Abstract
Introduction
While there is increasing evidence of the effects of cannabis‐based medicinal products (CBMPs) on health‐related quality of life (HRQoL), a major limitation of the current literature is the heterogeneity of studied CBMPs. This study aims to analyze changes in HRQoL in patients prescribed a homogenous selection of CBMPs.
Methods
Primary outcomes were changes in patient‐reported outcomes (PROMs) at 1, 3, 6, and 12 months from baseline. The secondary outcome was an adverse events analysis. Statistical significance was defined as p < 0.050.
Results
1378 patients prescribed Adven® CBMPs (Curaleaf International, Guernsey, UK) were included in the final analysis. 581 (42.16%) participants were current users of cannabis at baseline. 641 (46.51%), 235 (17.05%), and 502 (36.43%) patients were treated with oils, dried flowers, or a combination of the two, respectively. Improvements were found in all PROMs in each route of administration at 1, 3, 6, and 12 months from baseline (p < 0.010). Those prescribed dried flower only or both oils and dried flower experienced greater improvements in GAD‐7, SQS, and EQ‐5D‐5L index values at 12 months (p < 0.050). There was no difference in outcomes between those prescribed dried flower only or dried flower with oils (p > 0.050). 3663 (265.82%) adverse events were reported by 297 (21.55%) patients.
Conclusion
There was an associated improvement in self‐reported anxiety, sleep quality, and HRQoL in patients treated with the CBMPs. Those prescribed treatment formulations including dried flower were most likely to show a clinical improvement. However, these results must be interpreted with caution given the limitations of study design.
Keywords: anxiety, cannabidiol, cannabis, sleep, tetrahydrocannabinol
Data from the UK Medical Cannabis Registry assessed the change in health‐related quality of life in patients prescribed Adven(R) products. This found an associated improvement in anxiety, sleep and general health‐related quality of life. Those prescribed treatment formulations including dried flower were most likely to show a clinical improvement.
1. INTRODUCTION
Globally, access to cannabis‐based medicinal products (CBMPs) is increasing. 1 In the UK, while there are three licensed preparations of CBMPs, unlicensed CBMPs can only be initiated by doctors on the General Medical Council's specialist register in agreement with a multidisciplinary team. 2 In addition, patients are required to have trialed appropriate licensed therapies for the condition, as well off‐license medications, without sufficient clinical benefit prior to consideration of unlicensed CBMPs. 2 There is growing pre‐clinical evidence on CBMPs in chronic conditions. However, there is a paucity of high‐quality randomized controlled trials due to the limitations of studying their effects in this setting. 3
The cannabis plant contains over 400 chemical compounds with medical potential, 4 of which more than 144 are phytocannabinoids that provide clinical effects through interactions with the endocannabinoid system. 5 The most prevalent compounds by concentration in the cannabis flower are the cannabinoids (−)‐trans‐Δ9‐tetrahydrocannabinol (THC) and cannabidiol (CBD). 6 However, CBMPs are a heterogeneous group of medicines, including isolate formulations of cannabinoids, broad‐spectrum products 7 containing other compounds from the cannabis flower with potential therapeutic properties (minor cannabinoids, terpenes, flavonoids), 8 and full‐spectrum medicines containing the full complement of compounds extracted from the cannabis plant during its manufacture. 7
Cannabinoids exert most of their actions through G protein‐coupled type 1 (CB1) and type 2 (CB2) cannabinoid receptors. 9 The concentration of CB1 receptors is highest in the central nervous system. 10 Activation of CB1 receptors results in the blockade of calcium and activation of potassium channels, causing the downregulation of neurotransmission. 11 CB1 receptors are typically found on pre‐synaptic terminals and subsequently regulate the release of neurotransmitters, such as glutamate and γ‐aminobutyric acid (GABA), in the central nervous system resulting in inhibition of excitatory or inhibitory signals, respectively. 12 CB2 receptors are predominantly expressed in peripheral immune cells and the gastrointestinal system, regulating the release of inflammatory cytokines, including interleukin (IL)‐1β, IL‐6, and tumor necrosis factor (TNF)‐α. 13 THC is a partial agonist of CB1 and CB2 receptors. 9 The predominant mechanism of action of CBD, however, is inhibition of postsynaptic uptake and subsequent fatty acid amide hydrolase‐mediated catabolism of the endocannabinoid, anandamide. This results in the accumulation of anandamide, a CB1 receptor agonist, at synaptic junctions. 14 In addition, CBD is a negative allosteric modulator of CB1 receptors, therefore simultaneously limiting the activation of CB1 receptors by agonists. 15
Considering the overlapping role of the endocannabinoid system in the nervous, immune, and gastrointestinal systems, modulation of CB1 and CB2 receptor activity has been considered a novel target of interest in treating a range of chronic health conditions, particularly those with a psychological or neurological component. 10 , 11 , 12 With respect to chronic pain, the most common condition for which CBMPs are prescribed, cannabinoids have shown effects on nociceptive transmission through the peripheral and central nervous systems, in addition to affecting the cognitive manifestations of pain. 10 Interactions with serotoninergic pathways have been implicated in mood and anxiety disorders, with cannabinoids producing similar effects in pre‐clinical studies to monoamine reuptake inhibitors. 10 Meanwhile, the regulation of GABA and glutamate is implicated in its effects on seizure disorders. 12
In addition to pre‐clinical studies demonstrating an array of effects of CBD and THC on physiological processes, there is growing clinical evidence demonstrating effects on specific conditions. 16 , 17 , 18 , 19 An umbrella review encompassing both observational and randomized controlled trials has suggested that CBMPs are effective in conditions such as multiple sclerosis and chronic pain. 19 With respect to chronic pain, Wang and colleagues have previously modeled a 10% risk difference of individuals prescribed non‐inhaled CBMPs experiencing a clinically important improvement in pain severity. 16 There is a lack of randomized controlled trials which have examined the effects of inhaled CBMPs and considering the heterogeneity of chronic pain conditions, there are still unanswered questions with respect to the efficacy of CBMPs according to the different etiologies of chronic pain and the optimum doses of cannabinoids and mode of administration. 16 Concerns also remain about the use of cannabis in young people and people susceptible to severe psychiatric conditions. 19 Despite a paucity of randomized controlled trials reporting outcomes for CBMPs, there is also evidence of symptomatic benefit in conditions, such as sleep disorders and chemotherapy‐induced nausea and vomiting. 17 For other conditions, such as anxiety, where there has been promising pre‐clinical or observational data on the effects of CBMPs, there is still insufficient evidence to arrive at a consensus on its efficacy based on randomized controlled trial data. 17
Assessment of the impact of CBMPs on health‐related quality of life (HRQoL) has been identified by medical cannabis patients as being a key research priority. 20 Yet there remains a paucity of high‐quality clinical data on their effects on HRQoL. This is likely secondary to inherent challenges with studying the health effects of CBMPs within the context of randomized controlled trials. 3 Observational studies have therefore sought to bridge the gap in research on this topic. Data from the UK Medical Cannabis Registry (UKMCR) was developed to demonstrate the outcomes in patients prescribed CBMPs, with a focus on HRQoL. To date, improvements have been observed in patients with chronic pain, generalized anxiety disorder, autism spectrum disorder, post‐traumatic stress disorders, depression, headache disorders, and pediatric epilepsy. 21 , 22 , 23 , 24 , 25 , 26 , 27 There have also been successive evaluations of changes in HRQoL across patients with all conditions. 28 , 29 , 30 While these have helped to overcome some of the limitations of the present literature on CBMPs, they incorporate a homogeneous selection of CBMPs, which can affect both observational studies and randomized controlled trials. 31 As such, they are confounded by the effects of different CBMPs, with varying concentrations of CBD and THC, and routes of administration. The primary aim of the present study is to therefore examine the associated changes in HRQoL, opioid prescription, and adverse events in patients treated exclusively with a homogeneous selection of CBMPs (Adven®, Curaleaf International, Guernsey, UK) enrolled in the UKMCR. The secondary aim is to evaluate the impact of method of administration on the changes in HRQoL, opioid prescription, and incidence of adverse events.
2. METHODS
This was a prospective cohort study of patients enrolled in the UKMCR and prescribed Adven® CBMPs (Curaleaf International, Guernsey, UK), including inhaled dried flower, sublingual/oral medium‐chain triglyceride‐based oil, or a combination of two for any indication. All patients signed a formal, written consent form before the data collection. The UKMCR received formal ethical approval from the Central Bristol Research Ethics Committee (22/SW/0145). This study was reported with the following Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 32
The UKMCR is a patient registry that is privately owned and run by Sapphire Medical Clinics. It was established in December 2019 to gather prospective clinical data from patients prescribed CBMPs. An evaluation of the UKMCR has previously shown that over 90% of participants found the registry to be important in impacting the future of care of patients. 20
2.1. Patient and data selection
Patient data from those prescribed Adven® CBMPs (Curaleaf International, Guernsey, UK) for any indication were extracted from the UKMCR on January 9th 2023. Patients who had incomplete baseline PROMs data or had been enrolled on the UKMCR for fewer than 12 months were excluded. Specific cohorts were determined depending on how prescribed CBMPs were administered. There were three cohorts, including patients only prescribed oil‐based products (including reformulated oils in lozenges or capsules) for sublingual or oral administration, inhaled dried flower, and patients prescribed in a combination of both.
The decision to prescribe was made by consultant physicians with the appropriate specialist training in the indication for CBMPs, in conjunction with a multidisciplinary team. The choice of product was therefore dictated by clinical need on an individual patient basis.
2.2. Data collection
During the first appointment with patients, clinicians recorded patient demographics, medications, comorbidities, occupations, and drug and alcohol history. Occupations were categorized using the international standard classification of occupations. 33 Indications for therapy with CBMPs were also documented using primary, secondary, and tertiary diagnoses.
Comorbidities were recorded, and the Charlson comorbidity index was calculated for each patient. The Charlson comorbidity index is widely used to measure the risk of death and disease burden in observational studies. 34 Tobacco and alcohol status of patients were recorded as units per week and pack‐years, respectively. Before receiving a precription for CBMPs, patients were requested to provide the cannabis status for recreational or medical use, including ‘never used’, ‘current’, or ‘ex‐user’. For ‘current’ and ‘ex‐user’ cannabis status, cannabis grams years were calculated to quantify lifetime cannabis use. 35 Cannabis gram years are calculated by multiplying grams consumed per day by years of cannabis use. 35 Participants were counseled against continued illicit cannabis consumption, but they were not required to prove a period of abstinence from cannabis via a urine drug screen before receiving a prescription.
