Abstract
The aim of our study was to assess the relationship between the changes of antinuclear autoantibodies (ANAs) and autoantibodies to topoisomerase 1 (anti-Topo 1) in systemic sclerosis (SSs) patients on rituximab (RTX) therapy. The prospective study included 88 patients (73 women) with a mean age of 47 (17–71) years. The mean disease duration was 5.9 ± 4.8 years. The mean follow-up period was more than 2 years (27 (12–42) months). We documented a statistically significant change in skin score, the disease activity index, improvement of pulmonary function and reduction of mean dose of prednisolone after RTX treatment. There was a significant decrease in the number of patients with high levels of ANA and overall decrease of the ANA and anti-Topo 1 levels. A moderate positive statistically significant correlation was found between ANA and anti-Topo 1 (r = 0.403). In the group of patients positive for anti-Topo 1 there were a more pronounced depletion of B lymphocytes, significantly higher increase in forced vital capacity and diffusion capacity, decrease in the disease activity index, compared with patients negative for anti-Topo 1. We observed the decline in the level of ANA and anti-Topo 1 in SSc patients after RTX therapy, and it was correlated by an improvement of the main outcome parameters of the disease. Therefore, anti-Topo 1 positivity could be considered as a predictor of a better response to RTX treatment, especially in SSc patients with hyperproduction of anti-Topo 1.
Keywords: systemic sclerosis, antinuclear antibody, anti-topoisomerase 1 antibody, interstitial lung disease, rituximab
Systemic sclerosis (SSc) is a systemic immune-inflammatory (autoimmune) rheumatic disease (IIRD), the pathogenesis of which is based on immune disorders combined with vasospastic vascular reactions and leading to activation of fibrosis and uncontrolled deposition of extracellular matrix components in tissues [1]. SSc, as well as other IIRDs, is characterized by overproduction of autoantibodies to a wide range of nuclear and cytoplasmic molecules (ANAs, antinuclear autoantibodies), which are determined by the conventional methods such as indirect immunofluorescence analysis, as well as enzyme immunoassay, immunoblotting, etc. [2–4]. Along with the antibodies to the centromere (ACA) and RNA polymerase III, the antibodies that are specific for SSc include antibodies to the non-histone chromosomal protein Scl-70, which is the enzyme topoisomerase I with a molecular weight of 70 kDa (anti-Topo 1). In SSc, the detection of “sclerodermal” antibodies not only has a diagnostic significance (within the classification criteria for SSc) [5], but also makes it possible to identify the clinical and immunological subtypes that are characterized by different range of lesions of internal organs and determine the prognosis of the disease [6]. Anti-Topo 1, which are detected in one-third of SSc patients, is associated with the development of rapidly progressive skin fibrosis, interstitial lung disease (ILD), digital ulcers, and high mortality [7–9].
According to modern concepts, the disturbance of B-cell tolerance plays a fundamental role in the immunopathogenesis of SSc and other IIRDs [10, 11]. However, the specific mechanisms underlying the hyperproduction of anti-Topo 1 and their pathogenetic significance (as well as the autoantibodies detected in other IIRDs) are not completely clear [12]. The following facts testify to the potential pathogenetic significance of the immune response to Topo 1. According to experimental data, immunization of mice with Topo 1 induces the synthesis of anti-Topo 1 and the development of skin and lung fibrosis [13, 14]. In the lung tissue of SSc patients, an increase in the expression of Topo 1 was detected [15], and in the blood of patients with ILD, an increase in the number of autoreactive CD4+ T cells, which are specific for Topo 1 and have the Th17 “proinflammatory” phenotype, was noted [16]. In some SSc patients, decreased levels of anti-Topo 1 during treatment are associated with a milder course of the disease [17, 18]. However, according to other studies, the dynamics of the level of anti-Topo 1 during therapy, including after transplantation of autologous hematopoietic stem cells, is insignificant or absent [19–21].
A promising direction in the treatment of SSc is associated with anti-B-cell therapy with rituximab (RTX), which is chimeric monoclonal antibodies to B-cell CD20 [22, 23]. The clinical efficacy of RTX in SSc was demonstrated in many studies [24–29]. Preliminary results indicate that a decrease in ANA titers during RTX treatment in SSc patients is associated with a positive dynamics of skin count [30, 31]. In SSc patients in whose sera anti-Topo 1 was initially detected, the effectiveness of therapy with RTX was higher than that with cyclophosphamide [32].
All above served as a basis for a study aimed at investigating the relationship between the dynamics of the levels of antinuclear autoantibodies and autoantibodies to topoisomerase I in patients with systemic scleroderma and the effectiveness of rituximab therapy.
