Abstract
Aim
This study aims to analyze the health‐related quality of life (HRQoL) and safety outcomes in attention‐deficit/hyperactivity disorder (ADHD) patients treated with cannabis‐based medicinal products (CBMPs).
Methods
Patients were identified from the UK Medical Cannabis Registry. Primary outcomes were changes in the following patient‐reported outcome measures (PROMs) at 1, 3, 6, and 12 months from baseline: EQ‐5D‐5L index value, generalized anxiety disorder‐7 (GAD‐7) questionnaire, and the single‐item sleep quality score (SQS). Secondary outcomes assessed the incidence of adverse events. Statistical significance was defined as p < 0.050.
Results
Sixty‐eight patients met the inclusion criteria. Significant improvements were identified in general HRQoL assessed by EQ‐5D‐5L index value at 1, 3, and 6 months (p < 0.050). Improvements were also identified in GAD‐7 and SQS scores at 1, 3, 6, and 12 months (p < 0.010). 61 (89.71%) adverse events were recorded by 11 (16.18%) participants, of which most were moderate (n = 26, 38.24%).
Conclusion
An association between CBMP treatment and improvements in anxiety, sleep quality, and general HRQoL was observed in patients with ADHD. Treatment was well tolerated at 12 months. Results must be interpreted with caution as a causative effect cannot be proven. These results, however, do provide additional support for future evaluation within randomized controlled trials.
Keywords: attention deficit disorder with hyperactivity, cannabidiol, cannabis, psychiatry, tetrahydrocannabinol
Patients prescribed cannabis‐based medicinal products reported improvements in anxiety, sleep, and general health‐related quality of life. Fewer than one in five individuals reported an adverse event in 12 months of therapy.
1. INTRODUCTION
Attention‐deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders, with an estimated global prevalence of 5% in children and 2.5% in adults. 1 , 2 The estimated incidence of ADHD diagnosis has increased by approximately 42% in children between 2003 and 2011, and 123% in adults between 2007 and 2016 in the United States. 3 , 4 , 5 ADHD is characterized by symptoms of inattentiveness, hyperactivity, and impulsiveness causing functional impairment in two or more settings (e.g., work and home). 6 , 7 , 8 ADHD is often associated with psychosocial difficulties, such as relationship problems, unemployment, educational underachievement, and criminality. 9 Moreover, ADHD is also associated with a higher incidence of sleep disturbance and psychiatric co‐morbidities, including anxiety, substance misuse, and depression. 10 As a result, these issues can significantly reduce the quality of life for individuals with ADHD.
Current treatment for ADHD consists of a combination of psychological therapies and both stimulant and non‐stimulant medications. 11 Stimulants are the most commonly prescribed medications for ADHD and target executive and attentional function. 11 , 12 They are considered relatively safe and effective treatments, however, they are commonly associated with decreased appetite, insomnia, emotional dysregulation, irritability, and an increased risk of adverse cardiovascular events. 12 , 13 , 14 Non‐stimulant medications have been shown to reduce ADHD‐related functional impairments and co‐occurring mood disorders. 11 , 13 , 15 Despite their effectiveness, medication adherence rates are relatively low due to the adverse events that are commonly experienced. 15 , 16 , 17 This highlights the need for novel therapeutics for ADHD.
The endocannabinoid system (ECS) plays a vital role in cognitive function, motor coordination, and emotional homeostasis, in addition to the regulation of dopaminergic pathways in the brain. 18 , 19 , 20 , 21 , 22 , 23 The ECS is a signaling network consisting of endocannabinoids, enzymes, and cannabinoid receptors, including cannabinoid type 1 (CB1) receptors and cannabinoid type 2 (CB2) receptors. 21 , 22 Dysregulation in the ECS has been implicated in the pathophysiology of ADHD. 22 , 24 , 25 , 26 , 27 CB1 receptors are widely distributed throughout the central nervous system, with high levels found in regions associated with cognitive functioning and processing, such as the basal ganglia, cerebellum, neocortex, and hippocampus. 23 Anandamide (AEA) is an endogenous ligand of CB1 receptors, activation of which results in the modulation of neurotransmitter release and neuronal plasticity. 28 , 29 Importantly, AEA has been demonstrated to regulate dopamine transmission. 30 Therefore, activation of CB1 receptors directly or through increasing AEA has been postulated as a potential target for managing ADHD. 24 , 31
The flower of the cannabis plant contains many phytocannabinoids, the most abundant of which are (−)‐trans‐Δ9‐tetrahydrocannabinol (THC) and cannabidiol (CBD). THC, similar to AEA, is a partial agonist at CB1 receptors. 32 , 33 CBD is a negative allosteric modulator of CB1 and has been shown to enhance AEA levels indirectly. 34 , 35 , 36 CBD may also modulate gamma‐aminobutyric acid (GABA) activity, a neurotransmitter responsible for the inhibition of neuronal excitability, increasing dopamine production. 35 These phytocannabinoids are active ingredients of cannabis‐based medicinal products (CBMPs). 37 In the United Kingdom, CBMPs can be considered if current licensed treatments have previously been used and have failed to give sufficient benefit and to manage these symptoms. 38
Only one placebo‐controlled randomized controlled trial (RCT) on CBMPs has been conducted to date. Cooper et al. demonstrated a nominal improvement in symptoms of hyperactivity and impulsivity in ADHD patients treated with nabiximols (an oromucosal spray containing THC (2.7 mg/dose) and CBD (2.5 mg/dose)). 39 However, the limited sample size of this study restricts generalizability of the findings. A systematic review investigating cannabis and ADHD found 13 studies reporting improvements in symptoms including concentration, motivation, learning, memory, and impulsivity. 40 However, most studies found no associations or demonstrated negative effects on core ADHD symptoms. 40 , 41 , 42 , 43 Additionally, observational studies have generally identified a relationship between illicit cannabis use and the increased risk of neurocognitive impairment and addiction. 43 , 44 , 45 , 46 , 47 This has also been reflected in evidence from neuroimaging studies of those with ADHD. Structural abnormalities were found in certain brain regions involved with executive function and reward processing, particularly those with heavy cannabis use. 48 , 49 , 50 Interestingly, Silva et al. have presented neurobiological evidence for the tendency of ADHD individuals to self‐medicate with cannabis to alleviate their symptoms, with increased vulnerability to developing cannabis use disorder. 47 , 50 , 51
There is a paucity of high‐quality clinical evidence and prospective studies on CBMPs in the treatment of ADHD‐related symptoms. Importantly, there are no published clinical studies of long‐term outcomes to assess the efficacy and safety of adult patients treated with CBMPs. Herein, the primary aim of this study is to report the outcomes of patients who are prescribed CBMPs and have enrolled in the UK Medical Cannabis Registry (UKMCR) for the treatment of ADHD to evaluate changes in health‐related quality of life (HRQoL) and adverse event incidence associated with CBMPs to determine the safety of their use.
