Abstract
Pulmonary hypertension (PH) is the most severe complication in preterm infants with bronchopulmonary dysplasia (BPD) and associated with significant mortality. Diagnostic and treatment strategies, however, still lack standardization. By the use of a survey study (PH in BPD), we assessed clinical practice (diagnosis, treatment, follow‐up) in preterm infants with early postnatal persistent pulmonary hypertension of the newborn (PPHN) as well as at risk for or with established BPD‐associated PH between 06/2018 and 10/2020 in two‐thirds of all German perinatal centers with >70 very low birthweight infants/year including their cardiology departments and outpatient units. Data were analyzed descriptively by measures of locations and distributional shares. In routine postnatal care, clinical presentation and echocardiography were reported as the main diagnostic modalities to screen for PPHN in preterm infants, whereas biomarkers brain natriuretic peptide/N‐terminal pro b‐type natriuretic peptide were infrequently used. For PPHN treatment, inhaled nitric oxide was used in varying frequency. The majority of participants agreed to prescribe diuretics and steroids (systemic/inhaled) for infants at risk for or with established BPD‐associated PH and strongly agreed on recommending respiratory syncytial virus immunization and the use of home monitoring upon discharge. Reported oxygen saturation targets, however, varied in these patients in in‐ and outpatient care. The survey reveals shared practices in diagnostic and therapeutic strategies for preterms with PPHN and BPD‐associated PH in Germany. Future studies are needed to agree on detailed echo parameters and biomarkers to diagnose and monitor disease next to a much‐needed agreement on the use of pulmonary vasodilators, steroids, and diuretics as well as target oxygen saturation levels.
Keywords: bronchopulmonary dysplasia, chronic lung disease, clinical practice, preterm infant, pulmonary hypertension
INTRODUCTION
The most prevalent morbidity in the preterm infant is neonatal chronic lung disease (CLD), internationally known as Bronchopulmonary Dysplasia (BPD). The disease develops in preterm infants as a consequence of risk factors impacting on the immature lung such as growth restriction, pre‐ and postnatal infections as well as life‐saving, yet injury‐provoking postnatal treatments such as invasive or noninvasive ventilation and oxygen supplementation. 1 The subsequent development of a malstructured and malfunctional gas exchange area results in long‐term impairment of lung function and the risk to develop pulmonary hypertension (PH), associated with significant morbidity and mortality. 2 , 3 Postnatally, the failure to reduce pulmonary vascular resistance (PVR) during hemodynamic transition is intertwined with ventilation impairment presenting as respiratory distress syndrome and presents as persistent pulmonary hypertension of the newborn (PPHN) with right‐to‐left shunting via the foramen ovale, ductus arteriosus, and intrapulmonary shunts that further complicate the clinical picture. 4 , 5
In the preterm infant, pulmonary vascular disease (PVD) furthermore evolves when the functionally as well as structurally immature vascular bed is exposed to the above indicated pre‐ and postnatal hazards and characterizes the majority of infants with moderate and severe BPD. 6 , 7 When PVD progresses, significant vascular remodeling leads to the development of precapillary PH (Nice classification group 3) 8 , 9 , 10 , 11 , 12 in one out of four infants with moderate or severe BPD. 1 , 13
Despite the significant immediate and long‐term consequences of pathologic pulmonary blood flow in the preterm neonate, 14 strategies to diagnose, monitor, and treat early postnatal (PPHN) or later PH occurring in cases of developing or established BPD are limited. These limitations result from the lack of standardization and sensitive diagnostic tools, 7 , 14 , 15 complicated by the undefined transition from early postnatal PH, here referred to as PPHN, into PVD into fully established BPD‐associated PH.
As of today, the diagnostic process is dominated by clinical evaluation confronted with a broad variety of mostly unspecific clinical symptoms and the use of echocardiography. Infants present with signs of impaired gas exchange, e.g., hypoxemia, which typically persists despite the optimization of pulmonary ventilation and associated with the risk to culminate in arterial hypotension, shock and multiorgan failure. The clinical picture in BPD‐associated PH, both borderline or readily established, can include reduced exercise tolerance and symptom exacerbation during respiratory infections 16 , 17 , 18 with clinical symptoms only insufficiently discriminating between vascular, alveolar, and airway pathology and progression of PVD into fully established PH mostly going unnoticed.
As the lack of monitoring and treatment standards results in expert opinion and case‐to‐case‐based decisions that most likely vary between perinatal centers, we assessed current clinical practice for treatment and monitoring strategies in preterm infants with PPHN or infants at risk for or with established BPD‐associated PH by the means of a survey study (PUlmonary Hypertension as a Complication of Bronchopulmonary Dysplasia in Germany (PUsH BPD) that addressed a large number of perinatal centers in Germany from 06/2018 until 10/2020. The results are intended to specify the need for future clinical guidelines and their content, inform training programs as well as the design of clinical trials.
METHODS
Questionnaire and participants
We developed and piloted a questionnaire to portray routine diagnostic and treatment strategies for preterm infants with early postnatal PH, e.g., PPHN, and for infants at risk for or with established BPD‐associated PH in German perinatal centers. BPD was defined according to Jobe et al. (NIH consensus conference). 19
Besides the sociodemographic and occupational information of the respondents, the questionnaire comprised questions about first‐line diagnostic and treatment practices as well as the established structures and strategies for follow‐up care (online Supporting Information S1: Table E1). The questionnaire was distributed as an online version to physicians of >30 German perinatal centers in the time from 06/2018 until 10/2020. Informed consent was obtained from all study participants (EC# (LMU Munich) 21‐0753).
Data processing and analysis
A total of 54 individuals participated in the survey, thereby representing two‐thirds of all German perinatal centers with >70 very low birthweight infants/year including the respective pediatric cardiology departments. Coverage of perinatal centers was calculated according to the zip code provided by the participants for the working address and cross‐checked for the statements provided for numbers of infants treated per year. To fulfill the criteria of anonymity, employment was not related to specific perinatal centers so that the descriptive statistical analyses could not account for potential clustering.
A total of 42% (18/43) of the participants reported for their respective perinatal center to treat >100 preterm infants below 32 weeks gestational age (GA) per year, whereas 23% (10/43) reported 70–99 preterm infants per year and 21% (9/43) reported <70 preterm infants to be treated per year. The remaining six individuals did not answer the corresponding question.
Due to several missing answers, the following sample reduction rules were defined and applied: First, questionnaires, which only contained information on background characteristics of the respondents as well as questionnaires where all three sections of the questionnaire were left unanswered were excluded from analysis. In the second step, missingness per questionnaire and per item was analyzed to ensure validity and reliability of the data set. Questionnaires with >30% missingness (n = 11, 20% total) as well as items with a response rate below 30% (n = 49 items) were excluded from further analysis. The final analysis was therefore based on n = 43 questionnaires and n = 73 out of 122 items.
For data analysis and results presentation, the statements were divided into data addressing diagnostic procedures, therapeutic decision making and follow‐up care for (a) preterm infants with early postnatal PH, e.g., PPHN and (b) cases at risk for or with established BPD‐associated PH. 8 Data were analyzed descriptively by measures of locations and distributional shares. Descriptive data are presented as percentages and absolute numbers (categorical variables) or means and standard deviation (continuous variables), respectively using the Software SAS 9.4.
