Abstract
Agrobacterium tumefaciens causes crown gall disease by transferring oncogenic, single-stranded DNA (T strand), covalently attached to the VirD2 protein, across the bacterial envelope into plant cells where its expression results in tumor formation. The single-stranded DNA binding protein VirE2 is also transferred into the plant cell, though the location at which VirE2 interacts with the T strand is still under investigation. The movement of the transferred DNA and VirE2 from A. tumefaciens to the plant cell depends on the membrane-localized VirB and VirD4 proteins. Further, the movement of the IncQ broad-host-range plasmid RSF1010 between Agrobacterium strains or from Agrobacterium to plants also requires the virB-encoded transfer system. Our earlier studies showed that the presence of the RSF1010 plasmid in wild-type strains of Agrobacterium inhibits both their virulence and their capacity to transport VirE2, as assayed by coinfection with virE mutants. Here we demonstrate that the capacity to form a conjugal intermediate of RSF1010 is necessary for this inhibition, suggesting that the transferred form of the plasmid competes with the VirD2-T strand and/or VirE2 for a common export site.
The soil phytopathogen Agrobacterium tumefaciens transforms plant cells by transporting DNA, mobilized from a tumor-inducing (Ti) plasmid located in the virulent bacterium, into the plant cell nucleus. Expression of this transferred DNA (T-DNA) leads to the formation of crown gall tumors on most dicotyledonous plants (for a review, see reference 25). The Ti plasmid also contains the virulence (vir) region, which provides several gene products that mediate transformation. The T-DNA from the Ti plasmid undergoes site-specific nicking at the 23-bp border repeats by VirD2, and a single-stranded DNA intermediate, covalently bound at its 5′ end to VirD2, is formed (for reviews, see references 25, 38, and 51). A single-stranded DNA binding protein, VirE2, coats the DNA sometime during the transfer process, although this interaction may occur after the VirD2-T strand and VirE2 have been independently translocated to the plant cell (8, 13, 34, 42).
The processing of T-DNA and its movement from Agrobacterium to plants is similar to the conjugal transport of a variety of plasmids in gram-negative bacteria (reviewed in references 12, 29, and 48). During conjugal transfer, single-stranded plasmid DNA is thought to move from donor to recipient through a membrane-spanning pore encoded by the transfer (tra) genes of conjugative plasmids. Sequence comparisons have shown that the virB genes of A. tumefaciens, which most likely produce a membrane-localized multimeric protein channel for T-DNA export, are quite similar to several conjugal transport operons (12). The virB genes of A. tumefaciens also have significant sequence homology with the ptl genes of Bordetella pertussis (20, 39, 46), the products of which are required for the export of the six-subunit pertussis toxin. Given this similarity to a protein export system, it is perhaps not surprising that in addition to translocating DNA-protein complexes the VirB transport apparatus also appears to mediate the movement of proteins. Strains mutant in VirE2 (34) or VirF (30) can be complemented for tumorigenesis by coinfection with a helper strain that carries an intact vir region but lacks a T region, suggesting that both VirE2 and VirF proteins can be exported from Agrobacterium independently of the VirD2-T strand. Extracellular complementation assays have also shown that virE mutant strains are capable of moving an uncoated VirD2-T strand out of the bacterium into the plant cell (30, 34). To date, all of the VirB proteins tested, with the exception of VirB1, are essential for the movement of T-DNA (5, 17, 43), VirD2-T strands (8), VirE2 (8, 14), and VirF (30) from Agrobacterium to plant cells. These observations suggest that the VirB complex is a multifunctional translocation apparatus that recognizes and exports diverse substrates, most likely based on information contained within their protein component.
The hypothesis that the mechanisms of T-DNA transfer and conjugation are functionally related is further supported by the observation that plasmid RSF1010, a mobilizable, broad-host-range plasmid of the IncQ incompatibility group, can be transferred by A. tumefaciens to plant cells (10) in a process that requires the VirB proteins (44). Interestingly, the VirB and VirD4 proteins of A. tumefaciens can also direct the conjugative transfer of RSF1010 between agrobacteria (4). RSF1010, which lacks the border sequences upon which the VirD2 protein acts, carries genes which encode three proteins (MobA, MobB, and MobC) and carries an origin of transfer (oriT), all compactly organized within a 2.9-kb region of the plasmid. Each of these sequences is required for mobilization during conjugation (9, 19). Nicking of the DNA strand at oriT is carried out in a DNA-protein complex, called the relaxosome, by MobA and MobC (36, 49, 50). MobB increases the proportion of molecules specifically nicked at oriT, thereby increasing the efficiency of relaxosome formation (35). After site-specific nicking of one DNA strand, MobA becomes covalently attached to the 5′ end of the nicked strand and transfer of the single-stranded DNA into the recipient bacteria is initiated (6, 36). MobA and the oriT site are also essential for the transfer of RSF1010 into plant cells from Agrobacterium sp. strain LBA4404 (10). Apparently, nicking of the RSF1010 oriT by the Mob proteins is functionally equivalent to nicking at the Ti plasmid border sequences by VirD2, and the resultant complex is then translocated into plant cells via the normal T-DNA transfer process.
Our previous results (8, 44) have shown that pJW323, an RSF1010 derivative containing the nosP-nptII plant-selectable marker, inhibits the virulence of Agrobacterium sp. strain A348. Overexpression of virB9-11 in such a pJW323-containing strain restored virulence (44). Interestingly, as demonstrated by extracellular complementation experiments, pJW323 drastically reduces the ability of Agrobacterium strains to serve as VirE2 donors, while only partially inhibiting the capacity of virE2 mutant strains to act as VirD2-T strand donors (8). These findings suggest that pJW323 inhibits tumorigenesis by competing for a limiting factor essential for VirE2 transport, most likely the VirB complex. In this study, we tested the hypothesis that formation of an intermediate of RSF1010 capable of conjugal transfer is necessary for the inhibition of tumorigenesis and VirE2 movement.