2. METHODS
2.1. Study design and database
An uncontrolled case series of patients from the UKMCR of patients prescribed CBMPs for ADHD was extracted and analyzed. Participants were requested to complete questionnaires about patient‐reported outcome measures (PROMs) at baseline and after 1, 3, 6, and 12 months, as well as adverse events.
The UKMCR has collected prospective, clinical data on patients treated with CBMPs since 2019, and is privately owned and managed by Sapphire Medical Clinics. 52 All participants were recruited consecutively and completed written, formal informed consent prior to enrolment. The UKMCR has received a favorable ethical opinion from the Central Bristol Research Ethics Committee (reference: 22/SW/0145). The study was performed in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidance for reporting observational studies. 53
2.2. Setting and participants
The inclusion criteria for this study were patients who were receiving treatment with CBMPs where ADHD was the primary indication. Patients with incomplete baseline patient‐reported outcome measures (PROMs) or those who were enrolled in the UKMCR for less than 12 months prior to data extraction on 9th January 2023 were also excluded from this study.
2.3. Data selection
Demographic data and medical history of patients including age, gender, occupation, body mass index (BMI), alcohol consumption, cannabis, and smoking history were extracted at baseline, and recorded by clinicians. A novel metric, cannabis gram‐years, was used to quantify cannabis use in ex‐ and current users. 54 Information about concurrent ADHD treatment medication was recorded at baseline. Changes to information about the patient's medications between follow‐up appointments were self‐updated by the patients using an online data collection platform or updated by clinicians during routine follow‐up appointments.
Indications for CBMP prescription, including primary, secondary, and tertiary diagnoses were recorded by clinicians. The Charlson comorbidity index was recorded as a measure of population morbidity. 55 In addition, the incidence of hypertension, arthritis, epilepsy, venous thromboembolism, anxiety/depression, and endocrine/thyroid dysfunction was recorded.
CBMPs were recorded throughout enrollment. This included the route of administration, doses, and concentrations of cannabinoids, cannabis strains, and formulation. All CBMPs adhered to Good Manufacturing Practice (GMP) criteria. 38 Treatment options included sublingual medium‐chain triglyceride‐based oil preparations, inhaled dried flowers, or a combination of both. Utilizing this information the dose of CBD and THC were calculated in milligrams (mg) per day.
2.4. Outcome measures
The primary outcome was determined by changes in PROMs from baseline to 1, 3, 6, and 12 months. The following metrics were used to assess the patient's physical, psychological, and social well‐being: generalized anxiety disorder‐7 (GAD‐7), single‐item sleep quality scale (SQS), and EQ‐5D‐5L. 56 , 57 , 58 Furthermore, patient global impression of change (PGIC) values were collected at each follow‐up. 59
GAD‐7 is a validated scale that evaluates the severity of generalized anxiety disorder symptoms. This involves self‐reporting of the frequency of 7 different symptoms on a scale of 0 to 3, whereby 0 is “not at all” and 3 is “nearly every day” to generate a total score that ranges from 0 to 27. Scores of ≥5, ≥10, and ≥15 represent mild, moderate, and severe anxiety, respectively. 58 A clinically significant change was determined with a minimally clinically important difference (MCID) of 4 points or greater. 60
The Single‐Item Sleep Quality Scale (SQS) is a scale used to evaluate self‐reported sleep quality over the previous 7 days, with 0 being “terrible” sleep quality and 10 being “excellent”. 57
EQ‐5D‐5L is a global measure of the severity of HRQoL with a scale ranging from 1, representing “no problems,” to 5, which is equivalent to “extreme problems.” This scale is measured across five domains: mobility, self‐care, usual activities, pain or discomfort, and anxiety or depression. These scores collectively generate a health state mapped to a country‐specific EQ‐5D‐5L index value. An EQ‐5D‐5L index score of less than 0 represents an HRQoL which is worse in comparison to death, whilst 1 is the highest available score. 58 This is the preferred methodology to assess the HRQoL by the National Institute of Health and Care Excellence. 61
Patient Global Impression of Change (PGIC) assesses a patient's belief about the efficacy of treatment, to determine whether there has been an improvement or decline in a patient's quality of life since starting treatment. This uses a seven‐point scale, whereby there is “no change” at 1, to a “considerable improvement” at 7. 59
2.5. Missing data
The baseline‐observation‐carried‐forward (BOCF) approach was used to account for any missing follow‐up PROMs data. The results are biased to no significance and no positive benefit is assumed from the treatment with CBMPs. 62 Another method used was the last‐observation‐carried‐forward (LOCF) approach, in which the patient's previously observed value was used to replace missing data. 63
2.6. Medication data
Additional medications prescribed during enrollment on the UKMCR and relevant doses were recorded by patients between consultations or asynchronously during mandatory clinical follow‐up.
2.7. Adverse events
Secondary outcomes assessed the incidence of adverse events recorded. Adverse events were either self‐reported by patients when completing PROMs or reported during their follow‐up appointments to their clinician. Adverse events are classified using the Common Terminology Criteria for Adverse Events v4.0. 64
2.8. Statistical analysis
Data about patient demographics, medical history, CBMP prescriptions, and adverse events were analyzed using descriptive statistics. Unless otherwise stated, parametric data was presented as mean ± standard deviation (SD), and nonparametric data was presented as median and interquartile range (IQR). A repeated measures one‐way ANOVA was used to analyze changes in reported PROMs over time. Analysis was performed on PROMs outcomes having used both BOCF and LOCF methods for adjusting for missing data. All further analyses were conducted with the BOCF dataset to reduce an overestimation of effect size. Statistical significance was defined as p < 0.050. All analyses were conducted using Statistical Package for Social Sciences (SPSS) (IBM Statistics version 26 SPSS [New York, IL], USA).
3. RESULTS
3.1. Patient data
Following data extraction and application of inclusion criteria, 68 patients with ADHD were incorporated in the final analysis. The number of patients who had completed PROMs recorded after 1, 3, 6, and 12 months were 61 (89.71%), 53 (77.94%), 50 (73.53%), and 33 (48.53%), respectively.
Participant's clinicopathological characteristics at baseline, including patient demographics and medical history in this study were analyzed (Table 1). Among these patients, 55 (80.88%) were male and 13 (19.11%) were female, with a mean age of 35.62 (±10.23) years and a mean BMI of 25.26 (±4.77) kg/m2. In terms of occupation, “unemployed” was the most common category with 19 (27.94%) patients. Regarding cannabis history, a total of 55 (80.88%) patients were cannabis users at the point of initiating treatment.
TABLE 1.
