Abstract
Mycobacteroides abscessus (Mab, also known as Mycobacterium abscessus) can cause chronic pulmonary disease in the setting of structural lung conditions. Current treatment recommendations require at least one year of daily therapy with repurposed antibiotics. Yet these therapies are often ineffective and associated with significant adverse events. To address this challenge, research efforts are underway to develop new antibiotics and regimens. During the preclinical phase of treatment development, experimental agents require testing and comparison alongside positive controls that are known agents with clinical history. As there are no FDA approved treatments for this indication, here, we have considered repurposed antibiotics currently included in the recommendation for treating Mab disease as candidates for selection of an ideal standard comparator that can serve as a positive control in preclinical studies. Clofazimine meets the criteria for an ideal positive control as it can be administered via the least invasive route, requires only once-daily dosing, is well tolerated, and is widely available in high purity from independent sources. Using a mouse model of pulmonary Mab disease, we assessed for ideal dosages of clofazimine in C3HeB/FeJ and BALB/c mice in a six-week treatment window. Clofazimine, 25 mg/kg, once daily, produced desired reduction in Mab burden in the lungs of C3HeB/FeJ and BALB/c. Based on these findings, we conclude that clofazimine meets the criteria for a positive control comparator in mice for use in preclinical efficacy assessments of agents for treatment of Mab pulmonary disease. Although not included in the current standard-of-care for treating Mab disease, rifabutin, 20 mg/kg, also produced desired Mab lung burden in C3HeB/FeJ mice but not in BALB/c mice.
Keywords: M. abscessus, experimental therapeutics, clofazimine, rifabutin, positive control
1. INTRODUCTION
Mycobacteroides abscessus (Mab) can cause opportunistic pulmonary infections in patients with chronic lung conditions such as cystic fibrosis, bronchiectasis, and chronic obstructive pulmonary disease [1,2]. It is also known to cause diseases in the skin and soft tissues and can be fatal if it disseminates [3–7]. Mab lung disease is often misdiagnosed as tuberculosis as clinical presentations of Mab, and Mycobacterium tuberculosis lung diseases bear significant resemblance. Mab was recently reclassified into genus Mycobacteroides due to its distinct genetic differences from members of the Mycobacterium genus [8]. The Mab species is further classified into three sub-species- abscessus, massiliense, and bolletii [9]. A higher incidence of subsp. abscessus and subsp. massiliense was reported in the US and East Asia, while the incidence of infections with subsp. bolletii was rare [10]. Variations in the subsp. incidence rates between different geographical regions have also been reported [11]. The incidence of Mab infections has been gradually increasing in the US [12–14].
Mab infection, like tuberculosis, is a chronic infection and is treated with a regimen containing a cocktail of antibiotics given in phases that last for at least 12 months [15–17]. Despite the multi-drug therapy that is continually administered for a long duration, the treatment success rate is 30–50%, and the culture conversion rate is between 25% and 88% [11,18–22]. Several factors are considered pertinent to poor outcomes of current treatment regimens. First, the minimum inhibitory concentration (MIC) of most of the antibiotics available today is higher than what is physiologically attainable in human blood, and hence Mab is considered to be intrinsically resistant to most antibiotics [16,23–26]. Second, the current recommendations are not based on clinical trials and comprise of repurposed antibiotics that are not approved by the FDA for this indication [16,17]. Third, significant adverse events have been reported for several antibiotics included in the treatment regimens necessitating careful monitoring of patient response and revision of prescriptions [27,28]. Hence, patients with Mab infection have limited treatment options. To address this challenge, efforts at developing new antibiotics have begun in earnest.
Naturally, the first steps towards developing new treatments for Mab disease have focused on in vitro evaluations of activities of new agents and those approved for other indications. These studies have identified several new candidates such as PIPD1, an indole-2-carboxamide [29–32], epetraborole [33,34], oxazolidinones LCB01–0371 [35] and tedizolid [36], and a penem T405 [37–39]. In addition, tetracyclines such as omadacycline and eravacycline [40–45] and a spectrum of β-lactams have been evaluated for their activities against Mab [46–50]. β-lactamase inhibitors such as sulbactam, tazobactam, clavulanate, relebactam, vaborbactam, and avibactam have been evaluated as companions to β-lactams against Mab [51–55]. For some of these candidates, preclinical efficacy evaluations in animal models that mimic key features of Mab disease in humans have been described [31,33,34,45,47,56,57]. Recently, phage therapy has surfaced as a potential treatment option for multi-drug resistant Mab infections and is being tested in clinical settings [58–60]. New combination regimens comprising of repurposed drugs are also being evaluated against Mab [32,47,57,61]. It is necessary to compare efficacy of an experimental agent with that of a known standard-of-care drug to assess if desirable clinical activity can be expected from the experimental agent.
The following practical therapeutic attributes are desirable in a positive control comparator antibiotic for use in preclinical efficacy assessment studies [62,63]: (i) requires low dosing frequency (once daily or less): To reduce variability in the experiment due to multiple interferences during dosing and to introduce the least amount of stress to animals, once daily or lower frequency of administration would be ideal for preclinical use. A low dosing frequency is also less effort- and resource-intensive and, therefore, logistically desirable. (ii) produces tolerable adverse events in animal models: Antibiotics that cause adverse events in animal models make it difficult to delineate the desirable targeted effect of the drug from its undesirable in vivo toxicity. The use of such drugs would thus, reduce confidence in the experiment and interfere with the interpretation of the antibiotic efficacy. Therefore, such antibiotics would not be preferred as comparator control antibiotics. (iii) is reliably available from multiple independent sources: For the antibiotic to be adopted and used widely and sustainably as a comparator control, it must be reliably available in high purity from independent vendors. This enables independent laboratories to undertake efficacy evaluations and contribute to the development of new therapeutics to treat Mab disease. (iv) can be administered via the least invasive and most convenient route in the intended model animal: we reviewed the literature for precedent for the types of model animals used in mycobacterial research and for routes of drug administration. Although larger animals such as guinea pigs, rabbits and macaques are also used, mice have been the choice in overwhelming number of studies and have successfully predicted drug and regimen efficacies in humans [64–66].
There is rich literature for preclinical drug development for diseases caused by Mycobacterium tuberculosis, an organism related to Mab. In these studies, standard-of-care antibiotics like isoniazid, rifampin, and pyrazinamide have been effectively used as positive control comparators against experimental agents [67–71]. The use of such controls has enabled harmonizing of protocols across laboratories, which has allowed for a more reliable comparison of findings. These antibiotics are administered orally. While intravenous route bypasses the need for adsorption, and there are several anti-tuberculosis drugs that are administered intravenously in humans, such as amikacin, capreomycin, kanamycin and meropenem, intravenous administration in mice is extremely labor intensive as locating and reliably injecting via the tail vein in large numbers of mice, each day for several weeks is logistically impractical and undesirable. Other routes of injection such as subpleural and subcutaneous can be undertaken reliably, but daily injections over several weeks often leads to puncture wounds and ulcers due to repeated trauma at the sites of injection. Perhaps because of these reasons, oral routes have been used in the overwhelming majority of efficacy evaluations against mycobacterial diseases.
We have observed the disadvantages of long-term injections through our previous studies on a model of pulmonary Mab disease using the C3HeB/FeJ mouse strain [72]. In some of these studies, imipenem, a parenteral drug, was used and had to be administered via subcutaneous injections in mice, twice-daily for four-weeks [39,45]. While feasible, injecting large numbers of mice daily for several weeks was far more resource and time intensive compared to similar treatments delivered via oral gavage in mice. For instance, in evaluation of efficacy of an experimental agent T405 [39], 196 needle-syringe combinations (single-use) were required. In comparison, only one oral gavage needle would be sufficient for the same study if the drug could be administered orally as the gavage needle can be rinsed and sterilized in 70% ethanol after each use. We also observed fibrosis of skin tissue at injection sites, necessitating administration via other sites. Oral route of administration (voluntary consumption, orogastric or nasogastric gavage) overcomes these challenges, is less invasive and, less resource- and time-intensive.
Various antibiotics are repurposed as standard-of-care for Mab disease, including amikacin, azithromycin, bedaquiline, cefoxitin, ciprofloxacin, clarithromycin, clofazimine, doxycycline, ethambutol, imipenem, linezolid, minocycline, moxifloxacin, omadacycline, tigecycline, and trimethoprim [16,17]. Prior preclinical efficacy studies have used different comparator antibiotics in different dosages such as amikacin 150 mg/kg [31,57], or 250 mg/kg [33], clarithromycin 200 mg/kg [35], imipenem 200 mg/kg administered twice daily [39,45], linezolid 100 mg/kg, and rifabutin (10 mg/kg) [34]. Amikacin and imipenem are both injectable, and therefore, they also are less convenient than oral drugs for administration in mice. Clarithromycin and linezolid are used in very high doses and given twice daily, hence do not meet the criteria. The use of different antibiotics has made it challenging to harmonize protocols and compare efficacies of experimental agents against Mab across preclinical studies. Therefore, there is a need to identify a drug with ideal features of a positive control comparator that can be included in preclinical efficacy evaluations of experimental agents against Mab.
The aim of this study is to identify one standard-of-care drug that meets the above-mentioned criteria and can serve as a positive control comparator in efficacy studies against Mab disease. We considered all antibiotics included in the current standard-of-care for Mab disease treatment and assessed them against the above-mentioned criteria. Clofazimine and moxifloxacin meet all criteria. We evaluated the efficacy of clofazimine against Mab infections in two mouse strains that have been extensively used in preclinical studies of mycobacterial infections, including Mab: C3HeB/FeJ [47,57,72–75] and BALB/c [69,76]. Based on the findings, we describe clofazimine dose and dosing frequency for preclinical efficacy testing of experimental agents against Mab disease. This satisfied the overall aim of this study to identify one standard-of-care drug with ideal features to serve as a positive control comparator. Although rifabutin is not included in the current recommendations to treat Mab disease, there is an increasing interest in repurposing this drug to treat Mab disease [77]. Therefore, we also evaluated rifabutin against Mab pulmonary infections in C3HeB/FeJ and BALB/c mice to assess for attributes of an ideal positive control comparator.
