Abstract
Purpose
Non-tuberculous mycobacteria (NTM) infections in hematopoietic stem cell transplantation (HSCT) recipients represent a diagnostic and therapeutic challenge. Here, we aimed to review and analyze current literature on incidence, clinical presentation, and outcome of NTM infection after allogeneic HSCT.
Methods
We performed a systematic review and meta-analysis of available literature regarding NTM infection in children and adults receiving allogeneic HSCT.
Results
We identified 56 articles eligible for the analysis. Among 15 studies, describing 15,798 allogeneic HSCT, we estimated a prevalence of 1.26% (95% CI 0.72, 1.93) of NTM after transplant. Analysis of 175 patients with NTM infection showed a median time of diagnosis of 318 days after HSCT, an increased prevalence in adults (82.9%), and a most frequent pulmonary involvement (44%). Comparison between children and adults revealed an earlier post-transplant disease onset (median 130 days vs 287 days) and most frequent non-pulmonary presentation in children. A vast heterogeneity of therapeutic approach reflected the lack of universal recommendations regarding drug combination and duration of therapy. Overall, NTM-related mortality accounted for 33% in this systematic review.
Conclusion
Although rare, NTM infections can complicate post-transplant course with a high mortality rate in children and adults. The lack of prospective studies and guidelines prevents identification of risk factors and therapeutic recommendations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-023-01615-3.
Keywords: Non-tuberculous mycobacteria, NTM, Hematopoietic stem cell transplant, HSCT, Immunodeficiency, Infection
Introduction
Infections are among the most frequent complications occurring in patients receiving hematopoietic stem cell transplant (HSCT) [1]. As diagnostic techniques advanced, specific cultures are now able to identify a wide spectrum of pathogens causing infections post-HSCT, including non-tuberculous mycobacteria (NTM) [2]. Mycobacteria are responsible of several infectious manifestations in transplant recipients. However, while tuberculous mycobacteria infections/reactivation or Bacillus Calmette-Guerin (BCG) vaccine-associated complications have been widely described [3], there is limited data on the prevalence, clinical presentation, outcome, and risk factors associated with NTM infection post-HSCT. NTM are ubiquitous organisms that grow and persist in natural environments including water, household plumbing systems, soil, dust, foods, and a variety of animals [4]. More than 170 NTM species have been identified, most of them being commensal organisms colonizing skin, respiratory, and gastrointestinal tract in humans [5]. Although NTM are rarely pathogenic in immunocompetent subjects, approximately 20 NTM species have been reported as causative pathogens [6] especially in immunocompromised hosts [7].
Human disease due to NTM has been generally classified also into two distinct clinical manifestations: pulmonary disease and extra-pulmonary (including lymph node, cutaneous, soft tissue infections, joints, and gut infection) [8, 9]. Disseminated disease affecting more than one organ was seen, mainly among immunocompromised recipients [10, 11].
NTM pathogenicity depends on different factors namely site of infection, species-specific virulence, host factors, and interactions between the latter two [12]. The main prominent factors that increase the risk of NTM infection include pre-existing structural lung disease and genetic or secondary impairment of cell-mediated immunity [13]. In fact, a functional IL-12/23-IFN-γ integrity and a good interaction and cooperation between macrophages and T and natural killer (NK) lymphocytes are essential to control mycobacterial infections [11, 14, 15]. Recipients of HSCT have an impaired immune system with a noted delay in the immune reconstitution of T cells and cell-mediated immunity, in comparison to innate and B cell immunity, regardless of the source of stem cells [16]. Such a cohort is thus susceptible to NTM infection and is at risk of disseminated disease [17, 18]. Moreover, these patients might require immune suppressive therapy to control autoimmunity or graft versus host disease, increasing their risk for disseminated NTM infection [19]. It remains difficult to discern HSCT recipients who are at highest risk for NTM disease. Allograft T cell depletion and the allogeneic HSCT conditioning regimen (e.g., total body irradiation (TBI)) are potential risk factors for NTM infections [20].
Diagnosis of NTM in HSCT recipients is considered challenging with difficulty in distinguishing colonization from active infection and between infection and other infectious or non-infectious complications related to HSCT, leading to a similar clinical picture (e.g., lung GvHD) [17, 20, 21]. A high index of suspicion and improvement in diagnostic techniques are thus mandated for proper diagnosis and initiation of treatment for better outcome post-HSCT.
Moreover, the decision to start a treatment for NTM-associated disease is not easy among this cohort of patients, since multidrug regimens are required, with numerous toxicities and the potential metabolic interaction with other treatments [22]. Also, the optimal treatment and duration for NTM infections in HSCT recipients remain undefined [23].
This is a systematic review and meta-analysis research focusing on the prevalence and characteristics of NTM infection in recipients of allogeneic hematopoietic stem cell transplantation, with the aim to clarify the most encountered species, sites of disease, NTM-related mortality and differences between adults and pediatrics.
Methods
This is a systematic review and meta-analysis research focusing on NTM infection in recipients of allogeneic HSCT. A literature search was performed for all studies associated with NTM infections post-HSCT. Two reviewers independently extracted the data and assessed the quality of the studies. Meta-analyses of pre-specified outcomes were performed when deemed appropriate. The PICO statement was as follows: patient/population (P) = patients underwent allogeneic hematopoietic stem cell transplantation; intervention or exposure (I) = diagnosed with NTM infection after the transplantation; comparison or control (C) = children and adult cohort; outcomes (O) = prevalence, NTM infections, and risk factors.
Search Strategy
We performed a comprehensive systematic search of articles published in peer reviewed journals using PubMed/MEDLINE (from 1983 until 2020). A structured search was conducted with controlled vocabulary and relevant key terms to enhance sensitivity. Reference lists of included papers were hand searched for additional relevant studies. The search was restricted to articles in English language.
Study Selection
Two investigators performed an initial screening of identified titles and abstracts. Those deemed to be clearly irrelevant were removed on the initial screen. They also performed a second screen to identify potentially relevant studies. If no abstract was available, the full text was obtained unless the article could be confidently excluded by title alone. If there was any doubt as to whether or not a study could be excluded, a full-text screen was performed to reduce the likelihood of incorrect exclusion of a relevant study. Three reviewers obtained and independently screened full-text versions of potentially eligible studies to determine their eligibility based on the selection criteria. Any disagreements during the screening process were resolved through discussion among the authors in accordance with the selection criteria. We included cross-sectional, retrospective cohort studies that reported NTM infection in allogeneic HSCT recipients. To be included in the analysis, records must have described patients receiving allogeneic HSCT, without known pre-existing NTM infection. Studies describing patients with Mendelian susceptibility to mycobacterial disease (MSMD) or with NTM colonization (e.g., NTM infection detected in asymptomatic patients as infection screening) were also excluded.
Data Extraction and Synthesis
Three investigators independently extracted the data from each eligible study. Assessed variables related to the organization and outcome of the studies included study design, the year of reporting, countries, age, gender, number of study participants, NTM species, the timing to NTM infections in post-allogeneic transplant, and reporting of relevant outcomes. The primary objective was to estimate cumulative prevalence of NTM after allogeneic HSCT. The secondary objectives were to determine the characteristics of NTM infection post-HSCT and to assess the risk factors associated with NTM post HSCT. To calculate the prevalence of NTM among HSCT recipients, only studies reporting the total number of patients who have received allogeneic HSCT were thus included. Disseminated NTM was defined as including either blood stream infection or more than one organ affected with or without blood stream infection. The data analysis was performed by 3 investigators.
