Highlights
-
•
The taste quality of spring black tea exceeds that of summer black tea.
-
•
Glutamic acid and aspartic acid contents differ between spring and summer teas.
-
•
Color- and taste-contributing compounds are significantly correlated.
-
•
Chromaticity values may reflect the quality of Yinghong No. 9 black tea.
Keywords: Camellia sinensis, Quality, Season, Secondary metabolite, Differential contributor
Chemical compounds studied in this article: Epicatechin gallate (PubChem CID: 107905), caffeine (PubChem CID: 2519), l-theanine (PubChem CID: 439378), glutamic acid (PubChem CID: 33032)
Abstract
Spring green tea is usually considered to be better than summer green tea. Whether this phenomenon applies to black tea is unknown. Black tea produced using Camellia sinensis var. Yinghong No. 9 leaves is popular in South China and analyzed in the study. The taste and color quality of the infusion was higher for spring tea than for summer tea. Compared with summer tea, the main catechin contents were lower in spring tea, whereas caffeine and total amino acid contents were higher, especially glutamic acid, which may be responsible for the differences between teas. Moreover, spring tea had a higher theabrownin content and a lower L* value. The compounds contributing to the infusion taste and color were correlated with the chromaticity value (i.e., useful indicator of black tea quality). This study revealed the seasonal differences in Yinghong No. 9 black tea quality and the key underlying factors.
Introduction
Tea plants (Camellia sinensis) have been used for thousands of years, including as the raw materials for beverages for more than 2,400 years (Lu et al., 2021). Tea is one of the most consumed beverages worldwide, second only to water. The popularity of tea beverages is due to their refreshing taste, rich aroma, and health benefits. More than 66% of the global population consumes tea (Ho et al., 2015, Zhang et al., 2020). The unique flavor quality and health effects of tea are closely related to a considerable abundance of secondary metabolites. The core secondary metabolites influencing quality include tea polyphenols (TPP), total amino acids (TAA), alkaloids, and aroma substances, of which TPP, TAA, and alkaloids affect tea taste quality, while the products of TPP oxidation contribute to the infusion color and aroma substances influence aroma quality. The formation of these quality-related metabolites is regulated during the tea plant growth stage. Seasonal climatic conditions have important effects on tea quality at the pre-harvest stage. For example, tea quality is significantly higher in spring than in summer or autumn, especially for green tea (Guo et al., 2021). The main reason for this difference is the diversity in the light intensity, photoperiod, and temperature among seasons, which leads to obvious differences in the secondary metabolite contents in fresh tea leaves among seasons. Based on the required processing methods, tea products can be divided into green, white, yellow, oolong, black and dark tea (Xie et al., 2021). Because of how green tea is manufactured, its metabolite content is more similar to that of fresh leaves than the other tea types, especially in terms of the taste-related metabolites in spring tea, which has a fresh taste and is not bitter or astringent (Xu et al., 2007). However, in addition to the regulation during the pre-harvest stage, post-harvest processing also significantly affects tea quality (Wan & Xia, 2015). Whether seasonal changes significantly influence the quality of black tea, which requires a more complex manufacturing process than green tea, remains unclear.
Black tea is the most consumed tea (i.e., 75% of the tea consumed worldwide) (Gao et al., 2022, Sanlier et al., 2018). The fermentation process is considered to be the critical step. During the fermentation process, polyphenols, such as catechins, are mostly oxidized to tea pigments, including total theaflavins (TFs), thearubigins (TRs), and theabrownins (TBs) (Wan, 2003). High-quality black tea often has a bright red tea infusion, mellow taste, and rich aroma. A recent study involving a sensory evaluation and an examination of consumer preferences indicated infusion color and taste are important black tea indices among consumers (Cui et al., 2022). Current black tea products include small-leaf black tea (e.g., Keemun and Lapsang Souchong), large-leaf black tea (e.g., Indian Assam and Yinghong No. 9), and red crushed tea (e.g., crush-tear-curl). Yinghong No. 9, which was derived from the large-leaf group species in Yunnan (China), is a representative large-leaf black tea famous in South China (Qi et al., 2019, Zhang et al., 2021). In this study, Yinghong No. 9 was selected to explore whether seasonal changes affect the quality of large-leaf black tea. We collected Yinghong No. 9 samples of different seasons from 42 black tea-producing companies distributed in the core black tea-producing region for a systematic analysis of the differences between the quality of the spring and summer teas. Additionally, the quality-related metabolites responsible for the differences were identified. The study results have clarified the effects of the growing season on the quality of black tea, which may be useful for improving black tea quality to satisfy consumer demands.
Materials and methods
2.1. Chemicals and reagents
Gallic acid, caffeine, seven catechin monomers, caffeine and four TF monomers were purchased from Shanghai Zzbio Co., Ltd. (Shanghai, China). Methanol (≧98%), n-butanol (≧98%), ethyl acetate (≧98%) and ethanol (≧98%) were purchased from Tianjin Fine Chemical Co., Ltd. (Tianjin, China). Folin-Ciocalteu was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sodium carbonate (≧98%) and sodium hydrogen carbonate (≧98%) were purchased from Guangzhou Chemical Reagent Factory Co., Ltd. (Guangzhou, China). Sulfosalicylic acid (≧98%) was purchased from Shanghai Bioesn Biotechnology Co., Ltd. Oxalic acid was purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, China).
2.2. Plant materials
C. sinensis var. Yinghong No. 9 black tea products were collected in Guangdong Province, China in 2022. The main processing steps of black tea are withering, rolling, fermentation and drying. The samples processed within the three months from March to May were collected as spring teas, and the samples processed within the three months from June to September were collected as summer teas. Spring and summer tea were collected at the identical company. Eighty-four samples were collected from 42 different tea companies in Guangdong Province. All samples were stored in −80 °C.
2.3. Sensory quality evaluation of tea samples
Tea samples (3 g) were infused with boiled water (150 mL) for 5 min. The black tea quality was estimated by a group consisted of eight healthy assessors with extensive evaluation experience. The analysis group of tea sensory evaluation consisted with 6 males and 2 females. The total scores were 100 points, including 25 for appearance, 25 for aroma, 10 for infusion color, 30 for taste, and 10 for infused leaf. The operation of the sensory evaluation experiment was referred from evaluation standards in China (GB/T23776-2018). Before the evaluation, the assessors had been previously trained and signed the informed consent for the sensory quality evaluation of tea.
2.4. Analysis of total polyphenols
The total polyphenols were determined using the Folin-Ciocalteu method based on previous method (Zeng et al., 2017). Black tea powders (0.2 g) were extracted with 70% methanol (10 mL). The extraction time and temperature were 20 min and 70 °C, respectively. The extract was diluted to 10 mL and centrifuged. The extract was diluted 20-fold with distilled deionized water before the reaction. Folin-Ciocalteu reagent (10%, 500 μL) was added into diluted extract (200 μL) centrifuge tube for reacting 4 min. After that, 7.5 % Na2CO3 (400 μL) was added. After 60 min-reaction at room temperature, the absorbance was measured at 765 nm.
2.5. Analysis of amino acids
Black tea powders (0.2 g) were extracted with 10 mL boiled water for 15 min and the mixture was shaken per 5 min. The extracts were collected and diluted 10-fold with distilled deionized water for follow-up reaction. The extract (200 μL) was mixed with 100 μL phosphate buffer (pH = 8). Ninhydrin solution (100 μL) was added and heated in boiled water for 15 min. After reacting, the absorbance was measured at 540 nm. The method refers to the analysis standards in China (GBT8314-2013).
Analysis of free amino acids was performed by amino acid analyzer (Sykam, Eresing, Germany) with partial modifications of the method (Chen et al., 2017, Zeng et al., 2017). Tea powders (0.1 g) was extracted in boiling water (2 mL) for 10 min, and the mixture was centrifuged. Supernatant (0.5 mL) was mixed with 2 mL 5% sulfosalicylic acid, incubating for 1 h. The solution was filtered using 0.45 μm polyethersulfone membrane before analysis. The analysis was carried out on high-efficiency sodium cation exchange column (LCA K07/Li). The Sykam S433D Physiological Li C4 system used mobile phase. The UV–Vis detection wavelength was set at 570 and 440 nm. The flow rates of the mobile phase and ninhydrin (derivatization reagent) were 7.5 and 4.167 μL/s. The injection volume was 50 μL. The temperatures of column, reaction equipment (post-column) and auto-sampler are 38, 130 and 5 °C.
