Abstract
Objective
This study aims to conduct a meta-analysis to evaluate the short-term and long-term therapeutic effects of robot-assisted laparoscopic treatment in patients with mid and low rectal cancer.
Methods
A comprehensive search strategy was employed to retrieve relevant literature from PubMed, NCBI, Medline, and Springer databases, spanning the database inception until August 2023. The focus of this systematic review was on controlled studies that compared the treatment outcomes of robot-assisted (Rob) and conventional laparoscopy (Lap) in the context of mid and low rectal cancer. Data extraction and literature review were meticulously conducted by two independent researchers (HMW and RKG). The synthesized data underwent rigorous analysis utilizing RevMan 5.4 software, adhering to established methodological standards in systematic reviews. The primary outcomes encompass perioperative outcomes and oncological outcomes. Secondary outcomes include long-term outcomes.
Result
A total of 11 studies involving 2239 patients with mid and low rectal cancer were included (3 RCTs and 8 NRCTs); the Rob group consisted of 1111 cases, while the Lap group included 1128 cases. The Rob group exhibited less intraoperative bleeding (MD = −40.01, 95% CI: −57.61 to −22.42, P < 0.00001), a lower conversion rate to open surgery (OR = 0.27, 95% CI: 0.09 to 0.82, P = 0.02), a higher number of harvested lymph nodes (MD = 1.97, 95% CI: 0.77 to 3.18, P = 0.001), and a lower CRM positive rate (OR = 0.46, 95% CI: 0.23 to 0.95, P = 0.04). Additionally, the Rob group had lower postoperative morbidity rate (OR = 0.66, 95% CI: 0.53 to 0.82, P < 0.0001) and a lower occurrence rate of complications with Clavien–Dindo grade ≥ 3 (OR = 0.60, 95% CI: 0.39 to 0.90, P = 0.02). Further subgroup analysis revealed a lower anastomotic leakage rate (OR = 0.66, 95% CI: 0.45 to 0.97, P = 0.04). No significant differences were observed between the two groups in the analysis of operation time (P = 0.42), occurrence rates of protective stoma (P = 0.81), PRM (P = 0.92), and DRM (P = 0.23), time to flatus (P = 0.18), time to liquid diet (P = 0.65), total hospital stay (P = 0.35), 3-year overall survival rate (P = 0.67), and 3-year disease-free survival rate (P = 0.42).
Conclusion
Robot-assisted laparoscopic treatment for mid and low rectal cancer yields favorable outcomes, demonstrating both efficacy and safety. In comparison to conventional laparoscopy, patients experience reduced intraoperative bleeding and a lower incidence of complications. Notably, the method achieves comparable short-term and long-term treatment results to those of conventional laparoscopic surgery, thus justifying its consideration for widespread clinical application.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-023-04579-3.
Keywords: Robot-assisted, Mid and low rectal cancer, Short-term efficacy, Long-term efficacy, Meta-analysis
Background
Laparoscopic minimally invasive surgery has become widely accepted for the treatment of colorectal cancer globally [1]. However, when compared to colon cancer, surgical procedures for mid and low rectal cancer are inherently more intricate, presenting specific surgical demands and challenges within the operative environment. Total mesorectal excision (TME) stands as the gold standard for curative surgery in rectal cancer, ensuring the complete removal of the rectum and surrounding lymph nodes to achieve a circumferentially negative surgical margin [2]. Modern surgical approaches also emphasize the preservation of pelvic autonomic nerve function to enhance patients’ quality of life, requiring advanced surgical skills [3]. The complex structure of pelvic organs, distributed within a confined space containing numerous critical organs, vessels, and nerves, makes it challenging for laparoscopic instruments with elongated handles to navigate, thereby increasing the difficulty of the surgical procedure [4]. In recent years, the application of the da Vinci robotic surgical system (Intuitive Surgical Inc., Sunnyvale, CA) in rectal cancer has seen gradual growth. The advantages of the robotic system, such as a magnified 3D field of view, 10 times enlargement, and 7 degrees of freedom in wrist-like surgical instruments, have successfully overcome limitations in the laparoscopic approach, particularly in terms of surgical visibility and precise manipulation [2]. These features make the robotic system particularly suitable for performing surgery in the confined space of the pelvic cavity. While robotic surgery appears to be a safe and feasible surgical approach in colorectal cancer, however, there is still some controversy in clinical application. Therefore, we conducted a recent meta-analysis study, comparing the short-term and long-term treatment outcomes of robotic and laparoscopic surgery for mid and low rectal cancer. This research aims to provide a more comprehensive understanding of the relative effectiveness of these two surgical approaches, offering clearer guidance for treatment decisions in patients with rectal cancer.
Methods
Study design
The protocol was compiled and registered in PROSPERO (CRD42023466246). We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis: the PRISMA statement [5]. Methodological quality was ensured by following AMSTAR (assessing the methodological quality of systematic review) guidelines.
A systematic search for published articles was conducted in August 2023 in four electronic databases (PubMed, NCBI, Medline, and Springer databases,) from their inception till the end of July 2023. The keywords and Medical Subject Headings terms used for the search strategy were rectal neoplasm OR rectal cancer OR rectal carcinoma OR rectal tumor AND robotics OR robotic surgery OR robot assisted laparoscopy OR robot assisted total mesorectal excision AND laparoscopy OR laparoscopic surgery OR laparoscopic total mesorectal excision OR laparoscopic TME AND randomized controlled trial OR Retrospective study. References of accepted articles were also manually screened for potentially relevant studies to ensure that no additional publications were missed.
The selection criteria followed the PICOT. P (participants): patients over 18 years of age diagnosed with mid or low rectal cancer; I (intervention): robot-assisted laparoscopic resection; C (comparison): laparoscopic rectal resection; O (outcomes): short-term and long-term therapeutic effects; T (type of study design): randomized controlled trial and retrospective study.
The last search was performed in the end of August 2023, the search strategy was limited to papers written in English, and the reference lists of the eligible studies were tracked manually for other potentially relevant studies.