Patient Involvement
There was no patient involvement in this study.
Statistical Analysis
Descriptive methods were used to present the data by year of study, country, total transplant, total NTM patients, and prevalence based on reported study. Meta-analyses were performed using STATA SE16 software. Data from case studies were not pooled for prevalence study because of concerns about the validity of the results. The reporting of this systematic review is in accordance with PRISMA guidelines.
Results
Characteristics of Included Studies
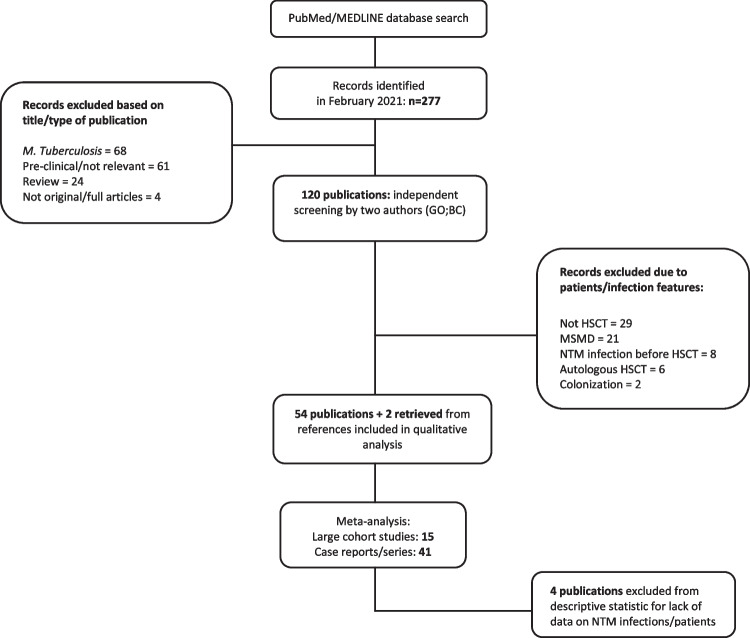
A total of 277 records were identified in February 2021.We excluded studies that showed only non-clinical data (in vitro studies or those performed on animal models) (n = 61), review articles (n = 24), articles on mycobacteria tuberculosis infection rather than NTM (n = 68) besides studies with non-original or incomplete data (n = 4). One hundred twenty publications were thus screened independently by 2 authors. A further 66 studies were excluded; NTM infections in autologous rather than allo-HSCT setting (n = 6), NTM infection but in non-HSCT setting (n = 29), reported NTM colonization rather than infection (n = 2), NTM infection acquired pre-HSCT (n = 8), and reports of NTM infection in patients with Mendelian Susceptibility to Mycobacteria Disease (MSMD) (n = 21). Overall, 54 publications were considered eligible for the analysis, and two further records were retrieved from the references of included articles (Fig. 1). The final analysis was conducted on 56 publications, divided in two categories: (i) large cohort studies (≥ 90 HSCT transplant recipients), included in the prevalence analysis, and (ii) case report/series.
Fig. 1.
PRISM flow chart for the systematic review
The Prevalence of NTM Infection Among Allogeneic HSCT Recipients
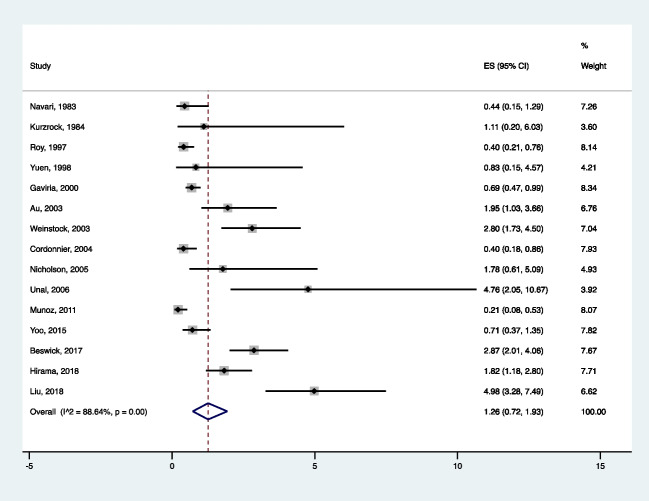
For the meta-analysis calculation for the prevalence of NTM post-HSCT, 15 studies fulfilled the inclusion criteria and were included in the analysis [19–21, 24–35] (Fig. 1, Supplementary tables 1 and 2). A total of 15,798 patients who underwent allogeneic HSCT were evaluated. The combined prevalence of NTM post-HSCT was 1.26% (95% CI 0.72, 1.93) (Fig. 2), estimated via random effect model. The heterogeneity of the included studies was I2 = 88.64%, p < 0.001. The Egger’s weighted regression test analysis suggested no small study effect noted in the meta-analysis (t = 0.72, p = 0.47) and there was no apparent bias in the studies included in the meta-analysis (Fig. 3).
Fig. 2.
Forest plot of the meta-analysis on prevalence of post-HSCT NTM infections
Fig. 3.
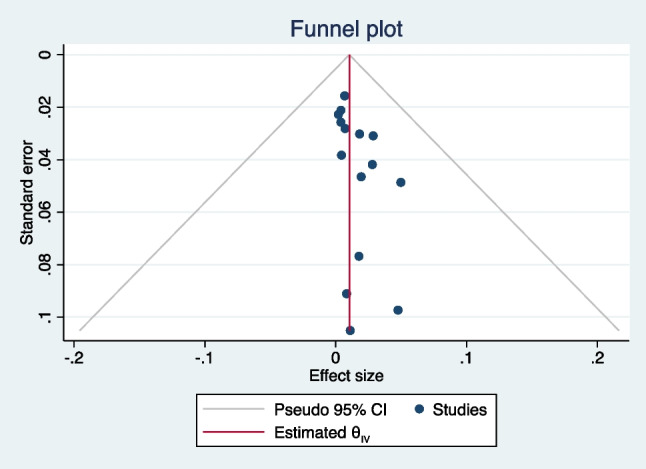
Funnel plot of the meta-analysis on prevalence of post-HSCT NTM infections
The Characteristics of NTM Infections Post-HSCT
A total of 175 cases of NTM infection post-allogeneic HSCT in 52 publications [both types of reporting (case reports and cohort studies)] were included in the analysis [20, 21, 24–27, 29, 31–33, 35–76]. Four publications (total studies 56) were ultimately excluded given lack of data on patients and NTM infection characteristics [19, 28, 30, 34]. All the papers were published between 1983 until 2020. The median age of reported cases was 35 years old (range 1–69 years old). Thirty cases (17.1%) were patients aged less than 18 years old, and 145 cases (82.9%) involved patients aged equal or more than 18 years old. Seventy-nine patients (45.1%) were female, and in 5%, gender was not reported. Cases were reported mainly from North America (110 cases, 62.9%), followed by from Asia (48 cases, 27.4%), Europe (10 cases, 5.7%), and Australia (7 cases, 4%).