MATERIALS AND METHODS
Bacterial strains and plasmid constructions.
Table 1 lists the bacterial strains and plasmid constructs used in this study. To create pAJ1, a frameshift mutation (mobA1) was made at the AccI site at the 5′ end of the mobA coding sequence (10) in pJB31, an IncQ Spr RSF1010 derivative (3). First, a 2.8-kb EcoRV fragment from pJB31 containing oriT and the 5′ end of the mobA gene was cloned into the Escherichia coli cloning vector pBluescript (Stratagene, La Jolla, Calif.). The resulting plasmid was then cut with AccI, the sticky ends were filled in with Klenow and deoxynucleoside triphosphates, and the DNA was religated to introduce an extra 2 bp. Next, the mutated 2.8-kb EcoRV piece was cloned into pJB31, replacing the wild-type EcoRV fragment and thus altering the reading frame of the 72-kDa MobA protein, creating pAJ1. In a second construct, pAJ6 was created to introduce point mutations in two bases flanking the nic site at oriT (oriT1) (7). To achieve this, the 2.8-kb EcoRV fragment in pBluescript, described above, was subjected to PCR-based site-directed mutagenesis according to the QuickChange protocol (Stratagene) by using two complementary 29-bp oligonucleotides containing G-to-A transitions flanking the oriT nic site at bp 3133 and 3135 (base pair numbering according to reference 37). At the same time, the C at position 3136 was changed to A and the A at position 3137 was changed to G to create a HindIII site for use in screening products of the mutagenesis reaction. The sequence of the mutated EcoRV fragment was then confirmed in its entirety by automated fluorescence sequencing reactions (PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing Kit, Perkin-Elmer) run on the Applied Biosystems, Inc., model 373A DNA sequencing system. Finally, the wild-type EcoRV fragment of pJB31 was replaced with the fragment containing the point mutations at oriT, yielding pAJ6. All other cloning was performed with standard protocols. Plasmids were introduced into E. coli by electroporation and into agrobacteria by either electroporation or conjugal transfer from E. coli S17-1 (40), which contains the tra genes of the RP4 plasmid integrated into the chromosome. The presence of the desired plasmid in transformed Agrobacterium strains was confirmed by restriction analysis of plasmid DNA isolated from clones preselected by plating on medium containing the appropriate antibiotic.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | λ− φ80d/lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA relA1 | GIBCO BRL |
| S17-1 | Chromosomally integrated tra genes of pRP4; Spr | 40 |
| HB101 | F−hsdS20 (rB− mB−) supE44 recA13 ara-14 λ− galK2 lacY1 proA2 rpsL120 (Smr) xyl-5 mtl-1 rB− mB− | Clontech |
| A. tumefaciens | ||
| A136 | C58 heat cured of pTiC58 | 45 |
| A348 | A136 containing pTiA6 | 23 |
| A348::virE2 | A348 carrying an insertion mutation in virE2 | 8 |
| 358mx | A348 containing pTiA6 virE::Tn3HoHo | 41 |
| LBA4404 | Ach5 chromosome; pTiAch5 carrying deletion of the T-DNA; Smr | 33 |
| Plasmids | ||
| pAD1285 | Broad-host-range vector derived from high-copy-number mutant of pTJS75; IncP Cbr Tetr | 17 |
| pAJ1 | mobA1 derivative of pJB31 | This study |
| pAJ5 | 2.9-kb AvaI fragment containing the mob region from pAJ1 cloned into pBluescript; Cbr | This study |
| pAJ6 | oriT1 derivative of pJB31 | This study |
| pBluescript | E. coli vector; Cbr | Stratagene |
| pEND4K | Binary vector with nos-nptII plant-selectable marker; IncP Cmr Kanr | 27 |
| pJB20 | Kans Sps derivative of broad-host-range pUCD2; IncW Cbr Tetr | 3 |
| pJB31 | RSF1010-derived broad-host-range vector; IncQ Spr | 3 |
| pKT231 | RSF1010 derivative; IncQ Kanr | 1 |
| pLS1 | 5.7-kb Xmn/Pst fragment containing the mob and rep regions from pKT231 cloned into pBluescript; Cbr | This study |
| pLS2 | 5.7-kb XmnI/PstI fragment containing the mob and rep regions from pAJ1 cloned into pBluescript; Cbr | This study |
| pLS7 | 2.9-kb AvaI fragment containing the mob region from pAJ6 cloned into pBluescript; Cbr | This study |
| pLS8 | 5.7-kb XmnI/PstI fragment containing the mob and rep regions from pAJ6 cloned into pBluescript; Cbr | This study |
| pLS9 | 2.9-kb AvaI fragment containing the wild-type mob region from pKT231 cloned into pBluescript; Cbr | This study |
| pLS17 | pAD1285 with nos-NPTII plant-selectable marker; IncP Cbr | This study |
| pLS18 | 2.9-kb AvaI fragment containing the wild-type mob region from pKT231 cloned into pLS17; Cbr | This study |
| pLS50 | PstI Gmr gene fragment from pML122 cloned into pJB20; IncW Tetr Gmr | This study |
| pML122 | RSF1010-derived broad-host-range vector; Gmr Tetr | 1 |
| pRK2013 | Self-transmissible, ColE1 replicon, tra functions of pRK2; IncP Kanr | 21 |
| pSW213 | Broad-host-range plasmid with lac promoter and lacIq; IncP Tetr | 11 |
| pUCD2 | Broad-host-range cloning vector; IncW Cbr Kanr Spr Tetr | 15 |
Conjugation assays.