Clinicopathological characteristics of study participants at baseline (n = 68).
| Demographic details | n (%)/mean ± SD |
|---|---|
| Gender | |
| Male | 55 (80.88%) |
| Female | 13 (19.11%) |
| Age (years) | 35.62 ± 10.23 |
| BMI (kg/m2) | 25.26 ± 4.77 |
| Occupation | |
| Craft and related trades workers | 3 (4.41%) |
| Elementary occupations | 6 (8.82%) |
| Managers | 3 (4.41%) |
| Other occupations | 8 (11.8%) |
| Professional | 13 (19.12%) |
| Service and sales workers | 6 (8.82%) |
| Skilled agricultural, forestry, and fishery workers | 1 (1.47%) |
| Technicians and associate professionals | 5 (7.35%) |
| Unemployed | 19 (27.94%) |
| Charlson Comorbidity Index | 0.00 [0.00–0.00] |
| Tobacco, alcohol, and cannabis history | n (%)/median [IQR] |
| Smoking status | |
| Never smoked | 14 (20.59%) |
| Ex‐smoker | 23 (33.82%) |
| Current smoker | 31 (45.59%) |
| Smoking pack years | 9.50 [2.00–17.25] |
| Weekly alcohol consumption, units | 0.00 [0.00–3.00] |
| Cannabis status | |
| Never used | 6 (8.82%) |
| Previous user | 7 (10.29%) |
| Current user | 55 (80.88%) |
| Cannabis gram years | 15.50 [4.75–34.25] |
Note: Parametric data are presented as mean ± standard deviation (SD) and non‐parametric data are presented as median [interquartile range, IQR].
Abbreviations: BMI, body mass index; IQR, interquartile range; SD, standard deviation.
The most common secondary indication for treatment with CBMPs was anxiety (n = 22, 32.4%) (Table S1). The median Charlson comorbidity index was 0.00 [0.00–0.00]. The prevalence of recorded co‐morbidities is detailed in Table S2.
3.2. CBMP dosing
The dosing and mode of administration of CBMP prescription were assessed and displayed in Table 2. Inhaled dried flower preparations alone were prescribed to 38 (55.88%) patients, sublingual median‐chain triglyceride‐based oil preparations alone were prescribed to 4 (5.88%) patients, and 26 (38.24%) were prescribed a combination of both. The median CBD dose for all patients per day was 15.0 [5.13–55.00] mg, while the median THC dose per day was 208.75 [120.63–291.43] mg. The most prescribed dried flower was Adven® EMT1 (Curaleaf International, United Kingdom). The most prescribed medium‐chain triglyceride oils were Adven® 50 mg/mL CBD (Curaleaf International, United Kingdom) and Adven® 20 mg/mL THC (Curaleaf International, United Kingdom).
TABLE 2.
Prescription information for study participants (n = 68).
| Prescription information | n (%)/median [IQR] |
|---|---|
| Oils | 4 (5.88%) |
| CBD, mg/24 h | 17.50 [1.25–48.75] |
| THC, mg/24 h | 5.00 [1.25–8.75] |
| Dried flower | 38 (55.88%) |
| CBD, mg/24 h | 10.00 [5.00–21.25] |
| THC, mg/24 h | 215.00 [165.00–282.50] |
| Oils and dried flower | 26 (38.24%) |
| CBD, mg/24 h | 30.00 [10.00–60.00] |
| THC, mg/24 h | 211.00 [106.88–301.20] |
Note: Data for patients prescribed cannabis‐based medicinal products (CBMPs). The daily prescribed dose of (−)‐trans‐Δ9‐tetrahydrocannabinol and cannabidiol for each type of preparation are presented as median [interquartile range, IQR].
Abbreviations: CBD, cannabidiol; IQR, interquartile range; THC, (−)‐trans‐Δ9‐tetrahydrocannabinol.
3.3. Patient‐reported outcome measures
Changes in baseline and follow‐up scores at 1, 3, 6, and 12 months for each PROM are outlined in Table 3 and further reporting of Bonferroni corrected p‐values for paired‐comparisons between baseline and each follow‐up time‐points are reported in Table 4. Analysis where the LOCF method was used to account for missing data is detailed in Tables S3 and S4.
TABLE 3.
One‐way repeated measures ANOVA analysis comparing patient‐reported outcome measures after 12 months in study participants (n = 68).
| PROM | Baseline | 1 month | 3 months | 6 months | 12 months | F‐value | p‐Value |
|---|---|---|---|---|---|---|---|
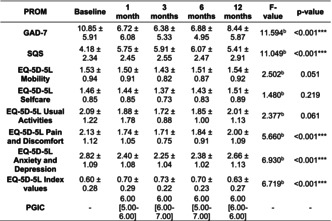
| GAD‐7 | 10.85 ± 5.91 | 6.72 ± 6.08 | 6.38 ± 5.33 | 6.88 ± 4.95 | 8.44 ± 5.87 | 11.594 | <0.001*** |
| SQS | 4.18 ± 2.34 | 5.75 ± 2.45 | 5.91 ± 2.55 | 6.07 ± 2.47 | 5.41 ± 2.91 | 11.049 | <0.001*** |
| EQ‐5D‐5L Mobility | 1.53 ± 0.94 | 1.50 ± 0.91 | 1.43 ± 0.82 | 1.51 ± 0.87 | 1.54 ± 0.92 | 2.502 | 0.051 |
| EQ‐5D‐5L Selfcare | 1.46 ± 0.85 | 1.44 ± 0.85 | 1.37 ± 0.73 | 1.43 ± 0.83 | 1.51 ± 0.89 | 1.480 | 0.219 |
| EQ‐5D‐5L Usual Activities | 2.09 ± 1.22 | 1.88 ± 1.78 | 1.72 ± 0.88 | 1.85 ± 1.00 | 2.01 ± 1.13 | 2.377 | 0.061 |
| EQ‐5D‐5L Pain and Discomfort | 2.13 ± 1.12 | 1.74 ± 1.05 | 1.71 ± 0.75 | 1.84 ± 0.91 | 2.00 ± 1.09 | 5.660 | <0.001*** |
| EQ‐5D‐5L Anxiety and Depression | 2.82 ± 1.09 | 2.40 ± 1.08 | 2.25 ± 1.04 | 2.38 ± 1.02 | 2.66 ± 1.13 | 6.930 | <0.001*** |
| EQ‐5D‐5L Index values | 0.60 ± 0.28 | 0.70 ± 0.29 | 0.73 ± 0.22 | 0.70 ± 0.23 | 0.63 ± 0.27 | 6.719 | <0.001*** |
| PGIC | – | 6.00 [5.00–6.00] | 6.00 [6.00–7.00] | 6.00 [5.00–7.00] | 6.00 [6.00–6.00] | – | – |
Note: Each patient‐reported outcome measure (PROM) is displayed in mean ± standard deviation (SD), except for PGIC which is presented as median [interquartile range, IQR].
Abbreviations: GAD‐7, generalized anxiety disorder‐7; PGIC, Patient Global Impression of Change; PROM, patient reported outcome measure; SD, standard deviation; SQS, Single‐Item Sleep Quality Scale.
*** p < 0.001.
TABLE 4.
p‐Values from pairwise comparison of the significant differences in baseline and all follow‐up scores for patient‐reported outcome measures of study participants (n = 68).
| PROMs | 1 month | 3 months | 6 months | 12 months |
|---|---|---|---|---|
| GAD‐7 | <0.001*** | <0.001*** | <0.001*** | 0.002** |
| SQS | <0.001*** | <0.001*** | <0.001*** | 0.002** |
| EQ‐5D‐5L index value | 0.008** | <0.001*** | 0.015* | 1.000 |
| EQ‐5D‐5L Pain and Discomfort | 0.001** | 0.001** | 0.077 | 1.000 |
| EQ‐5D‐5L Anxiety and Depression | 0.032* | <0.001*** | 0.001** | 1.000 |
Note: Significant p‐values after Bonferroni correction are indicated by *p < 0.050, **p < 0.010, ***p < 0.001.