With regards to the NTM species, the most frequently grown species were Mycobacterium Avium Complex (MAC) followed by M. haemophilum, M. chelonae, M. fortuitum, and M. abscesses. The timing of NTM infections in post-allogeneic transplant recipients varied according to the type of species as shown in Table 1. In most species, NTM was diagnosed at a median of 318 days post-HSCT.
Table 1.
Time interval from HSCT to the diagnosis of NTM based on species
| Species | Timing to NTM infections post allogeneic transplantation (days) Median (min, max) |
Number of patients |
|---|---|---|
| MAC | 364 (14, 3650) | 26 |
| M. haemophilum | 123 (90, 1460) | 19 |
| M. chelonae | 422 (7, 1642) | 18 |
| M. abscessus | 381 (10, 3285) | 14 |
| M. fortuitum | 301 (50, 1140) | 14 |
| M. kansasii | 120 (35, 1270) | 5 |
| M. immunogenum | 140 (130, 180) | 4 |
| M. xenopi | 306 (133, 649) | 4 |
| M. genavense | 264 (240, 253) | 2 |
| M. massiliense | 946 (3, 1890) | 2 |
| M. gordonae | 120 | 1 |
| M. marinum | 174 | 1 |
| M. mucogenicum | 180 | 1 |
| M. neoaurum | 2 | 1 |
| M. szulgai | 84 | 1 |
| M. scrofulaceum | 2040 | 1 |
M. Mycobacterium, MAC Mycobacterium avium complex
Most of the reported patients (77/175; 44%) had pulmonary NTM (with no other organ involvement) with MAC being the most frequently reported organism (23%). Forty-three patients (24.5%) had cutaneous NTM (with no other organ involvement); most predominant organism retrieved was M. haemophilum grown in 29 patients (67%). Disseminated NTM was seen in 38 patients (21.7%) with no noted predominant species (Table 2). Few cases had other sites of NTM infection including gastrointestinal in 6 patients, catheter related in 4 patients, musculoskeletal in 2 patients, and lymph node infiltration in 1 patient.
Table 2.
NTM site of infection and related species
| Infection sites | Number of patients affected for each species/number of total cases |
|---|---|
|
Pulmonary [20, 21, 25, 29, 32, 33, 35, 36, 38, 40, 41, 44, 48, 49, 59, 62, 66–68, 76] |
77/175 (44%) MAC, 18 M. abscessus, 10 M. chelonae, 8 M. haemophilum, 8 M. fortuitum, 6 M. xenopi, 4 M. kansasii, 2 M. fortuitum and M. chelonae, 1 M. massiliense, 1 M. scrofulaceum, 1 MAC and M. gordonae, 1 Unclassified, 17 |
|
Cutaneous [20, 24, 26, 31, 32, 37, 41–43, 45, 46, 50–52, 55, 58, 61, 63, 71, 76] |
43/175 (24.6%) M. haemophilum, 29 M. chelonae, 4 M. fortuitum, 3 M. kansasii, 2 M. abscessus, 1 M. marinum, 1 M. massiliense, 1 M. szulgai, 1 MAC, 1 |
|
Disseminated [20, 26, 29, 31, 32, 36, 38, 40, 42, 47, 53, 57, 59, 64, 69, 72–74, 76] |
38/175 (21.7%) M. mucogenicum, 8 M. fortuitum, 5 M. haemophilum, 5 MAC, 4 M. chelonae, 4 M. immunogenum, 4 M. abscessus, 3 M. fortuitum and M. haemophilum, 1 M. genavense, 1 M. gordonae, 1 M. massiliense, 1 MAC and M. haemophilum, 1 |
|
Others |
15/175 (8.6%) MAC, 4 M. chelonae, 4 M. haemophilum, 2 M. abscessus, 1 M. genavense, 1 M. neoaurum, 1 Unclassified, 2 |
|
Musculoskeletals |
2/175 (1.1%) M. kansasii, 2 |
M. mycobacterium, MAC Mycobacterium avium complex
NTM-directed therapy was initiated for 138 patients (78%), including at least two drug combinations. Data on specific treatment was not available for 18 reported cases. Moreover, 19 patients did not receive any NTM-directed therapy (10%): 4 of them passed away (1 died before initiating therapy and 3 had the diagnosis identified post-mortem) and 1 patient had resolution of infection after line removal. In the remaining 14 cases, the cause for not initiating NTM-directed therapy was not clear: 8 of them were alive at time of reporting while the other 6 patients died from NTM infection (data not shown). Median duration of therapy among 92 cases (where length of therapy was mentioned) was 9.2 months.
A total of 60 deaths (40%) were reported at the time of publications with NTM infection being the cause of death in 20 patients (33% of the total NTM cohorts).
Differences in NTM Infection Between Pediatric and Adult Cohort
For reported NTM cases affecting children, the median age at presentation was 9 years old (range 1.2–17 years old); the majority of patients were boys (18 cases, 60%). An equal number of cases received HSCT for malignant or non-malignant disease. Most patients received an HLA-matched graft (11 cases, 36.7%). Unfortunately, the type of graft source, conditioning regimens, and the presence of acute GVHD were not documented in most of the publications. The majority of this age group had non-disseminated (66.7%) and non-pulmonary (66.7%) NTM infection. Slowly growing NTM species were isolated in 53.3%. The most frequently isolated NTM species reported was MAC (8 cases, 26.7%) followed by M. chelonae (4 cases, 13.3%) and M. kansasii (4 cases, 13.3%). Five patients were reported to be dead at the time of publication of the articles (16.7%).
For reported NTM cases affecting adults, the median age at presentation was 42 years old (range 18–69). There were no gender differences noted, and the majority had a malignant disease as indication for HSCT (89%). Unfortunately, as seen among paediatric publications, the type of graft source, conditioning regimens, and the presence of acute GvHD were not documented in the majority of publications. The majority of adults—similarly to children—had a non-disseminated NTM infection (79.3%). However, in contrast with the paediatric cohort, there was no difference between pulmonary versus non-pulmonary NTM infection proportions among adults. M. haemophilum was the most reported species in this cohort (44 cases, 30.3%) followed by MAC (19 cases, 13.1%), M. chelonae (16 cases, 11%), M. abscessus (13 cases, 8.9%), and M. fortuitum (11 cases, 7.6%).
The time to NTM infection post-HSCT was noted to be longer among adults in comparison to children (median 287 days vs 130 days). The clinical characteristics of the NTM infections post-HSCT are listed in Table 3 and in details in Supplementary Table 2.
Table 3.