2. MATERIALS AND METHODS
2.1. Bacterial strains and in vitro growth conditions.
M. abscessus strain ATCC 19977, which has been historically considered a reference Mab strain, was used [78]. This strain was procured from ATCC (Manassas, VA) and authenticated by genome sequencing [48]. Middlebrook 7H9 broth (catalog no. 271310, Difco) supplemented with 0.5% glycerol, 10% albumin-dextrose-salt enrichment and 0.05% Tween-80 was used to culture Mab as described [79]. Mab cultures were grown in an orbital shaker at 220 RPM, 37 °C. Middlebrook 7H11 selective agar (catalog no. 283810, Difco), supplemented with 0.5% glycerol, 10% albumin-dextrose-salt enrichment, 0.05% Tween-80, 50 μg/ml carbenicillin (catalog no. C46000, Research Products International) and 50 μg/ml cycloheximide (catalog no. C7698, Sigma-Aldrich) was used to recover Mab from mouse lung homogenates. When grown on Middlebrook 7H11 agar, both rough and smooth colonies of Mab ATCC 19977 were observed.
2.2. Efficacy determination in mice.
C3HeB/FeJ mice (Jackson Laboratories, Bar Harbor, ME) and BALB/c mice (Charles River Laboratories), 5–6 weeks old, female, were used as described in the protocol for the mouse model of pulmonary Mab infection used in this study [72] and in studies in which imipenem was used as a positive control comparator [45,47]. Only female mice were used as in our experience male mice exhibit aggressive behavior including biting the handler. As the study requires daily administration of drugs in large numbers of mice, inclusion of only females allowed us to exclude avoidable challenges and undertake the studies in a safe manner. Beginning a week prior to infection and continuing throughout the study, 5 mg/kg/day dexamethasone was administered to each mouse as specified in the publication that described this mouse model of pulmonary Mab infection [37]. Dexamethasone (catalog no. D1756, Sigma-Aldrich) was dissolved in sterile 1x phosphate buffered saline, pH 7.4 (catalog no. 114-058-101CS, Quality Biologicals) to a concentration of 1.25 mg/ml and 0.1 ml bolus was administered once daily, seven days a week, by subcutaneous injection into the hind dorsal tissue using 27-gauge needle (catalog no. 305620, Beckton and Dickinson). Mab was grown to exponential phase in Middlebrook 7H9 broth and was used to prepare a 10 ml infecting suspension at an optical density, A600nm = 0.1. For the pilot study in which treatments were administered for three weeks, C3HeB/FeJ (n=60) were infected simultaneously with aerosolized suspension of Mab in an inhalation chamber according to manufacturer’s guidelines (Glas-Col, Terre Haute, IN). The infection cycle included preheating for 15 min, aerosol nebulization for 30 min, and cloud decay for 30 min, followed by surface decontamination for 15 min. Separately BALB/c (n=60) mice were infected using the identical protocol. Five mice were allocated for determination of Mab implantation following infection and five additional mice were allocated for determination of Mab burden at one week following infection, the time at which antibiotic treatment was initiated. One week following infection, mice were randomly allocated into five groups of 10 mice per group. Treatment was administered once daily, and 0.2 ml bolus of each agent was administered via oral gavage. Mice in the first group were administered 1x PBS, pH 7.4, as this buffer was used as the solvent for clofazimine and rifabutin, and therefore represent the negative control group. Mice in the second and third groups received 25 and 50 mg/kg clofazimine, respectively. Similarly, mice in the fourth and fifth groups received 10 and 20 mg/kg rifabutin. Mab burden in the lungs of mice were determined at 24 hours post-infection (designated week −1), one week following infection (week 0), and at one- and three weeks following treatment initiation (designated week +1 and +3, respectively).
Similarly, in the subsequent study in which treatments were administered for six weeks, C3HeB/FeJ (n=55) and BALB/c (n=55) were infected simultaneously with aerosol of Mab. Five mice were allocated for determination of Mab implantation following infection and five additional mice were allocated for determination of Mab burden at one week following infection. At this time, the remaining 45 mice were randomly allocated into three groups of 15 mice per group and antibiotic treatment was initiated. 1xPBS, pH 7.4, was administered to the first group, 25 mg/kg clofazimine was administered to the second group and 20 mg/kg rifabutin was administered to the third group.
Clofazimine was procured from Sigma-Aldrich (catalog no. C8895) and rifabutin was procured from Octagon Chemicals Limited (CAS# 72559-06-9). Suspensions of these drugs were prepared in 0.05% agarose as described [80]. To prepare 0.05% agarose, 500 mg Bacto Agar (catalog no. 214010, Becton Dickinson) was added to 1000 ml 1x PBS, pH 7.4, autoclaved for 10 minutes and cooled to room temperature. To deliver 25- and 50 mg/kg doses of clofazimine, 3.125- and 6.25 mg/ml suspensions were prepared, respectively, and 0.2 ml was administered to each mouse via oral gavage, once daily, seven days a week. Similarly, 1.25- and 2.5 mg/ml rifabutin suspensions prepared in 0.05% agarose were used to deliver 10 and 20 mg/kg rifabutin, respectively.
Five mice were sacrificed per time point per group, lungs were obtained and homogenized in a tube containing 900 μl 1x PBS, pH 7.4, and 0.2 mm glass beads in a mechanical homogenizer (Minilys, Bertin Technologies) at 5,000 RPM for 0.5 min. 100 μl of undiluted lung homogenates and 10-fold dilutions prepared in 1x PBS, pH 7.4 were inoculated onto Middlebrook 7H11 selective agar, incubated at 37 °C for 5 days and CFU was enumerated. . CFU counts from each mouse lung were converted into CFU per lung, comprising the average of three consecutive steps of a 10-fold dilution series of a given lung sample. Mean CFU ± standard error of the mean (SEM) in five mice per group per time point was plotted to determine the determine Mab burden in the lungs of mice.
2.3. Data and Statistics:
Mab CFU burden in the lungs of each mouse (n=5 per group, per time-point) was determined as described above. Mean Mab lung CFUs data was graphed as a function of time ± SEM. Statistical comparisons of CFUs at end time-point between different PBS vs treatment groups were performed by unpaired one-tailed t-test. Significance was determined at 95% confidence intervals (p<0.05 was considered significant). One-tailed t-tests were used to determine whether the performance of treated group was higher than the untreated control group (PBS), thus indicating the efficacy of the tested drugs (clofazimine or rifabutin).
2.4. Ethics.
Animal procedures used in the studies described here were performed in adherence to the Johns Hopkins University Animal Care and Use Committee and to the national guidelines.
3. RESULTS
3.1. Criterial for consideration as a positive control comparator:
The antibiotics used in the current regimens to treat Mab disease and whether they meet each of the above-mentioned criteria for an ideal positive control comparator are listed in Table 1 [16,17]. As clofazimine met all criteria and is one of the first-line antibiotics recommended to treat Mab disease, it was selected as a candidate for dose-efficacy evaluations in the pulmonary Mab infection models in C3HeB/FeJ and BALB/c mice [72]. Prior studies have reported once-daily 20 mg/kg clofazimine to be effective at reducing Mab burden in the lungs of mice [56,81]. A dose range of 25–100 mg/kg was demonstrated to be efficacious against M. tuberculosis infection in mice [82]. Independent studies have reported clofazimine MIC of 0.25–4.0 μg/ml against Mab isolates [25,83–87]. Current guidelines for treatment of Mab lung disease recommend once daily, 1.5 mg/kg, clofazimine in humans [88]. Using validated methods to determine animal equivalent dose (AED) [89], we determined that for clofazimine, a dose of ~19 mg/kg, once daily, is equivalent in C3HeB/FeJ and BALB/c mice. According to this method, AED = Human dose (mg/kg) x Km ratio (for mouse Km ratio is 12.3). Based on these prior findings, we selected two dosages of clofazimine for further evaluation: a lower dose of 25 mg/kg and a higher dose of 50 mg/kg. Evaluations of clofazimine in C3HeB/FeJ and BALB/c mice was undertaken in two phases. In the initial phase, the pilot study, we limited the duration of efficacy evaluation to three weeks of antibiotic treatment as the goal was to identify the lower dose of clofazimine, between 25- and 50 mg/kg, which would produce a reduction in Mab burden in the lungs of these mice.
Table 1:
Selection of ideal positive comparator control from antibiotics considered for treating Mab disease*
| Drug Name | Dosing Frequency | Adverse Events | Availability | Route of Administration |
|---|---|---|---|---|
| First-line antibiotics | ||||
| Amikacin | Once/Twice daily | High | Yes | Parenteral |
| Azithromycin† | Once daily | Moderate | Yes | Oral |
| Bedaquiline | Once daily | High | Yes | Oral |
| Cefazolin | >Twice daily | Low | Yes | Parenteral |
| Cefoxitin | >Twice daily | High | Yes | Parenteral |
| Clarithromycin† | Twice daily | High | Yes | Oral |
| Clofazimine | Once daily | Low | Yes | Oral |
| Imipenem | Twice daily | Low | Parenteral | |
| Linezolid | Once/Twice daily | High | Yes | Oral |
| Omadacycline | Once daily | Low | # | Oral |
| Rifampicin | Once daily | Moderate | Yes | Oral |
| Tedizolid | Once daily | High | Yes | Oral |
| Tigecycline | Twice daily | Moderate | Yes | Parenteral |
| Additional antibiotics | ||||
| Ceftazidime | Twice daily | High | Yes | Oral |
| Ciprofloxacin | Once daily | High | Yes | Oral |
| Doxycycline | Twice daily | Moderate | Yes | Oral |
| Ethambutol | Twice daily | High | Yes | Oral |
| Minocycline | Twice daily | High | Yes | Oral |
| Moxifloxacin | Once daily | Low | Yes | Oral |
| Rifabutin | Once daily | Low | Yes | Oral |
| Trimethoprim/co-trimoxazole | Twice daily | High | Yes | Oral |
Green color highlight indicates that antibiotic qualifies for the respective criteria
Red color highlight indicates disqualification
induces macrolide resistance in Mab subsp. abscessus and subsp. bolletii
Insufficent data
Information presented in this table have been obtained from published references [16,17,21,88,90]. The information presented in this table reflect activities in human populations. Low adverse events refer to tolerable effects like gastrointestinal discomfort, mild diarrhoea, that allow these drugs to be suitable for longer treatment durations. Drugs exhibiting moderate and high adverse events refer to side-effects that are intolerable (acute and chronic)- like renal, hepatic, vestibular, or auditory impairment, congenital effects (in pregnant women), hypersensitivity reactions, seizure, neuropathy (optic/peripheral).