Eligibility criteria and study selection
Two independent authors (HMW and RKG) meticulously sifted through articles retrieved from the initial literature search, meticulously removing duplicate studies and excluding those not directly pertinent to the research. The two authors then independently conducted a detailed review of studies that met the predetermined eligibility criteria, whether in abstract or full-text form, carefully evaluating their alignment with the specified standards. Any disparities in study selection were methodically addressed through thorough discussions, consensus-building, or seeking input from a third independent author (HYL). The inclusion criteria were thoughtfully outlined as follows: (1) the study designs were exclusively centered on comparing robotic-assisted and conventional laparoscopic treatments for rectal cancer; (2) each group consisted of a minimum of 10 patients; (3) all subjects were clinically and pathologically confirmed or diagnosed through laboratory examinations as having mid or low rectal (tumor located within 15 cm of the anus) cancer; (4) crucial data required for this study could be reliably obtained, and statistical methods were diligently applied; and (5) the literature had undergone prior public dissemination.
Data extraction
Data extraction from the enrolled studies was undertaken independently by two meticulous authors, HMW and RKG. Any disparities in the extraction process were diligently addressed through comprehensive discussions and, when needed, consultation with the third author (HYL):
Characteristics of included studies
Author’s name; year of publication; study design; sample size, age, sex; body mass index (BMI); American Society of Anesthesiologists (ASA) grading; cTMN stage; tumor size; tumor distance from the anal verge (Endoscopic diagnosis); neoadjuvant therapies; and follow-up duration.
-
2.
Primary outcomes
Conversion to open surgery rate, total hospital stay, postoperative complications, circumferential resection margin positive rate.
-
3.
Secondary outcomes
Operation time, operative blood loss, protective stoma rate, time to flatus, time to liquid diet, occurrence rate of complications with Clavien–Dindo grade ≥ 3 [6], harvested lymph nodes, proximal resection margin, distal resection margin, 3-year overall survival rate, 3-year disease-free survival rate.
Risk of bias assessment
The quality of the RCTs included in the analysis was assessed using the Cochrane Collaboration’s tool [7] for evaluating the risk of bias, while NRCTs were evaluated using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool.
Statistical analysis
Data organization and calculations were conducted using Excel 2016 software, and data analysis was performed using RevMan 5.4 software. For continuous variables, mean difference (MD) was employed. Given variations in assessment methods across included studies, standardized mean difference (SMD) was used as the composite effect measure to eliminate the impact of differing data scales. Categorical variables were addressed using odds ratio (OR). Heterogeneity analysis employed the χ2 test [8]. If P ≤ 0.1 and I2 ≥ 50%, it indicated significant heterogeneity among included studies, prompting the adoption of a random effects model (REM); otherwise, a fixed effects model (FEM) was chosen. A significance level of P ≤ 0.05 was considered statistically meaningful. A funnel plot was created to assess publication bias.
Trial sequential analysis (TSA)
To mitigate the risk of type I errors associated with repeated significance testing and minimize the potential for false positive results due to random errors, we employed trial sequential analysis (TSA) 0.9.5.10 Beta software to conduct sequential analyses on the advantageous outcomes identified in the robotic (Rob) group during this analysis process.
Result
Literature searching
During the initial screening, a total of 1389 studies were identified. After removing duplicates, we screened 1262 studies and identified 103 eligible studies by reviewing titles and abstracts. Among these 103 studies, 11 articles were determined to meet the inclusion criteria for the final analysis after a full-text evaluation. The study selection progress is presented in the PRISMA flow diagram (Fig. 1).
Fig. 1.
PRISMA flow chart of study selection. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71
Characteristics of the included studies
A total of 11 studies, involving 2239 patients with mid and low rectal cancer, were included—comprising 3 RCTs and 8 NRCTs. The robotic group consisted of 1111 cases, while the Lap group had 1128 cases. The mean age ranged from 53.1 to 66, and the male-to-female ratio was 1.7:1. The mean BMI varied from 21.9 to 26 kg/m2. Patients with ASA I scores accounted for 25.2 to 67.1%, ASA II accounted for 29 to 57.7%, ASA III accounted for 0 to 33.3%, and ASA IV accounted for 0 to 0%. Patients with TMN stage I accounted for 0 to 55.0%, TMN stage II accounted for 17.1 to 46.5%, TMN stage III accounted for 11 to 57.1%, and TMN stage IV accounted for 0 to 7.9%. The mean tumor size ranged from 2.5 to 3.6 cm. The mean distance from the anal verge ranged from 3.24 to 8 cm. Six studies described the follow-up duration. The characteristics of included studies are summarized in Table 1.
Table 1.
