Abstract
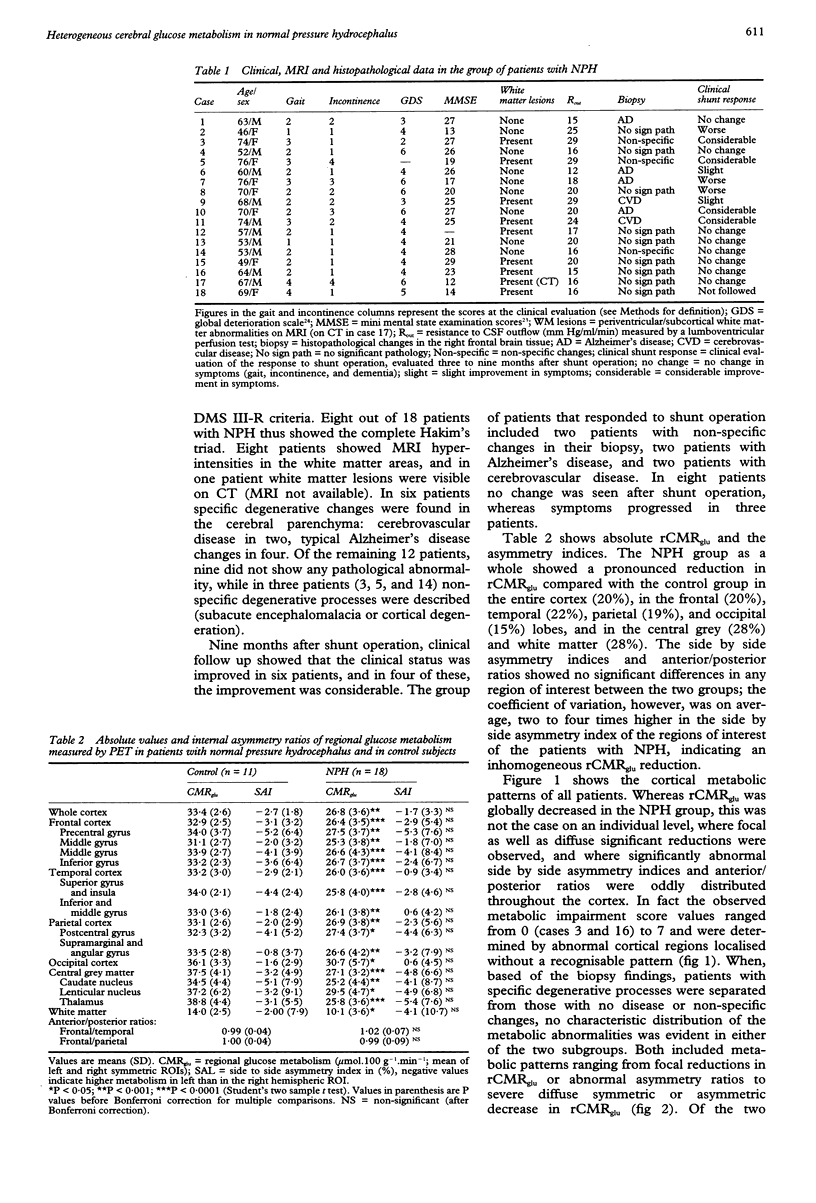
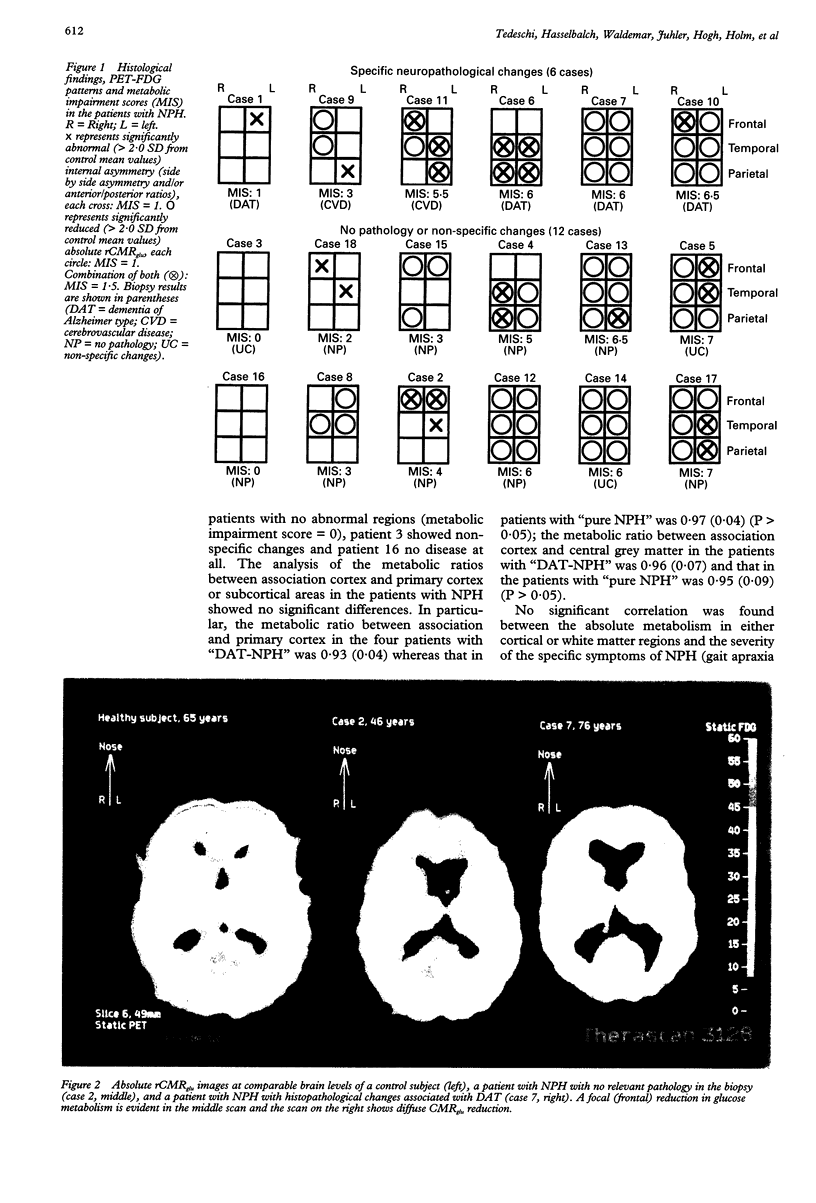
The regional cerebral metabolic rate for glucose (rCMRglu) has never been investigated in large consecutive groups of patients with normal pressure hydrocephalus (NPH), a potentially treatable form of dementia with an unpredictable outcome after shunt surgery. Using PET and 18F-2-fluorodeoxyglucose, rCMRglu was studied in 18 patients who fulfilled hydrodynamic criteria for NPH and in whom a biopsy of the frontal cortex was obtained. When compared with an age matched group of 11 healthy subjects, the patients with NPH showed a significant rCMRglu reduction in all cortical and subcortical regions of interest. Individual metabolic patterns, however, disclosed a large topographical heterogeneity. Furthermore, histopathological examination identified Alzheimer's disease or cerebrovascular disease in six cases, and no parenchymal disease or non-specific degenerative processes in the remaining 12. After separating the patients according to the histological diagnosis, the rCMRglu patterns were still heterogeneous, the abnormalities ranging from focal to diffuse in both subgroups. After shunt operation, 11 patients did not improve or worsened clinically. Six patients improved; of those, two had Alzheimer changes and two cerebrovascular changes in their biopsy. The metabolic pattern of these six patients did not differ from the rest of the NPH group. The results indicate that the NPH syndrome may be non-specifically associated with different degenerative disorders. The metabolic heterogeneity, together with the heterogeneous histopathological findings, indicate the necessity of reevaluating the pathogenesis of the NPH syndrome, and may account for the high variability in the success rate of shunt surgery series.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albeck M. J., Børgesen S. E., Gjerris F., Schmidt J. F., Sørensen P. S. Intracranial pressure and cerebrospinal fluid outflow conductance in healthy subjects. J Neurosurg. 1991 Apr;74(4):597–600. doi: 10.3171/jns.1991.74.4.0597. [DOI] [PubMed] [Google Scholar]
- Benson D. F., Kuhl D. E., Hawkins R. A., Phelps M. E., Cummings J. L., Tsai S. Y. The fluorodeoxyglucose 18F scan in Alzheimer's disease and multi-infarct dementia. Arch Neurol. 1983 Nov;40(12):711–714. doi: 10.1001/archneur.1983.04050110029003. [DOI] [PubMed] [Google Scholar]
- Brooks D. J., Beaney R. P., Powell M., Leenders K. L., Crockard H. A., Thomas D. G., Marshall J., Jones T. Studies on cerebral oxygen metabolism, blood flow, and blood volume, in patients with hydrocephalus before and after surgical decompression, using positron emission tomography. Brain. 1986 Aug;109(Pt 4):613–628. doi: 10.1093/brain/109.4.613. [DOI] [PubMed] [Google Scholar]
