M., Buckingham, M., and Cossu, G. (1999). Transplacental delivery of the Wnt antagonist Frzb1 inhibits development of caudal paraxial mesoderm and skeletal myogenesis in mouse embryos. Development 126, 4247-4255. [DOI] [PubMed] [Google Scholar]
- Brennan, T. J., Edmondson, D. G., and Olson, E. N. (1990). Aberrant regulation of MyoD1 contributes to the partially defective myogenic phenotype of BC3H1 cells. J. Cell Biol. 110, 929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, S. V., and Faulkner, J. A. (1994). Skeletal muscle weakness in old age: underlying mechanisms. Med. Sci. Sports Exerc. 26, 432-439. [PubMed] [Google Scholar]
- Buckingham, M., Bajard, L., Chang, T., Daubas, P., Hadchouel, J., Meilhac, S., Montarras, D., Rocancourt, D., and Relaix, F. (2003). The formation of skeletal muscle: from somite to limb. J. Anat. 202, 59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy, I. M., Conboy, M. J., Smythe, G. M., and Rando, T. A. (2003). Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575-1577. [DOI] [PubMed] [Google Scholar]
- Cossu, G., and Borello, U. (1999). Wnt signaling and the activation of myogenesis in mammals. EMBO J. 18, 6867-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csete, M., Walikonis, J., Slawny, N., Wei, Y., Korsnes, S., Doyle, J. C., and Wold, B. (2001). Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J. Cell. Physiol. 189, 189-196. [DOI] [PubMed] [Google Scholar]
- Cullen, M. J., and Mastaglia, F. L. (1980). Morphological changes in dystrophic muscle. Br. Med. Bull. 36, 145-152. [DOI] [PubMed] [Google Scholar]
- Dodson, M. V., and Allen, R. E. (1987). Interaction of multiplication stimulating activity/rat insulin-like growth factor II with skeletal muscle satellite cells during ageing. Mech. Ageing Dev. 39, 121-128. [DOI] [PubMed] [Google Scholar]
- Dulor, J. P., Cambon, B., Vigneron, P., Reyne, Y., Nougues, J., Casteilla, L., and Bacou, F. (1998). Expression of specific white adipose tissue genes in denervation-induced skeletal muscle fatty degeneration. FEBS Lett. 439, 89-92. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden, E. E., Houle, J. D., Gurley, C. M., and Peterson, C. A. (1998). Early changes in muscle fiber size and gene expression in response to spinal cord transection and exercise. Am. J. Physiol. 275, C1124-C1133. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden, E. E., Murphy, R.J.L., Houle, J. D., Gurley, C. M., and Peterson, C. A. (1999). Activated satellite cells fail to restore myonuclear number in spinal cord transected and exercised rats. Am. J. Physiol. 277, C589-C597. [DOI] [PubMed] [Google Scholar]
- Goichberg, P., Shtutman, M., Ben-Ze'ev, A., and Geiger, B. (2001). Recruitment of beta-catenin to cadherin-mediated intercellular adhesions is involved in myogenic induction. J. Cell Sci. 114, 1309-1319. [DOI] [PubMed] [Google Scholar]
- Goodpaster, B. H., Carlson, C. L., Visser, M., Kelley, D. E., Scherzinger, A., Harris, T. B., Stamm, E., and Newman, A. B. (2001). Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 90, 2157-2165. [DOI] [PubMed] [Google Scholar]
- Grimaldi, P. A., Teboul, L., Inadera, H., Gaillard, D., and Amri, E. Z. (1997). Trans-differentiation of myoblasts to adipoblasts: triggering effects of fatty acids and thiazolidinediones. Prostaglandins Leukot. Essent. Fatty Acids 57, 71-75. [DOI] [PubMed] [Google Scholar]
- Guan, Y., Taylor-Jones, J. M., Peterson, C. A., and McGehee, R. E., Jr. (2002). p130/p107 expression distinguishes adipogenic potential in primary myoblasts based on age. Biochem. Biophys. Res. Commun. 296, 1340-1345. [DOI] [PubMed] [Google Scholar]