Characteristics of the included studies
| Author | Year | Study design | Group | Patients | Mean age | Sex (M/F) | Mean BMI | ASA (I/II/III/IV) | TMN stage (I/II/III/IV) | Tumor size (cm) | Distance from the anal verge (cm) | Neoadjuvant therapies | Follow-up duration (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bedirli et al. [9] | 2016 | NRCT | Rob | 35 | 64.7 ± 8.5 | 24/11 | 24.7 ± 3.9 | 6/17/12/0 | 0/13/19/3 | ——— | ——— | 35 | —— |
| Lap | 28 | 60.4 ± 7.1 | 19/9 | 23.2 ± 3.2 | 4/15/9/0 | 0/9/17/2 | ——— | ——— | 28 | —— | |||
| Baek et al. [10] | 2013 | NRCT | Rob | 47 | 58.0 ± 12.9 | 31/16 | 23.37 ± 3.27 | 22/24/1/0 | 28/9/10/1 | ——— | 4.39 ± 2.25 | —— | 60 |
| Lap | 37 | 61.8 ± 12.8 | 28/9 | 23.4 ± 2.74 | 25/12/0/0 | 17/9/8/3 | ——— | 5.52 ± 3.74 | —— | 60 | |||
| Feng et al. [11] | 2021 | NRCT | Rob | 137 | 58.3 ± 11.2 | 75/62 | —— | 93/38/6/0 | 54/24/59/0 | 3.3 ± 1.1 | 6.7 ± 2.0 | 5 | 72 |
| Lap | 137 | 59.5 ± 11.2 | 81/56 | —— | 91/41/5/0 | 54/22/61/0 | 3.4 ± 1.0 | 6.9 ± 1.8 | 7 | 72 | |||
| Feng et al. [12] | 2022 | RCT | Rob | 586 | 59·1 ± 11·0 | 356/230 | 23.5 ± 3.3 | 324/230/32/0 | 205/192/189/0 | —— | 5.9 ± 2.4 | 254 | —— |
| Lap | 585 | 60.7 ± 9.8 | 354/231 | 23.5 ± 3.1 | 318/240/27/0 | 203/200/182/0 | —— | 5.8 ± 2.6 | 257 | —— | |||
| Feroci et al. [13] | 2016 | NRCT | Rob | 53 | 66 (33–80) | 42/16 | 24.6 (19–37) | 11/33/9/0 | 27/8/18/0 | —— | 8 (3–12) | 26 | 80 |
| Lap | 58 | 66 (42–84) | 27/26 | 24.6 (18–31) | 7/31/20/0 | 34/11/13/0 | —— | 8 (4–12) | 25 | 80 | |||
| Huang et al. [14] | 2017 | NRCT | Rob | 40 | 60.0 ± 12.2 | 25/15 | 23.0 ± 4.4 | —— | —— | 2.5 ± 1.5 | 6.8 ± 3.2 | —— | —— |
| Lap | 38 | 60.1 ± 14.2 | 28/10 | 24.3 ± 3.5 | —— | —— | 2.7 ± 1.6 | 6.3 ± 3.4 | —— | —— | |||
| Park et al. [15] | 2013 | NRCT | Rob | 40 | 57.3 ± 12.1 | 28/12 | 23.9 ± 2.4 | 27/9/4/0 | 1/19/19/1 | 2.8 ± 1.9 | 3.4 ± 1.1 | —— | 6 |
| Lap | 40 | 63.6 ± 10.6 | 25/15 | 24.3 ± 3.1 | 24/14/2/0 | 1/13/26/0 | 3.2 ± 1.9 | 3.6 ± 1.3 | —— | 6 | |||
| Serin et al. [16] | 2015 | NRCT | Rob | 14 | 54 (41–71) | 14/0 | 24.7 (2, 7–23, 23–27) | —— | —— | —— | —— | —— | —— |
| Lap | 65 | 57 (28–80) | 65/0 | 26 (21–32) | —— | —— | —— | —— | —— | —— | |||
| Tang et al. [17] | 2020 | RCT | Rob | 65 | 55.1 ± 12.1 | 36/29 | 22.0 ± 2.5 | 35/30/0/0 | 9/28/28/0 | 3.6 ± 1.1 | 6.0 ± 2.4 | 1 | 24 |
| Lap | 64 | 58.0 ± 9.7 | 36/28 | 22.1 ± 2.3 | 27/37/0/0 | 7/32/25/0 | 3.7 ± 1.0 | 5.8 ± 2.6 | 0 | 24 | |||
| Yoo et al. [18] | 2014 | NRCT | Rob | 44 | 59.77 ± 12.33 | 35/9 | 24.13 ± 3.33 | 26/17/1/0 | 2/15/22/5 | 2.97 ± 1.75 | 3.24 ± 0.78 | 24 | 60 |
| Lap | 26 | 60.5 ± 10.75 | 19/7 | 21.42 ± 3.13 | 15/11/0/0 | 3/4/18/1 | 3.62 ± 2.27 | 3.71 ± 0.89 | 7 | 60 | |||
| Zou et al. [19] | 2018 | RCT | Rob | 50 | 53.1 ± 11.8 | 29/21 | 22.0 ± 1.7 | 34/13/3/0 | 21/24/5/0 | 3.52 ± 1.15 | 5.9 ± 2.4 | —— | —— |
| Lap | 50 | 57.8 ± 9.5 | 26/24 | 21.9 ± 1.8 | 30/16/4/0 | 23/21/6/0 | 3.46 ± 12.1 | 5.8 ± 2.7 | —— | —— |
Risk of bias assessment
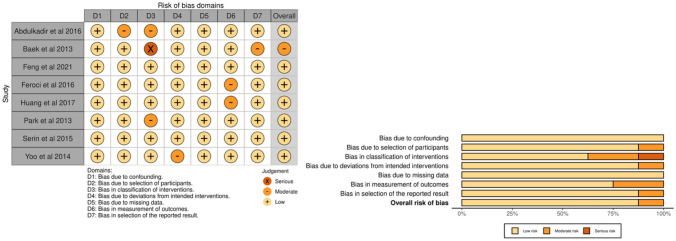
After assessing RCTs using the Cochrane Collaboration’s tool, it was found that one study had an unclear risk in random sequence generation, one study had a higher risk, and another study had an unclear risk in the blinding of outcome assessment. Additionally, there were two studies with an unclear risk in incomplete outcome data. The risk of bias assessment according to the Cochrane Collaboration’s tool is shown in Fig. 2. Applying the ROBINS-I tool to assess the risk of other NRCTs revealed that one study was at moderate risk, while the rest of the studies were at low risk.. The risk of bias assessment according to the ROBINS-I tool is shown in Fig. 3.
Fig. 2.
The risk of bias assessment according to the Cochrane Collaboration’s tool
Fig. 3.
The risk of bias assessment according to the ROBINS-I tool
Perioperative outcome
Operation time
A total of 5 study were included in the study, and there was significant heterogeneity among the included studies (P < 0.00001, I2 = 93%), necessitating the use of a random-effects model. The results indicated no significant difference between the two groups (MD = 11.10, 95% CI: −16.12 to 38.32, P = 0.42).
Operative blood loss
A total of 6 study were included in the study, and there was significant heterogeneity among the included studies (P = 0.001, I2 = 75%), necessitating the use of a random-effects model. The results indicate that the robotic group had less operative blood loss (MD = −40.01, 95% CI: −57.6 to −22.42, P < 0.00001).
