Abstract
The two components ArcB and ArcA play a crucial role in the signal transduction implicated in the complex transcriptional regulatory network that allows Escherichia coli to sense various respiratory growth conditions. ArcB is a hybrid sensor kinase having multiple phosphorylation sites in its primary amino acid sequence, including a transmitter, a receiver, and a histidine-containing phosphotransfer (HPt) domain. ArcA is a DNA-binding transcriptional regulator with a receiver domain. Results of recent in vitro studies revealed multistep His-to-Asp phosphotransfer circuitry in the ArcB-ArcA signaling system. For this report we conducted a series of in vivo experiments using a set of crucial ArcB mutants to evaluate the regulation of the sdh operon. The results suggested that the phosphorylated His-717 site in the HPt domain of ArcB is essential for anaerobic repression of sdh. Nonetheless, the ArcB mutant lacking this crucial His-717 site does not necessarily exhibit a null phenotype with respect to ArcB-ArcA signaling. The HPt mutant appears to maintain an ability to signal ArcA, particularly under aerobic conditions, which results in a significant repression of sdh. Based on these and other in vivo results, we propose a model in which ArcB functions in its own right as a dual-signaling sensor that is capable of propagating two types of stimuli through two distinct phosphotransfer pathways.
The two components ArcB and ArcA play a crucial role in the signal transduction implicated in the complex transcriptional regulatory network that allows Escherichia coli to sense various respiratory growth conditions (12, 13, 15). Under anoxic conditions, the ArcB sensor is stimulated to autophosphorylate and subsequently signal the ArcA response regulator through transphosphorylation (6). Thus, this system is seemingly a simple and classical example of two-component signaling through His-to-Asp (His→Asp) phosphotransfer (3, 8, 20). However, recent studies revealed that the reality is more complex, because ArcB is a hybrid sensor having multiple (at least three) phosphorylation sites in its primary amino acid sequence, including a transmitter, a receiver, and a histidine-containing phosphotransfer (HPt) domain at its C terminus (5). The newly discovered HPt domain is of particular interest, since a number of signal transducers containing similar HPt domains were recently found (14, 22, 25). For example, E. coli alone has five hybrid signal transducers that each contain an HPt domain (ArcB, BarA, EvgS, TorS, and YojN) (18). It is thus probable that these HPt domains function as a common device involved in multistep His→Asp phosphotransfer signal transduction (1). However, examination of the function of this newly emerging phosphotransfer signaling domain is still at a very early stage, even for the best-characterized ArcB sensor.
On the basis of recent intensive in vitro studies from our and other laboratories (2, 5, 24), one can propose a plausible scheme to explain the complex circuitry of ArcB-ArcA phosphotransfer signaling (see Fig. 4). First, His-292 in the ArcB transmitter acquires the γ-phosphoryl group from ATP through its own catalytic function (i.e., autophosphorylation). This reaction is essential for subsequent phosphotransfer, and in fact the phosphoryl group at His-292 moves onto its intrinsic phospho-accepting aspartate (Asp-576) in the ArcB receiver. His-717 in the HPt domain can also be modified by phosphorylation, in which His-292 and Asp-576 play crucial roles. The final destination of the phosphoryl group at His-717 is Asp-54 in the ArcA receiver. Surprisingly, however, ArcA can also receive the phosphoryl group directly from His-292. In other words, ArcA acquires the phosphoryl group from either His-292 or His-717, presumably at the same aspartate site, Asp-54 (2, 24). It should be emphasized that this scenario is based mainly on in vitro biochemical results. Furthermore, although the ArcB-ArcA signaling system has been characterized extensively during the last decade in terms of anaerobic regulation (6–13, 15, 16), the significance of the HPt domain containing His-717 has been recognized only recently. Therefore, the physiological (or in vivo) relevance of this multistep phosphotransfer signaling process has not yet been fully examined. Lin and his colleagues showed that a C-terminally truncated form of ArcB which lacks the HPt domain exhibits a null phenotype with regard to anaerobic regulation, suggesting that the HPt domain plays a role in vivo (13). In our study, we addressed this issue more closely. Furthermore, we characterized the ArcB-ArcA system to address the general issue of whether there is an advantage to multistep signaling through the HPt domain. Although this issue has been addressed recently, no clear view has emerged (1, 4, 26).
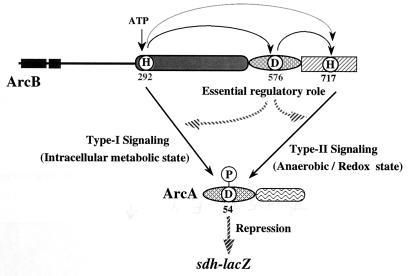
FIG. 4.
