Abstract
The Yersinia pestis low-Ca2+ response stimulon is responsible for the temperature- and Ca2+-regulated expression and secretion of plasmid pCD1-encoded antihost proteins (V antigen and Yops). We have previously shown that lcrD, yscC, yscD, yscG, and yscR encode proteins that are essential for high-level expression and secretion of V antigen and Yops at 37°C in the absence of Ca2+. In this study, we characterized yscO of the Yop secretion (ysc) operon that contains yscN through yscU by determining the localization of its gene product and the phenotype of an in-frame deletion. The yscO mutant grew and expressed the same levels of Yops as the parent at 37°C in the presence of Ca2+. In the absence of Ca2+, the mutant grew independently of Ca2+, expressed only basal levels of V antigen and Yops, and failed to secrete these. These defects could be partially complemented by providing yscO in trans in the yscO mutant. Overexpression of YopM and V antigen in the mutant failed to restore the export of either protein, showing that the mutation had a direct effect on secretion. These results indicated that the yscO gene product is required for high-level expression and secretion of V antigen and Yops. YscO was found by immunoblot analysis in the soluble and membrane fractions of bacteria growing at 37°C irrespective of the presence of Ca2+ and in the culture medium in the absence of Ca2+. YscO is the only mobile protein identified so far in the Yersinia species that is required for secretion of V antigen and Yops.
The genus Yersinia contains three species that are pathogenic for humans: Y. pestis, the causative agent of bubonic plague, and Y. pseudotuberculosis and Y. enterocolitica, which cause gastroenteritis and mesenteric lymphadenitis. A requirement for the pathogenesis of yersiniae is the ability to both express and secrete Yersinia outer proteins (Yops) and V antigen (also called LcrV) (10, 11, 50). The genes that encode Yops and V antigen, the proteins which regulate their expression, and the proteins of the Yop-specific secretion apparatus (Ysc) are carried on a ca. 70-kb plasmid found in all virulent isolates of pathogenic yersiniae (referred to as pCD1 in Y. pestis KIM) (6, 19). Yops and V antigen have antihost functions that subvert innate defenses (e.g., phagocytosis) and cellular signaling and cytokine expression necessary for the mobilization of an effective immune response (14). They are crucial for the survival and multiplication of yersiniae in lymphoid tissues (14, 50, 74). Yops exert their antihost effects within eukaryotic cells. When yersiniae contact a eukaryotic cell, at least four Yops (YopE, YopH, YopM, and YpkA) are vectorially targeted into the cell at the point of contact (9, 28, 52, 59, 71) by a process requiring YopB, YopD, and YopK (29, 31, 59, 71). In Y. pestis, the secreted Yops and V antigen are subject to degradation by Pla, a plasminogen activator protease, present on the bacterial surface and encoded by the Y. pestis-specific plasmid pPCP1 (69, 70). Despite its ability to rapidly degrade Yops on the cell surface (60, 61, 69), Pla has little effect on the expression, secretion, or vectorial targeting of Yops (46, 55, 56, 66–68, 72).
High-level expression of Yops and V antigen, concomitant with secretion, is called the low-Ca2+ response (LCR). In vitro this occurs at 37°C in the absence of Ca2+ and is accompanied by growth cessation (termed restriction). Ca2+ is required in millimolar concentrations for growth in certain media at temperatures above 34°C (80). The LCR is believed to be manifest in vivo upon yersinial contact with a eukaryotic cell (14). It is believed that growth restriction does not occur in vivo (23) but that Yops and V antigen expression and secretion are regulated and that the absence of Ca2+ in vitro mimics cell contact (14, 73). The genes that are coordinately downregulated (73) by Ca2+ are referred to as the LCRS (low-Ca2+ response stimulon) (72).
A cluster of genes occupying ca. 25 kb on the Yersinia LCR plasmid is responsible for the regulation of expression, secretion, and postsecretion targeting of Yops (13, 50). Most of these (at least 22 gene products) encode the type III, or contact-dependent Yop secretion mechanism, Ysc (13, 14, 43). This is one of the best characterized of a relatively new class of secretion mechanisms present in a wide variety of gram-negative pathogens of mammals and plants (37). The Ysc mechanism is environmentally modulated in its activity at 37°C, and this modulation is indirectly responsible for the Ca2+ regulation of yop transcription seen in vitro. A current model for this effect (14) holds that under conditions that do not activate the Ysc (presence of Ca2+ and absence of contact with a eukaryotic cell), proteins necessary for negative regulation (LcrQ [53, 58] and YopD [77]) are retained in the bacterial cytoplasm, where they permit only a low expression of Yops and V antigen. However, this small pool of virulence proteins is available for immediate targeting upon imposition of Ysc-activating conditions (5). Upon cell contact, the Ysc is activated locally between the eukaryotic and bacterial cells and the effector Yops are vectorially targeted into the eukaryotic cell by the postsecretion delivery mechanism containing YopB, YopD, and YopK. The negative regulator LcrQ also is secreted out of the bacterial cell (53, 58), and this permits upregulation of yop transcription (14, 53). In vitro, the absence of Ca2+ is thought to activate all secretion channels and cause strong yop induction, through the massive secretion of YopD and LcrQ. Upregulation of translation of at least some Yops may also occur upon the activation of the Ysc and be coordinated with targeting of the newly expressed Yops to the Ysc (5). This also would ensure a rapidly available supply of additional Yops for targeting and a greater degree of amplification of Yops expression than that expected from transcriptional induction alone (5).
The Ysc secretes Yops, without processing, through both bacterial membranes. Although some Yops can be obtained in small amounts in periplasmic fractions (46), there is no evidence for a true, obligatory periplasmic phase of Yop secretion. By analogy to the type III secretion system of centisome 63 in Salmonella typhimurium (35), the Ysc probably spans both membranes as a supramolecular complex. Three operons encode the secretion mechanism proper: lcrDR (55), yscA to yscM (27, 43), and yscN to yscU (3, 7, 20, 78); VirG appears to promote insertion of the secretin YscC into the outer membrane (1, 34). Additional genes, including those that encode LcrE (also called YopN), LcrG, LcrV, and TyeA, modulate the activity of the Ysc in response to environmental inputs such as Ca2+ and cell contact (22, 32, 46, 66, 67, 79). Finally, members of the lcrGVH-yopB-yopD operon function in the postsecretion targeting of Yops (14).
Homologies to components of the flagellar basal body have been noted for LcrD (54, 55) and all except YscP of the products of the eight-member yscN to yscU operon (3, 7, 20, 78). All of these except YscO, YscP, and YscQ have been shown or assumed on the basis of predicted sequences to be inner membrane proteins or inner membrane-associated proteins (in the case of the putative energizer protein YscN). The only other known inner membrane component is YscD (not a flagellar component homolog) (43, 56). It has been speculated that these homologies could reflect a supramolecular analogy between the flagellar basal structure and the inner membrane and membrane-spanning regions of the type III mechanism, and this is supported by the recently demonstrated shape of the Salmonella type III mechanism (35).
