Abstract
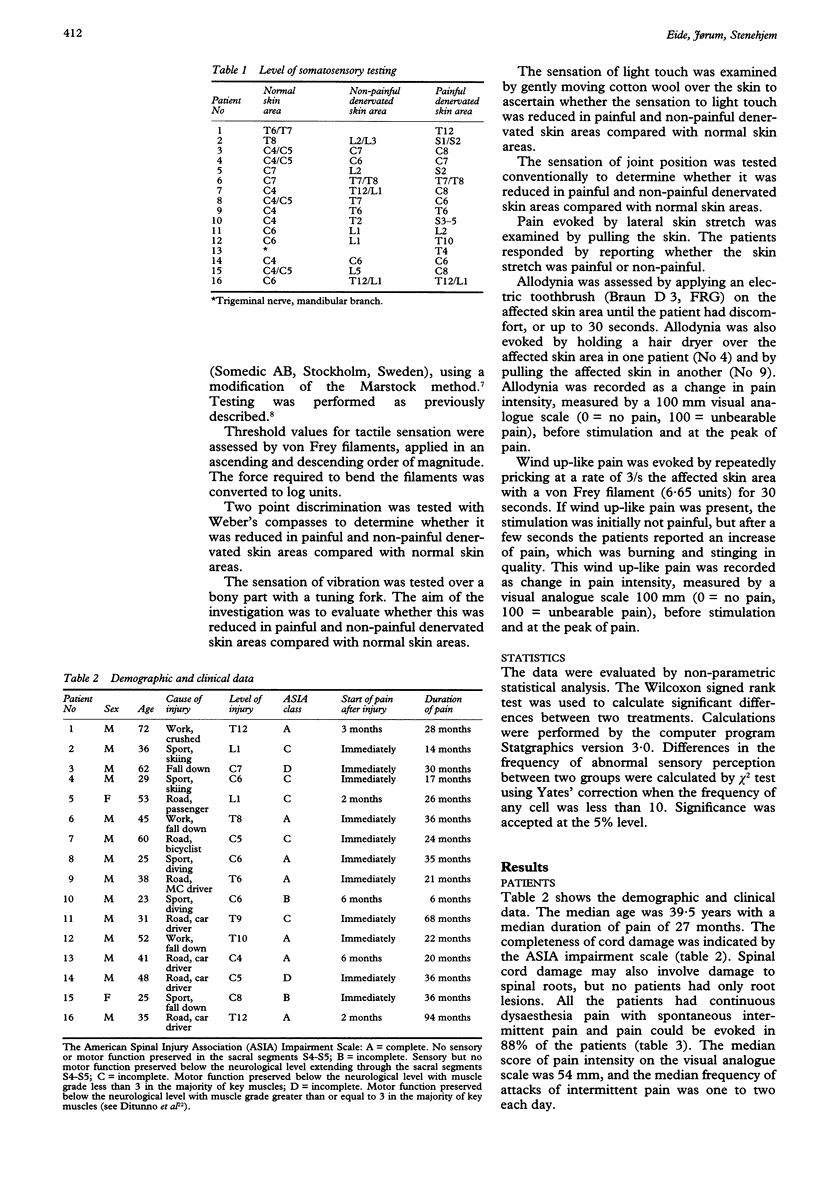
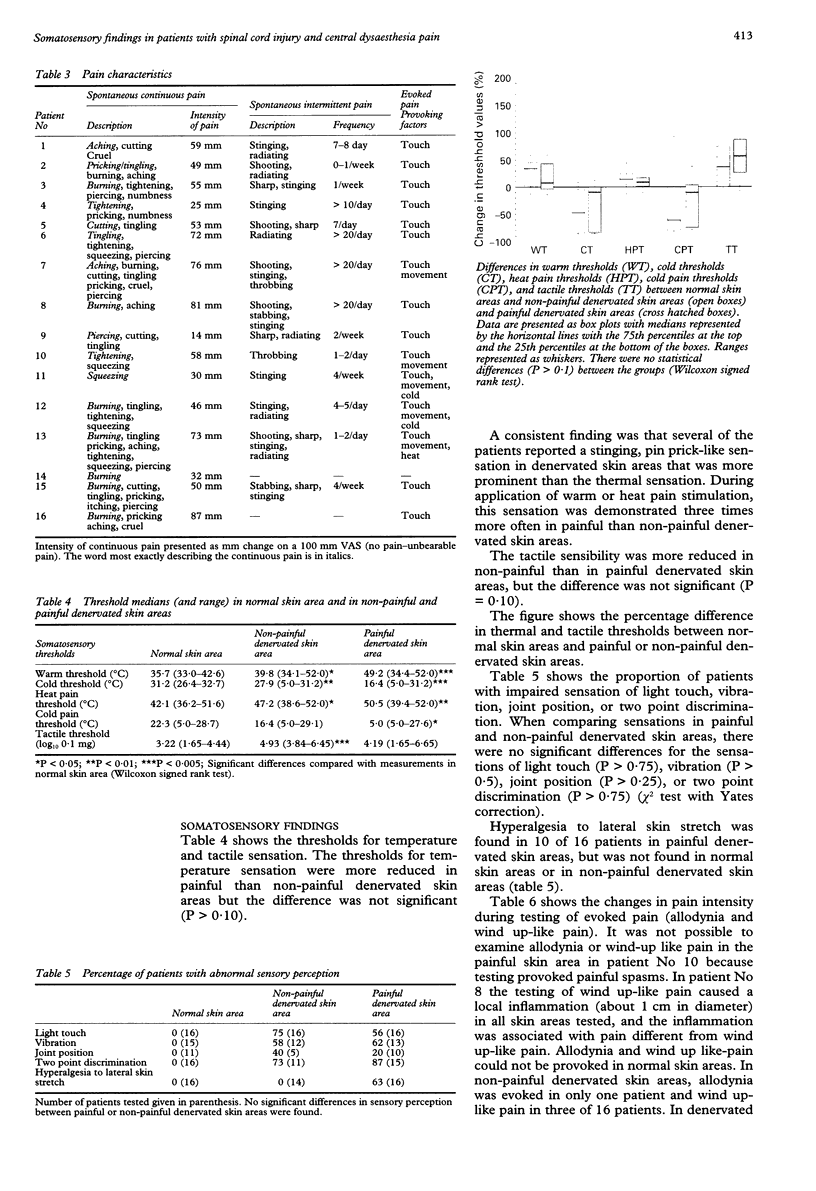
OBJECTIVE: To determine whether central pain in patients with spinal cord injury is only dependent on the lesioning of spinothalamic pathways. METHODS: In sixteen patients with spinal cord injury and central dysaesthesia pain, somatosensory abnormalities in painful denervated skin areas were compared with somatosensory findings in normal skin areas as well as in non-painful denervated skin areas. RESULTS: The threshold values for detection of thermal (heat, cold, heat pain, or cold pain) and tactile stimulation were significantly changed in denervated skin areas although there were no significant differences in the threshold values between painful and non-painful denervated skin areas. The reductions of sensations of touch, vibration, joint position, and two point discrimination in painful and non-painful denervated skin areas were not significantly different. Allodynia (pain caused by non-noxious stimulation) and wind up-like pain (pain caused by repeatedly pricking the skin) were significantly more common in painful than non-painful denervated skin areas. CONCLUSIONS: Because pain and thermal sensory perception are primarily mediated to the brain via spinothalamic pathways, whereas the sensations of touch, vibration and joint position are primarily mediated by dorsal column-medial lemniscal pathways, the results indicate that central pain is not only dependent on the lesioning of either dorsal column-medial lemniscal pathways or spinothalamic pathways. The findings of abnormal evoked pain (allodynia and wind up-like pain) may be consistent with the experimental findings of hyperexcitability in nociceptive spinothalamic tract neurons, that may be involved in the pathogenesis of central pain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berić A., Dimitrijević M. R., Lindblom U. Central dysesthesia syndrome in spinal cord injury patients. Pain. 1988 Aug;34(2):109–116. doi: 10.1016/0304-3959(88)90155-8. [DOI] [PubMed] [Google Scholar]