- Brooks R. A. Alternative formula for glucose utilization using labeled deoxyglucose. J Nucl Med. 1982 Jun;23(6):538–539. [PubMed] [Google Scholar]
- Børgesen S. E., Gjerris F., Sørensen S. C. Intracranial pressure and conductance to outflow of cerebrospinal fluid in normal-pressure hydrocephalus. J Neurosurg. 1979 Apr;50(4):489–493. doi: 10.3171/jns.1979.50.4.0489. [DOI] [PubMed] [Google Scholar]
- Børgesen S. E., Gjerris F. The predictive value of conductance to outflow of CSF in normal pressure hydrocephalus. Brain. 1982 Mar;105(Pt 1):65–86. doi: 10.1093/brain/105.1.65. [DOI] [PubMed] [Google Scholar]
- Coblentz J. M., Mattis S., Zingesser L. H., Kasoff S. S., Wiśniewski H. M., Katzman R. Presenile dementia. Clinical aspects and evaluation of cerebrospinal fluid dynamics. Arch Neurol. 1973 Nov;29(5):299–308. doi: 10.1001/archneur.1973.00490290039003. [DOI] [PubMed] [Google Scholar]
- Duara R., Barker W., Loewenstein D., Pascal S., Bowen B. Sensitivity and specificity of positron emission tomography and magnetic resonance imaging studies in Alzheimer's disease and multi-infarct dementia. Eur Neurol. 1989;29 (Suppl 3):9–15. doi: 10.1159/000116474. [DOI] [PubMed] [Google Scholar]
- Earnest M. P., Fahn S., Karp J. H., Rowland L. P. Normal pressure hydrocephalus and hypertensive cerebrovascular disease. Arch Neurol. 1974 Oct;31(4):262–266. doi: 10.1001/archneur.1974.00490400076009. [DOI] [PubMed] [Google Scholar]
- Fisher C. M. Hydrocephalus as a cause of disturbances of gait in the elderly. Neurology. 1982 Dec;32(12):1358–1363. doi: 10.1212/wnl.32.12.1358. [DOI] [PubMed] [Google Scholar]
- Fisher C. M. The clinical picture in occult hydrocephalus. Clin Neurosurg. 1977;24:270–284. doi: 10.1093/neurosurgery/24.cn_suppl_1.270. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frackowiak R. S., Pozzilli C., Legg N. J., Du Boulay G. H., Marshall J., Lenzi G. L., Jones T. Regional cerebral oxygen supply and utilization in dementia. A clinical and physiological study with oxygen-15 and positron tomography. Brain. 1981 Dec;104(Pt 4):753–778. doi: 10.1093/brain/104.4.753. [DOI] [PubMed] [Google Scholar]
- Friedland R. P. 'Normal'-pressure hydrocephalus and the saga of the treatable dementias. JAMA. 1989 Nov 10;262(18):2577–2581. [PubMed] [Google Scholar]
- Gjerris F., Børgesen S. E. Current concepts of measurement of cerebrospinal fluid absorption and biomechanics of hydrocephalus. Adv Tech Stand Neurosurg. 1992;19:145–177. doi: 10.1007/978-3-7091-6672-7_5. [DOI] [PubMed] [Google Scholar]