Other than VirG, which probably is located in the outer membrane, the only proven outer membrane component of the Ysc is the secretin YscC, which oligomerizes to form a ring-shaped structure with a central pore (34). This has been assumed to serve as a channel for Yop secretion through the outer membrane (34). Interestingly, the homolog in Salmonella, InvG, was found to be one of three main components of a needle-like projection from the putative outer membrane ring of the isolated type III complex (35).
In Y. pestis, the LcrD, YscC, YscD, YscG, and YscR products have been shown to be required for Yop secretion (20, 27, 56). In Y. pseudotuberculosis or Y. enterocolitica, YscC to YscG, YscI to YscL, YscN, YscQ, YscR, and YscU have been shown to be essential for the secretion of Yops (2, 3, 7, 43, 78). All are thought to be core (essential) components of the Ysc and to exert an indirect inductive effect on yop operon expression through their requirement for the secretion of negative regulators such as LcrQ and YopD out of the bacterial cell (14, 53, 77). Mutations in any of the essential Ysc components abolish the Ca2+ requirement for growth (a phenotype referred to as Ca2+ independence), limit the induction of LCRS operons at 37°C to the level typical for the presence of Ca2+, and block the secretion of LCRS proteins (20, 25, 55, 56).
yscN and yscQ through yscT have a high sequence similarity to counterparts in loci for virulence protein secretion in Salmonella pathogenicity island 1 (SPI1) at centisome 63 (spa or inv), SPI2 at centisome 30 (ssa), Shigella flexneri (spa), and enteropathogenic Escherichia coli (esc) (18, 24, 26, 30, 63). The Yersinia and both Salmonella loci are similar in the size and arrangement of the yscN to yscU homologs. However, the predicted sequences of yscO and yscP have at best weak similarity to homologs in type III systems and no similarity to any non-type III-related proteins outside the Yersinia spp. For example, YscO is 21% identical (37% similar) to SpaM/InvI of the Salmonella SPI1 and 20% identical (23% similar) to Shigella Spa13. This suggests that YscO and YscP may play a specific role in the recognition, secretion, or targeting of the Yersinia-specific Yops or V antigen and prompted us to initiate their characterization. In this study, we have focused on yscO. We constructed a mutant with essentially a complete deletion of yscO and characterized it for the expression and secretion of V antigen and Yops. The data show yscO to be essential for high-level expression and secretion of V antigen and Yops, with a direct effect on secretion. We show that under LCR-inductive conditions, YscO is weakly expressed and found in all bacterial fractions. To our knowledge, this is the first mobile core component of the Ysc.
MATERIALS AND METHODS
Bacteria, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study and their relevant properties are listed in Table 1, and some are shown in Fig. 1. Y. pestis KIM5-3001 (LCR+) was the parent strain (wild type [wt]) used in these studies. It contains three naturally occurring Y. pestis plasmids: pCD1 (containing the genes encoding the LCR phenotype) (19, 25), pPCP1 (encoding the plasminogen activator [Pla] responsible for the degradation of Yops [69]), and pMT1 (encoding the F1 capsular protein) (57). Y. pestis KIM8-3002 (LCR+, Pla−: wt Pla−) was used in experiments in which degradation of proteins would have affected the analysis of the results. E. coli strains were typically grown in Luria-Bertani (LB) broth or on LB agar (15). Y. pestis strains were routinely grown in heart infusion broth or on tryptose blood agar base plates (Difco Laboratories, Detroit, Mich.) at 26°C. For physiological studies, Y. pestis strains were grown in TMH defined liquid medium (72) supplemented with 2.5 mM CaCl2 as indicated. The medium was inoculated to an optical density at 620 nm of ca. 0.1 from a culture that had been growing exponentially at 26°C with shaking at 200 rpm for about seven generations. Cultures were started at 26°C and then shifted to 37°C when the optical density reached ca. 0.2. Cells and secreted proteins were harvested at 5 or 6 h after the temperature shift. All bacteria with antibiotic resistances were grown in the presence of the appropriate antibiotic(s) (100 μg/ml for ampicillin and streptomycin).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| E. coli | ||
| BMH 71-18 mutS | thi supE Δ(lac-proAB) [mutS::Tn10] [F′ proAB laqIqZΔM15] | Promega |
| DH5α | end-1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacIZYA-argF)U169 deoR[φ80dlacΔ(lacZ)M15] | GIBCO-BRL |
| Y. pestisa | ||
| KIM5-3001 (wt) | Smr; pCD1 (LCR+), pPCP1(Pla+) pMT1 | 38 |
| KIM5-3001.16 (ΔyscO) | Smr; pCD1 yscO(Δ6–151),b pPCP1(Pla+), pMT1 | This study |
| KIM6-3001 | Smr; pCD1− (LCR−), pPCP1(Pla+) | 38 |
| KIM8-3002 (wt Pla−) | Smr; pCD1, pPCP1−(Pla−), pMT1 | 75 |
| KIM8-3002.3 (ΔyscO Pla−) | Smr; pCD1 yscO(Δ6–151),b pPCP1−(Pla−), pMT1 | This study |
| Plasmids | ||
| pBluescript II SK+ | Apr; cloning vector | Stratagene |
| pBluescript II SK− | Apr; cloning vector | Stratagene |
| pYP-F2 | Apr; BamHI F fragment cloned from pBGCD1 carrying yscN′OPQRS, with frameshift mutation in yscR, was cloned into pBluescript II SK− with the insert oriented with lac promoter | 20 |
| pYscOP.2 | Apr; 2.38-kb Bpu 1102I fragment of pYP-F2 carrying yscO and yscP was filled in with Klenow and cloned into XhoI-digested/Klenow blunt-ended pBluescript II SK+ with the insert oriented with the lac promoter | This study |
| pYscOP | Apr; same as pYscOP.2 with insert oriented with the T7 promoter | This study |
| pYscO.2 | Apr; SphI-KpnI (KpnI site in vector) digestion of pYscOP.2 followed by blunt ending with T4 polymerase and religation resulting in the elimination of yscP and carrying yscO | This study |
| pYscO | Apr; ClaI (one site in vector) digest of pYscOP followed by religation of plasmid, carries yscO | This study |
| pΔyscO | Apr; AvrIIc-digested pyscOP.2 was filled in with Klenow and religated, resulting in a deletion of yscO (Δ6–151)b | This study |
| pYscN′ΔOP | Apr; 400-bp SacII fragment of pYP-F2 ligated with ∼5.0-kb SacII fragment of pΔyscO carrying yscN′, yscO (Δ6–151),b and yscP | This study |
| pYscP | Apr; 1.8-kb AvaI fragment of pYP-F1 (20) carrying yscP, filled in with Klenow and cloned into EcoRV site of pBluescript II SK+ with insert oriented with the T7 promoter | This study |
| pHTV | Apr; expression vector carrying lcrV; translationally fused to a leader encoding 19 residues, including 6 histidines | 21, 45 |
| pTRCM.2 | Apr; expression vector carrying yopM behind the trc promoter | 56 |
| pGEX-3X | Apr; GST fusion expression vector using the tac promoter | Pharmacia |
| pGST-YscO | Apr; 1.9-kb MluI-EcoRI (vector site) fragment of pyscO filled in with Klenow following MluI digestion and directionally cloned into SmaI-EcoRI-digested pGEX-3X (expresses fusion protein of GST and aa 13–154 of YscO) | This study |
| pUK4134 | Apr; suicide vector oriR6K oriT cos rpsL | 65 |
| pUKΔyscO | Apr; ∼2.3-kb XbaI (in vector site)-AgeI fragment of pyscN′ΔOP filled in with Klenow and cloned into the EcoRV site of pUK4134, carries yscO (Δ16–151)b | This study |
| pCVD442 | Apr Sucs; suicide vector | 16 |
| pCVD442ΔyscO | Apr Sucs; same insert as in pUKΔyscO cloned into the SmaI site of pCVD442 | This study |
All Y. pestis strains are Pgm− (76).
