Abstract
We constructed and characterized a Xanthomonas campestris pv. phaseoli oxyR mutant. The mutant was hypersensitive to H2O2 and menadione killing and had reduced aerobic plating efficiency. The oxidants’ induction of the catalase and ahpC genes was also abolished in the mutant. Analysis of the adaptive responses showed that hydrogen peroxide-induced protection against hydrogen peroxide was lost, while menadione-induced protection against hydrogen peroxide was retained in the oxyR mutant. These results show that X. campestris pv. phaseoli oxyR is essential to peroxide adaptation and revealed the existence of a novel superoxide-inducible peroxide protection system that is independent of OxyR.
Inducible stress responses are important components of bacterial survival under stressful conditions. Exposure to a low level of one stress can induce a protective response against subsequent exposure to lethal levels of the same (adaptive response) or unrelated (cross-protective response) stresses (3, 5, 7, 23, 32). OxyR, a global regulator for peroxide stress response, is a bifunctional protein that acts as a peroxide sensor and a transcription activator in response to oxidative stress (2, 31, 33). It regulates many genes involved in the scavenging of peroxides (i.e., catalase and alkyl hydroperoxide reductase [ahpR] [5, 30]) and the prevention and repair of oxidative damage for macromolecules (i.e., glutathione reductase and dps) (5, 17, 19, 29).
The inducible adaptive and cross-protective responses against peroxide killing could play important roles in plant-microbe interactions. Active plant defense response against microbes involves increased production of H2O2, organic peroxides, and superoxides (14). These reactive oxygen species can inhibit growth and kill invading microbes. During initial interactions, bacteria are exposed to low-concentration mixtures of superoxide anions and peroxides (14). These could induce protection against subsequent exposure to higher concentrations of reactive oxygen species that prolong bacterial survival in the plant and may affect disease progression. Moreover, normal aerobic metabolism also generates significant quantities of reactive oxygen species (8, 9), which have to be rapidly detoxified.
We have isolated and characterized an oxyR from Xanthomonas campestris pv. phaseoli (15, 22). The gene has unique organization and transcription regulation (1, 16, 23). This fact, coupled with observations that many aspects of Xanthomonas oxidative stress response differ from those of other bacteria (1, 16), leads us to investigate OxyR function in X. campestris pv. phaseoli.
Construction of the oxyR mutants.
Inactivation of the oxyR gene was achieved by insertion of a KpnI-digested gentamicin resistance gene from pUCGM (27) into a KpnI site located in the coding region of oxyR on plasmid pUC18 (15). The new recombinant plasmid, designated poxyR::Gm, was electroporated into X. campestris pv. phaseoli as previously described (21). Transformants were selected on SB (0.5% yeast extract, 0.5% peptone, 0.5% sucrose, 0.1% glutamic acid; pH 7.0) plates containing 15 μg of gentamicin per ml. Gmr colonies were subsequently scored for an Aps phenotype. Many colonies had Aps Gmr phenotypes, indicating an exchange of the mutated oxyR for its functional counterpart. These colonies were selected for further characterization by both Southern and Western analyses, which confirmed that the mutated oxyR had replaced the functional gene in these cells with an Aps Gmr phenotype (data not shown).
Physiological characterization of the mutant.