- Campbell J. N., Raja S. N., Meyer R. A., Mackinnon S. E. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988 Jan;32(1):89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Lodge D. Evidence for involvement of N-methylaspartate receptors in 'wind-up' of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987 Oct 27;424(2):402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Sullivan A. F. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987 Aug;26(8):1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Eide P. K., Jørum E., Stubhaug A., Bremnes J., Breivik H. Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain. 1994 Sep;58(3):347–354. doi: 10.1016/0304-3959(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H., Lindblom U., Schmidt W. C. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976 Nov;39(11):1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbaud G., Benoist J. M., Jazat F., Gautron M. Neuronal responsiveness in the ventrobasal thalamic complex of rats with an experimental peripheral mononeuropathy. J Neurophysiol. 1990 Nov;64(5):1537–1554. doi: 10.1152/jn.1990.64.5.1537. [DOI] [PubMed] [Google Scholar]
- Loeser J. D., Ward A. A., Jr, White L. E., Jr Chronic deafferentation of human spinal cord neurons. J Neurosurg. 1968 Jul;29(1):48–50. doi: 10.3171/jns.1968.29.1.0048. [DOI] [PubMed] [Google Scholar]
- Mariano A. J. Chronic pain and spinal cord injury. Clin J Pain. 1992 Jun;8(2):87–92. doi: 10.1097/00002508-199206000-00005. [DOI] [PubMed] [Google Scholar]
- Price D. D., Bennett G. J., Rafii A. Psychophysical observations on patients with neuropathic pain relieved by a sympathetic block. Pain. 1989 Mar;36(3):273–288. doi: 10.1016/0304-3959(89)90086-9. [DOI] [PubMed] [Google Scholar]
- Tasker R. R., DeCarvalho G. T., Dolan E. J. Intractable pain of spinal cord origin: clinical features and implications for surgery. J Neurosurg. 1992 Sep;77(3):373–378. doi: 10.3171/jns.1992.77.3.0373. [DOI] [PubMed] [Google Scholar]
- Yamashiro K., Iwayama K., Kurihara M., Mori K., Niwa M., Tasker R. R., Albe-Fessard D. Neurones with epileptiform discharge in the central nervous system and chronic pain. Experimental and clinical investigations. Acta Neurochir Suppl (Wien) 1991;52:130–132. doi: 10.1007/978-3-7091-9160-6_35. [DOI] [PubMed] [Google Scholar]



