Abstract
Purpose:
The purpose of this study was to investigate the social determinants of health for keratoconus.
Methods:
In this retrospective cohort study of patients with keratoconus, the electronic health record was reviewed for keratometry, treatments received, clinical comorbidities, and social characteristics. Outcomes included severe keratoconus at presentation (steep keratometry ≥52 diopters), disease progression (≥0.75 diopters increase from the first to the most recent clinical visit), and corneal transplantation. Logistic regression was used to evaluate factors associated with severity at presentation and corneal transplantation. Cox proportional hazards modeling was used to evaluate progression.
Results:
A total of 1038 patients with keratoconus were identified, 725 (70%) of whom had baseline imaging. Compared with commercially insured patients, Medicaid recipients were more likely to have severe keratoconus, independent of social and clinical confounders [odds ratio (OR) 1.94, 95% confidence interval (CI), 1.12–3.35, P = 0.017]. Male sex was independently associated with progression (hazard ratio = 1.38, 95% CI, 1.03–1.84, P = 0.030). Medicare and Medicaid recipients were more likely to require transplantation compared with commercially insured patients (OR 2.71, 95% CI, 1.65–4.46, P < 0.001 and OR 1.74, 95% CI, 1.08–2.80, P = 0.022, respectively). Other social determinants of health, including non-White race/ethnicity, limited English proficiency, and unemployment, were associated with the outcomes only in univariate analysis. Obstructive sleep apnea, atopy, body mass index, and tobacco use were not associated with any outcome.
Conclusions:
Socioeconomic factors were more consistent predictors of keratoconus severity and corneal transplantation compared with clinical factors that have received relatively greater attention in the keratoconus literature.
Keywords: keratoconus, social determinants of health, insurance, risk factors
Keratoconus is characterized by progressive thinning and steepening of the cornea. Progressive keratoconus may result in vision loss because of the development of irregular astigmatism and/or corneal scarring.1 The prevalence of keratoconus in the United States is 0.17 per 1000.2 Keratoconus is associated with allergic diseases,3 Down syndrome, collagen vascular disorders,4 obstructive sleep apnea (OSA),5,6 eye rubbing, and a family history of the condition,7 while diabetes mellitus may be protective.8–10 In the United States, eye care access and utilization vary by social status. Patients of racial and ethnic minority groups, those with lower incomes, and those who lack medical insurance are at higher risk of underutilizing eye care and losing vision from conditions, such as glaucoma, diabetic retinopathy, and cataracts.11 Few studies have explored the relationship between socioeconomic status and keratoconus. We aim to investigate the social factors influencing disease severity and progression of patients with keratoconus, with hopes that this insight may provide an opportunity to improve disease outcomes.
METHODS
In this study approved by the Institutional Review Board at the University of California San Francisco, all patients with an International Classification of Diseases (ICD) diagnosis of keratoconus (ICD-9 code 371.6, ICD-10 code H18.609) were retrospectively identified and screened for eligibility. Clinical documentation of keratoconus and complete data on 1 or more of the outcomes was required for inclusion. The retrospective period dated from 2012 [the implementation of Epic (Verona, WI), the electronic health record at the University of California San Francisco] to 2019. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Epic was reviewed for sociodemographic and socioeconomic traits, including race (White or non-White), primary language (English or non-English), insurance payer (commercial, Medicaid, or Medicare), and employment status (employed/retired or unemployed/disabled). Clinical characteristics (including age, sex, diabetes, Down syndrome, OSA, allergic rhinitis, asthma, eczema, dry eye, tobacco use, and body mass index [BMI]) were also collected. All characteristics were collected from the initial visit for each patient. Age was categorized into clinically meaningful groups, including pediatric patients (age ≤18 years), young adults (age 18–35 years), adults (35–50 years), and older adults (≥50 years). Atopy was defined as the presence of allergic rhinitis, asthma, or eczema. BMI was categorized as underweight, healthy, overweight, or obese using Centers for Disease Control and Prevention definitions.12
The primary outcomes were the severity of keratoconus at presentation, disease progression, and corneal transplantation. Severe keratoconus was defined as steep keratometry ≥52 diopters at the initial clinical visit, and progression was a ≥0.75 diopters increase in steep keratometry from the first to the most recent clinical visit (of either eye in bilateral keratoconus). Keratometry values were obtained from corneal tomography reports or, if unavailable, corneal topography reports. Corneal transplantation included full-thickness and partial-thickness transplants.