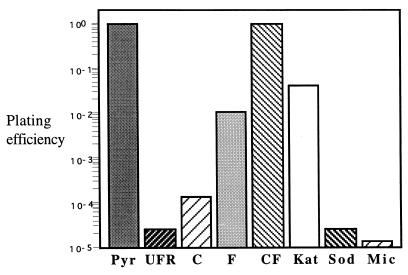
We noticed that the oxyR mutants formed smaller colonies than did the parental strain on SB plates. Mutations in genes involved in oxidative stress response often lead to defects in aerobic plating efficiency (18, 34). All of the X. campestris pv. phaseoli oxyR mutant strains tested showed a 104 decrease in aerobic plating efficiency on SB plates compared to that for the parental strain. This effect could be reversed by the addition of 10 mM sodium pyruvate (18, 24, 34) to SB plates (Fig. 1), suggesting that accumulation of peroxides in the oxyR mutants probably caused the defect. To test the hypothesis, plasmids containing Xanthomonas genes involved in oxidative stress protection were transformed into the mutant and their plating efficiency was determined. The results are shown in Fig. 1. A high level of superoxide dismutase (pUFR-SOD [28]) or microaerobic growth conditions had no effect on the plating efficiency of the mutant. An increased level of enzymes directly involved in peroxide metabolism (e.g., monofunctional catalase [pkat] [21] and AhpR subunits C and F [pUFR-ahpCF]) restored the plating efficiency of the mutant so that it was close to that of the parental strain. An increased level of catalase was less efficient than AhpR at complementing the defect, probably due to the inability of catalase to metabolize organic peroxide. Unexpectedly, increased levels of AhpF (pUFR-ahpF) alone restored the level of plating efficiency similar to the level attained by overexpression of catalase, while high levels of AhpC (pahpC [15]) alone were not as effective (Fig. 1). Purified AhpC and AhpF can use both H2O2 and organic peroxide as substrates (25, 26). On the other hand, we have observed in X. campestris pv. phaseoli that increased expression of either ahpC (15) or ahpC-ahpF in vivo does not increase resistance to H2O2 killing. We interpreted these data as evidence that oxyR mutants accumulate both H2O2 and organic peroxides, consistent with the observation in Escherichia coli that oxyR mutants have higher levels of peroxides than a wild-type strain (9). This fact and increased susceptibility to oxidative damage during the early stages of colony formation when bacterial density is low (17) could have been responsible for the lower aerobic plating efficiency seen for the mutants.
FIG. 1.
Plating efficiency of an oxyR mutant harboring various expression plasmids containing genes involved in oxidative stress response or conditions that affected oxidative stress. In all experiments, a mid-log-phase X. campestris pv. phaseoli oxyR mutant grown in SB was serially diluted and plated on SB plates with or without 10 mM pyruvate. Plating efficiency is defined as the number of cells on SB plates divided by the number of cells on SB plates with pyruvate. Pyr, X. campestris pv. phaseoli oxyR mutant on 10 mM pyruvate SB plates; Mic, the mutant was plated on SB plates and incubated in an anaerobic jar under microaerobic conditions (Oxoid gas generating kit); UFR, X. campestris pv. phaseoli oxyR mutant harboring only pUFR047 (4) expression vector; C, pahpC (15); F, pahpF (ahpF subunit of X. campestris pv. phaseoli [15] in pUFR047); CF, pahpCF (ahpC and ahpF [15] in pUFR047); Kat, pkat (21); Sod, psod (Xanthomonas sod [28] coding region in pUFR047).
Next we qualitatively determined the sensitivity of the log-phase oxyR mutant to killing concentrations of various oxidants by a killing zone method (15). Essentially, 6 μl of indicated concentrations of oxidants applied to 6-mm-diameter paper discs was subsequently placed on lawns of cells. Experiments were performed in triplicate. To ensure reproducibility, only log-phase cells were used. The killing zones for H2O2 (500 mM), menadione (MD) (500 mM), tert butyl hydroperoxide (tBOOH) (500 mM), and cumene hydroperoxide (CuOOH) (500 mM), respectively, were 13, 17, 11, and 16 mm for a wild-type X. campestris pv. phaseoli and 34, 42, 13, and 18 mm for an oxyR mutant. The oxyR mutant showed increased sensitivity to all oxidants tested, with MD and H2O2 causing the most severe effects. The high sensitivity of the oxyR mutant to H2O2 was expected, but the hypersensitivity to MD implied that its killing mechanism could partly be mediated via superoxide anion metabolism to H2O2 (11, 12). By contrast to an E. coli oxyR mutant, the X. campestris pv. phaseoli oxyR mutant had only a minor increase in sensitivity to organic peroxide killing. This could be due to presence of an additional novel organic peroxide-protective system (ohr) in X. campestris pv. phaseoli that may functionally compensate for regulatory defects of AhpC (20).
Regulation of oxidant induction of catalase and AhpC by oxyR.
