Abstract
The xylP gene of Lactobacillus pentosus, the first gene of the xylPQR operon, was recently found to be involved in isoprimeverose metabolism. By expression of xylP on a multicopy plasmid in Lactobacillus plantarum 80, a strain which lacks active isoprimeverose and d-xylose transport activities, it was shown that xylP encodes a transporter. Functional expression of the XylP transporter was shown by uptake of isoprimeverose in L. plantarum 80 cells, and this transport was driven by the proton motive force generated by malolactic fermentation. XylP was unable to catalyze transport of d-xylose.
Lactobacillus pentosus is a facultative heterolactic bacterium which is characteristically found during the natural fermentation of vegetables. L. pentosus develops predominantly in green olives, cucumbers, or cabbages in combination with yeasts and other heterolactic bacteria, such as species of the genus Leuconostoc or Pediococcus (4). In plant material fermentations, in which several microorganisms usually take part simultaneously and sequentially, the availability of nutrients is of crucial importance to the survival of one or more particular species of the microbial population. The ability to ferment the degradation products of the plant cell wall, a structure rich in polysaccharides, may be an important criterion of selection for the microflora adapted to growth on fermented vegetables. L. pentsus, for instance, is capable of fermenting isoprimeverose [α-d-xylopyranosyl-(1,6)-d-glucopyranose], the major end product of xyloglucan hydrolysis. Recently, we reported that isoprimeverose metabolism in L. pentosus involves the expression of an operon located within the xyl regulon, whose expression is inducible by xylose (2). The operon comprises three genes: xylP, encoding a putative permease; xylQ, encoding a membrane-associated α-xylosidase which is responsible for the hydrolysis of isoprimeverose into glucose and xylose; and xylR, encoding a negative transcriptional regulator of the regulon. The xylose formed by the activity of the α-xylosidase on isoprimeverose is further catabolized to xylulose 5-phosphate by d-xylose isomerase and d-xylulose kinase, encoded by the distal genes of the xyl regulon, xylA and xylB. Disruption of xylP did not abolish or reduce the ability of L. pentosus to take up and metabolize d-xylose, suggesting that XylP might be a transporter specific for the uptake of isoprimeverose (α-xyloside) rather than a transporter of the monosaccharide. However, the ΔxylP mutation exerted a polar effect on xylQ expression. Therefore, a role for XylP in isoprimeverose metabolism could not be assessed with certainty. A L. pentosus mutant carrying a mutation of the xylP gene without an effect on xylQ expression was not obtained. For this reason, we have chosen another strategy to assess the role of XylP in d-xylose and isoprimeverose uptake.
Knowledge about xyloside transporters in bacteria is scarce. So far, the only bacterial xyloside uptake system that has been characterized involves the product of the msiK gene of Streptomyces lividans (7). This gene encodes an ATP-binding protein required for the transport of cellobiose and xylobiose. The product of xylP does not belong to the family of ATP-binding proteins but shows similarity to the galactoside-pentose-hexuronide (GPH) translocators, a family of cation symporters which use the proton motive force (PMF) to drive the accumulation of di- or trisaccharides inside the cell (14). In this family, XylP is most closely related to XynC, a putative β-(1,4)-xyloside (oligomeric xylan) transporter encoded by a gene located in the xyl regulon of Bacillus subtilis. Its expression is induced by xylose (6). Although neither biochemical nor genetic evidence demonstrating a role for the product of xynC in β-xyloside transport has been obtained, the similarity between XylP and XynC suggested that these two proteins might constitute a group of bacterial cation symporters specific for the uptake of xylosides.
To investigate the activity and substrate specificity of XylP toward isoprimeverose and d-xylose, we attempted to express the xylP gene in a bacterial strain which was deficient in d-xylose transport. Since radiolabelled isoprimeverose was not available, the most straightforward strategy for measurement of the accumulation of this α-xyloside was to determine, after transport, the intracellular concentration of glucose liberated by hydrolysis of isoprimeverose by the L. pentosus MD353 α-xylosidase. Therefore, to avoid degradation of the disaccharide during the transport experiment, it was important that the bacterial strain used in the isoprimeverose transport lacked all α-xylosidase activity.
Expression of xylP in L. plantarum 80.