Logistic regression was used to evaluate the association between predictor variables and keratoconus severity and corneal transplantation. Cox proportional hazards modeling was used to account for variable follow-up duration in the evaluation of progression. The proportional hazards assumption was confirmed by calculating Schoenfeld residuals for each regression variable. Univariate analysis was performed for each factor–outcome pair. Factors associated with the outcome at P < 0.05 (2-sided) were included in a multivariate model. Stata software version 15.1 (StataCorp, College Station, TX) was used for statistical analysis.
RESULTS
A total of 1038 patients had an ICD-9 or ICD-10 diagnosis of keratoconus. Of these, 877 (84%) had clinical documentation consistent with keratoconus and 725 (70%) had baseline imaging data; these patients comprised the final study sample for the outcome of baseline keratoconus severity. The final keratoconus treatment information was available for 716 patients (68%) to evaluate for corneal transplantation. Imaging data at a subsequent clinical visit were available for 475 patients (46%); these patients comprised the study sample for the analysis of progression. Figure 1 shows derivation of the study sample.
FIGURE 1.
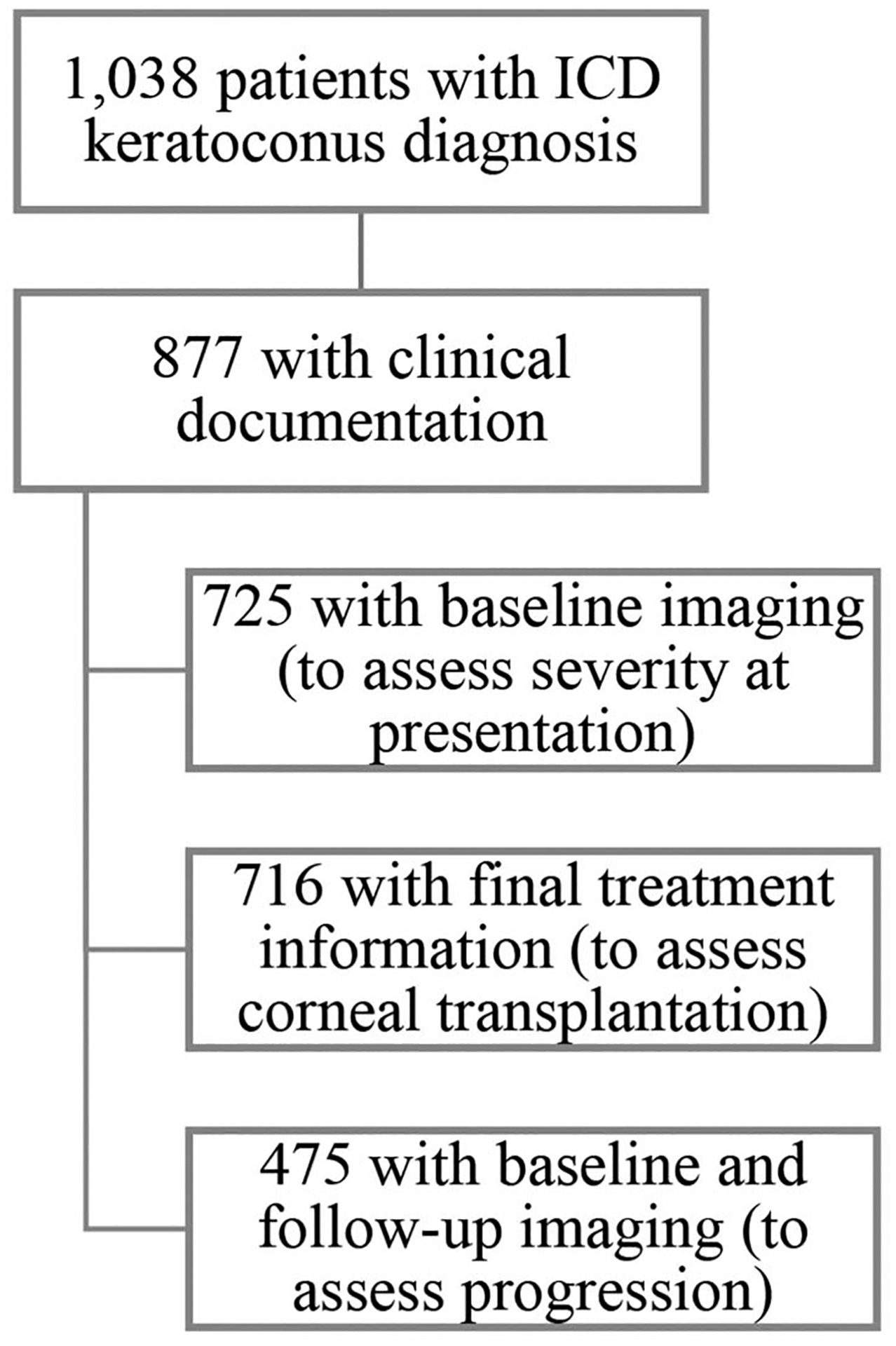
Derivation of the study sample. A total of 1,038 were initially identified using ICD codes, 877 of whom had clinical documentation consistent with keratoconus. The study sample was further narrowed for each outcome based on the number of patients with baseline imaging (725), final treatment information (716), and both baseline and follow-up imaging (475).