Protective stoma rate
A total of 7 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.10, I2 = 43%), necessitating the use of a fixed-effects model. The results indicated no significant difference between the two groups (OR = 0.95, 95% CI: 0.65 to 1.39, P = 0.81).
Conversion to open surgery rate
A total of 5 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.18, I2 = 38%), necessitating the use of a fixed-effects model. The robotic group had a lower rate of conversion to open surgery (OR = 0.27, 95% CI: 0.09 to 0.82, P = 0.02).
Time to flatus
A total of 5 study were included in the study and there was significant heterogeneity among the included studies (P = 0.004, I2 = 71%), necessitating the use of a random-effects model. The results indicated no significant difference between the two groups (SMD = −0.20, 95% CI: −0.49 to −0.09, P = 0.18).
Time to liquid diet
A total of 5 study specify this outcomes, and there was significant heterogeneity among the included studies (P = 0.0001, I2 = 82%), necessitating the use of a random-effects model. The results indicated no significant difference between the two groups (SMD = −0.11, 95% CI: −0.57 to 0.36, P = 0.65).
Total hospital stay
A total of 6 study were included in the study, and there was significant heterogeneity among the included studies (P = 0.04, I2 = 56%), necessitating the use of a random-effects model. The results indicated no significant difference between the two groups (MD = −0.30, 95% CI: −0.92 to 0.32, P = 0.35).
Postoperative morbidity rate
A total of 10 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.72, I2 = 0%), necessitating the use of a fixed-effects model. In terms of postoperative morbidity rate, the robotic group had a lower occurrence (OR = 0.66, 95% CI: 0.53 to 0.82, P < 0.0001). Subgroup analysis for postoperative morbidity revealed a lower anastomotic leakage rate in the robotic group (OR = 0.66, 95% CI: 0.45 to 0.97, P = 0.04), with no significant differences in abdominal or anastomotic bleeding (OR = 0.65, 95% CI: 0.32 to 1.31, P = 0.23), wound-related complications (OR = 0.66, 95% CI: 0.39 to 1.11, P = 0.12), ileus (OR = 0.54, 95% CI: 0.26 to 1.13, P = 0.10), urinary retention or infection (OR = 0.58, 95% CI: 0.32 to 1.05, P = 0.07), stoma-related complications (OR = 0.86, 95% CI: 0.40 to 1.85, P = 0.70), and pulmonary infection (OR = 0.60, 95% CI: 0.19 to 1.85, P = 0.37).
Clavien–Dindo grade
A total of 7 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.90, I2 = 0%), necessitating the use of a fixed-effects model. The results indicated the robotic group’s occurrence rate of complications with Clavien–Dindo grade ≥ 3 was lower (OR = 0.60, 95% CI: 0.39 to 0.90, P = 0.02).
Oncological outcomes
Harvested lymph nodes
A total of 4 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.54, I2 = 0%), necessitating the use of a fixed-effects model. The results indicated the robotic group had a higher number of harvested lymph nodes (MD = 1.97, 95% CI: 0.77 to 3.18, P = 0.001).
Proximal resection margin
A total of 4 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.45, I2 = 0%), necessitating the use of a random-effects model. The results indicated no significant difference between the two groups (MD = −1.11, 95% CI: −2.54 to 0.33, P = 0.13).
Distal resection margin
A total of 5 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.55, I2 = 0%), necessitating the use of a random-effects model. The results indicated no significant difference between the two groups (MD = 0.09, 95% CI: −0.008 to 0.26, P = 0.30).
Circumferential resection margin positive rate
A total of 7 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.91, I2 = 0%), necessitating the use of a fixed-effects model. The robotic group had a lower circumferential resection margin positive rate (OR = 0.46, 95% CI: 0.23 to 0.95, P = 0.04).
Long-term outcome
3-year overall survival rate
A total of 3 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.49, I2 = 0%), necessitating the use of a fixed-effects model. The results indicated no significant difference between the two groups (MD = 1.15, 95% CI: 0.60 to 2.22, P = 0.67).
3-year disease-free survival rate
A total of 3 study were included in the study, and there was no significant heterogeneity among the included studies (P = 0.90, I2 = 0%), necessitating the use of a fixed-effects model. The results indicated no significant difference between the two groups (MD = 1.23, 95% CI: 0.74 to 2.03, P = 0.42).
Publication bias
A funnel plot was generated using the postoperative overall complication rates for both groups as indicators. The results showed that the scatter points representing each included study were mostly within the funnel, and the majority of scatter points were symmetrically distributed along the central axis. This suggests a low risk of bias in the included studies (Fig. 4).
Fig. 4.
A funnel plot
Trial sequential analysis (TSA)
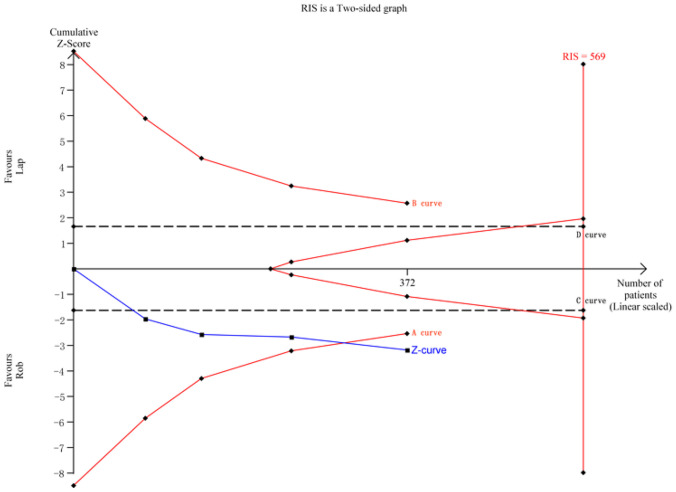
For operative blood loss, a meta-analysis of 6 studies with 514 cases was conducted. The required information size for the actual meta-analysis (RIS) was 407, estimated based on the following statistical indicators: type I error rate (α = 0.05) and type II error rate (β = 0.2). TSA results demonstrated that the cumulative Z-value (Z-curve) crossed both the conventional boundary (D-curve) and the TSA boundary (B-curve), providing evidence of the superiority of the robotic group, with the cumulative information size reaching the required level (Fig. 5).
