Abstract
Stalk formation is a novel pattern of multicellular organization. Yeast cells which survive UV irradiation form colonies that grow vertically to form very long (0.5 to 3.0 cm) and thin (0.5 to 4 mm in diameter) multicellular structures. We describe the conditions required to obtain these stalk-like structures reproducibly in large numbers. Yeast mutants, mutated for control of cell polarity, developmental processes, UV response, and signal transduction cascades were tested and found capable of forming stalk-like structures. We suggest a model that explains the mechanism of stalk formation by mechanical environmental forces. We show that other microorganisms (Candida albicans, Schizosaccharomyces pombe, and Escherichia coli) also form stalks, suggesting that the ability to produce stalks may be a general property of microorganisms. Diploid yeast stalks sporulate at an elevated frequency, raising the possibility that the physiological role of stalks might be disseminating spores.
In response to stress conditions, usually starvation and dryness, cells of some unicellular organisms (e.g., myxobacteria [10], filamentous fungi [15, 16], and the social amoeba Dictyostelium discoideum [9]) aggregate and differentiate to form structures such as stalks (conidiophores) and fruiting bodies (conidia). Elevation of cells above a surface may increase the efficiency of their dissemination. Diploid yeast cells also sporulate (following meiosis) under stress conditions, but unlike myxobacteria, filamentous fungi, and D. discoideum, they are not thought to have a device for disseminating their spores. In this report we show that yeast cells can form stalk-like structures (Fig. 1).
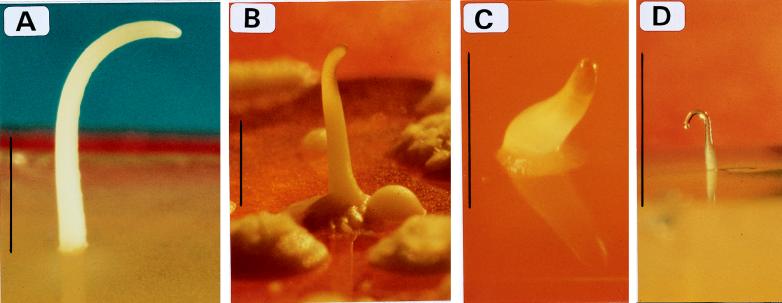
FIG. 1.
Examples of S. cerevisiae stalks. (A) Stalks of strain 419H; (B to D) stalks of the dun1Δ strain. Cells (2 × 106) were plated on SSP as described in the text. The plate in panel A was exposed to UV dose of 100 J/m2, and plates in panels B to D were exposed to 50 J/m2. Photographs were taken 4 days after irradiation. Bars, 0.5 cm.
The formation of stalk-like structures by strains of Saccharomyces has been observed as a rare event in many laboratories. We noticed that a dense lawn of cells produced a higher frequency of stalks after UV irradiation. To identify the parameters controlling the formation of stalks, and to optimize these conditions, we measured the effects of changing the UV dose, cell concentration, agar concentration, and composition and quality of the media. A high agar concentration (above 3.5%) dramatically increases the frequency of stalks. For example, we plated 2 × 106 cells on 2 and 4% agar plates and subsequently irradiated them with an identical UV dose. Similar rates of killing were observed on both types of plates. On 2% agar plates stalks appeared very rarely (<0.01% of the surviving colonies), whereas on the 4% agar plates 10 to 95% of the colonies formed stalks (the frequency depends upon the UV dose; see below and Fig. 2). The higher agar concentration gave more stalks on a variety of media (YPD, SC, and SD [14]) and with several different laboratory strains (SP1 [2], KY2002 [2], H4 [6], S288C [14], Y202 [18], SE6210 [17], A364A and W303 [both obtained from the Yeast Genetic Stock Center, University of California, Berkeley], L5366 [isogenic to Σ1287] [13], and 419H [see genotype below; G. Simchen’s stock]). Also, different lots of two types of agar (Bacto Agar and granular agar, both from Difco) were tested and all gave identical results. Dry plates (prepared by placing them with the lid removed at 37°C for 30 to 60 min prior to the plating of cells and UV irradiation) increased stalk formation fivefold.