Abbreviations: GAD‐7, generalized anxiety disorder‐7; PGIC, Patient Global Impression of Change; PROMs, patient‐reported outcome measures; SQS, Single‐Item Sleep Quality Scale.
There were improvements in anxiety severity and sleep quality, as assessed by the GAD‐7 and SQS, respectively, between baseline scores and across all follow‐up time periods at 1, 3, 6, and 12 months (p < 0.001). Additionally, clinically significant improvements in GAD‐7 score were observed in 50.00% (n = 34) of patients at 1 month, 42.65% (n = 29) at 3 months, 39.71% (n = 27) at 6 months and 26.47% (n = 18) at 12 months.
There were improvements in the general health‐related quality of life, as assessed by the EQ‐5D‐5L Index Value, at 1, 3, and 6 months compared to baseline (p < 0.050). However, there was no change at 12 months (p = 1.000). This improvement in the EQ‐5D‐5L was found in the domains of Pain and Discomfort at up to 3 months (p = 0.001), and Anxiety & Depression subscale at up to 6 months when compared to baseline (p < 0.050). An improvement was also observed between the baseline and 3‐month time‐point in the Usual Activities' subscale (p = 0.047). For PGIC, the median remained constant at 6.00 at 1, 3, 6, and 12 months.
There was no difference at 12 months between those who were current cannabis users at baseline and those who had never used cannabis or had used it previously in the GAD‐7 (−2.47 ± 5.28 vs. −2.15 ± 4.08; p = 0.839), SQS (1.09 ± 2.58 vs. 1.84 ± 2.67; p = 0.350) or EQ‐5D‐5L index value (0.01 ± 0.19 vs. 0.07 ± 0.13; p = 0.340).
3.4. Co‐administered medications
The most commonly co‐administered medications were methylphenidate (n = 20; 29.41%), lisdexamfetamine (n = 13; 19.11%), dexamfetamine (n = 7; 10.29%), and atomoxetine (n = 6; 8.82%) (Table 5). In all, 38.46% (n = 5), 15.00% (n = 3), and 14.29% (n = 1) of patients stopped taking lisdexamfetamine, methylphenidate, and dexamfetamine, respectively, during treatment with CBMPs (Table 5).
TABLE 5.
Changes in co‐administered medications throughout treatment with cannabis‐based medicinal products after 12 months in study participants (n = 68).
| Medication | Total | Stopped taking | Reduced dose | No change | Increased dose | New medication |
|---|---|---|---|---|---|---|
| Methylphenidate, n (%) | 20 | 3 (15.00%) | 0 (0.00%) | 14 (70.00%) | 0 (0.00%) | 3 (15.00%) |
| Lisdexamfetamine, n (%) | 13 | 5 (38.46%) | 0 (0.00%) | 8 (61.54%) | 0 (0.00%) | 0 (0.00%) |
| Atomoxetine, n (%) | 6 | 0 (0.00%) | 0 (0.00%) | 6 (100.00%) | 0 (0.00%) | 0 (0.00%) |
| Dexamfetamine, n (%) | 7 | 1 (14.29%) | 0 (0.00%) | 4 (57.14%) | 0 (0.00%) | 2 (28.57%) |
3.5. Adverse events
Table 6 outlines the adverse events reported by the patients. Eleven (16.18%) patients reported a total of 61 (89.71%) adverse events, with the most common severity class being moderate (n = 26, 38.24%). The most common adverse events reported were insomnia (n = 5, 7.35%), concentration impairment (n = 5, 7.35%), somnolence (n = 5, 7.35%), lethargy (n = 5, 7.35%), and dry mouth (n = 5, 7.35%). No incidences of life‐threatening or disabling adverse events were reported. There was no statistically significant difference in the proportion of patients prescribed oils only (n = 1; 25.00%), dried flower only (n = 9; 23.68%), or both formulations (n = 1; 3.85%; p = 0.095).
TABLE 6.
Adverse events reported by study participants (n = 11).
| Adverse events | Mild | Moderate | Severe | Total (%) |
|---|---|---|---|---|
| Abdominal pain | 0 | 2 | 0 | 2 (2.94%) |
| Amnesia | 0 | 1 | 1 | 2 (2.94%) |
| Anorexia | 2 | 0 | 0 | 2 (2.94%) |
| Anxiety | 0 | 1 | 1 | 2 (2.94%) |
| Cognitive disturbance | 1 | 2 | 1 | 4 (5.88%) |
| Concentration impairment | 0 | 4 | 1 | 5 (7.35%) |
| Confusion | 0 | 0 | 1 | 1 (1.47%) |
| Constipation | 0 | 1 | 1 | 2 (2.94%) |
| Delirium | 0 | 0 | 1 | 1 (1.47%) |
| Dizziness | 1 | 0 | 0 | 1 (1.47%) |
| Dry Mouth | 5 | 0 | 0 | 5 (7.35%) |
| Dyspepsia | 1 | 2 | 1 | 4 (5.88%) |
| Fatigue | 1 | 3 | 0 | 4 (5.88%) |
| Generalized muscle weakness | 1 | 1 | 0 | 2 (2.94%) |
| Headache | 3 | 0 | 1 | 4 (5.88%) |
| Insomnia | 0 | 0 | 5 | 5 (7.35%) |
| Lethargy | 1 | 4 | 0 | 5 (7.35%) |
| Nausea | 2 | 0 | 0 | 2 (2.94%) |
| Pharyngitis | 0 | 0 | 1 | 1 (1.47%) |
| Somnolence | 0 | 5 | 0 | 5 (7.35%) |
| Upper respiratory infection | 0 | 0 | 1 | 1 (1.47%) |
| Weight loss | 1 | 0 | 0 | 1 (1.47%) |
| Total (%) | 19 (27.94%) | 26 (38.24%) | 16 (23.53%) | 61 (89.71%) |
4. DISCUSSION
This study assessed an uncontrolled case series of patients with ADHD prescribed CBMPs from the UKMCR. The findings demonstrated an associated improvement in the general HRQoL, sleep quality, and anxiety severity in patients with ADHD following the initiation of CBMP treatment. A reduction of 38.46%, 15.00%, and 14.29% in the concomitant prescribing of lisdexamfetamine, methylphenidate, and dexamfetamine, respectively, was also demonstrated within this cohort. Adverse events were experienced by 16.18% of the cohort with a total adverse event incidence of 89.71%.