RESULTS
Results presented are based on n = 43 questionnaires and n = 73 out of 122 items (see methods for strategy of inclusion/exclusion).
Participants
Participants of whom results were included in the final analysis were physicians of different experience levels. A total of 33% (14/43) of the included study participants (n = 43) identified themselves as female, 63% (27/43) as male. A total of 9% (4/43) of the participants reported their age to range between 30 and 39 years, 47% (20/43) between 40 and 49 years, and 42% (18/43) as 50 years and older. A total of 44% (19/43) of the participants reported work experience between 11 and 20 years, 44% (19/43) reported work experience >20 years. A total of 91% (39/43) of the survey participants reported in a multiple selection item to be trained neonatologists, 49% (21/43) documented to be specialized in pediatric intensive care, and 26% (11/43) reported to be trained pediatric cardiologists. A total of 70% (30/43) reported to work in a university hospital, whereas 28% (12/43) reported to work in a nonuniversity hospital. A total of 93% (40/43) of all participants documented to work in neonatal intermediate or neonatal intensive care units (NICU), 49% (26/43) worked in general pediatric or mixed intensive care units.
The participating physicians characterized their respective perinatal centers as follows: 42% (18/43) reported to treat more than 100 preterm infants with and without BPD below 32 weeks GA per year, 23% (10/43) reported to treat 70–99 preterm infants per year, and 21% (9/43) reported to treat <70 preterm infants per year in their perinatal center. In 14% (6/43) of the cases, information on this measure was missing. A total of 81% (35/43) of the participants reported in‐patient care with 10–20 NICU beds, 12% (5/43) of cases reported >20 NICU beds, 7% (3/43) of cases reported <10 NICU beds.
Participant and perinatal center characteristics are displayed in Table 1. Supporting Information S2: Figure 1OS in the Online Supplement displays the distribution of the participating perinatal centers throughout Germany.
Table 1.
Participant characteristics.
| Parameter | n‐number of cases (percentage of total) |
|---|---|
| Study participants | 43 |
| Gender | |
| Female | 27 (62.8%) |
| Male | 14 (32.6%) |
| No specification | 2 (4.7%) |
| Age or participants in years | |
| 30–39 | 4 (9.3%) |
| 40–49 | 20 (46.5%) |
| ≥50 | 18 (41.9%) |
| No specification | 1 (2.3%) |
| Professional experience in years | |
| 5–10 | 3 (7.0%) |
| 11–20 | 19 (44.2%) |
| >20 | 19 (44.2%) |
| No specification | 2 (4.7%) |
| Qualification | |
| Neonatology | 39 |
| Pulmonology | 0 |
| Cardiology | 11 |
| Intensive care | 21 |
| Other | 5 |
| None | 15 |
| Professional environment | |
| University hospital | 28 (65.1%) |
| Hospital | 11 (25.6%) |
| Other | 2 (4.7%) |
| No specification | 2 (4.7%) |
Diagnostic and therapeutic strategies in preterm infants with early postnatal PH, that is, PPHN
Decisions for therapy initiation for preterm infants with PPHN were based on clinical presentation (93%, 40/43), such as the occurrence of periods of undersaturation or clinical signs of pulmonary hypertensive crisis, as well as echocardiographic findings (95%, 41/43). The following echocardiographic criteria were reported to be considered when diagnosing PPHN in the preterm infant: right ventricle (RV) dimension (74%, 32/43), tricuspid valve insufficiency (TI) (81%, 35/43), tricuspid regurgitation velocity (TRV) (79%, 34/43), right–left shunting (atrium) (77%, 33/43), right–left shunting (via the persistent ductus arteriosus) (84%, 36/43), and qualitative assessment of the right atrium (61%, 26/43). A total of 40% (17/43) of the participants reported to use the M‐mode in the parasternal short axis (PSAX) or a comparable other measurement for quantitative sizing of the RV and stated to include this measure in diagnosis. A total of 28% (12/43) of the participants, however, reported to not perform quantitative sizing as part of the diagnostic procedure (33%, 14/43 did not provide information). The use of pulmonary acceleration time in the PSAX axis measured by echocardiography was reported by 51% (22/43), whereas 21% (9/43) of the enrolled physicians stated to not use this echocardiographic measure for PH diagnosis (28%, 12/43 of the participants did not provide information). Qualitative assessment of RV contractility in echocardiography was used by 54% (23/43) of the participants to diagnose PH, whereas 23% (10/43) of the participants did not use this parameter (23%, 10/43 did not provide information). The RV/left ventricle (LV) ratio as an end‐systolic assessment in the PSAX to quantify the size of the RV was reported as a part/not a part of the diagnostic process in 30% (13/43)/37% (16/43); 33% (14/43) did not provide any information on that behalf. Measurement of tricuspid annular plane systolic excursion (TAPSE) was reported by 37% (16/43) of the participants to be used for the diagnosis of PH, 35% (15/43) did not use this measure (28%, 12/43 did not provide information) (Figure 1).
Figure 1.
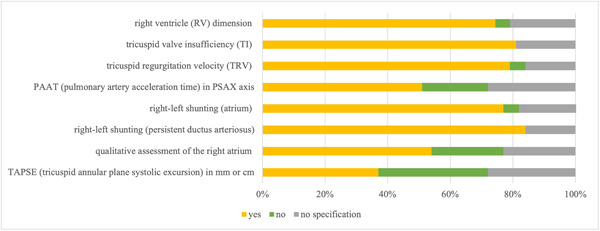
Echocardiographic parameters considered when diagnosing preterm infants with early postnatal (PPHN). The following echocardiographic criteria were reported to be considered when diagnosing PPHN in the preterm infant: right ventricle (RV) dimension (74%, 32/43), tricuspid valve insufficiency (81%, 35/43), tricuspid regurgitation velocity (79%, 34/43), right–left shunting (atrium) (77%, 33/43), right–left shunting (via persistent ductus arteriosus) (84%, 36/43), and qualitative assessment of the right atrium (61%, 26/43). A total of 40% (17/43) of the participants reported to use the M‐mode in the parasternal short axis (PSAX) or a comparable other measurement for quantitative sizing of the RV and stated to include this measure in the diagnosis. A total of 28% (12/43) of the participants, however, reported to not perform quantitative sizing as part of the diagnostic procedure (33%, 14/43 did not provide information). The use of pulmonary acceleration time in the PSAX axis measured by echocardiography was reported by 51% (22/43), whereas 21% (9/43) of the enrolled physicians stated to not use this echocardiographic measure for pulmonary hypertension (PH) diagnosis (28%, 12/43 of the participants did not provide information). Qualitative assessment of RV contractility in echocardiography was used by 54% (23/43) of the participants to diagnose PH, whereas 23% (10/43) of the participants did not use the parameter (23%, 10/43 did not provide information). The RV/left ventricle ratio as an end‐systolic assessment in the PSAX to quantify the size of the RV was reported as a part/not a part of the diagnostic process in 30% (13/43)/37% (16/43); 33% (14/43) did not provide any information on that behalf. Measurement of tricuspid annular plane systolic excursion was reported by 37% (16/43) of the participants to be used for the diagnosis of PH; 35% (15/43) did not use this measure (28%, 12/43 did not provide information). The data are provided as the percentage of the total number of participants (43) and items (73) in the final analysis as well as by total n‐numbers.