For conjugation between E. coli strains, donor strains, either S17-1 containing Ampr pBluescript derivatives or DH5α carrying these derivatives and pRK2013 as a helper plasmid, and the recipient strain HB101 (Strr) were grown to log phase, pelleted, resuspended in 0.9% NaCl at a ratio of 1:100 (donor to recipient) in a final volume of 10 μl, and spotted onto 1.5 ml of Luria-Bertani agar in 24-well plates. After 1 h the conjugation mix was resuspended in 500 μl of 0.9% NaCl, diluted appropriately, and plated onto medium containing carbenicillin and streptomycin to select for transconjugants.
For conjugation between Agrobacterium strains, Spr plasmids pJB31, pAJ1, and pAJ6 and Cbr plasmids pLS1, pLS2, and pLS8 were transformed into A348. These plasmids were then tested for their capacity to mobilize into recipient strain A348 that contained pLS50, a Gmr nonmobilizable plasmid, during incubations on minimal medium containing the vir inducer acetosyringone (AS) (Aldrich Chemical). The conjugation experiments were carried out as previously described (3). Bacteria were then washed from the agar and plated on medium containing spectinomycin and gentamicin or medium containing carbenicillin and gentamicin to select for transconjugants. Donors were quantitated by growth on spectinomycin- or carbenicillin-containing medium, and recipients were quantitated by growth on gentamicin-containing medium. Colonies were scored after 3 days.
Virulence assays.
Virulence assays using Kalanchoe daigremontiana were carried out as described previously (43) with strain A348 carrying the various RSF1010 derivatives and with A348 alone and with A348(pJB31) as positive and negative controls, respectively. All inoculations were scored for the level of tumor formation after 14, 21, and 28 days. Nicotiana tabacum cv. Havana 425 was used for all tobacco leaf square transformation assays as previously described (2). Leaf squares were scored for tumor growth 10 days after cocultivation with the Agrobacterium strains. The ability of various RSF1010 derivative plasmids to block the capacity of disarmed (no T-DNA) strain LBA4404 to serve as a VirE2 donor was assayed by infecting leaf square explants with a 1:1 mixture (each at an optical density at 600 nm [OD600] of 0.5) of LBA4404 carrying the various plasmids and virE2 mutant A348::virE2. After 2 days of cocultivation on hormone-free MS medium (31) containing 100 μM AS, the leaf squares were washed and transferred to selection (hormone-free MS) medium and were scored for tumor formation 10 days after cocultivation. In similar experiments, the abilities of various RSF1010 derivative plasmids to block the capacity of A348 to serve as a VirE2 donor were assayed by infecting leaf square explants with a 1:1 mixture (each at an OD600 of 0.5) of the various A348 strains and virE2 mutant strain 358mx carrying the binary vector pEND4K (8). After 2 days of cocultivation on hormone-free MS medium (32) containing 100 μM AS, the leaf squares were washed and transferred to MS selection medium containing the plant hormones kinetin (1 μM) and naphthalene acetic acid (10 μM), antibiotics to eliminate the bacteria (200 μg of timentin and 200 μg of vancomycin per ml), and 100 μg of kanamycin per ml. Kanamycin-resistant growth, resulting from the transfer of the pEND4K T-DNA into plant cells, was scored after 21 days.
RESULTS
The MobA protein and oriT nic site of pJB31 are necessary for plasmid movement between E. coli strains.
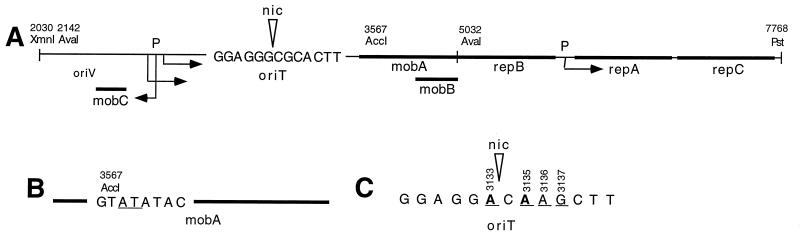
Our earlier results showed that the presence of an RSF1010 derivative in A348 blocked this strain’s ability to transform Kalanchoe and tobacco (44). We sought to test the hypothesis that the conjugal intermediate of this plasmid was responsible for the observed inhibition. Therefore, mutations predicted to abolish mobilization were constructed by altering either the mobA coding sequence or the nic site of oriT of RSF1010. Previously published data on RSF1010 (10) have shown that oriT and the entire mob region are within a 2.9-kb AvaI fragment (Fig. 1A) and that a mutation at the AccI site in the mobA gene leads to a nonfunctional mob region (Fig. 1B). In addition, Bhattacharjee et al. have shown, using in vitro assays for cleavage of single-stranded oriT sequence by purified MobA* β-galactosidase hybrid protein, that two G-to-A transitions in bases bracketing the nic site at the oriT of plasmid R1162 (nearly identical to RSF1010) decrease cleavage by at least 90% (7) (Fig. 1C). To confirm that mob regions with mutations at these sites no longer support conjugation between E. coli strains, the mob region of the RSF1010 derivative pJB31 was mutagenized (see Materials and Methods for details) so that it contained a frameshift mutation at the 5′ end of mobA (yielding mobA1 in pAJ1) or the transition mutations at oriT (yielding oriT1 in pAJ6). The 2.9-kb AvaI fragments carrying the mutagenized or wild-type mob regions were then cloned into pBluescript (Stratagene), a small nonmobilizable E. coli plasmid, to create pAJ5 (mobA1), pLS7 (oriT1), and pLS9 (wild-type mob region). Whereas pBluescript carrying the wild-type AvaI fragment (pLS9) is mobilizable at a high frequency from E. coli S17-1 (carrying the tra genes of RP4 in the chromosome) to HB101 (1.7 ± 0.2 transconjugants/donor input [5 × 106 bacteria]) pAJ5 (mobA1) is very poorly mobilized (4.8 × 10−3 ± 2 × 10−3 transconjugants/donor input [5 × 106 bacteria]) and pLS7 (oriT1) is essentially nonmobilizable (no transconjugants). (A total of 5 × 108 HB101 bacteria were used as the recipient cells for each experiment.) In the case of pAJ5 a very small number of transconjugants were observed, presumably because the function of MobA can be partially replaced by a gene encoded by the host bacterium (10). Similar results were observed when DHα strains containing the pBluescript derivatives, as well as the helper plasmid pRK2013 that contains the tra genes of RK2 (21), were used as the donor (data not shown).
