Abstract
tagA, tagD, and tuaA operons are responsible for the synthesis of cell wall anionic polymer, teichoic acid, and teichuronic acid, respectively, in Bacillus subtilis. Under phosphate starvation conditions, teichuronic acid is synthesized while teichoic acid synthesis is inhibited. Expression of these genes is controlled by PhoP-PhoR, a two-component system. It has been proposed that PhoP∼P plays a key role in the activation of tuaA and the repression of tagA and tagD. In this study, we demonstrated the role of PhoP∼P in the switch process from teichoic acid synthesis to teichuronic acid synthesis, by using an in vitro transcription system. The results indicate that PhoP∼P is sufficient to repress the transcription of the tagA and tagD promoters and also to activate the transcription of the tuaA promoter.
In Bacillus subtilis, the Pho regulon consists of genes whose expression is responsive to phosphate concentration and regulated by the PhoP-PhoR two-component regulator proteins (5, 6, 9, 22). PhoR, a histidine kinase, senses a signal and is autophosphorylated under phosphate starvation conditions, or in the presence of ATP in vitro, and it then transfers the phosphoryl group to PhoP, a response regulator. Phosphorylated PhoP (PhoP∼P) acts as a transcriptional activator or repressor of Pho regulon genes (operons), including phoA, phoB, pstS, phoD, tuaA, tagA, and tagD (1, 2, 8, 9, 11, 13, 20). DNase I footprinting data showed that both PhoP and PhoP∼P bound to a PhoP core binding region located between positions −22 and −60 in all known Pho regulon promoters (11–14), while PhoP∼P also bound to sites in the coding regions of some Pho regulon genes (14).
Products encoded by tuaA, tagA, and tagD operons are involved in anionic cell wall polymer synthesis. The tuaA operon is responsible for synthesis of teichuronic acid, a phosphate-free polymer synthesized during phosphate starvation (3, 4, 24). The tagA and tagD operons are divergently transcribed and are responsible for synthesis of teichoic acid, a phosphate-containing anionic polymer. Under phosphate-limiting conditions, the biosynthesis of teichoic acid ceases and is replaced by the synthesis of teichuronic acid (3, 10), thereby reducing the requirement for cell wall phosphate and saving phosphorus for cellular metabolism and DNA synthesis.
Genetic, promoter fusion, and footprinting data suggested that PhoP-PhoR controlled the switch from synthesis of one kind of anionic polymer to that of another under phosphate starvation conditions (11, 14, 18). Analysis of the cell wall composition in a phoR or phoP mutant strain showed that under these conditions teichuronic acid synthesis was not induced but teichoic acid was synthesized (18). The levels of transcription of the tagA and tagD promoters were dramatically decreased in the parent strain, but these promoters continued to be expressed in either the phoP or phoR strain under phosphate-limiting conditions (11, 17). Expression of the tuaA promoter from the parent strain increased under phosphate starvation conditions (13, 21). There was no tuaA promoter activity in a phoP mutant strain, but the promoter activity in a phoR mutant strain was about 10% of that in the parent strain. These data indicated that both PhoP and PhoR are required for the activation of the tuaA transcription and for the repression of the tagA and tagD promoter transcription under phosphate-limiting conditions. Under phosphate-replete conditions, PhoP and PhoR have no apparent role in the regulation of the tuaA, tagA, and tagD promoters. Binding of PhoP and PhoP∼P to the tuaA and tagA promoters (11, 13) suggested that PhoP-PhoR played a direct role in this transcription regulation. The observations that both PhoP and PhoP∼P bound to the tuaA promoter and that tuaA transcription continued at low levels in the phoR mutant strain raised the question of activation by unphosphorylated PhoP.
Here, we utilized an in vitro transcription system to analyze the roles of PhoP and PhoP∼P in the regulation of the tuaA, tagA, and tagD promoters in vitro. The results indicated that PhoP∼P is essential and sufficient to activate the transcription of tuaA and repress the transcription of tagA and tagD.
PhoP∼P represses both the tagA operon and the tagD operon.