The use of cardiac catheterization for PPHN preterms was reported by 56% (24/43) of the participants to not be used and by 12% (5/43) to be used to guide therapeutic decisions.
In ventilated preterm patients with PPHN as diagnosed by echocardiography, inhaled nitric oxide (iNO) was reported as the therapy of choice by 84% (36/43) of the participants, with half of the participants reporting to either use this therapeutic strategy regularly (18/36) or only in special cases (18/36). A total of 79% (34/43) of the participants reported to use other pulmonary vasodilators such as Sildenafil. A total of 74% (32/43) of the participants reported Sildenafil to be used for weaning off iNO therapy to prevent rebound PH. In this subgroup, Sildenafil was reported to be administered p.o. in 50% (16/32) and i.v. in 6% (2/32), whereas individual decisions between p.o. or i.v. therapy were reported to be made in 38% (12/32). A total of 65% (28/43) of the participants indicated that <10% of preterms below 32 weeks of GA receive therapy with specific pulmonary vasodilators according to individual treatment plans.
Diagnostic and therapeutic strategies in preterm infants at risk for or with established BPD‐associated PH
The participating physicians reported treating preterm infants below 32 weeks GA per year with respective BPD grades as follows: 28% (12/43) of the participants reported treating less than five, 2% (1/43) reported treating five to nine, 16% (7/43) treating 10–20, 12% (5/43) treating 21–30, and 7% (3/43) reported treating >30 preterm infants with mild BPD in their perinatal center. A total of 40% (17/43) of the participants reported treating less than five, 16% (7/43) reported treating five to nine, 9% (4/43) treating 10–20, and 2% (1/43) treating 21–30 preterm infants with moderate BPD in their perinatal center. A total of 50% (21/43) of the participants reported treating less than five, 7% (3/43) reported treating five to nine, 7% (3/43) treating 10–20, and 2% (1/43) treating 21–30 preterm infants with severe BPD in their perinatal center (Table 2).
Table 2.
Treated preterm infants below 32 weeks GA per year with respective BPD grades.
| Mild BPD | |
| <5 | 12 (27.9%) |
| 5–9 | 1 (2.3%) |
| 10–20 | 7 (16.3%) |
| 21–30 | 5 (11.6%) |
| >30 | 3 (7.0%) |
| No specification | 15 (34.9%) |
| Moderate BPD | |
| <5 | 17 (39.5%) |
| 5–9 | 7 (16.3%) |
| 10–20 | 4 (9.3%) |
| 21–30 | 1 (2.3%) |
| >30 | 0 (0.0%) |
| No specification | 14 (32.6%) |
| Severe BPD | |
| <5 | 21 (48.8%) |
| 5–9 | 3 (7.0%) |
| 10–20 | 3 (7.0%) |
| 21–30 | 1 (2.3%) |
| >30 | 0 (0.0%) |
| No specification | 15 (34.9%) |
Note: BPD is defined according to Jobe et al as follows: mild BPD with oxygen O2 supplementation for at least 28 days postnatally and breathing room air at 36 weeks GA/at discharge; moderate BPD with O2 supplementation <30% at 36 weeks GA/at discharge; and severe BPD O2 supplementation >30% and/or ventilator support at 36 weeks GA/at discharge. 19
Abbreviations: BPD, bronchopulmonary dysplasia; GA, gestational age.
During postnatal care for preterm infants at risk for or with established BPD‐associated PH, any PH‐related therapy was reported to be initiated in cases with (i) prolonged weaning from mechanical ventilation (84%, 36/43), (ii) signs of right heart strain as detected by echocardiography during weaning from the respirator (84%, 36/43), and (iii) a pathologic oxygen discharge test (72%, 31/43) as well as (iv) insufficient weight gain (49%, 21/43), and (v) chronic hypercapnia (53%, 23/43).
A total of 51% (22/43) of the participants reported that preterm infants at risk for or with established BPD‐associated PH with either signs of PH in echocardiography or at risk for PH as indicated by prolonged ventilation receive a pediatric cardiology consultation before therapy initiation with pulmonary vasodilators other than iNO as standard of care, whereas 26% (11/43) reported consultation with a pediatric cardiologist in individual cases only. The majority of participants (70%, 30/43) reported to not initiate invasive diagnostic procedures before the onset of pulmonary vasodilator therapy, whereas 14% (6/43) reported invasive diagnostics in individual cases and 16% (7/43) claimed to regularly perform catheterization before initiation of pulmonary vasodilatation therapy. The majority of participants (77%, 33/43) did report to not perform regular screening for l‐Arginine deficiency as a precursor of endogenous NO before the initiation of BPD‐associated PH therapy.
Regarding the use of biomarkers to monitor the course of disease during inpatient care, 40% (17/43) used N‐terminal pro b‐type natriuretic peptide (NT‐proBNP) in preterm infants at risk for or with established BPD‐associated PH, in contrast to 35% (15/43) of the participants that did not use brain natriuretic peptide (BNP) or NT‐proBNP for risk patient monitoring. More specifically, none of the participants reported using the combination of BNP and NT‐proBNP before the initiation of PH‐targeted therapy to monitor the course of disease, whereas single markers were used in 35% (15/43) (NT‐proBNP) or 16% (7/43) (BNP), respectively. A total of 40% (17/43) of the participants stated to assess neither BNP nor NT‐proBNP before the initiation of therapy (Figure 2a).
Figure 2.
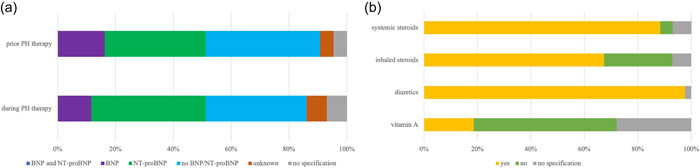
Diagnostic and therapy in premature infants with developing (at risk) or established bronchopulmonary dysplasia (BPD)‐associated pulmonary hypertension (PH). (a) The use of biomarkers BNP and NT‐proBNP in preterm infants with developing (at risk) or established BPD‐associated PH before initiation of therapy in contrast monitoring PH during therapy. None of the participants reported using the combination of BNP and NT‐proBNP before or during therapy. Before initiation of targeted PH therapy to monitor disease progression, individual markers were used in 35% (15/43) (NT‐proBNP), 16% (7/43) (BNP), and 40% (17/43) of participants reported not determining either BNP or NT‐proBNP before initiation of therapy. A total of 40% (17/43) used NT‐proBNP and 12% (5/43) used BNP in infants with developing (at risk) or established BPD‐associated PH during therapy, in contrast to 35% (15/43) of participants who used neither BNP nor NT‐proBNP to monitor high‐risk patients during therapy. (b) Prescription of therapy in preterm infants with developing (at risk) or established BPD‐associated PH. For infants with developing or established BPD‐associated PH before discharge, 98% (42/43) of participants reported the prescription diuretics in addition to systemic steroids (88%, 38/43), whereas prescription of inhalative steroids were reported by 67% (29/43). The recommendation of vitamin A prescription was reported by only 19% (8/43). Most participants (54%, 23/43) documented to not use vitamin A in infants with developing or established BPD‐associated PH, whereas inhalative and systemic steroids were reported by only 26% (11/43) and 5% (2/43). The data are provided as the percentage of the total number of participants (43) and items (73) in the final analysis as well as by total n‐numbers.