Numbers in parentheses give the amino acids deleted from the protein product.
AvrII sites introduced by site-directed mutagenesis (see Materials and Methods).
FIG. 1.
Physical and genetic map of the region of pCD1 that encompasses yscO and yscP. (A) Coding regions for yscO and yscP and parts of yscN and yscQ carried on pYscOP.2, as well as selected restriction sites. Asterisks denote restriction sites introduced by site-directed mutagenesis. (B) Regions included in selected clones.
DNA methods.
Cloning methods, including the use of restriction endonucleases, T4 DNA ligase, isolation of plasmid DNA (8, 33, 41), and electroporation (51), were as previously described. The PCR technique (44) was performed with 20 to 30 cycles of amplification; the denaturing, annealing, and extending conditions were 94, 55, and 72°C for 30 s each in a model 480 thermocycler (Perkin-Elmer Cetus, Norwalk, Conn.). Double-stranded DNA was sequenced by the method of Sanger et al. (62) with the Sequenase 2.0 sequencing kit (United States Biochemical, Cleveland, Ohio) and [α-35S]dATP (NEN Research Products, Boston, Mass.) or by the Macromolecular Structure Analysis Facility (University of Kentucky, Lexington).
Site-directed mutagenesis of yscO.
To study an effect of yscO on the LCR, we used a variation of two site-directed in vitro mutagenesis protocols (11a, 56a) to construct an in-frame deletion in yscO that effectively created a null mutation. A single-stranded DNA (ssDNA) template of pYscOP.2 (Table 1; Fig. 1) coding strand was prepared by using the helper phage M13K07, as described previously (41). The mutagenesis reaction involved annealing a selection oligonucleotide and two mutagenic oligonucleotides to the ssDNA template. The 5′-phosphorylated selection oligonucleotide (Trans Oligo AlwNI/SpeI; Clontech) changed AlwNI to SpeI in the vector region of pYscOP.2. The two mutagenic oligonucleotides used to introduce AvrII sites into yscO at bp 881 and 1321 were 5′-ACTTTAACCCGGCCTAGGCGGCGTATC-3′ and 5′-TCATTAGGCGTTCCTAGGATGCTGTAG-3′, respectively. (The underlined portions represent AvrII sites; the bold sequences represent bases that were changed during mutagenesis; nucleotide numbering corresponds to the numbering of GenBank accession no. L25667). The mutagenic oligonucleotides were 5′-end phosphorylated (Promega) and annealed to ssDNA at 100°C for 3 min with a 200-fold molar excess of each of the three oligonucleotides over the ssDNA template (Clontech). The mutant strand was synthesized with T4 DNA polymerase to prevent strand displacement and allow the annealing of all three oligonucleotides in one reaction (42, 48). After ligation (Clontech), any nonmutagenized parental pYscOP.2 DNA remaining in the mixture was selectively linearized by digestion with AlwNI to decrease its transformation efficiency, and the DNA was transformed into a repair-defective strain of E. coli (BMH 71-18 mutS). The use of BMH 71-18 mutS prevented repair of the newly synthesized unmethylated strand and provided high mutation efficiency. A 100-μl volume of the transformation mix was subjected to 60 min of incubation in LB broth without antibiotics and then to overnight selection in the presence of ampicillin, and plasmid DNA was isolated and subjected to a second digestion with AlwNI to enrich for mutant plasmid DNA (undigested). Following desalting with a Qiaex kit (Qiagen Inc., Studio City, Calif.), the plasmid digestion mix was introduced into E. coli DH5α. Plasmid DNA from selected transformants was sequenced to verify the introduction of AvrII sites at bp 881 and 1321. Plasmid DNA containing both AvrII sites was digested with AvrII, filled in with Klenow, and religated to form pΔyscO, which encoded only 9 of the original 154 amino acids of yscO but retained the ribosome binding site of the downstream yscP gene. This was transformed into E. coli DH5α. Again, use of the correct in-frame deletion was confirmed by sequencing the double-stranded DNA (dsDNA) of transformants.
DNA from a clone with the correct in-frame deletion was digested with SacII to produce a 72-bp fragment and a ∼5.0-kb fragment. The ∼5.0-bp fragment carried the 3′ end of yscN, the yscO deletion, and yscP. The dephosphorylated ∼5.0-kb SacII fragment and a 400-bp SacII fragment of pYP-F2, containing additional yscN sequences, were ligated to form pYscN′ΔOP. This Y. pestis DNA insert contained 870 bp upstream of the yscO start codon, the mutant yscO sequence, and the coding sequence of yscP. The resulting clones were checked for proper orientation of the SacII fragments by PCR amplification with primers that annealed to portions of both SacII fragments. The ends of a XbaI-AgeI fragment excised from pYscN′ΔOP were filled in with Klenow, and the resulting ∼2.3 kb fragment containing the mutant yscO sequence and ∼900 bp on either side of the sequence was cloned into the EcoRV site of pUK4134 to form pUKΔyscO and into the SmaI site of the suicide vector pCVD442 (16) to form pCVD442ΔyscO. Y. pestis KIM5-3001.16 (ΔyscO) was created by allelic exchange with pUKΔyscO, as previously described (65). Y. pestis KIM8-3002.3 (ΔyscO Pla−) was created by allelic exchange with pCVD442ΔyscO. Plasmid pCVD442ΔyscO was electroporated into Y. pestis KIM8-3002. Selection for resolvants of the plasmid was performed by growth on medium containing 5% sucrose. Allelic exchange was confirmed by checking the size of the ΔyscO-containing fragment on a HindIII digestion of pCD1 and by PCR analysis with primers outside the region mutagenized. Additionally, we sequenced a PCR fragment of the regions ∼500 bp upstream and downstream of ΔyscO in Y. pestis KIM5-3001.16 to verify that the deletion and adjacent sequences were correct after allelic exchange.