The concomitant medications patients were prescribed at baseline were recorded using the Systematized Nomenclature of Medicine – Clinical Terms. 36 Patients and/or clinicians recorded the dose of the medications being prescribed at the onset of CBMP therapy or any changes during follow‐up. Opioid medications were converted to oral morphine equivalents using conversion factors quoted by the British National Formulary and the General Practice notebook. 37 , 38
CBMP prescriptions at the end of the follow‐up period were recorded, including formulation, and THC and CBD doses (mg/24 h).
Patient‐reported outcome measures (PROMs) were completed electronically at baseline, and 1, 3, 6, and 12 months after initiation of treatment with CBMPs in line with clinical consultation frequency. Over 90% of patients found the online platform easy to use when completing health questionnaires. 20
2.3. PROMs
The PROMs collected during this study were the General Anxiety Disorder (GAD‐7), Single‐Item Sleep Quality Scale (SQS), EQ‐5D‐5L, and Patient Global Impression of Change (PGIC) questionnaires. In addition to these, each condition collected PROMs specific to their respective conditions; however, these measures were not included in the present analysis. Patients completed PROMs online; if the data were incomplete, electronic reminders were used to prompt patients to fill in any missing information until complete.
GAD‐7 consists of seven core symptoms of generalized anxiety disorder, and patients choose the frequency of being bothered by those symptoms over the last 14 days. Frequency options include ‘not at all’, ‘several days’, ‘more than half the days’, or ‘nearly every day’. Each represents 0, 1, 2, and 3, resulting in a total score from 0 to 21. The cut‐off scores for mild, moderate, and severe anxiety are ≥5, ≥10, and ≥15, respectively. A reduction of 4 points or more on the GAD‐7 scale from the baseline to the follow‐up is clinically significant and indicates an improvement in symptoms of anxiety. 39
SQS is a validated measure with a rating scale from 0 to 10, with 0 referring to ‘terrible’, 10 referring to ‘excellent’ sleep quality over the preceding week. An increase of 2.6 points is considered a clinically significant improvement. 40
EQ‐5D‐5L is a descriptive system that assesses HRQoL, consisting of 5 aspects: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. Every dimension comprises 5 levels, and each represents a number: no problems (1), slight problems (2), moderate problems (3), severe problems (4), and extreme problems (5). From this, one of 3125 health scores is generated. 41 Subsequently, the health state is mapped onto a UK‐specific index value in line with National Institute for Health and Care Excellence recommendations. 42 An index value of 1 refers to ‘full health’ while index value lower than 0 refers to worse than death. 43 There is no value that is deemed clinically significant across different medical conditions.
PGIC is a 7‐point scale, in which patients determine the difference in their health status before and after the start of treatment. 1 indicates ‘no change’ and 7 indicates ‘a great deal better’. 44
2.4. Missing data
Missing data were dealt with using the baseline observation carried forward, to provide a conservative estimate of true effects. Missing follow‐up values in any PROMs were therefore replaced with the value reported by a patient at baseline. 35
2.5. Adverse events
Adverse events were classified according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The incidence rate of the adverse events for each group was calculated as the percentage of adverse events per patient in each group.
2.6. Statistical analysis
Extracted patient data were analyzed using IBM Statistical Package for Social Sciences (version: 29.0.0.0 SPSS Inc., [New York IL], USA). p < 0.050 was determined as being statistically significant. Parametric and non‐parametric data were presented as mean ± standard deviation (SD) and median and interquartile range (IQR), respectively.
Repeated measures ANOVA was used to compare the mean difference of the PROMs and change in opioid prescriptions between each time period among patients treated with oils, dried flowers, or a combination of both. For statistically significant measures, a pairwise analysis with Bonferroni correction was performed. A sensitivity analysis was performed with analysis restricted to cannabis‐naïve patients. A one‐way ANOVA was used to compare the difference between each mode of administration at each follow‐up period. Tukey's honestly significant differences test was used to further evaluate any differences between treatments that showed statistically significant values from the one‐way ANOVA.
Univariate binary logistic regression was used to assess the effects of age, BMI, gender, treatment type, and prior cannabis exposure on adverse event likelihood using odds ratios (ORs) and 95% confidence intervals (CI). Additionally, a multivariate binary logistic regression model was applied to determine the impact of each variable on adverse event occurrence while considering the other included variables.
3. RESULTS
3.1. Patient demographics, clinical history, and prescription information
On January 9, 2023, patient data from the UKMCR were extracted. 9464 patients were enrolled at the time, of which 3058 were enrolled on the UKMCR for a minimum of 12 months and 2717 of these had completed the baseline PROM assessment. 1378 patients prescribed Adven® CBMPs were included in the final analysis.
Table 1 shows the patient demographics, clinical history, and prescription information. The mean age of patients was 46.31 ± 15.65 years. 645 (46.81%) patients were male, and 733 (53.19%) were female. The mean BMI of patients was 27.58 ± 7.21 kg/m2. The Charlson comorbidity index was 1.00 [0.00–6.00].
TABLE 1.
Patient baseline demographics, clinical history, and prescriptions.
| Demographic details | n (%)/mean ± SD/median [IQR] |
|---|---|
| Gender | |
| Female | 733 (53.19) |
| Male | 645 (46.81) |
| Age, years | 46.31 ± 15.65 |
| Body mass index, kg/m2 | 27.58 ± 7.21 |
| Occupations | |
| Unemployed | 579 (42.02) |
| Professional | 171 (12.41) |
| Other occupations | 162 (11.76) |
| Undisclosed | 117 (8.49) |
| Technicians and associate professionals | 77 (5.59) |
| Managers | 68 (4.93) |
| Elementary occupations | 65 (4.72) |
| Service and sales workers | 47 (3.41) |
| Craft and related trades workers | 41 (2.98) |
| Clerical support workers | 35 (2.54) |
| Plant and machine operators, and assemblers | 8 (0.58) |
| Skilled agricultural, forestry, and fishery workers | 4 (0.29) |
| Armed forces occupations | 4 (0.29) |
| Charlson comorbidity index | 1.00 [0.00–6.00] |
| Anxiety/depression | 610 (44.27) |
| Arthritis | 337 (24.45) |
| Hypertension | 185 (13.43) |
| Endocrine dysfunction | 113 (8.20) |
| Epilepsy | 48 (3.48) |
| Venous thromboembolism | 48 (3.48) |
| Prescription information | |
| Oils | 641 (46.52) |
| CBD, mg/24 h | 20.00 [20.00–50.00] |
| THC, mg/24 h | 10.00 [5.00–11.60] |
| Dried flower | 235 (17.05) |
| CBD, mg/24 h | 7.50 [5.00–15.00] |
| THC, mg/24 h | 167.50 [100.00–200.00] |
| Oils and dried flower | 502 (36.43) |
| CBD, mg/24 h | 27.50 [20.00–55.00] |
| THC, mg/24 h | 112.00 [105.00–195.00] |
Note: Demographic details, clinical history, and prescription of patients were collected at baseline by clinicians. Route of administration was reported as ‘oils’, ‘dried flower’, and ‘oils and dried flower’. The median CBD to THC dose was presented in mg/24 h.
Abbreviations: CBD, cannabidiol; IQR, interquartile range; n, number of patients; SD, standard deviation; THC, (−)‐trans‐∆9‐tetrahydrocannabinol.
641 (46.51%), 235 (17.05%), 502 (36.43%) patients were treated with oil, dried flower, and a combination of both CBMPs, respectively. The median CBD and THC doses for patients prescribed oils only were 20.00 [20.00–50.00] mg/24 h and 10.00 [5.00–11.60] mg/24 h, for patients prescribed dried flower only were 7.50 [5.00–15.00] mg/24 h and 167.50 [100.00–200.00] mg/24 h, and for patients prescribed both CBMPs were 27.50 [20.00–55.00] mg/24 h and 112.00 [105.00–195.00] mg/24 h respectively. The most frequently prescribed medium‐chain triglyceride oils were Adven® 50 mg/mL CBD (Curaleaf International, Guernsey, United Kingdom) and Adven® 20 mg/mL THC (Curaleaf International, Guernsey, United Kingdom). The most commonly prescribed dried flower was Adven® EMT1 200 mg/g THC < 10 mg/g CBD (Curaleaf International, Guernsey, United Kingdom).
Table 2 demonstrates the indications for CBMP treatment. There were a total of 32 indications reported in the analysis. For the primary indication, chronic non‐cancer pain (n = 394; 28.59%), neuropathic pain (n = 136; 9.87%), fibromyalgia (n = 162; 11.76%), and anxiety (n = 120, 8.71%) were the most common reasons for CBMP treatment. 528 (38.32%) patients had secondary diagnoses, and 180 (12.40%) were reported to have a tertiary diagnosis.
TABLE 2.
Primary, secondary, and tertiary diagnoses of patients.
| Diagnosis | Primary | Secondary | Tertiary |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Agoraphobia | 0 (0.00) | 3 (0.22) | 3 (0.22) |
| Anxiety | 120 (8.71) | 111 (8.06) | 25 (1.81) |
| Attention‐deficit/hyperactivity disorder | 22 (1.60) | 14 (1.02) | 3 (0.22) |
| Autistic spectrum disorder | 19 (1.38) | 8 (0.58) | 12 (0.87) |
| Cancer pain | 11 (0.80) | 7 (0.51) | 0 (0.00) |
| Chemotherapy‐induced nausea and vomiting | 6 (0.44) | 1 (0.07) | 1 (0.07) |
| Chronic non‐cancer pain | 394 (28.59) | 80 (5.81) | 1 (0.94) |
| Cluster headaches | 3 (0.22) | 1 (0.07) | 0 (0.00) |
| Complex regional pain syndrome | 8 (0.58) | 3 (0.22) | 2 (0.15) |
| Crohn's disease | 27 (1.96) | 5 (0.36) | 2 (0.15) |
| Depression | 54 (3.92) | 50 (3.63) | 34 (2.47) |
| Eating disorder | 1 (0.07) | 3 (0.22) | 4 (0.29) |
| Ehlers‐Danlos Syndrome | 42 (3.05) | 23 (1.67) | 9 (0.65) |
| Endometriosis | 1 (0.07) | 1 (0.07) | 0 (0.00) |
| Epilepsy adult | 26 (1.89) | 1 (0.07) | 1 (0.07) |
| Fibromyalgia | 162 (11.76) | 73 (5.30) | 7 (0.51) |
| Headache | 2 (0.15) | 8 (0.58) | 2 (0.15) |
| Inflammatory arthritis | 52 (3.77) | 0 (0.00) | 0 (0.00) |
| Insomnia | 30 (2.18) | 31 (2.25) | 22 (1.60) |
| Migraine | 38 (2.76) | 20 (1.45) | 8 (0.58) |
| Multiple sclerosis | 36 (2.61) | 3 (0.22) | 0 (0.00) |
| Neuropathic pain | 136 (9.87) | 49 (3.56) | 7 (0.51) |
| Obsessive‐compulsive disorder | 2 (0.15) | 3 (0.22) | 1 (0.07) |
| Osteoarthritis | 51 (3.70) | 5 (0.36) | 4 (0.29) |
| Palliative care | 55 (3.99) | 1 (0.07) | 0 (0.00) |
| Parkinson's | 12 (0.87) | 1 (0.07) | 0 (0.00) |
| Post‐traumatic stress disorder | 54 (3.92) | 3 (0.22) | 10 (0.73) |
| Rare and challenging skin condition | 5 (0.36) | 13 (0.94) | 0 (0.00) |
| Tourette's syndrome | 2 (0.15) | 3 (0.22) | 0 (0.00) |
| Trigeminal neuralgia | 1 (0.07) | 3 (0.22) | 1 (0.07) |
| Ulcerative colitis | 6 (0.44) | 3 (0.07) | 0 (0.00) |
Note: The indications for cannabis‐based medicinal product treatment and the number of patients with the corresponding indication were recorded.