For infants at risk for or with established BPD‐associated PH, 98% (42/43) of the participants reported the prescription of diuretics next to the recommendation of systemic steroids (88%, 38/43) whereas prescription of inhalative steroids were reported by 67% (29/43). The majority of participants (54%, 23/43) documented to not use vitamin A treatment in infants at risk for or with established BPD‐associated PH (Figure 2b).
Oxygen saturation levels for preterm patients with BPD without established PH in the hospital were reported to target >95% SpaO2 in 0% (0/43), >92% SpaO2 in 19% (8/43), >90% SpaO2 in 28% (12/43), or other oxygen saturation limits in 12% (5/43), while participants reported oxygen saturation levels for preterm patients at risk for or with established BPD‐associated PH in the hospital to target >95% SpaO2 in 12% (5/43), >92% SpaO2 in 51% (22/43), >90% SpaO2 in 9% (4/43), or other oxygen saturation limits in 12% (5/43). The indication for oxygen supplementation in infants at risk for or with established BPD‐associated PH before discharge was reported to be based on pulse oximetry alone without oxygen testing by 21% (9/43) of the participants, whereas 9% (4/43) stated to use pulse oximetry together with oxygen testing. In 7% (3/43) of the cases, pulse oximetry was reported to be combined with capnography, whereas 12% (5/43) of the participants stated the indication of oxygen therapy to be based on the results obtained by polysomnography and sleep monitoring. A total of 33% (14/43) of the participating physicians documented to not use one of the above measures (19%, 8/43 did not provide information). A total of 51% (22/43) of the physicians reported to use pulse oximetry with correction for movement artifacts.
Near term age, 47% (20/43) of the participating physicians stated to perform routine echocardiograms for infants born at <32 weeks GA before discharge home, including cases with and without the current or historic need for mechanical ventilation, respiratory support or oxygen supplementation. A total of 40% (17/43) reported to not perform routine echocardiograms before discharge. In case of successful treatment with pulmonary vasodilator therapy in a preterm infant, continuation of vasodilator therapy at discharge was reported as a standard in these cases by 61% (26/43) of the physicians. A total of 21% (9/43) of the participants reported to terminate pulmonary vasodilator therapy in these patients before discharge. After termination of vasodilator therapy, reassessment and elective presentation to a pediatric cardiologist was recommended by 65% (28/43) of the participants, whereas 9% (4/43) reported this recommendation only for individual cases (26%, 11/43 did not provide information). Patients discharged with pulmonary vasodilator therapy were referred to a special PH outpatient clinic by 65% (28/43) of the participants. Recommendations for these patients included the performance of routine echocardiograms in the majority of cases (77%, 33/43; 9/43 did not provide an answer).
For postdischarge care, oxygen saturation levels for outpatient care in BPD patients without established PH are stated to target >95% in 9% (4/43) of the cases, >92% in 9% (4/43), and >90% in 37% (16/43) of the cases (27.9%, 12/43 did not provide a statement). In preterm infants at risk for or with established BPD‐associated PH participants reported postdischarge oxygen saturation levels to target >95% SpaO2 in 16% (7/43), >92% SpaO2 in 35% (15/43), >90% SpaO2 in 16% (7/43), or other oxygen saturation limits in 5% (2/43) (12/43 did not provide an answer) (Figure 3).
Figure 3.
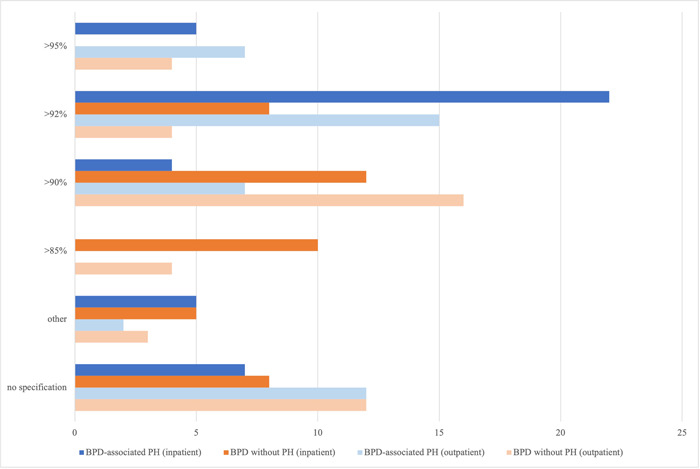
Oxygen saturation targets for preterm infants with bronchopulmonary dysplasia (BPD) (in‐ and outpatient) with or without associated pulmonary hypertension (PH). Oxygen saturation levels for preterm patients with BPD without associated PH in the hospital were reported to target >95% SpaO2 in 0% (0/43), >92% SpaO2 in 19% (8/43), >90% SpaO2 in 28% (12/43), or other oxygen saturation limits in 12% (5/43) while participants reported oxygen saturation levels for preterm patients with BPD‐associated PH in the hospital to target >95% SpaO2 in 12% (5/43), >92% SpaO2 in 51% (22/43), >90% SpaO2 in 9% (4/43), or other oxygen saturation limits in 12% (5/43). For postdischarge care, targeted oxygen saturation levels for outpatient care in BPD patients without established PH are stated to be set at >95% in 9% (4/43) of the cases, >92% in 9% (4/43), and >90% in 37% (16/43) of the cases (27.9%, 12/43 did not provide a statement) while participants reported oxygen saturation levels for preterm infants with BPD‐associated PH as outpatients to target >95% SpaO2 in 16% (7/43), >92% SpaO2 in 35% (15/43), >90% SpaO2 in 16% (7/43), or other oxygen saturation limits in 5% (2/43) (12/43 did not provide an answer). The data are provided as the percentage of the total number of participants (43) and items (73) in the final analysis as well as by total n‐numbers.
The initiation of home monitoring was reported to be based on the need for oxygen supplementation by 84% (36/43) of the participants (16%, 7/43 did not provide information). A total of 70% (30/43) of the participating physicians stated to initiate home monitoring in infants with BPD in combination with a risk for SIDS in the family history (21% [9/43] did not provide an answer). A total of 58% (25/43) reported the use of home monitoring in infants with BPD when occurring together with apnea‐bradycardia syndrome (19% [8/43] did not provide an answer). A total of 16% (7/43) of the participants documented to initiate home monitoring due to prematurity only, whereas 51% (22/43) reported to not follow this strategy (33% [14/43] did not provide an answer).
Overall, home monitoring was reported to be used for the surveillance of oxygen saturation by 81% (35/43) of the participants, heart rate by 77% (33/43) and respiratory rate by 51% (22/43) of the participants.