Fig. 5.
TSA for operative blood loss
For conversion to open surgery rate, a meta-analysis of 5 studies with 628 cases was performed. The RIS for the actual meta-analysis was 1373, estimated based on the specified statistical indicators. TSA results indicated that the cumulative Z-value (Z-curve) crossed the conventional boundary (D-curve) but did not surpass the TSA boundaries (A-curve and B-curve), suggesting a higher possibility of false positives. Further randomized controlled trials are needed to validate this outcome (Fig. 6).
Fig. 6.
TSA for conversion to open surgery rate
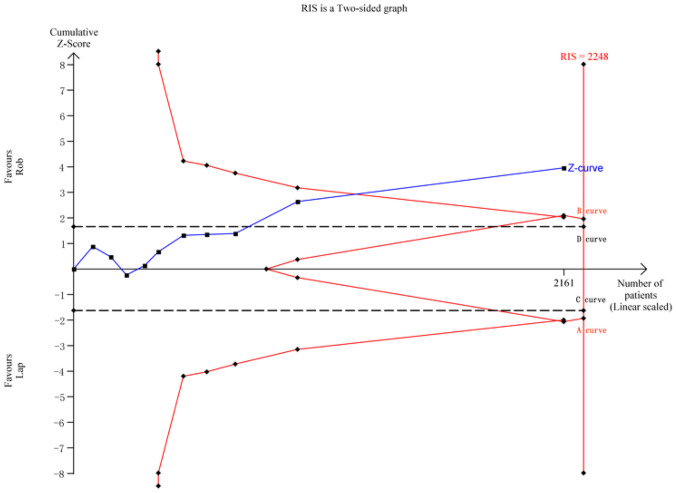
In the case of postoperative morbidity, a meta-analysis of 10 studies with 2161 cases was conducted. The RIS for the actual meta-analysis was 2248, estimated based on the predefined statistical indicators. TSA results demonstrated that the cumulative Z-value (Z-curve) crossed both the conventional boundary (D-curve) and the TSA boundary (B-curve), providing evidence of the superiority of the robotic group. However, the cumulative information size did not reach the required level (Fig. 7).
Fig. 7.
TSA for postoperative morbidity
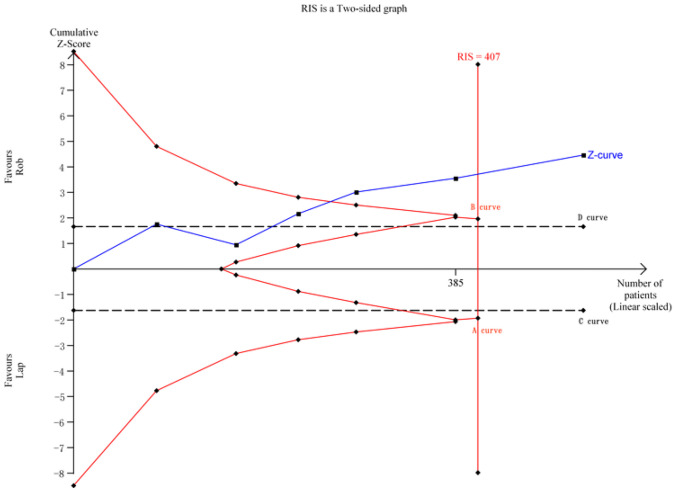
For harvested lymph nodes, a meta-analysis of 4 studies with 372 cases was performed. The RIS for the actual meta-analysis was 569, estimated based on the specified statistical indicators. TSA results indicated that the cumulative Z-value (Z-curve) crossed the conventional boundary (C-curve) and the TSA boundary (A-curve), providing evidence of the superiority of the robotic group. However, the cumulative information size did not reach the required level (Fig. 8).
Fig. 8.
TSA for harvested lymph nodes
Regarding circumferential resection margin positive rate, a meta-analysis of 7 studies with 1703 cases was conducted. The RIS for the actual meta-analysis was not specified in the provided text. TSA results showed that the cumulative Z-value (Z-curve) crossed the conventional boundary (D-curve) but did not surpass the TSA boundaries (A-curve and B-curve), indicating a higher possibility of false positives. Additional randomized controlled trials are required to further validate this outcome (Fig. 9).
Fig. 9.
TSA for circumferential resection margin positive rate
Discussion
Colorectal cancer (CRC) is one of the most prevalent malignant tumors globally, and its incidence is increasingly shifting toward a younger demographic [20, 21]. However, in contrast to colon cancer, surgery for middle and lower rectal cancer is more intricate, characterized by specific surgical requirements and operating environments [17, 22–24].
Total mesorectal excision (TME) is the gold standard for rectal cancer surgery, ensuring complete removal of the rectum and lymph nodes for a negative circumferential surgical margin [25]. Modern surgery prioritizes preserving pelvic nerve function, demanding exceptional skills due to the complex pelvic structure [26–29].
Entering the twenty-first century, minimally invasive surgery has become the main theme and inevitable trend in the field of colorectal surgery. Conventional laparoscopic systems have inherent drawbacks such as non-stereoscopic imaging technology, a steep learning curve, the “chopstick effect” leading to a phenomenon known as “clashing,” potential hand tremors, and the passive fatigue-inducing standing posture of the surgeon [30, 31]. These limitations have paved the way for the rise of robotic surgical systems, with the da Vinci Surgical System (DVSS) representing surgical robots that have gained global popularity due to their novel concepts and advanced technological advantages, overcoming the inherent deficiencies of laparoscopic technology [14, 32].
Controversies surround robot use in middle and lower rectal cancer treatment. Bulky robot size limits use to large operating rooms, posing challenges in surgeries on slender patients or involving multiple sites [33]. Lack of tactile feedback hinders surgeons’ sense of touch, risking tissue damage. Reliance on visual cues without tension control increases surgery complexity. Lengthy DVSS setup extends surgical time, reducing operating room efficiency. Wireless interference during surgery prolongs operation times [34, 35]. Long-term effects of robot-assisted therapy for rectal cancer are unclear. Our recent meta-analysis compared short-term and long-term outcomes of robot-assisted and laparoscopic surgery [36].