Abbreviation: n, number of patients.
Table 3 illustrates the tobacco, cannabis, and alcohol status of the patients at baseline. Most patients (n = 805; 58.42%) were currently using cannabis at baseline or had previously consumed cannabis, and the lifetime cannabis use was 5.00 [1.00–15.00] gram years. For the tobacco status, 872 (63.28%) participants were current or ex‐smokers, and the median of smoking pack years was 10.00 [3.00–20.00]. The weekly alcohol consumption of the participants in the analysis was 0.00 [0.00–5.00] units.
TABLE 3.
Cannabis, tobacco, and alcohol status.
| Cannabis, tobacco, and alcohol status | n (%)/median [IQR] |
|---|---|
| Cannabis status | |
| Current user | 581 (42.16) |
| Cannabis‐naïve | 573 (41.58) |
| Ex‐user | 224 (16.25) |
| Cannabis use, gram years | 5.00 [1.00–15.00] |
| Smoking status | |
| Ex‐smoker | 532 (38.61) |
| Non‐smoker | 506 (36.72) |
| Current smoker* | 340 (24.67) |
| Smoking pack years | 10.00 [3.00–20.00] |
| Weekly alcohol consumption, units | 0.00 [0.00–5.00] |
Note: *Frequency of use: Every day (n = 478; 82.27%), every other day (n = 48, 8.27%), 1–2 times per week (n = 41, 7.06%), >1 per month (n = 7; 1.20%), <1 per month (n = 7; 1.20%). Mean use per day 1.00 [0.50–2.00] g.
Abbreviations: IQR, interquartile range; n, number of patients.
3.2. Patient‐reported outcome measures
Table 4 displays the PROMs at baseline and each follow‐up, with mean values presented separately by route of administration. Overall, a significant improvement in HRQoL was observed compared to baseline, as indicated by changes in GAD‐7, SQS, and EQ‐5D‐5L index values (p < 0.001) (Figure 1). Subscale analysis of EQ‐5D‐5L also revealed improvements (p < 0.001) in all subscales, except for self‐care, where no improvement was observed in oils only (p = 0.193) and dried flower only (p = 0.110) groups.
TABLE 4.
Baseline and follow‐up patient‐reported outcome measures (PROMs).
| Patient‐reported outcome measures and administration route | Baseline | 1 month | 3 months | 6 months | 12 months | p‐value | F‐value | Partial η 2 |
|---|---|---|---|---|---|---|---|---|
| GAD‐7 | ||||||||
| Oils | 7.54 ± 6.31 | 6.13 ± 5.89 | 6.29 ± 6.00 | 6.34 ± 6.00 | 6.39 ± 5.60 | <0.001*** | 37.53 | 0.055 |
| Dried flower | 9.60 ± 6.65 | 6.60 ± 5.60 | 6.48 ± 5.63 | 6.37 ± 5.55 | 6.10 ± 5.36 | <0.001*** | 22.34 | 0.166 |
| Oils and dried flower | 8.59 ± 6.53 | 6.23 ± 5.65 | 6.15 ± 5.56 | 6.09 ± 5.43 | 6.10 ± 5.52 | <0.001*** | 31.93 | 0.117 |
| SQS | ||||||||
| Oils | 4.39 ± 3.53 | 5.31 ± 2.57 | 5.43 ± 2.65 | 5.36 ± 2.64 | 5.39 ± 2.64 | <0.001*** | 31.84 | 0.101 |
| Dried flower | 4.29 ± 2.59 | 5.86 ± 2.52 | 5.91 ± 2.63 | 5.90 ± 2.58 | 5.56 ± 2.55 | <0.001*** | 29.10 | 0.158 |
| Oils and dried flower | 3.95 ± 2.43 | 5.38 ± 2.54 | 5.49 ± 2.60 | 5.56 ± 2.56 | 5.53 ± 2.61 | <0.001*** | 53.24 | 0.173 |
| EQ‐5D‐5L mobility | ||||||||
| Oils | 2.58 ± 1.25 | 2.49 ± 1.22 | 2.48 ± 1.24 | 2.49 ± 1.23 | 2.52 ± 1.23 | <0.001*** | 4.75 | 0.009 |
| Dried flower | 2.03 ± 1.20 | 1.89 ± 1.11 | 1.91 ± 1.12 | 1.92 ± 1.13 | 1.95 ± 1.19 | <0.001*** | 2.60 | 0.013 |
| Oils and dried flower | 2.58 ± 1.20 | 2.42 ± 1.17 | 2.41 ± 1.17 | 2.36 ± 1.18 | 2.38 ± 1.15 | <0.001*** | 9.05 | 0.030 |
| EQ‐5D‐5L self‐care | ||||||||
| Oils | 2.04 ± 1.15 | 1.99 ± 1.14 | 2.02 ± 1.14 | 2.00 ± 1.14 | 2.01 ± 1.14 | 0.220 | 1.53 | 0.002 |
| Dried flower | 1.75 ± 0.97 | 1.65 ± 0.89 | 1.73 ± 0.97 | 1.69 ± 0.90 | 1.7 ± 1.00 | 0.094 | 1.91 | 0.009 |
| Oils and dried flower | 2.08 ± 1.09 | 2.02 ± 1.09 | 1.96 ± 1.05 | 1.96 ± 1.03 | 1.93 ± 1.07 | 0.002** | 4.24 | 0.014 |
| EQ‐5D‐5L usual activities | ||||||||
| Oils | 2.92 ± 1.22 | 2.70 ± 1.20 | 2.70 ± 1.20 | 2.68 ± 1.20 | 2.74 ± 1.19 | <0.001*** | 11.96 | 0.034 |
| Dried flower | 2.47 ± 1.20 | 2.03 ± 1.03 | 2.05 ± 1.11 | 2.05 ± 1.10 | 2.07 ± 1.12 | <0.001*** | 16.00 | 0.092 |
| Oils and dried flower | 2.86 ± 1.17 | 2.53 ± 1.10 | 2.51 ± 1.14 | 2.48 ± 1.12 | 2.52 ± 1.12 | <0.001*** | 18.86 | 0.062 |
| EQ‐5D‐5L pain and discomfort | ||||||||
| Oils | 3.34 ± 1.17 | 3.03 ± 1.13 | 2.98 ± 1.13 | 2.98 ± 1.10 | 3.00 ± 1.11 | <0.001*** | 30.04 | 0.091 |
| Dried flower | 2.81 ± 1.29 | 2.31 ± 1.17 | 2.34 ± 1.15 | 2.27 ± 1.13 | 2.29 ± 1.20 | <0.001*** | 23.50 | 0.141 |
| Oils and dried flower | 3.43 ± 1.13 | 2.91 ± 1.11 | 2.85 ± 1.12 | 2.87 ± 1.08 | 2.90 ± 1.10 | <0.001*** | 54.52 | 0.167 |
| EQ‐5D‐5L anxiety and depression | ||||||||
| Oils | 2.44 ± 1.18 | 2.26 ± 1.14 | 2.29 ± 1.16 | 2.28 ± 1.14 | 2.29 ± 1.15 | <0.001*** | 8.36 | 0.022 |
| Dried flower | 2.77 ± 1.26 | 2.33 ± 1.11 | 2.31 ± 1.10 | 2.31 ± 1.09 | 2.26 ± 1.08 | <0.001*** | 17.48 | 0.103 |
| Oils and dried flower | 2.62 ± 1.22 | 2.27 ± 1.13 | 2.25 ± 1.10 | 2.23 ± 1.09 | 2.25 ± 1.09 | <0.001*** | 24.09 | 0.076 |
| EQ‐5D‐5L index value | ||||||||
| Oils | 0.30 ± 0.32 | 0.47 ± 0.31 | 0.46 ± 0.31 | 0.47 ± 0.31 | 0.46 ± 0.31 | <0.001*** | 25.88 | 0.081 |
| Dried flower | 0.48 ± 0.32 | 0.61 ± 0.28 | 0.62 ± 0.28 | 0.62 ± 0.28 | 0.62 ± 0.29 | <0.001*** | 27.51 | 0.155 |
| Oils and dried flower | 0.37 ± 0.33 | 0.49 ± 0.31 | 0.51 ± 0.30 | 0.51 ± 0.30 | 0.50 ± 0.31 | <0.001*** | 44.25 | 0.144 |
| PGIC | ||||||||
| Oils | – | 4.31 ± 1.79 | 4.5 ± 1.76 | 4.58 ± 1.77 | 4.55 ± 1.84 | <0.001*** | 8.83 | 0.017 |
| Dried flower | – | 5.46 ± 1.39 | 5.64 ± 1.24 | 5.64 ± 1.30 | 5.80 ± 1.13 | <0.001*** | 5.96 | 0.027 |
| Oils and dried flower | – | 5.20 ± 1.38 | 5.33 ± 1.38 | 5.47 ± 1.26 | 5.14 ± 1.62 | <0.001*** | 14.04 | 0.034 |
Note: All patients treated with cannabis‐medicinal products recorded PROMs at regular intervals. Paired data were analyzed using repeated measures ANOVA. Patient‐reported outcome measures were reported as mean ± standard deviation.
Abbreviations: GAD‐7, generalized anxiety disorder‐7; PGIC, patient global impression of change; SQS, Single‐Item Sleep Quality Scale.
p < 0.050;
p < 0.010;
p < 0.001.
FIGURE 1.