Premature infants with BPD and established PH were reported to be provided with home oxygen therapy according to the following standards: case‐by‐case decisions in 47% (20/43), all cases regardless of therapy in 16% (7/43), when discharged with vasodilator therapy in 7% (3/43), or not at all in 9% (4/43) (21% [9/43] did not provide an answer).
Respiratory syncytial virus (RSV) immunization was reported to be recommended according to the following strategy: 72% (31/43) of the physicians stated to recommend RSV vaccination in their discharge reports as a standard in neonatal care, 16% (7/43) in individual cases, 2% (1/43) documented to not recommend RSV vaccination (9%, 4/43 did not provide information). For infants born preterm with a GA of 29 0/7–34 6/7 weeks GA and younger than 6 months of age 67% (29/43) of the participants reported to recommend RSV vaccination in case of at least two of the following risk factors: (a) discharge from neonatal care immediately before or during the RSV season (b) daycare attendance or older siblings in external child care, or (c) severe underlying neurological disease at younger than 6 months of age at the start of the RSV season. A total of 19% (8/43) stated to not recommend vaccination in the cases outlined above. In children under 12 months of age, 81% (35/43) reported to recommend RSV vaccination in case of hemodynamically relevant heart disease and/or severe heart failure under treatment at the start of the RSV season, 67% (29/43) of the participants reported to recommend vaccination at the start of the RSV season in case of preterm birth below 28 6/7 weeks GA and younger than 12 months of age, and 84% (36/43) of the physicians stated to recommend RSV immunization at the start of the RSV season in case of another underlying disease with severe impairment (e.g., persistent oxygen therapy) of respiratory capacity (e.g., neuromuscular disease, trisomy 21, diaphragmatic hernia) or severe immunodeficiency in children younger than 12 months. In children between 12 and 24 months of age, 74% (32/43) of the participants stated to recommend vaccination in case of hemodynamically relevant heart disease and severe heart failure under treatment, whereas 82% (35/43) of the physicians recommended RSV vaccination for infants younger than 24 months of age with BPD II° or III° with oxygen therapy until at least 6 months before RSV season.
Regarding institutionalized follow‐up, 61% (26/43) of the participants reported to not have access to a BPD outpatient clinic in their hospital, 33% (14/43) reported to have such a facility available. A total of 70% (30/43) reported that they provide home monitoring consultations, whereas 28% (12/43) did not report to provide this service. A total of 47% (20/43) of the participants reported to not offer specific follow‐up care (in the form of a consultation, outpatient clinic, or specialized physicians) for preterm infants below 32 weeks GA with established PH (7% [3/43] did not provide information). When PH outpatient clinics were available, 74% (17/23) stated that these clinics are run by pediatric cardiologists (resident in training), 17% (4/23) reported that this service is provided by physicians with pediatric cardiology experience. A total of 9% (2/23) of the physicians reported the background of the physicians working in their respective PH outpatient clinics as “unknown.”
DISCUSSION
Despite the significant immediate and long‐term consequences of pathologic pulmonary blood flow in the preterm neonate, 14 strategies to diagnose, treat, and monitor early postnatal PH, e.g., PPHN, as well as infants at risk for or with established BPD‐associated PH are limited due to the lack of standardization and sensitive diagnostic tools. 7 , 14 , 15 The lack of monitoring and treatment standards result in expert‐opinion‐based, case‐to‐case decisions that vary between perinatal centers.
In our survey study (PUsH BPD) we successfully assessed current clinical practice for treatment and monitoring strategies in preterm infants with PPHN or infants at risk for or with established BPD‐associated PH between 06/2018 and 10/2020 in two‐thirds of all German perinatal centers with >70 very low birthweight infants/year including their cardiology departments and outpatient units.
We thereby specified the topics that need to be addressed by clinical guidelines, training programs, and future clinical trials.
When evaluating the early form of PH in the preterm infant, e.g., PPHN, the PUsH survey confirmed that clinical presentation and echocardiography were used as the main diagnostic modalities to screen for right heart function abnormalities and presence of PPHN in preterm infants, 20 thereby in line with the recommendation of The American Heart Association (AHA) and American Thoracic Society (ATS). 21 Current clinical understanding considers the presence of hypoxemia especially as PH‐indicative when being disproportionate to the severity of parenchymal disease on radiograph as well as discrepancies between preductal and postductal arterial oxygenation, e.g., a difference in arterial PO2 of ≥20 mmHg or oxygen saturation of ≥5%–10%, indicate the presence of PH. 22
In PPHN, echocardiography detects right‐to‐left or bidirectional shunting via the foramen ovale and/or via the ductus arteriosus together with elevated pressure in the pulmonary artery or RV. In accordance with this, right‐left shunting in the atrium or via the PDA were described as main diagnostic criteria by study participants in the PUsH survey. The majority of the PUsH survey participants considered the dimension of the RV as an additional diagnostic criterion, in line with current recommendations 23 : The expert consensus statement on the diagnosis and treatment of pediatric PH by the European Pediatric Pulmonary Vascular Disease Network (EPPVDN) published in 2016 recommended several parameters in transthoracic echocardiography when diagnosing pediatric PH. 24 PUsH survey participants showed a strong agreement on some of those parameters. As such, the assessment of right ventricular pathologies in echocardiography is considered crucial in the evaluation of PH with RV dimension and contractility assessment in echocardiography indicating right heart strain in PH. 25 , 26 More than half of the PUsH study participants stated to assess right atrial dimensions in addition in accordance with data published for children with PH and with studies in adults, considering RA enlargement and interatrial septal bowing as indicators of poor RV compliance and increased mean RA pressure. 24 , 27 Although recommended, the assessment of RV/LV ratio is only used by one‐third of our survey participants when diagnosing PH. Further, the presence of TI have been previously described as relevant diagnostic parameters when diagnosing PH in the preterm infant. 28 However, it has also been shown that pressure estimations by echocardiography with the use of tricuspid valve jet velocity measurements in routine clinical settings do not necessarily correlate well with pressure assessment by catheterization as shown for PH in children with CLD. Qualitative echocardiographic markers such as right atrial enlargement, right ventricular hypertrophy or dilation, however, revealed relatively poor predictive value in the absence of a measurable TRV. 28 M‐mode measurements such as the TAPSE have been shown as a valuable tool when evaluating RV function with predictive value in PH, 29 , 30 contrasted by only less than half of the PUsH participants considering M‐mode measurements of TAPSE. Pulmonary acceleration time, recommended by the EPPVDN, was indicated by approximately half of the PUsH participants to be used in PH diagnostics. However, in preterm infants, PH screening by echocardiography has been shown to have several limitations, including image quality in off‐axis images as well as the dependability of qualitative parameter interpretation in the presence of TRV. 7 , 28
In any case, echocardiography is required to exclude cyanotic congenital heart disease, determine the degree of pulmonary pressure elevation as well as assess overall cardiac function before the initiation of pulmonary vasodilator therapy.