The median duration of follow-up from the first to the most recent clinical visit was 1.92 months (range: 0–42). Most patients were male (65%), English-speaking (94%), and commercially insured (63%). Forty-five percent identified as White. Social and clinical characteristics are summarized in Table 1.
TABLE 1.
Social and Clinical Characteristics of Patients With Keratoconus
| Frequency | Percentage | |
|---|---|---|
| Race | ||
| White | 412 | 45 |
| Non-White | 507 | 55 |
| Language | ||
| English | 971 | 94 |
| Non-English | 67 | 6 |
| Insurance | ||
| Private | 643 | 63 |
| Medicaid | 208 | 20 |
| Medicare | 169 | 17 |
| Employment | ||
| Employed/retired | 443 | 65 |
| Unemployed/disabled | 241 | 35 |
| Age (yr) | ||
| ≤18 | 80 | 9 |
| 18–35 | 293 | 34 |
| 35–50 | 241 | 28 |
| ≥50 | 259 | 30 |
| Sex | ||
| Female | 362 | 35 |
| Male | 674 | 65 |
| Diabetes | ||
| Yes | 89 | 9 |
| No | 879 | 91 |
| Down syndrome | ||
| Yes | 20 | 2 |
| No | 1018 | 98 |
| Obstructive sleep apnea | ||
| Yes | 62 | 6 |
| No | 906 | 94 |
| Atopy | ||
| Yes | 190 | 18 |
| No | 848 | 82 |
| Dry eye | ||
| Yes | 42 | 4 |
| No | 926 | 96 |
| Tobacco use | ||
| Yes | 95 | 10 |
| No | 873 | 90 |
| Body mass index | ||
| Underweight | 14 | 3 |
| Healthy | 182 | 35 |
| Overweight | 164 | 31 |
| Obese | 167 | 32 |
In univariate logistic regression, patients who were non-White [odds ratio (OR) 1.83, 95% confidence interval (CI) 1.33–2.52, P < 0.001], non-English–speaking (OR 2.39, 95% CI, 1.35–4.22, P = 0.003), unemployed/disabled (OR 1.66, 95% CI, 1.14–2.44, P = 0.009), and insured by Medicaid (OR 2.00, 95% CI, 1.39–2.87, P < 0.001) were significantly more likely to have severe keratoconus at presentation compared with White, English-speaking, employed/retired, and commercially insured patients, respectively. Compared with patients older than 50 years, younger patients were more likely to have severe keratoconus (OR 2.98, 95% CI, 1.71–5.22, P < 0.001 for ≤18 years; OR 1.96, 95% CI, 1.33–2.91, P = 0.001 for 18–35 years; and OR 1.66, 95% CI, 1.08–2.54, P = 0.02 for 35–50 years). Patients with a diagnosis of dry eye syndrome were significantly less likely to have severe keratoconus compared with patients without dry eye syndrome (OR 0.31, 95% CI, 0.10–0.93, P = 0.04). In a multivariate logistic regression analysis (including race, language, insurance, employment, age, and dry eye), only Medicaid-insured patients were significantly more likely to have severe keratoconus at presentation compared with commercially insured patients (OR 1.94, 95% CI, 1.12–3.35, P = 0.02).