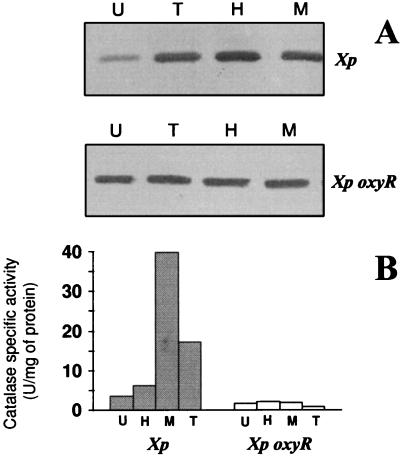
We have observed in Xanthomonas that the peroxide-scavenging enzymes, catalase and AhpC, are highly induced by low concentrations of peroxides and superoxide generators (1, 22). However, the regulator of these responses could not be identified. Experiments were performed to determine catalase and AhpC levels in response to low concentrations of oxidants in X. campestris pv. phaseoli and X. campestris pv. phaseoli oxyR. The results are shown in Fig. 2. In X. campestris pv. phaseoli, H2O2, tBOOH, and MD induced both catalase and AhpC to high levels, consistent with previous observations (1, 16, 21). However, induction of both enzymes by all oxidants tested did not occur in the oxyR mutant. This finding is consistent with a notion that OxyR is acting as a peroxide sensor and a transcription activator of genes for peroxide-scavenging enzymes. These functions are conserved for oxyR in all bacteria thus far studied (28, 31, 33, 34). An increase in the basal level of AhpC in the oxyR mutant was observed. This could be due to OxyR in its reduced form functioning as a repressor of ahpC; thus, in the absence of OxyR, this leads to an increase in ahpC expression (20). The induction of these peroxide-scavenging enzymes by a superoxide generator (MD) was likely to occur via the breakdown of superoxide anion to H2O2 that, in turn, activated OxyR, not via a superoxide sensor transcription activator protein such as SoxRS (11, 12).
FIG. 2.
Levels of AhpC and catalase activities in response to various oxidants in X. campestris pv. phaseoli (Xp) and an X. campestris pv. phaseoli oxyR mutant (Xp oxyR). Mid-log-phase X. campestris pv. phaseoli or an X. campestris pv. phaseoli oxyR mutant grown in SB was induced with 100 μM H2O2 (H) or tBOOH (T) or 20 μM MD (M) for 30 min. Various concentrations of oxidants were chosen to give maximum induction and minimal effects on X. campestris pv. phaseoli growth. Uninduced (U) and induced samples were collected by centrifugation, and lysates were prepared as previously described (21). AhpC levels (A) were determined by Western immunoblotting with an anti-E. coli AhpC (22, 30). Forty micrograms of total protein was loaded into each lane, and immunodetection was performed according to the method of Mongkolsuk et al. (22). At the right of each panel is indicated whether lysates were from X. campestris pv. phaseoli or an X. campestris pv. phaseoli oxyR mutant. Catalase levels were determined spectrophotometrically (21). (B) Closed and open bars represent catalase activities of X. campestris pv. phaseoli and the X. campestris pv. phaseoli oxyR mutant, respectively. Letters above the lanes (A) or below the bars (B) indicate that lysates were prepared from uninduced or oxidant-induced cultures, respectively. Experiments were performed three times, and typical results are shown.
Basal levels of catalase and AhpC in the mutant appeared to be sufficient for normal aerobic growth. The lack of an induction mechanism for peroxide-scavenging enzymes and the increased oxidant sensitivity of oxyR mutants support the interpretation that up-regulation of these scavenging enzymes is important to bacterial survival under stressful conditions. Consistent with this notion, oxyR suppressor mutants with high levels of AhpC-AhpF and catalases have been isolated (10).
oxyR roles in adaptive and cross-protective responses.
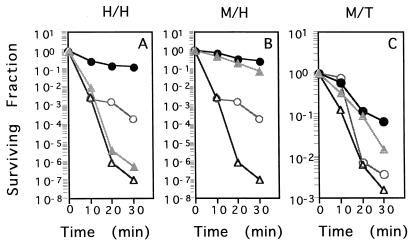
In Xanthomonas, peroxide and superoxide anions induce protective responses to peroxide killing (23). These responses are mediated by OxyR in E. coli (32), and the oxyR mutant was used to investigate whether the situation in Xanthomonas was similar. The results of the experiment are shown in Fig. 3. H2O2 induced protection against H2O2 killing in wild-type X. campestris pv. phaseoli. This response was abolished in the oxyR mutant (Fig. 3A). In contrast to previous observations with other bacteria (6, 10, 18), MD could induce protection against H2O2 and tBOOH killing in both the parental strain and the oxyR mutant (Fig. 3B and C). The data indicate that OxyR is essential to peroxide adaptation and also to the existence of a novel superoxide-inducible peroxide-protective system independent of OxyR. This novel peroxide-protective system does not depend on up-regulation of the well-known peroxide-scavenging enzymes catalase and AhpR, since their induction by superoxide anions was abolished in the oxyR mutant (Fig. 2).