3.2: Clofazimine, 25- and 50 mg/kg; treatment duration three weeks:
The mean Mab lung burdens at implantation (week −1) were 2.82 and 3.39 log10 CFU in C3HeB/FeJ and BALB/c mice, respectively (Figure 1A and 1B). One week following infection, when antibiotic treatment was initiated (week 0), the mean Mab lung burdens were 3.99 and 4.66 log10 CFU in C3HeB/FeJ and BALB/c mice, respectively. At this time, mice were randomly allocated to antibiotic treatment groups, with ten mice per group, and once-daily antibiotic treatment was initiated. Mice in the negative control group were administered PBS as it was used as the vehicle to prepare clofazimine formulations. Mice in the clofazimine treated group were administered either 25 or 50 mg/kg clofazimine. Mab lung burden steadily increased throughout the duration of the study in both C3HeB/FeJ and BALB/c mice treated with PBS, thereby reproducing prior observations [45,47,72].
Figure 1. Burden of M. abscessus in the lungs of C3HeB/FeJ and BALB/c mice treated with clofazimine for three weeks.
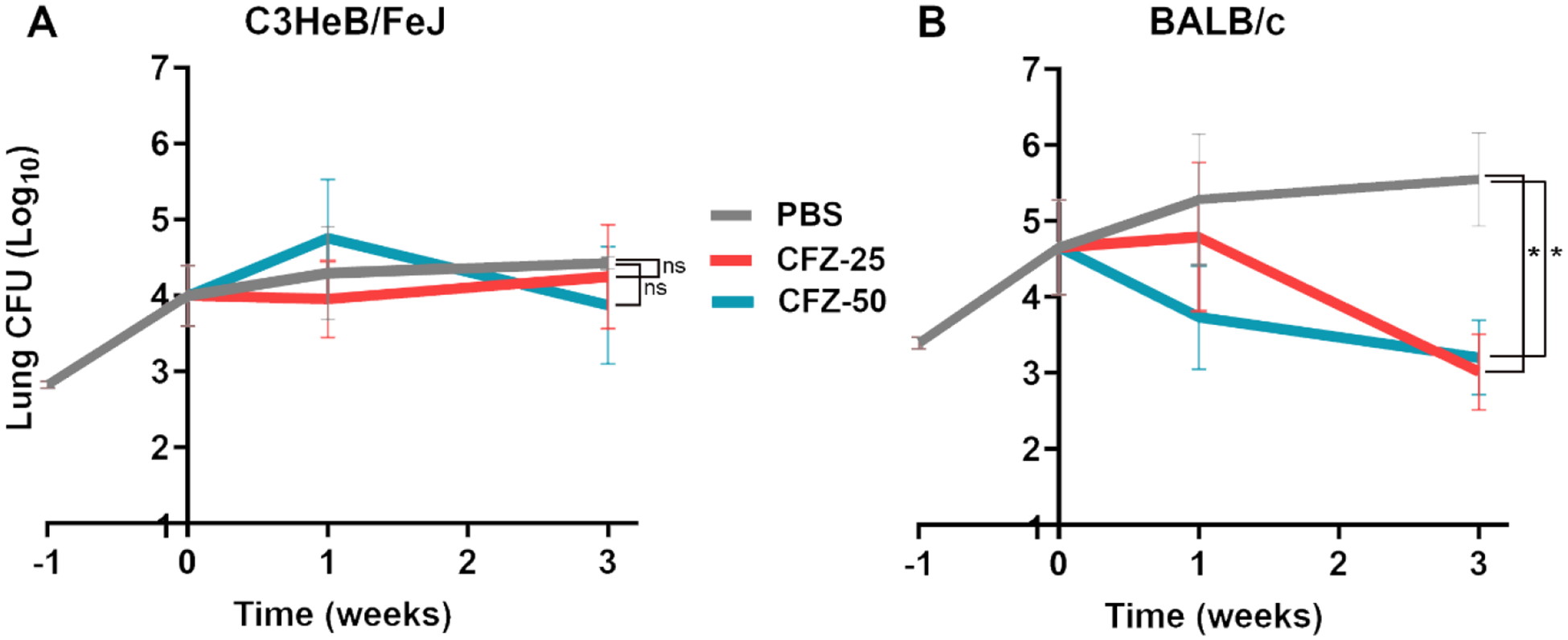
Mean (±SEM) Mab lung burden (log10 CFU) in A) C3HeB/FeJ and B) BALB/c mice, n=5 per time-point, per treatment group, treated with 25 or 50 mg/kg clofazimine are shown in red and light blue, respectively. Mean (±SEM) Mab lung burden in the negative control group, administered with phosphate buffered saline (PBS), is shown in grey. Time-point week −1 represents the day following infection with Mab. Time-point week 0 represents the day antibiotic administration was initiated. CFZ-25, once daily 25 mg/kg clofazimine; CFZ-50, once daily 50 mg/kg clofazimine. P-values of PBS vs. each treatment group are represented as stars: * represents p ≤0.05; ‘ns’ represents p>0.05 (not significant).
In C3HeB/FeJ mice treated with 25-or 50 mg/kg clofazimine, Mab lung burden failed to decline during the three-week treatment duration of this study (Figure 1A). At the three-week time point, the mean Mab lung burden of C3HeB/FeJ mice treated with 25- or 50 mg/kg clofazimine was statistically insignificant compared to that of PBS-treated mice as determined by one-tailed t-test with p=0.3973 and p=0.2444, respectively. In BALB/c mice treated with 25 mg/kg clofazimine, Mab lung burden remained steady at the end of first week but was ~1.64 log10 lower at the end of the third week of treatment. Whereas 50 mg/kg clofazimine produced a consistent reduction in lung Mab CFU throughout the treatment period (Figure 1B). At the end of third week, the mean Mab lung CFU in BALB/c mice treated with 25 mg/kg or 50mg/kg clofazimine group was statistically significant compared to the PBS group, as determined by one-tailed t-test with p=0.0159 and p= 0.0317, respectively.
Based on the Mab lung CFU reduction during the later stage of treatment in BALB/c mice treated with 25 mg/kg of clofazimine (Figure 1B), and prior reports of delayed bactericidal response in mice infected with M. tuberculosis [82], we hypothesized that the lower dose of clofazimine considered here, 25 mg/kg, might be sufficient to exhibit efficacy if administered for a longer treatment duration. Therefore, we considered 25 mg/kg clofazimine for further evaluation.
The existing recommendations for treating Mab lung disease require administration of antibiotics for one year or more [16,17]. For efficacy evaluations of experimental agents in mice to be informative for studies in humans, it is likely that Mab burden in the lungs of mice will need to be evaluated for an extended duration. Therefore, in the second phase of the study, clofazimine administration duration was extended to six week and the efficacy of once daily 25 mg/kg oral treatment was evaluated in both C3HeB/FeJ and BALB/c mice.
3.2. Clofazimine, 25 mg/kg; treatment duration six weeks:
In this study, the mean Mab lung burdens at implantation (week −1) were 3.21 and 3.15 log10 CFU in C3HeB/FeJ and BALB/c mice, respectively (Figure 2). One week following infection, when antibiotic treatment was initiated (week 0), the mean Mab lung burdens were 3.75 and 3.80 log10 CFU in C3HeB/FeJ and BALB/c mice, respectively. In BALB/c mice, compared to that in three-week study (Figure 1b), the initial inoculum was slightly lower and so was the CFU at the initiation of treatment. Although exact protocol was used for infection, this level of variation can be expected in biological repeats. At this time, mice were randomly allocated to different treatment groups, with 15 mice per group, and once-daily treatment was initiated. As in the first study, mice that were administered PBS represent the negative control group. To mice in the test group, 25 mg/kg clofazimine was administered once daily. Mab lung burden steadily increased throughout the duration of the study in both C3HeB/FeJ and BALB/c mice that received PBS (Figure 2A and 2B), thereby reproducing prior observations [45,47,72].
Figure 2. Burden of M. abscessus in the lungs of C3HeB/FeJ and BALB/c mice treated with 25mg/kg clofazimine for six weeks.
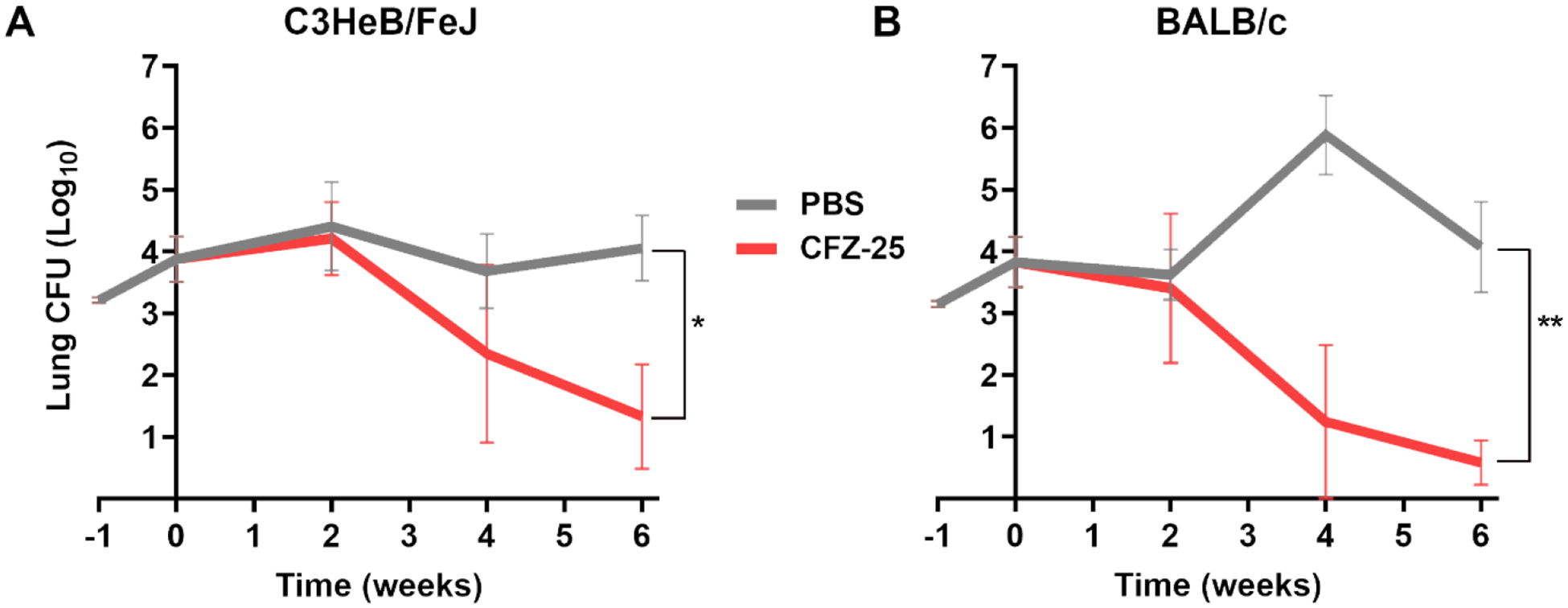
Mean (±SEM) Mab lung burden (log10 CFU) in A) C3HeB/FeJ and B) BALB/c mice, n=5 per time-point, per treatment group, treated with 25mg/kg clofazimine is shown in red. Mean (±SEM) Mab lung burden in the negative control group, administered with phosphate buffered saline (PBS), is shown in grey. Time-point week −1 represents the day following infection with Mab. Time-point week 0 represents the day antibiotic administration was initiated. CFZ-25, 25 mg/kg clofazimine. P-values of PBS vs each treatment group are represented as stars: * represents p ≤0.05; ** represents p ≤0.01.