Clinical characteristics of NTM infections among reported allogeneic HSCT recipients from 1983 to 2020
| Characteristics | Children n = 30 | Adults n = 145 | Total n = 175 |
|---|---|---|---|
| Age in years | |||
| Mean (SD) | 8.7 (4.5) | 40.9 (13.3) | |
| Median (min, max) | 9 (1.2, 17) | 42 (18, 69) | |
| Gender (n, %) | |||
| Male | 18 (60%) | 69 (47.6%) | 87 (50%) |
| Female | 11 (36.7%) | 68 (46.9%) | 79 (45%) |
| Not available | 1 (3.3%) | 8 (5.5%) | 9 (5%) |
| Diagnosis upon HSCT (n, %) | |||
| Malignant | 15 (50%) | 129 (89%) | 144 (82%) |
| Non-malignant | 15 (50%) | 8 (5.5%) | 23 (13%) |
| Not available | - | 8 (5.5%) | 8 (5%) |
| HLA matching (n, %) | |||
| Matched | 11 (36.7%) | 65 (44.9%) | 76 (43%) |
| Mismatched | 3 (10%) | 6 (4.1%) | 9 (5%) |
| Not available | 16 (53.3%) | 74 (51%) | 90 (52%) |
| Graft source (n, %) | |||
| BM | 2 (6.7%) | 6 (4.1%) | 8 (5%) |
| PBSC | 1 (3.3%) | 5 (3.5%) | 6 (3%) |
| UCB | 6 (20%) | 1 (0.7%) | 7 (4%) |
| Not available | 21 (70%) | 133 (91.7%) | 154 (88%) |
| Conditioning (n, %) | |||
| Myeloablative | 5 (16.7%) | 64 (44.1%) | 69 (40%) |
| Non-myeloablative | 3 (10%) | 3 (2.1%) | 6 (3%) |
| Not available | 22 (73.3%) | 78 (53.8%) | 100 (57) |
| Severe acute GVHD (n, %) | |||
| Yes | 11 (36.7%) | 60 (41.4%) | 71 (41%) |
| No | 5 (16.7%) | 24 (16.6%) | 29 (17%) |
| Not available | 14 (46.6%) | 61 (42%) | 75 (42%) |
| Location of NTM isolated (n, %) | |||
| Disseminated | 10 (33.3%) | 30 (20.7%) | 40 (23%) |
| Non-disseminated | 20 (66.7%) | 115 (79.3%) | 135 (77%) |
| Pulmonary | 10 (33.3%) | 72 (49.7%) | 82 (47%) |
| Non-pulmonary | 20 (66.7%) | 73 (50.3%) | 93 (53%) |
| Types of NTM (n, %) | |||
| Slowly growing NTM | 16 (53.3%) | 74 (51%) | 90 (52%) |
| Rapidly growing NTM | 14 (46.7%) | 52 (35.9%) | 66 (38%) |
| Unclassified | - | 19 (13.1%) | 19 (10%) |
| NTM species (n, %) | |||
| M. abscessus | 2 (6.7%) | 13 (8.9%) | 15 (9%) |
| M. chelonae | 4 (13.3%) | 16 (11%) | 20 (11%) |
| M. fortuitum | 3 (10%) | 11 (7.6%) | 14 (8%) |
| M. genavense | 1 (3.3%) | 1 (0.7%) | 2 (1%) |
| M. gordonae | - | 1 (0.7%) | 1 (0.5%) |
| M. haemophilum | - | 44 (30.3%) | 44 (25%) |
| M. immunogenum | 3 (10%) | 1 (0.7%) | 4 (2%) |
| M. kansasii | 4 (13.3%) | 2 (1.4%) | 6 (3%) |
| M. marinum | - | 1 (0.7%) | 1 (0.5%) |
| M. massiliense | - | 3 (2.1%) | 3 (1.5%) |
| M. mucogenicum | 2 (6.7%) | 6 (4.1%) | 6 (3%) |
| M. neoaurum | 1 (3.3%) | - | 1 (0.5%) |
| M. szulgai | 1 (3.3%) | - | 1 (0.5%) |
| MAC | 8 (26.7%) | 19 (13.1%) | 27 (15%) |
| M. xenopi | - | 4 (2.8%) | 4 (2%) |
| M. scrofulaceum | - | 1 (0.7%) | 1 (0.5%) |
| MAC and M. gordonae | 1 (3.3%) | - | 1 (0.5%) |
| M. fortuitum and M. chelonae | - | 1 (0.7%) | 1 (0.5%) |
| M. fortuitum and M. haemophilum | - | 1 (0.7%) | 1 (0.5%) |
| MAC and M. haemophilum | - | 1 (0.7%) | 1 (0.5%) |
| Unclassified | - | 19 (13.1%) | 19 (10%) |
| Timing to NTM infections post allogeneic transplantation (days) Median (min, max) | 130 (2,1642) | 287 (3,3650) | |
| Outcome summary | |||
| Alive | 20 (66.7%) | 68 (46.9%) | 88 (51%) |
| Dead | 5 (16.7%) | 55 (37.9%) | 60 (34%) |
| Missing data | 5 (16.7%) | 22 (15.2%) | 27 (15%) |
HSCT hematopoietic stem cell transplant, HLA human leukocyte antigen, BM bone marrow, PBSC peripheral blood stem cell, UCB umbilical cord blood, GVHD graft versus host disease, NTM non-tuberculous mycobacteria, M. Mycobacterium, MAC Mycobacterium avium complex
Discussion
To our knowledge, this is the first meta-analysis study investigating prevalence and characteristics of NTM infection post-allogeneic HSCT for both paediatric and adult cohorts. We reported a combined prevalence rate of NTM disease of 1.6%. This is significantly higher than what has been reported so far in both immunocompetent hosts and in patients with human immunodeficiency virus (HIV). Among immunocompetent hosts, NTM disease prevalence was noted to be 0.2–3.1 (2.9 in UK [77]) in Europe, in 8.6–9.08 in USA and Canada, and in Brazil is 0.25/100,000 individuals [78].
In pre-antiretroviral therapy (ART) era, it was reported that up to 43% of AIDS patients had disseminated NTM infections, caused in the 86% of cases by MAC [79]. Following the introduction of both ART and primary prophylaxis, the overall incidence of disseminated MAC infection in HIV-infected patients has decreased to about 2.5 cases per 1000 person-years [80], which is still far less than what is reported in our study.
Interestingly, in our meta-analysis, we noted more cases reported from North America (almost 60%) followed by Asia (27%) in comparison to few cases (< 10%) reported from Australia and Europe and none from Africa or South America. While this might reflect environmental, climate prevalence of NTM, there might be other factors. In resource-limited settings, diagnosis of NTM could be missed because of scarce access to adequate laboratory and differentiation between TB and NTM might be a challenge [81]. Patients are empirically treated as drug sensitive or resistant TB [82] rather than for NTM infection.
In the allogeneic HSCT setting, multiple factors could be responsible for the development of NTM: including impaired cell-mediated immunity with the use of serotherapy like ATG, TBI-based conditioning (associated with lymphotoxicity), during early phases before immune recovery in the context of GvHD, and the use of immune suppressive medications to prevent or treat GvHD[17, 20, 83]. Unfortunately, the graft source, conditioning regimens, in vivo lymphodepletion, and GvHD were not reported in most of the included studies; thus, we are not able to evaluate the role of these factors on the development of NTM among the reported cases.
NTM infection occurs mainly by inhalation, ingestion, or direct inoculation, leading to different sites of colonization [83]. Lymphadenitis is the most common manifestation of NTM disease in healthy childhood [6, 84] while pulmonary NTM is mainly seen in patients with underlying lung pathology such as cystic fibrosis, bronchopulmonary dysplasia, or primary ciliary dyskinesia [6, 8].
In the immunosuppressed population, extrapulmonary NTM disease is more common [11]. The infection can occur at any site and then become disseminated because the organisms that escape intracellular killing are able to multiply within macrophages and disseminate via lymphatics and the bloodstream (84).
