Abstract
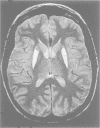
OBJECTIVE--To determine the roles of the putamen and pallidum in ocular motor control. METHODS--Eye movements were recorded electro-oculographically in nine patients with bilateral focal lesions affecting the lentiform nucleus, and in 12 age matched control subjects. Reflexive visually guided saccades (gap task), antisaccades, memorised sequences of saccades, memory guided saccades (with visual input only, and with both visual and vestibular inputs), and predictive saccades (with and without gap) were studied. RESULTS--Latency and accuracy of visually guided saccades were normal. The percentage of errors in the antisaccade task and latency of correct antisaccades did not differ significantly from the results of controls. The percentage of errors in saccade sequences was significantly increased. Accuracy of the two types of memory guided saccades was impaired bilaterally. The percentage of predictive saccades was significantly decreased when a gap existed, but unchanged without a gap, compared with controls. Therefore, saccades made immediately in response to an external target (reflexive visually guided saccades and antisaccades) were performed without difficulty, whereas those requiring an internal representation of such a target (such as memory guided saccades, predictive saccades, and saccade sequences) were performed with significant disturbances. CONCLUSIONS--The lentiform nucleus influences the cortical areas involved in the control of saccades when the experimental paradigm requires the use of an internal representation of the target for correct planning and execution of the ensuing saccade.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G. E., Crutcher M. D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990 Jul;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander G. E., DeLong M. R., Strick P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bhatia K. P., Marsden C. D. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994 Aug;117(Pt 4):859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- Brotchie P., Iansek R., Horne M. K. Motor function of the monkey globus pallidus. 2. Cognitive aspects of movement and phasic neuronal activity. Brain. 1991 Aug;114(Pt 4):1685–1702. doi: 10.1093/brain/114.4.1685. [DOI] [PubMed] [Google Scholar]
- Crawford T. J., Henderson L., Kennard C. Abnormalities of nonvisually-guided eye movements in Parkinson's disease. Brain. 1989 Dec;112(Pt 6):1573–1586. doi: 10.1093/brain/112.6.1573. [DOI] [PubMed] [Google Scholar]
- Crawford T., Goodrich S., Henderson L., Kennard C. Predictive responses in Parkinson's disease: manual keypresses and saccadic eye movements to regular stimulus events. J Neurol Neurosurg Psychiatry. 1989 Sep;52(9):1033–1042. doi: 10.1136/jnnp.52.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard B., Pierrot-Deseilligny C., Rivaud S. Impairment of sequences of memory-guided saccades after supplementary motor area lesions. Ann Neurol. 1990 Nov;28(5):622–626. doi: 10.1002/ana.410280504. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Role of basal ganglia in saccades. Rev Neurol (Paris) 1989;145(8-9):580–586. [PubMed] [Google Scholar]
- Jenkins I. H., Fernandez W., Playford E. D., Lees A. J., Frackowiak R. S., Passingham R. E., Brooks D. J. Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann Neurol. 1992 Dec;32(6):749–757. doi: 10.1002/ana.410320608. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Isaki K., Fukutani Y., Kurachi M., Eboshida A., Matsubara R., Yamaguchi N. CT findings of the interval form of carbon monoxide poisoning compared with neuropathological findings. Eur Neurol. 1984;23(1):34–43. doi: 10.1159/000115675. [DOI] [PubMed] [Google Scholar]
- Laplane D., Levasseur M., Pillon B., Dubois B., Baulac M., Mazoyer B., Tran Dinh S., Sette G., Danze F., Baron J. C. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study. Brain. 1989 Jun;112(Pt 3):699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- Lapresle J., Fardeau M. The central nervous system and carbon monoxide poisoning. II. Anatomical study of brain lesions following intoxication with carbon monixide (22 cases). Prog Brain Res. 1967;24:31–74. doi: 10.1016/s0079-6123(08)60181-8. [DOI] [PubMed] [Google Scholar]
- Leichnetz G. R., Spencer R. F., Hardy S. G., Astruc J. The prefrontal corticotectal projection in the monkey; an anterograde and retrograde horseradish peroxidase study. Neuroscience. 1981;6(6):1023–1041. doi: 10.1016/0306-4522(81)90068-3. [DOI] [PubMed] [Google Scholar]
- Lueck C. J., Tanyeri S., Crawford T. J., Henderson L., Kennard C. Antisaccades and remembered saccades in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1990 Apr;53(4):284–288. doi: 10.1136/jnnp.53.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushiake H., Inase M., Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991 Sep;66(3):705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- Ojemann J. G., Ojemann G. A., Lettich E. Neuronal activity related to faces and matching in human right nondominant temporal cortex. Brain. 1992 Feb;115(Pt 1):1–13. doi: 10.1093/brain/115.1.1. [DOI] [PubMed] [Google Scholar]
- Petit L., Orssaud C., Tzourio N., Salamon G., Mazoyer B., Berthoz A. PET study of voluntary saccadic eye movements in humans: basal ganglia-thalamocortical system and cingulate cortex involvement. J Neurophysiol. 1993 Apr;69(4):1009–1017. doi: 10.1152/jn.1993.69.4.1009. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Israël I., Berthoz A., Rivaud S., Gaymard B. Role of the different frontal lobe areas in the control of the horizontal component of memory-guided saccades in man. Exp Brain Res. 1993;95(1):166–171. doi: 10.1007/BF00229665. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Rivaud S., Gaymard B., Agid Y. Cortical control of memory-guided saccades in man. Exp Brain Res. 1991;83(3):607–617. doi: 10.1007/BF00229839. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Rivaud S., Gaymard B., Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991 Jun;114(Pt 3):1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Rivaud S., Gaymard B., Müri R., Vermersch A. I. Cortical control of saccades. Ann Neurol. 1995 May;37(5):557–567. doi: 10.1002/ana.410370504. [DOI] [PubMed] [Google Scholar]
- Rivaud S., Müri R. M., Gaymard B., Vermersch A. I., Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res. 1994;102(1):110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Schlag J., Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987 Jan;57(1):179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- Segraves M. A., Goldberg M. E. Functional properties of corticotectal neurons in the monkey's frontal eye field. J Neurophysiol. 1987 Dec;58(6):1387–1419. doi: 10.1152/jn.1987.58.6.1387. [DOI] [PubMed] [Google Scholar]
- Selemon L. D., Goldman-Rakic P. S. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985 Mar;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A. C., Van Gisbergen J. A. A short-latency transition in saccade dynamics during square-wave tracking and its significance for the differentiation of visually-guided and predictive saccades. Exp Brain Res. 1989;76(1):64–74. doi: 10.1007/BF00253624. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G. W., Yeterian E. H., Lavizzo-Mourey R. Widespread corticostriate projections from temporal cortex of the rhesus monkey. J Comp Neurol. 1981 Jun 20;199(2):205–219. doi: 10.1002/cne.901990205. [DOI] [PubMed] [Google Scholar]
- Yeterian E. H., Van Hoesen G. W. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res. 1978 Jan 6;139(1):43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]