In C3HeB/FeJ mice treated with 25 mg/kg clofazimine, there was a slight increase in mean lung Mab burden at two weeks followed by 1.41 log10 (compared to week 0) and an additional 1 log10 decline by four- and six-week time-points, respectively. Overall, 25 mg/kg clofazimine produced 2.42 log10 reduction in lung Mab burden in C3HeB/FeJ mice over the six-week treatment period (Figure 2A). In BALB/c mice, 25 mg/kg clofazimine produced 3.22 log10 reduction in Mab burden during the period and therefore exhibited activity similar to that in C3HeB/FeJ mice (Figure 2B). At six weeks, the average Mab lung CFU in mice treated with 25 mg/kg clofazimine in both mice strains was statistically significant compared to PBS group, as determined by one-tailed t-test with p= 0.0130 for C3HeB/FeJ mice and p= 0.0014 for BALB/c mice.
Apart from clofazimine, we also investigated, rifabutin, one of second-line antibiotics that met all four criteria of an ideal positive comparator (Table 1). A recent study demonstrated that 10 mg/kg rifabutin, administered once daily, reduces Mab burden in the lungs of mice [91]. Studies evaluating rifabutin efficacy against Mycobacterium avium have reported the effective dose range to be 5–40 mg/kg, once daily [92–94]. Independent publications have reported MIC of rifabutin of 0.25–32 μg/ml against Mab [61,91,95]. Based on the prior efficacy finding against Mab and M. avium infections and the wide range of MIC, we selected 10 mg/kg as the lower and 20 mg/kg as the higher dose of rifabutin for evaluation in C3HeB/FeJ and BALB/c mice infected with Mab. Similar to the clofazimine study, efficacies of these two doses were evaluated with a treatment duration of three weeks. One dose was selected from this study and subsequently evaluated in both mouse strains over a treatmen duration of six weeks.
3.3. Rifabutin, 10mg/kg and 20 mg/kg; treatment duration three weeks:
Mab lung burden failed to decrease in C3HeB/FeJ or BALB/c mice treated with 10 mg/kg rifabutin (Figure 3A & 3B). In C3HeB/FeJ mice, while the mean lung burden in 20 mg/kg rifabutin treated group was statistically insignificant at three-week time-point as determined by one-tailed t-test; p=0.0520, this was barely below the 95% confidence interval. Overall, a reduction of 1.58 log10 CFU occurred in the lungs of these mice at the culmination of three weeks of treatment (Figure 3A). In BALB/c mice treated with 20 mg/kg rifabutin, while lung Mab CFU increased at the end of the first week of treatment, in the following two weeks, there was a 1.83 log10 reduction in lung Mab CFU (Figure 3B). Mean Mab lung CFU of this group at the final time-point was statistically significant compared to PBS (one-tailed t-test, p=0.0196). To assess whether rifabutin showed delayed bactericidal effect like clofazimine in this study in C3HeB/FeJ mice and efficacy in BALB/c mice, 20 mg/kg dose was selected for assessment for a six-week treatment duration.
Figure 3. Burden of M. abscessus in the lungs of C3HeB/FeJ and BALB/c mice treated with rifabutin for three weeks.
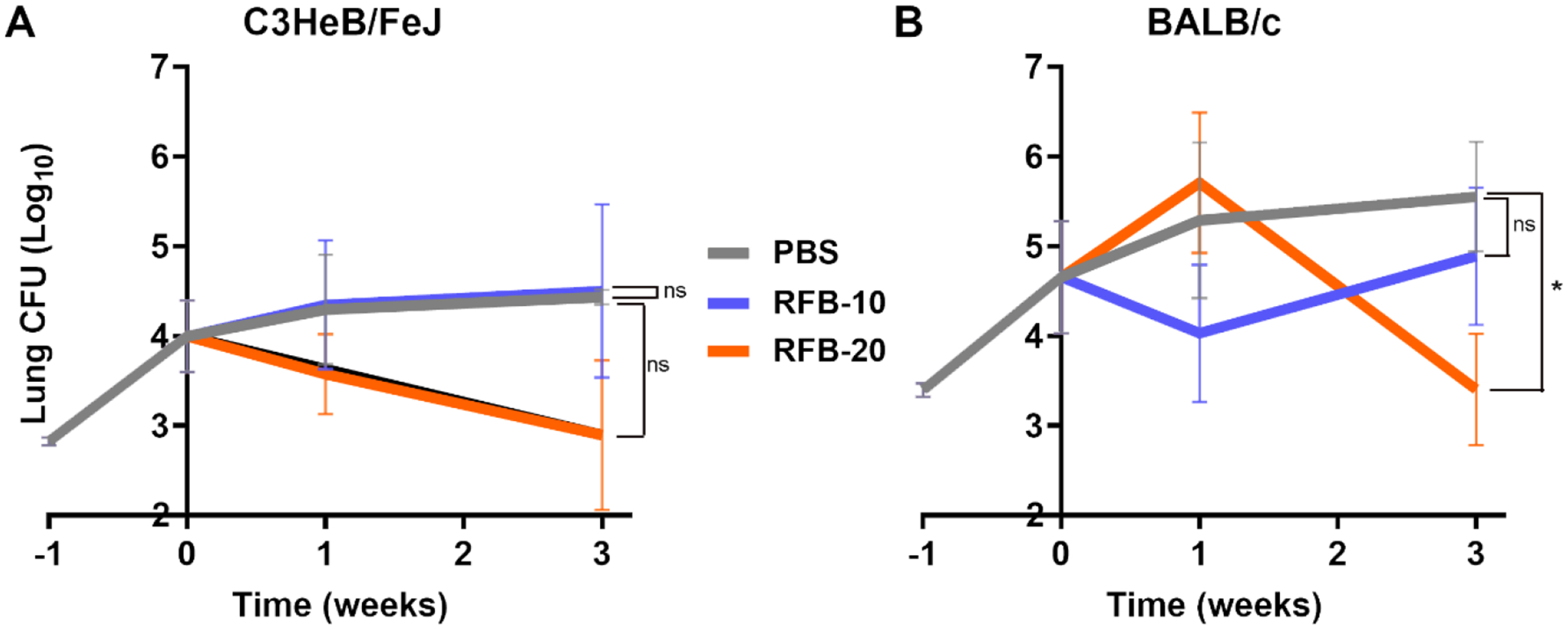
Mean (±SEM) Mab lung burden (log10 CFU) in A) C3HeB/FeJ and B) BALB/c mice, n=5 per time-point, per treatment group, treated with 10mg/kg and 20 mg/kg rifabutin are shown in purple and orange, respectively. Mean (±SEM) Mab lung burden in the negative control group, administered with phosphate buffered saline (PBS), is shown in grey. Time-point week −1 represents the day following infection with Mab. Time-point week 0 represents the day antibiotic administration was initiated. RFB-10, 10mg/kg of rifabutin; RFB-20, 20mg/kg of rifabutin. P-values of PBS vs each treatment group are represented as stars: * represents p ≤0.05; ‘ns’ represents p>0.05 (not significant).
3.4. Rifabutin, 20 mg/kg; treatment duration six weeks:
In C3HeB/FeJ mice, lung Mab burden remained unchanged at the end of the two-week time-point (Figure 4A). At the four- and six-week time-points, lung Mab burden was reduced by 0.84 log10 and an additional 1.16 log10 CFU, respectively. Overall, 20 mg/kg rifabutin produced 2 log10 reduction in lung Mab burden in C3HeB/FeJ mice over the six-week treatment period. At the six-week time-point, Mab lung burden in C3HeB/FeJ mice treated with 20 mg/kg rifabutin was statistically significant compared to the control group that received PBS treatment (one-tailed t-test; p=0.0196). However, in BALB/c mice, 20 mg/kg rifabutin did not produce reduction in lung Mab burden during the study period (Figure 4B). Instead, a steady increase in Mab lung burden was observed at four- and six-week time-points.
Figure 4. Burden of M. abscessus in the lungs of C3HeB/FeJ and BALB/c mice treated with 20mg/kg rifabutin for six weeks.