In line with the recommendations for infants with PPHN issued by the EPPVDN, 20 the majority of participants reported to not use cardiac catheterization for diagnosing PPHN in premature infants as it is associated with several risks in this patient group due to its invasive nature. 13
Regarding the use of biomarkers, that is, BNP and NT‐proBNP with the potential advantage of being less expensive, faster to perform, and not requiring specialized equipment 31 and recommended for diagnosis and monitoring of BPD infants at risk of PH by the AHA and ATS, 21 the PUsH survey indicated that these biomarkers are not consistently used in preterms at risk for or with established BPD‐associated PH but—in case considered—favored NT‐proBNP consistent with previous studies. 32
Administration of iNO was reported as the therapy of choice in PPHN by greater than two‐thirds of the PUsH participants, whereas other pulmonary vasodilators such as Sildenafil were reported to be used for weaning off iNO therapy to prevent rebound PH, in line with current recommendations. With iNO holding a prime role in the regulation of vascular muscle tone, 33 , 34 a systematic review of randomized iNO trials published by the Cochrane Collaboration provided evidence that iNO at doses ranging from 10 to 80 ppm significantly improved oxygenation, 35 as measured by the oxygenation index (OI = FiO2 × mean airway pressure × 100/PaO2), when compared to placebo or standard treatment, while also reducing the incidence of death or need for extracorporeal membrane oxygenation in mature infants with PPHN. 36 In preterm infants <34 weeks GA, however, iNO treatment is not generally recommended to date 37 but supported by statements from the AHA, the ATS 21 and the Pediatric Pulmonary Hypertension Network in certain cases when administered in NICU under strict adherence to protocols and continuous clinical monitoring. 38 Here, the initial recommended dose in preterm infants is 10 ppm, with gradual reductions as oxygenation improves, to achieve minimal toxicity. 39 , 40 , 41 Reflecting these supporting statements, an increase in the use of iNO was shown in a retrospective population study in NICU in England between 2010 and 2015. 37
Although no randomized studies currently compared different PaO2 levels in the management of PPHN in a term infant, hypoxia is known to increase PVR, whereas the brief exposure to 100% oxygen in newborn lambs resulted in increased contractility in pulmonary arteries and reduced the response to iNO. Reflecting the data obtained in animal models where oxygen saturations between 90% and 97% were demonstrated to result in low PVR, expert statements recommended maintaining preductal oxygen saturations between 91% and 95% in preterm infants with PPHN. 20 In line with these recommendations, more than half of the 43 study participants reported to target oxygen saturation levels of at least 92%.
While following the same diagnostic strategies for PH based on echocardiography, the care for infants at risk for or with established BPD‐associated PH especially takes specific therapeutic strategies as well as postdischarge monitoring regimen into consideration. The European Respiratory Society (ERS) guideline on the long‐term treatment of children with BPD recommended bronchodilator treatment for children with BPD only in subgroups, e.g., children with severe BPD and asthma‐like symptoms, repeated hospital admissions for respiratory morbidity, exercise intolerance or reversible lung dysfunction. 42 Further, the ERS guideline recommends no treatment with inhaled or systemic corticosteroids, however, PUsH participants indicated that inhalative and systemic steroids are frequently used in preterm infants with BPD in Germany. Diuretics were also reported to be frequently applied in preterm infants with evolving and established BPD, in contrast to the recommendations of the ERS, which recommended to use diuretics only in BPD cases with clinical evidence of fluid retention and while monitoring clinical effects. 42
Oxygen therapy in preterm infants at risk for or with established BPD‐associated PH before discharge was reported by very few PUsH participants to be based on pulse oximetry alone without oxygen testing or on pulse oximetry together with oxygen testing. There was considerable disagreement for the PUsH participants on targeted oxygen saturation levels. This likely reflects that despite German guidelines from 2010 stating that oxygen administration can prevent the development of PH in preterm infants with BPD, 43 oxygen saturation targets remained a matter of debate. 44 This discussion is further complicated by the aim to prevent chronic hypoxemia by oxygen therapy after discharge resulting from apnoeic episodes both in the presence or absence of BPD. In line with current recommendations, 45 our survey demonstrated that the majority of participating physicians prescribed home monitoring when supplemental oxygen is needed. In line with the recommendations provided by the Committee on fetus and newborn, 46 the majority of PUsH survey participants indicated to use home monitoring in infants with BPD in combination with a risk for SIDS in the family history or in combination with apnea‐bradycardia‐syndrome.
The recommendations of the German guideline on the prophylaxis for RSV in high‐risk children with palivizumab in infants with a suspected severe course of RSV infection such as in significant prematurity, CLD, congenital heart defects, neuromuscular disease, immunodeficiencies, or chromosomal aberrations 47 , 48 are reflected in the reports of the majority of physicians participating in the PUsH survey, who recommended RSV vaccination in cases of preterms with moderate or severe BPD and oxygen therapy in the last 3 months before the start of the RSV season. Participants disagree on the recommendation in cases, where the German guideline leaves the recommendation for RSV prophylaxis open to the expert's decision.
Although the majority of participants stated that preterms with BPD‐associated PH are recommended for cardiologic follow‐ups as well as future echocardiograms, the PUsH survey revealed the absence of a clear standard regarding follow‐up care for preterm infants at risk for or with established BPD‐associated PH.
In summary, the PUsH survey provided valuable insight into the challenging field of current clinical practices for preterm infants with PPHN and for infants at risk or with established BPD‐associated PH. The survey revealed shared practices in diagnostic and therapeutic strategies for preterms with PPHN and BPD‐associated PH in Germany's perinatal centers, specifically regarding clinical presentation and echocardiographic criteria in line with current recommendations. The lack of using biomarkers (BNP/NT‐proBNP) deviated from international guidelines. Regarding treatment regimen, strategies varied for iNO, whereas the majority of participants agreed to prescribe diuretics and steroids (systemic/inhaled) for infants at risk for or with established BPD‐associated PH, thereby only partially in line with current recommendations specifically when regarding the use of diuretics.
However, interpretation of the results has to consider study limitations such as the number of answers provided for each variable and/or the number of items excluded due to missingness considering topics of clinical interest such as diagnostic strategies and therapy proceedings in perinatal care.
Future studies are needed to enable deeper correlation analyses that target structural elements of the care pathway (e.g., availability of BPD‐/PH‐outpatient clinic) as well as diagnostic and treatment regimen, especially regarding the use of pulmonary vasodilators, steroids, and diuretics. The need to standardize oxygen saturation levels for infants with PPHN and developing or established BPD‐associated PH highlights the challenge to better distinguish between these disease entities or developing disease severities. As exemplified by the strong agreement on the practice for recommending RSV vaccination, guidelines show good potential to harmonize current practices. Here, standardization is also needed for postdischarge care and resident training.
The development and use of novel diagnostic tools such as lung and heart MRI, already applied in pediatric PH, side by side with the use of routine, quantifiable echocardiographic markers and liquid biopsies for diagnosing and monitoring PH in this patient population will help to inform standardization and improve comparability. 49 , 50 , 51 , 52
Future guidelines, training programs, and clinical trials need to aim for the replacement of expert‐based case‐to‐case decisions by comparable standards of care, accompanied by new surveys to monitor the success of these approaches.