FIG. 3.
Adaptive and cross-protective responses against peroxide killing in X. campestris pv. phaseoli and an X. campestris pv. phaseoli oxyR mutant. Log-phase uninduced X. campestris pv. phaseoli (○) and an X. campestris pv. phaseoli oxyR mutant (▵) and oxidant-induced (30-min treatment with either 100 μM H2O2 [A] or 50 μM MD [B and C]) X. campestris pv. phaseoli (•) and X. campestris pv. phaseoli oxyR mutant (▴) grown in SB were treated with killing concentrations of either 30 mM H2O2 (A and B) or 100 mM tBOOH (C) as previously described (15). At the indicated times, aliquots of cells were removed and washed twice before viable cells were counted (23). Experiments were repeated three times, and representative results are shown.
It is noteworthy that resistance levels to peroxide killing in the MD-induced oxyR strain were similar to those attained by the similarly induced parental strain, even though the uninduced oxyR mutant was more sensitive than the parental strain to peroxide killing. Thus, the novel superoxide-inducible peroxide-protective system is likely to play a crucial role in protection against peroxide killing in X. campestris pv. phaseoli. We believe this system differs from the starvation-induced or the general stress-protective systems (13). In Xanthomonas, MD does not induce protection against itself or against a nonoxidative stress such as heat killing (23). We are investigating the mechanism of this novel superoxide anion-induced peroxide-protective system.
Acknowledgments
We thank G. Storz for her helpful comments and an anti-AhpC antibody and T. Flegel for reviewing the manuscript. This research was supported by grants from Chulabhorn Research Institute, Thai Research Fund BRG-10-40, and a career development award, RCF 01-40-005, from NASTDA to S.M.
REFERENCES
- 1.Chamnongpol S, Vattanaviboon P, Loprasert S, Mongkolsuk S. Atypical oxidative stress regulation of a Xanthomonas oryzae pv. oryzae monofunctional catalase. Can J Microbiol. 1995;41:541–547. [Google Scholar]
- 2.Christman M F, Morgan R W, Jacobson F S, Ames B N. Positive control of a regulon for a defense against oxidative stress and some heat shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 3.Crockford A J, Behncke C, Williams H D. The adaptation of Rhizobium leguminosarum pv. phaseoli to oxidative stress and its overlap with other environmental stress responses. Microbiology. 1996;142:331–336. doi: 10.1099/13500872-142-2-331. [DOI] [PubMed] [Google Scholar]
- 4.DeFeyter R, Kado C I, Gabriel D W. Small, stable shuttle vectors for use in Xanthomonas. Gene. 1990;88:65–72. doi: 10.1016/0378-1119(90)90060-5. [DOI] [PubMed] [Google Scholar]
- 5.Demple B. Regulation of bacterial oxidative stress genes. Annu Rev Genet. 1991;25:315–337. doi: 10.1146/annurev.ge.25.120191.001531. [DOI] [PubMed] [Google Scholar]
- 6.Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr S B, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Flecha B, Demple B D. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J Biol Chem. 1995;270:13681–13687. doi: 10.1074/jbc.270.23.13681. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Flecha B, Demple B D. Homeostatic regulation of intracellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J Bacteriol. 1997;179:382–388. doi: 10.1128/jb.179.2.382-388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg J T, Demple B. Overproduction of superoxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR− mutants. EMBO J. 1988;7:2611–2617. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg J T, Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989;171:3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan H M, Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979;196:385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- 13.Kolter R, Siegele D A, Tormo A. The stationary phase of bacterial life cycle. Annu Rev Microbiol. 1993;48:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 14.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 15.Loprasert S, Atichartpongkun S, Whangsuk W, Mongkolsuk S. Isolation and analysis of the Xanthomonas alkyl hydroperoxide reductase gene and the peroxide sensor regulator genes ahpC and ahpF-oxyR-orfX. J Bacteriol. 1997;179:3944–3949. doi: 10.1128/jb.179.12.3944-3949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loprasert S, Vattanaviboon P, Praituan W, Chamnongpol S, Mongkolsuk S. Regulation of oxidative stress protective enzymes, catalase and superoxide dismutase in Xanthomonas—a review. Gene. 1996;179:33–37. doi: 10.1016/s0378-1119(96)00427-1. [DOI] [PubMed] [Google Scholar]
- 17.Ma M, Eaton J W. Multicellular oxidant defense in unicellular organisms. Proc Natl Acad Sci USA. 1992;89:7924–7928. doi: 10.1073/pnas.89.17.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maciver I, Hansen E J. Lack of expression of the global regulator OxyR in Haemophilus influenzae has a profound effect on growth phenotype. Infect Immun. 1996;64:4618–4629. doi: 10.1128/iai.64.11.4618-4629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA binding protein Dps. J Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mongkolsuk, S. Unpublished observations.