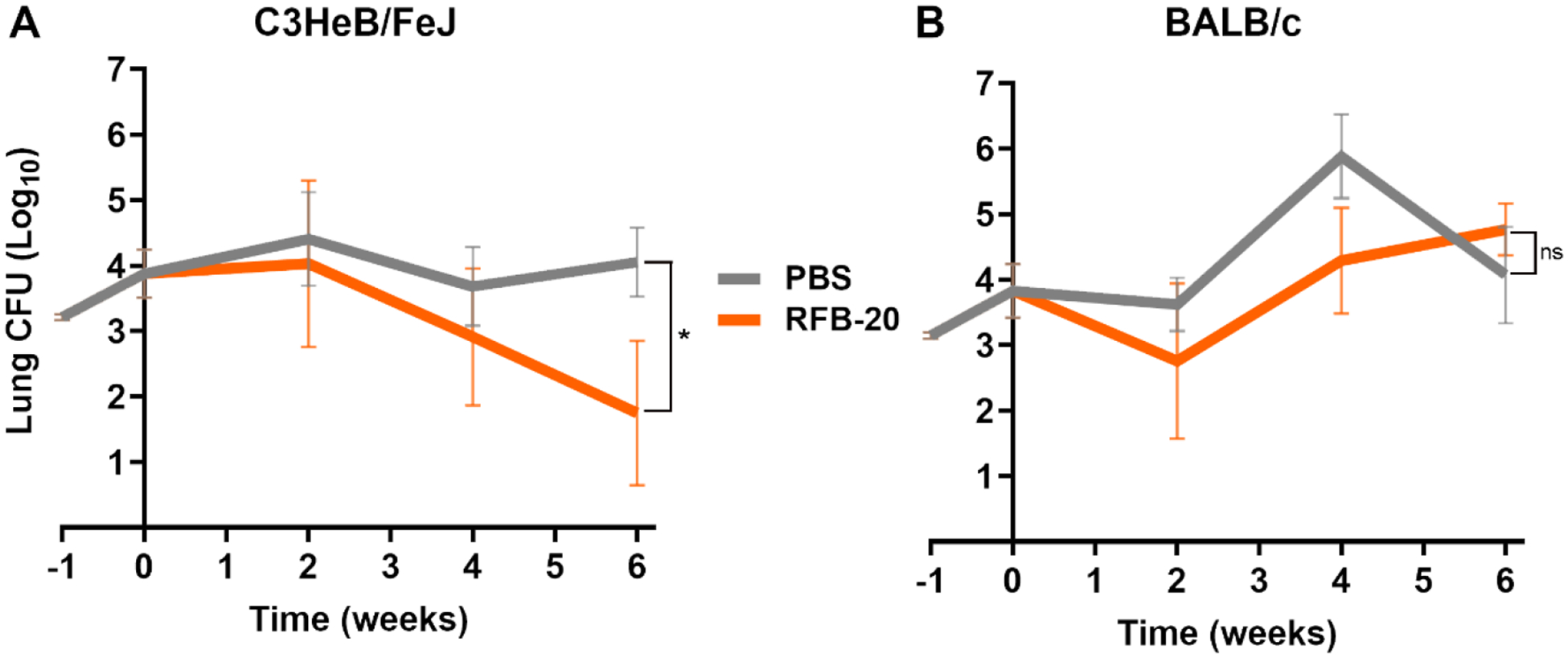
Mean (±SEM) Mab lung burden (log10 CFU) in A) C3HeB/FeJ and B) BALB/c mice, n=5 per time-point, per treatment group, treated with 20 mg/kg rifabutin is shown in orange. Mean (±SEM) Mab lung burden in the negative control group, administered with phosphate buffered saline (PBS), is shown in grey. Time-point week −1 represents the day following infection with Mab. Time-point week 0 represents the day antibiotic administration was initiated. RFB-20, 20mg/kg of rifabutin. P-values of PBS vs each treatment group are represented as stars: * represents p ≤0.05; ‘ns’ represents p>0.05 (not significant).
4. DISCUSSION
Developing a new drug is a long and expensive process as most candidates are rejected at various stages of development for not meeting criteria such as safety or efficacy. Prior to assessing safety and efficacy in a clinical trial, similar assessments are made in the preclinical and controlled laboratory setting [96]. In preclinical studies, the inclusion of a drug used as standard-of-care allows for direct comparison of activity of the experimental agent against the known activities of a drug with clinical experience. A standard-of-care drug serves as a benchmark for expected efficacy and safety and, therefore is a positive control comparator for a study. In addition, it serves as an instrument to measure reproducibility among different studies. This aspect is important as the same experimental agent is often evaluated by independent groups using protocols with multiple variables, such as different models of a disease or independent experimental setups. Inclusion of the same positive control comparator(s) as a constant across independent studies permits assessment of the reproducibility of studies, meaningful comparisons of studies, and subsequently combining findings and generating conclusions with higher statistical power. Therefore, the availability of robust positive control comparator(s) is vital to facilitate independent preclinical assessments of an experimental agent.
Preclinical efficacy evaluations of drugs against mycobacterial diseases require assessment over a long duration to mimic treatment durations of several months in humans. As mycobacterial burden needs to be assessed at multiple time-points over an extended duration, a large number of animals are required to complete these evaluations. The experimental drug, and the comparator need to be administered frequently (at least a few times a week, or daily or multiple times a day). For these requirements, the oral route is less invasive for mice and also more convenient for the personnel administering these treatments. Administration via the oral route also requires less resources as the gavage needle is cleaned and reused while the injection needle needs to be discarded after each use. There are numerous reports describing the preference of oral administration in humans with focus on benefits of switching from parenteral to oral treatment regimens [97–100]. This has been supported by several campaigns and initiatives as well, by regulatory bodies like American Board of Internal Medicine Foundation. Their “Choosing Wisely” campaign recommends preference be given to oral formulations of antimicrobials, subject to their high bioavailability [101]. In fact, most of these switches have been possible for oral antibiotics that achieve required bioavailability similar to parenteral and have thus been deemed effective for continued use. Some other advantages of oral antibiotics are: avoiding risk of cannula-related infections or thrombophlebitis, in addition to outright benefits of lower cost of drugs and treatment as it obviates the requirement of a health professional or equipment to administer intravenous antibiotics [102]. Oral antibiotic therapy also potentiates early discharge from the hospital or prevent hospitalization from emergency rooms [103].
Preclinical efforts to develop drugs and regimens to treat Mab disease are underway. As there are no FDA-approved drugs to treat this indication, there are no obvious candidates to be included in these studies as positive controls. We identified clofazimine as a candidate for positive control comparator as it is among the standard-of-care drugs for treating Mab disease and it met the criteria expected in an ideal positive control. Although clofazimine is not approved by the FDA for this indication, there is an increasing experience of its repurposing for treating Mab disease [16,104]. Additionally, there is a significant clinical experience with clofazimine to treat other mycobacterial diseases [105–107]. Among the antibiotics recommended for Mab treatment, moxifloxacin also met all four criteria for ideal positive comparators. As the main aim of our study was to identify one standard-of-care drug to treat Mab disease, and clofazimine exhibited the desired attributes of a positive control comparator in two strains of mice, it was beyond the scope of this study to consider additional standard-of-care drugs that met the initial screening criteria, such as moxifloxacin. Although we did not include cost of unit of a drug as a major criterion, clofazimine is currently available at $52 per gram (Sigma-Aldrich, catalog# C8995), whereas moxifloxacin is listed at $1000 per gram (Sigma-Aldrich, catalog# SML1581). As positive control comparators need to be included repeatedly, an agent that is significantly more affordable can be argued to be more accessible.
Azithromycin and rifampicin satisfied three out of four criteria for an ideal positive comparator antibiotic (Table 1). Another reason to exclude azithromycin was that two subspecies of Mab, subspecies abscessus and bolletii, are known to exhibit inducible resistance to macrolides [108]. We restricted our study to clofazimine as it is among the front-line drugs used in standard-of-care for Mab disease treatment today. Based on these precedents, studies were designed to identify the dose of clofazimine that would produce a reduction in Mab burden in the lungs of mice. Two different strains of mice, C3HeB/FeJ and BALB/c, were considered as they are often used in preclinical efforts to develop new drugs and regimens to treat Mab disease.
In summary, clofazimine exhibited bactericidal activity from the third and fourth weeks of treatment not only in C3Heb/FeJ (Figure 2A) but also in BALB/c mice (Figure 2B), indicating delayed bactericidal activity. Clofazimine is known to exhibit delayed in vivo bactericidal activity against M. tuberculosis, with reduction in lung burden observable only after two weeks of treatment [82,109]. In the pilot study of clofazimine, Mab lung burden remained steady at the end of three-week time-point in C3HeB/FeJ mice (Figure 1A). In the subsequent study, Mab lung burden in C3HeB/FeJ mice decreased at week-four and -six time-points (Figure 2A). It is likely that reduction in Mab burden began after three weeks of clofazimine treatment as delayed antimycobacterial activity has been reported for clofazimine. We have considered clofazimine carry-over from lung homogenates onto agar plates and assessed whether the carry-over occurs in sufficient concentration to affect growth of Mab and produce CFU that is lower than in the lungs. There are two aspects to clofazimine carry-over during the experiment. First is whether clofazimine accumulates in the lungs to a level greater than its MIC vs Mab. This would produce fewer Mab CFUs on agar plates than in the lungs. Clofazimine is known to accumulate in macrophages and fatty tissue in mice, but its concentration in serum of C3HeB/FeJ and BALB/c mice only increases from 1 μg/ml to <2 μg/ml from 4 to 12 weeks of treatment [80]. the MIC of clofazimine vs. Mab is 0.25–4.0 μg/ml. The second aspect to this is whether the concentration of clofazimine in the lung homogenates that are inoculated onto agar plates to recover Mab is >MIC to affect Mab growth and recovery. As each mouse lung was homogenized in nine-fold excess PBS, clofazimine becomes 10-fold diluted in the homogenate. This homogenate is further diluted 10-fold serially prior to inoculating on agar plates. As only 100 μl of pure or diluted lung homogenate is inoculated onto 25 ml agar, the effective concentration of clofazimine on the agar is diluted at least 100-fold compared to that in the lungs. Therefore, the final concentrations of clofazimine on agar plates is several folds below its MIC to affect Mab growth. Agar containing activated charcoal have been used to absorb excess drugs [80], but this was deemed unnecessary in our study as 25 mg/kg clofazimine treatment in C3HeB/FeJ mice resulted in slight increase in Mab CFU burden in the initial pilot study (Figure 1), which provided evidence that clofazimine tissue accumulation and carryover from lung homogenates does not reduce Mab CFU on agar plates. At the time of this publication, two clinical trials aimed at evaluating efficacy of clofazimine to treat Mab disease in humans were underway. Efficacy of regimen containing clofazimine to treat Mab disease, but not as a single agent, is being evaluated in a clinical trial NCT04310930 [110]. Another study, NCT05294146 [111], is aimed at optimizing the dose of clofazimine to treat nontuberculous mycobacteria infections, including but not limited to Mab. Findings from these and relevant future studies will provide insight into the role of clofazimine in treatment of Mab disease in humans.