Improvements in general HRQoL are supported by previous evaluations of CBMPs in the setting of ADHD. Findings from Cooper et al. identified improvements in behavioral symptoms and emotional liability. 39 Despite not being direct measures of HRQoL, Escobar et al. demonstrated a direct correlation between improved ADHD symptom‐severity and HRQoL. 65 Positive symptomatic differences were also presented in various case reports. Many of these identified improvements in at least one of the DSM‐5 ADHD symptoms, emotional regulation, and sleep, especially when used adjunctive to medication. 66 , 67 , 68 The associated change in HRQoL and CBMPs is further supplemented by similar findings from several other studies on psychiatric conditions published using data from the UKMCR, where improved HRQoL was achieved following treatment with CBMPs, as measured by EQ‐5D‐5L Index values. 69 , 70 , 71 , 72 This study demonstrated improvements in the EQ‐5D‐5L Index value at 1, 3, and 6 months, but this was not present at 12 months. The divergence at 12 months appears to be due to the methods used in the study design, whereby a conservative approach was used to account for the reduction in the number of patients followed up to 12 months, as results using the LOCF approach demonstrated statistically significant improvements across all follow‐up months in the EQ‐5D‐5L index values.
Comorbid sleep disorder is common in adults with ADHD, with many experiencing poor sleep quality and insomnia. 73 In this study, an associated improvement in self‐reported sleep quality was found within these participants at up to 12 months. The ECS is implicated with the regulation of sleep, thus, CBMPs may play an important role in targeting sleep disorders. 74 The results of improved sleep quality in the present study are consistent with the outcomes of a study on autism spectrum disorder patients treated with CBMPs from the UKMCR, which identified similar improvements. 69 However, current evidence on the effect of CBMPs on sleep is inconsistent, with studies suggesting a possible risk of developing tolerance associated with long‐term use of the sleep‐promoting effects of CBMPs. 75 , 76 , 77 Despite the overall positive results on sleep quality, insomnia was also one of the most frequently reported adverse events. As adverse events were not assessed to determine whether they were treatment‐related, this could be secondary to the inherent relationship between ADHD and sleep disorders, or secondary to co‐administered stimulants. This highlights the importance of using RCTs to evaluate the benefits and risks of long‐term use of CBMPs. 78
Improvements in the severity of anxiety symptoms at all follow‐up intervals after initiation of CBMP therapy were identified in this study. Results demonstrated that 25% or more participants reported clinically significant improvements in generalized anxiety symptoms between baseline and all follow‐up timepoints. These clinically significant improvements were also found in the results of a study on GAD patients from the UKMCR, which further supports the associated significant reductions in mean GAD‐7 scores with CBMP treatment. 79 The symptomatic improvement is suggested to be associated with the anxiolytic properties possessed by the CBMPs. 80 , 81
There was a reduction in concomitant ADHD medication use during treatment. As previously stated, ADHD medications, particularly stimulants, are associated with a high incidence of negative side effects. 12 , 13 , 14 Additionally, there are safety concerns regarding long‐term treatment as stimulants may increase the risks of psychosis, cardiovascular diseases, and substance use disorders. 13 , 14 Case reports involving patients with ADHD have similarly found that administration of CBMPs can result in the discontinuation of stimulants, as well as other associated medications. 66 However, further assessment is still required to determine if CBMPs are a suitable substitute for licensed medications used currently in the treatment of ADHD.
This study was one of the first to investigate the adverse events of CBMPs in ADHD, and currently, no other studies have reported the follow‐up of ADHD patients prescribed CBMPs for up to 12 months. The total incidence of adverse events reported in the present study was 61 (89.71%). Adverse events were reported by 11 (16.18%) of the participants, which was relatively lower than reported in previous observational studies, and there were no disabling or life‐threatening adverse events reported. 69 A similar incidence of adverse events was reported in the RCT conducted by Cooper et al. where adverse events were reported by 26.7% of CBMP users with ADHD. 39 Collectively, these findings indicate that CBMPs are well tolerated in the short term. The most frequent adverse events reported in this study were dry mouth, insomnia, somnolence, lethargy, and concentration impairment. Although concentration impairment is a common result of cannabis use, it is also a common clinical manifestation of ADHD. 6 As this study sought to collect all adverse events and these were not assessed by clinicians to determine if they were treatment‐related it is not possible to identify whether these were due to CBMPs, underlying symptoms of ADHD, or another confounding factor.
There were notable limitations to this study. As a case series, it cannot determine if CBMPs were the causative mechanism for improvements noted in participants in this study. It was not possible to control for confounding factors and determine if the observed effects were secondary to these or phenomena such as regression to the mean. There is significant heterogeneity in CBMPs prescribed within this study making it even more challenging to directly study the effect of specific regimens. Whilst attempts were made to analyze the differences between adverse events according to each route of administration, underlying confounders limit the ability to interpret this in full. There is also a noteworthy selection bias. Whilst there is a higher incidence of ADHD in males, 82 this cohort is over‐represented by male participants (80.88%). The underlying physiological differences between males and females may impact how ADHD manifests, which could affect the outcome of treatment with CBMPs. 83 Although research has indicated that males are more frequently diagnosed with ADHD, the gender disparity in diagnosis is likely exacerbated by social factors. 82 Despite illicit cannabis being commonly used by ADHD patients to self‐medicate, most patients (80.88%) were already current cannabis consumers at the point of starting treatment. Repeated cannabis administration may lead to the development of pharmacological tolerance, 84 potentially reducing the effectiveness of CBMPs. Moreover, patients were recruited from a private clinic and therefore, this cohort could be socioeconomically skewed. However, a sizeable proportion of these patients were unemployed (27.94%), indicating that the cost was not restrictive to starting treatment. Future studies should be conducted through RCTs with a diverse patient population, whilst also mitigating potential confounding factors that could interact with CBMPs.
5. CONCLUSION
This case series is the first of its kind in assessing the clinical outcome of patients from the UKMCR with a primary diagnosis of ADHD prescribed CBMPs for up to 12 months. This study reports that treatment with CBMPs was associated with improvements in general HRQoL after 1, 3, and 6, months, in addition to anxiety and sleep quality after 1, 3, 6, and 12 months (p < 0.050). These results suggest that CBMPs may play a role in alleviating symptoms and co‐morbid anxiety and sleep disruption associated with ADHD, though these are preliminary findings. CBMPs were well‐tolerated throughout this study and the majority of patients (83.82%) did not report any adverse events. Due to limitations in study design, a causal relationship cannot be determined, thus, a definite conclusion cannot be drawn from these results. The findings from this study guide further investigation to assess the therapeutic efficacy and long‐term safety profile of CBMPs. Comparative analysis should be performed on patients in the UKMCR for future evaluations, and importantly, it is essential to conduct high‐quality RCTs for the treatment of ADHD whilst controlling for potential confounding factors.
AUTHOR CONTRIBUTIONS
Study conception and design: PI, SE, CH, RC, JRR, MHS; Acquisition of data: PI, SE, CH, RC, JRR; Analysis and interpretation of data: PI, SE, MHS; Drafting of manuscript: PI, SE, MHS; Critical revision: PI, SE, CH, RC, JRR, MHS; All authors have contributed to and approved the final manuscript. The authors confirm that the PI for this paper is Mikael H Sodergren and that he had direct clinical responsibility for patients.
FUNDING INFORMATION
There was no external or commercial funding associated with this paper.