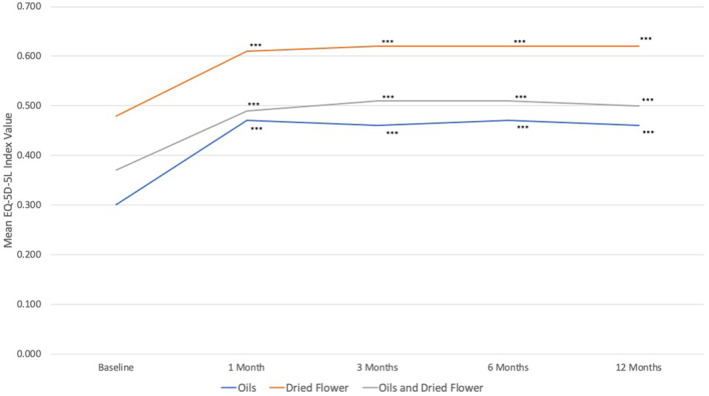
Baseline and follow‐up EQ‐5D‐5L in individuals receiving treatment with cannabis‐based medicinal products (CBMPs). Paired data were analyzed using repeated measures ANOVA. *p < 0.050, **p < 0.010, ***p < 0.001 on pairwise analysis against baseline with Bonferroni correction.
A sensitivity analysis where analysis was restricted to cannabis‐naïve patients is detailed in Table S1. Similar to the all‐patient analysis, statistically significant improvements were noted in GAD‐7, SQS, and EQ‐5D‐5L index values (p < 0.050). Subscale analysis of EQ‐5D‐5L similarly revealed improvements (p < 0.050) in most subscales, except for self‐care (p > 0.050) or mobility (p > 0.050).
Overall, the number of patients with clinically significant improvements was 406 (29.46%) and 410 (29.75%) in GAD‐7 and SQS at 12 months respectively (Table 5). For GAD‐7, the number of patients experiencing clinically significant improvements was as follows: oils (n = 138; 21.53%), dried flower (n = 93; 39.57%), and dried flower and oils (n = 175; 34.86%; p < 0.001). Similarly, for SQS, the numbers were: oils (n = 154; 24.02%), dried flower (n = 80; 34.04%), and dried flower and oils (n = 176; 35.06%; p < 0.001).
TABLE 5.
Reported proportion of patients experiencing clinically significant improvements in anxiety and sleep quality after receiving treatment with cannabis‐based medicinal products (CBMPs).
| PROM | Follow‐up interval | Oils | Dried flower | Oils and dried flower | p‐Value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| GAD‐7 | 1 month | 142 (22.15) | 94 (40.00) | 169 (33.67) | <0.001*** |
| GAD‐7 | 3 months | 143 (22.31) | 92 (39.15) | 178 (35.46) | <0.001*** |
| GAD‐7 | 6 months | 143 (22.31) | 90 (38.30) | 181 (36.06) | <0.001*** |
| GAD‐7 | 12 months | 138 (21.53) | 93 (39.57) | 175 (34.86) | <0.001*** |
| SQS | 1 month | 144 (22.46) | 75 (31.91) | 161 (32.07) | <0.001*** |
| SQS | 3 months | 153 (23.87) | 76 (32.34) | 170 (33.86) | <0.001*** |
| SQS | 6 months | 155 (24.18) | 84 (35.74) | 180 (35.86) | <0.001*** |
| SQS | 12 months | 154 (24.02) | 80 (34.04) | 176 (35.06) | <0.001*** |
Note: Clinically significant improvements in GAD‐7 and SQS were assessed at regular intervals, and the data were analyzed using the chi‐squared test.
Abbreviations: GAD‐7, generalized anxiety disorder‐7; n, number of patients; PROM, patient‐reported outcome measures; SQS, Single‐Item Sleep Quality Scale.
p < 0.050;
p < 0.010;
p < 0.001.
3.3. Effect of CBMP treatment type
Comparison of the mean difference for each PROM according to treatment group at the follow‐up intervals relative to baseline is detailed in Table 6. Differences were observed between groups at 1, 3, 6, and 12 months follow‐up in GAD‐7 (p < 0.001), SQS (p < 0.001), the EQ‐5D‐5L index value (p < 0.001), and EQ‐5D‐5L subscales, including usual activities (p < 0050), pain and discomfort (p < 0.001), anxiety and depression (p < 0.001), and the EQ‐5D‐5L index value (p < 0.001), at all timepoints. At 6 and 12 months, differences were observed in the EQ‐5D‐5L subscale for mobility (p < 0.050).
TABLE 6.
One‐way ANOVA analysis comparing patient‐reported outcome measures (PROMs) at follow‐up intervals relative to baseline for each treatment type.
| PROM | Follow‐up interval | Oils | Dried flower | Oils and dried flower | p‐Value | |||
|---|---|---|---|---|---|---|---|---|
| Mean difference | SD | Mean difference | SD | Mean difference | SD | |||
| GAD‐7 | 1 month | 1.41 | 4.08 | 3.00 | 5.34 | 2.35 | 4.85 | <0.001*** |
| GAD‐7 | 3 months | 1.25 | 4.32 | 3.12 | 5.70 | 2.44 | 5.39 | <0.001*** |
| GAD‐7 | 6 months | 1.20 | 4.47 | 3.22 | 5.72 | 2.50 | 5.60 | <0.001*** |
| GAD‐7 | 12 months | 1.15 | 4.43 | 3.49 | 5.98 | 2.48 | 5.56 | <0.001*** |
| SQS | 1 month | −0.92 | 2.36 | −1.57 | 2.42 | −1.43 | 2.58 | <0.001*** |
| SQS | 3 months | −1.04 | 2.40 | −1.62 | 2.53 | −1.55 | 2.63 | <0.001*** |
| SQS | 6 months | −0.97 | 2.47 | −1.61 | 2.71 | −1.61 | 2.63 | <0.001*** |
| SQS | 12 months | −1.01 | 2.44 | −1.55 | 2.86 | −1.61 | 2.63 | <0.001*** |
| EQ‐5D‐5L mobility | 1 month | 0.10 | 0.68 | 0.13 | 0.68 | 0.16 | 0.74 | 0.347 |
| EQ‐5D‐5L mobility | 3 months | 0.10 | 0.74 | 0.12 | 0.65 | 0.17 | 0.77 | 0.297 |
| EQ‐5D‐5L mobility | 6 months | 0.09 | 0.75 | 0.11 | 0.79 | 0.21 | 0.83 | 0.021* |
| EQ‐5D‐5L mobility | 12 months | 0.06 | 0.77 | 0.07 | 0.82 | 0.20 | 0.81 | 0.012* |
| EQ‐5D‐5L self‐care | 1 month | 0.05 | 0.63 | 0.11 | 0.67 | 0.06 | 0.72 | 0.543 |
| EQ‐5D‐5L self‐care | 3 months | 0.02 | 0.72 | 0.02 | 0.64 | 0.12 | 0.74 | 0.057 |
| EQ‐5D‐5L self‐care | 6 months | 0.04 | 0.72 | 0.06 | 0.69 | 0.12 | 0.76 | 0.218 |
| EQ‐5D‐5L self‐care | 12 months | 0.03 | 0.74 | 0.05 | 0.70 | 0.14 | 0.78 | 0.051 |
| EQ‐5D‐5L usual activities | 1 month | 0.22 | 0.88 | 0.44 | 0.90 | 0.33 | 0.93 | 0.004** |
| EQ‐5D‐5L usual activities | 3 months | 0.22 | 0.92 | 0.42 | 0.93 | 0.34 | 1.00 | 0.009** |
| EQ‐5D‐5L usual activities | 6 months | 0.24 | 0.95 | 0.42 | 0.94 | 0.37 | 1.08 | 0.019* |
| EQ‐5D‐5L usual activities | 12 months | 0.19 | 0.91 | 0.40 | 1.00 | 0.34 | 1.02 | 0.004** |
| EQ‐5D‐5L pain and discomfort | 1 month | 0.31 | 0.85 | 0.50 | 0.91 | 0.52 | 0.91 | <0.001*** |
| EQ‐5D‐5L pain and discomfort | 3 months | 0.36 | 0.87 | 0.48 | 0.88 | 0.58 | 0.94 | <0.001*** |
| EQ‐5D‐5L pain and discomfort | 6 months | 0.35 | 0.85 | 0.54 | 0.94 | 0.56 | 0.94 | <0.001*** |
| EQ‐5D‐5L pain and discomfort | 12 months | 0.34 | 0.85 | 0.52 | 0.93 | 0.54 | 0.94 | <0.001*** |
| EQ‐5D‐5L anxiety and depression | 1 month | 0.18 | 0.79 | 0.44 | 0.89 | 0.36 | 0.87 | <0.001*** |
| EQ‐5D‐5L anxiety and depression | 3 months | 0.15 | 0.85 | 0.46 | 1.04 | 0.37 | 0.97 | <0.001*** |
| EQ‐5D‐5L anxiety and depression | 6 months | 0.16 | 0.85 | 0.46 | 1.02 | 0.39 | 1.02 | <0.001*** |
| EQ‐5D‐5L anxiety and depression | 12 months | 0.14 | 0.87 | 0.51 | 1.06 | 0.37 | 1.03 | <0.001*** |
| EQ‐5D‐5L index value | 1 month | −0.08 | 0.20 | −0.13 | 0.21 | −0.12 | 0.23 | <0.001*** |
| EQ‐5D‐5L index value | 3 months | −0.08 | 0.22 | −0.12 | 0.21 | −0.13 | 0.24 | <0.001*** |
| EQ‐5D‐5L index value | 6 months | −0.08 | 0.22 | −0.14 | 0.22 | −0.14 | 0.26 | <0.001*** |
| EQ‐5D‐5L index value | 12 months | −0.07 | 0.22 | −0.14 | 0.23 | −0.14 | 0.25 | <0.001*** |
Note: A one‐way ANOVA compared the difference in means at follow‐up intervals relative to baseline for each treatment type to analyze differences between the route of administration and PROM scores in all patients treated with cannabis‐based medicinal products.
Abbreviations: GAD‐7, generalized anxiety disorder‐7; PROM, patient‐reported outcome measure; SD, standard deviation; SQS, Single‐Item Sleep Quality Scale.
p < 0.050;
p < 0.010;
p < 0.001.
Table 7 displays the results of statistically significant mean differences obtained from the one‐way ANOVA, which were assessed using Tukey's honestly significant difference post hoc test.
TABLE 7.