Divergently transcribed tagA and tagD have been cloned on plasmid pSE90 as described previously (11). The transcription start sites of the tagA and tagD operons have been identified (15). In this study, linear DNA fragments were used as templates in in vitro transcription assays. All conditions for the in vitro transcription were the same as those described previously (19). The transcription reaction mixture (20-μl final volume) consisted of 0.08 pmol of template, 5 pmol of PhoP and/or 5 pmol of PhoR, 50 nmol of ATP, and 0.4 pmol of purified B. subtilis RNA polymerase (19). The transcription buffer contained 100 mM potassium glutamate, 10 mM Tris (pH 8.0), 0.1 mM EDTA, 50 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 10 μg of bovine serum albumin per ml, 1 mM dithiothreitol, and 5% glycerol. Either PhoP alone, PhoP and PhoR together, or a PhoP-PhoR-ATP mixture was preincubated with the template at 37°C for 10 min. The RNA polymerase was then added to the reaction mixture, and incubation continued at 37°C for 15 min. A single round of transcription was initiated by the addition of 5 μl of a transcription buffer containing ATP, GTP, and CTP at 100 μM each, 10 μM UTP, 5 μCi of [α-32P]UTP (Amersham), and 50 μg of heparin per ml. After incubation at 37°C for 15 min, reactions were stopped by the addition of 10 μl of loading dye (7 M urea, 100 mM EDTA, 5% glycerol, 0.05% xylene cyanol, and 0.05% [wt/vol] bromophenol blue). Samples were analyzed on 8 M urea–6% polyacrylamide gels. Dried gels were analyzed by a PhosphorImager.
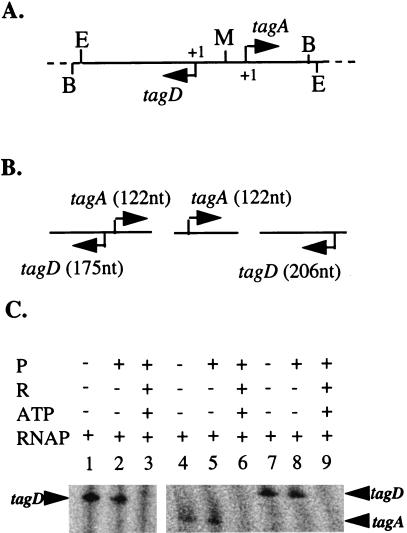
A 503-bp DNA fragment, containing both tagA and tagD promoters, was isolated from pSE90 digested by EcoRI and BamHI and used as a template in in vitro transcription assays (Fig. 1A and B). The results showed that only one transcript, with the expected size of 175 nucleotides (nt), generated from the tagD promoter, was produced in the absence of PhoP or in the presence of unphosphorylated PhoP (Fig. 1C, lanes 1 and 2). It appeared that these two promoters compete for transcription reagents and RNA polymerase in the reaction mixture, suggesting that tagD had a stronger promoter than tagA. This is consistent with in vivo data which showed that the level of transcription of tagD was two- to threefold higher than that of tagA (11, 15, 17).
FIG. 1.
Repression of tagA and tagD transcription by PhoP∼P. (A) Map of the plasmid containing divergently transcribed tagA and tagD promoters. The solid horizontal line represents the promoter sequences. The dashed lines represent pCRII vector sequences. The arrows indicate the gene transcription direction. +1 marks the transcriptional start sites. Restriction sites: B, BamHI; E, EcoRI; M, MunI. (B) Diagram of templates containing tagA and/or tagD promoters. The horizontal lines represent DNA fragments used for templates. The arrows indicate the gene transcription direction. Expected sizes of transcripts from the promoters are indicated in parentheses. (C) In vitro transcription of tagA and tagD promoters. Phosphorylation of PhoP and in vitro transcription reactions were carried out as described in the text. The results of coelectrophoresis of Perfect RNA marker template mix (Novagen, Madison, Wis.) are not shown. P, PhoP; R, PhoR; RNAP, purified B. subtilis RNA polymerase. −, absent; +, present.
pSE90 was digested with BamHI and MunI, resulting in three DNA fragments: one, which contains the tagA promoter region, extending from −82 to +122; a second, which contains the tagD promoter region, extending from −124 to +206 (Fig. 1A and B); and the vector. A transcript of the expected size, 122 nt, was synthesized from the tagA promoter by B. subtilis RNA polymerase, either in the absence of PhoP (Fig. 1C, lane 4), in the presence of PhoP (Fig. 1C, lane 5), or in the presence of PhoP and PhoR without ATP (data not shown). When PhoP was phosphorylated by PhoR in the presence of ATP, no transcript was produced, indicating that PhoP∼P repressed the transcription of tagA promoter (Fig. 1C, lane 6). Similar results were observed with the tagD promoter (Fig. 1C, lanes 7 to 9). A 206-nt transcript was generated in the absence of PhoP or in the presence of unphosphorylated PhoP, while no transcript was observed in the presence of PhoP∼P.