- Hamm, M., Hackel, T., Wawroschek, F., Knopfle, E., Hauser, H., Weckermann, D., and Harzmann, R. (2001). Unenhanced spiral computerized tomography in acute diagnosis of flank pain. Examination in contraindications for contrast medium administration. Urologe A 40, 388-393. [DOI] [PubMed] [Google Scholar]
- Hoppler, S., Brown, J. D., and Moon, R. T. (1996). Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 10, 2805-2817. [DOI] [PubMed] [Google Scholar]
- Hu, E., Tontonoz, P., and Spiegelman, B. M. (1995). Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc. Natl. Acad. Sci. USA 92, 9856-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken, J., and Behrens, J. (2002). The Wnt signalling pathway. J. Cell Sci. 115, 3977-3978. [DOI] [PubMed] [Google Scholar]
- Iezzi, S., DiPadova, M., Serra, C., Caretti, G., Simone, C., Maklan, E., Minetti, G., Zhao, P., Hoffman, E. P., Puri, P. L., and Sartorelli, V. (2004). Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev. Cell 6, 673-684. [DOI] [PubMed] [Google Scholar]
- Ikeya, M., and Takada, S. (1998). Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development 125, 4969-4976. [DOI] [PubMed] [Google Scholar]
- Johnson, S. E., and Allen, R. E. (1993). Proliferating cell nuclear antigen (PCNA) is expressed in activated rat skeletal muscle satellite cells. J. Cell. Physiol. 154, 39-43. [DOI] [PubMed] [Google Scholar]
- Kallman, D. A., Plato, C. C., and Tobin, J. D. (1990). The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J. Gerontol. 45, M82-M88. [DOI] [PubMed] [Google Scholar]
- Kelley, D. E., and Goodpaster, B. H. (2001). Skeletal muscle triglyceride. Diabetes Care 24, 933-941. [DOI] [PubMed] [Google Scholar]
- Kikuchi, A. (2000). Regulation of β-catenin signaling in the Wnt pathway. Biochem. Biophys. Res. Commun. 268, 243-248. [DOI] [PubMed] [Google Scholar]
- Kiyokawa, H., Richon, V. M., Rifkind, R. A., and Marks, P. A. (1994). Suppression of cyclin-dependent kinase 4 during induced differentiation of erythroleukemia cells. Mol. Cell Biol. 11, 7195-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup, S., and Lane, M. D. (1997). Regulating adipogenesis. J. Biol. Chem. 272, 5367-5370. [DOI] [PubMed] [Google Scholar]
- Martin, B., Schneider, R., Janetzky, S., Waibler, Z., Pandur, P., Kuhl, M., Behrens, J., von der Mark, K., Starinski-Powitz, A., and Wixler, V. (2002). The LIM-only protein FHL2 interacts with beta-catenin and promotes differentiation of mouse myoblasts. J. Cell Biol. 159, 113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry, J. D. (2002). Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51, 7-18. [DOI] [PubMed] [Google Scholar]
- Moldes, M., Zuo, Y., Morrison, R. F., Silva, D., Park, B. H., Liu, J., and Farmer, S. R. (2003). Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem. J. 376, 607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsterberg, A. E., Kitajewski, J., Bumcrot, D. A., McMahon, A. P., and Lassar, A. B. (1995). Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 9, 2911-2922. [DOI] [PubMed] [Google Scholar]
- Nelson, W. J., and Nusse, R. (2004). Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor, M., and Kritchevsky, S. (1998). Research hypotheses on muscle wasting, aging, loss of function and disability. J. Nutr. Health Aging 2, 97-100. [PubMed] [Google Scholar]
- Polesskaya, A., Seale, P., and Rudnicki, M. A. (2003). Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113, 841-852. [DOI] [PubMed] [Google Scholar]