Tukey's honestly significant difference test for statistically significant ANOVA outcomes.
| PROM | Follow‐up interval | Oils vs dried flower | Oils vs oils and dried flower | Oils and dried flower vs dried flower | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% confidence interval | p adj | Mean difference | 95% confidence interval | p adj | Mean difference | 95% confidence interval | padj | ||
| GAD‐7 | 1 month | 1.58 | [0.76–2.41] | <0.001*** | 0.94 | [0.30–1.59] | 0.002** | 0.64 | [−0.21–1.49] | 0.185 |
| GAD‐7 | 3 months | 1.86 | [0.97–2.76] | <0.001*** | 1.19 | [0.49–1.88] | <0.001*** | 0.68 | [−0.25–1.60] | 0.197 |
| GAD‐7 | 6 months | 2.03 | [1.11–2.95] | <0.001*** | 1.31 | [0.59–2.03] | <0.001*** | 0.72 | [−0.23–1.67] | 0.177 |
| GAD‐7 | 12 months | 2.34 | [1.42–3.26] | <0.001*** | 1.33 | [0.61–2.05] | <0.001*** | 1.01 | [0.05–1.96] | 0.035* |
| SQS | 1 month | −0.65 | [−1.08 – −0.21] | 0.002** | −0.51 | [−0.86 – −0.17] | 0.001** | −0.13 | [−0.59–0.32] | 0.780 |
| SQS | 3 months | −0.58 | [−1.03 – −0.13] | 0.007** | −0.51 | [−0.86 – −0.15] | 0.002** | −0.07 | [−0.54–0.39] | 0.930 |
| SQS | 6 months | −0.64 | [−1.10 – −0.18] | 0.003** | −0.64 | [−1.00 – −0.28] | <0.001*** | 0.00 | [−0.48–0.48] | 1.000 |
| SQS | 12 months | −0.54 | [−1.00 – −0.08] | 0.017* | −0.60 | [−0.97 – −0.24] | <0.001*** | 0.06 | [−0.42–0.54] | 0.950 |
| EQ‐5D‐5L mobility | 6 months | 0.01 | [−0.13–0.15] | 0.969 | 0.12 | [0.01–0.23] | 0.03* | −0.10 | [−0.25–0.04] | 0.210 |
| EQ‐5D‐5L mobility | 12 months | 0.01 | [−0.13–0.15] | 0.985 | 0.13 | [0.02–0.25] | 0.012* | −0.12 | [−0.27–0.02] | 0.114 |
| EQ‐5D‐5L usual activities | 1 month | 0.22 | [0.06–0.38] | 0.004** | 0.11 | [−0.02–0.23] | 0.109 | 0.11 | [−0.06–0.28] | 0.261 |
| EQ‐5D‐5L usual activities | 3 months | 0.20 | [0.03–0.37] | 0.014* | 0.12 | [−0.01–0.26] | 0.073 | 0.08 | [−0.10–0.26] | 0.548 |
| EQ‐5D‐5L usual activities | 6 months | 0.18 | [0.00–0.36] | 0.046* | 0.13 | [−0.01–0.27] | 0.068 | 0.05 | [−0.14–0.23] | 0.810 |
| EQ‐5D‐5L usual activities | 12 months | 0.21 | [0.04–0.38] | 0.012* | 0.15 | [0.02–0.29] | 0.022* | 0.06 | [−0.12–0.24] | 0.736 |
| EQ‐5D‐5L pain and discomfort | 1 month | 0.19 | [0.03–0.35] | 0.012* | 0.21 | [0.08–0.33] | <0.001*** | −0.02 | [−0.18–0.15] | 0.972 |
| EQ‐5D‐5L pain and discomfort | 3 months | 0.12 | [−0.04–0.28] | 0.180 | 0.23 | [0.10–0.35] | <0.001*** | −0.11 | [−0.27–0.06] | 0.285 |
| EQ‐5D‐5L pain and discomfort | 6 months | 0.19 | [0.03–0.35] | 0.017* | 0.21 | [0.08–0.33] | <0.001*** | −0.02 | [−0.18–0.15] | 0.968 |
| EQ‐5D‐5L pain and discomfort | 12 months | 0.19 | [0.03–0.35] | 0.017* | 0.20 | [0.07–0.33] | <0.001*** | −0.01 | [−0.18–0.15] | 0.983 |
| EQ‐5D‐5L anxiety and depression | 1 month | 0.26 | [0.11–0.41] | <0.001*** | 0.18 | [0.06–0.29] | 0.001** | 0.08 | [−0.07–0.24] | 0.415 |
| EQ‐5D‐5L anxiety and depression | 3 months | 0.32 | [0.15–0.48] | <0.001*** | 0.22 | [0.09–0.35] | <0.001*** | 0.09 | [−0.08–0.26] | 0.429 |
| EQ‐5D‐5L anxiety and depression | 6 months | 0.30 | [0.13–0.47] | <0.001*** | 0.23 | [0.10–0.37] | <0.001*** | 0.07 | [−0.11–0.24] | 0.624 |
| EQ‐5D‐5L anxiety and depression | 12 months | 0.37 | [0.20–0.54] | <0.001*** | 0.23 | [0.09–0.36] | <0.001*** | 0.14 | [−0.04–0.32] | 0.148 |
| EQ‐5D‐5L index value | 1 month | −0.05 | [−0.09 – −0.01] | 0.005** | −0.04 | [−0.07 – −0.01] | 0.002** | ‐0.01 | [−0.05–0.03] | 0.906 |
| EQ‐5D‐5L index value | 3 months | −0.05 | [−0.09 – −0.01] | 0.023* | −0.05 | [−0.09 – −0.02] | <0.001*** | 0.01 | [−0.03–0.05] | 0.858 |
| EQ‐5D‐5L index value | 6 months | −0.05 | [−0.10 – −0.01] | 0.007** | −0.05 | [−0.09 – −0.02] | <0.001*** | 0.00 | [−0.04–0.04] | 0.998 |
| EQ‐5D‐5L index value | 12 months | −0.06 | [−0.10 – −0.02] | 0.002** | −0.06 | [−0.09 – −0.03] | <0.001*** | 0.00 | [−0.04–0.04] | 0.998 |
Note: Tukey honestly significant difference post hoc test was conducted on the statistically significant mean differences identified from the one‐way ANOVA to determine statistically significant differences in outcome scores based on the type of cannabis‐based medicinal product patients received.
Abbreviations: GAD‐7, generalized anxiety disorder; p adj, adjusted p‐value; PROM, patient‐reported outcome measure; SQS, Single‐Item Sleep Quality Scale.
p < 0.050;
p < 0.010;
p < 0.001.
3.4. Change in opioid medications
Table 8 illustrates the change in opioid medication following CBMP treatment in 427 (30.99%) patients who were prescribed opioids either at baseline or at other time periods in the present analysis. Overall, there was a reduction in opioid prescriptions at 1 month (140.53 mg/24 h ± 616.97 mg/24 h), 3 months (133.65 mg/24 h ± 594.06 mg/24 h), 6 months (132.07 mg/24 h ± 594.13 mg/24 h), and 12 months (132.91 mg/24 h ± 594.61 mg/24 h) compared to the baseline (140.89 mg/24 h ± 616.60 mg/24 h; p = 0.039). This represents a total reduction in mean opioid dose of 0.25%, 5.14%, 6.26%, and 5.66% at 1, 3, 6, and 12 months, respectively.
TABLE 8.
Reported opioid medications in patients across all conditions following the cannabis‐based medicinal products (CBMP) treatment.
| Baseline | 1 month | 3 months | 6 months | 12 months | p‐Value | F‐value | |
|---|---|---|---|---|---|---|---|
| Oils | 140.16 ± 656.68 | 139.74 ± 656.74 | 133.90 ± 618.97 | 133.22 ± 619.08 | 133.47 ± 619.18 | 0.374 | 0.82 |
| Dried flower | 102.19 ± 436.83 | 102.23 ± 436.82 | 100.96 ± 437.09 | 96.22 ± 436.02 | 98.67 ± 435.57 | 0.316 | 1.07 |
| Oils and dried flower | 151.27 ± 607.30 | 150.90 ± 607.37 | 141.38 ± 599.67 | 139.51 ± 599.87 | 140.64 ± 600.96 | 0.223 | 1.51 |
| Total | 140.89 ± 616.60 | 140.53 ± 616.97 | 133.65 ± 594.06 | 132.07 ± 594.13 | 132.91 ± 594.61 | 0.039* | 2.52 |
Note: Paired patient opioid usage in oral morphine equivalents was analyzed using repeated measures ANOVA. Opioid medications were reported as mean ± standard deviation.
p < 0.050;
p < 0.010;
p < 0.001.
3.5. Adverse events
Table 9 provides a comprehensive overview of the quantity and severity of adverse events. Overall, 3663 (265.82%) adverse events were reported by 297 (21.55%) patients. The incidence of adverse events for patients prescribed oils only (n = 2008; 313.26%) and dried flower only (n = 687; 292.34%) was higher than the incidence of patients prescribed oils and dried flower (n = 968; 192.83%).
TABLE 9.
Incidence of adverse events and the list of top five most common adverse event incidence in each category.
| Treatment type | Adverse event | n (%) |
|---|---|---|
| Adverse event category | ||
| Mild | ||
| Oils | 826 (41.14) | |
| Dried flower | 268 (39.01) | |
| Oils and dried flower | 466 (48.14) | |
| All treatments | 1560 (42.59) | |
| Moderate | ||
| Oils | 898 (44.72) | |
| Dried flower | 306 (44.54) | |
| Oils and dried flower | 380 (39.26) | |
| All treatments | 1584 (43.24) | |
| Severe | ||
| Oils | 283 (14.09) | |
| Dried flower | 112 (16.30) | |
| Oils and dried flower | 122 (12.60) | |
| All treatments | 517 (14.11) | |
| Life threatening/Severe | ||
| Oils | 1 (0.05) | |
| Dried flower | 1 (0.15) | |
| Oils and dried flower | 0 (0.00) | |
| All treatments | 2 (0.05) | |
| Adverse event incidence | ||
| Oils | 2008 (313.26) | |
| Dried flower | 687 (292.34) | |
| Oils and dried flower | 968 (192.83) | |
| All treatments | 3663 (265.82) | |
| Patients with adverse events | ||
| Oils | 159 (11.54) | |
| Dried flower | 42 (3.05) | |
| Oils and dried flower | 96 (6.97) | |
| All treatments | 297 (21.55) | |
| Top five most common adverse event incidence | ||
| Oils | Dizziness | 117 (18.26) |
| Dry mouth | 121 (18.88) | |
| Lethargy | 125 (19.50) | |
| Somnolence | 131 (20.44) | |
| Fatigue | 151 (23.56) | |
| Dried flower | Headache | 38 (16.17) |
| Fatigue | 42 (17.87) | |
| Concentration impairment | 50 (21.28) | |
| Somnolence | 50 (21.28) | |
| Dry mouth | 55 (23.40) | |
| Oils and dried flower | Headache | 54 (10.76) |
| Lethargy | 62 (12.35) | |
| Somnolence | 69 (13.75) | |
| Dry mouth | 69 (13.75) | |
| Fatigue | 78 (15.50) | |
| All treatments | Headache | 205 (14.88) |
| Lethargy | 221 (16.04) | |
| Dry mouth | 246 (17.85) | |
| Somnolence | 250 (18.14) | |
| Fatigue | 271 (19.67) | |
Note: Adverse event incidence was the division of the total number of adverse events experienced by the number of patients in the corresponding treatment groups.