Antibody preparation.
To determine the location of YscO in bacterial fractions, antibody was raised in rabbits against a fusion protein of glutathione S-transferase (GST) and amino acids 12 to 154 of YscO. Plasmid pGST-YscO was transformed into E. coli DH5α (Table 1; Fig. 1). The soluble GST-YscO fusion protein was expressed and purified as specified by the manufacturer for use of the pGEX-3X vector (Pharmacia Biotech Inc., Piscataway, N.J.) and used to raise antibodies in New Zealand White rabbits as previously described (54).
Cell fractionation and immunoblot analysis.
Cells were pelleted by centrifugation, and the top half of the culture medium containing secreted proteins was removed from the tubes. The bacterial pellet was washed and resuspended in 100 mM Tris-HCl (pH 7.4)–1 mM EDTA and lysed by a single passage through a chilled French pressure cell at 20,000 lb/in2. Unlysed cells and cellular debris were removed by centrifugation at 8,800 × g for 5 min at 4°C. Total soluble proteins (cytoplasmic plus periplasmic) were separated from membranes of the cleared lysates by ultracentrifugation at 417,000 × g for 15 min in a TLA 100.4 rotor (Beckman, Inc., Palo Alto, Calif.). The membranes were resuspended in 100 mM Tris-HCl (pH 7.4)–1 mM EDTA. Secreted proteins were precipitated with 5% (vol/vol) trichloroacetic acid for 2 h to overnight on ice. After centrifugation (14,000 × g for 30 min at 4°C) to pellet the precipitated proteins, the pellet was neutralized with 1 M Tris-HCl (pH 8.0), resuspended in electrophoresis sample buffer (60 mM Tris-Cl [pH 6.8], 2.3% [wt/vol] sodium dodecyl sulfate [SDS], 20% [vol/vol] glycerol, 5% [vol/vol] β-mercaptoethanol, 0.01% [wt/vol] bromophenol blue), and stored at −20°C. Bacterial fractions were analyzed on denaturing SDS-polyacrylamide gels (12 to 15% [wt/vol] acrylamide) (36) followed by transfer to Immobilon-P (Millipore Corp., Bedford, Mass.) using Towbin transfer buffer (75). The gels were loaded such that each lane contained proteins corresponding to equal numbers of bacteria. Y. pestis LCR proteins were visualized on the membranes by using protein-specific primary antibody and a secondary antibody (goat anti-rabbit or goat anti-mouse [Sigma]) conjugated to either alkaline phosphatase or horseradish peroxidase. The primary rabbit polyclonal antibodies included anti-ECP, against total proteins in the culture medium of Y. pseudotuberculosis 43 (pCD1 yopKL::Mu d1 [Apr lac]) (45); anti-HTV, against full-length V antigen having a 19-residue leader containing 6 His residues fused to its N terminus (21, 45); anti-YopM, against purified YopM (45); anti-LcrD, against an LcrD peptide coupled to the carrier protein bovine serum albumin (54); anti-YscD, against a fusion protein of GST and YscD (56); and anti-YscP, against a fusion protein of GST and amino acids (aa) 328 to 455 of YscP (49). Rabbit anti-YopE was a generous gift from Gregory V. Plano (University of Miami, Miami, Fla.). Mouse anti-YopE and anti-YopH were a generous gift from Gerard P. Andrews and Arthur M. Friedlander (U.S. Army Medical Research Institute for Infectious Diseases, Ft. Dietrick, Md.).
DNA sequence analysis.
DNA and predicted protein sequences were analyzed with PCGene (IntelliGenetics, Inc., Mountain View, Calif.), IntelliGenetics Suite (IntelliGenetics, Inc.), and Coils 2.1 (39, 40). The deduced amino acid sequences were compared with available sequences in the GenBank database via the National Center for Biotechnology Information BLAST (4) mail server. The yscQRS nucleotide sequence of Y. pestis (20) has been updated to include the sequences of yscO and yscP (accession no. L25667).
RESULTS
Analysis of yscO of Y. pestis KIM.
We extended the sequence upstream of yscQRS (20) in Y. pestis. This encompassed the 3′ end of yscN and the 5′ end of yscQ. The predicted amino acid sequences of YscO and YscP were 98 and 100% identical to those predicted for Y. pseudotuberculosis but not significantly similar to those outside the Yersinia spp. Computer analysis of yscO predicted a 154-residue protein with a molecular mass of 18.9 kDa and a predicted isoelectric point of 7.89. The predicted protein sequence does not contain either a putative signal sequence or any hydrophobic domain. Secondary-structure analysis predicted a predominantly alpha-helical structure with a high probability of existing as a coiled-coil protein. Interestingly, Salmonella SpaM (also called InvI) (12) and Shigella Spa13 are also predicted by the same analysis to have a large percentage of charged residues and potentially exist as coiled-coil proteins. The similarity of size, charges, and potential secondary structure suggests that these proteins may have similar functions in their respective bacterial strains but have no similarity in sequence because of the differences in the proteins with which they interact.
Mutational analysis.
To determine the role of YscO in the LCR and its presumed role in secretion, we compared the growth (Fig. 2) and secretion (Fig. 3) of Y. pestis KIM5-3001 (wt), KIM5-3001.16 (ΔyscO), and KIM5-3001.16 carrying yscO or yscO and yscP in trans (ΔyscO/O or ΔyscO/OP) in TMH defined medium under both inductive and noninductive conditions of the LCR.
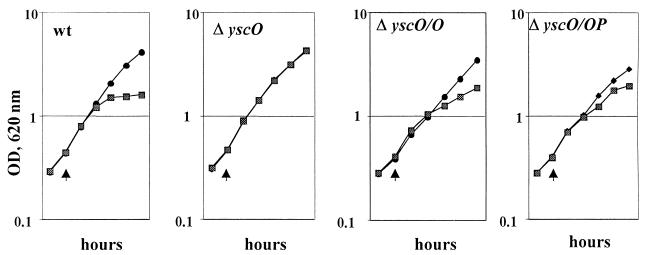
FIG. 2.
Growth of Y. pestis KIM5-3001 (wt); KIM5-3001.16, the yscO mutant (ΔyscO); and the mutant carrying yscO in trans (ΔyscO/O) and (ΔyscO/OP). Y. pestis strains were grown at 37°C in the presence or absence of Ca2+ in TMH defined medium. The temperature was shifted from 26 to 37°C (temperature shifts are denoted by arrowheads). Symbols: •, +Ca2+; ▩, −Ca2+.