CONFLICT OF INTEREST STATEMENT
Pim Ittiphakorn is a biomedical sciences student at Imperial College London. Pim Ittiphakorn has no shareholdings in pharmaceutical companies. Simon Erridge is a junior doctor and is the Head of Research at Sapphire Medical Clinics. Simon Erridge is an honorary clinical research fellow at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS. Simon Erridge has no shareholdings in pharmaceutical companies. Carl Holvey is the Chief Clinical Pharmacist at Sapphire Medical Clinics. Carl Holvey has no shareholdings in pharmaceutical companies. Ross Coomber is a Consultant Orthopedic Surgeon, Operations Director at Sapphire Medical Clinics, and a consultant at St George's Hospital, London. The views expressed are those of the author(s) and not necessarily those of the NHS. Ross Coomber has no shareholdings in pharmaceutical companies. James Rucker is a Consultant Psychiatrist and a Former Director at Sapphire Medical Clinics (London). James Rucker is an Honorary Consultant Psychiatrist at The South London & Maudsley NHS Foundation Trust, and an NIHR Clinician Scientist Fellow at the Centre for Affective Disorders at King's College London. James Rucker is funded by a fellowship (CS‐2017‐17‐007) from the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. James Rucker has no shareholdings in pharmaceutical companies. James Rucker reviewed this article and made comments. Mikael Sodergren is a consultant hepatopancreatobiliary surgeon at Imperial College NHS Trust. He is the Chief Medical Officer at Curaleaf International. He is a senior clinical lecturer at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: Ethical approval provided by South West–Central Bristol Research Ethics Committee (Reference: 22/SW/0145).
Informed Consent: All participants completed written, informed consent prior to enrolment in the registry.
Registry and the registration No. of the Study/Trial: N/A.
Animal Studies: N/A.
Supporting information
Table S1
Ittiphakorn P, Erridge S, Holvey C, Coomber R, Rucker JJ, Sodergren MH. UK Medical Cannabis Registry: An analysis of clinical outcomes of medicinal cannabis therapy for attention‐deficit/hyperactivity disorder. Neuropsychopharmacol Rep. 2023;43:596–606. 10.1002/npr2.12400
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available from the UK Medical Cannabis Registry for organizations with an appropriate data safe haven. Restrictions apply to the availability of pseudonymized data in accordance with the ethical approval provided by South West–Central Bristol Research Ethics Committee (Reference: 22/SW/0145). Data specifications and applications are available from the corresponding author.
REFERENCES
- 1. Wilens TE, Spencer TJ. Understanding attention‐deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010;122(5):97–109. 10.3810/pgm.2010.09.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Song P, Zha M, Yang Q, Zhang Y, Li X, Rudan I. The prevalence of adult attention‐deficit hyperactivity disorder: a global systematic review and meta‐analysis. J Glob Health. 2021;11:4009. 10.7189/jogh.11.04009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM et al. Trends in the Parent‐Report of Health Care Provider‐Diagnosis and Medication Treatment for ADHD disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. 2014,53(1):34–46.e2. [DOI] [PMC free article] [PubMed]
- 4. Chung W, Jiang S‐F, Paksarian D, Nikolaidis A, Castellanos FX, Merikangas KR, et al. Trends in the prevalence and incidence of attention‐deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdelnour E, Jansen MO, Gold JA. ADHD diagnostic trends: increased recognition or overdiagnosis? Mo Med. 2022;119(5):467–473. [PMC free article] [PubMed] [Google Scholar]
- 6. DSM‐5 changes: Implications for child serious emotional disturbance. 2016. [cited 2023 Feb 7]. Available from: https://pubmed.ncbi.nlm.nih.gov/30199184/ [PubMed]
- 7. Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos‐Quiroga JA, et al. Attention‐deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization . ICD‐11: International Classification of Diseases 11th Revision. 2018. Available from: https://icd.who.int/. Accessed January 2021.
- 9. Martínez L, Prada E, Satler C, Tavares MCH, Tomaz C. Executive dysfunctions: The role in attention deficit hyperactivity and post‐traumatic stress neuropsychiatric disorders. Front Psychol. 2016;7:1230. 10.3389/fpsyg.2016.01230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen CM, Steinhausen H‐C. Comorbid mental disorders in children and adolescents with attention‐deficit/hyperactivity disorder in a large nationwide study. Atten Defic Hyperact Disord. 2015;7(1):27–38. 10.1007/s12402-014-0142-1 [DOI] [PubMed] [Google Scholar]
- 11. Briars L, Todd T. A review of pharmacological management of attention‐deficit/hyperactivity disorder. J Pediatr Pharmacol Ther. 2016;21(3):192–206. 10.5863/1551-6776-21.3.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention‐deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255–270. 10.1016/j.neubiorev.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown KA, Samuel S, Patel DR. Pharmacologic management of attention deficit hyperactivity disorder in children and adolescents: a review for practitioners. Transl Pediatr. 2018;7(1):36–47. 10.21037/tp.2017.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang Z, Ghirardi L, Quinn PD, Asherson P, D'Onofrio BM, Larsson H. Risks and benefits of attention‐deficit/hyperactivity disorder medication on behavioral and neuropsychiatric outcomes: a qualitative review of pharmacoepidemiology studies using linked prescription databases. Biol Psychiatry. 2019;86(5):335–343. 10.1016/j.biopsych.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stueber A, Cuttler C. Self‐reported effects of cannabis on ADHD symptoms, ADHD medication side effects, and ADHD‐related executive dysfunction. J Atten Disord. 2022;26(6):942–955. 10.1177/10870547211050949 [DOI] [PubMed] [Google Scholar]
- 16. Emilsson M, Gustafsson PA, Öhnström G, Marteinsdottir I. Beliefs regarding medication and side effects influence treatment adherence in adolescents with attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2017;26(5):559–571. 10.1007/s00787-016-0919-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184–191. 10.3810/pgm.2010.01.2112 [DOI] [PubMed] [Google Scholar]
- 18. Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. 10.3389/fnbeh.2011.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernández‐Ruiz J, Hernández M, Ramos JA. Cannabinoid‐dopamine interaction in the pathophysiology and treatment of CNS disorders: cannabinoid‐dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16(3):e72–e91. 10.1111/j.1755-5949.2010.00144.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carvalho AF, Van Bockstaele EJ. Cannabinoid modulation of noradrenergic circuits: implications for psychiatric disorders. Prog Neuro‐Psychopharmacol Biol Psychiatry. 2012;38(1):59–67. 10.1016/j.pnpbp.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grinspoon P. The endocannabinoid system: essential and mysterious [Internet]. Harvard Health Publishing; 2021. [cited 2023 Feb 7]. Available from: https://www.health.harvard.edu/blog/the‐endocannabinoid‐system‐essential‐and‐mysterious‐202108112569 [Google Scholar]