Abbreviation: n, number of patients.
Most adverse events were moderate (n = 1584; 43.24%) or mild (n = 1560; 42.59%). The most common adverse events were fatigue (n = 271; 19.67%), somnolence (n = 250; 18.14%), dry mouth (n = 246; 17.85%), lethargy (n = 221; 16.04%), and headache (n = 205; 14.88%). There was one reported episode of psychosis (0.07%) and two (0.15%) incidences of euphoria.
Table 10 presents the findings from a univariate logistic regression, which examined factors contributing to the likelihood of adverse events. The analysis revealed that both cannabis‐naïve users (OR = 2.406; 95% CI: 1.792–3.230; p < 0.001) and cannabis ex‐users (OR = 1.769; 95% CI: 1.198–2.611; p = 0.004) exhibited a higher incidence of adverse events compared to current users. Moreover, male patients (OR = 0.426; 95% CI: 0.324–0.559; p < 0.001) and those prescribed with dried flower (OR = 0.679; 95% CI: 0.466–0.989; p = 0.044), as well as a combination of oils and dried flower (OR = 0.717; 95% CI: 0.539–0.954; p = 0.022), demonstrated a reduced likelihood of experiencing adverse events.
TABLE 10.
Univariate logistic regression model assessing factors contributing to the likelihood of experiencing adverse events.
| Variable | Odds ratio [95% confidence interval] | p‐value |
|---|---|---|
| Age, years | ||
| 18–30 | – | Ref |
| 31–40 | 0.736 [0.480–1.126] | 0.158 |
| 41–50 | 0.846 [0.558–1.285] | 0.434 |
| 51–60 | 1.193 [0.777–1.833] | 0.420 |
| 61–70 | 1.307 [0.806–2.120] | 0.277 |
| 71–80 | 1.067 [0.591–1.926] | 0.830 |
| 80+ | 1.224 [0.516–2.904] | 0.646 |
| BMI, kg/m2 | ||
| <20 | 0.839 [0.517–1.361] | 0.477 |
| 20–25 | – | Ref |
| 25–30 | 0.735 [0.521–1.036] | 0.079 |
| 30–35 | 0.845 [0.559–1.276] | 0.423 |
| 35+ | 1.046 [0.685–1.597] | 0.835 |
| Cannabis status | ||
| Current users | – | Ref |
| Ex‐users | 1.769 [1.198–2.611] | 0.004** |
| Naïve | 2.406 [1.792–3.230] | <0.001*** |
| Gender | ||
| Female | – | Ref |
| Male | 0.426 [0.324–0.559] | <0.001*** |
| Treatment type | ||
| Oils | – | Ref |
| Dried flower | 0.679 [0.466–0.989] | 0.044* |
| Oils and dried flower | 0.717 [0.539–0.954] | 0.022* |
Note: A univariate logistic regression model assessed the relationship of age, body mass index (BMI), cannabis status, gender, and treatment type on the probability of experiencing adverse events by the calculation of odd ratios and 95% confidence intervals.
Abbreviation: Ref, reference group.
p < 0.050;
p < 0.010;
p < 0.001.
Table 11 demonstrates the results of the multivariate analysis examining factors contributing to adverse event incidence. Compared to current users, cannabis‐naïve individuals (OR = 2.290; 95% CI: 1.583–3.313; p < 0.001) and ex‐users (OR = 1.760; 95% CI: 1.158–2.674; p = 0.008) displayed higher probabilities of experiencing adverse events. Furthermore, males (OR = 0.477; 95% CI: 0.352–0.645; p < 0.001) exhibited a reduced likelihood of adverse events.
TABLE 11.
Multivariate logistic regression model assessing factors contributing to the likelihood of experiencing adverse events.
| Variable | Odds ratio [95% confidence interval] | p‐Value |
|---|---|---|
| Age, years | ||
| 18–30 | – | Ref |
| 31–40 | 0.771 [0.490–1.212] | 0.259 |
| 41–50 | 0.894 [0.571–1.398] | 0.622 |
| 51–60 | 0.978 [0.611–1.565] | 0.926 |
| 61–70 | 1.039 [0.604–1.789] | 0.889 |
| 71–80 | 0.776 [0.395–1.523] | 0.461 |
| 80+ | 1.270 [0.489–3.304] | 0.624 |
| BMI, kg/m2 | ||
| <20 | 0.670 [0.402–1.117] | 0.124 |
| 20–25 | – | Ref |
| 25–30 | 0.794 [0.556–1.133] | 0.204 |
| 30–35 | 0.762 [0.498–1.166] | 0.211 |
| 35+ | 0.834 [0.538–1.293] | 0.417 |
| Cannabis status | ||
| Current users | – | Ref |
| Ex‐users | 1.760 [1.158–2.674] | 0.008** |
| Naïve | 2.290 [1.583–3.313] | <0.001*** |
| Gender | ||
| Female | – | Ref |
| Male | 0.477 [0.352–0.645] | <0.001*** |
| Treatment type | ||
| Oils | – | Ref |
| Dried flower | 1.486 [0.940–2.349] | 0.090 |
| Oils and dried flower | 1.193 [0.847–1.680] | 0.314 |
Note: A multivariate logistic regression model was used to assess the relationship between age, body mass index (BMI), cannabis status, gender, and treatment type on the likelihood of experiencing adverse events by the calculation of odd ratios and 95% confidence intervals.
Abbreviation: Ref, reference group.
p < 0.050;
p < 0.010;
p < 0.001.
4. DISCUSSION
The results of this study show an associated improvement in self‐reported anxiety, sleep quality, HRQoL, and a reduction in opioid prescription in patients treated with oil‐based, dried flower, and a combination of both CBMPs at 1, 3, 6, and 12 months from baseline. The magnitude of the improvements was static following the changes seen at 1 month, with no statistically significant differences in each primary outcome noted between each follow‐up on post hoc analysis, except in comparison to baseline. Patients prescribed CBMP treatment formulations with dried flower were more likely to show statistically significant improvements in GAD‐7, SQS, and EQ‐5D‐5L index value. Moreover, there was no significant difference in the PROMs between patients prescribed dried flower and both oils and dried flower. Individuals who were not already consuming cannabis at baseline were found to have a higher likelihood of experiencing adverse events than current users. Meanwhile, males exhibited a reduced likelihood of reporting adverse events.
The study revealed significant improvement in anxiety symptoms for all patients, with approximately one‐third experiencing clinically significant improvements at 12 months. Among these patients, the cohort prescribed dried flower exhibited the most substantial improvement. Improvements were also observed in a study of 5075 patients from Canada, but only 3.7% of the patients experienced a clinically significant decrease in GAD‐7 scores. 45 Conversely, another study did not find a significant change in the anxiety subscale of the DASS‐21. 46 The evidence regarding CBMPs' efficacy in treating anxiety symptoms is unclear, possibly due to heterogeneity in the studies conducted. 47 Moreover, self‐reported sleep quality significantly improved among patients prescribed CBMPs via any route of administration, with the dried flower and oils/dried flower routes demonstrating greater improvements compared to oils alone. Improvements in sleep quality with the use of cannabis have also been shown in randomized clinical studies. 48 , 49 , 50 These have, however, been affected by small sample sizes, limited follow‐up or both. This study, which focused on a homogeneous selection of CBMPs (Adven®), demonstrates associated improvements up to 12 months, with the greatest improvements seen in those prescribed dried flower preparations. While the limitations of study design limit direct inferences of effectiveness to be made, these results support the further evaluation of Adven® CBMPs in randomized controlled trials.
In addition, improvements in HRQoL were evident across all administration routes, as demonstrated by the EQ‐5D‐5L index value. The dried flower and the oils and dried flower cohorts exhibited superior improvements to the oils‐only cohort. These findings align with those of QUEST Initiative 51 and are substantiated in a study involving 3184 patients treated at Emerald Clinics, Australia, where CBMPs were associated with an improved HRQoL across a spectrum of medical conditions. 52 The differences between products may be secondary to the pharmacokinetic differences associated with each method of administration. Moreover, the dose of THC was higher in those prescribed dried flower in isolation or in combination with oils. This may have contributed to both objective and subjective improvements in specific symptoms and HRQoL. 53 Moreover, as the assignment to each treatment was not randomized these differences may reflect underlying patient characteristics. In contrast, other studies have reported a reduced HRQoL in patients prescribed CBMPs. 54 The variability in the outcomes regarding HRQoL through CBMPs is likely due to the heterogeneity of the studied population, emphasizing the need for further investigation using a homogenous sample of CBMPs within separate populations to determine the most appropriate medications to take forward into randomized controlled trials. 55
The study observed a decrease in daily opioid consumption of 0.25%, 5.14%, 6.26%, and 5.66% after 1, 3, 6, and 12 months respectively. A recent study indicated a clinically significant reduction required a 28.2% decrease. 56 However, findings from pooled observational studies reported a much higher reduction of 78% in oral morphine equivalents compared to the results reported here. 57 Multiple factors contribute to the relatively low decrease in opioid consumption within the study. Notably, 42.16% of patients were current cannabis consumers at the point of enrolment, suggesting they might have already utilized cannabis in reducing their opioid consumption. Furthermore, patients might have reduced their regular opioid use after starting CBMP treatment, yet this alteration might not have been accurately recorded. Indeed, a patient and public evaluation of the data collection platform used by participants in the UKMCR highlighted ‘difficulty adding as required medication’. 20 This is most likely to affect pain medications, such as opioids, which may be used for breakthrough pain, in addition to chronic use. 58
Most reported adverse events were mild to moderate, consistent with prior assessments of safety. 59 The highest incidence of adverse events was observed among patients prescribed oils (n = 2008; 313.26%), with a similar rate observed among patients prescribed the combination of oils and dried flowers (n = 687; 292.34%). These results may be attributed to the fact that most patients prescribed with oils were cannabis‐naïve. 60 , 61 On multivariate analysis, individuals who were cannabis‐naïve were demonstrated to have an increased likelihood of experiencing an adverse event, while differing routes of administration of CBMPs were not associated with the incidence of adverse events. This finding may be secondary to increased pharmacological tolerance in prior users. 62
This study has several limitations. The most crucial limitation of an observational study is that it is not possible to determine whether CBMPs were responsible for the observed effects or whether they were secondary to confounding factors and/or regression to the mean. There is a sampling bias associated with the present cohort. A higher proportion of participants were currently consuming or had previously consumed cannabis at baseline. These individuals may be self‐selecting as responders to CBMPs through previous consumption and therefore their inclusion could lead to an enhanced response rate. For these individuals, there may also be an expectancy bias considering their knowledge of the effects of cannabis, which could lead to reporting greater improvements in symptoms or HRQoL and reduced reporting of adverse events. However, a sensitivity analysis including only cannabis‐naïve patients demonstrated similar results to the primary analysis suggesting these effects are still seen in patients who had not previously been exposed to the effects of cannabis. All prescriptions for CBMPs were obtained privately, which may enhance responder bias. Moreover, the vasoactive and psychoactive effects of CBMPs have been demonstrated to cause an enhanced placebo effect. CBD and THC doses were calculated from prescriptions, rather than patient‐reported data to ensure completeness of data, so this may not represent the doses administered in all cases. These doses were also calculated from the optimally titrated dose at the end of the study period, rather than the dose prescribed throughout and therefore may be higher than those prescribed at earlier time points. There is loss of participants to follow‐up, leading to attrition bias. To counteract this, the present analysis uses a baseline observation carried forward approach to manage missing data, which likely leads to a more conservative estimate of the actual effect of CBMPs, with the results being biased towards a null finding. There is a significant amount of heterogeneity among CBMP formulations studied; however, to mitigate this, the studied population was only prescribed products from one manufacturer. Finally, while the length of the present analysis helps identify short‐term outcomes, future studies should aim to have longer follow‐up periods to determine long‐term adverse events and if patients develop tolerance to the effects of CBMPs.