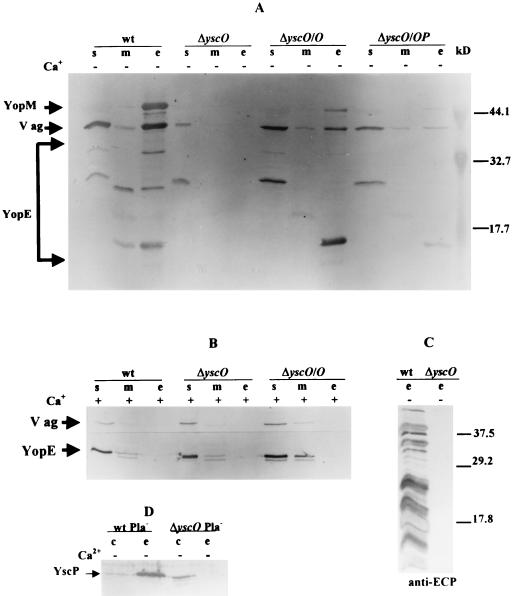
FIG. 3.
Secretion profile of ΔyscO Y. pestis grown in the presence or absence of Ca2+. Shown is an immunoblot analysis of proteins expressed and secreted from Y. pestis KIM5-3001 (wt); KIM5-3001.16, the yscO mutant (ΔyscO); and the mutant carrying pYscO.2 or pYscOP.2 in trans (ΔyscO/O and ΔyscO/OP). Bacteria were grown in TMH with (+) or without (−) Ca2+, and proteins from bacterial fractions were separated by SDS-PAGE (A, B, and D, 12% [wt/vol] acrylamide; C, 15% [wt/vol] acrylamide). Proteins from soluble (s), membrane (m), whole-cell (c), and culture medium (e) fractions were visualized with polyclonal antibodies specific to YopE, YopM, V antigen, and YscP and with antibody raised to a mixture of extracellular Yersinia proteins (ECP). The secondary antibody used was conjugated to alkaline phosphatase. Arrows denote the positions of proteins. (A) YopE was visualized with mouse anti-YopE (YopE and its Pla-generated degradation products are enclosed in brackets). (B) YopE was visualized with rabbit anti-YopE. (C) The complexity of the protein pattern reflects degradation products from multiple Yops due to the Pla protease. (D) YscP was visualized with antibody to a GST-YscP fusion protein. Molecular masses (in kilodaltons) of prestained molecular mass standards (Bio-Rad) are denoted to the right in panels A and C.
The yscO mutant has a similar growth phenotype and secretion pattern to that in previously characterized Y. pestis Ysc mutants (20, 25, 55, 56). In the presence of Ca2+, all strains exhibited full growth yield at 37°C (Fig. 2). The mutant and parent displayed similar expression patterns and low levels of V antigen and YopE (Fig. 3B); YopM was undetectable in both strains (data not shown). Additionally, there was no secretion in the presence of Ca2+, which was expected for the parent. Y. pestis KIM5-3001 (wt) displayed a normal Ca2+-dependent phenotype in which bacteria undergo restriction at 37°C in the absence of Ca2+ (Fig. 2), and the growth restriction was accompanied by expression and secretion of YopE, YopM, and V antigen (Fig. 3A, lanes wt). Expression was higher for YopE, YopM, and V antigen than in the presence of Ca2+ (compare Fig. 3A and B, lanes wt). The yscO mutant (ΔyscO) failed to show growth restriction irrespective of the presence of Ca2+ and was therefore characterized as having Ca2+-independent growth (Fig. 2). Under LCR-inductive conditions (without Ca2+), the mutant failed to secrete YopM, V antigen, YopE, or YopD and expressed clearly reduced levels of these proteins compared to the parent (Fig. 3A, compare ΔyscO and wt [not shown for YopD]). Significantly, YscP was detected in the whole-cell fraction of the mutant (Fig. 3D). As with Yops and V antigen, its expression was lower in the mutant than in the parent, and it was not secreted in the mutant as it was by the parent (49). This indicated that the yscO mutation did not have a polar effect on the expression of yscP.
We wanted to know whether the mutant was selectively impaired in the secretion of some but not all LCR proteins, as reported for both a virG mutant and a yscF mutant (1, 2). Therefore, we analyzed the culture medium of the mutant for secreted proteins by using antibody raised against a mixture of secreted Yersinia proteins (anti-ECP). No proteins were detected, although the parent (wt) secreted several (Fig. 3C). These results show that yscO is required for the secretion of V antigen and Yops.
Growth restriction and secretion were partially restored to the mutant by providing yscO in trans (Fig. 2 and 3A, lanes ΔyscO/O). The extent of complementation varied from experiment to experiment, and we were unable to determine what variables were responsible for these differences. We tried providing yscO and yscP together in trans on pYscOP-2 to see if the complementation would be stronger. However, the complementation of growth and secretion phenotypes turned out to be weaker (Fig. 2 and 3A, lanes ΔyscO/OP), probably due to an effect of the extra copies of yscP (49). There was no complementation by a plasmid carrying yscP in trans (data not shown). Accordingly, we believe that the phenotype of the yscO mutant is due to the absence of yscO and not to a polar effect on downstream genes in the operon.
Secretion of V antigen and YopM expressed from plasmids with non-LCR, inducible promoters.
To test the hypothesis that yscO affects secretion directly and to show that low levels of substrate available in the mutant were not responsible for the lack of secretion, we analyzed secretion in Y. pestis KIM5-3001 (wt) and Y. pestis KIM5-3001.16 (ΔyscO), each carrying V antigen or YopM expressed in trans from an inducible, non-LCR-regulated promoter. Plasmid pHTV, which encodes histidine-tagged V antigen (HTV), and pTRCM.2, which encodes YopM, were introduced into wt and ΔyscO Y. pestis strains. Both plasmids contain an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter. At 5 h before harvest, the expression of YopM or HTV was induced by the addition of IPTG (1 mM). The expression and secretion of YopM and HTV were monitored by immunoblot analysis (Fig. 4).
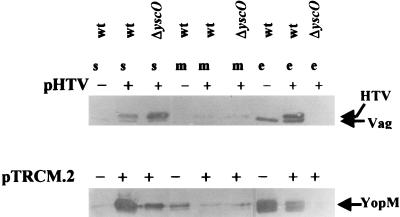
FIG. 4.