2.6. Analysis of tea pigments
The contents of TRs and TBs were detected according to spectrophotometry method (NY/T3675-2020). Black tea powders (0.3 g) were extracted with boiling water (20 mL) for 20 min. The mixture was centrifuged and the supernatant was diluted to 50 mL. Preparation of colorimetric solution A was as following: 2 mL saturated oxalic acid solution was added to 2 mL tea infusion, and the mixture was diluted to 25 mL with 95% ethanol. Preparation of colorimetric solution B was as following: 5 mL butanol was added to 5 mL extract. After vortexing for 2 min, the mixture was centrifuged. Then 2 mL of the lower layer solution was mixed with 2 mL saturated oxalic acid solution and 6 mL water. The mixture was diluted to 25 mL with 95% ethanol. Preparation of colorimetric solution C was as following: 7 mL ethyl acetate was added to 7 mL tea extract. After vortexing for 2 min, the mixture was centrifuged. Then, 6 mL upper layer (ethyl acetate layer) were taken into a 15 mL centrifuge tube and was added with 6 mL 25 g/L NaHCO3 with fully shaking for 30 s. The solution was centrifuged, and the upper layer (ethyl acetate layer, 4 mL) were diluted to 25 mL with 95% ethanol. The absorbance values were measured at 380 nm using a UV–vis spectrometer (UV-5100, Shanghai Yuanxi Instrument Co., Ltd., China) and the contents of TRs and TBs were calculated as follows:
| XTB=(16.944*Ab)/(100*0.3); XTR=(16.944*(Aa-0.5Ac-Ab))/(100*0.3) |
2.7. Chromatic difference analysis
The chromatic difference analysis was carried out in YS6010 Desktop Spectrophotometer (Shenzhen Sanenshi Technology Co., Ltd., Shenzhen, China). The white plate supplied by the YS6010 Desktop Spectrophotometer was used as background. White board and black board provided by the colorimeter were used to calibrate the instrument, and the measured value of distilled water was confirmed as the calibration. The L* value represents the lightness of the sample solution ranging from 0 (black) to 100 (white), while the a* and b* values represent the green (-) / red (+) and blue (-) / yellow (+) of the sample solution, respectively. The C* value represents the color saturation, and the hue angle (h° value) reflects the chroma or hue, ranging from 0° (red) −90° (yellow) − 180° (green) − 270° (blue) − 360° (red). And these values can be calculated as follows:
| C* = (a*2 + b*2)1/2; h° = arctan (b*/a*) |
2.8. Analysis of catechins, caffeine and TFs
Black tea powders (0.2 g) were extracted with 70% methanol (8 mL) for 20 min in 70 °C. The extract was diluted to 8 mL and filtered at 0.22 μm membrane after centrifugation. The catechins, caffeine and TFs were detected using HPLC analyzer (Alliance, Waters, Milford, MA, USA) equipped with ZORBAX Eclipse C18 column (4.6 mm × 150 mm, 5 μm). The injection volume was 10 μL, and column temperature was 35 °C. Mobile phase A was distilled water contained 9% acetonitrile and 2% glacial acetic acid, and mobile phase B was acetonitrile contained 2% glacial acetic acid. The linear gradient of the solvent was 0–22 min, 100% A; 22.1–52 min, 100%-70% A; 52.1–67 min, 70%-0% A; 67.1–97 min, 0% A; 0–32 min, 0%-30% B; 32.1–52 min, 30% B; 52–52.1 min, 30%-100% B; 67–67.1 min, 100%-0% B. Flow rate was 1 mL/min. The ultraviolet absorption wavelength was 278 nm. Chromatographic peaks were identified by UV spectroscopy using a diode array detector, the qualitative and quantitative analysis were based on authentic standards.
2.9. Statistical analysis
Scatterplots were drawn by SigmaPlot 12.5. Significant analysis was performed by IBM SPSS Statistics 2017 and Microsoft Excel 2016 software, and the difference between the two groups was determined by two-tailed t test. Partial least square discriminant analysis (PLS-DA) and random forest model analysis were performed at https://www.omicstudio.cn/tool using the OmicStudio tools. Heat map correlation analysis is performed on https://hiplot.com.cn/cloud-tool/drawing-tool/list. Pearson correlation was used to analyze the correlation between metabolites and chromaticity values in IBM SPSS Statistics 2017, and p < 0.05 was considered as significant.
3. Results
3.1. Sensory evaluation of Yinghong no. 9 black tea in different seasons
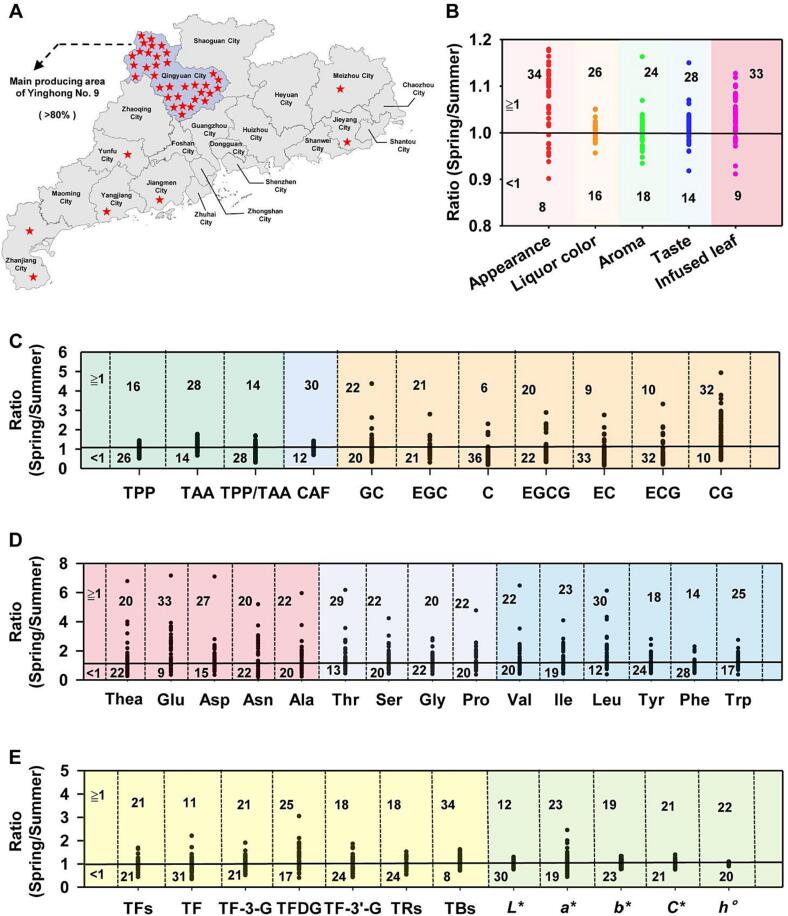
Because of the considerable diversity in the collected tea samples, there were significant differences in the pre-harvest management practices and post-harvest processing among the tea companies. Therefore, the scores for the spring and summer teas produced by each company were converted to a ratio. The 42 tea companies were located in seven cities in Guangdong province (China), with 33 of them in Yingde county, Qingyuan city (Fig. 1A). The output of the main black tea-producing areas accounted for more than 80% of the total output for the province. The scatter plot distribution of the spring and summer tea score ratios revealed that the biggest differences between the spring and summer teas were in the appearance and infused leaf scores (Fig. 1B). The specific scores for the analyzed indices are provided in Table S1. The appearance scores were higher for spring tea than for summer tea for 34 tea companies. The infused leaf scores were higher for spring tea than for summer tea for 33 tea companies. In terms of the sensory factors related to tea quality, the indices with the largest differences between the spring and summer teas were taste and infusion color. The taste scores were higher for spring tea than for summer tea for 28 tea companies. The infusion color scores were higher for spring tea than for summer tea for 26 companies.
Fig. 1.
Distribution of Camellia sinensis var. Yinghong No. 9 black tea-producing companies and scatter plot of the sensory evaluation scores and metabolite ratios. (A) Sampling enterprise distribution map; (B) Ratio of sensory evaluation scores; (C) Ratio of total polyphenols, total amino acids, caffeine and catechin monomers; (D) Ratio of free amino acid; (E) Ratio of tea pigment and color chromaticity. Ratio represents the value of spring tea to the summer tea. TPP, total polyphenols; TAA, total amino acids; GA, gallic acid; GC, gallocatechin; EGC, epigallocatechin; C, catechin; CAF, caffeine; EC, epicatechin; EGCG, epigallocatechin gallate; ECG, epicatechin gallate; CG, catechin gallate; Thea, theanine; Glu, glutamic acid; Asp, aspartic acid; Asn, asparagine; Ala, alanine; Thr, threonine; Ser, serine; Gly, glycine; Pro, proline; Val, valine; Ile, isoleucine; Leu, leucine; Tyr, tyrosine; Phe, phenylalanine; Trp, tryptophane; Arg, argnine; TRs, thearubigins; TBs, theabrownins; TFs, total theaflavins; TF, theaflavin; TF-3-G, theaflavin-3-gallate; TFDG, theaflavin-3,3′-gallate; TF-3′-G, theaflavin-3′-gallate; L*, lightness; a* represents the degree of greenness (negative)- redness (positive); b* represents the degree of blueness (negative)-yellowness (positive); C* value represents color saturation, h° value reflects the chromaticity or tone of color.