In univariate Cox proportional hazards regression, patients who were non-White [hazard ratio (HR) 1.34, 95% CI, 1.02–1.75, P = 0.04], insured by Medicaid (HR 1.38, 95% CI, 1.00–1.90, P = 0.05), younger than 35 years (HR 1.64, 95% CI, 1.03–2.62, P = 0.04 for ≤18 years and HR 1.56, 95% CI, 1.13–2.16, P = 0.007 for 18–35 years), and male (HR = 1.41, 95% CI, 1.07–1.86, P = 0.02) were significantly more likely to develop progression compared with patients who were White, commercially insured, older than 50 years, and female, respectively. In a multivariate analysis (including race, insurance, age, and sex), only male sex was significantly associated with progression (HR = 1.38, 95% CI, 1.03–1.84, P = 0.03).
In univariate logistic regression, corneal transplantation was far less likely in younger patients compared with patients older than 50 years (OR 0.14, 95% CI, 0.06–0.38, P < 0.001 for ≤18 years and OR 0.39, 95% CI, 0.25–0.59, P < 0.001 for 18–35 years), including in multivariate analysis (OR 0.28, 95% CI, 0.10–0.79, P = 0.02 for ≤18 years and OR 0.59, 95% CI, 0.36–0.96, P = 0.04 for 18–35 years), and patients with limited English proficiency compared with English-speaking patients (OR 0.44, 95% CI, 0.19–0.99, P = 0.05). Diabetic patients (OR = 2.51, 95% CI, 1.50–4.18, P < 0.001) and smokers (OR 1.90, 95% CI, 1.13–3.20, P = 0.02) were significantly more likely to undergo transplantation compared with patients without these conditions. Patients insured by Medicare were significantly more likely than commercially insured patients to undergo transplantation (OR 3.38, 95% CI, 2.21–5.16, P < 0.001). Insurance status remained a significant predictor of transplantation in a multivariate analysis (including language, age, diabetes, and tobacco use), with patients insured by both Medicare (OR 2.71, 95% CI, 1.65–4.46, P < 0.001) and Medicaid (OR 1.74, 95% CI, 1.08–2.80, P = 0.02) significantly more likely to require transplantation compared with commercially insured patients. Table 2 demonstrates the odds and hazard ratios for the study outcomes in univariate and multivariate analysis. Comorbid conditions, such as OSA, atopy, BMI, and tobacco use, were not associated with keratoconus severity, disease progression, or corneal transplantation.
TABLE 2.
Association of Social and Clinical Variables With Keratoconus Severity, Progression, and Need for Transplantation
| Steep K ≥ 52 D | Steep K Increase ≥0.75 D | Corneal Transplantation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| OR (95% CI) | P | OR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Race (ref. White) | ||||||||||||
| Non-White | 1.83 (1.33–2.52) | <0.001 | 1.15 (0.74–1.80) | 0.53 | 1.34 (1.02–1.75) | 0.04 | 1.19 (0.89–1.60) | 0.25 | 0.94 (0.66–1.33) | 0.72 | — | — |
| Language (ref. English) | 2.39 (1.35–4.22) | 0.003 | 0.97 (0.35–2.67) | 0.95 | 1.71 (0.92–3.16) | 0.09 | — | — | 0.44 (0.19–0.99) | 0.05 | 0.74 (0.30–1.85) | 0.53 |
| nsurance (ref. commercial) | ||||||||||||
