Abstract
The influence of extracytoplasmic proteases on the resistance of Escherichia coli to the antimicrobial peptide protamine was investigated by testing strains with deletions in the protease genes degP, ptr, and ompT. Only ΔompT strains were hypersusceptible to protamine. This effect was abolished by plasmids carrying ompT. Both at low and at high Mg2+ concentrations, ompT+ strains cleared protamine from the medium within a few minutes. By contrast, at high Mg2+ concentrations, protamine remained present for at least 1 h in the medium of an ompT strain. These data indicate that OmpT is the protease that degrades protamine and that it exerts this function at the external face of the outer membrane.
In animals, bacteria are often exposed to defensins and other cationic antimicrobial peptides produced by the host (6, 11, 12). Groisman and coworkers have introduced the strongly cationic antimicrobial peptide protamine as a model compound for the action of defensins on cells of Salmonella typhimurium (9, 18, 19). The site of action of protamine is the cytoplasmic membrane, where it causes membrane permeabilization, possibly by creating a large pore in the membrane (3, 10, 20). At low protamine doses, growing cells of Escherichia coli slowly recover from protamine treatment (20). This effect is probably due to protamine degradation by cell proteases, since the susceptibility of growing cells of E. coli toward protamine increases by fivefold in a strain lacking the extracytoplasmic proteases DegP, OmpT, and protease III (20). Here, we examine which of the above proteases is responsible for protamine inactivation and whether this inactivation is due to protamine degradation. For this purpose, we used strains possessing only a single extracytoplasmic protease (16) and employed high-performance liquid chromatography (HPLC) to monitor the fate of protamine in the medium of growing E. coli cells. We show that OmpT is the protease responsible for protamine inactivation by degradation of the toxic peptide at the external face of the cell envelope. Our data suggest that a major function of OmpT is to protect the cells against antimicrobial cationic peptides from the medium.
The strains and plasmids used in this study are listed in Table 1. Studies of protamine susceptibility of E. coli cells growing in shaking culture flasks were carried out as described elsewhere (20). Peptide contents and protamine concentrations in the supernatant of centrifuged cells were determined by reverse-phase HPLC separation on a C18-column with an acetonitrile-H2O gradient in a 140 apparatus from Applied Biosystems (Foster City, Calif.). The N-terminal sequences of proteins or peptides were determined by microsequencing after automatic Edman degradation in an ABI-473A Instrument (Perkin-Elmer Applied Biosystems, Weiterstadt, Germany). Protamine sulfate (from salmon milt; Calbiochem, Bad Soden, Germany) is a mixture of compounds (2). One of its major compounds, salmine AI, has the primary sequence MPRRRRSSSRPVRRRRRPRVSRRRRRRGGRRRR (1).
TABLE 1.
E. coli K-12 strains and plasmids used in this study
| Strain or plasmida | Genotype or property | Reference |
|---|---|---|
| KS272 | F− ΔlacX74 galE galK thi rpsL ΔphoA | 16 |
| KS474 | KS272 degP | 16 |
| SF103 | KS272 ptr | 16 |
| SF110 | KS272 degP ompT | 16 |
| SF115 | KS272 degP ptr | 16 |
| SF120 | KS272 depP ptr ompT | 16 |
| UT5600 | F−ara-14 leuB6 azi-6 lacY1 proC14 tsx67 Δ(ompT-fepC266) entA403 trpE38 rfbD1 rpsL109 xyl-5 mtl-1 thi-1 | 4 |
| pDS319 | Tetr; carries an ompT containing 2-kb E. coli chromosomal EcoRI-PstI fragment ligated with the large EcoRI-PstI fragment from plasmid pBR322 | |
| pML19 | Ampr; carries an ompT containing 2-kb E. coli chromosomal EcoRI-PstI fragment ligated with the large EcoRI-PstI fragment from plasmid pUC19 | 7, 8 |
The source for all strains and plasmids was G. Georgiou.