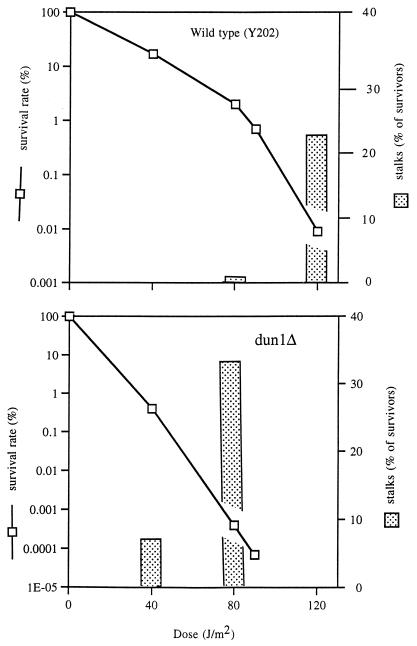
FIG. 2.
The optimal UV dose for stalk production of a given strain is inversely correlated with the UV sensitivity of that strain. An example is given for the UV-sensitive strain (dun1Δ strain) and its isogenic wild type (Y202). Cultures of both strains (2 × 106 cells in each sample) were plated on SSP which were subsequently UV irradiated with the indicated doses. Plates were incubated at 30°C. Colonies and stalks were counted 4 days later.
Although high agar concentrations and dryness are very important for stalk formation, they are not sufficient. In the absence of UV no induction of stalks was observed. Analysis of the frequency of stalk induction in many strains leads to the conclusion that the optimal UV dose for stalk formation is the dose required to kill about 99.95% of the cells. Therefore, the optimal UV dose for stalk production of a given strain is inversely correlated with the UV sensitivity of that strain; for a UV-sensitive strain, high stalk frequency is obtained at a low UV dose. This correlation is demonstrated in Fig. 2: for the UV-sensitive dun1Δ strain (18), low UV doses induce stalk formation quite efficiently. However, much higher doses are required to obtain stalks of the isogenic wild-type strain.
No stalks are observed if the culture is irradiated in solution (medium or water) prior to plating. Also, stalks have never been observed if plates are irradiated prior to plating of cells.
We standardized conditions as follows: experiments were carried out on “standard stalk plates” (SSP), which consist of well-dried medium containing 4% agar that has been coated with a lawn of 2 × 106 yeast cells. Using SSP with UV irradiation, we could obtain plates on which the majority of the surviving colonies were stalks. The maximum percentage of stalks occurs when the number of surviving colonies is very low (about 0.002% survival rate). However, even under optimal conditions, the frequency of stalks varies from experiment to experiment (0.05 to 95% of the survivors). The inability to obtain a uniform population of stalks impeded any hunt for mutants.
We tested whether chemical mutagens or toxins could be as effective as UV in the induction of stalks. SSP were supplemented with various concentrations of ethyl methanesulfonate (from 0.2 to 5.0 mg/ml), 4-nitroquinoline N-oxide (from 0.05 to 2.0 μg/ml), or hydroxyurea (from 0.6 to 26 mg/ml). Although killing rates in those plates were similar to those obtained with UV (between 1 and 99.9% depending on the drug concentration), no stalks appeared. Many stalks appeared on UV-irradiated control plates. These experiments have been repeated with several yeast strains with identical results. UV radiation of a dense lawn appears to be essential for the elevated frequency of stalk formation.
We examined the structure of stalks on SSP. Most stalks are similar in overall appearance (Fig. 1A; a typical stalk is shown in Fig. 1B). Some (∼10%) develop an unusual structure in their tip (e.g., Fig. 1C) while others (10 to 70%, depending on the strain) develop a ball-like tip (Fig. 1A). Most of the stalks grow singly, but about 20% grow in clusters of two stalks or more (Fig. 1D). Typical stalks continue to grow for about a week and reach an average length of about 1 cm (some are 3 cm long). Stalks grow rapidly for the first 36 h after irradiation, during which about 75% of the maximal length is obtained.