Immunoblot analysis of YopM, V antigen, and HTV in the soluble (s), membrane (m), and culture medium (e) fractions from Y. pestis KIM5-3001 (wt) and KIM5-3001.16 (ΔyscO) with (+) or without (−) pHTV or pTRCM.2. The Y. pestis strains were grown at 37°C in the absence of Ca2+. Expression of HTV from pHTV and YopM from pTRCM.2 was induced by the addition of IPTG to 1 mM 5 h prior to harvest. The proteins were separated by SDS-PAGE (12% [wt/vol] acrylamide). Polyclonal antibody to HTV or YopM was used to detect HTV and V antigen, and YopM, respectively. Secondary antibody was conjugated to alkaline phosphatase. In the lower panel, YopM was detected as two closely migrating species.
The parent strain expressed and secreted YopM and V antigen. When carrying pTRCM.2, the parent expressed and secreted higher levels of YopM. The parent strain carrying pHTV expressed both native V antigen and HTV, which ran slightly above the native V antigen due to its His6-containing leader sequence. These data indicate that YopM and HTV expressed from plasmid pTRCM.2 and pHTV, respectively, were competent for secretion. We think the extra protein expressed in the parent carrying either pTRCM.2 or pHTV overwhelmed the secretion system and resulted in a backup of the respective proteins (especially of YopM) in the soluble fraction of these strains in comparison to the situation in the parent not carrying a plasmid in trans.
The yscO mutant carrying pHTV expressed high levels of HTV but was unable to secrete any. A very small amount of the strongly expressed YopM was seen in the culture medium of the mutant carrying pTRCM.2. This may indicate that the yscO mutant is not 100% blocked in secretion but that the small amount of Yops that gets through is not visible unless a huge amount is overexpressed. Our results suggest that the low LCRS protein expression in the yscO mutant was secondary to the secretion defect in this mutant and was not the primary cause of the lack of secretion.
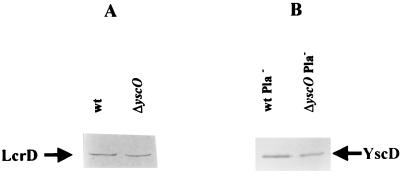
Since it is possible that the secretion defect is due to an indirect effect of yscO on other Ysc proteins, we analyzed membrane fractions of the yscO mutant for two Ysc components, LcrD and YscD, each encoded by one of the other two operons encoding components of the Ysc. We saw comparable levels of the two proteins in the mutant compared to the parent (Fig. 5). We were not able to analyze the expression of all known Ysc proteins; therefore, we cannot rule out the possibility that YscO affects the expression or localization of a component not tested. However, these results indicate that the yscO mutant is defective in some aspect of the secretion process, and they support a direct role for yscO in the secretion of LCR virulence proteins by Y. pestis.
FIG. 5.
Detection of LcrD and YscD in ΔyscO Y. pestis. Y. pestis strains were grown in TMH at 37°C in the absence of Ca2+. The proteins in membrane fractions were separated by SDS-PAGE (12% [wt/vol] acrylamide), transferred to Immobilon P, and analyzed by immunoanalysis with antibodies specific to LcrD or YscD. (A) Y. pestis KIM5-3001 (wt) and Y. pestis KIM5-3001.16 (ΔyscO) were analyzed with anti-LcrD. (B) Y. pestis KIM8-3002 (wt Pla−) and Y. pestis KIM8-3002.3 (ΔyscO Pla−) were analyzed with anti-YscD. The secondary antibody used was conjugated to alkaline phosphatase. Arrows indicate each protein.
Identification of the yscO gene product.
Since we had established a direct role for yscO in secretion, we wanted to gain insight into its possible function by determining its bacterial location. YscO was visualized on immunoblots of SDS-polyacrylamide gel electrophoresis (PAGE)-separated proteins from fractionated Y. pestis cultures by using a polyclonal antiserum that was directed against the GST-YscO fusion protein containing aa 13 to 154 of YscO (Fig. 6).
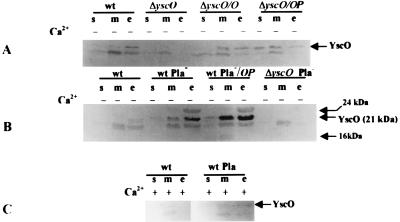
FIG. 6.
Localization of YscO by immunoblot analysis. Y. pestis KIM5-3001 (wt), KIM5-3001.16 (ΔyscO), KIM8-3002 (wt Pla−), and KIM8-3002.3 (ΔyscO Pla−) are shown. Strains carrying plasmids are denoted /O or /OP for pYscO.2 or pYscOP.2, respectively. Bacteria were grown at 37°C in TMH with (+) or without (−) Ca2+ for 5 h prior to harvest. Proteins from bacterial fractions were separated by SDS-PAGE (15% [wt/vol] acrylamide). Antibody raised against a GST-YscO fusion protein was used to detect YscO in soluble (s), total membrane (m), whole-cell (wc), or culture medium (e) proteins. All panels were analyzed with alkaline phosphatase. Arrows indicate the various proteins that were potential candidates for YscO.
YscO detection proved to be difficult, because the protein is expressed very weakly and the antiserum reacted with several proteins in the parent and yscO mutant strains (data not shown). The antibody identified a 21-kDa protein in all fractions of the parent, Y. pestis KIM5-3001 (wt) grown at 37°C in the absence of Ca2+ (Fig. 6A, lanes wt), and this was approximately the size expected (19 kDa) for YscO. We hypothesize that this is YscO, since it was not observed in the yscO mutant under the same conditions (lanes ΔyscO) but was seen in the mutant complemented with yscO (lanes ΔyscO/O and ΔyscO/OP). Furthermore, the intensity of the band was increased when yscO was expressed in trans in the parent (Fig. 6B, compare wt Pla− and wt Pla−/OP), although Yop expression was not changed in wt/OP (49). This supports the idea that the 21-kDa band represents YscO and is not a cross-reacting Yop. Less YscO was expressed in the presence of Ca2+ (compare Fig. 6C with Fig. 6A and B), which is consistent with previous observations of weak Ca2+ regulation in this operon (20). It is expected that a core secretion component would have to be present under noninductive conditions (in the presence of Ca2+) and be ready to function rapidly upon contact induction. Indeed, in the presence of Ca2+, YscO was localized to the membranes of KIM5-3001 (Fig. 6C).
In the course of visualizing YscO, we determined that it was subject to degradation by Pla. When grown at 37°C in the absence of Ca2+, the Y. pestis parent lacking Pla, KIM8-3002 (wt Pla−), yielded more YscO than did the parent containing Pla, KIM5-3001 (wt) (Fig. 6B, compare lanes wt to lanes wt Pla−), presumably due to the absence of Pla activity in Y. pestis KIM8-3002. Overall, the distribution pattern of YscO resembled that of Yops: more YscO was present in the culture medium than in the cellular fraction whether Pla was present or not. In some experiments, we unexpectedly detected YscO (Fig. 6C) as well as low levels of some Yops (not shown) in the culture medium in the presence of Ca2+, if the Y. pestis strain was Pla−. We believe that the absence of Pla unmasks a very low level of Yop secretion which occurs in the presence of Ca2+ but is not detected in Pla+ yersiniae due to degradation of these very small amounts by Pla.