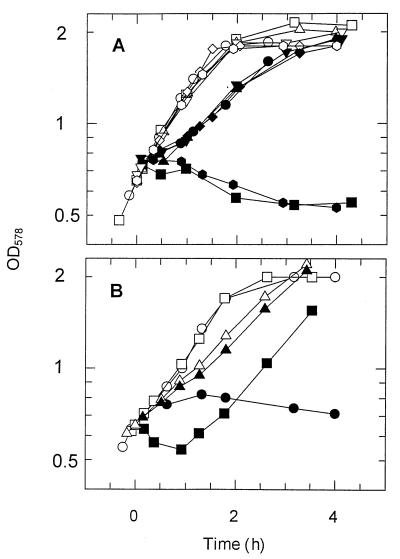
Protamine susceptibility of strains lacking extracytoplasmic proteases was tested in growth experiments (20). Protamine was added at a concentration of 100 mg/liter to a cell suspension with an optical density at 578 nm (OD578) of 0.65, and the cells were grown in a minimal mineral medium containing 20 mM K+ ions with 10 mM glucose as the carbon source. Figure 1A shows that strain SF115 lacking protease III and DegP but not OmpT was as susceptible as the wild-type strain. These strains resumed growth at approximately 1 h after the addition of the toxic peptide. In contrast, the ompT strains SF110 and SF120 did not recover from protamine treatment within 6 h (Fig. 1A). Mutations in either extracytoplasmic protease gene ptr or degP in an ompT+ background exerted no effect (strains SF103, SF115, and KS474 [Fig. 1A]). This suggests that OmpT alone is sufficient for protection of cells against the toxic peptide. Further information was obtained with the nonisogenic ΔompT strain UT5600 (4). As expected, this strain was hypersusceptible to protamine (Fig. 1B, closed circles). However, two types of ompT-containing plasmids rescued these cells. Cells harboring plasmid pML19 (an ompT-containing derivative of pUC19 [7, 8]) grew relatively slowly (Fig. 1B, closed triangles). Addition of protamine to these cells hardly exerted any effect on cell growth (Fig. 1B, triangles). The poor growth of UT5600(pML19) is due to the overexpression of OmpT from the high-copy-number pML19 plasmid. Therefore, we also tested the effect of ompT expression from the lower-copy-number plasmid pSD319, which is a pBR332 derivative. Strain UT5600(pSD319) still grew more slowly than did the control strain UT5600(pBR322) (data not shown). Moreover, addition of protamine to cells of strain UT5600(pSD319) led to considerable cell lysis, as indicated by the trough in the growth curve of this strain (Fig. 1B). This effect was only partially due to the presence of tetracycline in the growth medium of this strain and did not occur after protamine addition to cells of strain UT5600(pBR322) growing under similar conditions (data not shown). Still, the presence of ompT on the plasmid rescued the cells of strain UT5600(pSD319), since these cells resumed growth after approximately 2 h (Fig. 1B), whereas the control culture of strain UT5600(pBR322) did not resume growth within 5 h (data not shown). Taken together, the data from Fig. 1 show that OmpT is the extracytoplasmic protease that protects E. coli cells against protamine.
FIG. 1.
Effect of ompT and other extracytoplasmic protease genes on protamine susceptibility of E. coli cells. Protamine (100 mg/liter) was added at t = 0 to a suspension of growing cells. Closed symbols, protamine-treated cells; open symbols, control cells. (A) Circles, strain KS272; squares, strain SF110; triangles, strain SF103; diamonds, strain KS474; inverted triangles, strain SF115; and octagons, strain SF120; (B) circles, strain UT5600; triangles, strain UT5600(pML19); and squares, strain UT5600(pDS319). The last two strains grew in the presence of 100 mg of carbenicillin and 12.5 mg of tetracycline per liter, respectively.