In our study, the majority of cases were adults rather than pediatrics, with only one-fifth of the reported cases being 18 years old or less. Interestingly, rates of disseminated NTM were 33% and 20.7% among pediatrics and adults, respectively, with no predominant causative pathogen. We noted a difference in the timing of NTM infection post-HSCT, between the pediatric and adult cohort where NTM occurred earlier among children rather than adults (4 months vs 9 months post-HSCT). There is no clear explanation for this observation. The late occurrence of NTM among adults might be in part related to impaired recovery of cell-mediated immunity in adults in the context of thymus involution by age and need for thymus gland to support T cell development, maturation, and education [85, 86]. Also, as in adults the majority of patients received HSCT for malignant conditions, impact of pre-HSCT chemotherapy might have contributed to a slower immune recovery. Again, the lack of extensive details on conditioning, donor, stem cell source, and GvHD prophylaxis, precludes any further comparison between the two cohorts.
There are multiple guidelines set for adults to support a diagnosis of NTM including the American and British Thoracic Societies guidelines [87–89]. However, none of these are validated in children. Across the selected studies and case reports, we noticed that clinicians relied on clinical and pathogen recognition rather than using any of the published guidelines. Finally, most likely because we excluded patients with MSMD and pre-transplant NTM infections, no cases of immune reconstitution inflammatory syndrome (IRIS), that could guide diagnostic and therapeutic considerations, were reported in the analyzed studies.
In general, empiric treatment is usually begun before results on susceptibility testing are available [90]. Treatment of NTM disease requires the use of two or more antimicrobial agents for prolonged periods, to achieve microbiologic cure or radiologic improvement while avoiding the emergence of resistant strains [23]. The choice of antimicrobial therapy for transplant recipients is similar to that for patients without systemic immune suppression. However, in addition to well-documented toxicities and interactions of drugs used to treat NTM infection, interactions between medications for NTM infection and immunosuppressive agents (and other medications) are frequent and need to be considered especially in a transplant setting [18]. Moreover, a careful evaluation of the balance between risks and benefits is warranted for each patient, taking into account the clinical course of the disease, the pathogenicity of the isolated NTM species, and the underlying pathologies of the patient [22]. Several schedules of treatment are employed to contrast NTM infections in immunocompromised patients based on combination of antitubercular drugs, macrolides, aminoglycosides, and quinolones. International guidelines for therapy in adults with NTM lung infections have been published, while definite management in immunocompromised children has not yet been formulated and the optimal regimen is yet to be determined [91].
There is no clear recommendation for duration of treatment for NTM infection in the immunocompromised host, including HSCT recipients [23]. Besides antimicrobial NTM-targeted therapy, other interventions including foreign body removal in catheter-related infections and surgical resection or debridement of infected collections or devitalized tissue should be achieved early to allow improved penetration of drugs, to decrease the burden of disease, and to minimize the risk of developing resistance [29, 39, 42, 45, 53, 65, 69, 73, 74].
There are scarce data regarding outcome of NTM infections and related mortality among HSCT recipients. In our study, data on the patient’s status at time of reporting was missing for 27 cases. Among the remaining cases; 88 patients (88/145; 59%) were alive at time of reporting. Mortality rate of 40%, attributed to NTM in 33% of cases, is similar to what has been reported among patients with NTM infection in the setting of HIV in the pre-ART era. [92].
There are a number of limitations for this study including heterogeneity of the data and possible publication bias where some species tend to be reported more. Moreover, all analyzed studies only reported retrospective data on NTM infections, and differences in diagnostic procedures might have contributed to different incidences reported. Finally, our analysis was restricted to the English language literature, and therefore, we might have not included publications from Africa or South America.
Conclusions
NTM infection is not rare among HSCT recipients and is associated with NTM-related mortality rates of 33%. Active surveillance and early initiation of therapy are mandated to improve outcome. So far, there are no guidelines on diagnosis and management of NTM infection particularly in children. Further work is needed to set guidance to support early diagnosis and active management for best outcome.
Supplementary Information
(DOCX 121 kb)
Author Contribution
Conceptualization: RE; methodology: RE, GO, and BLC; formal analysis and investigation: IH, ZZ, IJH, GO, BLC, and RE; writing—original draft preparation: BLC, GO, ZZ, and IH; writing—review and editing: RE and IJH; All authors contributed to the article and approved the submitted version.
Data Availability
The raw data supporting the conclusions of the article will be made available by the authors, without undue reservation.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bianca Laura Cinicola and Giorgio Ottaviano contributed equally and shared first authorship. Intan Juliana Abd Hamid and Reem Elfeky contributed equally and shared last authorship.
Contributor Information
Intan Juliana Abd Hamid, Email: intanj@usm.my.
Reem Elfeky, Email: reem.elfeky@nhs.net.
References
- 1.Sahu KK. Infectious disease in hematopoietic stem cell transplantation. Ther Adv Infect Dis. 2021;19:8:20499361211005600. [DOI] [PMC free article] [PubMed]
- 2.Forbes BA, Hall GS, Miller MB, Novak SM, Rowlinson MC, Salfinger M, et al. Practical guidance for clinical microbiology laboratories: mycobacteria. Clin Microbiol Rev. 2018;31:31(2):e00038-17. [DOI] [PMC free article] [PubMed]
- 3.NaserEddin A, Dinur-Schejter Y, Shadur B, Zaidman I, Even-Or E, Averbuch D, et al. Bacillus Calmette–Guerin (BCG) vaccine-associated complications in immunodeficient patients following stem cell transplantation. J Clin Immunol. 2021;41:147–162. doi: 10.1007/s10875-020-00892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkinham JO. Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23:529–551. doi: 10.1016/S0272-5231(02)00014-X. [DOI] [PubMed] [Google Scholar]
- 5.Claeys TA, Robinson RT. The many lives of nontuberculous mycobacteria. J Bacteriol. 2018;26;200(11):e00739-17. [DOI] [PMC free article] [PubMed]