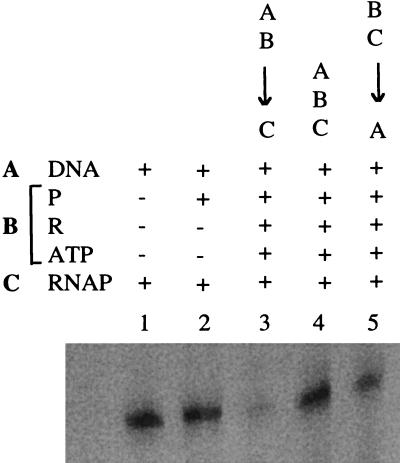
In vivo and in vitro data indicate that PhoP∼P is a repressor of the tagA and the tagD promoters. Although the mechanism of PhoP∼P repression is unknown, the pattern of PhoP∼P binding to these promoters extends from the promoter region well into the coding region of the genes, suggesting that PhoP∼P binding may interfere with the transcription initiation and/or elongation. In order to explore the mechanism of the transcriptional repression, we performed in vitro transcription assays and varied the order of component addition. The templates used in this experiment were DNA fragments containing the tagD promoter. The results showed that PhoP∼P repressed tagD transcription in vitro only when PhoP∼P was first preincubated with template DNA, before the addition of the RNA polymerase (Fig. 2, lane 3). This was the order of component addition used for the reactions in Fig. 1C. No repression was observed when PhoP∼P, RNA polymerase, and DNA were incubated together (Fig. 2, lane 4), and preincubation of the PhoP∼P and RNA polymerase, before addition of DNA, decreased the repression (Fig. 2, lane 5). Figure 2, lanes 1 and 2, shows the transcript produced in the absence of PhoP and in the presence of unphosphorylated PhoP. Combined, these data suggested that PhoP∼P and the RNA polymerase compete for the same binding site in the tagD promoter region and that the binding of PhoP∼P in the promoter region may exclude the RNA polymerase binding to the promoter.
FIG. 2.
Dependence of tagD transcription on RNA polymerase binding before PhoP∼P. Reactions as described for Fig. 1 except that the order of component addition was different and some reaction mixtures were preincubated. DNA, tagD template (Fig. 1B); P, PhoP; R, PhoR; RNAP, purified B. subtilis RNA polymerase. −, absent; +, present; ↓, preincubation at 37°C for 10 min. A, DNA template; B, mixture of PhoP, PhoR, and ATP; C, RNA polymerase. The order of component addition is indicated proceeding from top to bottom above lanes 3 to 5.
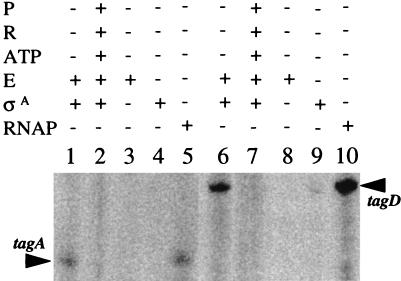
It has been suggested that tagA and tagD are possibly ςA-dependent promoters (16, 23). We performed in vitro transcription experiments using purified B. subtilis RNA polymerase core enzyme (19) and/or purified ςA (provided by John Helmann, Cornell University). The templates used in this experiment were DNA fragments containing either the tagA or the tagD promoter. The reactions were performed in the presence of either unphosphorylated PhoP or PhoP∼P. As shown in Fig. 3, the core enzyme alone was not able to transcribe either the tagA or the tagD promoter (lanes 3 and 8). However, the core enzyme combined with ςA produced the same transcripts as the RNA polymerase holoenzyme (Fig. 3, lane 1 compared to lane 5 and lane 6 compared to lane 10). This transcript was not observed in the presence of PhoP∼P (Fig. 3, lanes 2 and 7), and ςA alone did not transcribe either promoter (Fig. 3, lanes 4 and 9). The data indicate that the tagA and tagD promoters are ςA dependent and that PhoP∼P is sufficient to repress the transcription of the tagA and tagD operons in vitro. These results support the theory that any repression of tag gene expression by a metabolite, dependent on teichuronic acid synthesis (18), must require PhoP, acting through PhoP∼P (11).
FIG. 3.