- 22. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kendall DA, Yudowski GA. Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci. 2016;10:294. 10.3389/fncel.2016.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dawson DA, Persad CP. Targeting the endocannabinoid system in the treatment of ADHD [Internet]. Scivisionpub.com. [cited 2023 Feb 7]. Available from: https://scivisionpub.com/pdfs/targeting‐the‐endocannabinoid‐system‐in‐the‐treatment‐of‐adhd‐1604.pdf
- 25. Haspula D, Clark MA. Cannabinoid receptors: an update on cell signaling, pathophysiological roles and therapeutic opportunities in neurological, cardiovascular, and inflammatory diseases. Int J Mol Sci. 2020;21(20):7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu AT, Ogdie MN, Järvelin M‐R, Moilanen IK, Loo SK, McCracken JT, et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post‐traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1488–1494. 10.1002/ajmg.b.30693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castelli M, Federici M, Rossi S, De Chiara V, Napolitano F, Studer V, et al. Loss of striatal cannabinoid CB1 receptor function in attention‐deficit / hyperactivity disorder mice with point‐mutation of the dopamine transporter: cannabinoid receptors in ADHD. Eur J Neurosci. 2011;34(9):1369–1377. [DOI] [PubMed] [Google Scholar]
- 28. Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83(3):1017–1066. 10.1152/physrev.00004.2003 [DOI] [PubMed] [Google Scholar]
- 29. Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. [DOI] [PubMed] [Google Scholar]
- 30. Laksmidewi AAAP, Soejitno A. Endocannabinoid and dopaminergic system: the pas de deux underlying human motivation and behaviors. J Neural Transm (Vienna). 2021;128(5):615–630. 10.1007/s00702-021-02326-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98(2):408–419. [DOI] [PubMed] [Google Scholar]
- 32. Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241–254. 10.1177/2045125312457586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernández‐Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid?: cannabidiol and neurodegenerative disorders. Br J Clin Pharmacol. 2013;75(2):323–333. 10.1111/j.1365-2125.2012.04341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laprairie RB, Bagher AM, Kelly MEM, Denovan‐Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor: negative allosteric modulation of CB1 by cannabidiol. Br J Pharmacol. 2015;172(20):4790–4805. 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ibeas Bih C, Chen T, Nunn AVW, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12(4):699–730. 10.1007/s13311-015-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14(3):237–246. 10.2174/092986607780090829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cannabis‐based medicinal products. National Institute for Health and Care Excellence; 2021. Available from: https://www.nice.org.uk/guidance/ng144 [PubMed] [Google Scholar]
- 38. The CP. NICE guideline on medicinal cannabis: keeping Pandora's box shut tight? Med Law Rev. 2020;28(2):401–411. [DOI] [PubMed] [Google Scholar]
- 39. Cooper RE, Williams E, Seegobin S, Tye C, Kuntsi J, Asherson P. Cannabinoids in attention‐deficit/hyperactivity disorder: a randomised‐controlled trial. Eur Neuropsychopharmacol. 2017;27(8):795–808. [DOI] [PubMed] [Google Scholar]
- 40. Francisco AP, Lethbridge G, Patterson B, Goldman Bergmann C, Van Ameringen M. Cannabis use in attention ‐ deficit/hyperactivity disorder (ADHD): a scoping review. J Psychiatr Res. 2023;157:239–256. [DOI] [PubMed] [Google Scholar]
- 41. Ly C, Gehricke J‐G. Marijuana use is associated with inattention in men and sleep quality in women with attention‐deficit/hyperactivity disorder: a preliminary study. Psychiatry Res. 2013;210(3):1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pardini D, White HR, Xiong S, Bechtold J, Chung T, Loeber R, et al. Unfazed or dazed and confused: does early adolescent marijuana use cause sustained impairments in attention and academic functioning? J Abnorm Child Psychol. 2015;43(7):1203–1217. 10.1007/s10802-015-0012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. MacDonald B, Sadek J. Naturalistic exploratory study of the associations of substance use on ADHD outcomes and function. BMC Psychiatry. 2021;21(1):251. 10.1186/s12888-021-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duperrouzel JC, Granja K, Pacheco‐Colón I, Gonzalez R. Adverse effects of cannabis use on neurocognitive functioning: a systematic review of meta‐ analytic studies. J Dual Diagn. 2020;16(1):43–57. 10.1080/15504263.2019.1626030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bourque J, Potvin S. Cannabis and cognitive functioning: from acute to residual effects, from randomized controlled trials to prospective designs. Front Psychiatry. 2021;12:596601. 10.3389/fpsyt.2021.596601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soler Artigas M, Sánchez‐Mora C, Rovira P, Richarte V, Garcia‐Martínez I, Pagerols M, et al. Attention‐deficit/hyperactivity disorder and lifetime cannabis use: genetic overlap and causality. Mol Psychiatry. 2020;25(10):2493–2503. 10.1038/s41380-018-0339-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention‐deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta‐analytic review. Clin Psychol Rev. 2011;31(3):328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lisdahl KM, Tamm L, Epstein JN, Jernigan T, Molina BSG, Hinshaw SP, et al. The impact of ADHD persistence, recent cannabis use, and age of regular cannabis use onset on subcortical volume and cortical thickness in young adults. Drug Alcohol Depend. 2016;161:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amen DG, Waugh M. High resolution brain SPECT imaging of marijuana smokers with AD/HD. J Psychoactive Drugs. 1998;30(2):209–214. 10.1080/02791072.1998.10399692 [DOI] [PubMed] [Google Scholar]
- 50. Silva N Jr, Szobot CM, Shih MC, Hoexter MQ, Anselmi CE, Pechansky F, et al. Searching for a neurobiological basis for self‐medication theory in ADHD comorbid with substance use disorders: an in vivo study of dopamine transporters using (99m)Tc‐TRODAT‐1 SPECT. Clin Nucl Med. 2014;39(2):e129–e134. [DOI] [PubMed] [Google Scholar]
- 51. Notzon DP, Pavlicova M, Glass A, Mariani JJ, Mahony AL, Brooks DJ, et al. ADHD is highly prevalent in patients seeking treatment for cannabis use disorders. J Atten Disord. 2020;24(11):1487–1492. 10.1177/1087054716640109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Erridge S, Salazar O, Kawka M, Holvey C, Coomber R, Usmani A, et al. An initial analysis of the UK Medical Cannabis Registry: outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. 2021;41(3):362–370. 10.1002/npr2.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbrouckef JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. The Lancet. 2007;370(9596):1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wetherill RR, Hager N, Guthier E, Franklin TR. Gram years: a method to standardize and quantify lifetime cannabis consumption. Cannabis Cannabinoid Res. 2016;1(1):216–217. 10.1089/can.2016.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 56. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD‐7. Arch Intern Med. 2006;166(10):1092–1097. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 57. Snyder E, Cai B, DeMuro C, Morrison MF, Ball W. A new single‐item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018;14(11):1849–1857. 10.5664/JCSM.7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20(10):1727–1736. 10.1007/S11136-011-9903-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Norton C, Clark M, Haley JA, Law LF, Graven‐Nielsen T, Mcmullen T, et al. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J Pain. 2009;10(4):S73. 10.1016/J.JPAIN.2009.01.258 [DOI] [Google Scholar]