Proposed model to explain the dual-signaling mechanism underlying signal transduction in ArcB-ArcA multistep phosphotransfer. This model is based on the in vivo findings of this study, together with those of previous in vivo and in vitro studies (2, 13, 15, 25). Previous in vitro studies suggested that an unprecedented phosphotransfer from His-292 to His-717 may also occur (2, 24). Other details are given in the text.
Experimental design and viewpoint.
Succinate dehydrogenase of E. coli, an enzyme complex of the tricarboxylic acid cycle, participates in the aerobic electron-transport pathway and is encoded by the sdhCDAB operon. Previous studies have established well that the expression of sdhCDAB is markedly elevated by aerobiosis and severely suppressed during growth under anaerobic conditions, mainly through the ArcB-ArcA signaling system (8, 19). To gain insight into the physiological relevance of the in vitro-observed multistep phosphotransfer circuitry of ArcB-ArcA, we employed an E. coli strain (named DAC903) carrying an sdh-lacZ transcriptional fusion gene on its chromosome. This strain also contains an arcB null mutation (i.e., arcB::Cmr) and is a derivative of OG903 carrying the wild-type arcB allele. We also employed a set of plasmids (24), each of which carries a certain mutant gene of arcB (these low-copy-number plasmids were designated as members of the pLIA series). ArcB consists of 778 amino acids, among which His-292, Asp-576, and His-717 are involved in phosphotransfer circuitry, as mentioned above. Each of these amino acids was replaced by an altered one to create a set of mutant ArcB proteins, ArcB-ΔH1 (His-292 to Leu) in pLIA004, ArcB-ΔD (Asp-576 to Gln) in pLIA003, and ArcB-ΔH2 (His-717 to Leu) in pLIA002, although mutant plasmids were constructed by oligonucleotide-directed mutagenesis by using pLIA001, which specifies the wild-type protein (ArcB-W). These mutant ArcB proteins were expressed from the plasmids in a stable form and were incorporated into the cytoplasmic membrane in nearly equal amounts. These sets of hosts and plasmids allowed us to examine the in vivo relevance of the ArcB-ArcA signaling system, with special reference to multistep phosphotransfer circuitry.
Regulation of the expression of sdh-lacZ through the ArcB-ArcA signaling system.
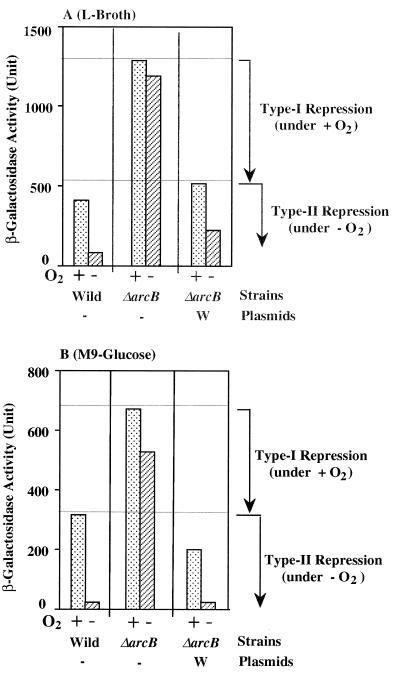
Strain OG903 carrying the sdh-lacZ fusion gene, otherwise wild type with respect to the arcB gene, was grown in Luria broth under both aerobic and anaerobic conditions, and β-galactosidase activities expressed in these cells were measured (Fig. 1A, bars denoted by “Wild”). As expected, the expression of sdh-lacZ was enhanced under the aerobic growth conditions and markedly repressed under the anaerobic growth conditions (8, 19). The results support the view that the ArcB sensor kinase is activated under anaerobic conditions and, consequently, that the phosphorylated form of ArcA functions as a DNA-binding repressor for sdh (6). Then the expression of sdh-lacZ was examined in DAC903 lacking the functional arcB gene. As expected, the anaerobic repression of sdh-lacZ was completely abolished (Fig. 1A, bars denoted by “ΔarcB”). However, when pLIA001, which encompasses the wild-type arcB gene, was introduced into DAC903, the regulatory profile of sdh-lacZ reverted to that exhibited by the original wild-type OG903 strain (Fig. 1A, bars denoted by “W”). These observations indicate that our experimental design, employing DAC903 and the pLIA series, is appropriate to explore the in vivo ArcB-ArcA signaling system.