The delayed activity of clofazimine versus Mab observed herein makes them suitable for long-term efficacy studies but limits their usage in short-term studies. Since the duration of Mab disease treatment in humans often extends beyond 12 months, preclinical studies with extended treatment durations are likely to be more informative for treatment outcomes in humans. Similar to clofazimine, 20 mg/kg dose of rifabutin also showed a delayed bactericidal activity in C3Heb/FeJ mice, indicating its use in long-term studies in this mice strain (Figure 3A and 4A). However, it is evident from literature that short-term studies are performed especially for proof-of-concept or when new candidate agents are available in quantities that are sufficient only for limited study durations, as is often the case for agents that are difficult to scale in academic settings. In such instances, a positive control comparator that produces reduction in Mab burden immediately following treatment is necessary. Prior studies have demonstrated that administration of imipenem in mice infected with Mab results in immediate and remarkable reduction in lung CFU burden [39,45,47]. However, imipenem does not meet two out of the four criteria for an ideal positive comparator, as it is needs to be administered twice a day and via injection.
5. CONCLUSION
In summary, our studies demonstrated that 25 mg/kg of clofazimine, administered once daily via oral gavage, reduces Mab burden in the lungs of both C3HeB/FeJ and BALB/c mice over six weeks. Rifabutin, 20 mg/kg, once daily, administered by the same method exhibits bactericidal activity in C3HeB/FeJ mice but fails to reduce Mab lung burden in BALB/c mice over the six-week period. In addition to their efficacies against Mab, there were no noticeable adverse events in mice associated with these two drugs at the specified dosages. Based on these findings, we conclude that clofazimine meets the criteria for an ideal positive control and, therefore, propose that it be considered as comparator in future efforts to develop new drugs and regimens to treat Mab disease.
HIGHLIGHTS.
Existing antibiotics to treat M. abscessus (Mab) disease are not optimal.
There is an urgent need for new effective treatments for Mab disease.
New treatments need to be compared with existing antibiotics.
An existing antibiotic that can serve as a comparator is needed.
Clofazimine has ideal attributes to serve as a positive control comparator.
IMPORTANCE.
Mycobacteroides abscessus can cause life-threatening infections in patients with chronic lung conditions. New treatments are needed as cure rate using existing drugs is low. During pre-clinical phase of treatment development, it is important to compare the efficacy of the experimental drug against to existing ones with known history. Here, we demonstrate that clofazimine, one of the standard-of-care antibiotics used for treating Mab disease, can serve as a positive control comparator for efficacy assessments of experimental drugs and regimens to treat M. abscessus disease in mice.
ACKNOWLEDGMENTS
This study was supported by NIH awards R01 AI155664 and R21 AI153201. ECM was supported by NIH F31 award HL147392.
ABBREVIATIONS
- Mab
Mycobacteroides abscessus
- CFZ
clofazimine
- RFB
rifabutin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF COMPETING INTEREST
All authors declare no conflict of interest.
REFERENCES
- [1].Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- [2].Adjemian J, Olivier KN, Prevots DR. Nontuberculous mycobacteria among patients with cystic fibrosis in the United States: screening practices and environmental risk. Am J Respir Crit Care Med 2014;190:581–6. 10.1164/RCCM.201405-0884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and Soft Tissue Infections Due to Rapidly Growing Mycobacteria: Comparison of Clinical Features, Treatment, and Susceptibility. Arch Dermatol 2006;142:1287–92. 10.1001/ARCHDERM.142.10.1287. [DOI] [PubMed] [Google Scholar]
- [4].Kwon YH, Lee GY, Kim WS, Kim KJ. A Case of Skin and Soft Tissue Infection Caused by Mycobacterium abscessus. Ann Dermatol 2009;21:84. 10.5021/AD.2009.21.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fukui S, Sekiya N, Takizawa Y, Morioka H, Kato H, Aono A, et al. Disseminated Mycobacterium abscessus Infection Following Septic Arthritis: A Case Report and Review of the Literature. Medicine (Baltimore) 2015;94. 10.1097/MD.0000000000000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee M-R, Sheng W-H, Hung C-C, Yu C-J, Lee L-N, Hsueh P-R. Mycobacterium abscessus Complex Infections in Humans. Emerg Infect Dis 2015;21. 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tsuruyama Y, Mori N, Fujisawa T, Katayama M. Disseminated Mycobacterium abscessus subspecies massiliense infection and subsequent prosthetic joint infection in a hemodialysis patient: A case report. J Infect Chemother 2021;27:1504–7. 10.1016/J.JIAC.2021.05.003. [DOI] [PubMed] [Google Scholar]
- [8].Gupta RS, Lo B, Son J. Phylogenomics and Comparative Genomic Studies Robustly Support Division of the Genus Mycobacterium into an Emended Genus Mycobacterium and Four Novel Genera. Front Microbiol 2018;9:67. 10.3389/fmicb.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tortoli E, Kohl TA, Brown-Elliott BA, Trovato A, Leão SC, Garcia MJ, et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. nov. Int J Syst Evol Microbiol 2016;66:4471–9. 10.1099/IJSEM.0.001376. [DOI] [PubMed] [Google Scholar]
- [10].Victoria L, Gupta A, Gómez JL, Robledo J. Mycobacterium abscessus complex: A Review of Recent Developments in an Emerging Pathogen. Front Cell Infect Microbiol 2021;11. 10.3389/FCIMB.2021.659997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 2011;183:405–10. 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- [12].Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous Mycobacterial Lung Disease Prevalence at Four Integrated Health Care Delivery Systems. Am J Respir Crit Care Med 2010;182:970–6. 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Adjemian J, Olivier KN, Prevots DR. Epidemiology of pulmonary nontuberculous mycobacterial sputum positivity in patients with cystic fibrosis in the United States, 2010–2014. Ann Am Thorac Soc 2018;15:817–25. 10.1513/ANNALSATS.201709-727OC/SUPPL_FILE/DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johansen MD, Herrmann J-L, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 2020;18:392–407. 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- [15].Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin Infect Dis 2020;71:e1–36. 10.1093/cid/ciaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Griffith DE, Daley CL. Treatment of Mycobacterium abscessus Pulmonary Disease. Chest 2022;161:64–75. 10.1016/J.CHEST.2021.07.035. [DOI] [PubMed] [Google Scholar]
- [17].Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017;72:ii1–64. 10.1136/THORAXJNL-2017-210927. [DOI] [PubMed] [Google Scholar]
- [18].Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 2011;52:565–71. 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- [19].Diel R, Ringshausen F, Richter E, Welker L, Schmitz J, Nienhaus A. Microbiological and Clinical Outcomes of Treating Non-Mycobacterium Avium Complex Nontuberculous Mycobacterial Pulmonary Disease: A Systematic Review and Meta-Analysis. Chest 2017;152:120–42. 10.1016/j.chest.2017.04.166. [DOI] [PubMed] [Google Scholar]
- [20].Pasipanodya JG, Ogbonna D, Ferro BE, Magombedze G, Srivastava S, Deshpande D, et al. Systematic Review and Meta-analyses of the Effect of Chemotherapy on Pulmonary Mycobacterium abscessus Outcomes and Disease Recurrence. Antimicrob Agents Chemother 2017;61:9561. 10.1128/AAC.01206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kwak N, Dalcolmo MP, Daley CL, Eather G, Gayoso R, Hasegawa N, et al. M ycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J 2019;54. 10.1183/13993003.01991-2018. [DOI] [PubMed] [Google Scholar]
- [22].Flight W, Hough N, Chapman S. Outcomes of pulmonary Mycobacterium abscessus infection. Int J Mycobacteriology 2020;9:48–52. 10.4103/IJMY.IJMY_3_20. [DOI] [PubMed] [Google Scholar]
- [23].Brown-Elliott BA, Nash KA, Wallace RJ. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev 2012;25:545–82. 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: A new antibiotic nightmare. J Antimicrob Chemother 2012;67:810–8. 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- [25].Schwartz M, Fisher S, Story-Roller E, Lamichhane G, Parrish N. Activities of dual combinations of antibiotics against multidrug-resistant nontuberculous mycobacteria recovered from patients with cystic fibrosis. Microb Drug Resist 2018;24:1191–7. 10.1089/mdr.2017.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lopeman RC, Harrison J, Desai M, Cox JAG. Mycobacterium abscessus: Environmental Bacterium Turned Clinical Nightmare. Microorganisms 2019;7:90. 10.3390/microorganisms7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen J, Zhao L, Mao Y, Ye M, Guo Q, Zhang Y, et al. Clinical efficacy and adverse effects of antibiotics used to treat Mycobacterium abscessus pulmonary disease. Front Microbiol 2019;10. 10.3389/FMICB.2019.01977/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Novosad SA, Beekmann SE, Polgreen PM, Mackey K, Winthrop KL. Treatment of Mycobacterium abscessus Infection - Volume 22, Number 3—March 2016 - Emerging Infectious Diseases journal - CDC. Emerg Infect Dis 2016;22:511–4. 10.3201/EID2203.150828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dupont C, Viljoen A, Dubar F, Blaise M, Bernut A, Pawlik A, et al. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol Microbiol 2016;101:515–29. 10.1111/MMI.13406. [DOI] [PubMed] [Google Scholar]
- [30].Kozikowski AP, Onajole OK, Stec J, Dupont C, Viljoen A, Richard M, et al. Targeting Mycolic Acid Transport by Indole-2-carboxamides for the Treatment of Mycobacterium abscessus Infections. J Med Chem 2017;60:5876–88. 10.1021/ACS.JMEDCHEM.7B00582. [DOI] [PubMed] [Google Scholar]