MATERIALS AND METHODS
The study included 88 patients (age, 17 to 71 years; disease duration, 1 to 30 years) with a reliable diagnosis of SSc according to the criteria of ACR/EULAR (American College of Rheumatology/European League Against Rheumatism) of 2013 [5], who were treated with RTX (Table 1).
Table 1.
Characteristics of patients included in the study (n = 88)
| Parameters | Value |
|---|---|
| Age, years, M ± σ | 47 ± 13 |
| Sex, n (%) | |
| – female | 73 (83) |
| – male | 15 (17) |
| Disease subset, n (%) | |
| – limited | 30 (34) |
| – diffuse | 50 (57) |
| – overlap | 8 (9) |
| Interstitial lung disease, n (%) | 70 (80) |
| Disease duration, years, M ± σ | 5.9 ± 4.8 |
| Follow up, month, M ± σ | 26.3 ± 10.7 |
| Prednisolone mg/day, M ± σ | 11.7 ± 4.4 |
| Immunosupressants at baseline, n (%) | 37 (42) |
| Cumulative mean dose of RTX (gr), M ± σ | 2.9 ± 1.1 |
| ANA HEp-2, n (%) | 88 (100) |
| A-Topo-1 positivity, n (%) | 63 (75) |
| ACA positivity, n (%) | 3 (3.4) |
RTX, rituximab; ANA, antinuclear antibody; A-Topo-1, anti-topoisomerase 1 antibody; ACA, anti-centromere antibody.
The basis for prescribing RTX was the severe course of the disease, the presence of unfavorable prognosis factors, or the insufficient effectiveness of standard therapy [26, 33]. To assess the effectiveness of RTX therapy, along with the assessment of the main parameters characterizing the activity of the disease [34], we determined the skin score [35], forced vital capacity (FVC) and diffusion lung capacity (DLCO) using spirometry (Master Screen PFT, Viasys, Germany). The results of functional pulmonary tests are given as a percentage of the expected values. Values of 80–120% of the due value were taken as the norm for both FVC and DLCO. Interstitial pneumonia was diagnosed on the basis of data from multislice computed tomography of the chest organs (MSCT of the chest). ANA was determined by indirect immunofluorescence using Hep-2 cells (Immco, United States). ANA titers ≤1 : 160 were taken as the upper limit of the norm. Anti-Topo 1 and ACA were detected by enzyme-linked immunosorbent assay (ORGENTEC Diagnostika, Germany). The upper limit of the norm for anti-Topo 1 was 25 U/mL; for ACA, 10.0 U/mL (according to the manufacturer’s instructions). The number of CD19+ B cells in peripheral blood was determined by flow cytometry (Cytomics FC 500 analyzer, Beckman Coulter, United States). The normal level of cells in the peripheral blood was 6–19%, 0.1– 0.5 × 109/L. A complete depletion of CD19+ B cells was considered a decrease in their absolute number in the blood to a level of ≤0.005 × 109/L. The results of the study were processed using the Statistica 10.0 statistical software package (StatSoft Inc., United States). To analyze the statistical significance of differences in parametric indices at a normal distribution of the studied parameter, Student’s t test was used. Differences were considered statistically significant at p < 0.05.
RESULTS AND DISCUSSION
During therapy, a significant improvement in the main clinical parameters of the disease was observed (Table 2). The severity of dermal fibrosis (skin score) and the disease activity index decreased statistically significantly, lung function parameters improved, and the daily dose of prednisolone was reduced.
Table 2.
Changes of clinical parameters of the disease during RTX (n = 88)
| Parameters | Before RTX therapy | During RTX therapy | р |
|---|---|---|---|
| Rodnan skin score, M ± σ | 11.21 ± 9.33 | 6.19 ± 4.74 | 0.001 |
| Activity index (EScSG-AI), M ± σ | 2.9 ± 1.74 | 1.36 ± 1.15 | 0.001 |
| FCV (% predicted), M ± σ | 76.35 ± 19.65 | 84.37 ± 21.04 | 0.001 |
| DLCO (% predicted), M ± σ | 45.56 ± 17.72 | 47.62 ± 16.96 | 0.019 |
| B-lymphocytes (absolute count) (×109/L), M ± σ | 0.224 ± 0.19 | 0.0175 ± 0.058 | 0.001 |
| Prednisolone (mg/day), M ± σ | 11.7 ± 4.4 | 9.2 ± 3.2 | 0.001 |
RTX, rituximab; FVC, forced vital capacity % predicted; DLCO, diffusion capacity for carbon monoxide % predicted.