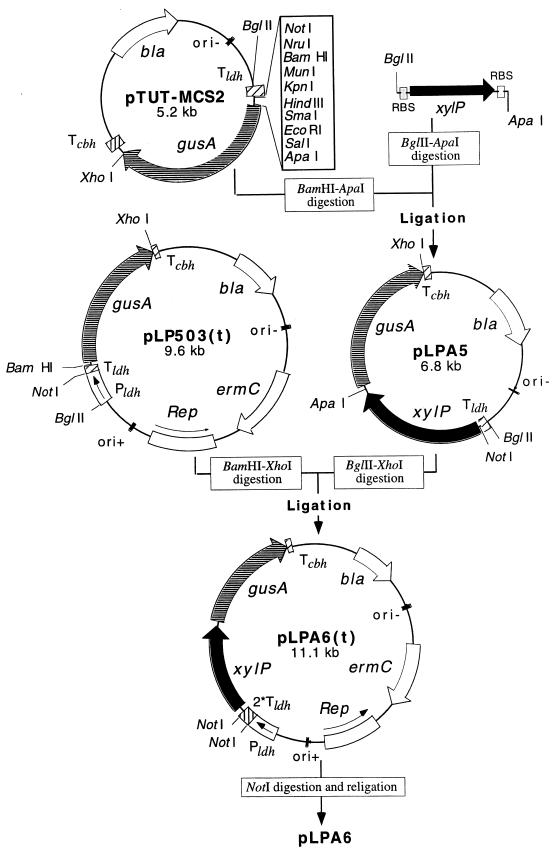
The xylP gene was cloned in a recently developed Lactobacillus expression vector [pLP503(t) (15)]. The gene was expressed in Lactobacillus plantarum 80 (16), a strain which is phylogenetically closely related to L. pentosus (5) but lacks the ability to ferment both isoprimeverose and xylose. The lack of xylP and xylQ (α-xylosidase) sequences in L. plantarum 80 was confirmed by Southern hybridization of L. plantarum 80 chromosomal DNA with a xylP or xylQ probe under heterologous conditions (data not shown). For a number of Lactobacillus strains, electrotransformation with plasmid DNA is inefficient and cloning of genes in Lactobacillus vectors requires a subcloning step in Escherichia coli. Thus, the construction of an xylP expression vector, pLPA6, was achieved by a multistep process (Fig. 1). Briefly, the xylP gene, which contained its original ribosome binding site (RBS) and that of the xylQ gene, was amplified from chromosomal DNA by PCR (using the ULTma enzyme [Perkin-Elmer]) and cloned into pTUT-MCS2 (9) downstream from a strong terminator (Tldh from Lactobacillus casei ATCC 393 [15]). The cassette of the resulting plasmid, pLPA5, was isolated and cloned in the hybrid Lactobacillus-E. coli shuttle expression vector pLP503(t), downstream from a second Tldh site, yielding pLPA6(t). The presence of two Tldh sequences blocked the transcription from the strong promoter (Pldh from L. casei ATCC 393) and circumvented instability of the expression vector in E. coli (15). Finally, the two Tldh sequences were eliminated by digestion of the plasmid with NotI and religation, yielding pLPA6. As a result, a TAA stop codon located 10 nucleotides upstream of the original xylP RBS was in frame with the first few codons of the ldh gene and allowed translation of the native XylP protein. The cloning of the PCR fragment in the ApaI site of pTUT-MCS2 placed the original xylQ RBS 10 nucleotides upstream of the ATG codon of gusA, constituting a translation initiation site for this reporter gene. L. plantarum 80 was transformed with plasmid pLPA6 as described elsewhere (8), and the transformants were selected on M medium (10) agar plates containing 25 mM glucose, 60 μg of 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-Gluc; Sigma Chemical Co., St. Louis, Mo.)/ml, 100 mM potassium phosphate buffer (pH 7.4), and 5 μg of erythromycin/ml. The transformants expressing the gusA reporter gene (i.e., colonies with a blue phenotype) were selected for the uptake studies.
FIG. 1.
Strategy for the construction of the Lactobacillus-E. coli shuttle plasmid pLPA6(t) and the expression vector pLPA6. The forward primer (5′-TTCTAAGATCTAGGTACCATTAATTGAATTCAGAAAGAAGGC-3′) used in the PCR amplification of xylP generated BglII and KpnI sites (underlined) and a stop codon (indicated in boldface) upstream of the original xylP RBS. The reverse primer (5′-TTAAGGGCCCTCCTTTCTTATCCCATCTTAC-3′) generated an ApaI site (underlined) downstream of the original xylQ RBS. The expression cassette of pLPA6 was derived from plasmid pLP503(t) (a broad-host-range expression vector) (15), which contains the constitutive promoter Pldh of L. casei ATCC 393, the β-glucuronidase gene (gusA) from E. coli, and the terminator Tcbh of L. plantarum 80 (3). bla, β-lactamase (ampicillin resistance) determinant; ermC, erythromycin resistance determinant; ori−, origin of replication of pGEM; ori+, origin of replication of Lactobacillus plasmid pLP3537 (10); Rep, replication protein gene of pLP3537; 2*Tldh, two tandemly arranged transcription terminators of the ldh gene of L. casei.