- 6.Zimmermann P, Curtis N, Tebruegge M. Nontuberculous mycobacterial disease in childhood—update on diagnostic approaches and treatment. J Infect. 2017;74:S136–S142. doi: 10.1016/S0163-4453(17)30204-9. [DOI] [PubMed] [Google Scholar]
- 7.Simner PJ, Woods GL, Wengenack NL. Mycobacteria. Microbiol Spectr. 2016. [DOI] [PubMed] [Google Scholar]
- 8.Koh W-J, Kwon OJ, Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol. 2002;3:145. doi: 10.3348/kjr.2002.3.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piersimoni C, Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351–1358. doi: 10.3201/eid1509.081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Anazi KA, Al-Jasser AM, Al-Anazi WK. Infections caused by non-tuberculous mycobacteria in recipients of hematopoietic stem cell transplantation. Front Oncol. 2014;10:4:311. [DOI] [PMC free article] [PubMed]
- 11.Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36:91–99. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss CH, Glassroth J. Pulmonary disease caused by nontuberculous mycobacteria. Expert Rev Respir Med. 2012;6:597–613. doi: 10.1586/ers.12.58. [DOI] [PubMed] [Google Scholar]
- 13.Mortaz E, Moloudizargari M, Varahram M, Movassaghi M, Garssen J, Kazempour Dizagie M, et al. What immunological defects predispose to non-tuberculosis mycobacterial infections? Iran J Allergy Asthma Immunol. 2018;17:100–109. [PubMed] [Google Scholar]
- 14.Lake MA, Ambrose LR, Lipman MCI, Lowe DM. ‘”Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med. 2016;14:54. doi: 10.1186/s12916-016-0606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu U-I, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15:968–980. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]
- 16.Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, et al. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2016;17:7:507. [DOI] [PMC free article] [PubMed]
- 17.Kang JY, Ha JH, Kang HS, Yoon H-K, Kim H-J, Lee S, et al. Clinical significance of nontuberculous mycobacteria from respiratory specimens in stem cell transplantation recipients. Int J Hematol. 2015;101:505–513. doi: 10.1007/s12185-015-1745-9. [DOI] [PubMed] [Google Scholar]
- 18.Doucette K, Fishman JA. Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis. 2004;38:1428–1439. doi: 10.1086/420746. [DOI] [PubMed] [Google Scholar]
- 19.Beswick J, Shin E, Michelis FV, Thyagu S, Viswabandya A, Lipton JH, et al. Incidence and risk factors for nontuberculous mycobacterial infection after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24:366–372. doi: 10.1016/j.bbmt.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Weinstock DM, Feinstein MB, Sepkowitz KA, Jakubowski A. High rates of infection and colonization by nontuberculous mycobacteria after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31:1015–1021. doi: 10.1038/sj.bmt.1704043. [DOI] [PubMed] [Google Scholar]
- 21.Hirama T, Brode SK, Beswick J, Law AD, Lam W, Michelis FV, et al. Characteristics, treatment and outcomes of nontuberculous mycobacterial pulmonary disease after allogeneic haematopoietic stem cell transplant. Eur Respir J. 2018;51:1702330. doi: 10.1183/13993003.02330-2017. [DOI] [PubMed] [Google Scholar]
- 22.Guglielmetti L, Mougari F, Lopes A, Raskine L, Cambau E. Human infections due to nontuberculous mycobacteria: The infectious diseases and clinical microbiology specialists’ point of view. Future Microbiol. 2015;10:1467–1483. doi: 10.2217/fmb.15.64. [DOI] [PubMed] [Google Scholar]
- 23.Anjan S, Morris MI. How can we improve the outcome for transplant patients with nontuberculous mycobacterial infections? Future Microbiol. 2018;13:903–914. doi: 10.2217/fmb-2018-0006. [DOI] [PubMed] [Google Scholar]
- 24.Navari RM, Sullivan KM, Springmeyer SC, Siegel MS, Meyers JD, Buckner CD, et al. Mycobacterial infections in marrow transplant patients. Transplantation. 1983;36:509–513. doi: 10.1097/00007890-198311000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Kurzrock R, Zander A, Vellekoop L, Kanojia M, Luna M, Dicke K. Mycobacterial pulmonary infections after allogeneic bone marrow transplantation. Am J Med. 1984;77:35–40. doi: 10.1016/0002-9343(84)90432-7. [DOI] [PubMed] [Google Scholar]
- 26.Roy V, Weisdorf D. Mycobacterial infections following bone marrow transplantation: A 20 year retrospective review. Bone Marrow Transplant. 1997;19:467–470. doi: 10.1038/sj.bmt.1700686. [DOI] [PubMed] [Google Scholar]
- 27.Yuen KY, Woo PC, Liang RH, Chiu EK, Chen FF, Wong SS, et al. Clinical significance of alimentary tract microbes in bone marrow transplant recipients. Diagn Microbiol Infect Dis. 1998;30:75–81. doi: 10.1016/S0732-8893(97)00213-7. [DOI] [PubMed] [Google Scholar]
- 28.Gaviria JM, Garcia PJ, Garrido SM, Corey L, Boeckh M. Nontuberculous mycobacterial infections in hematopoietic stem cell transplant recipients: Characteristics of respiratory and catheter-related infections. Biol Blood Marrow Transplant. 2000;6:361–369. doi: 10.1016/S1083-8791(00)70012-7. [DOI] [PubMed] [Google Scholar]
- 29.Au WY, Cheng VCC, Ho PL, Yuen KY, Hung I, Ma SY, et al. Nontuberculous mycobacterial infections in Chinese hematopoietic stem cell transplantation recipients. Bone Marrow Transplant. 2003;32:709–714. doi: 10.1038/sj.bmt.1704210. [DOI] [PubMed] [Google Scholar]
- 30.Cordonnier C, Martino R, Trabasso P, Held TK, Akan H, Ward MS, et al. Mycobacterial infection: A difficult and late diagnosis in stem cell transplant recipients. Clin Infect Dis. 2004;38:1229–1236. doi: 10.1086/383307. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson O, Feja K, LaRussa P, George D, Unal E, Della Latta P, et al. Nontuberculous mycobacterial infections in pediatric hematopoietic stem cell transplant recipients: Case report and review of the literature. Pediatr Infect Dis J. 2006;25:263–267. doi: 10.1097/01.inf.0000202119.75623.f6. [DOI] [PubMed] [Google Scholar]
- 32.Unal E, Yen C, Saiman L, George D, Della-Latta P, van de Ven C, et al. A low incidence of nontuberculous mycobacterial infections in pediatric hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2006;12:1188–1197. doi: 10.1016/j.bbmt.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz A, Gonzalez-Vicent M, Badell I, Diaz de Heredia C, Martinez A, Maldonado MS. Mycobacterial diseases in pediatric hematopoietic SCT recipients. Bone Marrow Transplant. 2011;46:766–768. doi: 10.1038/bmt.2010.183. [DOI] [PubMed] [Google Scholar]
- 34.Yoo JW, Jo KW, Kim SH, Lee SO, Kim JJ, Park SK, et al. Incidence, characteristics, and treatment outcomes of mycobacterial diseases in transplant recipients. Transpl Int. 2016;29:549–558. doi: 10.1111/tri.12752. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y-C, Wu C-J, Ko P-S, Chien S-H, Fan N-W, Wang H-Y, et al. Mycobacterial infections in adult recipients of allogeneic hematopoietic stem cell transplantation: A cohort study in a high endemic area. J Microbiol Immunol Infect. 2020;53:274–282. doi: 10.1016/j.jmii.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Ozkaynak MF, Lenarsky C, Kohn D, Weinberg K, Parkman R. Mycobacterium avium-intracellulare infections after allogeneic bone marrow transplantation in children. Am J Pediatr Hematol Oncol. 1990;12:220–224. doi: 10.1097/00043426-199022000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Wallace RJ, Tanner D, Brennan PJ, Brown BA. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482–486. doi: 10.7326/0003-4819-119-6-199309150-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kiehn TE, White M, Pursell KJ, Boone N, Tsivitis M, Brown AE, et al. A cluster of four cases of Mycobacterium haemophilum infection. Eur J Clin Microbiol Infect Dis. 1993;12:114–118. doi: 10.1007/BF01967586. [DOI] [PubMed] [Google Scholar]