AUTHOR CONTRIBUTIONS
Conception and design of the manuscript was done by Anne Hilgendorff. Conception of the survey was performed by Kai Förster and Sabine Witt and data were acquired by Friederike Häfner, Kai Förster, and Yvonne Kraus. Data analysis and interpretation were performed by Larissa Schwarzkopf, Lars Schwettmann, Anne Hilgendorff, Friederike Häfner, and Caroline Johansson. Figures were done by Caroline Johansson. Drafting and writing of the manuscript was performed by Friederike Häfner, Caroline Johansson, Larissa Schwarzkopf, Lars Schwettmann, Kai Förster, and Anne Hilgendorff. The manuscript was drafted for important intellectual content and reviewed by Anne Hilgendorff, Andreas W. Flemmer, Georg Hansmann, Hannes Sallmon, and Ursula Felderhoff‐Müser.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
EC# (LMU Munich) 21‐0753.
Supporting information
Supporting information.
Supporting information: Figure 1OS
ACKNOWLEDGMENTS
The present study was supported by the Young Investigator "Molecular Mechanisms of Neonatal Chronic Lung Disease" Grant NWG VH‐NG‐829 by the Helmholtz Foundation and the Helmholtz Zentrum Muenchen, Germany, the International Research Group “Role of BMP signaling” (01KI07110), Helmholtz Foundation (Federal Ministry of Education and Research in Germany [BMBF]), and the German Centre for Lung Research [DZL] [BMBF]) as well as the Research Training Group "Targets in Toxicology" (GRK2338) of the German Science and Research Organisation (DGF). We sincerely thank the survey participants from the different perinatal centers throughout Germany for their invaluable input. As a member of the PVRI GoDeep Registry (PVRI Global Deep Phenotyping PH Meta‐Registry), the study was supported through intellectual contribution. Open Access funding enabled and organized by Projekt DEAL.
Häfner F, Johansson C, Schwarzkopf L, Förster K, Kraus Y, Flemmer AW, Hansmann G, Sallmon H, Felderhoff‐Müser U, Witt S, Schwettmann L, Hilgendorff A. Current diagnosis and treatment practice for pulmonary hypertension in bronchopulmonary dysplasia—A survey study in Germany (PUsH BPD). Pulm Circ. 2023;13:e12320. 10.1002/pul2.12320
Friederike Häfner and Caroline Johansson wish to be known that they contributed equally to this study.
REFERENCES
- 1. Evans NJ, Archer LN. Doppler assessment of pulmonary artery pressure during recovery from hyaline membrane disease. Arch Dis Child. 1991;66(7 Spec No):802–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Subhedar NV. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2000;82(3):243F–247F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120(6):1260–1269. [DOI] [PubMed] [Google Scholar]
- 4. Krishnan U, Feinstein JA, Adatia I, Austin ED, Mullen MP, Hopper RK, Hanna B, Romer L, Keller RL, Fineman J, Steinhorn R, Kinsella JP, Ivy DD, Rosenzweig EB, Raj U, Humpl T, Abman SH, Coulson J, Collaco M, Grenolds A. Evaluation and management of pulmonary hypertension in children with bronchopulmonary dysplasia. J Pediatr. 2017;188:24–34.e1. [DOI] [PubMed] [Google Scholar]
- 5. Khan S, Concina VA, Schneider D, Westgate P, Arriagada S, Bada H. Role of NT‐proBNP in the prediction of moderate to severe bronchopulmonary dysplasia in preterm infants. Pediatr Pulmonol. 2020;55(2):376–382. [DOI] [PubMed] [Google Scholar]
- 6. Seo YH, Choi HJ. Clinical utility of echocardiography for early and late pulmonary hypertension in preterm infants: relation with bronchopulmonary dysplasia. J Cardiovasc Ultrasound. 2017;25(4):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlton EF, Sontag MK, Younoszai A, DiMaria MV, Miller JI, Poindexter BB, Abman SH, Mourani PM. Reliability of echocardiographic indicators of pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. J Pediatr. 2017;186:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101(1):40–46. [DOI] [PubMed] [Google Scholar]
- 9. Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, Poindexter BB, Ingram DA, Abman SH. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arjaans S, Zwart EAH, Ploegstra MJ, Bos AF, Kooi EMW, Hillege HL, Berger RMF. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: a systematic review and meta‐analysis. Paediatr Perinat Epidemiol. 2018;32(3):258–267. [DOI] [PubMed] [Google Scholar]
- 11. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abman SH. Monitoring cardiovascular function in infants with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 2002;87(1):15F–18F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Apitz C, Hansmann G, Schranz D. Hemodynamic assessment and acute pulmonary vasoreactivity testing in the evaluation of children with pulmonary vascular disease. expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102(Suppl 2):ii23–ii29. [DOI] [PubMed] [Google Scholar]
- 14. Jain D, Bancalari E. Bronchopulmonary dysplasia: clinical perspective. Birth Defects Res A Clin Mol Teratol. 2014;100(3):134–144. [DOI] [PubMed] [Google Scholar]
- 15. Mourani PM, Abman SH. Pulmonary hypertension and vascular abnormalities in bronchopulmonary dysplasia. Clin Perinatol. 2015;42(4):839–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hilgendorff A, Apitz C, Bonnet D, Hansmann G. Pulmonary hypertension associated with acute or chronic lung diseases in the preterm and term neonate and infant. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102(Suppl 2):ii49–ii56. [DOI] [PubMed] [Google Scholar]
- 17. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–1955. [DOI] [PubMed] [Google Scholar]
- 18. Clark RH, Ursprung RL, Walker MW, Ellsbury DL, Spitzer AR. The changing pattern of inhaled nitric oxide use in the neonatal intensive care unit. J Perinatol. 2010;30(12):800–804. [DOI] [PubMed] [Google Scholar]
- 19. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. [DOI] [PubMed] [Google Scholar]
- 20. Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, Budts W, D'Alto M, Gatzoulis MA, Hasan BS, Kozlik‐Feldmann R, Kumar RK, Lammers AE, Latus H, Michel‐Behnke I, Miera O, Morrell NW, Pieles G, Quandt D, Sallmon H, Schranz D, Tran‐Lundmark K, Tulloh RMR, Warnecke G, Wåhlander H, Weber SC, Zartner P. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38(9):879–901. [DOI] [PubMed] [Google Scholar]
- 21. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037–2099. [DOI] [PubMed] [Google Scholar]
- 22. Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am. 2009;56(3):579–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar S, Vadlamudi K, Kaddoura T, Bobhate P, Goot BH, Elgendi M, Jain S, Colen T, Khoo NS, Adatia I. Active right atrial emptying fraction predicts reduced survival and increased adverse events in childhood pulmonary arterial hypertension. Int J Cardiol. 2018;271:306–311. [DOI] [PubMed] [Google Scholar]
- 24. Koestenberger M, Apitz C, Abdul‐Khaliq H, Hansmann G. Transthoracic echocardiography for the evaluation of children and adolescents with suspected or confirmed pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and D6PK. Heart. 2016;102(Suppl 2):ii14–ii22. [DOI] [PubMed] [Google Scholar]