- 21.Mongkolsuk S, Loprasert S, Vattanaviboon P, Chanvanichayachai C, Chamnongpol S, Supsamran N. Heterologous growth phase- and temperature-dependent expression and H2O2 toxicity protection of a superoxide-inducible monofunctional catalase gene from Xanthomonas oryzae pv. oryzae. J Bacteriol. 1996;178:3578–3584. doi: 10.1128/jb.178.12.3578-3584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mongkolsuk S, Loprasert S, Whangsuk W, Fuangthong M, Atichartpongkun S. Characterization of transcription organization and analysis of unique expression patterns of an alkyl hydroperoxide reductase C gene (ahpC) and the peroxide regulator operon ahpF-oxyR-orfX from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1997;179:3950–3955. doi: 10.1128/jb.179.12.3950-3955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mongkolsuk S, Vattanaviboon P, Praituan W. Induced adaptive and cross-protection responses against oxidative stress killing in a bacterial phytopathogen. Xanthomonas oryzae pv. oryzae. FEMS Microbiol Lett. 1997;146:212–217. [Google Scholar]
- 24.Nath K A, Ngo E O, Hebbel R P, Croatt A J, Zhou B, Nutter L M. Alpha-ketoacids scavenge H2O2 in vitro and in vivo and reduced menadione-induced DNA injury and cytotoxicity. Am J Physiol. 1995;268:C227–C236. doi: 10.1152/ajpcell.1995.268.1.C227. [DOI] [PubMed] [Google Scholar]
- 25.Niimura Y, Poole L B, Massey V. Amphibacillus xylanus NADH oxidase and Salmonella typhimurium alkyl hydroperoxide reductase flavoprotein components show extremely high scavenging activity for both alkyl hydroperoxide and hydrogen peroxide in the presence of S. typhimurium alkyl hydroperoxide reductase 22 kDa protein component. J Biol Chem. 1995;270:25645–25650. doi: 10.1074/jbc.270.43.25645. [DOI] [PubMed] [Google Scholar]
- 26.Poole L B, Ellis H R. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry. 1996;35:56–64. doi: 10.1021/bi951887s. [DOI] [PubMed] [Google Scholar]
- 27.Schweizer H D. Small broad-host-range gentamycin resistance cassettes for site-specific insertion and deletion mutagenesis. BioTechniques. 1993;15:831–834. [PubMed] [Google Scholar]
- 28.Smith S G, Wilson T J, Dow J M, Daniels M J. A gene for superoxide dismutase from Xanthomonas campestris pv. campestris and its expression analysis during bacterial-plant interactions. Mol Plant-Microbe Interact. 1996;9:584–593. doi: 10.1094/mpmi-9-0584. [DOI] [PubMed] [Google Scholar]
- 29.Storz G, Altuvia S. OxyR regulon. Methods Enzymol. 1994;234:217–223. doi: 10.1016/0076-6879(94)34088-9. [DOI] [PubMed] [Google Scholar]
- 30.Storz G, Jacobson F S, Tartaglia L A, Morgan R W, Silveira L A, Ames B N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J Bacteriol. 1989;171:2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storz G, Tartaglia L A, Ames B N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990;248:189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- 32.Tartaglia L A, Storz G, Farr S B, Ames B N. The bacterial adaptation to hydrogen peroxide stress. In: Sies H, editor. Oxidative stress, oxidants and antioxidant. New York, N.Y: Academic Press; 1991. pp. 155–169. [Google Scholar]
- 33.Tolendano M B, Kullik I, Trinh F, Baird P T, Scheider T D, Storz G. Redox-dependent shift of OxyR-DNA contacts along an extended DNA binding site: a mechanism for differential promoter selection. Cell. 1994;78:897–909. doi: 10.1016/s0092-8674(94)90702-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Dhandayuthapani S, Deretic V. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc Natl Acad Sci USA. 1996;93:13212–13216. doi: 10.1073/pnas.93.23.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]