In conclusion, the CBMPs studied in this analysis were associated with an improvement in self‐reported anxiety, sleep quality, and HRQoL, consistent with existing literature on CBMPs. Patients prescribed treatment formulations, including dried flowers, were most likely to show clinical improvement, although caution must be taken in interpreting these results due to the study's limitations. Furthermore, the study highlights the low incidence of severe or disabling adverse events linked to extended use of CBMP. It demonstrates a higher occurrence of adverse events among females and individuals who are cannabis‐naïve. Therefore, future studies with active comparators should investigate the effect of CBMPs on HRQoL, account for confounders, and assess long‐term safety. This study does however provide support for the further examination of Adven® CBMPs (Curaleaf International, Guernsey, UK) in randomized controlled trials.
AUTHOR CONTRIBUTIONS
Study conception and design: SE, CH, RC, SB, SK, MWW, JRR, MWP, MHS; Acquisition of data: SE, OL, CH, RC, SB, SK, MWW, JRR, MWP; Analysis and interpretation of data: SE, OL, MHS; Drafting of manuscript: SE, OL, MHS; Critical revision: SE, OL, CH, RC, SB, SK, MWW, JRR, MWP, MHS. All authors have contributed to and approved the final manuscript. The authors confirm that the PI for this paper is Mikael H Sodergren and that he had direct clinical responsibility for patients.
FUNDING INFORMATION
There was no external or commercial funding associated with this paper.
CONFLICT OF INTEREST STATEMENT
Simon Erridge is a junior doctor and is the Head of Research at Sapphire Medical Clinics. Simon Erridge is an honorary clinical research fellow at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS. Simon Erridge has no shareholdings in pharmaceutical companies. Ophilia Leung is a biomedical sciences student at Imperial College London. Ophilia Leung has no shareholdings in pharmaceutical companies. Carl Holvey is Chief Clinical Pharmacist at Sapphire Medical Clinics. Carl Holvey has no shareholdings in pharmaceutical companies. Ross Coomber is a consultant orthopedic surgeon, Operations Director at Sapphire Medical Clinics, and a consultant at St George's Hospital, London. The views expressed are those of the author(s) and not necessarily those of the NHS. Ross Coomber has no shareholdings in pharmaceutical companies. Sushil Beri is a consultant neurologist at Sapphire Medical Clinics and Imperial College NHS Healthcare Trust. The views expressed are those of the author(s) and not necessarily those of the NHS. Sushil Beri has no shareholdings in pharmaceutical companies. Shaheen Khan is a consultant palliative care physician at Sapphire Medical Clinics and Guy's & St. Thomas' NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS. Shaheen Khan has no shareholdings in pharmaceutical companies. Mark Weatherall is a consultant palliative care physician at Sapphire Medical Clinics and Buckinghamshire Healthcare NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS. Mark Weatherall has no shareholdings in pharmaceutical companies. James Rucker is a consultant psychiatrist and a former director at Sapphire Medical Clinics (London). James Rucker is an honorary consultant psychiatrist at The South London & Maudsley NHS Foundation Trust, and an NIHR Clinician Scientist Fellow at the Centre for Affective Disorders at King's College London. James Rucker is funded by a fellowship (CS‐2017‐17‐007) from the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. James Rucker has no shareholdings in pharmaceutical companies. James Rucker reviewed this article and made comments. Michael Platt is a consultant pain physician at Sapphire Medical Clinic. Michael Platt has no shareholdings in pharmaceutical companies. Mikael Sodergren is a consultant hepatopancreatobiliary surgeon at Imperial College NHS Trust. He is the Chief Medical Officer at Curaleaf International. He is a senior clinical lecturer at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS.
ETHICS APPROVAL
Ethical approval provided by South West–Central Bristol Research Ethics Committee (Reference: 22/SW/0145).
Informed Consent: All participants completed written, informed consent prior to enrolment in the registry.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
N/A.
CLINICAL TRIAL REGISTRATION
N/A.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors have nothing to report.
Erridge S, Leung O, Holvey C, Coomber R, Beri S, Khan S, et al. An observational study of clinical outcome measures in patients treated with cannabis‐based medicinal products on the UK Medical Cannabis Registry. Neuropsychopharmacol Rep. 2023;43:616–632. 10.1002/npr2.12403
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available from the UK Medical Cannabis Registry. Restrictions apply to the availability of these data. Data specifications and applications are available from the corresponding author.
REFERENCES
- 1. Freeman TP, Hindocha C, Green SF, Bloomfield MAP. Medicinal use of cannabis based products and cannabinoids. BMJ. 2019;1141:365. 10.1136/bmj.l1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Case P. The NICE guideline on medicinal cannabis: keeping Pandora's box shut tight? Med Law Rev. 2020;28(2):401–411. [DOI] [PubMed] [Google Scholar]
- 3. Banerjee R, Erridge S, Salazar O, Mangal N, Couch D, Pacchetti B, et al. Real world evidence in medical cannabis research. There Innov Regul Sci. 2021;56(2):8–24. 10.1007/s43441-021-00346-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Center for Biotechnology Information . PubChem Compound Summary for CID 644019, Cannabidiol. https://pubchem.ncbi.nlm.nih.gov/compound/Cannabidiol [Accessed Feb 25, 2023].
- 5. Berman P, Futon K, Lewitus GM, Much D, Benami M, Shlomi T, et al. A new ESI‐LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in cannabis. Sci Rep. 2018;8:14280. 10.1038/s41598-018-32651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atakan Z. Cannabis, a Complex Plant: Different Compounds and Different Effects on Individuals. Ther Adv Psychopharmacol. 2012;2(6):241–254. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3736954/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cogan PS. The ‘entourage effect’ or ‘Hodge‐podge hashish’: the questionable rebranding, marketing, and expectations of cannabis polypharmacy. Expert Rev Clin Pharmacol. 2020;13(8):835–845. [DOI] [PubMed] [Google Scholar]
- 9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9‐tetrahydrocannabinol, cannabidiol and Δ9‐tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. 10.1038/sj.bjp.0707442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brunt TM, Bossong M. The neuropharmacology of cannabinoid receptor ligands in central signaling pathways. Eur J Neurosci. 2020;55(4):909–921. 10.1111/ejn.14982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murillo‐Rodríguez E. The role of the CB1 receptor in the regulation of sleep. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1420–1427. 10.1016/j.pnpbp.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 12. Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16(1):9–29. 10.1038/s41582-019-0284-z [DOI] [PubMed] [Google Scholar]
- 13. Jean‐Gilles L, Fahey AJ, Braitch M, Edwards LJ, Robins RA, Showe L, Latif ML, Alexander SP, Chapman V, Kendall DA, Constantinescu CS. Effects of pro‐inflammatory cytokines on cannabinoid CB1 and CB2 receptors in immune cells of Normal subjects and patients with multiple sclerosis. In Neurology 2010. 74, No. 9, pp. A418‐A418 Philadelphia, USA: Lippincott Williams & Wilkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deutsch DG. A personal retrospective: elevating anandamide (AEA) by targeting fatty acid amide hydrolase (FAAH) and the fatty acid binding proteins (FABPs). Front Pharmacol. 2016;7:370. 10.3389/fphar.2016.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laprairie RB, Bagher AM, Kelly ME, Denovan‐Wright E. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang L, Hong PJ, May C, Rehman Y, Oparin Y, Hong CJ, et al. Medical cannabis or cannabinoids for chronic non‐cancer and cancer related pain: a systematic review and meta‐analysis of randomised clinical trials. BMJ. 2021;9:374. [DOI] [PubMed] [Google Scholar]
- 17. Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta‐analysis. JAMA. 2015;313(24):2456–2473. [DOI] [PubMed] [Google Scholar]
- 18. Filippini G, Minozzi S, Borrelli F, Cinquini M, Dwan K. Cannabis and cannabinoids for symptomatic treatment for people with multiple sclerosis. Cochrane Database Syst Rev. 2022;5:CD013444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solmi M, De Toffol M, Kim JY, Choi MJ, Stubbs B, Thompson T, et al. Balancing risks and benefits of cannabis use: umbrella review of meta‐analyses of randomised controlled trials and observational studies. BMJ. 2023;382:e072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tait J, Erridge S, Sodergren MH. UK medical cannabis registry: a patient evaluation. J Pain Palliat Care Pharmacother. 2023;37:170–177. 10.1080/15360288.2023.2174633 [DOI] [PubMed] [Google Scholar]
- 21. Bapir L, Erridge S, Nicholas M, Pillai M, Dalavaye N, Holvey C, et al. Comparing the effects of medical cannabis for chronic pain patients with and without co‐morbid anxiety: a cohort study. Expert Rev Neurother. 2023;23(3):281–295. [DOI] [PubMed] [Google Scholar]
- 22. Rifkin‐Zybutz R, Erridge S, Holvey C, Coomber R, Gaffney J, Lawn W, et al. Clinical outcome data of anxiety patients treated with cannabis‐based medicinal products in the United Kingdom: a cohort study from the UK medical cannabis registry. Psychopharmacology (Berl). 2023;240:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erridge S, Kerr‐Gaffney J, Holvey C, Coomber R, Barros DA, Bhoskar U, et al. Clinical outcome analysis of patients with autism spectrum disorder: analysis from the UK medical cannabis registry. Ther Adv Psychopharmacol. 2022;12:20451253221116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pillai M, Erridge S, Bapir L, Nicholas M, Dalavaye N, Holvey C, et al. Assessment of clinical outcomes in patients with post‐traumatic stress disorder: analysis from the UK medical cannabis registry. Expert Rev Neurother. 2022;22(11–12):1009–1018. [DOI] [PubMed] [Google Scholar]