FIG. 1.
Structures of RSF1010 derivatives. Base pair numbering is according to reference 38. (A) The mobilization region and replication genes of RSF1010. The approximate positions of the genes and DNA sites discussed in this paper are indicated as are the locations and orientations of the corresponding promoters and the DNA sequence surrounding the nic site (arrowhead) at oriT. Relevant restriction sites are shown. (B) Frameshift mutation in mobA. Two base pairs at the AccI site in mobA (underlined) were added by digestion with AccI, blunt-ending with Klenow and deoxynucleoside triphosphates and religation. (C) Transition mutations in oriT. Two base pairs (boldface and underlined) were changed by oligonucleotide-directed mutagenesis to destroy the nic site at oriT; an additional 2 bp were changed (underlined) to create a HindIII site.
The MobA protein and oriT nic site of pJB31 are necessary for plasmid movement between Agrobacterium strains.
In addition to being mobilizable between E. coli strains, RSF1010 can be transferred between Agrobacterium strains in a process that is mediated by the Ti-borne VirB and VirD4 proteins (4, 22) rather than the Ti-borne Tra proteins (16). To demonstrate that pJB31 derivatives with mutations in mobA or oriT are no longer capable of using the virB-encoded transfer system, strains carrying the mutated RSF1010 derivatives, pAJ1 (mobA1) and pAJ6 (oriT1), were tested for VirB-dependent movement between Agrobacterium strains. In these experiments, spectinomycin-resistant pJB31, pAJ1, and pAJ6 were transformed into the tumorigenic strain A348 and tested for their ability to be mobilized to a gentamicin-resistant recipient. Table 2 shows that little if any pAJ1 or pAJ6 transfer was observed compared to that of the wild-type pJB31.
TABLE 2.
Mobilization of RSF1010 derivatives between Agrobacterium strains
| Donor strain | Feature(s) | Donor output (109) | No. of trans- conjugants | Mean no. (±SD) of transconjugants/ output donor | % of A348(pJB31) transconjugants |
|---|---|---|---|---|---|
| A348(pJB31) | Wild-type mob region | 5.2 | 210,000.0 | 4.0 × 10−5 ± 0.4 × 10−5 | 100.0 |
| A348(pAJ1) | pJB31 with mobA1 | 10.3 | 4.7 | 4.6 × 10−10 ± 6.5 × 10−10 | 0.001 |
| A348(pAJ6) | pJB31 with oriT1 | 12.0 | 0.0 | 0.0 ± 0 | 0.0 |
| A348(pLS1) | IncQ mob-rep region in pBS | 6.3 | 205,000.0 | 3.3 × 10−5 ± 0.3 × 10−5 | 82.5 |
| A348(pLS2) | pLS1 with mobA1 | 8.0 | 3.3 | 4.2 × 10−10 ± 5.2 × 10−10 | 0.001 |
| A348(pLS8) | pLS1 with oriT1 | 1.4 | 1.7 | 1.0 × 10−9 ± 1.8 × 10−9 | 0.002 |
| A348(pLS17) | IncP vector | 2.1 | 0.7 | 3.4 × 10−10 ± 2.9 × 10−10 | 0.001 |
| A348(pLS18) | pLS17 and RSF1010 mob region | 0.4 | 31.0 | 7.3 × 10−8 ± 0.8 × 10−8 | 0.2 |
Data are the means of triplicate determinations from a single experiment. A total of three independent experiments with similar results were performed. Totals of 2 × 105 donors and 106 recipients (A348[pLS50]) were used as input in the conjugation mix. The number of recovered (output) recipients after 3 days’ growth at 25°C on induction medium was approximately 4 × 1010 cells.
A third set of plasmids was constructed to determine which regions of RSF1010 are sufficient to create a plasmid that is mobilizable through the Agrobacterium VirB transfer apparatus. The 2.9-kb AvaI fragment (Fig. 1A) that conferred to pBluescript the capacity to be mobilized between E. coli strains was cloned into an IncP broad-host-range vector, pLS17, which cannot be mobilized between agrobacteria via the VirB proteins. Surprisingly, the resultant fusion plasmid, pLS18, could not be efficiently mobilized between agrobacteria. The transfer frequency was approximately 500 times less than that of the wild-type RSF1010 derivative pJB31 (Table 2). While extremely inefficient, this transfer was reproducible and required AS (data not shown).
To determine the minimum amount of RSF1010 required for high-efficiency transfer between Agrobacterium strains, a 5.7-kb PstI/XmnI fragment (Fig. 1A), containing the mob region along with the rep genes necessary to create a plasmid that could be maintained in agrobacteria, was cloned into pBluescript to create pLS1. The mob and rep regions of pAJ1(mobA1) and pAJ6(oriT1) were also cloned into pBluescript to create pLS2 and pLS8, respectively. These hybrid plasmids were then tested for their ability to mobilize between agrobacteria. Only pLS1, which carries the wild-type mob region, was capable of conjugal movement (Table 2).
pJB31 MobA protein and the nic site at oriT are necessary for inhibition of tumorigenesis.