Microscopic analysis of stalks revealed that the cells were in the yeast form (no indication of pseudohyphal growth). A single stalk consists of 0.5 × 106 to 3 × 106 cells. Most of the cells are unbudded (>95% in a week-old stalk) and appear to be arrested in G1. Viability tests (replating of stalk cells) carried out with cells of 4-day-old stalks showed that nearly 100% of the cells are viable. All of these cells retained the genetic markers of the original strain. Some of the stalks made from diploid strains contained asci, suggesting that some of the stalk cells underwent meiosis and sporulation on SSP medium (Table 1). As observed by both light and electron microscopy, asci obtained from stalks are identical to asci obtained on SPO medium (14) (not shown). To estimate the level of sporulation on stalks, we used the diploid strain 419H (MATa/MATα ade2/ade2-R8 ura3-52/ura3-52 his4-912/HIS4 lys2-201/lys2 trp5-d/TRP5 leu2-3,112/LEU2 his7/HIS7 metX/MET can1/CAN). This CAN1/can1 heterozygote is unable to grow when plated on canavanine, unless it undergoes meiosis to produce haploid spores, one-half of which carry the can1 allele and are therefore resistant to the drug (8). To test for meiosis in strain 419H, we induced stalks on YPD plates and monitored the kinetics of sporulation. High rates of sporulation occur on the stalks relative to control colonies (Table 1). Nevertheless, the sporulation frequency on stalks (11%) is far below the maximal level obtained with this strain on sporulation medium (80%; Table 1).
TABLE 1.
Sporulation on stalksa
| Time (days) | % sporulation
|
|||
|---|---|---|---|---|
| Stalks | Control colonies (from stalk plates) | Colonies from 2% agar plates | SPO medium | |
| 2 | 3.1 | 0 | 0 | 21 |
| 4 | 4.0 | 0.1 | 0 | 60 |
| 5 | 7.0 | 1.5 | 0.3 | 80 |
| 10 | 9.0 | 1.8 | 0.8 | |
| 14 | 11.0 | 4.6 | 2.0 | |
Stalks and colonies (five at each time point) of strain 419H were collected from YPD plates and suspended in water. Equal sample volumes of cells were plated on canavanine-containing medium and on complete medium to determine the kinetic of appearance of Canr colonies. The percent sporulation was calculated from the ratio of Canr colonies to total colonies. Shown are the averages of the five samples. Standard deviations were below 25%. Controls were colonies of more regular appearance which grew on the same plates near stalks. The sporulation frequency on these colonies was lower than that of stalks, but still quite high, maybe a result of dryness and high agar concentration. Also, these control colonies, considered as normal on the irradiated plates, were somewhat more elevated than those on unirradiated plates. Therefore, colonies from unirradiated 2% agar plates were also used as controls. Asci were also counted microscopically with essentially the same results.
Stalk formation is not the consequence of a mutation. First, when stalks are suspended in liquid and replated, the cells from a stalk grew as colonies and failed to generate any stalks. To test whether the stalks arose from a mutation that increased the tendency of a cell to form stalks in response to UV irradiation, we replated and irradiated cells derived from a stalk. We suspended the cells from each of 10 stalks (obtained from the wild-type strain H4) (6), dispersed the cells by agitating them on a vortex mixer, grew them in YPD, spread a sample of the culture (2 × 106 cells) on SSP, and exposed the cells to UV. These cells, obtained from stalks, formed stalks no more frequently than did cells of the progenitor H4 strain.
The oriented growth of the stalk led us to examine the effects of genes involved in the control of cell polarity on stalk formation. We tested bud1, bud2, bud3, ste20, ste11, and ste7 mutants defective in bud site selection and pseudohyphal growth (1, 4, 5, 13) and found that all could give rise to stalk-like structures on SSP. We further tested hog1, pbs2, mpk1, mkk1, mkk2, bck1, ste4, ste5, and yap1 mutants defective in various signal transduction cascades (7, 11, 13); rad6, rad52, rad2, and dun1 mutants defective in DNA repair and checkpoint control (12, 18); ras1, ras2, CRI4, tpk1w, RAS2val19, bcy1, and gcn4 mutants defective in the UV-activated Ras/Gcn4 pathway (2, 3); and mutants defective in cell wall glycosylation and mutants defective in agglutination processes (aga2, agα, aga1, kre6, kre1, pmt1, mnn2, mnn1, and mnn4 mutants). We found that on SSP, UV radiation induced stalk-like structures in all the strains and mutants tested.
Microscopic inspection of whole stalks on plates showed that most stalks are “rooted” inside tiny pits caused by air bubbles in the agar. If these pits are required, then plates lacking these pits should fail to form stalks. Plates with fewer air bubbles were made by flaming the molten agar surface of the SSP medium just before it solidified. SSP with extra bubbles were produced by vigorously shaking the medium prior to pouring. The number of pits in solid plates was related to the presence or absence of bubbles. Plates from which bubbles were removed did not give rise to stalks, whereas those with increased pits gave rise to a high frequency of stalks (5 to 99% depending on the UV dose used). This experiment was repeated with three different strains (H4 [6] and 419H and F1D [3]) on YPD and SD plates, and in all cases no stalks were observed on plates treated to remove bubbles. These results show that the pits in the plates are necessary for stalk formation.