3.2. Analysis of the polyphenol and caffeine contents in Yinghong no. 9 black tea in different seasons
The bitterness of the tea infusion is mainly due to polyphenols and alkaloids. Tea polyphenols, which are the main secondary metabolites, include catechins, flavonoids and their glycosides, anthocyanins, phenolic acids, and other compounds that contribute to the astringency of tea infusions (Wan, 2003). The indexes with significant difference were shown in the manuscript and the others were provided in Supplementary and Materials. The average TPP content in spring tea was 9.22%, whereas it was 10.24% in summer tea (Table S2). To reduce the of background differences due to the different pre-harvest management practices and post-harvest processing among different tea companies, the comparison was only performed between the spring and summer teas from the same company. For 26 of the 42 tea companies, the TPP content was higher in summer tea than in spring tea (Table S2), which may be related to the accelerated carbon cycle and TPP synthesis due to the higher temperatures in summer than in spring. Catechins are flavanol compounds that account for 70% of the TPP content. According to their structures, they are designated as galloylated catechins and non-galloylated catechins. The catechin contents, especially catechin (C), epicatechin (EC), and epicatechin gallate (ECG), were significantly higher in summer tea than in spring tea (Fig. 1C and Table S3). The average total catechin contents in spring tea and summer tea were 30.01 mg/g and 38.28 mg/g, respectively. The C, EC, and ECG contents were higher for summer tea than for spring tea for 36, 33, and 32 tea companies, respectively. The caffeine (CAF) content was significantly higher in spring tea than in summer tea because of the increase in nitrogen metabolism in spring, which is conducive to CAF synthesis. The average CAF contents in spring tea and summer tea were 39.31 and 35.25 mg/g, respectively.
The tea companies located outside Qingyuan city, which is the main production area, were tea company No. 29, 30, 31, 32, 33, 40, and 42 (Fig. 1A). The total catechin contents of the spring and summer teas produced by these companies were 19.09–51.36 mg/g and 21.95–51.36 mg/g, respectively (Table S3). The TPP contents of these spring and summer teas were 5.29 %–14.90% and 6.67%–13.56%, respectively. Of these seven tea companies, the total catechin content and TPP content were lower in spring tea than in summer tea for 5 and 6 companies, respectively, which was consistent with the results for the companies in Qingyuan. Therefore, the high bitterness of summer tea was mainly due to seasonal factors rather than geographical factors.
3.3. Analysis of the amino acid contents in Yinghong No. 9 black tea in different seasons
Amino acids are one of the main chemical components in tea. The TAA composition and content and the degradation and transformation products directly affect tea quality. High TAA levels increased the freshness of tea infusions (Fig. 1C and Table S2). The comparison of TAA contents indicated the TAA content was higher for spring tea than for summer tea for 28 tea companies. The average TAA contents for spring tea and summer tea were 3.08% and 2.80%, respectively. The freshness of spring tea was greater than that of summer tea. The detailed data of umami, sweet and bitter amino acids in spring and summer tea are shown in Tables S4, S5 and S6, respectively. For the tea companies in Qingyuan, the TAA content was higher for spring tea than for summer tea, which was consistent with the differences in the TAA contents of the tea samples produced by the companies outside Qingyuan.
3.4. Analysis of the tea infusion color and main contributing compounds in Yinghong no. 9 black tea in different seasons
Seasonal changes will affect the tea infusion color, even for the same black tea product. In terms of the chromaticity values, the L*, a*, b*, C*, and h° values were higher for summer tea than for spring tea for 30, 19, 23, 21, and 20 tea companies, respectively. The L* value was significantly lower for spring tea than for summer tea, reflecting the decrease in the brightness and intensification of the coloration of the spring tea infusion. The average L*, a*, and b* values for spring tea were respectively 41.97, 17.70, and 62.20 for spring tea and 43.92, 17.42, and 62.16 for summer tea (Table S7). Tea pigments, which are the products of the oxidation of TPP, are important components in the black tea infusion. The main tea pigment components were TFs, TRs, and TBs. The TFs, TRs, and TBs contents were higher in spring tea than in summer tea for 21, 18, and 34 tea companies, respectively (Fig. 1E and Table S7). The brightness of the spring tea infusion was lower than that of the summer tea infusion, likely because of the darkening of the color due to the longer fermentation period in spring than in summer. For five of the seven tea companies outside Qingyuan, the TBs content was higher in spring tea than in summer tea, which was in accordance with the corresponding trend for the tea samples produced in Qingyuan.
3.5. Determination of key differential metabolites
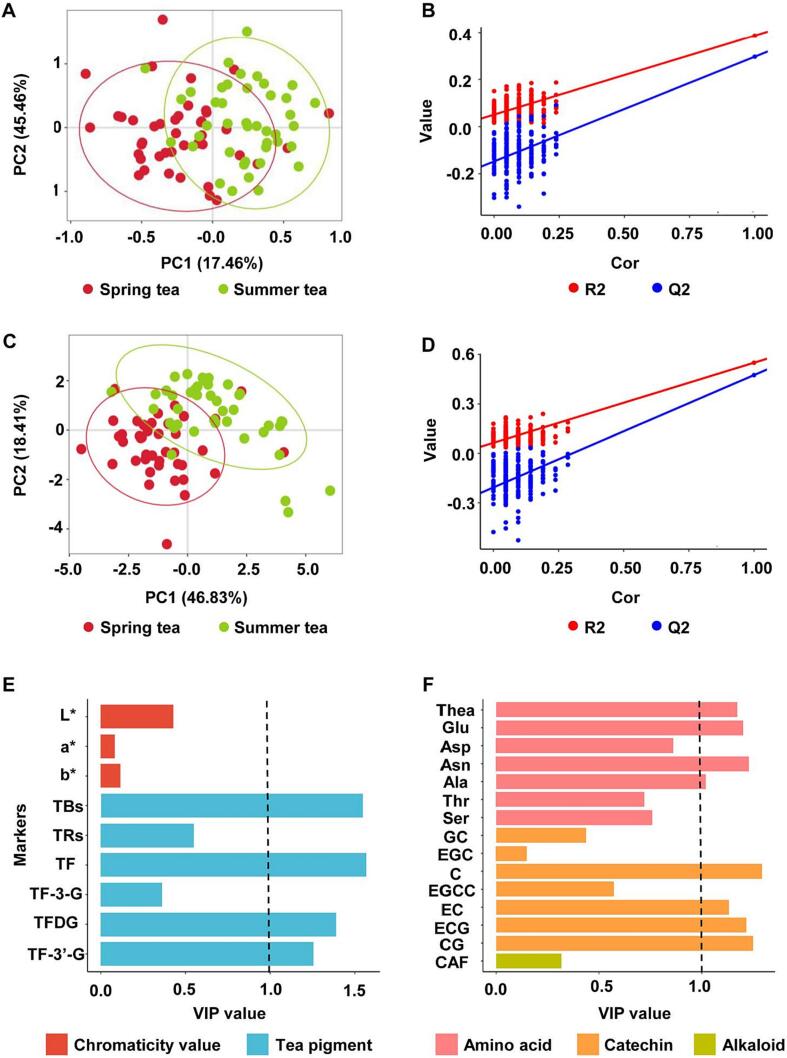
Principal component analysis (PCA) achieves dimensionality reduction of high-dimensional data by extracting the main characteristic components of the data. We found that the PCA model was not applicable and it was difficult to separate the tea samples. PLS-DA analysis was performed on metabolites contributing to infusion color and taste (Fig. 2A-D). In Fig. 2A, principal components 1 (PC1) and 2 (PC2) accounted for 17.46% and 45.46%. Variable Important for the Projection (VIP) value is calculated based on the PLS-DA model and is often used to analyze the correlation between metabolites and sample classification. Metabolites with VIP ≥ 1 were considered marker metabolites during the screening process. Substances with VIP values ≥ 1 are TF, TBs, TFDG and TF-3′-G, and their VIP values are 1.57, 1.55, 1.40 and 1.25, respectively (Fig. 2E). It suggested these tea pigments is the key different compounds between spring and summer black tea infusion color. As shown in Fig. 2C, principal components 1 (PC1) and 2 (PC2) accounted for 46.83 and 18.41%. Taste-related metabolites with VIP ≥ 1 are C, catechin gallate (CG), asparagine (Asn), ECG, glutamic acid (Glu), theanine (Thea), EC, phenylalanine (Phe) and alanine (Ala). Those VIP values are 1.29, 1.25, 1.23, 1.22, 1.20, 1.17, 1.13, 1.10 and 1.02, respectively (Fig. 2F). It indicated that these metabolites might be the reason for difference between spring and summer black tea taste quality.