FIG. 1.
β-Galactosidase activity expressed by the sdh-lacZ transcriptional fusion gene. Strains OG903 (bars denoted by “Wild” [arcB+ sdh-lacZ]) and DAC903 (bars denoted by “ΔarcB” [arcB::Cmr sdh-lacZ]) were grown in either Luria (L) broth (A) or M9-glucose minimal medium (B), under both aerobic (+ O2) and anaerobic (−O2) conditions. DAC903 carrying plasmid pLIA001 containing the wild-type arcB gene was also grown under the same conditions as those described above (bars denoted by “ΔarcB” and “W”). The harvested cells were assayed for β-galactosidase, according to the method of Miller (17). The same experiments were repeated more than three times, and the means were plotted with Miller units. Note that error bars are omitted for clarity (the deviations were less than 7%). The horizontal lines are placed to indicate the highest levels of β-galactosidase activities that were observed in the presence of O2 for the type I and type II repression phenomena.
However, upon close inspection of the results shown in Fig. 1A, we noticed that the level of the expression of sdh-lacZ was significantly higher (threefold) in the ΔarcB background even under aerobic conditions (bars indicating the presence of O2) than in the arcB+ background. This observation was confirmed with a more defined growth medium, i.e., M9-glucose minimal medium (Fig. 1B). Essentially the same regulatory profiles were observed for the cells grown in M9-glucose. We interpreted these observations by assuming that, even under aerobic conditions, the arcB sensor may be partially activated so as to signal ArcA through phosphorylation. As schematically shown in Fig. 1, we can distinguish the presumed repression observed under aerobic conditions (type I repression) from the classical one observed under anaerobic conditions (type II repression), both of which are apparently mediated by the ArcB-ArcA signaling system. Keeping this assumption in mind, we specifically asked whether the phosphorylated His-717 site in the HPt domain is crucial for the ArcB-ArcA signaling system.
The phosphorylated His-717 site is crucial for anaerobic signaling through the ArcB-ArcA system; nonetheless, the His-717 mutation does not necessarily result in a null phenotype.
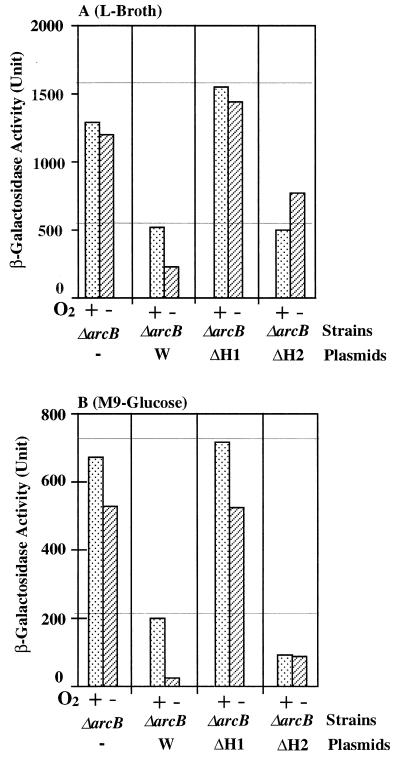
Plasmids pLIA004, which specifies ArcB-ΔH1, and pLIA002, which specifies ArcB-ΔH2, as well as pLIA001, which specifies ArcB-W, were introduced into the ΔarcB host. β-Galactosidase activities expressed by these transformants were measured under both aerobic and anaerobic conditions, in either Luria broth (Fig. 2A) or M9-glucose (Fig. 2B). Cells producing ArcB-ΔH1 exhibit a null phenotype (Fig. 2, bars denoted by “ΔH1”), as with ΔArcB cells, as far as the expression of sdh-lacZ is concerned. These results support the idea that the phosphorylated His-292 site in the transmitter is essential for the signaling function of ArcB. With the ArcB-ΔH2 cells, the anaerobic repression of sdh-lacZ was completely abolished in both media (Fig. 2, bars denoted by “ΔH2”). Thus, phosphorylated His-717 is crucial, as far as anaerobic (type II) repression is concerned. Surprisingly, however, the presumed type I repression was fully maintained in the ArcB-ΔH2 cells, in marked contrast to the situation with ArcB-ΔH1. These results suggest that the His-717 site is essential for typical anaerobic signaling through the ArcB-ArcA system, leading us to conclude that multistep His→Asp→His→Asp phosphotransfer is required for ArcB to finally phosphorylate ArcA, which results in the anaerobic repression of sdh. However, the His-717 mutation does not necessarily result in a null phenotype in terms of the ArcB function, suggesting that ArcB lacking the phosphorylated His-717 site appears to still be functional with regard to its signaling ability, as far as the presumed type I repression is concerned.