- Graff-Radford N. R., Rezai K., Godersky J. C., Eslinger P., Damasio H., Kirchner P. T. Regional cerebral blood flow in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 1987 Dec;50(12):1589–1596. doi: 10.1136/jnnp.50.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado J. M., Diaz F., Alday R. Evaluation of brain SPECT in the diagnosis and prognosis of the normal pressure hydrocephalus syndrome. Acta Neurochir (Wien) 1991;112(3-4):88–91. doi: 10.1007/BF01405132. [DOI] [PubMed] [Google Scholar]
- Greenberg J. O., Shenkin H. A., Adam R. Idiopathic normal pressure hydrocephalus-- a report of 73 patients. J Neurol Neurosurg Psychiatry. 1977 Apr;40(4):336–341. doi: 10.1136/jnnp.40.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb R. L., Jr, Raichle M. E., Gado M. H., Eichling J. O., Hughes C. P. Cerebral blood flow, oxygen utilization, and blood volume in dementia. Neurology. 1977 Oct;27(10):905–910. doi: 10.1212/wnl.27.10.905. [DOI] [PubMed] [Google Scholar]
- Hakim S., Adams R. D. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965 Jul-Aug;2(4):307–327. doi: 10.1016/0022-510x(65)90016-x. [DOI] [PubMed] [Google Scholar]
- Hoffman J. M., Guze B. H., Baxter L. R., Mazziotta J. C., Phelps M. E. [18F]-fluorodeoxyglucose (FDG) and positron emission tomography (PET) in aging and dementia. A decade of studies. Eur Neurol. 1989;29 (Suppl 3):16–24. doi: 10.1159/000116476. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Conti D., Kinkel W. R., Manning E. J. "Normal-pressure" hydrocephalus. Relationship of clinical and radiographic findings to improvement following shunt surgery. JAMA. 1976 Feb 2;235(5):510–512. doi: 10.1001/jama.235.5.510. [DOI] [PubMed] [Google Scholar]
- Jagust W. J., Friedland R. P., Budinger T. F. Positron emission tomography with [18F]fluorodeoxyglucose differentiates normal pressure hydrocephalus from Alzheimer-type dementia. J Neurol Neurosurg Psychiatry. 1985 Nov;48(11):1091–1096. doi: 10.1136/jnnp.48.11.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J. A., Grady C. L., Haxby J. V., Moore A., Friedland R. P. Plasticity in the aging brain. Reversibility of anatomic, metabolic, and cognitive deficits in normal-pressure hydrocephalus following shunt surgery. Arch Neurol. 1990 Dec;47(12):1336–1341. doi: 10.1001/archneur.1990.00530120082014. [DOI] [PubMed] [Google Scholar]
- Kimura M., Tanaka A., Yoshinaga S. Significance of periventricular hemodynamics in normal pressure hydrocephalus. Neurosurgery. 1992 May;30(5):701–705. [PubMed] [Google Scholar]
- Kirkpatrick J. B., Hayman L. A. White-matter lesions in MR imaging of clinically healthy brains of elderly subjects: possible pathologic basis. Radiology. 1987 Feb;162(2):509–511. doi: 10.1148/radiology.162.2.3797666. [DOI] [PubMed] [Google Scholar]