Fig. 2.
Screening of important differential metabolites between spring and summer tea. Score plot of partial least square discriminant analysis (PLS-DA) plot based on tea pigments and chromaticity values; (B) Validation model of PLS-DA with 200 permutation tests (R2 = 0.39, Q2 = 0.30, Intercept R2 = 0.05, Intercept Q2 = -0.15); (C) PLS-DA plot based on tea taste metabolites; (D) Validation model of PLS-DA with 200 permutation tests (R2 = 0.55, Q2 = 0.47, Intercept R2 = 0.06, Intercept Q2 = -0.21); (E) VIP values of PLS-DA based on tea pigments and chromaticity values; (F) VIP values of PLS-DA based on tea taste metabolites. GC, gallocatechin; EGC, epigallocatechin; C, catechin; CAF, caffeine; EC, epicatechin; EGCG, epigallocatechin gallate; ECG, epicatechin gallate; CG, catechin gallate; Thea, theanine; Glu, glutamic acid; Asp, aspartic acid; Asn, asparagine; Ala, alanine; Thr, threonine; Ser, serine; Gly, glycine; Pro, proline; Val, valine; Ile, isoleucine; Leu, leucine; Tyr, tyrosine; Phe, phenylalanine; Trp, tryptophane; Arg, argnine; TRs, thearubigins; TBs, theabrownins; TF, theaflavin; TF-3-G, theaflavin-3-gallate; TFDG, theaflavin-3,3′-gallate; TF-3′-G, theaflavin-3′-gallate; L*, lightness; a* represents the degree of greenness (negative)-redness (positive); b* represents the degree of blueness (negative)-yellowness (positive).
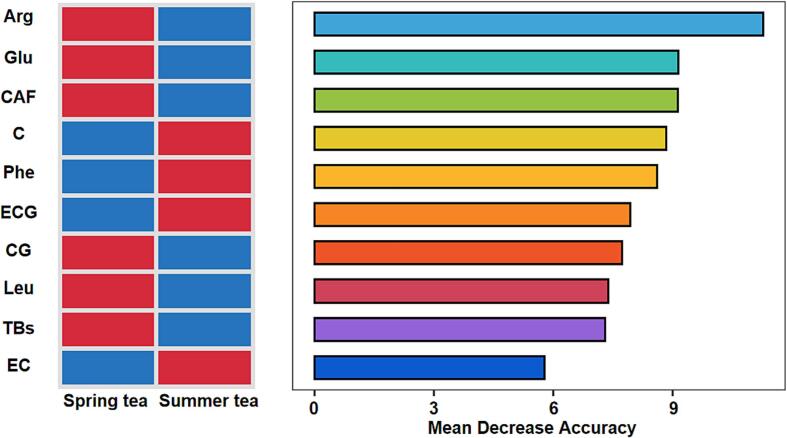
The random forest machine learning method is a method of analyzing data using decision trees, and is often used as a method for finding biomarkers in ecological research. We analyzed the taste metabolites, infusion color metabolites and chromaticity values of 84 Yinghong No. 9 black tea products from 42 tea companies through the random forest model. In addition, we also discovered differences in ten important metabolites between Yinghong No. 9 spring and summer tea (Fig. 3). Mean decrease accuracy (MDA) indicates the degree of reduction in the prediction accuracy of the random forest (Han et al., 2016). Variables with larger MDA values are more important. According to MDA value, we screened out ten important difference substances. They are Arg, Glu, CAF, C, Phe, ECG, CG, leucine (Leu), TBs and EC. Differences in ten marker metabolites between spring and summer tea are presented in a heat map in Fig. 3. C, Phe, ECG, and EC in summer tea are more than in spring tea.
Fig. 3.
Random forest model analysis of quality metabolites in 84 tea samples from 42 tea companies. Arg, argnine; Glu, glutamic acid; CAF, caffeine; C, catechin; Phe, phenylalanine; ECG, epicatechin gallate; CG, catechin gallate; Leu, leucine; TBs, theabrownins; EC, epicatechin.
3.6. Correlations among different substances contributing to infusion taste
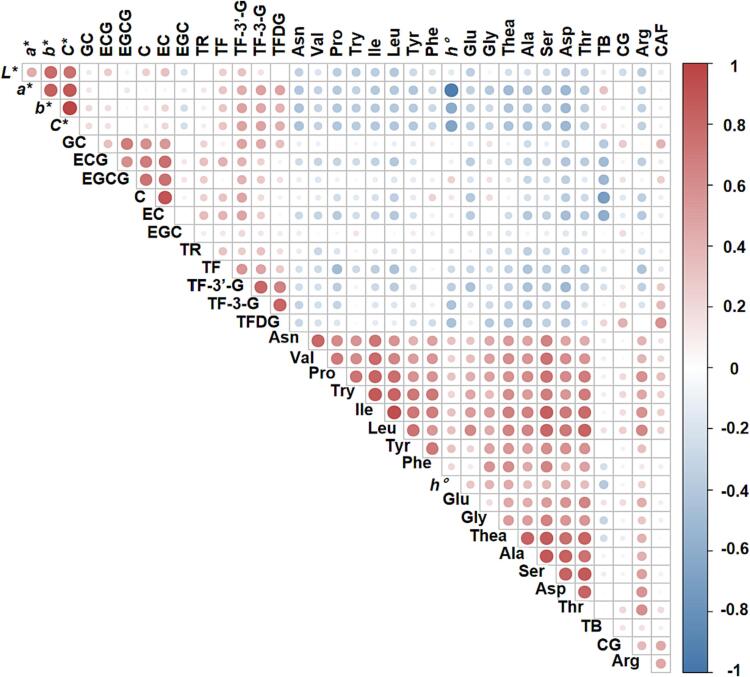
The taste metabolites, infusion color metabolites and chromaticity values of 84 Yinghong No. 9 black tea products were investigated using correlation analysis (Fig. 4). According to the characteristics of taste-related compounds, the correlations among catechins, CAF, and amino acids were determined (Table S8). There was a negative correlation between some of the catechins (C, EC, and ECG) and amino acids, whereas gallocatechin (GC), CG, and epigallocatechin gallate (EGCG) were not highly correlated with amino acids. Both EC and ECG were negatively correlated with umami and sweet amino acids, including Thea, Glu, aspartic acid (Asp), asparagine (Asn), alanine (Ala), threonine (Thr), serine (Ser), and proline (Pro), with correlation coefficients ranging from −0.246 to −0.478. Similarly, C was significantly negatively correlated with Glu, Asp, Ala, Thr, and Pro, with correlation coefficients ranging from −0.224 to −0.399. CAF was not highly correlated with amino acids, but it was positively correlated with the sweet amino acid Pro and the bitter amino acids isoleucine (Ile), Leu, tryptophan (Trp), and arginine (Arg), with correlation coefficients ranging from 0.230 to 0.463. The results were suggestive of metabolic competition between catechins (carbon source) and amino acids (nitrogen source).
Fig. 4.
Correlation analysis of quality metabolites in 84 tea samples from 42 tea companies. GC, gallocatechin; EGC, epigallocatechin; C, catechin; CAF, caffeine; EC, epicatechin; EGCG, epigallocatechin gallate; ECG, epicatechin gallate; CG, catechin gallate; Thea, theanine; Glu, glutamic acid; Asp, aspartic acid; Asn, asparagine; Ala, alanine; Thr, threonine; Ser, serine; Gly, glycine; Pro, proline; Val, valine; Ile, isoleucine; Leu, leucine; Tyr, tyrosine; Phe, phenylalanine; Trp, tryptophane; Arg, argnine; TRs, thearubigins; TBs, theabrownins; TFs, total theaflavins; TF, theaflavin; TF-3-G, theaflavin-3-gallate; TFDG, theaflavin-3,3′-gallate; TF-3′-G, theaflavin-3′-gallate; L*, lightness; a* represents the degree of greenness (negative)- redness (positive); b* represents the degree of blueness (negative)-yellowness (positive).