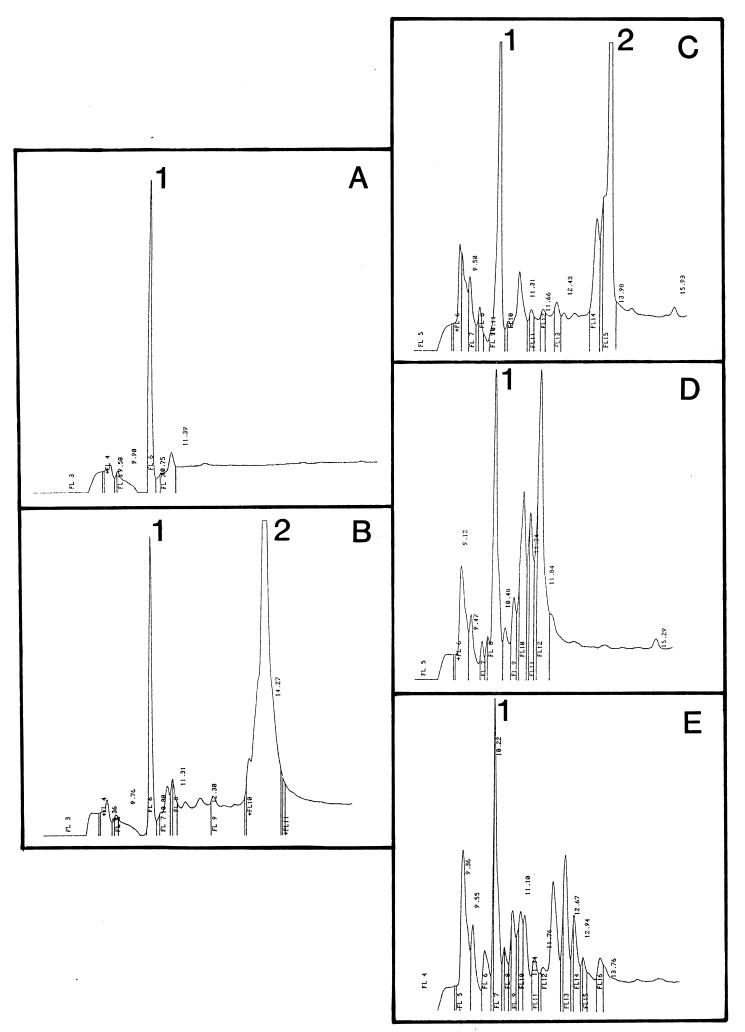
More information about the fate of protamine in the cell suspension came from HPLC analysis of the peptide contents of supernatants from centrifuged cells. Growth medium contained several compounds, including thiamine, with signals in the HPLC chromatogram, with a retention time of 10 to 11 min (Fig. 2A, peak 1). Addition of 100 mg of protamine per liter to this medium gave a major additional peak with a retention time centered at 13 to 14 min (Fig. 2B, peak 2). The form of this peak confirms that protamine consists of a mixture of compounds (2). Addition of protamine to a suspension of growing cells of the ompT+ strain KS272 led to a rapid removal of the toxic peptide from the medium (Fig. 2, traces C to E). After 75 s, the protamine peak was already much smaller (Fig. 2C), and at 450 s, it had vanished from the medium (see Fig. 4D). Several new products appeared in the medium (Fig. 2D and E). Below, we show that some of them were protamine degradation products. From these data, it is concluded that the cells removed protamine with a half-life of 1 to 2 min from the medium. However, we cannot differentiate whether this removal from the medium represents protamine adsorption to the cells (10, 23), proteolysis of the compound, or both. The ΔompT strain SF110 removed protamine as rapidly from the medium as did strain KS272 (data not shown), suggesting that adsorption to the outer membrane is important in the process of protamine removal from the medium.
FIG. 2.
Protamine vanishes rapidly from the medium of growing cells of E. coli KS272. The peptide content of the supernatant from centrifuged cells was analyzed by reverse-phase HPLC. (A) Growth medium; (B) growth medium plus 100 mg of protamine per liter; (C to E) chromatograms of the supernatants taken at 75 s, 7 min and 30 s and 30 min, respectively, after addition of 100 mg of protamine per liter to the cell suspension at time zero. Peaks 1 and 2 represent thiamine and protamine, respectively.