During RTX treatment, the number of patients with high ANA titers decreased. For example, before treatment, low ANA titers (from 1/160 to 1/320) were detected in 18 patients; high (≥1/640), in 70 patients. After treatment, the number of patients with low titers almost doubled (to 47), and the number of patients with high titers decreased to 41 (p = 0.00001). Simultaneously with the decrease in ANA titers (data not shown), in patients positive for anti-Topo 1, a decrease in the concentration of these antibodies from 174.2 ± 50.1 to 148.1 ± 66.1 U/mL (p = 0.0009) was detected (Fig. 1). Of the 63 patients initially positive for anti-Topo 1, the level of these antibodies decreased to normal values in 5 (7.9%) patients. The absence of anti-Topo 1 was accompanied by a clear improvement in the skin score (–7.4 points). A decrease in ANA titers correlated with a decrease in the severity of dermal fibrosis (skin score reduction) (r = 0.26; p = 0.014).
Fig. 1.
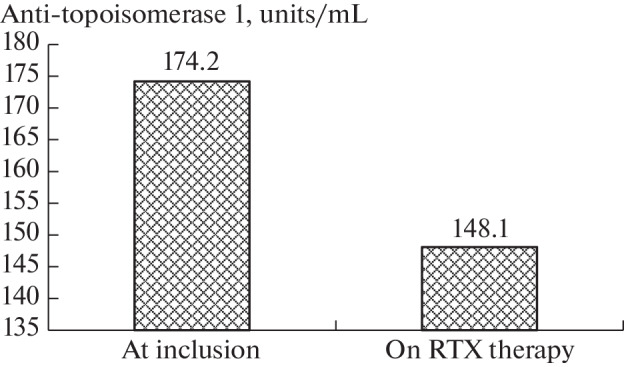
Changes of anti-topoisomerase 1 during rituximab (RTX) therapy (n = 63).
Data regarding the effectiveness of RTX therapy depending on the initial detection of anti-Topo 1 are presented in Table 3. As can be seen from Table 3, the effectiveness of RTX therapy in terms of the dynamics of the activity index, lung function, and skin lesions in the group of anti-Topo 1-positive patients was statistically significantly higher than in anti-Topo 1-negative patients.
Table 3.
Changes (Δ) of the main outcome parameters depending on the presence of anti-topoisomerase 1 (a-Topo-1) on rituximab (RTX) therapy (n = 88)
| Parameters | a-Topo-1 positive (n = 63) | a-Topo-1 negative (n = 25) | p |
|---|---|---|---|
| Δ Activity index (EScSG-AI) | 1.79 | 0.9 | 0.001 |
| Δ Rodnan skin score | 4.9 | 5.2 | NS |
| Δ FVC, % predicted | 8.64 | 6.46 | 0.001 |
| Δ DLCO, % predicted | 2.86 | 0.032 | 0.001 |
NS, nonsignificant; FVC, forced vital capacity, % predicted; DLCO, diffusion capacity for carbon monoxide, % predicted.
The obtained data supplement and expand the results of our previous studies [25, 26] and studies of other authors [27, 28, 36–38], indicating the effectiveness of RTX in relation to the overall activity of SSc, as well as skin and lung fibrosis. Of particular interest are data on the decrease in the level of anti-Topo 1 and a higher efficacy of RTX in the patients with anti-Topo 1-positive SSc subtype. This is somewhat consistent with the data on the effects of RTX in other IIRDs. For example, in patients with systemic lupus erythematosus, treatment with RTX leads to a decrease in the titers of antibodies to double-stranded DNA, antibodies to cardiolipin [39–41], and antibodies to C1q [42]. In patients with rheumatoid arthritis (RA), treatment with RTX led to a decrease in the titers of rheumatoid factors (RFs), antibodies to vimentin, and, to a lesser extent, antibodies to cyclic citrullinated proteins (ACCPs) [43–45]. Similar results on the dynamics of antineutrophil cytoplasmic antibodies were obtained in patients with systemic vasculitis [46, 47]; antibodies to the glomerular basement membrane, in patients with Goodpasture’s syndrome [48, 49]; antibodies to the phospholipase A2 receptor, in patients with membranous nephropathy [50, 51]; antibodies to platelets, in patients with immune thrombocytopenia [52]; antibodies to erythrocytes, in patients with autoimmune hemolytic anemia [53]; antibodies to pancreatic islet cells, in patients with diabetes mellitus [54]. In RA, a high basal level of RFs and ACCPs is associated with the effectiveness of RTX therapy [55, 56]. In patients with immune thrombocytopenia, the absence of antibodies to platelets correlated with resistance to RTX therapy [57], and ANA positivity, on the contrary, was a predictor of a good response to RTX [58]. The clinical efficacy of RTX therapy in IIRDs in general and SSc in particular and the decrease in autoantibody titers correlate with the severity of B-cell depletion [59], which is consistent with our results. According to the literature, in patients with diffuse SSc who were treated with RTX for 5 years, an increase in FVC and a decrease in skin score correlated with a decrease in ANA and anti-Topo 1 titers [30]. Temporary cancellation of RTX led to an increase in the level of anti-Topo 1 and the severity of skin fibrosis, and the resumption of therapy led to a positive dynamics in the clinical manifestations