3.7. Correlations between the substances contributing to infusion colors and the chromaticity values
The chromaticity value measured using the colorimeter reflects the tea infusion color, with the color level mainly determined by tea pigments. We analyzed the correlation between tea pigments and chromaticity values (Table S9). The significant correlation between the TFs and L*, a*, b*, C*, and h° reflected the association between the TFs and the redness and yellowness of the tea infusion. These correlations were greater than the correlation between the TFs and brightness. The TFs were significantly correlated with the chromaticity values, with similar trends among the TFs. Specifically, theaflavin-3-gallate (TF-3-G) was not significantly correlated with L*, but it was significantly correlated with a*, b*, C*, and h°, implying TF-3-G may primarily contribute to the redness, yellowness, and saturation of the tea infusion, while having relatively little effect on the brightness. In addition, theaflavin-3-3′-digallate (TFDG), which is similar to TF-3-G, was significantly correlated with a*, b*, C*, and h°, but not with L*. There was also a significant correlation between theaflavin-3′-gallate (TF-3′-G) and L*, a*, b*, C*, and h°. Thus, all of the TF monomers contributed to the redness and yellowness of the tea infusion, but only TF and TF-3′-G appeared to contribute significantly to brightness. There were no significant correlations between the TRs and L*, a*, b*, C*, and h°, whereas the TBs were correlated with a* and h°, with correlation coefficients of 0.317 and −0.385, respectively. shown at Table 1, we used the tea pigment of samples from 35 tea companies as the dependent variable, and the chromaticity value significantly correlated with the tea pigment as the independent variable to fit the linear regression equation. The sample’s data of the remaining 7 tea companies were substituted into the model for verification.
Table 1.
Fitting model for theaflavins and colorimetric values of 35 companies.
| Marker compounds | Fitting model | Accuracy (%) |
|---|---|---|
| TFs | y = 0.125–0.121L*-0.096a*+0.222b* | 84.46 |
| TF | y = 0.012–0.012L*-0.01a*+0.022b* | 84.43 |
| TF-3-G | y = 0.421 + 0.019a*+0.021b* | 85.51 |
| TFDG | y = 0.243 + 0.033a*+0.005b* | 83.84 |
| TF-3′-G | y = -0.267–0.01L*-0.012a*+0.029b* | 83.34 |
Accuracy, fitting model to verify the accuracy of tea samples from the remaining 7 tea companies.
3.8. Correlations among infusion taste-contributing and color-contributing substances
To identify the factors contributing to the black tea color, we analyzed the correlations between the core secondary metabolites in the tea infusion and the color attributes of the tea infusion (Fig. 4 and Table S10). Tea pigments, which were the main compounds affecting the infusion color, are the result of the oxidation of TPP. Because of the metabolic competition between TPP (carbon source) and amino acids (nitrogen source), we speculated that tea pigments are correlated with TPP, TAA, CAF, and other substances. There was a positive correlation between TPP and TF, TF-3-G, TF-3′-G, L*, b*, and C* of the tea infusion, whereas TPP were negatively correlated with TBs. Accordingly, TPP positively affect the brightness, redness, yellowness, and saturation of the tea infusion, indicative of their beneficial effects on tea color quality. There was a negative correlation between TAA and tea pigments, although the correlation was not significant for the TBs. These results reflected the adverse effects of TAA on the black tea infusion quality-related characteristics (e.g., brightness, redness, and yellowness).
Similar to the TPP, the catechins (especially C, EC, and ECG) were positively correlated with TFs and TRs, but negatively correlated with TBs. There were no significant correlations between the catechins and chromaticity values. The umami amino acids (Thea, Glu, Asp, and Ala) and sweet amino acids (Thr, Ser, and Pro) were negatively correlated with the tea pigments and chromaticity values. These findings suggested the presence of the main umami taste-related compounds did not enhance the redness, yellowness, and saturation of the tea infusion. CAF was positively correlated with TFs, especially TF-3-G, TFDG, and TF-3′-G. CAF was not significantly correlated with TRs, TBs, and chromaticity values, implying it may not substantially affect the tea infusion color.
As shown in Table S11, we fit the linear regression equation using the taste quality contributions of samples from 35 tea companies as dependent variables and the colorimetric values significantly related to metabolites as independent variables. The sample data of the remaining seven tea companies were substituted into the model for verification. The prediction accuracy rates of TPP, TAA, Ser, Ile, Phe and Trp are all higher than 80%, which are 85.62%, 82.03%, 80.93%, 81.45%, 84.77% and 85.28%, respectively.
4. Discussion
4.1. Similar to green tea, spring Yinghong no. 9 black tea has a lower catechin content, but a higher amino acid content, than summer Yinghong no. 9 black tea
Yinghong No. 9 black tea samples were obtained from companies mainly located in Qingyuan city, Guangdong province, which is the main black tea-producing region in China. Samples from seven tea companies were collected in other cities. The spring tea samples produced by the companies outside Qingyuan were less bitter and astringent (related to the TPP content) and more refreshing (related to the TAA content) than the summer tea samples, which is consistent with the trends in the main black tea-producing region (Fig. 1). Therefore, the differences in the Yinghong No. 9 black tea quality were likely unrelated to geographical differences. The catechins in tea are mainly galloylated catechins. They contribute to the astringency of tea infusions, while their antioxidant and tumor prevention properties have beneficial effects on human health (Kerio et al., 2013, Long et al., 2023, Zeng et al., 2021). CAF accounts for 2%–4% of the tea dry weight. Yinghong No. 9 is a high-caffeine tea variety and average CAF content of spring tea and summer tea are 39.31 and 35.25 mg/g, respectively (Table S3). Moreover, it is the main contributor to the bitterness of tea infusions. The synthesis of CAF involves nitrogen metabolism. Earlier research showed CAF accumulates in tea leaves during the pre-harvest stage, with little change in the CAF content in the post-harvest stage (Wan, 2003). CAF with bitter-tasting influences other taste (e.g., umami), while also decreasing blood pressure and anxiety. It can also enhance tea plant resistance to pests and diseases (Hewavitharanage et al., 1999, Mohanpuria et al., 2010, Wan, 2003).
The difference in the quality of spring and summer teas is mainly caused by the differences in environmental conditions, which affect the synthesis of secondary metabolites (Huang et al., 2022, Xu et al., 2012). Light intensity and temperature can synergistically regulate catechin synthesis (Wang et al., 2022). In summer, catechins may function as antioxidants that protect tea plants from oxidative damage caused by intense light. Moreover, the accumulation of catechins is related to photosynthetic capacity. An exposure to intense light increases the photosynthetic rate of tea plants and induces the synthesis of galloylated catechin precursors (Xiang et al., 2021). In addition, the high temperatures in summer affect the synthesis of enzymes related to the phenylpropanoid pathway. The subsequent increase in catechin synthesis leads to increases in the TPP content of fresh tea leaves. Thus, tea beverages made using these leaves will tend to have a bitter taste. TPP and catechins in summer tea were higher than those in spring tea (Tables S2 and S3). CAF metabolism is associated with nitrogen metabolism. Therefore, increases in light intensity will increase the use of purine nucleotides for nucleic acid and energy metabolism in plants. A decrease in light intensity (e.g., under shade) reportedly inhibits the degradation of CAF, leading to the accumulation of CAF (Suzuki and Waller, 1985). CAF production is also influenced by temperature. More specifically, cool conditions will decrease the CAF content. In contrast, an exposure to strong sunlight and high temperatures in summer will promote CAF synthesis in fresh leaves (Wang et al., 2022).
Green tea, which is the most commonly consumed tea in China, accounts for 60% of the total tea yield. Additionally, because its production does not require the fermentation step, the quality of the fresh tea leaves may be maintained. Green tea produced using fresh leaves picked in spring is generally considered to be better than green tea produced using leaves harvested in summer or autumn (Ji et al., 2018). Summer green tea has a higher TPP content and a lower TAA content than spring green tea. These differences explain why summer green tea is less desirable (i.e., more bitter and less umami taste) than spring green tea (Dai et al., 2015). The diverse environmental conditions in different seasons influence the quality of green tea, which is not fermented. This shows similar results in fermented teas (Fig. 1A). The spring tea of Yinghong No. 9 black tea contains higher amino acid content than summer tea. The average amino acid content of spring tea and summer tea is 3.08 and 2.80%, respectively (Table S2). The complex processes required to produce black tea, especially fermentation, results in the oxidative degradation and conversion of a considerable proportion of the TPP to tea pigments. The complex processes also affect amino acids. These changes ultimately decrease the astringency of the tea infusion.
In this study, the catechin content of spring black tea was significantly lower than that of summer black tea (Table S3). This difference was greatest for ECG, which has the lowest bitterness detection threshold and the most intense taste among catechins (Narukawa et al., 2010). Therefore, spring tea was less astringent than summer tea. According to random forest model, dihydroxycatechin C, ECG and EC, which have lower bitterness threshold and higher taste intensity, are all marker compounds to distinguish the quality of spring and summer tea, and the trend of summer tea is higher than that of spring tea. The CAF content was significantly higher in spring tea than in summer tea (Fig. 2E and Table S3), which is in accordance with the effects of different seasons on fresh leaves and green tea. Black tea contains large amounts of TFs, TRs, and other tea pigments, which can combine with CAF to form compounds that increase tea infusion freshness (Mo et al., 2011). The final tea quality is typically positively correlated with the CAF content (Wan & Xia, 2015). Hence, spring Yinghong No. 9 black tea was less bitter and astringent than summer tea and had a fresher taste.