Core enzyme plus ςA is sufficient for in vitro transcription of the tagA and tagD promoters. Reactions and sample analysis were as described for Fig. 1. RNA markers are not shown. P, PhoP; R, PhoR; RNAP, purified B. subtilis RNA polymerase ςA holoenzyme; E, isolated core enzyme; ςA, purified ςA. −, absent; +, present.
PhoP∼P activates the transcription of tuaA promoter.
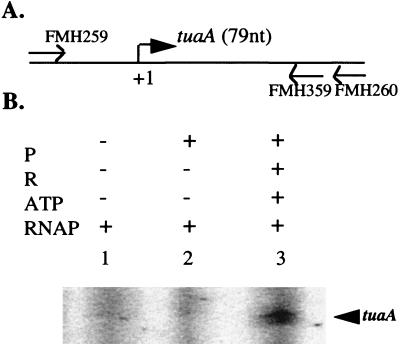
It has been reported that both PhoP and PhoP∼P bound to the tuaA promoter (13). Therefore, low levels of tuaA transcription from a tuaA-lacZ fusion in the phoR mutant strain may have resulted from the activation by unphosphorylated PhoP. To understand how PhoP and PhoP∼P regulate the tuaA promoter in vitro, we carried out in vitro transcription experiments using a PCR product containing the tuaA promoter region as a template. Primers FMH259 and FMH260, described previously (13), were used to amplify a 245-bp DNA fragment containing the tuaA promoter from JH642 chromosomal DNA (Fig. 4A). A transcript was produced in the presence of PhoP∼P (Fig. 4B, lane 3) but not in the absence of PhoP or in the presence of unphosphorylated PhoP (Fig. 4B, lanes 1 and 2), indicating that PhoP∼P is essential for the activation of the tuaA promoter transcription in vitro.
FIG. 4.
Activation of the tuaA transcription by PhoP∼P. (A) Physical map of the tuaA promoter region. The horizontal line represents the DNA fragment used for template. The bent arrow indicates the tuaA transcription direction. +1 marks the transcriptional start site. The expected transcript size of the promoter is indicated in parentheses. (B) In vitro transcription of tuaA promoter. All reactions and sample analysis are as described for Fig. 1. Abbreviations are the same as in the legend for Fig. 1.
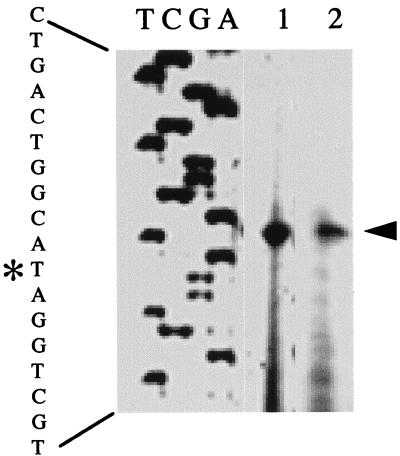
In order to determine if the in vivo and in vitro transcription start sites for the tuaA promoter were identical, we first performed in vivo primer extension experiments as described previously (1). RNA was isolated from the wild-type strain, JH642, grown in low-phosphate defined medium (LPDM) (7) or Luria-Bertani medium until late exponential phase. Primer FMH359 (5′2452CCTCACGATTTGTTTGGGATG2431-3′) was used for mapping the start site. The numbers at the 5′ and 3′ ends of the primer refer to numbers in the cwlB gene with the GenBank database accession no. M61747. A transcription start site was observed for RNA isolated in LPDM (Fig. 5, lane 1) but not in Luria-Bertani medium (data not shown). The tuaA transcriptional start site was 3 bp downstream of the putative tuaA transcriptional start site previously indicated (13). Therefore, the size of the in vitro transcript produced in the presence of PhoP∼P was 79 nt.
FIG. 5.
Primer extension analysis of the tuaA promoter in vivo and in vitro. The end-labeled primer FMH359 was annealed to RNA and then extended with avian myeloblastosis virus reverse transcriptase. Lane 1, RNA isolated from late-exponential-phase cells grown in LPDM; lane 2, RNA synthesized from the in vitro transcription reaction mixture containing PhoP∼P (products of the primer extension reactions are marked by an arrow); lanes A, G, C, and T are a sequencing ladder generated by annealing the same end-labeled primer to a plasmid, pES69, containing the tuaA promoter region (13) and extending it with Sequenase. The sequence of the region is indicated on the left. The asterisk indicates the base to which the primer extension product maps.