In the Pla− (but not Pla+) parent and the parent carrying yscO in trans, we visualized a 24-kDa protein more strongly in the culture medium than in the membrane fraction (Fig. 6B, lanes wt Pla− and wt Pla−/OP). This band is of similar intensity in the two strains, and we think it represents a rapidly degraded LCRS protein (which would be expressed more strongly in strains having a functional secretion system than in the yscO mutant). In some experiments, we also saw a 16-kDa protein in either the soluble and/or membrane fraction but never in the culture medium (Fig. 6B).
DISCUSSION
The present study characterized yscO of the Y. pestis yscN to yscU operon. The lack of significant similarity of YscO to the corresponding type III proteins in Salmonella and Shigella suggests that YscO could function in recognition of Yersinia secreted effector proteins, and our data point to a role for YscO in Yop secretion.
At 37°C in the absence of Ca2+, our yscO mutant failed to undergo growth restriction and was unable to export LCRS proteins (V antigen and Yops). We believe that this phenotype was an effect of the loss of yscO only, since we observed expression of YscP in the mutant and since provision of only yscO in trans complemented the mutant. A His-tagged fusion of V antigen was not secreted when overexpressed from pHTV in the yscO mutant, and only a small amount of YopM was seen in the culture medium when overexpressed in the mutant from pTRCM.2. These results indicated that the yscO deletion directly prevented secretion of LCRS proteins in Y. pestis. This type of defect is similar to those described for other Yersinia proteins found to be components of the Ysc secretion mechanism, including the known or predicted inner membrane proteins, LcrD (55), YscD (43, 55), and YscR, YscS, and YscU (3, 7, 20); the outer membrane PulD homolog YscC (56); the lipoprotein YscJ (2); and the cytoplasmic protein YscN (78), thought to be associated with the inner membrane and a likely candidate for providing the energy needed for secretion. Other proteins that are essential for secretion but whose function and subcellular location are not known include YscE to YscG and YscI to YscL (2, 27, 43). For all of these essential Ysc components, mutations block the secretion of LCRS proteins and prevent full transcriptional induction of LCRS operons, probably by blocking release of the LCR negative regulator(s) from the cytosol through the type III secretion system. The similarity in growth and secretion defects in the yscO mutant suggests that yscO also is a core (essential) component of the Y. pestis LCRS export apparatus.
Surprisingly, we found that YscO was present in the culture medium in the absence of Ca2+. This differs from the localization of the corresponding type III protein in Salmonella called SpaM or InvI. SpaM/InvI was not detected in the culture medium but is essential for Salmonella entry into cultured epithelial cells (12). The corresponding protein in Shigella, Spa13, has been shown to be essential for invasion and presumably for the secretion of Ipa proteins, but its bacterial location has not been determined (63).
Secretion and translocation of Yops have been shown to occur in distinct steps (64) and to require recognition by some component of the type III secretion apparatus. There is no picture yet for how the ca. 22 Ysc components are arranged and function to move Yops through the two bacterial membranes. We would expect that each type III system would have unique proteins or adapted domains of conserved proteins for the recognition and/or transport of the system-specific secreted proteins. If YscO serves this purpose, it is unlikely that it interacts and moves with Yops in a 1:1 ratio, because YscO does not appear to be abundant enough for such a role. YscO evidently is a peripheral protein that is largely associated with or released from the membrane, suggesting that its function does directly correlate with Yop conduction through the membranes. Consistent with this idea, no YscO was found in the culture medium at 26°C, a situation in which no bulk Yop secretion was taking place (data not shown). YscO was sometimes observed in culture medium of the Pla− parent at 37°C in the presence of Ca2+, a condition in which low levels of Yops were also seen in the culture medium. Our findings suggest the possibility that the type III Ysc mechanism has a dynamic moving core and that YscO is part of this. Such an image is consistent with the developing picture for protein secretion through the Sec system, in which cycles of the piston-like pumping of SecA and the orientation inversion of SecG are essential to the mechanism of protein secretion (17, 47). We do not yet know the role of YscO release in the Yops secretion process. It is conceivable that this is an artifact of our in vitro system, which lacks the localized activation of secretion upon contact with a mammalian cell and the coupling of secretion with translocation into the cell. However, it is also possible that the release of YscO into the medium in our studies reflects a role that links the secretion of Yops with their subsequent targeting into a host cell.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant AI21017.
We acknowledge Mike Russ, University of Kentucky Macromolecular Structure Analysis Facility, for the synthesis of some of the oligonucleotides used in this study.
REFERENCES
- 1.Allaoui A, Scheen R, Lambert de Rouvroit C, Cornelis G R. VirG, a Yersinia enterocolitica lipoprotein involved in Ca2+ dependency, is related to ExsB of Pseudomonas aeruginosa. J Bacteriol. 1995;177:4230–4237. doi: 10.1128/jb.177.15.4230-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscHencoding YopR. Mol Microbiol. 1995;18:343–355. doi: 10.1111/j.1365-2958.1995.mmi_18020343.x. [DOI] [PubMed] [Google Scholar]
- 3.Allaoui A, Woestyn S, Sluiters C, Cornelis G R. YscU, a Yersinia enterocoliticainner membrane protein involved in Yop secretion. J Bacteriol. 1995;176:4534–4542. doi: 10.1128/jb.176.15.4534-4542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Gurion R, Shafferman A. Essential virulence determinants of different Yersiniaspecies are carried on a common plasmid. Plasmid. 1981;5:183–187. doi: 10.1016/0147-619x(81)90019-6. [DOI] [PubMed] [Google Scholar]
- 7.Bergman T, Erickson K, Galyov E, Persson C, Wolf-Watz H. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol. 1994;176:2619–2626. doi: 10.1128/jb.176.9.2619-2626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocoliticais internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:1591–5201. [PMC free article] [PubMed] [Google Scholar]
- 10.Bolin I D, Portnoy D A, Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985;48:234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows T W, Bacon G A. The basis of virulence in Pasteurella pestis: comparative behaviour of virulent and avirulent strains in vivo. Br J Exp Pathol. 1954;35:134–143. [PMC free article] [PubMed] [Google Scholar]
- 11a.Clontech Laboratories. Transformer site-directed mutagenesis kit. Palo Alto, Calif: Clontech Laboratories; 1994. [Google Scholar]
- 12.Collazo C M, Zierler M K, Galan J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the invlocus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 13.Cornelis G R. Yersinia pathogenicity factors. In: Dangl J Y, editor. Bacterial pathogenesis of plants and animals. Molecular and cellular mechanisms. New York, N.Y: Springer-Verlag; 1994. pp. 243–263. [Google Scholar]
- 14.Cornelis G R, Wolf-Watz H. The YersiniaYop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 15.Davis R H, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 16.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coliby using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 18.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y, Lai L-C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coliE2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferber D M, Brubaker R. Plasmids in Yersinia pestis. Infect Immun. 1981;27:839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields K A, Plano G V, Straley S C. A low-Ca2+ response (LCR) secretion (ysc) locus lies within the lcrB region of the LCR plasmid in Yersinia pestis. J Bacteriol. 1994;176:569–579. doi: 10.1128/jb.176.3.569-579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields, K. A., and S. C. Straley. Unpublished data.