In analyzing the results, we found that robotic surgery does not increase the surgical time, achieving comparable postoperative recovery outcomes to pure laparoscopic surgery. Additionally, robotic surgery results in less intraoperative bleeding and a lower conversion rate to open surgery. On both proximal and distal resection margins, the robotic approach demonstrates similar effectiveness to laparoscopic surgery. Moreover, the robotic group exhibits a higher lymph node clearance, a lower circumferential resection margin positive rate, indicating that robotic rectal cancer surgery has better curative effects compared to laparoscopic surgery. The analysis also reveals that robotic surgery can achieve a 3-year overall survival rate and 3-year disease-free survival rate similar to laparoscopic surgery.
The robotic surgery system’s superiority lies in its simulated multi-degree-of-freedom mechanical arms, replicating human wrist articulation. The end effector’s unrestricted rotation in Rx.Ry.Rz directions enhances operational flexibility [37–39]. Automatic filtering of surgeon hand tremors and a motion calibration system ensure instrument stability, reinforcing surgical operation stability. In confined spaces, the system enables precise tasks like dissection, hemostasis, and suturing. A stable 10 × high-definition magnification offers a three-dimensional visual field, improving hand–eye coordination [40]. Ergonomic design follows human engineering principles, reducing fatigue, maintaining focus, and lowering error rates during complex procedures [41].
Overall, postoperative complications are crucial indicators for assessing the safety and feasibility of surgical procedures. Therefore, we further discussed the advantages and disadvantages of robotic and laparoscopic surgeries from the perspective of postoperative complications. The meta-analysis results indicate a lower incidence of postoperative complications and a lower rate of complications with Clavien–Dindo grade ≥ 3 for robotic surgery, consistent with findings from multiple studies. Consequently, we conclude that, compared to laparoscopic surgery, robotic rectal cancer surgery holds distinct advantages. Further subgroup analysis reveals that the robotic group has a lower rate of anastomotic leakage, a critical complication after radical rectal resection. Anastomotic leakage-induced acute diffuse peritonitis is the most severe complication following rectal surgery, leading to reoperation or even death [42]. Anastomotic leakage, usually caused by low anastomotic position, poor blood flow, tension, and local infection [43], occurs at a rate of 5.6% in the robotic group and 8.3% in the laparoscopic group in this meta-analysis. DVSS’s low anastomotic leakage is due to precise robotic arms, advanced imaging, and stability, ensuring accurate and stable anastomosis during the procedure.
Moreover, it is noteworthy that urinary complications are one of the parameters used to assess the protection of pelvic autonomous nerves during surgery. Although urinary complications are considered to be caused by various factors, surgical injury during the procedure is considered a major contributor, significantly impacting postoperative quality of life. Previous studies [44–47] suggested that robotic rectal surgery can significantly protect pelvic autonomous nerves due to its 10-fold magnification of the surgical field, leading to a substantial reduction in the incidence of postoperative urinary complications. However, in our meta-analysis, no statistically significant difference was observed between robotic and laparoscopic rectal surgeries regarding urinary complications.
This study has some limitations that should be noted. Firstly, the relatively small sample size included in the literature may limit a comprehensive evaluation of robotic treatment for low rectal cancer. Due to the limited sample size, the observation period of the study is relatively short, providing limited insight into long-term treatment outcomes. Long-term follow-up is crucial for evaluating the sustained effects of surgery and factors such as patient survival. Therefore, future research should focus on expanding the sample size and adopting a more extended observation period to obtain more comprehensive and reliable data.
Secondly, the lack of a large number of high-quality RCTs is also a limitation of the study. RCTs are generally considered the gold standard for assessing the effectiveness of treatment methods. Still, in this study, relevant RCT studies were relatively limited. This may introduce potential bias and uncertainty, affecting the accurate assessment of the effectiveness of robotic treatment. To draw more confident and reliable conclusions about the effectiveness of robotic treatment for low rectal cancer, future research needs more support from high-quality RCT study.
Given these limitations, we hope that future studies can overcome these challenges by expanding the sample size, extending the observation period, and increasing the number of RCT studies, providing more reliable and comprehensive clinical evidence. This will contribute to a deeper understanding of the actual effects of robotic treatment for low rectal cancer and offer more scientific and reliable guidance for clinical practice.
Conclusion
Robot-assisted laparoscopic treatment for mid and low rectal cancer yields favorable outcomes, demonstrating both efficacy and safety. In comparison to conventional laparoscopy, patients experience reduced intraoperative bleeding and a lower incidence of complications. Notably, the approach achieves comparable short-term and long-term treatment results to those of conventional laparoscopic surgery, thus justifying its consideration for widespread clinical application.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Huiming Wu and Renkai Guo (study supervision, study design); Huiming Wu (drafting the manuscript, statistical analysis acquisition of data, analysis and interpretation of data); Huiming Wu, Renkai Guo, and Huiyu Li (acquisition of data, analysis and interpretation of data). The authors read and approved the final manuscript.
Funding
This study was funded by “Key Research and Development (R&D) Projects of Shanxi Province” (2021XM22) and “Fundamental Research Program of Shanxi Province” (202103021224346) and Shanxi Province “136 Revitalization Medical Project Construction Funds” (2019XY005).