of SSc. In another study, SSc patients treated with RTX showed a decrease in the level of ANA and “sclerodermal” autoantibodies, which correlated with the positive dynamics of SSc activity and a decrease in skin fibrosis [31]. It should be emphasized that, during treatment with RTX, a decrease in anti-Topo 1 titers is not associated with a simultaneous decrease in the concentration of IgG and IgG antibodies to the Epstein–Barr virus [31]. This fact indicates the relative specificity of the RTX effect in relation to the suppression of autoantibody synthesis. Important results were obtained by Boonstra et al. [60], who found an association between the SSc progression and an increase in the concentration of anti-Topo 1 of both IgG and IgM isotypes, whereas the detection of only IgG anti-Topo 1 did not correlate with the course of the disease. It should be noted that IgG anti-Topo 1 titers were stable and did not depend on SSc progression, whereas IgM anti-Topo 1 titers significantly fluctuated both upwards and downwards. This may reflect the development of two types of immune response to Topo 1 in SSc. One type is associated with T-cell-dependent activation of long-lived plasma cells (PCs) that synthesize IgG anti-Topo 1 in the absence of additional antigenic stimuli. The second type, which depends on the permanent activation of Toll-like receptors in short-lived plasma cells synthesizing IgM anti-Topo 1, more adequately reflects the current immune-inflammatory process. However, the nature of external stimuli that induce the activation of Toll-like receptors is not clear and requires special study. On the basis on these data, it can be assumed that the point of application of RTX in SSc is a subpopulation of autologous autoantibody-synthesizing short-lived B cells with the CD20+ CD19med+ IgD-CD27hi CD39hi phenotype and/or activated switch memory B cells (CD19+ IgD-CD27+ CD38-CD95+) [61], which are “sensitive” to RTX depletion [62]. It is noteworthy that an increase in the level of these B cells in the peripheral blood is statistically significantly associated with a high concentration of anti-Topo 1 and the development of pulmonary fibrosis [61]. Another potential mechanism of action of RTX may be associated with the suppression of the T-cell immune response. The level of CD4+ T cells autoreactive to Topo 1 is higher in the SSc patients whose sera were found to contain anti-Topo 1 than in the patients with anti-Topo 1 negative results [16]. It should be noted that Topo 1+ T cells have a Th17 “proinflammatory” phenotype, and an increase in their level is associated with the development of ILD and a decrease in FVC and DLCO. In this regard, it is of interest that, in IIRDs (and, in particular, in RA), CD20 expression is observed not only on B but also on T [63] and Th17 [64] cells and that RTX induces depletion of T [63] and Th17 [65] cells and a decrease in the Th17cell immune response, which manifests itself in the synthesis of interleukin (IL) 22 and IL-17 [65]. Finally, since the synthesis of IL-6 by Topo 1-specific T cells in SSc patients is of key importance for the effective activation and production of anti-Topo 1 by autologous B cells [66], it can be assumed that a decrease in the level of anti-Topo 1 during RTX treatment may be associated with depletion of B cells synthesizing IL-6 [67, 68]. It should be recalled that IL-6 exhibits a pronounced profibrotic activity [69], and its inhibition is considered a promising method of therapy for SSc [70, 71].
Thus, the use of RTX in SSc patients leads to a decrease in ANA and anti-Topo 1 titers, which is associated with the clinical effectiveness of therapy. It can be assumed that RTX therapy may be especially relevant for the SSc subtype associated with overproduction of anti-Topo 1.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Footnotes
Translated by M. Batrukova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/24/2024
An Erratum to this paper has been published: 10.1134/S1607672923050022
REFERENCES
- 1.Denton C.P., Khanna D., Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 2.Mehra S., Walker J., Patterson K., Fritzler M.J. Autoantibodies in systemic sclerosis. Autoimmun Rev. 2013;12:340–354. doi: 10.1016/j.autrev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Ananyeva L.P., Aleksandrova E.N. Autoantibodies in systemic sclerosis: Spectrum, clinical associations, and prognostic value, Nauchcno-Prakt. Revmatol. 2016;54:86–99. [Google Scholar]