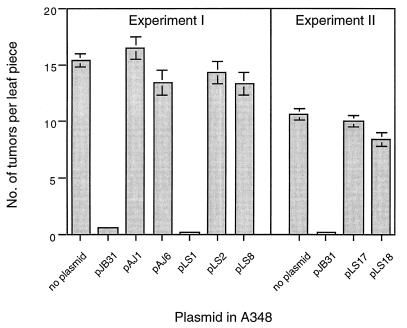
We next tested the effect of the RSF1010 derivative plasmids pAJ1 (mobA1) and pAJ6 (oriT1) on the virulence of wild-type strain A348. Assays using K. daigremontiana leaves (data not shown) as well as tobacco leaf disk assays (Fig. 2) showed that virulence, as demonstrated by tumor formation, was inhibited by the wild type (pJB31) but not by the nonmobilizable (pAJ1 and pAJ6) RSF1010 derivatives. These results indicate that both the MobA protein and the oriT nic site are essential to the inhibition of virulence. Because the nonmobilizable plasmid pAJ6 is mutant for oriT but wild type for MobA, these results also rule out the possibility that the MobA protein itself can inhibit virulence independent of the transferred intermediate. Therefore, by separately mutating the MobA coding sequence and the oriT nic site of pJB31, we have shown that a functional mob region is necessary for the inhibition of virulence and that inhibition of virulence correlates with the capacity of the plasmid to be mobilized between A. tumefaciens strains via the VirB pore.
FIG. 2.
Virulence assay of N. tabacum. N. tabacum cv. Havana 425 leaf explants were infected with A348 strains without and with RSF1010 derivative plasmids for 2 days and were then transferred to antibiotic-containing medium as described in Materials and Methods. The mean numbers of tumors ± standard errors (n = 15 to 17 leaf pieces) were determined after 10 days of incubation.
We also determined that tumor formation was not affected by pLS18, a fusion of the mob region of RSF1010 with the IncP plasmid pLS17. In contrast, the mobilizable plasmid pLS1, consisting of the wild-type mob region plus rep genes of RSF1010 in pBluescript, did prevent tumorigenesis (Fig. 2), further suggesting that the capacity to be efficiently mobilized through the VirB transfer apparatus correlates directly with virulence inhibition.
Both MobA and the oriT nic site of pJB31 are necessary for inhibition of VirE2 transfer.
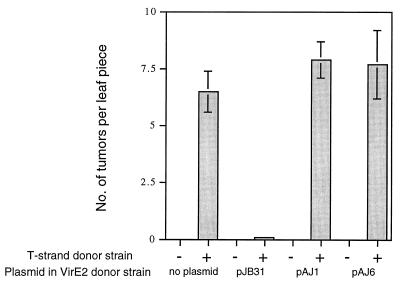
Having demonstrated that a mobilizable version of the RSF1010 derivative pJB31 was necessary to inhibit virulence, we next sought to determine whether the capacity for mobilization was necessary to inhibit the capacity of an Agrobacterium strain to serve as a VirE2 donor in the extracellular complementation of virE2 mutants (8, 34). In the assays used in this study, plant tissues are exposed to a mixture of two strains, a virE mutant strain containing the wild-type T-DNA or a standard binary vector, pEND4K, carrying a plant-expressible neomycin phosphotransferase gene (nosP-nptII) and a strain producing VirE2 but lacking the T-DNA or the binary vector. The stable transformation of plant cells by the T-DNA from either of the virE2 mutant T strand donors requires coinfection with the helper strain wild type for virE. Using such extracellular complementation assays, we previously demonstrated that mobilizable RSF1010 derivatives block the capacity of either LBA4404 or A348 to serve as a VirE2 donor, resulting in little transformation of the leaf pieces (8). Here we tested the ability of LBA4404 carrying either nonmobilizable pAJ1 or pAJ6, or wild-type pJB31 as a control, to function as a VirE2 donor in extracellular complementation assays when mixed with the virE mutant strain A348::virE2′. After coinfection, the leaf explants were monitored for transformation by scoring for the number of tumors formed. The results shown in Fig. 3 clearly indicate that pJB31 greatly inhibits the capacity of LBA4404 to serve as a VirE2 donor whereas pAJ1 and pAJ6 have no such effect. Similar results were observed when A348 carrying an RSF1010 derivative was tested for its capacity to serve as a VirE2 donor when mixed with the virE2 mutant 358mx (41) carrying pEND4K. In this case, the leaf explants were monitored for transformation by pEND4K, as assayed by the growth of kanamycin-resistant calli. The nonmobilizable RSF1010 derivatives did not inhibit the capacity of the A348 strain to serve as a VirE2 donor (data not shown). Thus, these results demonstrate that a functional mob region is needed for RSF1010 to inhibit not only tumorigenesis but also the capacity of an Agrobacterium strain to serve as a VirE2 donor in extracellular complementation assays.
FIG. 3.
Effects of different RSF1010 derivatives on the capacity of Agrobacterium sp. strain LBA4404 to serve as a VirE2 donor. N. tabacum cv. Havana 425 leaf explants were infected with a VirE2 donor strain (LBA4404 without or with RSF1010 derivative plasmids) either in the absence (−) or presence (+) of the T-strand donor strain A348::virE2 for 2 days, as described in Materials and Methods, and then transferred to selection medium. The mean numbers of tumors per explant ± standard errors (n = 18 leaf pieces) were determined after a 10-day incubation on selection medium.
The capacity of virE2 mutants, carrying either pJB31 or its mutant derivatives, to deliver their T-DNA was tested by extracellular complementation assays using strain LBA4404 as the VirE2 donor. Consistent with our previously published results (8), the wild-type RSF1010 derivative pJB31 only moderately inhibited VirD2-T strand transfer from the virE2 mutant A348::virE2 (50 to 75% reduction of the tumors compared to control) (data not shown). When this strain carried the mutant derivative pAJ1 (mobA1) or pAJ6 (oriT1), this inhibitory effect was not observed (data not shown). Thus, a functional mob region is needed in order to inhibit, albeit moderately, the transfer of the VirD2-T strand from virE2 mutant strains of Agrobacterium to plant cells.