- Porter, M. M., Vandervoort, A. A., and Lexell, J. (1995). Aging of human muscle: structure, function and adaptability. Scand. J. Med. Sports 5, 129-142. [DOI] [PubMed] [Google Scholar]
- Rando, T. A., and Blau, H. M. (1994). Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault, V., Piron-Hamelin, G., Forestier, C., DiDonna, S., Decary, S., Hentati, F., Saillant, G., Butler-Browne, G. S., and Mouly, V. (2000). Skeletal muscle regeneration and the mitotic clock. Exp. Gerontol. 35, 711-719. [DOI] [PubMed] [Google Scholar]
- Ridgeway, A. G., Petropoulos, H., Wilton, S., and Skerjanc, I. S. (2000). Wnt signaling regulates the function of MyoD and myogenin. J. Biol. Chem. 275, 32398-32405. [DOI] [PubMed] [Google Scholar]
- Rosen, E. D., Walkey, C. J., Puigserver, P., and Spiegelman, B. M. (2000). Transcriptional regulation of adipogenesis. Genes Dev. 14, 1293-1307. [PubMed] [Google Scholar]
- Ross, S. E., Hemati, N., Longo, K. A., Bennett, C. N., Lucas, P. C., Erickson, R. L., and MacDougald, O. A. (2000). Inhibition of adipogenesis by Wnt signaling. Science 289, 950-953. [DOI] [PubMed] [Google Scholar]
- Schultz, E., and Lipton, B. H. (1982). Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech. Ageing Dev. 20, 377-383. [DOI] [PubMed] [Google Scholar]
- Seale, P., and Rudnicki, M. A. (2000). A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 218, 115-124. [DOI] [PubMed] [Google Scholar]
- Shimizu, H., Julius, M. A., Giarre, M., Zheng, Z., Borwn, A. M., and Kitajewski, J. (1997). Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 8, 1349-1358. [PubMed] [Google Scholar]
- Stern, H. M., Brown, A. M., and Hauschka, S. D. (1995). Myogenesis in paraxial mesoderm: preferential induction by dorsal neural tube and by cells expressing Wnt-1. Development 121, 3675-3686. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh, S., Borello, U., Vivarelli, E., Kelly, R., Papkoff, J., Duprez, D., Buckingham, M., and Cossu, G. (1998). Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development 125, 4155-4162. [DOI] [PubMed] [Google Scholar]
- Taylor-Jones, J. M., McGehee, R. E., Rando, T. A., Lecka-Czernik, B., Lipschitz, D. A., and Peterson, C. A. (2002). Activation of an adipogenic program in adult myoblasts with age. Mech. Ageing Dev. 123, 649-661. [DOI] [PubMed] [Google Scholar]
- Teboul, L., Gaillard, D., Staccini, L., Inadera, H., Ez-Zoubir, A., and Grimaldi, P. A. (1995). Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. J. Biol. Chem. 270, 28183-28187. [DOI] [PubMed] [Google Scholar]
- Tontonoz, P., Hu, E., and Spiegelman, B. M. (1994). Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79, 1147-1156. [DOI] [PubMed] [Google Scholar]
- Unger, R. H. (2003). Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144, 5159-5165. [DOI] [PubMed] [Google Scholar]
- Willert, K., Brown, J. D., Danenberg, E., Duncan, A. W., Weissman, I. L., Reya, T., Yates, J. R., 3rd, and Nusse, R. (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448-452. [DOI] [PubMed] [Google Scholar]
- Yeow, K., Phillips, B., Dani, C., Cabane, C., Amri, E. Z., and Derijard, B. (2001). Inhibition of myogenesis enables adipogenic trans-differentiation in the C2C12 myogenic cell line. FEBS Lett. 506, 157-162. [DOI] [PubMed] [Google Scholar]
- Young, C. S., Kitamura, M., Hardy, S., and Kitajewksi, J. (1998). Wnt-1 induces growth, cytosolic beta-catenin, and Tcf/Lef transcriptional activation in rat-1 fibroblasts. Mol. Cell. Biol. 18, 2474-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S., Eid, K., and Glowacki, J. (2004). Cooperation between TFG-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J. Bone Miner. Res. 19, 463-470. [DOI] [PubMed] [Google Scholar]