- 4.Bossuyt X., De Langhe E., Borghi M.O., Meroni P.L. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2020;16:715–726. doi: 10.1038/s41584-020-00522-w. [DOI] [PubMed] [Google Scholar]
- 5.van den Hoogen F., Khanna D., Fransen J., Johnson S.R., Baron M., Tyndall A. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiniakou E., Crawford J., Darrah E. Insights into origins and specificities of autoantibodies in systemic sclerosis. Curr. Opin. Rheumatol. 2021;33:486–494. doi: 10.1097/BOR.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 7.Nihtyanova S.I., Denton C.P. Autoantibodies as predictive tools in systemic sclerosis. Nat. Rev. Rheumatol. 2010;6:112–116. doi: 10.1038/nrrheum.2009.238. [DOI] [PubMed] [Google Scholar]
- 8.Nihtyanova S.I., Sari A., Harvey J.C., Leslie A., Derrett-Smith E.C., Fonseca C. Using autoantibodies and cutaneous subset to develop outcome-based disease classification in systemic sclerosis. Arthritis Rheumatol. 2020;72:465–476. doi: 10.1002/art.41153. [DOI] [PubMed] [Google Scholar]
- 9.Kuwana, M., Gil-Vila, A., and Selva-O’Callaghan, A., Role of autoantibodies in the diagnosis and prognosis of interstitial lung disease in autoimmune rheumatic disorders, Ther. Adv. Musculoskelet. Dis., 2021, vol. 13, p. 1759720X211032457. [DOI] [PMC free article] [PubMed]
- 10.Yoshizaki A., Pathogenic roles of B lymphocytes in systemic sclerosis. Immunol. Lett. 2018;195:76–82. doi: 10.1016/j.imlet.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Melissaropoulos K., Daoussis D. B cells in systemic sclerosis: from pathophysiology to treatment. Clin. Rheumatol. 2021;40:2621–2631. doi: 10.1007/s10067-021-05665-z. [DOI] [PubMed] [Google Scholar]
- 12.Burbelo P.D., Iadarola M.J., Keller J.M., Warner B.M. Autoantibodies targeting intracellular and extracellular proteins in autoimmunity. Front. Immunol. 2021;12:548469. doi: 10.3389/fimmu.2021.548469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshizaki A., Yanaba K., Ogawa A., Asano Y., Kadono T., Sato S. Immunization with DNA topoisomerase I and Freund’s complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheum. 2011;63:3575–3585. doi: 10.1002/art.30539. [DOI] [PubMed] [Google Scholar]
- 14.Mehta H., Goulet P.O., Nguyen V., Pérez G., Koenig M., Senécal J.L. Topoisomerase I peptide-loaded dendritic cells induce autoantibody response as well as skin and lung fibrosis. Autoimmunity. 2016;49:503–513. doi: 10.1080/08916934.2016.1230848. [DOI] [PubMed] [Google Scholar]
- 15.Cottrell T.R., Askin F., Halushka M.K., Casciola-Rosen L., McMahan Z.H. Expression of the autoantigen topoisomerase-1 is enriched in the lung tissues of patients with autoimmune interstitial lung disease: a case control study. ACR Open Rheumatol. 2020;2:657–661. doi: 10.1002/acr2.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fava A., Cimbro R., Wigley F.M., Liu Q.R., Rosen A., Boin F. Frequency of circulating topoisomerase-I-specific CD4 T cells predicts presence and progression of interstitial lung disease in scleroderma. Arthritis Res. Ther. 2016;18:99. doi: 10.1186/s13075-016-0993-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwana M., Kaburaki J., Mimori T., Kawakami Y., Tojo T. Longitudinal analysis of autoantibody response to topoisomerase I in systemic sclerosis. Arthritis Rheum. 2000;43:1074–1084. doi: 10.1002/1529-0131(200005)43:5<1074::AID-ANR18>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Einhaus J., Pecher A.C., Asteriti E., Schmid H., Secker K.A., Duerr-Stoerzer S. Inhibition of effector B cells by ibrutinib in systemic sclerosis. Arthritis Res. Ther. 2020;22:66. doi: 10.1186/s13075-020-02153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu P.Q., Fertig N., Medsger T.A., Wright T.M. Correlation of serum anti-DNA topoisomerase I antibody levels with disease severity and activity in systemic sclerosis. Arthritis Rheum. 2003;48:1363–1373. doi: 10.1002/art.10977. [DOI] [PubMed] [Google Scholar]
- 20.Sato S., Hamaguchi Y., Hasegawa M., Takehara K. Clinical significance of anti-topoisomerase I antibody levels determined by ELISA in systemic sclerosis. Rheumatology. 2001;40:1135–1140. doi: 10.1093/rheumatology/40.10.1135. [DOI] [PubMed] [Google Scholar]
- 21.Henes J., Glaeser L., Kötter I., Vogel W., Kanz L., Klein R. Analysis of anti-topoisomerase I antibodies in patients with systemic sclerosis before and after autologous stem cell transplantation. Rheumatology. 2017;56:451–456. doi: 10.1093/rheumatology/kew319. [DOI] [PubMed] [Google Scholar]