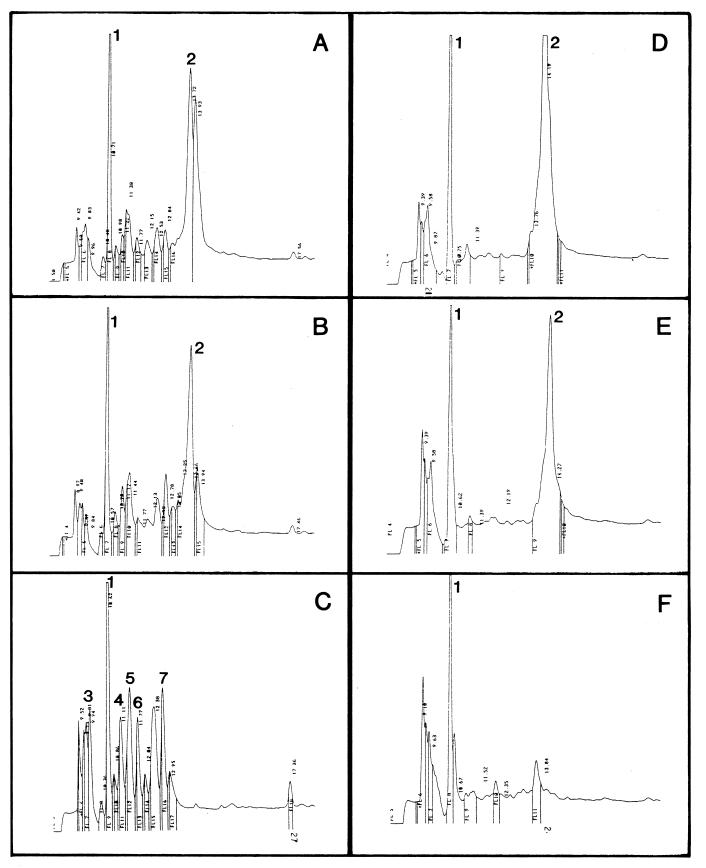
FIG. 4.
Fate of protamine in the high-Mg2+ medium of cells of strains KS272 and SF110. Protamine (100 mg/liter) was added at time zero to the suspension of cells growing at 5 mM Mg2+. (A to C) HPLC chromatograms of the supernatant of cells of strain KS272 centrifuged at 3 min, 7 min and 30 s, and 60 min, respectively, after addition of protamine to the suspension; (D to F) HPLC chromatograms of the supernatant of cells of strain SF110 centrifuged at 30, 60, and 180 min, respectively, after addition of protamine to the medium. Peaks 1 and 2 are the same as those described in the legend to Fig. 2. The N termini of peptides from peaks 3 to 7 in panel C were determined by Edman degradation (see the text for details).
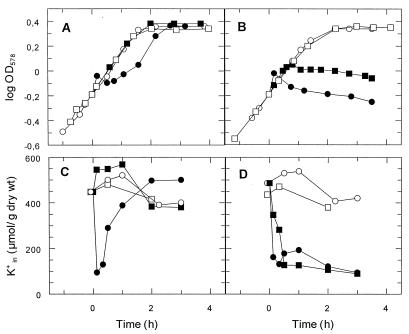
High Mg2+ concentrations prevent adsorption of protamine to the cell wall (10, 23). In order to obtain more information on the fate of protamine under these conditions, the experiments were repeated at 5 mM Mg2+. At this [Mg2+], protamine did not inhibit cell growth of the ompT+ strain KS272 (Fig. 3A). Moreover, these cells did not lose K+ (Fig. 3C), suggesting that under these conditions protamine did not reach the cytoplasmic membrane. By contrast, at 5 mM Mg2+, growth of the ΔompT strain SF110 slowed down gradually and stopped completely at approximately 1 h after protamine addition (Fig. 3B). These cells lost K+, although much more slowly than at 0.4 mM Mg2+ (half-lives for K+ loss, 20 min and a few minutes, respectively [Fig. 3D]), suggesting that at 5 mM Mg2+, protamine slowly reached the cytoplasmic membrane of the ompT strain.
FIG. 3.