4.2. Spring black tea had a fresher taste than summer black tea, possibly because of differences in the Glu
Amino acids are the main taste-related compounds associated with freshness. They have been divided into proteinaceous and nonproteinaceous amino acids. Thea, which is the main amino acid in tea, contributes to the umami taste of the tea infusion and can offset the astringency and bitterness of catechins and CAF, while also having a calming effect and promoting sleepiness in humans (Yu and Yang, 2020, Yang et al., 2021). Tea also contains many proteinaceous amino acids. According to the differences in their taste characteristics, the proteinaceous amino acids have been categorized as follows: umami amino acids (e.g., Glu, Asp, and Ala), sweet amino acids (e.g., Thr, Ser, and Pro), and bitter amino acids (e.g., Val, Leu, and Trp) (Fig. 1D) (Scharbert and Hofmann, 2005). Because of their relatively low abundance and high taste detection thresholds, the bitter amino acids generally do not contribute to tea taste (Scharbert and Hofmann, 2005). Umami amino acids, such as Glu, have a low taste detection threshold, enabling them to increase tea taste freshness (Liu et al., 2014). Accordingly, they are positively correlated with tea quality. We also found that the taste quality of spring tea of Yinghong No. 9 black tea is generally higher than that of summer tea, and Glu is the umami amino acid with the greatest difference between spring tea and summer tea (Fig. 1B and D).
An increase in light intensity in summer promotes carbon metabolism, which will decrease the synthesis of proteinaceous amino acids (e.g., Glu), while also inhibiting the hydrolysis of proteins and the degradation of chloroplasts (Chen et al., 2017, Wan and Xia, 2015). In the present study, the TAA content was significantly lower in summer tea than in spring tea. The summer tea also had a weak umami taste (Fig. 1B). During the pre-harvest stage, shading is often used to decrease carbon assimilation and increase the amino acid content (Chen et al., 2021). In addition, darkness can promote chloroplast degradation and protein hydrolysis, which is conducive to the formation of umami amino acids, thereby enhancing the umami taste of summer tea (Chen et al., 2017). The umami taste was greater in spring tea than in summer tea, which may be related to the greater abundance of Glu rather than Thea (Fig. 1D). The Thea content did not differ significantly between the spring and summer teas of 42 tea companies, whereas there were highly significant differences in the Glu (Table S4). Meanwhile, based on the analysis of PLS-DA and random forest model, Glu may be an important metabolite to distinguish spring and summer tea quality (Fig. 2F and Fig. 3). Due to the high threshold of bitter amino acids, the contribution of Arg to tea infusion is generally not considered. Notably, Glu is an important precursor of Thea and a key contributor to the umami taste. Among the 42 tea companies, 33 have higher Glu content in spring tea than in summer tea, while only 20 of them have higher levels of Thea in spring tea than in summer tea (Fig. 1D). Scharbert and Hofmann (2005) suggested that the taste detection threshold for Thea is relatively high, implying it has no significant effect on the umami taste. In contrast, compared with its umami and sweetness detection thresholds, Thea has a much lower astringency detection threshold (Scharbert and Hofmann, 2005). Moreover, compared with Thea, the umami taste of Glu and Asp is more easily detected (i.e., lower taste detection threshold). This is consistent with the results of our sensory evaluation, the taste score of spring tea is higher than that of summer tea (Fig. 1B). After Thea, Glu is the second most important umami amino acids in tea. All three of these amino acids can form hydrogen bonds with galloylated catechins, thereby decreasing the astringency of the galloylated catechins. However, compared with Thea, Glu requires less energy to form hydrogen bonds with galloylated catechins. Thus, it tends to decrease the astringency of galloylated catechins more than Thea (Liu et al., 2023).
During the withering and fermentation steps of the black tea production process, Leu and glutamine can be converted to α-isohexanoic acid and Glu via a reaction catalyzed by a transaminase (Wan, 2003). In addition, during the withering process, Thea levels decrease significantly, mainly because of enzymatic hydrolysis (Wan & Xia, 2015). The relatively low temperatures in spring typically necessitate a long withering stage during the production of Yinghong No. 9 black tea. The degradation of Thea and the synthesis of Glu tend to be greater in spring tea than in summer tea and this is consistent with our results (Table S4).
4.3. There is a correlation between color- and taste-contributing substances in the Yinghong no. 9 black tea infusion
The positive correlations among TPP, TFs, and chromaticity values, including L*, a*, b*, and C*, imply TPP may significantly influence the yellowness, redness, brightness, and saturation of tea infusions (Fig. 4 and Table S10). In addition, TAA and free amino acids were negatively correlated with the color-contributing compounds. The color-contributing compounds in black tea, which is a typical fully fermented tea, were mainly tea pigments, including TFs, TRs, and TBs. Tea pigments are derived from the oxidation of TPP. Black tea, which has a considerable abundance of TPP, often contains large amounts of tea pigments, which can enhance the tea infusion quality (i.e., increased redness and yellowness). The tea organic matter content can be divided into the following two categories: nitrogen-containing compounds and carbon-containing compounds. The carbon:nitrogen ratio can directly affect tea quality. Furthermore, TPP are important products of the carbon metabolism cycle. Catechins, which are the main TPP, are synthesized by the shikimic acid pathway and the phenylpropane pathway. In addition to the synthesis of catechins, intermediate compounds, such as α-ketoglutarate, oxaloacetate, and pyruvate, are also produced and Glu, Asp, Ala, Ser, and other amino acids are subsequently synthesized. A branch upstream of the TPP synthesis pathway synthesizes aromatic amino acids (Wan, 2003). Therefore, there is a competitive relationship between the synthesis of TPP and TAA. The increase of TPP production will lead to the decrease of TAA synthesis, and TPP is beneficial to the synthesis of tea infusion color contributors. Therefore, TAA is also negatively correlated with TAA and color substances. This is similar to our findings (Table S10).
4.4. The chromaticity value may be used as an indicator of the quality of the infusion color and taste of Yinghong no. 9 black tea
The degree of fermentation during the production of tea affects the tea infusion and the color-contributing compounds. Because of the fixation during the processing of non-fermented teas, polyphenol oxidase (PPO) and peroxidase (POD) are inactivated at high temperatures and the oxidation of catechins is terminated. Non-fermented teas maintain the quality of fresh tea leaves better than fermented teas. Flavonoid glycosides and the remaining chlorophyll are the main contributors to the coloration of non-fermented tea infusions (Zhang et al., 2019). In high-quality fermented tea, including black tea, PPO and POD remain active for a relatively long time and the conversion of TPP to other compounds continues. As the products of TPP oxidation, tea pigments are the primary contributor to fermented tea infusion colors (Chen et al., 2023).
During the oxidation of catechins, catechins are first oxidized to o-quinones, which are unstable and mostly reddish-brown or red. Additionally, they tend to polymerize and form intermediate products (i.e., diphenol quinones). Because of the instability of biphenol quinones, they are reduced to diflavanols via a disproportionation reaction; the other product of this reaction is subsequently condensed and oxidized to form phenol ketones and TF, TF-3-G, TF-3′-G, and TFDG, among which TFDG has the lowest astringency detection threshold. TFs are generally considered to be positively correlated with the quality of black tea. TF, TF-3-G, TF-3′-G, and TFDG are significantly positively correlated with the a* and b* values of tea infusion, which shows that they have important contributions to the redness and yellowness of black tea infusion color (Fig. 4). More specifically, they can enhance the brightness and yellowness of the black tea infusion, while also increasing the strength and freshness of the taste. Earlier research identified TFs as the core components responsible for the excellent quality-related characteristics of black tea (Wan, 2003). Thearubigins, which are the products of the oxidation of TFs, are the most abundant polyphenolic oxides in black tea, accounting for approximately 5%–19% (dry weight). The following are the three main pathways for the synthesis of TRs: direct oxidation of non-galloylated catechins and galloylated catechins; oxidation of the intermediates of the synthesis of TFs; and auto-oxidation and the oxidative coupling of TFs. According to the differences in molecular weight, TRs have been divided into three categories, namely TRs-SI (high molecular weight), TRs-SII (moderate molecular weight), and TRs-SIII (low molecular weight). Recent research confirmed TRs produce a brownish-red color and are the main contributors to the redness of the black tea infusion as well as an important contributor to the intensity of the taste (Long et al., 2023). The astringency of TRs is not as high as that of TFs, resulting in a sweeter taste. In contrast, TBs are associated with a dark brown color and a flat and slightly sweet taste. They are the main compounds responsible for the darkening of black tea infusions, generally accounting for 4%–9% (dry weight) (Table S7).