- 22.Nasonov E.L., editor. Anti-V-kletochnaya terapiya v revmatologii: fokus na rituksimab (Anti-B Cell Therapy in Rheumatology: Focus on Rituximab) Moscow: IMA-PRESS; 2012. [Google Scholar]
- 23.Lee D.S.W., Rojas O.L., Gommerman J.L. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nat. Rev. Drug Discov. 2021;20:179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasonov E.L., Beketova T.V., Ananyeva L.P., Va-silyev V.I., Solovyev S.K., Avdeeva A.S. Prospects for anti-B cell therapy in immuno-inflammatory rheumatic diseases, Nauchcno-Prakt. Revmatol. 2019;57:1–40. [Google Scholar]
- 25.Ananyeva L.P., Desinova O.V., Koneva O.A., Starovoitova M.N., Yutkina N.N., Volkov A.V. Rituximab treatment for interstitial lung injury in scleroderma systematica, Nauchcno-Prakt. Revmatol. 2013;51:514–523. [Google Scholar]
- 26.Ananyeva L.P., Koneva O.A., Desinova O.V., Garzanova L.A., Glukhova S.I., Starovoitova M.N. Effect of rituximab on the manifestations of activity and pulmonary function in patients with systemic sclerosis: one-year follow-up evaluation, Nauchcno-Prakt. Revmatol. 2019;57:265–273. [Google Scholar]
- 27.Moradzadeh M., Aghaei M., Mehrbakhsh Z., Arab-Bafrani Z., Abdollahi N. Efficacy and safety of rituximab therapy in patients with systemic sclerosis disease (SSc): systematic review and meta-analysis. Clin. Rheumatol. 2021;40:3897–3918. doi: 10.1007/s10067-021-05698-4. [DOI] [PubMed] [Google Scholar]
- 28.Goswami R.P., Ray A., Chatterjee M., Mukherjee A., Sircar G., Ghosh P. Rituximab in the treatment of systemic sclerosis-related interstitial lung disease: a systematic review and meta-analysis. Rheumatology. 2021;60:557–567. doi: 10.1093/rheumatology/keaa550. [DOI] [PubMed] [Google Scholar]
- 29.de Figueiredo Caldas M.M.V., de Azevedo K.P.M., de França Nunes A.C., de Oliveira V.H., Pimen-ta I.D.S.F., de Araújo I.D.T. Is rituximab effective for systemic sclerosis? A systematic review and meta-analysis. Adv. Rheumatol. 2021;61:15. doi: 10.1186/s42358-021-00170-y. [DOI] [PubMed] [Google Scholar]
- 30.Moazedi-Fuerst F.C., Kielhauser S.M., Hermann J., Meilinger M., Demel U., Stradner M.H. Decrease in autoantibody titres during long-term treatment of scleroderma with rituximab: a promising surveillance marker of therapy? Scand. J. Rheumatol. 2015;44:519–520. doi: 10.3109/03009742.2015.1069888. [DOI] [PubMed] [Google Scholar]
- 31.Bonroy C., Smith V., Deschepper E., De Keyser F., Devreese K., Specific antinuclear antibody level changes after B cell depletion therapy in systemic sclerosis are associated with improvement of skin thickening. J. Rheumatol. 2016;43:247–249. doi: 10.3899/jrheum.150105. [DOI] [PubMed] [Google Scholar]
- 32.Ebata S., Yoshizaki A., Fukasawa T., Miura S., Takahashi T., Sumida H. Rituximab therapy is more effective than cyclophosphamide therapy for Japanese patients with anti-topoisomerase I-positive systemic sclerosis-associated interstitial lung disease. J. Dermatol. 2019;46:1006–1013. doi: 10.1111/1346-8138.15079. [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Codina A., Walker K.M., Pope J.E., Scleroderma Algorithm Group Treatment algorithms for systemic sclerosis according to experts. Arthritis Rheumatol. 2018;70:1820–1828. doi: 10.1002/art.40560. [DOI] [PubMed] [Google Scholar]
- 34.Valentini G., Della Rossa A., Bombardieri S., Bencivelli W., Silman A.J., D’Angelo S. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann. Rheum. Dis. 2001;60:592–598. doi: 10.1136/ard.60.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahaleh M.B., Sultany G.L., Smith E.A., Huffstutter J.E., Loadholt C.B., LeRoy E.C. A modified scleroderma skin scoring method. Clin. Exp. Rheumatol. 1986;4:367–369. [PubMed] [Google Scholar]
- 36.Elhai M., Boubaya M., Distler O., Smith V., Matucci-Cerinic M., Alegre Sancho J.J. for EUSTAR network: Outcomes of patients with systemic sclerosis treated with rituximab in contemporary practice: a prospective cohort study. Ann. Rheum. Dis. 2019;78:979–987. doi: 10.1136/annrheumdis-2018-214816. [DOI] [PubMed] [Google Scholar]
- 37.Hughes M., Denton C.P., Khanna D. Rituximab for the treatment of systemic sclerosis-interstitial lung disease. Rheumatology. 2021;60:489–491. doi: 10.1093/rheumatology/keaa675. [DOI] [PubMed] [Google Scholar]