High concentration of Mg2+ protects ompT+ cells from toxic effects of protamine. Protamine (100 mg/liter) was added at time zero to growing cells of the wild-type strain KS272 (A and C) or the ompT strain SF110 (B and D). (A and B) Growth curves; (C and D) K+ contents. Closed symbols, protamine-treated cells; open symbols, control cells; circles, cells growing at 0.4 mM Mg2+; and squares, cells growing at 5 mM Mg2+.
Figure 4A to C shows that at 5 mM Mg2+, protamine removal by strain KS272 is almost as rapid as that in the absence of Mg2+ (half-lives of 1 to 2 min in both cases [Fig. 4A to C and Fig. 2, respectively]). At high Mg2+ concentrations, several new peptides with retention times of between 9.20 and 12.67 min were formed (e.g., Fig. 4C). These compounds remained at constant concentrations for a period of 3 h in the medium (data not shown). The peptides from peaks 3 to 7 in Fig. 4C all contained several arginines at their N terminus, suggesting that they are protamine degradation products. Peptide 6 in Fig. 4C had an N-terminal sequence, XSSRRPVRRR, indicating that it is similar to protamine 2c from rainbow trout (15). It was not yet known that salmon protamine contains this type of compound. These data suggest that at 5 mM Mg2+, protamine degradation products do not adsorb to the cell wall of the ompT+ strain.
Protamine removal from the medium by the ΔompT strain SF110 was extremely slow (half-life of approximately 30 to 60 min [Fig. 4D to F]). Moreover, the protamine hydrolysis products observed at high Mg2+ concentrations with strain KS272 did not appear in the medium of strain SF110 (Fig. 4D to F). We assume that this removal of protamine from the medium represents uptake by the cells, since its time course correlated well with inhibition of growth and K+ loss from these cells (Fig. 3B and D, respectively). It is not known whether under these conditions protamine is degraded at all and, if this is the case, in which cellular compartment proteolysis occurs.
Since protamine remained in the medium at high Mg2+ concentrations with the ΔompT strain (Fig. 4D to F), we assume that under these conditions the major portion of protamine is not rapidly adsorbed by the cells. The observations that under these conditions the isogenic ompT+ strain removed protamine rapidly from the medium and that its degradation products appeared in the medium (Fig. 4A to C) suggest that OmpT cleaves protamine at the external surface of the outer membrane. This idea, i.e., that the active center of OmpT is directed outward, is supported by observations that in intact E. coli cells OmpT degrades T7 RNA polymerase added to the medium (8) and that it activates plasminogen (13). However, the question concerning the orientation of the active site of OmpT in the outer membrane remains controversial, since other data suggest that OmpT is active in the degradation of some periplasmic proteins that come into contact with the outer membrane (17).
OmpT is known to degrade peptides between cationic residues and between cationic residues and several apolar residues (8, 14, 17, 21, 22). Therefore, it was not unexpected that OmpT is the extracytoplasmic protease that inactivates the highly cationic peptide protamine (Fig. 1, 2, and 4). We have preliminary evidence that, in addition to protamine, OmpT detoxifies the cationic peptide melittin (data not shown). Moreover, OmpT is essential for the virulence of E. coli strains in the human urinary tract (5). We speculate that in this situation OmpT is active in the degradation of cationic peptides (defensins) excreted by epithelial cells from the urinary tract. However, at present no data that either support or contradict this hypothesis are available.