- Lying-Tunell U., Lindblad B. S., Malmlund H. O., Persson B. Cerebral blood flow and metabolic rate of oxygen, glucose, lactate, pyruvate, ketone bodies and amino acids. Acta Neurol Scand. 1981 Jun;63(6):337–350. doi: 10.1111/j.1600-0404.1981.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Tachibana H., Hardenberg J. P., Dowell R. E., Jr, Kitagawa Y., Mortel K. F. Normal pressure hydrocephalus. Influences on cerebral hemodynamic and cerebrospinal fluid pressure--chemical autoregulation. Surg Neurol. 1984 Feb;21(2):195–203. doi: 10.1016/0090-3019(84)90342-2. [DOI] [PubMed] [Google Scholar]
- Powers W. J., Perlmutter J. S., Videen T. O., Herscovitch P., Griffeth L. K., Royal H. D., Siegel B. A., Morris J. C., Berg L. Blinded clinical evaluation of positron emission tomography for diagnosis of probable Alzheimer's disease. Neurology. 1992 Apr;42(4):765–770. doi: 10.1212/wnl.42.4.765. [DOI] [PubMed] [Google Scholar]
- Reisberg B., Ferris S. H., de Leon M. J., Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982 Sep;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Sohn R. S., Siegel B. A., Gado M., Torack R. M. Alzheimer's disease with abnormal cerebrospinal fluid flow. Neurology. 1973 Oct;23(10):1058–1065. doi: 10.1212/wnl.23.10.1058. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Vanneste J., Augustijn P., Dirven C., Tan W. F., Goedhart Z. D. Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? A multicenter study and literature review. Neurology. 1992 Jan;42(1):54–59. doi: 10.1212/wnl.42.1.54. [DOI] [PubMed] [Google Scholar]
- Vorstrup S., Christensen J., Gjerris F., Sørensen P. S., Thomsen A. M., Paulson O. B. Cerebral blood flow in patients with normal-pressure hydrocephalus before and after shunting. J Neurosurg. 1987 Mar;66(3):379–387. doi: 10.3171/jns.1987.66.3.0379. [DOI] [PubMed] [Google Scholar]
- Waldemar G., Bruhn P., Kristensen M., Johnsen A., Paulson O. B., Lassen N. A. Heterogeneity of neocortical cerebral blood flow deficits in dementia of the Alzheimer type: a [99mTc]-d,l-HMPAO SPECT study. J Neurol Neurosurg Psychiatry. 1994 Mar;57(3):285–295. doi: 10.1136/jnnp.57.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldemar G., Hasselbalch S. G., Andersen A. R., Delecluse F., Petersen P., Johnsen A., Paulson O. B. 99mTc-d,l-HMPAO and SPECT of the brain in normal aging. J Cereb Blood Flow Metab. 1991 May;11(3):508–521. doi: 10.1038/jcbfm.1991.95. [DOI] [PubMed] [Google Scholar]
- Waldemar G., Schmidt J. F., Delecluse F., Andersen A. R., Gjerris F., Paulson O. B. High resolution SPECT with [99mTc]-d,l-HMPAO in normal pressure hydrocephalus before and after shunt operation. J Neurol Neurosurg Psychiatry. 1993 Jun;56(6):655–664. doi: 10.1136/jnnp.56.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]