DISCUSSION
We demonstrated previously that the IncQ broad-host-range plasmid RSF1010 inhibits the virulence of strain A348 despite the fact that it can, itself, be transferred from these Agrobacterium cells to plant cells. Additionally, we found that this plasmid also blocks the capacity of this strain to serve as a VirE2 donor in extracellular complementation assays (8, 44) but barely inhibits the transfer of the VirD2-T strand from virE2 mutants into plant cells. These results suggest that disruption of VirB-mediated VirE2 transfer by RSF1010 is largely responsible for this plasmid’s effects on virulence. Here we show that RSF1010 derivatives with mutations in either the mobA coding sequence or the nic site at oriT cannot be mobilized between agrobacteria in a virB-dependent manner (Table 2) and no longer inhibit virulence (Fig. 2) or the transfer of VirE2 (Fig. 3) from agrobacteria to plants. We have also shown that a small nonmobilizable E. coli plasmid carrying the rep genes as well as the mob region of RSF1010 (Fig. 1) is efficiently mobilized between Agrobacterium strains (Table 2) and inhibits Agrobacterium virulence (Fig. 2). Taken together, these findings support the hypothesis that it is the conjugal intermediate of RSF1010 that inhibits virulence and the movement of VirE2, most likely by competing for the multisubstrate VirB transport apparatus.
Intriguingly, we were unable to prove that the mobilization region of RSF1010 is sufficient to inhibit tumorigenesis or VirE2 movement when it is carried in an IncP broad-host-range vector. The promoters and coding sequences of MobA, MobB, and MobC, as well as the sequences for oriT, the origin of transfer, and oriV, the vegetative origin of replication, were isolated as a 2.9-kb AvaI fragment (Fig. 1A) and cloned into pLS17, a high-copy-number IncP plasmid (18) carrying a plant-selectable marker, to create pLS18. In contrast to the RSF1010 derivative pJB31, however, the hybrid plasmid was mobilizable only at a very low frequency between Agrobacterium strains (virB-dependent conjugation rates were at least 500-fold less than that of pJB31) (Table 2). Similar results were obtained when the 2.9-kb mob region was cloned into two other broad-host-range vectors, pSW213 (IncP) and pUCD2 (IncW) (data not shown). It is possible that local conformation of the hybrid plasmid helix affects the activity of the RSF1010 oriT or of the adjacent mob gene promoters (30a). Because vegetative and transfer modes of replication may be coordinated (26, 32), competition between oriV or oriT from RSF1010 with those of the IncW and IncP plasmids may prevent efficient relaxosome assembly and formation of conjugal intermediates. None of the three RSF1010-broad-host-range hybrid plasmids tested demonstrated an effect on virulence or VirE2 transfer (Fig. 2 and data not shown), again supporting the hypothesis that the conjugal intermediates are required for inhibition of virB-mediated transport.
Plant transformation assays did, however, show that pLS18, but not pLS17, was capable of movement into plant cells (data not shown). These results confirm the findings of Buchanan-Wollaston et al. (10), who showed that the mob region of RSF1010 can mediate the transfer of an IncP broad-host-range plasmid from Agrobacterium into plant cells. The level of conjugal efficiency exhibited by pLS18 is evidently sufficient to form complexes necessary for transfer to plants but is not sufficient to outcompete VirE2 for available export sites.
How could the RSF1010 transfer intermediate block or disrupt access of VirE2, and to a lesser extent that of the VirD2-T strand, to the VirB pore? Certainly the relative abundance of RSF1010 in agrobacteria (at least 20 copies/cell) may contribute to this. However, we have shown that high copy number of the plasmid alone, without the capacity to mobilize efficiently via the VirB transport apparatus, is not sufficient for inhibition. The mutant high-copy-number RSF1010 derivatives pAJ1 and pAJ6 do not affect A348 virulence. Additionally, the poorly mobilizable hybrid plasmid pLS18, which also had no effect on A348 virulence, is most likely present in agrobacteria at a very high copy number, around 30 to 45 copies per cell (18). An alternative is that the transferred intermediate of RSF1010 has advantages over VirE2 (or the VirD2-T strand) in interacting with the VirB pore. Mobilizable plasmids such as RSF1010 can be isolated from donor cells as relaxosomes, DNA-protein complexes that exist in an equilibrium of cleaved and uncleaved nic sites at the oriT. The formation of the relaxosome complex appears to be constitutive and does not depend on the presence of a recipient cell or any other conjugative trigger (reviewed in reference 28). Free, single-stranded DNA transfer intermediates of mobilizable broad-host-range plasmids have not been found in donor cells, suggesting that plasmid DNA movement is coupled to processing of the transferred intermediate (28). In contrast, vir-inducing conditions are required for the production of Vir proteins and the formation of single-stranded T-DNA transfer intermediates that have been detected in the donor A. tumefaciens cell (47). Thus, the relaxosome may be more readily available as potentially limiting VirB pore components are assembled after vir induction. The relatively smaller effect of RSF1010 on VirD2-T strand transfer (compared to VirE2) may reflect the possibility that less of this intermediate than VirE2 needs to be transferred into plant cells as long as VirE2 is available from another source.
Another important factor allowing RSF1010 to interfere with the interaction of other substrates and the VirB pore may be that the movement of RSF1010 through the VirB system is very inefficient. For example, VirB-mediated mobilization efficiencies are usually on the order of 10−3 to 10−5 transconjugants per donor cell, a process that takes three days (references 3 and 22 and this study). In contrast, mobilization through broad-host-range plasmid conjugal transfer systems in E. coli yields about 100 to 10−1 transconjugants per donor or about 100 to 10,000 times more transconjugants (24). Amazingly, this transfer takes place in 1 h rather than 3 days. In addition, the transfer of RSF1010 derivatives out of agrobacteria into plant cells is less efficient than the transfer of wild-type T-DNA (8). The results of this and previous studies suggest that inefficient utilization of the VirB pore by the RSF1010 conjugal intermediate may limit the access of VirE2, particularly, to this transfer complex.