- 39.Holland DJ, Chen SC, Chew WW, Gilbert GL. Mycobacterium neoaurum infection of a Hickman catheter in an immunosuppressed patient. Clin Infect Dis. 1994;18:1002–1003. doi: 10.1093/clinids/18.6.1002. [DOI] [PubMed] [Google Scholar]
- 40.Straus WL, Ostroff SM, Jernigan DB, Kiehn TE, Sordillo EM, Armstrong D, et al. Clinical and epidemiologic characteristics of Mycobacterium haemophilum, an emerging pathogen in immunocompromised patients. Ann Intern Med. 1994;120:118–125. doi: 10.7326/0003-4819-120-2-199401150-00004. [DOI] [PubMed] [Google Scholar]
- 41.White MH, Papadopoulos EB, Small TN, Kiehn TE, Armstrong D. Mycobacterium haemophilum infections in bone marrow transplant recipients. Transplantation. 1995;60:957–960. doi: 10.1097/00007890-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Ward MS, Lam KV, Cannell PK, Herrmann RP. Mycobacterial central venous catheter tunnel infection: A difficult problem. Bone Marrow Transplant. 1999;24:325–329. doi: 10.1038/sj.bmt.1701895. [DOI] [PubMed] [Google Scholar]
- 43.Busam KJ, Kiehn TE, Salob SP, Myskowski PL. Histologic reactions to cutaneous infections by Mycobacterium haemophilum. Am J Surg Pathol. 1999;23:1379–1385. doi: 10.1097/00000478-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Mohite U, Das M, Saikia T, Parikh P, Gopal R, Kelkar R, et al. Mycobacterial pulmonary infection post allogeneic bone marrow transplantation. Leuk Lymphoma. 2001;40:675–678. doi: 10.3109/10428190109097667. [DOI] [PubMed] [Google Scholar]
- 45.Okano A, Shimazaki C, Ochiai N, Hatsuse M, Takahashi R, Ashihara E, et al. Subcutaneous infection with Mycobacterium fortuitum after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;28:709–711. doi: 10.1038/sj.bmt.1703211. [DOI] [PubMed] [Google Scholar]
- 46.Frisk P, Boman G, Pauksen K, Petrini B, Lönnerholm G. Skin infection caused by Mycobacterium szulgai after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2003;31:511–513. doi: 10.1038/sj.bmt.1703861. [DOI] [PubMed] [Google Scholar]
- 47.Kline S, Cameron S, Streifel A, Yakrus MA, Kairis F, Peacock K, et al. An outbreak of bacteremias associated with Mycobacterium mucogenicum in a hospital water supply. Infect Control Hosp Epidemiol. 2004;25:1042–1049. doi: 10.1086/502341. [DOI] [PubMed] [Google Scholar]
- 48.Simmon KE, Pounder JI, Greene JN, Walsh F, Anderson CM, Cohen S, et al. Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J Clin Microbiol. 2007;45:1978–1980. doi: 10.1128/JCM.00563-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peres E, Khaled Y, Krijanovski OI, Mineishi S, Levine JE, Kaul DR, et al. Mycobacterium chelonae necrotizing pneumonia after allogeneic hematopoietic stem cell transplant: Report of clinical response to treatment with tigecycline. Transpl Infect Dis. 2009;11:57–63. doi: 10.1111/j.1399-3062.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- 50.Lu KJ, Grigg A, Leslie D, Finlay M, Sasadeusz J. Mycobacterium genavense duodenitis following allogeneic peripheral blood stem cell transplantation. Transpl Infect Dis. 2009;11:534–536. doi: 10.1111/j.1399-3062.2009.00431.x. [DOI] [PubMed] [Google Scholar]
- 51.Morales P, Gil A, Santos M. Mycobacterium abscessus infection in transplant recipients. Transplant Proc. 2010;42:3058–3060. doi: 10.1016/j.transproceed.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Saruwatari H, Yoshifuku A, Kawai K, Kanekura T. Cutaneous Mycobacterium intracellulare infection in a bone marrow transplantation recipient. J Dermatol. 2010;37:185–187. doi: 10.1111/j.1346-8138.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 53.Shachor-Meyouhas Y, Sprecher H, Eluk O, Ben-Barak A, Kassis I. An outbreak of Mycobacterium mucogenicum bacteremia in pediatric hematology-oncology patients. Pediatr Infect Dis J. 2011;30:30–32. doi: 10.1097/INF.0b013e3181ee31d7. [DOI] [PubMed] [Google Scholar]
- 54.Yamazaki R, Mori T, Nakazato T, Aisa Y, Imaeda H, Hisamatsu T, et al. Non-tuberculous mycobacterial infection localized in small intestine developing after allogeneic bone marrow transplantation. Intern Med. 2010;49:1191–1193. doi: 10.2169/internalmedicine.49.3288. [DOI] [PubMed] [Google Scholar]
- 55.Jacobs S, George A, Papanicolaou GA, Lacouture ME, Tan BH, Jakubowski AA, et al. Disseminated Mycobacterium marinum infection in a hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2012;14:410–414. doi: 10.1111/j.1399-3062.2011.00681.x. [DOI] [PubMed] [Google Scholar]
- 56.Rogers JT, Procop GW, Steelman CK, Abramowsky CR, Tuohy MT, Shehata BM. Clinical utility of DNA amplification and sequencing to identify a strain of Mycobacterium avium in paraffin-embedded, formalin-fixed biopsies from an immunosuppressed child. Pediatr Dev Pathol. 2012;15:315–317. doi: 10.2350/11-07-1061-CR.1. [DOI] [PubMed] [Google Scholar]
- 57.Brissot E, Gomez A, Aline-Fardin A, Lalande V, Lapusan S, Isnard F, et al. Report of disseminated Mycobacterium haemophilum infection after double cord blood allo-SCT. Bone Marrow Transplant. 2014;49:1347–1348. doi: 10.1038/bmt.2014.144. [DOI] [PubMed] [Google Scholar]
- 58.Ferry C, Saussine A, Bouaziz JD, Xhaard A, Peffault de Latour R, Ribaud P, et al. Disseminated cutaneous infection due to Mycobacterium chelonae following hematopoietic stem cell transplantation. IDCases. 2014;1:68–69. doi: 10.1016/j.idcr.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iroh Tam P-Y, Kline S, Wagner JE, Guspiel A, Streifel A, Ward G, et al. Rapidly growing mycobacteria among pediatric hematopoietic cell transplant patients traced to the hospital water supply. Pediatr Infect Dis J. 2014;33:1043–1046. doi: 10.1097/INF.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 60.Miyashita E, Yoshida H, Mori D, Nakagawa N, Miyamura T, Ohta H, et al. Mycobacterium avium complex-associated peritonitis with CAPD after unrelated bone marrow transplantation. Pediatr Int. 2014;56:e96–e98. doi: 10.1111/ped.12463. [DOI] [PubMed] [Google Scholar]
- 61.Moreno-Bonilla G, Choy B, Fernandez-Peñas P. Cutaneous non-tuberculous mycobacterial infection in patients with chronic graft-versus-host disease: A case series. Australas J Dermatol. 2015;56:124–127. doi: 10.1111/ajd.12269. [DOI] [PubMed] [Google Scholar]
- 62.Noguchi S, Yatera K, Yamasaki K, Kawanami T, Takahashi T, Shimabukuro I, et al. A case of rapid exacerbation of pulmonary mycobacterium avium complex infection mimicking pulmonary aspergillosis. J UOEH. 2015;37:177–183. doi: 10.7888/juoeh.37.177. [DOI] [PubMed] [Google Scholar]