- 38.Tang R., Yu J., Shi Y., Zou P., Zeng Z., Tang B. Safety and efficacy of Rituximab in systemic sclerosis: a systematic review and meta-analysis. Int. Immunopharmacol. 2020;83:106389. doi: 10.1016/j.intimp.2020.106389. [DOI] [PubMed] [Google Scholar]
- 39.Cambridge G., Leandro M.J., Teodorescu M., Manson J., Rahman A., Isenberg D.A. B cell depletion therapy in systemic lupus erythematosus: Effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum. 2006;54:3612–3622. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 40.Lazarus M.N., Turner-Stokes T., Chavele K.M., Isenberg D.A., Ehrenstein M.R. B-cell numbers and phenotype at clinical relapse following rituximab therapy differ in SLE patients according to anti-dsDNA antibody levels. Rheumatology. 2012;51:1208–1215. doi: 10.1093/rheumatology/ker526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tew G.W., Rabbee N., Wolslegel K., Hsieh H.J., Monroe J.G., Behrens T.W. Baseline autoantibody profiles predict normalization of complement and anti-dsDNA autoantibody levels following rituximab treatment in systemic lupus erythematosus. Lupus. 2010;19:146–157. doi: 10.1177/0961203309350752. [DOI] [PubMed] [Google Scholar]
- 42.Tsanian M.É., Torgashina A.V., Aleksandrova E.N., Solov’ev S.K., Nasonov E.L. Anti-C1q antibodies in patients with systemic lupus erythematosus treated by rituximab. Ter. Arkh. 2013;85:53–59. [PubMed] [Google Scholar]
- 43.Cambridge G., Leandro M.J., Lahey L.J., Fairhead T., Robinson W.H., Sokolove J. B cell depletion with rituximab in patients with rheumatoid arthritis: Multiplex bead array reveals the kinetics of IgG and IgA antibodies to citrullinated antigens. J. Autoimmun. 2016;70:22–30. doi: 10.1016/j.jaut.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Modi S., Soejima M., Levesque M.C. The effect of targeted rheumatoid arthritis therapies on anti-citrullinated protein autoantibody levels and B cell responses. Clin. Exp. Immunol. 2013;173:8–17. doi: 10.1111/cei.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindenberg L., Spengler L., Bang H., Dorner T., Maslyanskiy A.L., Lapin S.V. Restrictive IgG antibody response against mutated citrullinated vimentin predicts response to rituximab in patients with rheumatoid arthritis. Arthritis Res. Ther. 2015;17:206. doi: 10.1186/s13075-015-0717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortazar F.B., Pendergraft W.F., Wenger J., Owens C.T., Laliberte K., Niles J.L. Effect of continuous B cell depletion with rituximab on pathogenic autoantibodies and total IgG levels in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2017;69:1045–1053. doi: 10.1002/art.40032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dam L.S., Dirikgil E., Bredewold E.W., Ray A., Bakker J.A., van Kooten C. PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab. Nephrol. Dial Transplant. 2021;36:1408–1417. doi: 10.1093/ndt/gfaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heitz M., Carron P.L., Clavarino G., Jouve T., Pinel N., Guebre-Egziabher F. Use of rituximab as an induction therapy in anti-glomerular basement-membrane disease. BMC Nephrol. 2018;19:241. doi: 10.1186/s12882-018-1038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uematsu-Uchida M., Ohira T., Tomita S., Sato-naka H., Tojo A., Ishimitsu T. Rituximab in treatment of anti-GBM antibody glomerulonephritis: A case report and literature review. Medicine. 2019;98:e17801. doi: 10.1097/MD.0000000000017801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck L.H., Fervenza F.C., Beck D.M., Bonegio R.G., Malik F.A., Erickson S.B. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J. Am. Soc. Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruggenenti P., Debiec H., Ruggiero B., Chianca A., Pellé T., Gaspari F. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J. Am. Soc. Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnold D.M., Vrbensky J.R., Karim N., Smith J.W., Liu Y., Ivetic N. The effect of rituximab on anti-platelet autoantibody levels in patients with immune thrombocytopenia. Br. J. Haematol. 2017;178:302–307. doi: 10.1111/bjh.14664. [DOI] [PubMed] [Google Scholar]
- 53.Dierickx D., Kentos A., Delannoy A. The role of rituximab in adults with warm antibody autoimmune hemolytic anemia. Blood. 2015;125:3223–3229. doi: 10.1182/blood-2015-01-588392. [DOI] [PubMed] [Google Scholar]