Tea pigment contents and ratios are often used as important indicators of black tea quality. High TFs and TRs contents, a TR:TF ratio of 10–15, and a low TB content are reportedly characteristics of high-quality tea infusions (Wan, 2003). Low TFs and TRs contents adversely affect the brightness and redness of tea infusions, suggestive of insufficient fermentation. Excessive fermentation is typically reflected by a dark tea infusion that has a high TBs content. The L*, a*, and b* values represent the brightness, redness, and yellowness of the tea infusion, respectively. Changes in chromaticity values are closely related to the tea pigment content (Table S9). According to their correlations, increases in the a* and b* values are indicative of increases in the polyphenol content and decreases in the amino acid content of black tea and this is consistent with our correlation analysis (Table S10).
Moreover, the linear regression equation fitted based on the correlation analysis of tea pigment and chromaticity value has a good predictive effect.The prediction accuracy of the equations fitted with TFs, TF, TF-3-G, TFDG and TF-3′-G as dependent variables were 84.46%, 84.43%, 85.51%, 83.84% and 83.34%, respectively (Table 1). As for taste-contributing compounds, the prediction accuracy of TPP, TAA, Ser, Ile, Phe and Trp is over 80% (Table S11). The results suggested that we can quickly predict the tea pigment by measuring the chromaticity value of the tea infusion and taste with a color difference meter. This provides new insights into rapid analysis of black tea quality.
5. Conclusions
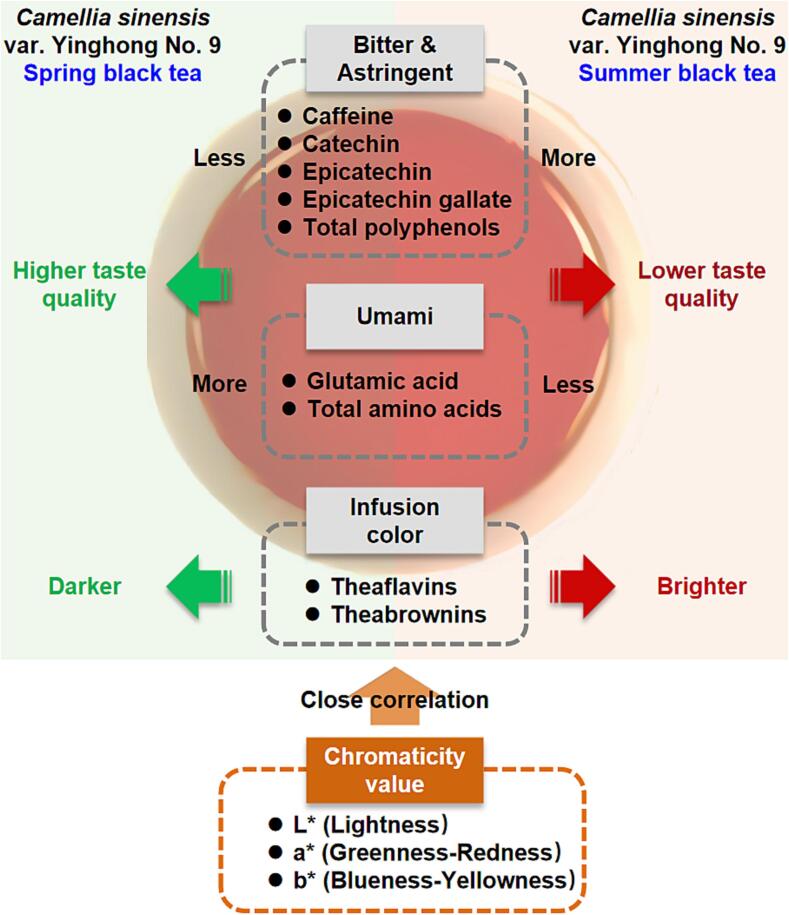
The effects of seasonal conditions on the quality of fresh tea leaves and non-fermented teas, such as green tea, have been reported, but the effects on teas that require a more complex production process, including black tea, remain unclear. On the basis of the results of a sensory evaluation, this study focused on the taste and infusion color of black tea produced using the leaves of Yinghong No. 9 plants (large-leaf variety). The differences in the spring and summer teas were consistent with the reported differences in the fresh leaves and green tea between the spring and summer. The compounds associated with astringency and bitterness were less abundant in spring tea than in summer tea. Additionally, the Glu, which contribute to the umami taste (sweetness), were higher in spring tea than in summer tea (Fig. 5). The analysis of the main components contributing to the black tea infusion color indicated that the TBs content was significantly higher in spring tea than in summer tea. In terms of chromaticity, the L* value was lower for spring tea than for summer tea. Furthermore, the L*, a*, b*, C*, and h° values were correlated with many compounds contributing to the taste and infusion color. Accordingly, these values may be useful for rapid evaluations of the quality of Yinghong No. 9 black tea (Fig. 5). In this study, we preliminarily analyzed the key compounds responsible for the differences between spring and summer Yinghong No. 9 black teas, while also identifying candidate marker compounds relevant for developing a rapid quality-evaluation system.
Fig. 5.
Summary of the differences between spring and summer black tea made from Camellia sinensis var. Yinghong No. 9.
CRediT authorship contribution statement
Chengshun Liu: Methodology, Data curation, Validation, Investigation, Writing – original draft, Writing – review & editing. Jianlong Li: Resources, Data curation, Investigation, Funding acquisition. Hanxiang Li: Data curation, Investigation. Jinghua Xue: Investigation. Miao Wang: Investigation. Guotai Jian: Investigation. Chen Zhu: Investigation. Lanting Zeng: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was supported by from the Guangdong Natural Science Foundation for Distinguished Young Scholar (2023B1515020107), South China Botanical Garden, Chinese Academy of Sciences (QNXM-202302), Guangdong Forestry Bureau (Key Laboratory of Plant Ex Situ Protection and Utilization in South China) (E336030011), Guangdong Provincial Department of Science and Technology Rural Science and Technology Commissioner Project (KTP20210351), the Foundation of Science and Technology Program of Guangzhou (2023A04J0717), the Science and Technology Project of Yunfu (2022020201), the fund for China Agriculture Research System (CARS-19), the special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (R2023PY-JG022).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100998.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Chen J.M., Wu S.H., Dong F., Li J.L., Zeng L.T., Tang J.C., Gu D.C. Mechanism underlying the shading-induced chlorophyll accumulation in tea leaves. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.779819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang H.J., Ye Y., Wang Y.F., Xu P. Structural insight into polyphenol oxidation during black tea fermentation. Food Chemistry: X. 2023;17 doi: 10.1016/j.fochx.2023.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.Y., Fu X.M., Mei X., Zhou Y., Cheng S.H., Zeng L.T.…Yang Z.Y. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. Journal of Proteomics. 2017;157:10–17. doi: 10.1016/j.jprot.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Cui Y.Q., Lai G.P., Wen M.C., Han Z.S., Zhang L. Identification of low-molecular-weight color contributors of black tea infusion by metabolomics analysis based on UV−visible spectroscopy and mass spectrometry. Food Chemistry. 2022;386 doi: 10.1016/j.foodchem.2022.132788. [DOI] [PubMed] [Google Scholar]
- Dai W.D., Qi D.D., Yang T., Lv H.P., Guo L., Zhang Y.…Lin Z. Nontargeted analysis using ultraperformance liquid chromatography−quadrupole time-of-flight mass spectrometry uncovers the effects of harvest season on the metabolites and taste quality of tea (Camellia sinensis L.) Journal of Agricultural and Food Chemistry. 2015;63:9869–9878. doi: 10.1021/acs.jafc.5b03967. [DOI] [PubMed] [Google Scholar]
- Gao C.X., Huang Y., Li J., Lyu S.H., Wang Z.H., Xie F.…Sun W.J. Relationship between the grade and the characteristic flavor of PCT (Panyong Congou Black Tea) Foods. 2022;11:2815. doi: 10.3390/foods11182815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.Y., Ho C.T., Schwab W., Wan X.C. Aroma profiles of green tea made with fresh tea leaves plucked in summer. Food Chemistry. 2021;363 doi: 10.1016/j.foodchem.2021.130328. [DOI] [PubMed] [Google Scholar]
- Han H., Guo X.L., Yu H. Variable selection using mean decrease accuracy and mean decrease Gini based on Random Forest. IEEE. 2016;2016 doi: 10.1109/ICSESS.2016.7883053. [DOI] [Google Scholar]
- Hewavitharanage P., Karunaratne S., Kumar N.S. Effect of caffeine on shot-hole borer beetle (Xyleborusfornicatus) of tea (Camellia sinensis) Phytochemistry. 1999;51:35–41. doi: 10.1016/S0031-9422(98)00610-4. [DOI] [Google Scholar]
- Ho C.T., Zheng X., Li S. Tea aroma formation. Food Science and Human Wellness. 2015;4:9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- Huang F.F., Li Y., Yang P.D., Liu Z.H., Huang J.A., Xiong L.G., Li J. Relationship between theanine, catechins and related genes reveals accumulation mechanism during spring and summer in tea plant (Camellia sinensis L.) Scientia Horticulturae. 2022;302 doi: 10.1016/j.scienta.2022.111142. [DOI] [Google Scholar]