| Medicaid | 2.00 (1.39–2.87) | <0.001 | 1.94 (1.12–3.34) | 0.02 | 1.38 (1.00–1.90) | 0.05 | 1.20 (0.83–1.72) | 0.33 | 1.29 (0.83–1.99) | 0.26 | 1.74 (1.08–2.80) | 0.02 |
| Medicare | 0.87 (0.56–1.34) | 0.53 | 1.67 (0.88–3.16) | 0.11 | 0.69 (0.49–0.98) | 0.04 | 0.73 (0.49–1.08) | 0.12 | 3.38 (2.21–5.16) | <0.001 | 2.71 (1.65–4.46) | <0.001 |
| Employment (ref. employed/retired) | ||||||||||||
| Unemployed/disabled Age (ref. ≥50) | 1.66 (1.14–2.44) | 0.009 | 1.09 (0.66–1.82) | 0.73 | 1.29 (0.94–1.78) | 0.11 | 0.73 (0.48–1.10) | 0.13 | — | — | ||
| ≤18 | 2.98 (1.71–5.22) | <0.001 | 2.58 (0.97–6.84) | 0.06 | 1.64 (1.03–2.62) | 0.04 | 1.16 (0.68–1.97) | 0.59 | 0.14 (0.06–0.38) | <0.001 | 0.28 (0.10–0.79) | 0.02 |
| 18–35 | 1.96 (1.33–2.91) | 0.001 | 1.77 (0.95–3.29) | 0.07 | 1.56 (1.13–2.16) | 0.007 | 1.32 (0.92–1.89) | 0.13 | 0.39 (0.25–0.59) | <0.001 | 0.59 (0.36–0.96) | 0.04 |
| 35–50 | 1.66 (1.08–2.54) | 0.02 | 1.33 (0.71–2.48) | 0.38 | 0.95 (0.67–1.36) | 0.79 | 0.81 (0.55–1.20) | 0.29 | 0.84 (0.56–1.27) | 0.42 | 1.27 (0.79–2.03) | 0.32 |
| Sex (ref. female) | 1.04 (0.76–1.42) | 0.78 | — | — | 1.41 (1.07–1.86) | 0.02 | 1.38 (1.03–1.84) | 0.03 | 0.87 (0.62–1.23) | 0.43 | — | — |
| Diabetes | 0.72 (0.41–1.24) | 0.24 | — | — | 0.73 (0.49–1.09) | 0.13 | — | — | 2.51 (1.50–4.18) | <0.001 | 1.56 (0.91–2.68) | 0.11 |
| Down syndrome | 1.15 (0.34–3.95) | 0.82 | — | — | 0.64 (0.13–3.04) | 0.58 | — | — | 0.15 (0.02–1.41) | 0.10 | — | — |
| Obstructive sleep apnea | 0.78 (0.42–1.47) | 0.45 | — | — | 0.53 (0.28–1.00) | 0.05 | — | — | 1.72 (0.90–3.28) | 0.10 | — | — |
| Atopy | 0.84 (0.57–1.23) | 0.37 | — | — | 1.11 (0.81–1.52) | 0.52 | — | — | 0.75 (0.48–1.18) | 0.22 | — | — |
| Dry eye | 0.31 (0.10–0.93) | 0.04 | 0.38 (0.11–1.37) | 0.14 | 0.64 (0.35–1.19) | 0.16 | — | — | 1.72 (0.77–3.86) | 0.19 | — | — |
| Body mass index (ref. healthy) | ||||||||||||
| Underweight | 1.25 (0.34–4.53) | 0.73 | — | — | 0.64 (0.09–4.69) | 0.66 | — | — | 0.94 (0.18–4.76) | 0.94 | — | — |
| Overweight | 0.95 (0.57–1.57) | 0.83 | — | — | 0.97 (0.60–1.55) | 0.89 | — | — | 1.44 (0.82–2.55) | 0.21 | — | — |
| Obese | 0.87 (0.53–1.45) | 0.61 | — | — | 0.77 (0.49–1.21) | 0.26 | — | — | 1.24 (0.70–2.21) | 0.46 | — | — |
| Tobacco use | 0.70 (0.41–1.18) | 0.18 | — | — | 0.94 (0.63–1.40) | 0.76 | — | — | 1.90 (1.13–3.20) | 0.02 | 1.20 (0.69–2.09) | 0.51 |
Bold indicates statistical significance at P < 0.05.
D, diopters; K, keratometry; ref., reference category.
Given the strength of association between insurance status and corneal transplantation, a post hoc analysis was conducted to examine the relationship between insurance status and other treatments of keratoconus. Compared with commercially insured patients, patients insured by Medicaid were significantly more likely to undergo corneal collagen cross-linking (CXL) (OR 2.09, 95% CI, 1.34–3.26, P = 0.001) and significantly less likely to receive rigid gas permeable (RGP) or scleral lenses (OR 0.51, 95% CI, 0.35–0.75, P = 0.001).