- 60. Toussaint A, Hüsing P, Gumz A, Wingenfeld K, Härter M, Schramm E, et al. Sensitivity to change and minimal clinically important difference of the 7‐item generalized anxiety disorder questionnaire (GAD‐7). J Affect Disord. 2020;265:395–401. [DOI] [PubMed] [Google Scholar]
- 61. Position statement on use of the EQ‐5D‐5L value set for England (updated October 2019)|Technology appraisal guidance|NICE guidance|Our programmes|What we do|About|NICE. [cited 2023 May 2]. Available from: https://www.nice.org.uk/about/what‐we‐do/our‐programmes/nice‐guidance/technology‐appraisal‐guidance/eq‐5d‐5l
- 62. Liu‐Seifert H, Zhang S, D'Souza D, Skljarevski V. A closer look at the baseline‐observation‐carried‐forward (BOCF). Patient Prefer Adherence. 2010;4:11–16. 10.2147/ppa.s8135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lachin JM. Fallacies of last observation carried forward analyses. Clin Trials. 2016;13(2):161–168. 10.1177/1740774515602688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and reliability of the US National Cancer Institute's patient‐reported outcomes version of the common terminology criteria for adverse events (PRO‐CTCAE). JAMA Oncol. 2015;1(8):1051–1059. 10.1001/JAMAONCOL.2015.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Escobar R, Montoya A, Polavieja P, Cardo E, Artigas J, Hervas A, et al. Evaluation of patients' and parents' quality of life in a randomized placebo‐controlled atomoxetine study in attention‐deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):253–263. 10.1089/cap.2008.0109 [DOI] [PubMed] [Google Scholar]
- 66. Mansell H, Quinn D, Kelly LE, Alcorn J. Cannabis for the treatment of attention deficit hyperactivity disorder: a report of 3 cases. Med Cannabis Cannabinoids. 2022;5(1):1–6. 10.1159/000521370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hasan A, Rothenberger A, Münchau A, Wobrock T, Falkai P, Roessner V. Oral delta 9‐tetrahydrocannabinol improved refractory Gilles de la Tourette syndrome in an adolescent by increasing intracortical inhibition: a case report: a case report. J Clin Psychopharmacol. 2010;30(2):190–192. [DOI] [PubMed] [Google Scholar]
- 68. Strohbeck‐Kuehner P, Skopp G, Mattern R. Cannabis improves symptoms of ADHD [Internet]. 2008. Free.fr [cited 2023 Feb 7]. Available from: http://mcforadhd.free.fr/ARTICLE%20ADHD%20DRIVING%20GERMANY.pdf
- 69. Erridge S, Kerr‐Gaffney J, Holvey C, Coomber R, Barros D, Bhoskar U, et al. Clinical outcome analysis of patients with autism Spectrum disorder – analysis from the UK medical cannabis registry. Therapeut Adv Psychopharmacol. 2022;12:204512532211162. 10.1177/20451253221116240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pillai M, Erridge S, Bapir L, Nicholas M, Dalavaye N, Holvey C, et al. Assessment of clinical outcomes in patients with post‐traumatic stress disorder: analysis from the UK medical cannabis registry. Expert Rev Neurother. 2022;22:1009–1018. 10.1080/14737175.2022.2155139 [DOI] [PubMed] [Google Scholar]
- 71. Mangoo S, Erridge S, Holvey C, Coomber R, Barros D, Bhoskar U, et al. Assessment of clinical outcomes of medicinal cannabis therapy for depression: analysis from the UK medical cannabis registry. Expert Rev Neurother. 2022;22:995–1008. 10.1080/14737175.2022.2161894 [DOI] [PubMed] [Google Scholar]
- 72. Rifkin‐Zybutz R, Erridge S, Holvey C, Coomber R, Gaffney J, Lawn W, et al. Clinical outcome data of anxiety patients treated with cannabis‐based medicinal products in the United Kingdom: a cohort study from the UK medical cannabis registry. Psychopharmacology (Berl). 2023;240:1735–1745. 10.1007/s00213-023-06399-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wajszilber D, Santiseban JA, Gruber R. Sleep disorders in patients with ADHD: impact and management challenges. Nat Sci Sleep. 2018;10:453–480. 10.2147/NSS.S163074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kesner AJ, Lovinger DM. Cannabinoids, endocannabinoids and sleep. Front Mol Neurosci. 2020;13:125. 10.3389/fnmol.2020.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kaul M, Zee PC, Sahni AS. Effects of cannabinoids on sleep and their therapeutic potential for sleep disorders. Neurotherapeutics. 2021;18(1):217–227. 10.1007/s13311-021-01013-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sznitman SR, Vulfsons S, Meiri D, Weinstein G. Medical cannabis and insomnia in older adults with chronic pain: a cross‐sectional study. BMJ Support Palliat Care. 2020;10(4):415–420. [DOI] [PubMed] [Google Scholar]
- 77. Vlessides M. Cannabis for sleep: short‐term benefit, long‐term disruption? [Internet]. 2020. Mdedge.com. Frontline Medical Communications Inc. [cited 2023 May 2]. Available from: https://www.mdedge.com/chestphysician/article/216153/sleep‐medicine/cannabis‐sleep‐short‐term‐benefit‐long‐term‐disruption
- 78. Cassoff J, Wiebe ST, Gruber R. Sleep patterns and the risk for ADHD: a review. Nat Sci Sleep. 2012;4:73–80. 10.2147/NSS.S31269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ergisi M, Erridge S, Harris M, Kawka M, Nimalan D, Salazar O, et al. UK medical cannabis registry: an analysis of clinical outcomes of medicinal cannabis therapy for generalized anxiety disorder. Expert Rev Clin Pharmacol. 2022;15(4):487–495. [DOI] [PubMed] [Google Scholar]
- 80. Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32(3):549–575. 10.1016/j.psc.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Guimarães FS, Chiaretti TM, Graeff FG, Zuardi AW. Antianxiety effect of cannabidiol in the elevated plus‐maze. Psychopharmacology (Berl). 1990;100(4):558–559. [DOI] [PubMed] [Google Scholar]
- 82. Skogli EW, Teicher MH, Andersen PN, Hovik KT, Øie M. ADHD in girls and boys – gender differences in co‐existing symptoms and executive function measures. BMC Psychiatry. 2013;13(1):298. 10.1186/1471-244X-13-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stibbe T, Huang J, Paucke M, Ulke C, Strauss M. Gender differences in adult ADHD: cognitive function assessed by the test of attentional performance. PLoS One. 2020;15(10):e0240810. 10.1371/journal.pone.0240810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Colizzi M, Bhattacharyya S. Cannabis use and the development of tolerance: a systematic review of human evidence. Neurosci Biobehav Rev. 2018;93:1–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data that support the findings of this study are available from the UK Medical Cannabis Registry for organizations with an appropriate data safe haven. Restrictions apply to the availability of pseudonymized data in accordance with the ethical approval provided by South West–Central Bristol Research Ethics Committee (Reference: 22/SW/0145). Data specifications and applications are available from the corresponding author.