Isoprimeverose transport in L. plantarum 80 cells harboring pLPA6 and in wild-type L. plantarum 80.
The strategy used to investigate the accumulation of nonradiolabelled isoprimeverose in L. plantarum was as follows. (i) L. plantarum 80 cells in the exponential phase of growth were harvested by centrifugation (5,000 × g, 4°C, 10 min), washed twice with 0.9% NaCl, and resuspended in KPM buffer (50 mM KH2PO4, 2 mM MgSO4), pH 4.5, at a concentration of 5 mg (dry weight)/ml. Subsequently, 500 μl of the cell suspension was incubated for 2 min at 37°C and energized with 50 mM l-malate. At a high concentration of l-malate (>5 mM), L. plantarum cells take up this dicarboxylic acid by a low-affinity system which generates a large PMF (∼160 mV) (11). Transport was initiated by adding isoprimeverose (purified from Tamarind seed xyloglucan [2]) at a final concentration of 0.5 mM. After incubation, the reaction was quenched by addition of 2 ml of ice-cold 0.1 M LiCl and the cells were pelleted within a few seconds (15,000 rpm in a tabletop centrifuge). (ii) The pellet was resuspended in 200 μl of ice-cold KPM buffer (pH 6.5), and the cells were disrupted by shaking them at full speed (IKA-VIBRAX-VXR; IKA-Labortechnik) for 1 h at 4°C with 50 mg of glass beads (0.1- to 0.3-mm diameter; Pertorp Analytical). After centrifugation (15,000 × g, 4°C, 15 min), the supernatant was boiled for 10 min at 100°C and denatured proteins were precipitated by centrifugation (15,000 × g, 4°C, 15 min). (iii) The supernatant was then incubated for 1 h with 20 μg of an L. pentosus MD353 membrane fraction containing α-xylosidase (2). The glucose concentration in each of these samples was determined as described elsewhere (1), and the intracellular concentration of glucose was calculated by assuming an average intracellular volume of 3 μl/mg (dry weight).
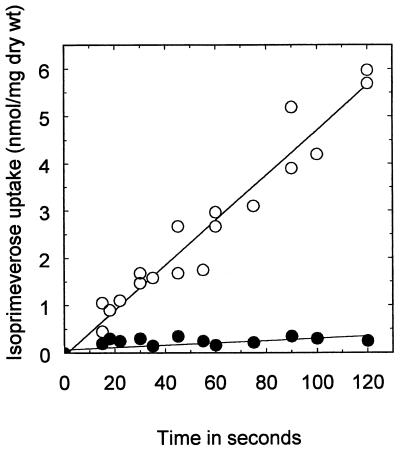
In a control experiment, the α-xylosidase was omitted to determine the concentration of glucose in L. plantarum cells. A second control experiment in which no l-malate was added prior to the transport reaction was carried out. The results are shown in Table 1. L. plantarum 80/pLPA6 took up and accumulated isoprimeverose against a concentration gradient. This uptake did not occur in the untransformed wild-type strain. Therefore, the product of the xylP gene is an isoprimeverose transporter. The uptake of isoprimeverose appeared to be driven by the PMF created by malolactic fermentation, since in the absence of l-malate the same amount of glucose (<0.2 mM) was found in transformed and untransformed bacteria (Table 1, reaction 1). The role of the PMF in the accumulation of isoprimeverose was further demonstrated when the extracellular pH was increased to 6.5, resulting in a strong decrease in both l-malate transport and the PMF resulting from it (11). Under these conditions, isoprimeverose could accumulate only to a concentration of 0.5 mM in L. plantarum 80/pLPA6 after 2 min. The uptake of isoprimeverose was linear for at least 2 min (Fig. 2).
TABLE 1.