DISCUSSION
We demonstrate the association between keratoconus and various clinical factors and social determinants of health. Medicaid recipients were more likely than commercially insured patients to have severe keratoconus at initial presentation and to undergo corneal transplantation, independent of clinical and social confounders. Other socioeconomic factors, including non-White race, limited English proficiency, and unemployment, were associated with worse disease in univariate but not multivariate analysis. Age and sex were the only biologic variables consistently associated with any of our study outcomes. Overall, socioeconomic factors were more consistent predictors of keratoconus severity on presentation, progression, and corneal transplantation compared with clinical factors that have received relatively greater attention in the keratoconus literature.
Socioeconomic status is a social construct inferred by income, employment, and education, which are tied to race. Racial and ethnic health disparities seem driven by socioeconomic status because socioeconomic health disparities are larger within racial groups than they are across racial groups.11 Still, non-White race and low socioeconomic status are both independently associated with vision impairment, putting poor ethnic minorities at highest risk.13,14 These populations tend to underutilize preventive eye care and bear a disproportionate burden of preventable, blinding conditions, such as glaucoma and diabetic retinopathy.11,15
Although the global prevalence of keratoconus varies by race and ethnicity, this is believed to be due to geographic and genetic ancestral variability.16 Few studies have explored the influence of race and ethnicity in the United States, where race is a social construct.17 A recent study in the United States identified higher odds of keratoconus among Black and Latino patients but no association with the education level or income.6 A study of patients with keratoconus in New Zealand identified a disproportionately high rate of transplantation among patients with keratoconus of Māori and Pacific ethnicities, which—similar to marginalized populations in the United States—have a high burden of disease and lower life expectancy.18 In this study, non-White race, limited English proficiency, and unemployment were associated with severe keratoconus and disease progression, but these differences were better explained by insurance status.
Medicaid is a federally funded health insurance program originally designed for low-income children. In 2013, the Affordable Care Act expanded coverage to low-income adults and now covers more than 70 million people.19 Although Medicaid is a financial need-based program, Medicare is based on age and disability. Racial and ethnic minorities remain more likely to be uninsured or to receive Medicaid than their White counterparts.19 The visual acuity is one of the few health outcomes shown to be affected by the generosity of insurance coverage.20 In this study, Medicaid patients were significantly less likely to receive RGP or scleral lenses and more likely to undergo CXL or transplantation. One likely explanation for this is that CXL is currently covered by Medicaid, whereas RGP and scleral lenses are not. Because CXL was only recently approved in the United States, lenses were the primary nonsurgical means of correcting vision in patients with keratoconus. Another possible explanation is that Medicaid patients presented with more severe keratoconus disease, necessitating surgical intervention. Additional barriers to care for Medicaid recipients include lower physician reimbursements, a limited supply of Medicaid providers (resulting in care delays or lack of access), difficulty with transportation to and from appointments, and greater medical comorbidities.21–23 Medicaid recipients also tend to be less educated and to have lower health literacy, potentially rendering them less aware of the importance of eye care.21,24 Although Medicare patients were similarly more likely to undergo corneal transplantation, this likely represents a selection bias. Patients receiving care for keratoconus in late adulthood are likely being followed for severe disease and corneal transplantation.
Younger patients were less likely to undergo corneal transplantation. The rates of transplantation for keratoconus have been declining with newer therapies, such as CXL.25 Men were significantly more likely than women to have progression, which is consistent with several studies reporting an earlier disease onset and faster progression among men,26,27 although some studies have shown no sex difference.1,28 Diabetic patients were two-and-a-half times more likely than nondiabetic patients to require corneal transplantation in univariate analysis; however, this association diminished to statistical insignificance with the inclusion of age and insurance status. Biomechanically, diabetes is believed to strengthen the cornea through glycosylation of corneal fibers, providing an auto–cross-linking effect and reducing the risk of keratoconus.8–10 However, clinical evidence is mixed, with some studies actually showing increased risk.29 A recent study by Woodward et al6 showed lower odds of a keratoconus diagnosis among diabetic patients but more severe disease when present. This may be consistent with the trend toward corneal transplantation among diabetic patients in our study.