Acknowledgments
Eva Uhlemann and Eva Limpinsel are thanked for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB171, Teilprojekte B5 and C1) and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Ando T, Watanabe S. A new method for fractionation of protamines and the amino acid sequences of one compound of salmine and three compounds of iridine. Int J Protein Res. 1969;1:221–224. doi: 10.1111/j.1399-3011.1969.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 2.Ando T, Yamasaki M, Suzuki S. Protamines; isolation, characterization and function. New York, N.Y: Springer Verlag; 1973. pp. 58–80. [PubMed] [Google Scholar]
- 3.Aspedon A, Groisman E A. The antibacterial action of protamine: evidence for disruption of cytoplasmic membrane energization in Salmonella typhimurium. Microbiology. 1996;142:3389–3397. doi: 10.1099/13500872-142-12-3389. [DOI] [PubMed] [Google Scholar]
- 4.Elish M E, Pierce J R, Earhart C F. Biochemical analysis of spontaneous fepA mutants of Escherichia coli. J Gen Microbiol. 1988;134:1355–1364. doi: 10.1099/00221287-134-5-1355. [DOI] [PubMed] [Google Scholar]
- 5.Foxman B, Zhang L, Palin K, Tallmann P, Marrs C F. Bacterial virulence characteristics of Escherichia coli isolates from first time urinary tract infection. J Infect Dis. 1995;171:1514–1521. doi: 10.1093/infdis/171.6.1514. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Biosynthesis of defensins and other antimicrobial peptides. Ciba Found Symp. 1994;186:62–76. doi: 10.1002/9780470514658.ch4. [DOI] [PubMed] [Google Scholar]
- 7.Gayda R C, Markovitz A. Cloned DNA fragment specifying major outer membrane protein a in Escherichia coli K-12. J Bacteriol. 1978;136:369–380. doi: 10.1128/jb.136.1.369-380.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grodberg J, Dunn J J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988;170:1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groisman E A, Parra-Lopez C, Salcedo M, Lipps C J, Heffner F. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11939–11943. doi: 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen C, Verheul A, Gram L, Abee T. Protamine-induced permeabilization of cell envelopes of gram-positive and gram-negative bacteria. Appl Environ Microbiol. 1997;63:1155–1159. doi: 10.1128/aem.63.3.1155-1159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreil G. Antimicrobial peptides from amphibian skin: an overview. Ciba Found Symp. 1994;186:77–79. doi: 10.1002/9780470514658.ch5. [DOI] [PubMed] [Google Scholar]
- 12.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 13.Lundrigan M D, Webb R M. Prevalence of OmpT among Escherichia coli isolates from human origin. FEMS Microbiol Lett. 1992;97:51–56. doi: 10.1016/0378-1097(92)90362-r. [DOI] [PubMed] [Google Scholar]
- 14.Maurer J, Jose J, Meyer T F. Autodisplay: one component system for efficient surface display and release of soluble recombinant proteins from Escherichia coli. J Bacteriol. 1977;179:794–804. doi: 10.1128/jb.179.3.794-804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKay D J, Renaux B S, Dixon G H. Rainbow trout protamines. Amino acid sequence of six distinct proteins from a single testis. Eur J Biochem. 1986;158:361–366. doi: 10.1111/j.1432-1033.1986.tb09759.x. [DOI] [PubMed] [Google Scholar]
- 16.Meerman H J, Georgiou G. Construction and characterization of a set of E. coli strains defective in all known loci effecting the proteolytic stability of secreted recombinant proteins. Bio/Technology. 1994;12:1107–1110. doi: 10.1038/nbt1194-1107. [DOI] [PubMed] [Google Scholar]
- 17.Miller C G. Protein degradation and proteolytic modification. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: ASM Press; 1996. pp. 938–954. [Google Scholar]
- 18.Parra-Lopez C, Baer M T, Groisman E A. Molecular genetic analysis of a locus required for resistance to antimicrobial peptides. EMBO J. 1993;12:4053–4062. doi: 10.1002/j.1460-2075.1993.tb06089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra-Lopez C, Lin R, Aspedon A, Groisman E A. A Salmonella protein that is required for resistance to antimicrobial peptides in Salmonella typhimurium. EMBO J. 1994;13:3964–3972. doi: 10.1002/j.1460-2075.1994.tb06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stumpe S, Bakker E P. Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch Microbiol. 1997;167:126–136. [PubMed] [Google Scholar]
- 21.Sugimura K, Higashi N. A novel outer-membrane-associated protease in Escherichia coli. J Bacteriol. 1988;170:3650–3654. doi: 10.1128/jb.170.8.3650-3654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimura K, Nishihara T. Purification, characterization, and primary structure of Escherichia coli protease VII with specificity for paired basic residues: identity of protease VII and OmpT. J Bacteriol. 1988;170:5625–5632. doi: 10.1128/jb.170.12.5625-5632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaara M, Vaara T. Polycations as outer membrane disorganizing agents. Antimicrob Agents Chemother. 1983;24:114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]