Accumulation of isoprimeverose
| Reaction no. | Uptake of isoprimeverose with protocol involvinga:
|
Intracellular glucose concn (mM) inc:
|
|||||
|---|---|---|---|---|---|---|---|
| Uptake treatment with:
|
Postuptake treatment with α-xylosidaseb |
L. plantarum 80/pLPA6
|
L. plantarum 80 (wild type)
|
||||
| l-Malate, 50 mM | Isoprimeverose, 0.5 mM | At 2 min | At 10 min | At 2 min | At 10 min | ||
| 1 | − | + | + | <0.2 | <0.2 | <0.2 | <0.2 |
| 2 | + | + | + | 2.01 ± 0.02 | 5.00 ± 0.25 | <0.2 | <0.2 |
| 3 | + | + | − | <0.2 | <0.2 | <0.2 | <0.2 |
+, uptake occurred; −, uptake did not occur. All experiments were performed in duplicate.
Corresponding to 20 μg of xylose-grown L. pentosus MD353 membrane protein.
The transport reaction was stopped at 2 min and at 10 min. All experiments were performed in duplicate.
FIG. 2.
Time course for isoprimeverose uptake by L. plantarum 80/pLPA6 (○) and L. plantarum 80 wild-type cells (•) at an extracellular pH of 4.5. Cells were energized with l-malate (50 mM), and transport was assayed as described in the text. The amount of isoprimeverose taken up was obtained by calculating the difference of the amount of glucose measured in the presence of l-malate (Table 1, reaction 2) and the amount of glucose measured in the absence of l-malate (Table 1, reaction 1).
d-Xylose transport in L. plantarum 80 cells harboring pLPA6 and in wild-type L. plantarum 80.
We have also tested the ability of L. plantarum 80/pLPA6 and L. plantarum 80 wild-type cells to accumulate d-[U-14C]xylose (specific activity, 0.4 μCi/mmol; Amersham). The transport experiments were conducted as described above for the uptake of isoprimeverose. The final concentration of d-xylose was 0.5 mM. In this case, the cells were rapidly filtered through glass fiber filters (GF/F; Whatman) after quenching and washed with 2 ml of ice-cold 0.1 M LiCl. No uptake or accumulation of d-[U-14C]d-xylose by the two strains tested could be detected (data not shown). The use of radiolabelled xylose also enabled us to study the accumulation of pentose under different PMF-generating conditions, mediated by the fermentation of 5 mM glucose, added 5 min prior to the transport reaction. In this case, the reaction was performed at an extracellular pH of 6.5. Also under those conditions, xylose uptake by the two strains could not be detected (data not shown). These results demonstrate that XylP does not mediate uptake of xylose.
Final conclusions.
This study has provided data confirming that the L. pentosus xylP gene encodes an isoprimeverose cation symporter. Under the conditions used for the transport experiment, the rate of isoprimeverose uptake was in the range of 3 nmol/min/mg (dry weight). It is possible that the rate of uptake may be higher under other conditions, especially at different pH values. Indeed, the extracellular pH can affect the activity of H+ symporters. The activity of the lactose H+ symporter LacS of Streptococcus thermophilus, for instance, is pH dependent, with an optimal activity at pH 6 and reduced activity at a lower or higher pH (13). Therefore, the extracellular pH of 4.5 used in the isoprimeverose transport assay, conditions required to generate a PMF via l-malate transport and metabolism, might not be an optimal pH for the activity of the XylP transporter. The transport of isoprimeverose by XylP was found to be dependent on the generation of a PMF, although the nature of the cation transported in symport with isoprimeverose is not yet known.
As mentioned above, XylP shows sequence similarity to the GPH family of translocators, especially to XynC of B. subtilis. Little is known about the XynC transporter, but Schmiedel and Hillen (17) have shown that B. subtilis expressing xynC could not take up d-xylose, indicating that XynC is not a d-xylose transporter. Similarly, our results show that XylP does not transport the monosaccharide. These observations provide the important finding that XylP and XynC are presumably transporters specific for the uptake of xylosides (with α and/or β linkages) but are not transporters of the pentose, although the designation of the GPH family had initially suggested otherwise (14).
Obviously, more-detailed studies of the XylP transporter could be conducted if radiolabelled xylosides were to become available. Recently, however, chromogenic substrate analogs have been used to assess uptake activity (12). Indeed, the design of chromogenic α/β-xyloside analogs may provide useful tools for the continued study of XylP and other xyloside transporters in bacteria.
Acknowledgments
We thank Rob J. Leer (TNO Nutrition and Food Research Institute) for suggestions concerning the construction of plasmid pLPA6.
This work was supported by EC grant BIO2-CT92-0137.