- 54.Yu L., Herold K., Krause-Steinrauf H., McGee P.L., Bundy B., Pugliese A. Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab selectively suppresses specific islet antibodies. Diabetes. 2011;60:2560–2565. doi: 10.2337/db11-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatzidionysiou K., Lie E., Nasonov E., Lukina G., Hetland M.L., Tarp U. Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann. Rheum. Dis. 2011;70:1575–1580. doi: 10.1136/ard.2010.148759. [DOI] [PubMed] [Google Scholar]
- 56.Isaacs J.D., Cohen S.B., Emery P., Tak P.P., Wang J., Lei G. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann. Rheum. Dis. 2013;72:329–336. doi: 10.1136/annrheumdis-2011-201117. [DOI] [PubMed] [Google Scholar]
- 57.Porcelijn L., Huiskes E., Schipperus M., van der Holt B., de Haas M., Zwaginga J.J., Dutch HOVON 64 Study Group Lack of detectable platelet autoantibodies is correlated with nonresponsiveness to rituximab treatment in ITP patients. Blood. 2017;129:3389–3391. doi: 10.1182/blood-2016-11-751719. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y.M., Yu Y.F., Liu Y., Liu S., Hou M., Liu X.G. The association between antinuclear antibody and response to rituximab treatment in adult patients with primary immune thrombocytopenia. Hematology. 2020;25:139–144. doi: 10.1080/16078454.2020.1740430. [DOI] [PubMed] [Google Scholar]
- 59.Crickx E., Weill J.C., Reynaud C.A., Mahévas M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: Successes, failures and future perspectives. Kidney Int. 2020;97:885–893. doi: 10.1016/j.kint.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 60.Boonstra M., Bakker J.A., Grummels A., Ninaber M.K., Ajmone Marsan N., Wortel C.M. Association of anti-topoisomerase I antibodies of the IgM isotype with disease progression in anti-topoisomerase I-positive systemic sclerosis. Arthritis Rheumatol. 2020;72:1897–1904. doi: 10.1002/art.41403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon D., Balogh P., Erdő-Bonyár S., Böröcz K., Minier T., Czirják L. Increased frequency of activated switched memory B cells and its association with the presence of pulmonary fibrosis in diffuse cutaneous systemic sclerosis patients. Front. Immunol. 2021;12:686483. doi: 10.3389/fimmu.2021.686483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leandro M.J. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res. Ther. 2013;15:S3. doi: 10.1186/ar3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilk E., Witte T., Marquardt N., Horvath T., Kalippke K., Scholz K. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum. 2009;60:3563–3571. doi: 10.1002/art.24998. [DOI] [PubMed] [Google Scholar]
- 64.Eggleton P., Bremer E., Tarr J.M., de Bruyn M., Helfrich W., Kendall A. Frequency of Th17 CD20+ cells in the peripheral blood of rheumatoid arthritis patients is higher compared to healthy subjects. Arthritis Res. Ther. 2011;13:R208. doi: 10.1186/ar3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van de Veerdonk F.L., Lauwerys B., Marijnissen R.J., Timmermans K., Di Padova F., Koenders M.I. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum. 2011;63:1507–1516. doi: 10.1002/art.30314. [DOI] [PubMed] [Google Scholar]
- 66.Kuwana M., Medsger T.A., Wright T.M. Analysis of soluble and cell surface factors regulating anti-DNA topoisomerase I autoantibody production demonstrates synergy between Th1 and Th2 autoreactive T cells. J. Immunol. 2000;164:6138–6146. doi: 10.4049/jimmunol.164.12.6138. [DOI] [PubMed] [Google Scholar]
- 67.Hasegawa M., Hamaguchi Y., Yanaba K., Bouaziz J.D., Uchida J., Fujimoto M. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am. J. Pathol. 2006;169:954–966. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bosello S., De Santis M., Lama G., Spanò C., Angelucci C., Tolusso B. B cell depletion in diffuse progressive systemic sclerosis: safety, skin score modification and IL-6 modulation in an up to thirty-six months follow-up open-label trial. Arthritis Res. Ther. 2010;12:R54. doi: 10.1186/ar2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.She Y.X., Yu Q.Y., Tang X.X. Role of interleukins in the pathogenesis of pulmonary fibrosis. Cell Death Discov. 2021;7:52. doi: 10.1038/s41420-021-00437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ananyeva L.P. Prospects for using tocilizumab in systemic sclerosis, Nauchcno-Prakt. Revmatol. 2015;53:632–640. [Google Scholar]
- 71.Shima Y. The benefits and prospects of interleukin-6 inhibitor on systemic sclerosis. Mod. Rheumatol. 2019;29:294–301. doi: 10.1080/14397595.2018.1559909. [DOI] [PubMed] [Google Scholar]