The start site of the in vitro-generated transcript was mapped to determine if the in vitro transcription system was initiating correctly from the tuaA promoter. RNA templates for primer extension studies were prepared with the buffer and temperature used for in vitro transcription but with 10-fold-greater amounts of template DNA, RNA polymerase, and PhoP and/or PhoR (e.g., 0.8 pmol of template, 50 pmol of PhoP or a mixture of 50 pmol of PhoP, 50 pmol of PhoR, and 0.5 mmol of ATP, and 4 pmol of RNA polymerase) in a 100-μl volume. The reaction mixture was incubated at 37°C for 15 min. ATP, GTP, CTP, and UTP (250 μM each) were added to a final volume of 125 μl. Heparin (50 μg per ml) was added to a final volume of 150 μl after 30 min. After incubation for 15 min, reactions were stopped by the addition of 5 U of RNase-free DNase I (Boehringer Mannheim), and incubation was continued for 20 min. RNA transcripts from the in vitro transcription reaction, which was carried out with a mixture containing either PhoP or PhoP∼P, were extracted with phenol. The primer extension reaction mixtures were the same as for in vivo primer extension. A transcriptional start site observed in products of reactions carried out with PhoP∼P (Fig. 5, lane 2) corresponded to the start site for tuaA transcription in vivo, indicating that PhoP∼P stimulated the transcription of the tuaA promoter in vitro. There were no products from the reaction with unphosphorylated PhoP (data not shown).
Previous in vitro transcription data showed that PhoP∼P is an activator of the Pho regulon genes phoA and pstS and that the RNA polymerase ςA holoenzyme and PhoP∼P are sufficient for the transcription of both genes in vitro (19). The results of the present study show that PhoP∼P is sufficient to repress both tagA and tagD operons and is essential for the activation of the tuaA operon in vitro. These data suggest that the minor level of transcription from the tuaA promoter in a phoR mutant strain is not due to unphosphorylated PhoP but depends on low levels of PhoP∼P. This conclusion suggests that PhoP can be phosphorylated by other kinases or small phosphodonors, albeit PhoP cannot be phosphorylated by small phosphodonors in vitro (12). It is interesting that among the Pho promoters analyzed to date, the only ones having measurable activity in a phoR mutant strain are the tuaA promoter and the promoter for the pstS operon, the operon encoding components for the high-affinity Pi transport system. Whether this is due to promoter strength or promoters with higher affinity for PhoP∼P, it does indicate that the cell’s first responses to a phosphate deficiency are to take up the remaining Pi from the environment and to start making an anionic cell wall polymer which does not contain phosphate.
Acknowledgments
This work was supported by National Institutes of Health grant GM33471 to F.M.H.
REFERENCES
- 1.Chesnut R S, Bookstein C, Hulett F M. Separate promoters direct expression of phoAIII, a member of the Bacillus subtilis alkaline phosphatase multigene family, during phosphate starvation and sporulation. Mol Microbiol. 1991;5:2181–2190. doi: 10.1111/j.1365-2958.1991.tb02148.x. [DOI] [PubMed] [Google Scholar]
- 2.Eder S, Shi L, Jensen K, Yamane K, Hulett F M. A Bacillus subtilis secreted phosphodiesterase/alkaline phosphatase is the product of a Pho regulon gene, phoD. Microbiology. 1996;142:2041–2047. doi: 10.1099/13500872-142-8-2041. [DOI] [PubMed] [Google Scholar]
- 3.Ellwood D C, Temoest D W. Control of teichoic acid and teichuronic acid biosyntheses in chemostat cultures of Bacillus subtilis var. Niger. Biochem J. 1969;111:1–5. doi: 10.1042/bj1110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellwood D C, Temoest D W. Effect of environment on bacterial wall content and composition. Adv Microb Physiol. 1972;7:83–117. [Google Scholar]
- 5.Hulett F M. Complex phosphate regulation by sequential switches in Bacillus subtilis. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 289–302. [Google Scholar]
- 6.Hulett F M. The signal-transduction network for Pho regulation in Bacillus subtilis. Mol Microbiol. 1996;19:933–939. doi: 10.1046/j.1365-2958.1996.421953.x. [DOI] [PubMed] [Google Scholar]