REFERENCES
- 1.Bergmeyer H U. Methods of enzymatic analysis. Deerfield Beach, Fla: VCH Publishers; 1985. [Google Scholar]
- 2.Chaillou S, Lokman B C, Leer R J, Posthuma C, Postma P W, Pouwels P H. Cloning, sequence analysis, and characterization of the genes involved in isoprimeverose metabolism in Lactobacillus pentosus. J Bacteriol. 1998;180:2312–2320. doi: 10.1128/jb.180.9.2312-2320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christiaens H, Leer R J, Pouwels P H, Verstraete W. Cloning and expression of a conjugated bile acid hydrolase gene from Lactobacillus plantarum by using a direct plate assay. Appl Environ Microbiol. 1992;58:3792–3798. doi: 10.1128/aem.58.12.3792-3798.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daeschel M A, Anderson R E, Fleming H P. Microbial ecology of fermenting plant material. FEMS Microbiol Rev. 1987;46:357–367. [Google Scholar]
- 5.Hammes W P, Vogel R F. The genus Lactobacillus. In: Wood B J B, Holzapfel W H, editors. The genera of lactic acid bacteria. Glasgow, Scotland: Blackie Academic & Professional Publishers; 1995. pp. 18–54. [Google Scholar]
- 6.Hastrup S. Analysis of the Bacillus subtilis xylose regulon. In: Cramson A T, Hoch J A, editors. Genetics and biotechnology of bacilli. New York, N.Y: Academic Press, Inc.; 1988. pp. 79–84. [Google Scholar]
- 7.Hurtubisse Y, Sharek F, Kluepfel D, Morosoli R. A cellulase/xylanase-negative mutant of Streptomyces lividans 1326 defective in cellobiose and xylobiose uptake is mutated in a gene encoding a protein homologous to ATP-binding proteins. Mol Microbiol. 1995;17:367–377. doi: 10.1111/j.1365-2958.1995.mmi_17020367.x. [DOI] [PubMed] [Google Scholar]
- 8.Josson K, Scheirlinck T, Michiels F, Platteeuw C, Stanssens P, Joos H, Dhaese P, Zabeau M, Mahillon J. Characteristics of a Gram-positive broad host range plasmid isolated from Lactobacillus hilgardii. Plasmid. 1989;11:9–20. doi: 10.1016/0147-619x(89)90082-6. [DOI] [PubMed] [Google Scholar]
- 9.Leer, R. J. Personal communication.
- 10.Lokman B C, Leer R J, van Sorge R, Pouwels P H. Promoter analysis and transcriptional regulation of Lactobacillus pentosus genes in xylose catabolism. Mol Gen Genet. 1994;245:117–125. doi: 10.1007/BF00279757. [DOI] [PubMed] [Google Scholar]
- 11.Olsen E B, Russell J B, Henick-Kling T. Electrogenic l-malate transport by Lactobacillus plantarum: a basis for energy derivation from malolactic fermentation. J Bacteriol. 1991;173:6199–6206. doi: 10.1128/jb.173.19.6199-6206.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poolman, B. Personal communication.
- 13.Poolman B, Knol J, Lolkema J S. Kinetics analysis of lactose and proton coupling in Glu379 mutants of the lactose transport protein of Streptococcus thermophilus. J Biol Chem. 1995;270:12995–13003. doi: 10.1074/jbc.270.22.12995. [DOI] [PubMed] [Google Scholar]
- 14.Poolman B, Knol J, van der Does C, Henderson P J F, Liang W J, Leblanc G, Pourcher T, Mus-Veteau I. Cation and sugar selectivity determinants in a novel family of transport proteins. Mol Microbiol. 1996;19:911–922. doi: 10.1046/j.1365-2958.1996.397949.x. [DOI] [PubMed] [Google Scholar]
- 15.Pouwels P H, Leer R J, Boersma W J A. The potential of Lactobacillus as a carrier for oral immunization: development and preliminary characterization of vector systems for targeted delivery of antigens. J Biotechnol. 1996;44:183–192. doi: 10.1016/0168-1656(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 16.Scheirlinck T, Mahillon J, Joos H, Dhaese P, Michiels F. Integration and expression of α-amylase and endoglucanase genes in the Lactobacillus plantarum chromosome. Appl Environ Microbiol. 1989;55:2130–2137. doi: 10.1128/aem.55.9.2130-2137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmiedel D, Hillen W. A Bacillus subtilis 168 mutant with increased xylose uptake can utilize xylose as sole carbon source. FEMS Microbiol Lett. 1996;135:175–178. [Google Scholar]