FIG. 2.
β-Galactosidase activity expressed by the sdh-lacZ transcriptional fusion gene. Strain DAC903 (arcB::Cmr sdh-lacZ) was transformed with the following plasmids: the vector (bars denoted by “ΔarcB”), pLIA001 (bars denoted by “W”), pILA004 (bars denoted by “ΔH1”), and pILA002 (bars denoted by “ΔH2”). These transformants were grown in either Luria (L) broth (A) or M9-glucose minimal medium (B) under both aerobic (+ O2) and anaerobic (−O2) conditions. The harvested cells were assayed for β-galactosidase, according to the method of Miller (17), as described for Fig. 1.
The putative type I repression is physiologically meaningful.
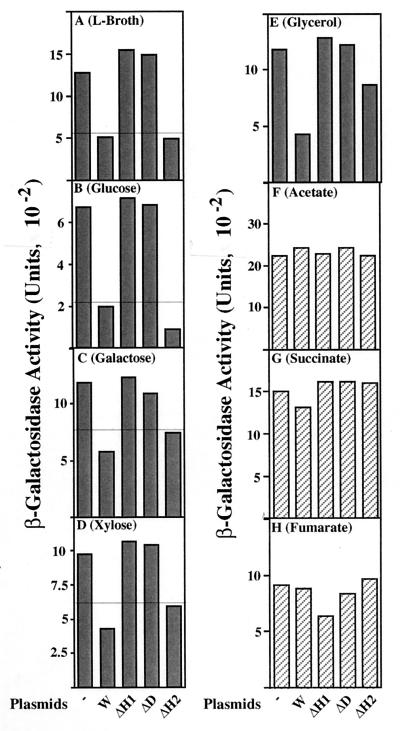
We then focused our attention on the presumed type I repression observed under fully aerobic growth conditions. Is this ArcB-dependent and His-717-independent repression physiologically meaningful? To address this issue, type I repression was characterized for the cells grown in different kinds of M9-based media, under aerobic conditions (Fig. 3). We found that when cells were grown in either M9-galactose or M9-xylose (Fig. 3C and D), type I repression was clearly observed (Fig. 3, bars denoted by “W”), as occurred with Luria broth and M9-glucose (Fig. 3A and B). In this respect, both the ArcB-ΔH1 and the ArcB-ΔD cells exhibited a null phenotype. It should be emphasized, however, that the ArcB-ΔH2 cells showed a repression ability essentially indistinguishable from that of ArcB-W, confirming the above-described notion (Fig. 2). A more intriguing finding was that when the cells were grown in either M9-acetate, M9-succinate, or M9-fumarate, type I repression was completely eliminated (Fig. 3F to H). In other words, in all of the ArcB mutants, the expression of sdh-lacZ was fully derepressed to the same maximum level as that in the ΔarcB background, when the cells were grown with these particular carbon and energy sources. M9-glycerol gave an ambiguous result (Fig. 3E).
FIG. 3.
β-Galactosidase activity expressed by the sdh-lacZ transcriptional fusion gene. Strain DAC903 (arcB::Cmr sdh-lacZ) was transformed with the following plasmids: the vector (bars denoted by “−”), pLIA001 (bars denoted by “W”), pILA004 (bars denoted by “ΔH1”), pLIA003 (bars denoted by “ΔD”), and pILA002 (bars denoted by “ΔH2”). These transformants were grown in either Luria (L) broth (A) or M9-based minimal media, each containing the indicated carbon and energy source (B to H) under the aerobic conditions. The harvested cells were assayed for β-galactosidase, according to the method of Miller (17), as described for Fig. 1.
These results suggest that aerobic type I repression mediated by ArcB is a physiological event, presumably reflecting the nature of the carbon and energy sources used. In addition, the putative stimulus that activates the ArcB sensor appears to be vital in the cells grown with certain types of carbon and energy sources (glucose, galactose, and xylose) but not in the cells grown with other types of carbon and energy sources (acetate, succinate, and fumarate). Considering carbon metabolism in E. coli, we assume a priori that the stimulus for type I repression must be related to the intracellular metabolic state, as will be discussed below.