- 7.Hulett F M, Bookstein C, Jensen K. Evidence for two structural genes for alkaline phosphatase in Bacillus subtilis. J Bacteriol. 1990;172:735–740. doi: 10.1128/jb.172.2.735-740.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulett F M, Lee J, Shi L, Sun G, Chesnut R, Sharkova E, Duggan M F, Kapp N. Sequential action of two-component genetic switches regulates the PHO regulon in Bacillus subtilis. J Bacteriol. 1994;176:1348–1358. doi: 10.1128/jb.176.5.1348-1358.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulett F M, Sun G, Liu W. The Pho regulon of Bacillus subtilis is regulated by sequential action of two genetic switches. In: Torriani-Gorini A, Yagil E, Silver S, editors. Phosphate in microorganisms: cellular and molecular biology. Washington, D. C: American Society for Microbiology; 1994. pp. 50–54. [Google Scholar]
- 10.Lang W K, Glassey K, Archibald A R. Influence of phosphate supply on teichoic acid and teichuronic acid content of Bacillus subtilis cell walls. J Bacteriol. 1982;151:367–375. doi: 10.1128/jb.151.1.367-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Eder S, Hulett F M. Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role for PhoP∼P. J Bacteriol. 1998;180:753–758. doi: 10.1128/jb.180.3.753-758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Hulett F M. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J Bacteriol. 1997;179:6302–6310. doi: 10.1128/jb.179.20.6302-6310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, W., and F. M. Hulett. Comparison of PhoP binding to the tuaA promoter with PhoP binding to other Pho regulon promoters establishes a Bacillus subtilis Pho box. Microbiology, in press. [DOI] [PubMed]
- 14.Liu W, Qi Y, Hulett F M. Sites internal to the coding regions of phoA and pstS bind PhoP and are required for full promoter activity. Mol Microbiol. 1998;28:119–130. doi: 10.1046/j.1365-2958.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 15.Mauël C, Bauduret A, Chervet C, Beggah S, Karamata D. In Bacillus subtilis 168, teichoic acid of the cross-wall may be different from that of the cylinder: a hypothesis based on transcription analysis of tag genes. Microbiology. 1995;141:2379–2389. doi: 10.1099/13500872-141-10-2379. [DOI] [PubMed] [Google Scholar]
- 16.Mauël C, Young M, Karamata D. Genes concerned with synthesis of poly(glycerol phosphate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J Gen Microbiol. 1991;137:929–941. doi: 10.1099/00221287-137-4-929. [DOI] [PubMed] [Google Scholar]
- 17.Mauël C, Young M, Monsutti-Grecescu A, Marriott S A, Karamata D. Analysis of Bacillus subtilis tag gene expression using transcription fusion. Microbiology. 1994;140:2279–2288. doi: 10.1099/13500872-140-9-2279. [DOI] [PubMed] [Google Scholar]
- 18.Muller J P, An Z, Merad T, Hancock I C, Harwood C R. Influence of Bacillus subtilis phoR on cell wall anionic polymers. Microbiology. 1997;143:947–956. doi: 10.1099/00221287-143-3-947. [DOI] [PubMed] [Google Scholar]
- 19.Qi Y, Hulett F M. PhoP∼P and RNA polymerase ςA holoenzyme are sufficient for transcription of Pho regulon promoters in Bacillus subtilis: PhoP∼P activator sites within the coding region stimulate transcription in vitro. Mol Microbiol. 1998;28:1187–1198. doi: 10.1046/j.1365-2958.1998.00882.x. [DOI] [PubMed] [Google Scholar]
- 20.Qi Y, Kobayashi Y, Hulett F M. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the Pho regulon. J Bacteriol. 1997;179:2534–2539. doi: 10.1128/jb.179.8.2534-2539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soldo B, Lazarevic V, Pagnl M, Mauël C, Karamata D. Abstracts for the 8th International Conference on Bacilli. 1995. Transcriptional regulation of teichuronic acid expression; p. 77. Stanford, Calif. [Google Scholar]
- 22.Sun G, Birkey B M, Hulett F M. Three two-component signal-transduction systems interact for Pho regulation in Bacillus subtilis. Mol Microbiol. 1996;19:941–948. doi: 10.1046/j.1365-2958.1996.422952.x. [DOI] [PubMed] [Google Scholar]
- 23.Wagner P M, Stewart G C. Role and expression of the Bacillus subtilis rodC operon. J Bacteriol. 1991;173:4341–4346. doi: 10.1128/jb.173.14.4341-4346.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward J B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981;45:211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]