- 25. Mangoo S, Erridge S, Holvey C, Coomber R, Barros DA, Bhoskar U, et al. Assessment of clinical outcomes of medicinal cannabis therapy for depression: analysis from the UK medical cannabis registry. Expert Rev Neurother. 2022;22(11–12):995–1008. [DOI] [PubMed] [Google Scholar]
- 26. Nicholas M, Erridge S, Bapir L, Pillai M, Dalavaye N, Holvey C, et al. UK medical cannabis registry: assessment of clinical outcomes in patients with headache disorders. Expert Rev Neurother. 2023;23(1):85–96. [DOI] [PubMed] [Google Scholar]
- 27. Erridge S, Holvey C, Coomber R, Hoare J, Khan S, Platt MW, et al. Clinical outcome data of children treated with cannabis‐based medicinal products for treatment resistant epilepsy—analysis from the UK medical cannabis registry. Neuropediatrics. 2023;30:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erridge S, Salazar O, Kawka M, Holvey C, Coomber R, Usmani A, et al. An initial analysis of the UK medical cannabis registry: outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. 2021;41(3):362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ergisi M, Erridge S, Harris M, Kawka M, Nimalan D, Salazar O, et al. An updated analysis of clinical outcome measures across patients from the UK medical cannabis registry. Cannabis Cannabinoid Res. 2023;8(3):557–566. [DOI] [PubMed] [Google Scholar]
- 30. Olsson F, Erridge S, Tait J, Holvey C, Coomber R, Beri S, et al. An observational study of safety and clinical outcome measures across patient groups in the United Kingdom medical cannabis registry. Expert Rev Clin Pharmacol. 2023;16(3):257–266. [DOI] [PubMed] [Google Scholar]
- 31. Banerjee R, Erridge S, Salazar O, Mangal N, Couch D, Pacchetti B, et al. Real world evidence in medical cannabis research. Ther Innov Regul Sci. 2022;56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ev E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 33. International Labour Organisation . The International Standard Classification of Occupations‐ ISCO‐08. https://isco‐ilo.netlify.app/en/isco‐08/ [Accessed 20 Feb 2023].
- 34. Roffman C, Buchanan J, Allison GT. Charlson comorbidities index. J Physiother. 2016;62(3):171. 10.1016/j.jphys.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 35. Wetherill RR, Hager N, Guthier E, Franklin TR. Gram years: a method to standardize and quantify lifetime cannabis consumption. Cannabis Cannabinoid Res. 2016;1(1):216–217. 10.1089/can.2016.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. NHS Digital . SNOMED CT. https://digital.nhs.uk/services/terminology‐and‐classifications/snomed‐ct [Accessed 20 Feb 2023].
- 37. National Institute for Health and Care Excellence . BNF British National Formulary – NICE. [Online] Available from: https://bnf.nice.org.uk/?utm_source=evidence_bnf_redirect&utm_medium=(other)&utm_campaign=old_site_redirect [Accessed: 7 Aug 2023]
- 38. General Practice Notebook . morphine to tramadol equivalence in chronic usage – General Practice Notebook. [Online] Available from: https://gpnotebook.com/simplepage.cfm?ID=x20041106080808159860 [Accessed: 7 Aug 2023]
- 39. Swinson RP. The GAD‐7 scale was accurate for diagnosing generalised anxiety disorder. BMJ Evid Based Med. 2006;11:184. [DOI] [PubMed] [Google Scholar]
- 40. Synder E, Cai B, DeMuro C, Morrison MF, Ball W. A new single‐item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018;14(11):1849–1857. 10.5664/jcsm.7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gerlinger C, Bamber L, Leverkus F, Schwenke C, Haberland C, Schmidt G, et al. Comparing the EQ‐5D‐5L utility index based on value sets of different countries: impact on the interpretation of clinical study results. BMC Res Notes. 2019;12:18. 10.1186/s13104-019-4067-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Institute for Health and Care Excellence . Position statement on use of the EQ‐5D‐5L value set for England. https://www.nice.org.uk/about/what‐we‐do/our‐programmes/nice‐guidance/technology‐appraisal‐guidance/eq‐5d‐5l [Accessed 20 Feb 2023].
- 43. Gandhi M, Rand K, Luo N. Valuation of health states considered to Be worse than death—an analysis of composite time trade‐off data from 5 EQ‐5D‐5L valuation studies. Value Health. 2019;22(3):370–376. 10.1016/j.jval.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 44. Rampakakis E, Ste‐Marie PA, Sampalis JS, Karellis A, Shir Y, Fitzcharles M. Real‐life assessment of the validity of patient global impression of change in fibromyalgia. RMD Open. 2015;1(1):e000146. 10.1136/rmdopen-2015-000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee C, Round JM, Hanlon JG, Hyshka E, Dyck JRB, Eurich DT. Generalized anxiety disorder 7‐item (GAD‐7) scores in medically authorized cannabis patients—Ontario and Alberta. Can J Psychiatry. 2022;67(7):470–480. 10.1177/07067437211043393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hser YI, Mooney LJ, Huang D, Zhu Y, Tomko RL, McClure E, et al. Reductions in cannabis use are associated with improvements in anxiety, depression, and sleep quality, but not quality of life. J Subst Abuse Treat. 2017;1(81):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cahill SP, Lunn SE, Diaz P, Page JE. Evaluation of patient reported safety and efficacy of cannabis from a survey of medical cannabis patients in Canada. Front Public Health. 2021;9:626853. 10.3389/fpubh.2021.626853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tervo‐Clemmens B, Schmitt W, Wheeler G, Cooke ME, Schuster RM, Hickey S, et al. Cannabis use and sleep quality in daily life: an electronic daily diary study of adults starting cannabis for health concerns. Drug Alcohol Depend. 2023;243:109760. 10.1016/j.drugalcdep.2022.109760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ried K, Tamanna T, Matthews S, Sali A. Medicinal cannabis improves sleep in adults with insomnia: a randomised double‐blind placebo‐controlled crossover study. J Sleep Res. 2022;32:e13793. 10.1111/jsr.13793 [DOI] [PubMed] [Google Scholar]
- 50. Walsh JH, Maddison KJ, Rankin T, Murray K, McArdle N, Ree MJ, et al. Treating insomnia symptoms with medicinal cannabis: a randomized, crossover trial of the efficacy of a cannabinoid medicine compared with placebo. Sleep. 2021;44(21):1–8. 10.1093/sleep/zsab149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tait M, Costa DSJ, Campbell R, Norman R, Warne LN, Schug S, et al. Health‐related quality of life in patients accessing medicinal cannabis in Australia: the QUEST initiative results of a 3‐month follow‐up observational study. PLoS One. 2023;18(9):e0290549. 10.1371/journal.pone.0290549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arkell TR, Downey LA, Hayley AC, Roth S. Assessment of medical cannabis and health‐related quality of life. JAMA Netw Open. 2023;6(5):e2312522. 10.1001/jamanetworkopen.2023.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peterson AM, Le C, Dautrich T. Measuring the change in health‐related quality of life in patients using marijuana for pain relief. Med Cannabis Cannabinoids. 2021;4:114–120. 10.1159/000517857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buonomano LS, Mitnick MM, McCalmont TR, Syracuse P, Dugosh KL, Festinger DS, et al. Clinical characteristics and quality of life in adults initiating medical marijuana treatment. Med Cannabis Cannabinoids. 2022;5(1):95–101. 10.1159/000524831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee S, Lee DK. What is the proper way to apply the multiple comparison test? Korean J Anesthesiol. 2018;71(5):353–360. 10.4097/kja.d.18.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goudman L, Smedt AD, Forget P, Moens M. Determining the minimal clinical important difference for medication quantification scale III and morphine milligram equivalents in patients with failed Back surgery syndrome. J Clin Med. 2020;9(11):3747. 10.3390/jcm9113747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lucas P, Boyd S, Milloy M, Walsh Z. Cannabis significantly reduces the use of prescription opioids and improves quality of life in authorized patients: results of a large prospective study. Pain Med. 2021;22(3):729–739. 10.1093/pm/pnaa396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nicholson B. Responsible prescribing of opioids for the management of chronic pain. Drugs. 2003;63:17–32. [DOI] [PubMed] [Google Scholar]
- 59. Ware MA, Wang T, Shapiro S, Collet J. Cannabis for the Management of Pain: assessment of safety study (COMPASS). J Pain. 2015;16(12):1233–1242. 10.1016/j.jpain.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 60. Kawka M, Erridge S, Holvey C, Coomber R, Usmani A, Sajad M, et al. Clinical outcome data of first cohort of chronic pain patients treated with cannabis‐based sublingual oils in the United Kingdom: analysis from the UK medical cannabis registry. J Clin Pharmacol. 2021;61(12):1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tait J, Erridge S, Holvey C, Coomber R, Usmani A, Sajad M, et al. Clinical outcome data of chronic pain patients treated with cannabis‐based oils and dried flower from the UK medical cannabis registry. Expert Rev Neurother. 2023;23(4):413–423. 10.1080/14737175.2023.2195551 [DOI] [PubMed] [Google Scholar]
- 62. Ramaekers JG, Van Wel JH, Spronk DB, Toennes SW, Kuypers KP, Theunissen EL, et al. Cannabis and tolerance: acute drug impairment as a function of cannabis use history. Sci Rep. 2016;6(1):26843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data that support the findings of this study are available from the UK Medical Cannabis Registry. Restrictions apply to the availability of these data. Data specifications and applications are available from the corresponding author.