- [31].Pandya AN, Prathipati PK, Hegde P, Li W, Graham KF, Mandal S, et al. Indole-2-carboxamides are active against Mycobacterium abscessus in a mouse model of acute infection. Antimicrob Agents Chemother 2019;63:1–16. 10.1128/AAC.02245-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Raynaud C, Daher W, Roquet-Banères F, Johansen MD, Stec J, Onajole OK, et al. Synergistic interactions of indole-2-carboxamides and β-lactam antibiotics against Mycobacterium abscessus. Antimicrob Agents Chemother 2020;64:e02548–19. 10.1128/aac.02548-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ganapathy US, Gengenbacher M, Dick T. Epetraborole Is Active against Mycobacterium abscessus. Antimicrob Agents Chemother 2021;65:e0115621. 10.1128/AAC.01156-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sullivan JR, Lupien A, Kalthoff E, Hamela C, Taylor L, Munro KA, et al. Efficacy of epetraborole against Mycobacterium abscessus is increased with norvaline. PLOS Pathog 2021;17:e1009965. 10.1371/JOURNAL.PPAT.1009965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim TS, Choe JH, Kim YJ, Yang CS, Kwon HJ, Jeong J, et al. Activity of LCB01–0371, a Novel Oxazolidinone, against Mycobacterium abscessus. Antimicrob Agents Chemother 2017;61:e02752–16. 10.1128/AAC.02752-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Le Run E, Arthur M, Mainardia JL. In Vitro and Intracellular Activity of Imipenem Combined with Tedizolid, Rifabutin, and Avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother 2019;63:e01915–18. 10.1128/AAC.01915-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Batchelder HR, Story-Roller E, Lloyd EP, Kaushik A, Bigelow KM, Maggioncalda EC, et al. Development of a penem antibiotic against Mycobacteroides abscessus. Commun Biol 2020;3:741. 10.1038/s42003-020-01475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kumar G, Galanis C, Batchelder HR, Townsend CA, Lamichhane G. Penicillin Binding Proteins and β-Lactamases of Mycobacterium tuberculosis: Reexamination of the Historical Paradigm. MSphere 2022;7:e0003922. 10.1128/MSPHERE.00039-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rimal B, Batchelder HR, Story-Roller E, Panthi CM, Tabor C, Nuermberger EL, et al. T405, a New Penem, Exhibits In Vivo Efficacy against M. abscessus and Synergy with β-Lactams Imipenem and Cefditoren. Antimicrob Agents Chemother 2022;66:e0053622. 10.1128/aac.00536-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bax HI, de Vogel CP, Mouton JW, de Steenwinkel JEM. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J Antimicrob Chemother 2019;74:2930–3. 10.1093/jac/dkz267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shoen C, Benaroch D, Sklaney M, Cynamon M. In Vitro Activities of Omadacycline against Rapidly Growing Mycobacteria. Antimicrob Agents Chemother 2019;63:e02522–18. 10.1128/AAC.02522-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kaushik A, Ammerman NC, Martins O, Parrish NM, Nuermberger EL. In vitro activity of new tetracycline analogs omadacycline and eravacycline against drug-resistant clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 2019;63:1–14. 10.1128/AAC.00470-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bich Hanh BT, Quang NT, Park Y, Heo BE, Jeon S, Park J-W, et al. Omadacycline Potentiates Clarithromycin Activity Against Mycobacterium abscessus. Front Pharmacol 2021;12:790767. 10.3389/fphar.2021.790767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brown-Elliott BA, Wallace RJ. In Vitro Susceptibility Testing of Omadacycline against Nontuberculous Mycobacteria. Antimicrob Agents Chemother 2021;65:e01947–20. 10.1128/AAC.01947-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nicklas DA, Maggioncalda EC, Story-Roller E, Eichelman B, Tabor C, Serio AW, et al. Potency of Omadacycline against Mycobacteroides abscessus Clinical Isolates In Vitro and in a Mouse Model of Pulmonary Infection. Antimicrob Agents Chemother 2022;66:e0170421. 10.1128/AAC.01704-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Story-Roller E, Maggioncalda EC, Lamichhane G. Select β-Lactam Combinations Exhibit Synergy against Mycobacterium abscessus In Vitro. Antimicrob Agents Chemother 2019;63:e02613–18. 10.1128/AAC.02613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Story-Roller E, Maggioncalda EC, Lamichhane G. Synergistic efficacy of β-lactam combinations against Mycobacterium abscessus pulmonary infection in mice. Antimicrob Agents Chemother 2019;63:e00614–19. 10.1128/AAC.00614-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Story-Roller E, Galanis C, Lamichhane G. β-lactam combinations that exhibit synergy against Mycobacteroides abscessus clinical isolates. Antimicrob Agents Chemother 2021;65:e02545–20. 10.1128/AAC.02545-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pandey R, Chen L, Manca C, Jenkins S, Glaser L, Vinnard C, et al. Dual β-lactam combinations highly active against Mycobacterium abscessus complex in vitro. MBio 2019;10:1–14. 10.1128/mBio.02895-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nguyen DC, Dousa KM, Kurz SG, Brown ST, Drusano G, Holland SM, et al. “One-Two Punch”: Synergistic ß-Lactam Combinations for Mycobacterium abscessus and Target Redundancy in the Inhibition of Peptidoglycan Synthesis Enzymes. Clin Infect Dis 2021;73:1532–6. 10.1093/CID/CIAB535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dubée V, Bernut A, Cortes M, Lesne T, Dorchene D, Lefebvre AL, et al. β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother 2015;70:1051–8. 10.1093/JAC/DKU510. [DOI] [PubMed] [Google Scholar]
- [52].Kaushik A, Gupta C, Fisher S, Story-Roller E, Galanis C, Parrish N, et al. Combinations of avibactam and carbapenems exhibit enhanced potencies against drug-resistant Mycobacterium abscessus. Future Microbiol 2017;12:473–80. 10.2217/fmb-2016-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lefebvre AL, Le Moigne V, Bernut A, Veckerlé C, Compain F, Herrmann JL, et al. Inhibition of the β-Lactamase Bla Mab by Avibactam Improves the In Vitro and In Vivo Efficacy of Imipenem against Mycobacterium abscessus. Antimicrob Agents Chemother 2017;61:e02440–16. 10.1128/AAC.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Soroka D, Ourghanlian C, Compain F, Fichini M, Dubée V, Mainardi JL, et al. Inhibition of β-lactamases of mycobacteria by avibactam and clavulanate. J Antimicrob Chemother 2017;72:1081–8. 10.1093/jac/dkw546. [DOI] [PubMed] [Google Scholar]
- [55].Kaushik A, Ammerman NC, Lee J, Martins O, Kreiswirth BN, Lamichhane G, et al. In vitro activity of the new -lactamase inhibitors relebactam and vaborbactam in combination with -lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob Agents Chemother 2019;63:e02623–18. 10.1128/AAC.02623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Obregón-Henao A, Arnett KA, Henao-Tamayo M, Massoudi L, Creissen E, Andries K, et al. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother 2015;59:6904–12. 10.1128/AAC.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Le Moigne V, Raynaud C, Moreau F, Dupont C, Nigou J, Neyrolles O, et al. Efficacy of Bedaquiline, Alone or in Combination with Imipenem, against Mycobacterium abscessus in C3HeB/FeJ Mice. Antimicrob Agents Chemother 2020;64:e00114–20. 10.1128/AAC.00114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019;25:730–3. 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Johansen MD, Alcaraz M, Dedrick RM, Roquet-Banères F, Hamela C, Hatfull GF, et al. Mycobacteriophage-antibiotic therapy promotes enhanced clearance of drug-resistant Mycobacterium abscessus. Dis Model Mech 2021;14:dmm049159. 10.1242/dmm.049159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dedrick RM, Freeman KG, Nguyen JA, Bahadirli-Talbott A, Smith BE, Wu AE, et al. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat Med 2021;27:1357–61. 10.1038/s41591-021-01403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cheng A, Tsai YT, Chang SYSC, Sun HY, Wu UI, Sheng WH, et al. In Vitro Synergism of Rifabutin with Clarithromycin, Imipenem, and Tigecycline against the Mycobacterium abscessus Complex. Antimicrob Agents Chemother 2019;63. 10.1128/AAC.02234-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J Am Assoc Lab Anim Sci 2011;50:600. [PMC free article] [PubMed] [Google Scholar]
- [63].Zhao M, Lepak AJ, Andes DR. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg Med Chem 2016;24:6390–400. 10.1016/J.BMC.2016.11.008. [DOI] [PubMed] [Google Scholar]
- [64].Bryda EC. The Mighty Mouse: the impact of rodents on advances in biomedical research. Mo Med 2013;110:207–11. [PMC free article] [PubMed] [Google Scholar]
- [65].Zuberi A, Lutz C. Mouse Models for Drug Discovery. Can New Tools and Technology Improve Translational Power? ILAR J 2016;57:178–85. 10.1093/ilar/ilw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sarkar S, Heise MT. Mouse Models as Resources for Studying Infectious Diseases. Clin Ther 2019;41:1912–22. 10.1016/j.clinthera.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mitchison DA, Davies GR. Assessment of the Efficacy of New Anti-Tuberculosis Drugs. Open Infect Dis J 2008;2:59–76. 10.2174/1874279300802010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Grosset JH, Singer TG, Bishai WR. New drugs for the treatment of tuberculosis: hope and reality [State of the Art Series. New tools. Number 2 in the series]. Int J Tuberc Lung Dis 2012;16:1005–14. 10.5588/ijtld.12.0277. [DOI] [PubMed] [Google Scholar]