- 22.Forsberg A, Viitanen A-M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 23.Fowler J M, Brubaker R R. Physiological basis of the low calcium response in Yersinia pestis. Infect Immun. 1994;62:5234–5241. doi: 10.1128/iai.62.12.5234-5241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galan J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goguen J D, Yother J, Straley S C. Genetic analysis of the low calcium response in Yersinia pestis Mu d1 (Ap lac) insertion mutants. J Bacteriol. 1984;160:842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groisman E A, Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993;12:3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddix P L, Straley S C. The structure and regulation of the Yersinia pestis yscBCDEFoperon. J Bacteriol. 1992;174:4820–4828. doi: 10.1128/jb.174.14.4820-4828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The YersiniaYpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 29.Hakansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosisis essential for the translocation of Yop effector proteins across the target cell plasma membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 30.Hensel M, Shea J E, Raupach B, Monack D, Falkow S, Gleeson C, Kubo T, Holden D W. Functional analysis of ssaJ and ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonellapathogenicity island 2. Mol Microbiol. 1997;24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 31.Holmstroem A, Pettersson J, Rosqvist R, Hakansson S, Tafazoli F, Faellman M, Magnusson K E, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosiscontrols translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 32.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of YersiniaYop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocoliticaforms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 35.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan J E, Aizawa S-I. Supramolecular structure of the Salmonella typhimuriumtype III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 38.Lindler L E, Klempner M S, Straley S C. Yersinia pestispH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect Immun. 1990;58:2569–2577. doi: 10.1128/iai.58.8.2569-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupas A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996;226:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- 40.Lupas A, Dyke M V, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 41.Maniatis T E, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 42.Masamune Y, Richardson C C. Strand displacement during deoxyribonucleic acid synthesis at single strand breaks. J Biol Chem. 1971;246:2692–2701. [PubMed] [Google Scholar]
- 43.Michiels T, Vanooteghem J-C, Lambert de Rouvroit C, China B, Gustin A, Boudry P, Cornelis G. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991;173:4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullis K B, Faloona F A. Specific synthesis of DNA in vitrovia a polymerase catalyzed chain reaction. Methods Enzymol. 1978;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 45.Nemeth J, Straley S C. Effect of Yersinia pestisYopM on experimental plague. Infect Immun. 1997;65:924–930. doi: 10.1128/iai.65.3.924-930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishiyama K, Suzuki T, Tokuda H. Inversion of the membrane topology of SecG, coupled with SecA-dependent preprotein translocation. Cell. 1996;85:71–81. doi: 10.1016/s0092-8674(00)81083-1. [DOI] [PubMed] [Google Scholar]
- 48.Nossal N G. DNA synthesis on a double-stranded DNA template by the T4 bacteriophage DNA polymerase and the T4 gene 32 DNA unwinding protein. J Biol Chem. 1974;249:5668–5676. [PubMed] [Google Scholar]
- 49.Payne, P. L., and S. C. Straley. YscP of Yersinia pestis is a secreted component of the Yop secretion system. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 50.Perry R D, Fetherston J E. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry R D, Pendrak M L, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Persson C, Nordfelth R, Holmström N, Hakansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersiniatranslocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 53.Pettersson J R, Nordfelth E, Dubinina T, Bergman T, Gustavsson M, Magnusson K-E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 54.Plano G V, Barve S S, Straley S C. LcrD, a membrane-bound regulator of the Yersinia pestislow-calcium response. J Bacteriol. 1991;173:7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plano G V, Straley S C. Multiple effects of lcrD mutations in Yersinia pestis. J Bacteriol. 1993;175:3536–3545. doi: 10.1128/jb.175.11.3536-3545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56a.Promega Corp. Promega protocol and application guide. 2nd ed. Madison, Wis: Promega Corp.; 1991. [Google Scholar]
- 57.Protsenko O A, Anisimov P I, Mosharov O T, Konnov N P, Popov Y A, Kokushkin A M. Detection and characterization of Yersinia pestisplasmids determining pesticin I, fraction I antigen, and “mouse” toxin synthesis. Sov Genet. 1983;19:838–846. [PubMed] [Google Scholar]
- 58.Rimpilainen M, Forsberg A, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis, shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of YersiniaYopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sample A K, Brubaker R R. Post-translational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb Pathog. 1987;3:239–248. doi: 10.1016/0882-4010(87)90057-x. [DOI] [PubMed] [Google Scholar]
- 61.Sample A K, Fowler J M, Brubaker R R. Modulation of the low-calcium response in Yersinia pestisvia plasmid-plasmid interaction. Microb Pathog. 1987;2:443–453. doi: 10.1016/0882-4010(87)90051-9. [DOI] [PubMed] [Google Scholar]
- 62.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri2a. J Bacteriol. 1993;175:2334–2346. doi: 10.1128/jb.175.8.2334-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schesser K, Frithz-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosisYopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skrzypek E, Haddix P L, Plano G V, Straley S C. New suicide vector for gene replacement in yersiniae and other gram-negative bacteria. Plasmid. 1993;29:160–163. doi: 10.1006/plas.1993.1019. [DOI] [PubMed] [Google Scholar]
- 66.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skrzypek, E., and S. C. Straley. Unpublished data.
- 69.Sodeinde O A, Sample A K, Brubaker R R, Goguen J D. Plasminogen activator/coagulase gene of Yersinia pestisis responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988;56:2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sodeinde O A, Subrahmanyam Y V B K, Stark K, Quan T, Bao Y, Goguen J D. A surface protease and the invasive character of plague. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 71.Sory M-P, Cornelis G. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocoliticainto HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 72.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestisinclude structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 74.Straley S C, Skrzypek E, Plano G V, Bliska J B. Yops of Yersiniaspp. pathogenic for humans. Infect Immun. 1993;61:3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Une T, Brubaker R R. In vivo comparison of avirulent Vwa− and Pgm− or Pstrphenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams A W, Straley S C. YopD of Yersinia pestisplays a role in the negative regulation of the low-calcium response in addition to its role in the translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woestyn S, Allaoui A, Wattiau P, Cornelis G R. YscN, the putative energizer of the YersiniaYop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zahorchak R J, Charnetzky W T, Little R V, Brubaker R R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979;139:792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]