We have tried to simulate the pits made by air bubbles by punching holes in the agar with pipette tips, toothpicks, applicators, and syringe needles of various bore sizes. Yeast cells manipulated into holes made with these implements produced colonies but no stalks. Perhaps pits made by air bubbles are unique in some property (size, shape, or surface structure) that is difficult to reproduce by impaling the agar. If so, then these pits should be foci for stalk development. To test this, we spread 2 × 106 cells on SSP containing pits, allowed the cells to settle (30 min), and then scraped the surface layer of cells off the plates by using a rubber policeman. Miniature stalks (1 to 3 mm in height) appeared at the sites of bubbles, but none of the large stalks (1.0 cm long, or longer) observed by plating and irradiation (Fig. 1) were observed, suggesting that UV irradiation is required to obtain large stalks.
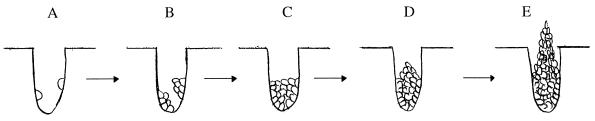
Based on our observations, we suggest a mechanical model for the formation of stalks (depicted in Fig. 3). When a dense layer of cells is spread on a plate, some drop into tiny pits in the agar. Upon heavy UV irradiation, most of the cells on the surface are killed, but some of the cells in the pits survive because they are protected (Fig. 3A). The surviving cells in the pits grow normally (Fig. 3B) until the space below the surviving cells is filled (Fig. 3C). The cells in the center of the colony are not in contact with nutrients and arrest in G1. Those in contact with the walls of the pit continue to divide but are constrained by the surrounding walls. As they divide, they extrude the cells in the center of the colony out of the pit just as toothpaste is squeezed out of the tube (Fig. 3D). The continued extrusion from the base forms the stalk (Fig. 3E). This model has both nutritional and environmental components. UV is required to kill the surface cells so that they do not compete or overgrow the pit cells. The pits, dryness, and agar concentration create the correct geometry and mold for the stalk to form.
FIG. 3.
A model for formation of yeast stalks in response to UV. A side view of a hole in an agar plate is shown. (A) Following UV radiation of a layer of cells, some of those which happen to be in holes survive. The survivors grow normally, form colonies inside the hole (B), and fill the bottom of the hole (C). (D) Cells in contact with medium continue to divide but cannot spread or invade the hard agar. Therefore, cells at the center are pushed. (E) The process continues, with cells at the center of the colony being continuously extruded.
This mechanical model suggested that other unicellular organisms might form stalks under similar conditions. To test this idea, we spread cells of Schizosaccharomyces pombe, Candida albicans, and the bacterium Escherichia coli on SSP and irradiated them. All three organisms were able to form stalks (Fig. 4). The stalks of S. pombe were similar in pattern and size to those obtained with Saccharomyces cerevisiae (Fig. 4A). Those of C. albicans were somewhat different, having a wide base (Fig. 4B). The E. coli stalks were of two types (Fig. 4C and D). Both were very small (1 to 4 mm in height and 0.5 to 2.0 mm in diameter) relative to the yeast stalks.
FIG. 4.
Examples of stalks of S. pombe 972 (A), a C. albicans clinical isolate (B), and E. coli DH5 (C and D). Cells of S. pombe and C. albicans were plated and irradiated on SSP just like S. cerevisiae cells, except that C. albicans cultures and plates were incubated at 37°C. E. coli cells were grown on Luria-Bertani (LB) medium and irradiated on LB plates supplemented with 4% agar. Bars, 0.5 cm.
The biological significance of these stalks is unclear. Strains of yeast with completely different patterns of cell division form stalks, as do several other unrelated microorganisms. These observations combined with the conditions used to produce the stalks led us to propose a completely mechanical mechanism for stalk production. Our observation that the stalk cells of diploid yeast cells show a high frequency of meiotic spores invites a comparison with the aerial structures of other microorganisms, such as social ameobas, filamentous fungi, and bacteria. In these organisms the stalks aid in the dissemination of spores to new substrates. Perhaps in yeast the stalks also provide a mechanism by which cells are dispersed. The assignment of a function to this process is made difficult because none of the yeast mutants we investigated affect stalk formation. Nevertheless, there may be genes that control the formation of these structures. If so, their identity could help to unravel the role of these structures in yeast biology.