BMI, OSA, atopy, and Down syndrome were not associated with any outcome in our study. Although several studies have demonstrated an association between obesity and keratoconus through OSA,30 a recent study demonstrated a strong independent correlation between BMI and keratoconus incidence.31 The lack of an association with OSA in our study may be related to the low prevalence in our data set, only 6%. Many studies have shown patients with asthma, allergy, and eczema to have increased incidence and severity of keratoconus.6,32–34 This was not observed in our study. Owing to the very low prevalence of asthma (n = 6), we considered asthma in combination with eczema and allergic rhinitis operationalized as ‟atopy.” Several studies have similarly failed to identify a correlation between atopy and keratoconus,35,36 believed to be due to differences in definitions and a potentially indirect relationship through eye rubbing.7
There are several study limitations. The single-institution design limits generalizability, and the retrospective nature is limited by the quality of the available data, any changes in reporting over time, and differential loss to follow-up. Furthermore, there is no gold-standard classification system for keratoconus severity; we used steep keratometry to define baseline severity and progression. As previously mentioned, some clinical and social variables had large differences in the numbers within the comparison groups. Baseline data were collected at the first visit at our institution, which, for patients diagnosed elsewhere, may not represent the initial clinical presentation. Nevertheless, as a large academic tertiary care center, barring—or in light of—care delays, data at the initial visit likely reflect the clinical and social context. Finally, although we searched for a family history of keratoconus and eye rubbing in our chart review of clinical notes, in most cases there was no such documentation. Thus, despite these being strong risk factors for keratoconus, they were not included in the analysis.7
In summary, socioeconomically marginalized patients are at higher risk for having severe keratoconus and requiring corneal transplantation. Social factors seem to be at least as important as clinical ones in keratoconus severity and progression. This underscores the importance of considering the social determinants of health when seeking to understand and address the drivers of disease outcomes in ophthalmology.
Acknowledgments
Supported by the University of California San Francisco (UCSF) Inquiry Funding Office and was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR001872. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This project was also made possible in part by unrestricted grant funding from That Man May See (San Francisco, CA) and Research to Prevent Blindness (New York, NY) to the University of California San Francisco, Department of Ophthalmology.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. [DOI] [PubMed] [Google Scholar]
- 2.Reeves SW, Ellwein LB, Kim T, et al. Keratoconus in the Medicare population. Cornea. 2009;28:40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemet AY, Vinker S, Bahar I, et al. The association of keratoconus with immune disorders. Cornea. 2010;29:1261–1264. [DOI] [PubMed] [Google Scholar]
- 4.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. [DOI] [PubMed] [Google Scholar]
- 5.Gupta PK, Stinnett SS, Carlson AN. Prevalence of sleep apnea in patients with keratoconus. Cornea. 2012;31:595–599. [DOI] [PubMed] [Google Scholar]
- 6.Woodward MA, Blachley TS, Stein JD. The association between sociodemographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide heath care claims database. Ophthalmology. 2016;123:457–465. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashemi H, Heydarian S, Hooshmand E, et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39:263–270. [DOI] [PubMed] [Google Scholar]
- 8.Kuo IC, Broman A, Pirouzmanesh A, et al. Is there an association between diabetes and keratoconus? Ophthalmology. 2006;113:184–190. [DOI] [PubMed] [Google Scholar]
- 9.Seiler T, Huhle S, Spoerl E, et al. Manifest diabetes and keratoconus: a retrospective case-control study. Graefes Arch Clin Exp Ophthalmol. 2000;238:822–825. [DOI] [PubMed] [Google Scholar]
- 10.Goldich Y, Barkana Y, Gerber Y, et al. Effect of diabetes mellitus on biomechanical parameters of the cornea. J Cataract Refract Surg. 2009; 35:715–719. [DOI] [PubMed] [Google Scholar]