Availability of data and materials
Data sharing was not applicable to this article, as no datasets were generated or analyzed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zawadzki M, Rząca M, Czarnecki R, Obuszko Z, Jacyna K, Stewart L, Witkiewicz W (2014) Beginning robotic assisted colorectal surgery – it’s harder than it looks! Wideochir Inne Tech Maloinwazyjne 9(4):562–568. 10.5114/wiitm.2014.45494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park S, Kim NK (2015) The role of robotic surgery for rectal cancer: overcoming technical challenges in laparoscopic surgery by advanced techniques. J Korean Med Sci 30(7):837–846. 10.3346/jkms.2015.30.7.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu LD, Li XF, Wang XY, Guo TK (2016) Robotic versus laparoscopic gastrectomy for gastric carcinoma: a meta-analysis of efficacy and safety. Asian Pac J Cancer Prev 17(9):4327–4333 [PubMed] [Google Scholar]
- 4.Marano A, Hyung WJ (2012) Robotic gastrectomy: the current state of the art. J Gastric Cancer 12(2):63–72. 10.5230/jgc.2012.12.2.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J et al (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341 [DOI] [PubMed] [Google Scholar]
- 6.Clavien PA, Sanabria JR, Strasberg SM (1992) Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 111(5):518–526 [PubMed] [Google Scholar]
- 7.Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 20(5):13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedirli A, Salman B, Yuksel O (2016) Robotic versus laparoscopic resection for mid and low rectal cancers. JSLS 20(1):e2015.00110. 10.4293/JSLS.2015.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek SJ, Al-Asari S, Jeong DH, Hur H, Min BS, Baik SH, Kim NK (2013) Robotic versus laparoscopic coloanal anastomosis with or without intersphincteric resection for rectal cancer. Surg Endosc 27(11):4157–4163. 10.1007/s00464-013-3014-4 [DOI] [PubMed] [Google Scholar]
- 11.Feng Q, Ng SSM, Zhang Z, Lin S, Niu Z, Wei Y, He G, Chang W, Zhu D, Xu J (2021) Comparison between robotic natural orifice specimen extraction surgery and traditional laparoscopic low anterior resection for middle and low rectal cancer: a propensity score matching analysis. J Surg Oncol 124(4):607–618. 10.1002/jso.26552 [DOI] [PubMed] [Google Scholar]
- 12.Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y, Zhang W, Zhao R, Zhang C, Cheng L, Zhang X, Liang F, He G, Wei Y, Xu J, REAL Study Group (2022) Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): Short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 7(11):991–1004. 10.1016/S2468-1253(22)00248-5 [DOI] [PubMed] [Google Scholar]
- 13.Feroci F, Vannucchi A, Bianchi PP, Cantafio S, Garzi A, Formisano G, Scatizzi M (2016) Total mesorectal excision for mid and low rectal cancer: laparoscopic vs robotic surgery. World J Gastroenterol 22(13):3602–3610. 10.3748/wjg.v22.i13.3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YM, Huang YJ, Wei PL (2017) Outcomes of robotic versus laparoscopic surgery for mid and low rectal cancer after neoadjuvant chemoradiation therapy and the effect of learning curve. Medicine (Baltimore) 96(40):e8171. 10.1097/MD.0000000000008171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP (2013) Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc 27(1):48–55. 10.1007/s00464-012-2405-2 [DOI] [PubMed] [Google Scholar]
- 16.Serin KR, Gultekin FA, Batman B, Ay S, Kapran Y, Saglam S, Asoglu O (2015) Robotic versus laparoscopic surgery for mid or low rectal cancer in male patients after neoadjuvant chemoradiation therapy: comparison of short-term outcomes. J Robot Surg 9(3):187–194. 10.1007/s11701-015-0514-3 [DOI] [PubMed] [Google Scholar]
- 17.Tang B, Gao GM, Zou Z, Liu DN, Tang C, Jiang QG, Lei X, Li TY (2020) Efficacy comparison between robot-assisted and laparoscopic surgery for mid-low rectal cancer: a prospective randomized controlled trial. Zhonghua Wei Chang Wai Ke Za Zhi 23(4):377–383. 10.3760/cma.j.cn [DOI] [PubMed] [Google Scholar]
- 18.Yoo BE, Cho JS, Shin JW, Lee DW, Kwak JM, Kim J, Kim SH (2015) Robotic versus laparoscopic intersphincteric resection for low rectal cancer: comparison of the operative, oncological, and functional outcomes. Ann Surg Oncol 22(4):1219–1225. 10.1245/s10434-014-4177-5 [DOI] [PubMed] [Google Scholar]
- 19.Zou Z et al (2018) Short-term outcomes of robotic versus laparoscopic radical resection for middle and low rectal cancer: a single-center randomized, controlled study. Chin J Gen Surg 27(4):408–413 [Google Scholar]
- 20.Nishimura K (2015) Current status of robotic surgery in Japan. Korean J Urol 56(3):170–8. 10.4111/kju.2015.56.3.170. Epub 2015 Mar 3. PMID: 25763120; PMCID: PMC4355427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papanikolaou IG (2014) Robotic surgery for colorectal cancer: systematic review of the literature. Surg Laparosc Endosc Percutan Tech 24(6):478–483. 10.1097/SLE.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 22.Kim JC, Yang SS, Jang TY, Kwak JY, Yun MJ, Lim SB (2012) Open versus robot-assisted sphincter-saving operations in rectal cancer patients: techniques and comparison of outcomes between groups of 100 matched patients. Int J Med Robot 8(4):468–475. 10.1002/rcs.1452 [DOI] [PubMed] [Google Scholar]
- 23.Becker T, Egberts JE, Schafmayer C, Aselmann H (2016) Roboterassistierte Rektumchirurgie: Hype oder Fortschritt? [Robot-assisted rectal surgery: hype or progress?]. Chirurg 87(7):567–572. German. 10.1007/s00104-016-0220-3 [DOI] [PubMed]
- 24.Wang X, Cao G, Mao W, Lao W, He C (2020) Robot-assisted versus laparoscopic surgery for rectal cancer: a systematic review and meta-analysis. J Cancer Res Ther 16:979–989 [DOI] [PubMed] [Google Scholar]
- 25.Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J (2015) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta- analysis of eight studies. J Gastrointest Surg 19:516–526 [DOI] [PubMed] [Google Scholar]