ACKNOWLEDGMENTS
We thank Richard Meyer for discussions and Lois Banta and Mark Jacobs for reading earlier versions of the manuscript.
This work was supported by the National Science Foundation through a grant to A.N.B. (MCB95-13662).
REFERENCES
- 1.Bagdasarian M, Lurz R, Franklin F C H, Bagdasarian M M, Frey J, Timmis K N. Specific purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 2.Banta L M, Joerger R D, Howitz V R, Campbell A M, Binns A N. Glu-255 outside the predicted ChvE binding site in VirA is crucial for sugar enhancement of acetosyringone perception by Agrobacterium tumefaciens. J Bacteriol. 1994;176:3242–3249. doi: 10.1128/jb.176.11.3242-3249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaupré C F, Bohne J, Dale E M, Binns A N. Interactions between VirB9 and VirB10 proteins involved in movement of DNA from Agrobacterium tumefaciens to plant cells. J Bacteriol. 1997;179:78–89. doi: 10.1128/jb.179.1.78-89.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beijersbergen A, Dulk-Ras A D, Schilperoort R A, Hooykaas P J J. Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science. 1992;256:1324–1327. doi: 10.1126/science.256.5061.1324. [DOI] [PubMed] [Google Scholar]
- 5.Berger B R, Christie P J. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994;176:3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee M K, Meyer R J. A segment of a plasmid gene required for conjugal transfer encodes a site-specific, single-strand DNA endonuclease and ligase. Nucleic Acids Res. 1991;19:1129–1137. doi: 10.1093/nar/19.5.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharjee M K, Rao X-M, Meyer R J. Role of the origin of transfer in termination of strand transfer during bacterial conjugation. J Bacteriol. 1992;174:6659–6665. doi: 10.1128/jb.174.20.6659-6665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binns A N, Beaupré C F, Dale E M. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J Bacteriol. 1995;177:4890–4899. doi: 10.1128/jb.177.17.4890-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasch M A, Meyer R J. A 38 base-pair segment of DNA is required in cis for conjugative mobilization of broad host-range plasmid R1162. J Mol Biol. 1987;198:361–369. doi: 10.1016/0022-2836(87)90286-5. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan-Wollaston V, Passiatore J E, Cannon F. The mob and oriT mobilization functions of a bacterial plasmid promote its transfer to plants. Nature (London) 1987;328:172–175. [Google Scholar]
- 11.Chen C-Y, Winans S C. Controlled expression of the transcriptional activator gene virG in Agrobacterium tumefaciens by using the Escherichia coli lac promoter. J Bacteriol. 1991;173:1139–1144. doi: 10.1128/jb.173.3.1139-1144.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie P J, Ward J E, Winans S C, Nester E W. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J Bacteriol. 1988;170:2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Citovsky V, DeVos G, Zambryski P. A novel single stranded DNA binding protein, encoded by the virE locus, is produced following activation of the A. tumefaciens T-DNA transfer process. Science. 1988;240:501–504. doi: 10.1126/science.240.4851.501. [DOI] [PubMed] [Google Scholar]
- 15.Close T J, Zaitlin D, Kado C I. Design and development of amplifiable broad-host-range cloning vectors: analysis of the vir region of Agrobacterium tumefaciens plasmid pTiC58. Plasmid. 1984;12:111–118. doi: 10.1016/0147-619x(84)90057-x. [DOI] [PubMed] [Google Scholar]
- 16.Cook D M, Farrand S K. The oriT region of Agrobacterium tumefaciens Ti plasmid pTiC58 shares DNA sequence identity with the transfer origins of RSF1010 and RK2/RP4 and with T-region borders. J Bacteriol. 1992;174:6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale E M, Binns A N, Ward J E., Jr Construction and characterization of Tn5virB, a transposon that generates nonpolar mutations, and its use to define virB8 as an essential virulence gene in Agrobacterium tumefaciens. J Bacteriol. 1993;175:887–891. doi: 10.1128/jb.175.3.887-891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das A, Xie Y-H. Replication of the broad-host-range plasmid RK2: isolation and characterization of a spontaneous deletion mutant that can replicate in Agrobacterium tumefaciens but not in Escherichia coli. Mol Gen Genet. 1995;246:309–315. doi: 10.1007/BF00288603. [DOI] [PubMed] [Google Scholar]
- 19.Derbyshire K M, Hatfull G, Willets N S. Mobilization of the nonconjugative plasmid RSF1010: a genetic and DNA sequence analysis of the mobilization region. Mol Gen Genet. 1987;206:161–168. doi: 10.1007/BF00326552. [DOI] [PubMed] [Google Scholar]
- 20.Farizo K, Cafarella T, Burns D. Evidence for a ninth gene, ptlI, in the locus encoding the pertussis toxin secretion system of Bordetella pertussis and formation of a PtlI-PtlF complex. J Biol Chem. 1996;271:31643–31649. doi: 10.1074/jbc.271.49.31643. [DOI] [PubMed] [Google Scholar]
- 21.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fullner K J, Nester E W. Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J Bacteriol. 1996;178:1498–1504. doi: 10.1128/jb.178.6.1498-1504.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfinkel D J, Simpson R B, Ream L W, White F F, Gordon M P, Nester E W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981;27:143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- 24.Haase J, Lurz R, Grahn A M, Bamford D H, Lanka E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage production, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J Bacteriol. 1995;177:4779–4791. doi: 10.1128/jb.177.16.4779-4791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooykaas P J J, Beijersbergen A G M. The virulence system of Agrobacterium tumefaciens. Annu Rev Phytopathol. 1994;32:157–179. [Google Scholar]
- 26.Jagura-Burdzy G, Khanim F, Smith C, Thomas C. Crosstalk between plasmid vegetative replication and conjugative transfer: repression of the trfA operon by trbA of broad host range plasmid RK2. Nucleic Acids Res. 1992;20:3939–3944. doi: 10.1093/nar/20.15.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klee H J, Yanofsky M F, Nester E W. Vectors for transformation of higher plants. Bio/Technology. 1985;3:637–642. [Google Scholar]
- 28.Lanka E, Wilkins B. DNA processing reactions in bacterial conjugation. Annu Rev Biochem. 1995;64:141–169. doi: 10.1146/annurev.bi.64.070195.001041. [DOI] [PubMed] [Google Scholar]
- 29.Lessl M, Lanka E. Common mechanisms in bacterial conjugation and Ti-mediated T-DNA transfer to plant cells. Cell. 1994;77:321–324. doi: 10.1016/0092-8674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 30.Melchers L S, Maroney M J, den Dulk-Ras A, Thompson D V, van Vuuren H A J, Schilperoort R A, Hooykaas P J J. Octopine and nopaline strains of Agrobacterium tumefaciens differ in virulence; molecular characterization of the virF locus. Plant Mol Biol. 1990;14:249–259. doi: 10.1007/BF00018565. [DOI] [PubMed] [Google Scholar]
- 30a.Meyers, Richard. Personal communication.