Acknowledgments
We are grateful to David Darom, head of the scientific photography unit of The Hebrew University, who took most of the photos shown in this work. We thank Ami Aronheim, William F. Loomis, Riki Perlman, and Amir Sherman for advice, support, and encouragement during early stages of this study. We are also thankful to numerous colleagues who shared with us their strains, mutants, and thoughts.
Work in D.E.’s laboratory was supported by grants from the Israel Cancer Research Fund and the German-Israel Foundation for Scientific Research and Development. Work in G.R.F.’s laboratory is supported by NIH grant GM3501.
REFERENCES
- 1.Chant J. Cell polarity in yeast. Trends Genet. 1994;10:328–333. doi: 10.1016/0168-9525(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 2.Engelberg D, Klein C, Martinetto H, Struhl K, Karin M. The UV response involving the Ras signaling pathway and AP-1 transcription factors is conserved between yeast and mammals. Cell. 1994;77:381–390. doi: 10.1016/0092-8674(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 3.Fasano O, Crechet J B, De Vendittis E, Zahn R, Feger G, Vitelli A, Parmeggiani A. Yeast mutants temperature-sensitive for growth after random mutagenesis of the chromosomal RAS2 gene and deletion of the RAS1 gene. EMBO J. 1988;7:3375–3383. doi: 10.1002/j.1460-2075.1988.tb03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimeno C J, Ljungdahl P, Styles C A, Fink G R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 5.Gimeno C J, Fink G R. Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulation of fungal development. Mol Cell Biol. 1994;14:2100–2112. doi: 10.1128/mcb.14.3.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harashima S, Hannig E M, Hinnebusch A G. Interaction between positive and negative regulators of GCN4 controlling gene expression and entry into the yeast cell cycle. Genetics. 1987;117:409–419. doi: 10.1093/genetics/117.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irie K, Takase M, Lee K S, Levin D E, Araki H, Matsumoto K, Oshima Y. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homolog, function in the pathway mediated by protein kinase C. Mol Cell Biol. 1993;13:3076–3083. doi: 10.1128/mcb.13.5.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassir Y, Simchen G. Monitoring meiosis and sporulation in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:94–110. doi: 10.1016/0076-6879(91)94009-2. [DOI] [PubMed] [Google Scholar]
- 9.Kessin R H, Van Lookeren Campagne M M. The development of social amoeba. Sci Am. 1992;80:556–565. [Google Scholar]
- 10.Kim S K, Kaiser D. Control of cell density and pattern by intracellular signaling in Myxococcus development. Annu Rev Microbiol. 1992;46:117–139. doi: 10.1146/annurev.mi.46.100192.001001. [DOI] [PubMed] [Google Scholar]
- 11.Lee K S, Irie K, Gotoh Y, Watanabe Y, Araki H, Nishida E, Matsumoto K, Levin D. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol Cell Biol. 1993;13:3067–3075. doi: 10.1128/mcb.13.5.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash S, Sung P, Prakash L. DNA repair genes and proteins of Saccharomyces cerevisiae. Annu Rev Genet. 1993;27:33–70. doi: 10.1146/annurev.ge.27.120193.000341. [DOI] [PubMed] [Google Scholar]
- 13.Roberts R L, Fink G R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 14.Rose M D, Winston F, Hieter P. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 15.Springer M L, Yanofsky C A. A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 1989;3:559–571. doi: 10.1101/gad.3.4.559. [DOI] [PubMed] [Google Scholar]
- 16.Timberlake W E. Molecular genetics of Aspergillus development. Annu Rev Genet. 1990;24:5–36. doi: 10.1146/annurev.ge.24.120190.000253. [DOI] [PubMed] [Google Scholar]
- 17.Wiederrecht G, Seto D, Parker C S. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988;54:841–853. doi: 10.1016/s0092-8674(88)91197-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, Elledge S J. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75:1119–1127. doi: 10.1016/0092-8674(93)90321-g. [DOI] [PubMed] [Google Scholar]