- [69].Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, et al. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother 2012;56:4331–40. 10.1128/AAC.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].De Groote MA, Gilliland JC, Wells CL, Brooks EJ, Woolhiser LK, Gruppo V, et al. Comparative Studies Evaluating Mouse Models Used for Efficacy Testing of Experimental Drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 2011;55:1237–47. 10.1128/AAC.00595-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mudde SE, Ayoun Alsoud R, van der Meijden A, Upton AM, Lotlikar MU, Simonsson USH, et al. Predictive modeling to study the treatment-shortening potential of novel tuberculosis drug regimens, towards bundling of preclinical data. J Infect Dis 2021;225:1876–85. 10.1093/INFDIS/JIAB101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Maggioncalda EC, Story-Roller E, Mylius J, Illei P, Basaraba RJ, Lamichhane G. A mouse model of pulmonary Mycobacteroides abscessus infection. Nat Sci Reports 2020;10:1–8. 10.1038/s41598-020-60452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Driver ER, Ryan GJ, Hoff DR, Irwin SM, Basaraba RJ, Kramnik I, et al. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 2012;56:3181–95. 10.1128/AAC.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Irwin SM, Driver E, Lyon E, Schrupp C, Ryan G, Gonzalez-Juarrero M, et al. Presence of multiple lesion types with vastly different microenvironments in C3HeB/FeJ mice following aerosol infection with Mycobacterium tuberculosis. Dis Model Mech 2015;8:591–602. 10.1242/dmm.019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lanoix J-P, Lenaerts AJ, Nuermberger EL. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis Model Mech 2015;8:603–10. 10.1242/dmm.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lerat I, Cambau E, Roth Dit Bettoni R, Gaillard JL, Jarlier V, Truffot C, et al. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 2014;209:905–12. 10.1093/infdis/jit614. [DOI] [PubMed] [Google Scholar]
- [77].Dick T Rifabutin: A Repurposing Candidate for Mycobacterium abscessus Lung Disease. Front Microbiol 2020;11:371. 10.3389/fmicb.2020.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Moore M, Frerichs JB. An Unusual Acid-Fast Infection of the Knee with Subcutaneous, Abscess-Like Lesions of the Gluteal Region. J Invest Dermatol 1953;20:133–69. 10.1038/jid.1953.18. [DOI] [PubMed] [Google Scholar]
- [79].Larsen M Some Common Methods in Mycobacterial Genetics. In: Hatfull GF, Jacobs WRJ, editors. Mol. Genet. Mycobact, Washington DC: American Society for Microbiology; 2000, p. 313–20. [Google Scholar]
- [80].Saini V, Ammerman NC, Chang YS, Tasneen R, Chaisson RE, Jain S, et al. Treatment-Shortening Effect of a Novel Regimen Combining Clofazimine and High-Dose Rifapentine in Pathologically Distinct Mouse Models of Tuberculosis. Antimicrob Agents Chemother 2019;63:e00388–19. 10.1128/AAC.00388-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Liu Y, Tan Y, Islam MM, Cao Y, Lu X, Zeng S, et al. Assessment of Clofazimine and TB47 Combination Activity against Mycobacterium abscessus Using a Bioluminescent Approach. Antimicrob Agents Chemother 2020;64:e01881–19. 10.1128/AAC.01881-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ammerman NC, Swanson RV, Tapley A, Moodley C, Ngcobo B, Adamson J, et al. Clofazimine has delayed antimicrobial activity against Mycobacterium tuberculosis both in vitro and in vivo. J Antimicrob Chemother 2017;72:455–61. 10.1093/jac/dkw417. [DOI] [PubMed] [Google Scholar]
- [83].Shen GH, Wu B Da, Hu ST, Lin CF, Wu KM, Chen JH. High efficacy of clofazimine and its synergistic effect with amikacin against rapidly growing mycobacteria. Int J Antimicrob Agents 2010;35:400–4. 10.1016/J.IJANTIMICAG.2009.12.008. [DOI] [PubMed] [Google Scholar]
- [84].van Ingen J, Totten SE, Helstrom NK, Heifets LB, Boeree MJ, Daley CL. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother 2012;56:6324–7. 10.1128/AAC.01505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Singh S, Bouzinbi N, Chaturvedi V, Godreuil S, Kremer L. In vitro evaluation of a new drug combination against clinical isolates belonging to the Mycobacterium abscessus complex. Clin Microbiol Infect 2014;20:O1124–7. 10.1111/1469-0691.12780. [DOI] [PubMed] [Google Scholar]
- [86].Luo J, Yu X, Jiang G, Fu Y, Huo F, Ma Y, et al. In Vitro Activity of Clofazimine against Nontuberculous Mycobacteria Isolated in Beijing, China. Antimicrob Agents Chemother 2018;62:e00072–18. 10.1128/AAC.00072-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kwak N, Whang J, Yang JS, Kim TS, Kim SA, Yim J-J. Minimal Inhibitory Concentration of Clofazimine Among Clinical Isolates of Nontuberculous Mycobacteria and Its Impact on Treatment Outcome. Chest 2021;159:517–23. 10.1016/j.chest.2020.07.040. [DOI] [PubMed] [Google Scholar]
- [88].Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016;71:i1–22. 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016;7:27–31. 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kurz SG, Zha BS, Herman DD, Holt MR, Daley CL, Ruminjo JK, et al. Summary for Clinicians: 2020 Clinical Practice Guideline Summary for the Treatment of Nontuberculous Mycobacterial Pulmonary Disease. Ann Am Thorac Soc 2020;17:1033–9. 10.1513/ANNALSATS.202003-222CME. [DOI] [PubMed] [Google Scholar]
- [91].Dick T, Shin SJ, Koh WJ, Dartois V, Gengenbacher M. Rifabutin Is Active against Mycobacterium abscessus in Mice. Antimicrob Agents Chemother 2020;64:e01943–19. 10.1128/AAC.01943-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gangadharam PRJ, Perumal VK, Jairam BT, Rao PN, Nguyen AK, Farhi DC, et al. Activity of rifabutin alone or in combination with clofazimine or ethambutol or both against acute and chronic experimental Mycobacterium intracellulare infections. Am Rev Respir Dis 1987;136:329–33. 10.1164/AJRCCM/136.2.329. [DOI] [PubMed] [Google Scholar]
- [93].Lazard T, Perronne C, Grosset J, Vilde JL, Pocidalo JJ. Clarithromycin, minocycline, and rifabutin treatments before and after infection of C57BL/6 mice with Mycobacterium avium. Antimicrob Agents Chemother 1993;37:1690–2. 10.1128/AAC.37.8.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Schön T, Chryssanthou E. Minimum inhibitory concentration distributions for Mycobacterium avium complex—towards evidence-based susceptibility breakpoints. Int J Infect Dis 2017;55:122–4. 10.1016/J.IJID.2016.12.027. [DOI] [PubMed] [Google Scholar]
- [95].Aziz DB, Low JL, Wu ML, Gengenbacher M, Teo JWP, Dartois V, et al. Rifabutin Is Active against Mycobacterium abscessus Complex. Antimicrob Agents Chemother 2017;61:e00155–17. 10.1128/AAC.00155-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hefti FF. Requirements for a lead compound to become a clinical candidate. BMC Neurosci 2008;9 Suppl 3:S7. 10.1186/1471-2202-9-S3-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Béïque L, Zvonar R. Addressing Concerns about Changing the Route of Antimicrobial Administration from Intravenous to Oral in Adult Inpatients. Can J Hosp Pharm 2015;68. 10.4212/cjhp.v68i4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jorgensen SCJ, Lagnf AM, Bhatia S, Shamim M-D, Rybak MJ. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: one step closer. J Antimicrob Chemother 2019;74:489–98. 10.1093/jac/dky452. [DOI] [PubMed] [Google Scholar]
- [99].Willekens R, Puig-Asensio M, Ruiz-Camps I, Larrosa MN, González-López JJ, Rodríguez-Pardo D, et al. Early Oral Switch to Linezolid for Low-risk Patients With Staphylococcus aureus Bloodstream Infections: A Propensity-matched Cohort Study. Clin Infect Dis 2019;69:381–7. 10.1093/cid/ciy916. [DOI] [PubMed] [Google Scholar]
- [100].Tamma PD, Conley AT, Cosgrove SE, Harris AD, Lautenbach E, Amoah J, et al. Association of 30-Day Mortality With Oral Step-Down vs Continued Intravenous Therapy in Patients Hospitalized With Enterobacteriaceae Bacteremia. JAMA Intern Med 2019;179:316. 10.1001/jamainternmed.2018.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lehmann C, Berner R, Bogner JR, Cornely OA, de With K, Herold S, et al. The “Choosing Wisely” initiative in infectious diseases. Infection 2017;45:263–8. 10.1007/s15010-017-0997-0. [DOI] [PubMed] [Google Scholar]
- [102].Patel AR, Patel AR, Singh S, Singh S, Khawaja I. Central Line Catheters and Associated Complications: A Review. Cureus 2019;11:e4717. 10.7759/cureus.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother 2014;5:83–7. 10.4103/0976-500X.130042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ganapathy US, Dartois V, Dick T. Repositioning rifamycins for Mycobacterium abscessus lung disease. Expert Opin Drug Discov 2019;14:867–78. 10.1080/17460441.2019.1629414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Garrelts JC. Clofazimine: A Review of its Use in Leprosy and Mycobacterium Avium Complex Infection. DICP 1991;25:525–31. 10.1177/106002809102500513. [DOI] [PubMed] [Google Scholar]
- [106].Gopal M, Padayatchi N, Metcalfe JZ, O’Donnell MR. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis [Review article]. Int J Tuberc Lung Dis 2013;17:1001–7. 10.5588/ijtld.12.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].McGuffin SA, Pottinger PS, Harnisch JP. Clofazimine in Nontuberculous Mycobacterial Infections: A Growing Niche. Open Forum Infect Dis 2017;4:ofx147. 10.1093/ofid/ofx147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Maurer FP, Castelberg C, Quiblier C, Bottger EC, Somoskovi A. Erm(41)-dependent inducible resistance to azithromycin and clarithromycin in clinical isolates of Mycobacterium abscessus. J Antimicrob Chemother 2014;69:1559–63. 10.1093/jac/dku007. [DOI] [PubMed] [Google Scholar]
- [109].Swanson RV, Adamson J, Moodley C, Ngcobo B, Ammerman NC, Dorasamy A, et al. Pharmacokinetics and Pharmacodynamics of Clofazimine in a Mouse Model of Tuberculosis. Antimicrob Agents Chemother 2015;59:3042–51. 10.1128/AAC.00260-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Finding the Optimal Regimen for Mycobacterium Abscessus Treatment (FORMaT) n.d. https://clinicaltrials.gov/ct2/show/NCT04310930.
- [111].Pharmacokinetic Study With a Loading Dose of Clofazimine in Adult Patients With Nontuberculous Mycobacterial Disease (C-LOAD) n.d. https://clinicaltrials.gov/ct2/show/NCT05294146?term=NCT05294146&draw=2&rank=1.