- 31.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–496. [Google Scholar]
- 32.Nordheim A, Hashimoto-Gotoh T, Timmis K N. Location of two relaxation nick sites in R6K and single sites in pSC101 and RSF1010 close to origins of vegetative replication: implication for conjugal transfer of plasmid deoxyribonucleic acid. J Bacteriol. 1980;144:923–932. doi: 10.1128/jb.144.3.923-932.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ooms G, Hooykaas P J J, Van Veen R, Van Beelen P, Regensburg-Tuink T J G, Schilperoort R A. Octopine Ti-plasmid deletion mutants of Agrobacterium tumefaciens with emphasis on the right side of the T-region. Plasmid. 1982;7:15–29. doi: 10.1016/0147-619x(82)90023-3. [DOI] [PubMed] [Google Scholar]
- 34.Otten L, De Greve H, Leemans J, Hain R, Hooykaas P, Schell J. Restoration of virulence of vir region mutants of Agrobacterium tumefaciens strain B6S3 by coinfection with normal and mutant Agrobacterium strains. Mol Gen Genet. 1984;195:159–163. [Google Scholar]
- 35.Perwez T, Meyer R. MobB protein stimulates nicking at the R1162 origin of transfer by increasing the proportion of complexed plasmid DNA. J Bacteriol. 1996;178:5762–5767. doi: 10.1128/jb.178.19.5762-5767.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherzinger E, Lurz R, Otto S, Dobrinski B. In vitro cleavage of double- and single-stranded DNA by plasmid RSF1010-encoded mobilization proteins. Nucleic Acids Res. 1992;20:41–48. doi: 10.1093/nar/20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholtz P, Volker H, Wittmann-Liebold B, Ashman K, Bagdasarian M, Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989;75:271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- 38.Sheng J, Citovsky V. Agrobacterium-plant cell DNA transport: have virulence proteins, will travel. Plant Cell. 1996;8:1699–1710. doi: 10.1105/tpc.8.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirasu K, Kado C I. The virB operon of the Agrobacterium tumefaciens virulence regulon has sequence similarities to B, C, and D open reading frames downstream of the pertussis toxin-operon and to the DNA transfer-operons of broad host-range conjugative plasmids. Nucleic Acids Res. 1993;21:353–354. doi: 10.1093/nar/21.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 41.Stachel S E, Nester E W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986;5:1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundberg C, Meek L, Carroll K, Das A, Ream W. VirE1 protein mediates export of the single-stranded DNA-binding protein VirE2 from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1996;178:1207–1212. doi: 10.1128/jb.178.4.1207-1212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward J E, Jr, Dale E M, Christie P J, Nester E W, Binns A N. Complementation analysis of Agrobacterium tumefaciens Ti plasmid virB genes by use of a vir promoter expression vector: virB9, virB10, and virB11 are essential virulence genes. J Bacteriol. 1990;172:5187–5199. doi: 10.1128/jb.172.9.5187-5199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward J E, Dale E M, Binns A N. Activity of the Agrobacterium T-DNA transfer machinery is affected by virB gene products. Proc Natl Acad Sci USA. 1991;88:9350–9354. doi: 10.1073/pnas.88.20.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson B, Currier T C, Gordon M P, Chilton M-D, Nester E W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975;123:255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winans S C. Two-way chemical signaling in Agrobacterium-plant interactions. Microbiol Rev. 1992;56:12–31. doi: 10.1128/mr.56.1.12-31.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winans S C, Burns D L, Christie P J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Meyer R. The relaxosome protein MobC promotes conjugal plasmid mobilization by extending DNA strand separation to the nick site at the origin of transfer. Mol Microbiol. 1997;25:509–516. doi: 10.1046/j.1365-2958.1997.4861849.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Meyer R J. Localized denaturation of oriT DNA within relaxosomes of the broad host-range plasmid R1162. Mol Microbiol. 1995;17:727–735. doi: 10.1111/j.1365-2958.1995.mmi_17040727.x. [DOI] [PubMed] [Google Scholar]
- 51.Zupan J R, Zambryski P. Transfer of T-DNA from Agrobacterium to plant cell. Plant Physiol. 1995;107:1041–1047. doi: 10.1104/pp.107.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]