- 25. de Boode WP, Singh Y, Molnar Z, Schubert U, Savoia M, Sehgal A, Levy PT, McNamara PJ, El‐Khuffash A. Application of neonatologist performed echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn. Pediatr Res. 2018;84(Suppl 1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Broderick‐Forsgren K, Davenport CA, Sivak JA, Hargett CW, Foster MC, Monteagudo A, Armour A, Rajagopal S, Arges K, Velazquez EJ, Samad Z. Improving on the diagnostic characteristics of echocardiography for pulmonary hypertension. Int J Cardiovasc Imaging. 2017;33(9):1341–1349. [DOI] [PubMed] [Google Scholar]
- 27. Koestenberger M, Burmas A, Ravekes W, Avian A, Gamillscheg A, Grangl G, Grillitsch M, Hansmann G. Echocardiographic reference values for right atrial size in children with and without atrial septal defects or pulmonary hypertension. Pediatr Cardiol. 2016;37(4):686–695. [DOI] [PubMed] [Google Scholar]
- 28. Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121(2):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koestenberger M, Nagel B, Avian A, Ravekes W, Sorantin E, Cvirn G, Beran E, Halb V, Gamillscheg A. Systolic right ventricular function in children and young adults with pulmonary artery hypertension secondary to congenital heart disease and tetralogy of fallot: tricuspid annular plane systolic excursion (TAPSE) and magnetic resonance imaging data: TAPSE and RVEF, and in PAH‐CHD and TOF patients. Congenit Heart Dis. 2012;7(3):250–258. [DOI] [PubMed] [Google Scholar]
- 30. Forfia PR, Fisher MR, Mathai SC, Housten‐Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034–1041. [DOI] [PubMed] [Google Scholar]
- 31. Xiong T, Kulkarni M, Gokulakrishnan G, Shivanna B, Pammi M. Natriuretic peptides in bronchopulmonary dysplasia: a systematic review. J Perinatol. 2020;40(4):607–615. [DOI] [PubMed] [Google Scholar]
- 32. Amdani SM, Mian MUM, Thomas RL, Ross RD. NT‐pro BNP‐A marker for worsening respiratory status and mortality in infants and young children with pulmonary hypertension. Congenit Heart Dis. 2018;13(4):499–505. [DOI] [PubMed] [Google Scholar]
- 33. Nelin LD, Potenziano JL. Inhaled nitric oxide for neonates with persistent pulmonary hypertension of the newborn in the CINRGI study: time to treatment response. BMC Pediatr. 2019;19(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martinho S, Adão R, Leite‐Moreira AF, Brás‐Silva C. Persistent pulmonary hypertension of the newborn: pathophysiological mechanisms and novel therapeutic approaches. Front Pediatr. 2020;8:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barrington KJ, Finer N, Pennaforte T, Altit G. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2017;1(1):Cd000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2006;4:Cd000399. [DOI] [PubMed] [Google Scholar]
- 37. Subhedar NV, Jawad S, Oughham K, Gale C, Battersby C. Increase in the use of inhaled nitric oxide in neonatal intensive care units in England: a retrospective population study. BMJ Paediatrics Open. 2021;5(1):e000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kinsella JP, Steinhorn RH, Krishnan US, Feinstein JA, Adatia I, Austin ED, Rosenzweig EB, Everett AD, Fineman JR, Hanna BD, Hopper RK, Humpl T, Ivy DD, Keller RL, Mullen MP, Raj JU, Wessel DL, Abman SH. Recommendations for the use of inhaled nitric oxide therapy in premature newborns with severe pulmonary hypertension. J Pediatr. 2016;170:312–314. [DOI] [PubMed] [Google Scholar]
- 39. Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2017;1(1):Cd000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kinsella J. Low‐dose inhalational nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340(8823):819–820. [DOI] [PubMed] [Google Scholar]
- 41. Roberts J. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340(8823):818–819. [DOI] [PubMed] [Google Scholar]
- 42. Duijts L, van Meel ER, Moschino L, Baraldi E, Barnhoorn M, Bramer WM, Bolton CE, Boyd J, Buchvald F, del Cerro MJ, Colin AA, Ersu R, Greenough A, Gremmen C, Halvorsen T, Kamphuis J, Kotecha S, Rooney‐Otero K, Schulzke S, Wilson A, Rigau D, Morgan RL, Tonia T, Roehr CC, Pijnenburg MW. European Respiratory Society guideline on long‐term management of children with bronchopulmonary dysplasia. Eur Respir J. 2020;55(1):1900788. [DOI] [PubMed] [Google Scholar]
- 43.Gesellschaft für Neonatologie und Pädiatrische Intensivmedizin DGfKuJ. Prävention und Therapie der bronchopulmonalen Dysplasie Frühgeborener. AWMF‐Leitlinien‐Register‐Nr 024/014 AWMF, Düsseldorf. 2010. [Google Scholar]
- 44. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network , Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, Yoder BA, Faix RG, Das A, Poole WK, Donovan EF, Newman NS, Ambalavanan N, Frantz 3rd, ID , Buchter S, Sánchez PJ, Kennedy KA, Laroia N, Poindexter BB, Cotten CM, Van Meurs KP, Duara S, Narendran V, Sood BG, O'Shea TM, Bell EF, Bhandari V, Watterberg KL, Higgins RD. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nassi N, Piumelli R, Lombardi E, Landini L, Donzelli G, de Martino M. Comparison between pulse oximetry and transthoracic impedance alarm traces during home monitoring. Arch Dis Child. 2008;93(2):126–132. [DOI] [PubMed] [Google Scholar]
- 46. Committee on Fetus and Newborn. American Academy of Pediatrics . Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111(4 Pt 1):914–917. [PubMed] [Google Scholar]
- 47. Sommer C. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J. 2011;5:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deutsche Gesellschaft für Pädiatrische Infektiologie (DGPI) . Leitlinie zur Prophylaxe von schweren Erkrankungen durch Respiratory Syncytial Virus (RSV) bei Risikokindern. S2k‐Leitlinie.
- 49. Weatherley ND, Eaden JA, Stewart NJ, Bartholmai BJ, Swift AJ, Bianchi SM, Wild JM. Experimental and quantitative imaging techniques in interstitial lung disease. Thorax. 2019;74(6):611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hatabu H, Ohno Y, Gefter WB, Parraga G, Madore B, Lee KS, Altes TA, Lynch DA, Mayo JR, Seo JB, Wild JM, van Beek EJR, Schiebler ML, Kauczor HU. Expanding applications of pulmonary MRI in the clinical evaluation of lung disorders: fleischner society position paper. Radiology. 2020;297(2):286–301. [DOI] [PubMed] [Google Scholar]
- 51. Venner C, Odille F, Voilliot D, Chaouat A, Chabot F, Felblinger J, Bonnemains L. Can MRI detect pulmonary hypertension in a population pre‐selected by echocardiography? Acta Radiol. 2018;59(2):180–187. [DOI] [PubMed] [Google Scholar]
- 52. Critser PJ, Higano NS, Tkach JA, Olson ES, Spielberg DR, Kingma PS, Fleck RJ, Lang SM, Moore RA, Taylor MD, Woods JC. Cardiac magnetic resonance imaging evaluation of neonatal bronchopulmonary dysplasia‐associated pulmonary hypertension. Am J Respir Crit Care Med. 2020;201(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information: Figure 1OS