- 11.Elam AR, Lee PP. High-risk populations for vision loss and eye care underutilization: a review of the literature and ideas on moving forward. Surv Ophthalmol. 2013;58:348–358. [DOI] [PubMed] [Google Scholar]
- 12.Defining adult overweight and obesity. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/obesity/adult/defining.html. Accessed June 7, 2021. [Google Scholar]
- 13.Tielsch JM, Sommer A, Katz J, et al. Socioeconomic status and visual impairment among urban Americans. Baltimore Eye Survey Research Group. Arch Ophthalmol. 1991;109:637–641. [DOI] [PubMed] [Google Scholar]
- 14.Tielsch JM, Sommer A, Witt K, et al. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108:286–290. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–825. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Amero KK, Al-Muammar AM, Kondkar AA. Genetics of keratoconus: where do we stand? J Ophthalmol. 2014;2014:641708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanagin A, Frey T, Christiansen SL. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326: 621–627. [DOI] [PubMed] [Google Scholar]
- 18.Crawford AZ, McKelvie J, Craig JP, et al. Corneal transplantation in Auckland, New Zealand, 1999–2009: indications, patient characteristics, ethnicity, social deprivation, and access to services. Cornea. 2017;36: 546–552. [DOI] [PubMed] [Google Scholar]
- 19.Artiga S, Hill L, Orgera K, et al. Health Coverage by Race and Ethnicity, 2010–2019. Kaiser Family Foundation. Available at: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity/. Accessed July 16, 2021. [Google Scholar]
- 20.Manning WG, Newhouse JP, Duan N, et al. Health insurance and the demand for medical care: evidence from a randomized experiment. Am Econ Rev. 1987;77:251–277. [PubMed] [Google Scholar]
- 21.Elam AR, Andrews C, Musch DC, et al. Large disparities in receipt of glaucoma care between enrollees in Medicaid and those with commercial health insurance. Ophthalmology. 2017;124:1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawn AG, Santiago-Turla C, Lee PP. Patient expectations regarding eye care: focus group results. Arch Ophthalmol. 2003;121:762–768. [DOI] [PubMed] [Google Scholar]
- 23.Owsley C, McGwin G, Scilley K, et al. Perceived barriers to care and attitudes about vision and eye care: focus groups with older African Americans and eye care providers. Invest Ophthalmol Vis Sci. 2006;47: 2797–2802. [DOI] [PubMed] [Google Scholar]
- 24.Sentell T Implications for reform: survey of California adults suggests low health literacy predicts likelihood of being uninsured. Health Aff (Millwood). 2012;31:1039–1048. [DOI] [PubMed] [Google Scholar]
- 25.Sarezky D, Orlin SE, Pan W, et al. Trends in corneal transplantation in keratoconus. Cornea. 2017;36:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fink BA, Wagner H, Steger-May K, et al. Differences in keratoconus as a function of gender. Am J Ophthalmol. 2005;140:459–468. [DOI] [PubMed] [Google Scholar]
- 27.Ertan A, Muftuoglu O. Keratoconus clinical findings according to different age and gender groups. Cornea. 2008;27:1109–1113. [DOI] [PubMed] [Google Scholar]
- 28.Ziaei H, Jafarinasab MR, Javadi MA, et al. Epidemiology of keratoconus in an Iranian population. Cornea. 2012;31:1044–1047. [DOI] [PubMed] [Google Scholar]
- 29.Kosker M, Suri K, Hammersmith KM, et al. Another look at the association between diabetes and keratoconus. Cornea. 2014;33: 774–779. [DOI] [PubMed] [Google Scholar]
- 30.Pihlblad MS, Schaefer DP. Eyelid laxity, obesity, and obstructive sleep apnea in keratoconus. Cornea. 2013;32:1232–1236. [DOI] [PubMed] [Google Scholar]
- 31.Eliasi E, Bez M, Megreli J, et al. The association between keratoconus and body mass index: a population-based cross-sectional study among half a million adolescents. Am J Ophthalmol. 2021;224:200–206. [DOI] [PubMed] [Google Scholar]
- 32.Bak-Nielsen S, Ramlau-Hansen CH, Ivarsen A, et al. A nationwide population-based study of social demographic factors, associated diseases and mortality of keratoconus patients in Denmark from 1977 to 2015. Acta Ophthalmol. 2019;97:497–504. [DOI] [PubMed] [Google Scholar]
- 33.Millodot M, Shneor E, Albou S, et al. Prevalence and associated factors of keratoconus in Jerusalem: a cross-sectional study. Ophthalmic Epidemiol. 2011;18:91–97. [DOI] [PubMed] [Google Scholar]
- 34.Mukhtar S, Ambati BK. Pediatric keratoconus: a review of the literature. Int Ophthalmol. 2018;38:2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merdler I, Hassidim A, Sorkin N, et al. Keratoconus and allergic diseases among Israeli adolescents between 2005 and 2013. Cornea. 2015;34: 525–529. [DOI] [PubMed] [Google Scholar]