It is also worth mentioning here that the carbon and energy sources affected significantly the absolute levels of the sdh-lacZ (e.g., 669 U for glucose versus 2,091 U for acetate). Thus, factors other than the ArcB-ArcA system must be implicated in the regulation of sdh (e.g., Fnr, cAMP, Cra, and perhaps other two-component systems), as has been emphasized previously (19).
Implication.
A well-defined scenario as to the molecular mechanism underlying the ArcB-ArcA signaling system has been proposed inductively from the elegant studies of Iuchi and colleagues (6–13) and Lynch and Lin (15, 16). Although the design of our in vivo experiments in this study is very simple, the results shed new light and allow us to propose a refined model, which presents results consistent with most of the previous in vitro results with regard to the ArcB-ArcA signaling system, as discussed below. Furthermore, the model provides new insight into the possible mechanistic advantage of multistep His→Asp phosphotransfer signal transduction.
Our revised model is presented in Fig. 4. ArcA is phosphorylated through two distinct phosphotransfer pathways: one directly from His-292 and the other through the multistep His→Asp phosphotransfer mediated by His-717. In any case, the resulting phospho-ArcA functions as the transcriptional repressor for the sdhCDAB operon. Important findings are that the His-717–to–ArcA (type II signaling) pathway is mainly responsible for the adaptation to anoxic conditions and that the shortcut His-292–to–ArcA (type I signaling) pathway appears to operate even under fully aerobic conditions in response to a presumed metabolic state. It has previously been proposed that a redox state, perhaps an element of the electron-transport chain or proton motive force, may be a primary anoxic stimulus for the kinase activity of ArcB, since both physiological and genetic experiments excluded O2 itself as the signal (13, 15). The ArcB sensor, stimulated by such a putative anoxic stimulus, may signal ArcA through the type II pathway involving the HPt domain. It has also been proposed that a certain set of cytosolic effectors, such as d-lactate, acetate, pyruvate, and NADH, may affect the kinase activity of ArcB (13, 15). Consistent with this notion, our results support the idea that the type I signaling pathway may be involved in this particular response to the presumed intracellular metabolic state, operating in E. coli even under aerobic conditions (Fig. 3). As a whole, a dual-signaling model, proposed here for the ArcB sensor, is consistent with a number of previous notions with regard to the ArcB-ArcA system, made by Iuchi and Lin (13).
In this model, at present, the function of the internal ArcB receiver domain containing the phosphorylated Asp-576 site is somewhat unclear. However, it should be emphasized that Asp-576 plays an essential role in these two signaling pathways under both aerobic (Fig. 2) and anaerobic (data not shown) conditions. Results of previous in vitro studies showed that the phosphorylation of His-292 and His-717 in ArcB and the phosphotransfer to Asp-54 in ArcA appear to occur at a certain level in an Asp-576-independent manner (2, 24). It is conceivable that such a contradiction between the phosphotransfer model based on the in vitro studies with purified proteins and the proposed in vivo signaling pathway model may be due to additional factors absent in the purified system. In any event, this issue remains to be addressed, in order to further refine the model in Fig. 4. The ArcB receiver domain may function as an essential self-controlling molecular switch in such a manner that it makes interplay between the two signaling pathways possible. This view is consistent with the assumption recently made by Georgellis et al., based on their in vitro experiments (2). Namely, ArcA receives the phosphoryl group from either His-292 or His-717, the relative contribution of which is regulated through a function of the ArcB receiver.