- Ji H.G., Lee Y.R., Lee M.S., Hwang K.H., Park C.Y., Kim E.H.…Hong Y.S. Diverse metabolite variations in tea (Camellia sinensis L.) leaves grown under various shade conditions revisited: A metabolomics study. Journal of Agricultural and Food Chemistry. 2018;66:1889–1897. doi: 10.1021/acs.jafc.7b04768. [DOI] [PubMed] [Google Scholar]
- Kerio L.C., Wachira F.N., Wanyoko J.K., Rotich M.K. Total polyphenols, catechin profiles and antioxidant activity of tea products from purple leaf coloured tea cultivars. Food Chemistry. 2013;136:1405–1413. doi: 10.1016/j.foodchem.2012.09.066. [DOI] [PubMed] [Google Scholar]
- Liu P.P., Deng Y.L., Yin J.F., Zhang Y.N., Chen G.S., Wang F.…Xu Y.Q. Quantitative analysis of the taste and its correlation research of chemical constitutes of green tea. Journal of Chinese Institute of Food Science and Technology. 2014;14:173–181. doi: 10.16429/j.1009-7848.2014.12.029. In Chinese. [DOI] [Google Scholar]
- Liu Z.Y., Ran Q.S., Li Q., Yang T., Dai Y.Q., Zhang T.…Long L. Interaction between major catechins and umami amino acids in green tea based on electronic tongue technology. Journal of Food Science. 2023;2023:1–14. doi: 10.1111/1750-3841.16543. [DOI] [PubMed] [Google Scholar]
- Long P.P., Rakariyatham K., Ho C.T., Zhang L. Thearubigins: Formation, structure, health benefit and sensory property. Trends in Food Science & Technology. 2023;133:37–48. doi: 10.1016/j.tifs.2023.01.013. [DOI] [Google Scholar]
- Lu G.Q., Jiang J.R., Wang Q., Wei S.Y. Analysis of tea remains from the warring states period tomb No. 1 in Xigang Cemetery, the old city of Zoucheng City. Shandong Provence. Archeology and Cultural Relics. 2021;247:118–122. In Chinese. [Google Scholar]
- Mo T., Zhang W.L., Li P. Changes and regulation mechanisms of key quality components in tea processing. Journal of Chinese Institute of Food Science and Technology. 2011;11:176–180. doi: 10.16429/j.1009-7848.2011.09.010. In Chinese. [DOI] [Google Scholar]
- Mohanpuria P., Kumar V., Yadav S.K. Tea caffeine: Metabolism, functions, and reduction strategies. Food Science and Biotechnology. 2010;19:275–287. doi: 10.1007/s10068-010-0041-y. [DOI] [Google Scholar]
- Narukawa M., Kimata H., Noga C., Watanabe T. Taste characterisation of green tea catechins. International Journal of Food Science & Technology. 2010;45:1579–1585. doi: 10.1111/j.1365-2621.2010.02304.x. [DOI] [Google Scholar]
- Qi D.D., Li J.X., Qiao X.Y., Lu M.L., Chen W., Miao A.Q.…Ma C.Y. Non-targeted metabolomic analysis based on ultra-high-performance liquid chromatography quadrupole time-of-flight tandem mass spectrometry reveals the effects of grafting on non-volatile metabolites in fresh tea leaves (Camellia sinensis L.) Journal of Agricultural and Food Chemistry. 2019;67:6672–6682. doi: 10.1021/acs.jafc.9b01001.y. [DOI] [PubMed] [Google Scholar]
- Sanlier N., Gokcen B.B., Altuğ M. Tea consumption and disease correlations. Trends in Food Science & Technology. 2018;78:95–106. doi: 10.1016/j.tifs.2018.05.026. [DOI] [Google Scholar]
- Scharbert S., Hofmann T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. Journal of Agricultural and Food Chemistry. 2005;53:5377–5384. doi: 10.1021/jf050294d. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Waller G.R. Effects of light on the production and degradation of caffeine in Camellia sinensis L. seedlings. Plant and Cell Physiology. 1985;26:765–768. doi: 10.1093/oxfordjournals.pcp.a076968. [DOI] [Google Scholar]
- Wan X.C., editor. Tea biochemistry. 3rd ed. China Agriculture Press; Beijing: 2003. (in Chinese) [Google Scholar]
- Wan X.C., Xia T., editors. Secondary metabolism of tea plant. 1st ed. Science Press; Beijing: 2015. (in Chinese) [Google Scholar]
- Wang M., Yang J., Li J.L., Zhou X.C., Xiao Y.Y., Liao Y.Y.…Zeng L.T. Effects of temperature and light on quality-related metabolites in tea [Camellia sinensis (L.) Kuntze] leaves. Food Research International. 2022;161 doi: 10.1016/j.foodres.2022.111882. [DOI] [PubMed] [Google Scholar]
- Xiang P., Wilson I.W., Huang J.X., Zhu Q.F., Tan M., Lu J.…Lin J.K. Co-regulation of catechins biosynthesis responses to temperature changes by shoot growth and catechin related gene expression in tea plants (Camellia sinensis L.) The Journal of Horticultural Science and Biotechnology. 2021;96:228–238. doi: 10.1080/14620316.2020.1830721. [DOI] [Google Scholar]
- Xie G.H., Yan J.N., Lu A.X., Kun J.R., Wang B., Song C.D.…Meng Q. Characterizing relationship between chemicals and in vitro bioactivities of teas made by six typical processing methods using a single Camellia sinensis cultivar, Meizhan. Bioengineered. 2021;12:1251–1263. doi: 10.1080/21655979.2021.1903237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W.P., Song Q.S., Li D.X., Wan X.C. Discrimination of the production season of Chinese green tea by chemical analysis in combination with supervised pattern recognition. Journal of Agricultural and Food Chemistry. 2012;60:7064–7070. doi: 10.1021/jf301340z. [DOI] [PubMed] [Google Scholar]
- Xu X.Q., Mo H.Z., Yan M.C., Zhu Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick−tea by gas chromatography/mass spectrophotometry. Journal of the Science of Food and Agriculture. 2007;87:1502–1504. doi: 10.1002/jsfa.2874. [DOI] [Google Scholar]
- Yang T.Y., Xie Y.X., Lu X., Yan X.M., Wang Y., Ma J.Z.…Zhang Z.L. Shading promoted theanine biosynthesis in the roots and allocation in the shoots of the tea plant (Camellia sinensis L.) cultivar Shuchazao. Journal of Agricultural and Food Chemistry. 2021;69:4795–4803. doi: 10.1021/acs.jafc.1c00641. [DOI] [PubMed] [Google Scholar]
- Yu Z.M., Yang Z.Y. Understanding different regulatory mechanisms of proteinaceous and nonproteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Critical Reviews in Food Science and Nutrition. 2020;60:844–858. doi: 10.1080/10408398.2018.1552245. [DOI] [PubMed] [Google Scholar]
- Zeng L.T., Zhou Y., Fu X.M., Mei X., Cheng S.H., Gui J.D.…Yang Z.Y. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chemistry. 2017;237:488–498. doi: 10.1016/j.foodchem.2017.05.137. [DOI] [PubMed] [Google Scholar]
- Zeng L.T., Zhou X.C., Liao Y.Y., Yang Z.Y. Roles of specialized metabolites in biological function and environmental adaptability of tea plant (Camellia sinensis) as a metabolite studying model. Journal of Advanced Research. 2021;34:159–171. doi: 10.1016/j.jare.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ho C.T., Zhou J., Santos J.S., Armstrong L., Granato D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Comprehensive Reviews in Food Science and Food Safety. 2019;18:1474–1495. doi: 10.1111/1541-4337.12479. [DOI] [PubMed] [Google Scholar]
- Zhang T., Fang K., Ni H., Li T., Li L.J., Li Q.B., Chen F. Aroma enhancement of instant green tea infusion using β-glucosidase and β-xylosidase. Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2020.126287. [DOI] [PubMed] [Google Scholar]
- Zhang W.J., Cao J.X., Li Z.G., Li Q.H., Lai X.F., Sun L.L.…Lai Z.X. HS-SPME and GC/MS volatile component analysis of Yinghong No. 9 dark tea during the pile fermentation process. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.