- 26.Hoshino N, Sakamoto T, Hida K, Sakai Y (2019) Robotic versus laparoscopic surgery for rectal cancer: an overview of systematic reviews with quality assessment of current evidence. Surg Today 49:556–570 [DOI] [PubMed] [Google Scholar]
- 27.Jayne D, Pigazzi A, Marshall H et al (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 318:1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu H, Yu D, Ye S, Shan R, Ai J, Shi J (2020) Long-term oncological outcomes in robotic versus laparoscopic approach for rectal cancer: a systematic review and meta-analysis. Int J Surg 80:225–230 [DOI] [PubMed] [Google Scholar]
- 29.Glynne-Jones R, Wyrwicz L, Tiret E et al (2017) Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl 4):iv22–40 [DOI] [PubMed] [Google Scholar]
- 30.Kuo LJ, Lin YK, Chang CC, Tai CJ, Chiou JF, Chang YJ (2014) Clinical outcomes of robot-assisted intersphincteric resection for low rectal cancer: comparison with conventional laparoscopy and multifactorial analysis of the learning curve for robotic surgery. Int J Colorectal Dis 29(5):555–562. 10.1007/s00384-014-1841-y [DOI] [PubMed] [Google Scholar]
- 31.Barrie J, Jayne DG, Wright J, Murray CJ, Collinson FJ, Pavitt SH (2014) Attaining surgical competency and its implications in surgical clinical trial design: a systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann Surg Oncol 21(3):829–840. 10.1245/s10434-013-3348-0 [DOI] [PubMed] [Google Scholar]
- 32.Sawada H, Egi H, Hattori M, Suzuki T, Shimomura M, Tanabe K, Okajima M, Ohdan H (2015) Initial experiences of robotic versus conventional laparoscopic surgery for colorectal cancer, focusing on short-term outcomes: a matched case-control study. World J Surg Oncol 12(13):103. 10.1186/s12957-015-0517-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HJ, Choi GS, Park JS, Park SY (2014) Comparison of surgical skills in laparoscopic and robotic tasks between experienced surgeons and novices in laparoscopic surgery: an experimental study. Ann Coloproctol 30(2):71–76. 10.3393/ac.2014.30.2.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber PA, Merola S, Wasielewski A, Ballantyne GH (2002) Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 45(12):1689–1694; discussion 1695–1696. 10.1007/s10350-004-7261-2 [DOI] [PubMed]
- 35.Gladyshev DV, Kovalenko SA, Moiseev ME, Gnedash SS, Karachun AM, Kotiv BN, Shelegetov DS (2015) Comparative analysis of immediate results of surgery for colon cancer using laparoscopic and robot-assisted surgical interventions. Vopr Onkol 61(6):937–940. Russian [PubMed]
- 36.Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP (2012) Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 99(9):1219–1226. 10.1002/bjs [DOI] [PubMed] [Google Scholar]
- 37.Rondelli F, Balzarotti R, Villa F, Guerra A, Avenia N, Mariani E, Bugiantella W (2015) Is robot-assisted laparoscopic right colectomy more effective than the conventional laparoscopic procedure? A meta-analysis of short-term outcomes. Int J Surg 18:75–82. 10.1016/j.ijsu.2015.04.044 [DOI] [PubMed] [Google Scholar]
- 38.Lim DR, Min BS, Kim MS, Alasari S, Kim G, Hur H, Baik SH, Lee KY, Kim NK (2013) Robotic versus laparoscopic anterior resection of sigmoid colon cancer: comparative study of long-term oncologic outcomes. Surg Endosc 27(4):1379–1385. 10.1007/s00464-012-2619-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pai A, Marecik SJ, Park JJ, Melich G, Sulo S, Prasad LM (2015) Oncologic and clinicopathologic outcomes of robot-assisted total mesorectal excision for rectal cancer. Dis Colon Rectum 58(7):659–667. 10.1097/DCR.0000000000000385 [DOI] [PubMed] [Google Scholar]
- 40.Mirnezami AH, Mirnezami R, Venkatasubramaniam AK, Chandrakumaran K, Cecil TD, Moran BJ (2010) Robotic colorectal surgery: hype or new hope? A systematic review of robotics in colorectal surgery. Colorectal Dis 12(11):1084–1093. 10.1111/j.1463-1318.2009.01999.x [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Kim MJ, Park SC, Sohn DK, Kim DY, Chang HJ, Nam BH, Oh JH (2016) Robotic versus laparoscopic surgery for rectal cancer after preoperative chemoradiotherapy: case-matched study of short-term outcomes. Cancer Res Treat 48(1):225–231. 10.4143/crt.2014.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Xu H, Li Z, Han J, Song W, Wang J, Xu Z (2016) Robotic versus laparoscopic low anterior resection for rectal cancer: a meta-analysis. World J Surg Oncol 1(14):61. 10.1186/s12957-016-0816-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Zhao GH, Yang H, Lin J (2016) A pooled analysis of robotic versus laparoscopic surgery for total mesorectal excision for rectal cancer. Surg Laparosc Endosc Percutan Tech 26(3):259–264. 10.1097/SLE.0000000000000263 [DOI] [PubMed] [Google Scholar]
- 44.Xu JM, Wei Y, Wang XY, Fan H, Chang WJ, Ren L, Jiang W, Fan J, Qin XY (2015) Robot-assisted one-stage resection of rectal cancer with liver and lung metastases. World J Gastroenterol 21(9):2848–2853. 10.3748/wjg.v21.i9.2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bedirli A, Salman B (2015) Robotic surgery for rectosigmoid junction tumor with ovarian metastases. J Minim Access Surg 11(1):99–102. 10.4103/0972-9941.147720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marecik SJ, Zawadzki M, Desouza AL, Park JJ, Abcarian H, Prasad LM (2011) Robotic cylindrical abdominoperineal resection with transabdominal levator transection. Dis Colon Rectum 54(10):1320–1325. 10.1097/DCR.0b013e31822720a2 [DOI] [PubMed] [Google Scholar]
- 47.Liao G, Zhao Z, Lin S, Li R, Yuan Y, Du S, Chen J, Deng H (2014) Robotic-assisted versus laparoscopic colorectal surgery: a meta-analysis of four randomized controlled trials. World J Surg Oncol 26(12):122. 10.1186/1477-7819-12-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing was not applicable to this article, as no datasets were generated or analyzed during the current study.