Finally, our model should be discussed with special reference to the general issue regarding the multistep phosphotransfer mechanism. The recent discovery of the multistep phosphotransfer mechanism raised the general question of what is the advantage of such a signaling mechanism through the additional HPt domain. This mechanism does not necessarily serve to amplify signals, in contrast to the eukaryotic signaling cascades involving a set of Ser/Thr kinases and/or Tyr kinases (26). Why should a phosphoryl group travel along a long railroad with extra stations? The extra His→Asp phosphotransfer components may serve as multiple regulatory checkpoints in a given signaling pathway (21). They may also provide the potential for an integration of multiple signals at the intermediate steps (23). Our model provides an alternative view with regard to this general issue, namely, the dual-signaling mechanism makes it possible for a hybrid sensor to function as a sophisticated device exhibiting the ability to propagate multiple signals in its own right. A number of hybrid sensor kinases, each of which has a structural design very similar to that of ArcB, have been recently uncovered (14, 18, 22, 25). They may also function in similar manners.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Appleby J L, Parkinson J S, Bourret R B. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 2.Georgellis D, Lynch S, Lin E C C. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J Bacteriol. 1997;179:5429–5435. doi: 10.1128/jb.179.17.5429-5435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoch J A, Silhavy T J. Two-component signal transduction. Washington, D.C: ASM Press; 1995. [Google Scholar]
- 4.Inouye M. His-Asp phosphorelay; two components or more? Cell. 1996;85:13–14. [Google Scholar]
- 5.Ishige K, Nagasawa S, Tokishita S, Mizuno T. A novel device of bacterial signal transducers. EMBO J. 1994;13:5195–5202. doi: 10.1002/j.1460-2075.1994.tb06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iuchi S. Phosphorylation/dephosphorylation of the receiver module at the conserved aspartate residue controls transphosphorylation activity of histidine kinase in sensor protein ArcB of Escherichia coli. J Biol Chem. 1993;268:23972–23980. [PubMed] [Google Scholar]
- 7.Iuchi S, Lin E C C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci USA. 1988;85:1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iuchi S, Matsuda Z, Fujiwara T, Lin E C C. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol Microbiol. 1990;4:715–727. doi: 10.1111/j.1365-2958.1990.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 9.Iuchi S, Chepuri V, Fu H-A, Gennis R B, Lin E C C. Requirement for the terminal cytochromes in generation of the aerobic signal for the arc regulatory system in Escherichia coli: study utilizing deletions and lac fusions of cyo and cyd. J Bacteriol. 1990;172:6020–6025. doi: 10.1128/jb.172.10.6020-6025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iuchi S, Lin E C C. Mutational analysis of signal transduction by ArcB, a membrane sensor protein responsible for anaerobic repression of operons involved in the central aerobic pathways in Escherichia coli. J Bacteriol. 1992;174:3972–3980. doi: 10.1128/jb.174.12.3972-3980.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iuchi S, Lin E C C. Purification and phosphorylation of the Arc regulatory components of Escherichia coli. J Bacteriol. 1992;174:5617–5623. doi: 10.1128/jb.174.17.5617-5623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iuchi S, Lin E C C. Adaptation of Escherichia coli to redox environments by gene expression. Mol Microbiol. 1993;9:715–727. doi: 10.1111/j.1365-2958.1993.tb01664.x. [DOI] [PubMed] [Google Scholar]
- 13.Iuchi S, Lin E C C. Signal transduction in the Arc system for control of operons encoding aerobic respiratory enzymes. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 223–231. [Google Scholar]
- 14.Kato M, Mizuno T, Shimizu T, Hakoshima T. Insights into multistep phosphorelay from the crystal structure of the C-terminal HPt domain of ArcB. Cell. 1997;88:717–723. doi: 10.1016/s0092-8674(00)81914-5. [DOI] [PubMed] [Google Scholar]
- 15.Lynch A S, Lin E C C. Responses to molecular oxygen. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1526–1538. [Google Scholar]
- 16.Lynch A S, Lin E C C. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J Bacteriol. 1996;178:6238–6249. doi: 10.1128/jb.178.21.6238-6249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. p. 356. [Google Scholar]
- 18.Mizuno T. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res. 1997;4:161–168. doi: 10.1093/dnares/4.2.161. [DOI] [PubMed] [Google Scholar]
- 19.Park S-J, Tseng C-P, Gunsalus R P. Regulation of succinate dehydrogenase (sdhCDAB) operon expression in Escherichia coli response to carbon supply and anaerobiosis: role of ArcA and Fnr. Mol Microbiol. 1995;15:473–482. doi: 10.1111/j.1365-2958.1995.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 21.Perego M, Hanstein C, Welsh K M, Djavakhishvili T, Glaser P, Hoch J A. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell. 1994;79:1047–1055. doi: 10.1016/0092-8674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 22.Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thai T C, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 23.Trach K A, Hoch J A. Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway. Mol Microbiol. 1993;8:69–79. doi: 10.1111/j.1365-2958.1993.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsuzuki M, Ishige K, Mizuno T. Phosphotransfer circuitry of the putative multi-signal transducer, ArcB, of Escherichia coli: in vitro studies with mutants. Mol Microbiol. 1995;18:953–962. doi: 10.1111/j.1365-2958.1995.18050953.x. [DOI] [PubMed] [Google Scholar]
- 25.Uhl M A, Miller J F. Integration of multiple domains in a two component sensor protein, the Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 26.Wurgler-Murphy S M, Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem Sci. 1997;22:172–176. doi: 10.1016/s0968-0004(97)01036-0. [DOI] [PubMed] [Google Scholar]