- 63.Baluch A, Pasikhova Y, Snyder M. Successful management of Mycobacterium haemophilum lower extremity cutaneous infection in a matched-unrelated donor stem cell transplant recipient. Transpl Infect Dis. 2017;19(1). [DOI] [PubMed]
- 64.Coelho R, Hanna R, Flagg A, Stempak LM, Ondrejka S, Procop GW, et al. Mycobacterium genavense-induced spindle cell pseudotumor in a pediatric hematopoietic stem cell transplant recipient: Case report and review of the literature. Transpl Infect Dis. 2017;19(2). [DOI] [PubMed]
- 65.Khalid M, Ali SA. Mycobacterium abscessus lymphadenitis in bone marrow transplant patient. J Pak Med Assoc. 2017;67:1615–1617. [PubMed] [Google Scholar]
- 66.Saeki K, Watanabe S, Waseda Y, Kasahara K. Endobronchial lesions of Mycobacterium abscessus infection in an immunocompromised patient. Am J Respir Crit Care Med. 2017;195:e37–e38. doi: 10.1164/rccm.201610-1973IM. [DOI] [PubMed] [Google Scholar]
- 67.Miyake N, Chong Y, Nishida R, Takenaka K, Kato K, Miyamoto T, et al. Mycobacterium abscessus and massiliense lung infection during macrolide treatment for bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. 2018;24:78–81. doi: 10.1016/j.jiac.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Salvator H, Berti E, Catherinot E, Rivaud E, Chabrol A, Nguyen S, et al. Pulmonary alveolar proteinosis and Mycobacterium abscessus lung infection related to ruxolitinib after allogeneic stem cell transplantation. Eur Respir J. 2018;51(5):1701960. [DOI] [PubMed]
- 69.Dávila Saldaña BJ, Keller M, Hanisch BR, Song X. Tap water: A possible source of nontuberculous mycobacterial infection in patients with T cell deficiency. Am J Infect Control. 2019;47:834–836. doi: 10.1016/j.ajic.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 70.Hou R, Nayak R, Pincus SM, Lai J, Omran LM, Alkaade S, et al. Esophageal Mycobacterium avium-intracellulare infection in a bone marrow transplant patient: Case report and literature review. Transpl Infect Dis. 2019;21(1):e13019. [DOI] [PubMed]
- 71.Musharbash M, Para A, Choi J. Widespread cutaneous nontuberculous mycobacterial infection in the absence of bacteremia mimicking leukemia cutis. JAAD Case Rep. 2019;5:679–681. doi: 10.1016/j.jdcr.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toyama T, Mori T, Kato J, Sugita K, Hasegawa N, Nakata N, et al. Disseminated Mycobacterium massiliense infection in a patient with myelodysplastic syndrome undergoing allogeneic bone marrow transplantation. Transpl Infect Dis. 2020;22(3):e13278. [DOI] [PubMed]
- 73.Xie O, Khan S, Globan M, Lea K, Bajel A, Slavin M. Mycobacterium abscessus bloodstream infection: Unexpected catheter tunnel infection localized by PET/CT. Transpl Infect Dis. 2019;21(5):e13147. [DOI] [PubMed]
- 74.Nagata A, Sekiya N, Najima Y, Horiuchi M, Fukushima K, Toya T, et al. Nontuberculous mycobacterial bloodstream infections after allogeneic hematopoietic stem cell transplantation. Int J Infect Dis. 2020;97:131–134. doi: 10.1016/j.ijid.2020.05.079. [DOI] [PubMed] [Google Scholar]
- 75.Sugiyama M, Terashita Y, Hara K, Cho Y, Asano T, Iguchi A. Septic arthritis caused by Mycobacterium Kansasii in a bone marrow transplant recipient. J Pediatr Hematol Oncol. 2020;42:e791–e794. doi: 10.1097/MPH.0000000000001746. [DOI] [PubMed] [Google Scholar]
- 76.Shah MK, Sebti A, Kiehn TE, Massarella SA, Sepkowitz KA. Mycobacterium haemophilum in immunocompromised patients. Clin Infect Dis. 2001;33:330–337. doi: 10.1086/321894. [DOI] [PubMed] [Google Scholar]
- 77.Moore RD, Chaisson RE. Natural history of opportunistic disease in an HIV-infected urban clinical cohort. Ann Intern Med. 1996;124:633–642. doi: 10.7326/0003-4819-124-7-199604010-00003. [DOI] [PubMed] [Google Scholar]
- 78.Sharma S, Upadhyay V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J Med Res. 2020;152:185. doi: 10.4103/ijmr.IJMR_902_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ristola MA, von Reyn CF, Arbeit RD, Soini H, Lumio J, Ranki A, et al. High rates of disseminated infection due to non-tuberculous mycobacteria among AIDS patients in Finland. J Infect. 1999;39:61–67. doi: 10.1016/S0163-4453(99)90104-4. [DOI] [PubMed] [Google Scholar]
- 80.Buchacz K, Baker RK, Palella FJ, Chmiel JS, Lichtenstein KA, Novak RM, et al. AIDS-defining opportunistic illnesses in US patients, 1994-2007: A cohort study. AIDS. 2010;24:1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 81.Baldwin SL, Larsen SE, Ordway D, Cassell G, Coler RN. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Negl Trop Dis. 2019;13:e0007083. doi: 10.1371/journal.pntd.0007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sarro YD, Kone B, Diarra B, Kumar A, Kodio O, Fofana DB, et al. Simultaneous diagnosis of tuberculous and non-tuberculous mycobacterial diseases: Time for a better patient management. Clin Microbiol Infect Dis. 2018;3(3):10.15761/CMID. [DOI] [PMC free article] [PubMed]
- 83.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: A changing epidemiology. Clin Infect Dis. 2009;49:e124–e129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 84.Reuss AM, Wiese-Posselt M, Weimann B, Siedler A, Zuschneid I, an der Heiden M, et al. Incidence rate of nontuberculous mycobacterial disease in immunocompetent children. Pediatr Infect Dis J. 2009;28:642–644. doi: 10.1097/INF.0b013e3181978e8e. [DOI] [PubMed] [Google Scholar]
- 85.Palmer DB. The effect of age on thymic function. Front Immunol. 2013;7:4:316. [DOI] [PMC free article] [PubMed]
- 86.Hino C, Xu Y, Xiao J, Baylink DJ, Reeves ME, Cao H. The potential role of the thymus in immunotherapies for acute myeloid leukemia. Front Immunol. 2023;6:14:1102517. [DOI] [PMC free article] [PubMed]
- 87.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 88.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71:e1–36. doi: 10.1093/cid/ciaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) Thorax. 2017;72:ii1–i64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 90.McGrath EE, Anderson PB. The therapeutic approach to non-tuberculous mycobacterial infection of the lung. Pulm Pharmacol Ther. 2010;23(5):389–96. [DOI] [PubMed]
- 91.Arlotta A, Cefalo MG, Maurizi P, Ruggiero A, Dodi I, Riccardi R. Critical pulmonary infection due to nontuberculous mycobacterium in pediatric leukemia: Report of a difficult diagnosis and review of pediatric series. J Pediatr Hematol Oncol. 2014;36:66–70. doi: 10.1097/MPH.0b013e3182841737. [DOI] [PubMed] [Google Scholar]
- 92.Wetzstein N, Geil A, Kann G, Lehn A, Schüttfort G, Kessel J, et al. Disseminated disease due to non-tuberculous mycobacteria in HIV positive patients: A retrospective case control study. PLoS One. 2021;16:e0254607. doi: 10.1371/journal.pone.0254607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 121 kb)
Data Availability Statement
The raw data supporting the conclusions of the article will be made available by the authors, without undue reservation.