Abstract
Background
Vitamin B12 and folate are essential micronutrients important for normal infant growth and development.
Objectives
The aims were to describe vitamin B12 and folate status in pregnant females and their infants according to commonly used status cutoffs and examine the associations between maternal status, maternal supplement use, and breastfeeding and infant status.
Methods
Pregnant females were recruited at 18 wk gestation in Bergen, Norway. Maternal vitamin B12 and folate status were measured at gestational weeks 18 (n = 136) and 36 (n = 116), and infant status was measured at ages 3 (n = 73) and 6 (n = 74) mo.
Results
At gestational weeks 18 and 36, respectively, 4.4% and 2.6% of the mothers had plasma cobalamin concentrations <148 pmol/L, 0.7% and 6.9% had methylmalonic acid (MMA) concentrations >0.26 μmol/L, and 3.7% and 30% had folate concentrations <10 nmol/L. None of the females had total homocysteine (t-Hcy) concentrations >13 μmol/L or 3 combined indicator of vitamin B12 (cB12) < −0.5. At 3 and 6 mo, respectively, 4.1% and 5.4% of the infants had cobalamin concentrations <148 pmol/L, 63% and 74% had t-Hcy concentrations >6.5 μmol/L, 59% and 66% had MMA concentrations >0.26 μmol/L, and 47% and 60% had cB12 > −0.5. None of the infants had folate concentrations <10 nmol/L. Several of the vitamin B12 biomarkers in infants were associated with maternal vitamin B12 status during pregnancy. Breastfed infants had lower vitamin B12 status (as indicated by plasma cobalamin, t-Hcy, and cB12) than nonbreastfed infants at both 3 and 6 mo. Use of supplements during pregnancy was associated with better vitamin B12 status among infants at 3 and 6 mo, as indicated by infants’ cobalamin and t-Hcy concentrations.
Conclusions
Subclinical vitamin B12 deficiency among infants was common and associated with maternal vitamin B12 status during pregnancy and breastfeeding. Among the mothers, an increase in biochemical folate deficiency was discovered toward the end of gestation. Further studies are needed to investigate clinical consequences.
This trial was registered at clinicaltrials.gov as NCT02610959.
Keywords: infants, pregnancy, breastfeeding, cobalamin, folate, homocysteine, methylmalonic acid, cB12, Mommy’s Food Study, supplement, micronutrient status
Introduction
The micronutrients vitamin B12 (cobalamin) and folate are closely related metabolically and involved in several processes in the body, including maturation and division of cells and myelination of the nervous system [1,2]. These vitamins are therefore essential for normal growth and development of the fetus during pregnancy and in the first years of life [2,3]. Pregnant females and infants are recognized to be at particular risk of vitamin B12 deficiency [4]. Studies from high- and low-income settings around the world have reported high prevalences of biochemical signs of subclinical vitamin B12 deficiency in infants [[5], [6], [7], [8], [9], [10]]. Furthermore, a recent meta-analysis including 57 studies worldwide estimated that 25% of pregnant females had poor vitamin B12 status during gestation [11]. The importance of folate in pregnancy is well recognized, and fortification programs and supplementation of folate before and during early pregnancy are implemented globally [12,13]. In Norway, it is advised to take a daily folate supplement of 400 μg from preconception through the first 12 wk of pregnancy [14].
Despite the reports of the high prevalence of poor vitamin B12 status among infants, overt and subclinical deficiency may be difficult to detect. Signs and symptoms of vitamin B12 deficiency in infants are often diffuse and overlap with other conditions. Symptoms may include failure to thrive, irritability, refusal of solid foods, megaloblastic anemia, and developmental regression [15]. Furthermore, subclinical vitamin B12 status may be asymptomatic. Therefore, the diagnosis of deficiency and subclinical vitamin B12 status is often dependent on biochemical testing.
Several biochemical indicators of vitamin B12 and folate status are available. Total cobalamin is a widely used direct measure reflecting total circulating vitamin B12 status [16]. Methylmalonic acid (MMA) is a product of a reaction that requires cobalamin as a cofactor, where circulating MMA concentrations rise with poor vitamin B12 status [17]. When assessing vitamin B12 status, it is recommended to use more than one biomarker [18], reflected by the development of the combined indicator of vitamin B12 (cB12) status developed by Fedosov et al. [19]. cB12 includes 2 to 4 biomarkers of vitamin B12 accounting for folate status and age when estimating vitamin B12 status [16,19]. Conversion of homocysteine (Hcy) in 1-carbon metabolism is dependent on both vitamin B12 and folate. Therefore, total Hcy (t-Hcy) is used as a functional biomarker of both vitamin B12 and folate status [17], where deficiency or low status of either of these vitamins may lead to elevated t-Hcy concentrations [16,17]. In adults, t-Hcy is primarily considered an indicator of folate status [20], while in infants, there is a consensus that t-Hcy is the preferred indicator of vitamin B12 status [16]. In addition, folate may be measured using serum/plasma folate concentrations, which is widely used for evaluating folate status [21].
Little is known about vitamin B12 and folate status in Norwegian pregnant females and their infants. Therefore, we aimed to describe vitamin B12 and folate status among pregnant females and their infants in Norway using indicators of vitamin B12 (plasma cobalamin, t-Hcy, MMA, and cB12) and folate status (plasma folate and t-Hcy). We also examined the relationship between maternal status in pregnancy and infant status during their first 6 mo of life and investigated whether maternal dietary supplement use during pregnancy and breastfeeding affected infants’ vitamin B12 or folate status.
Methods
Study design and participants
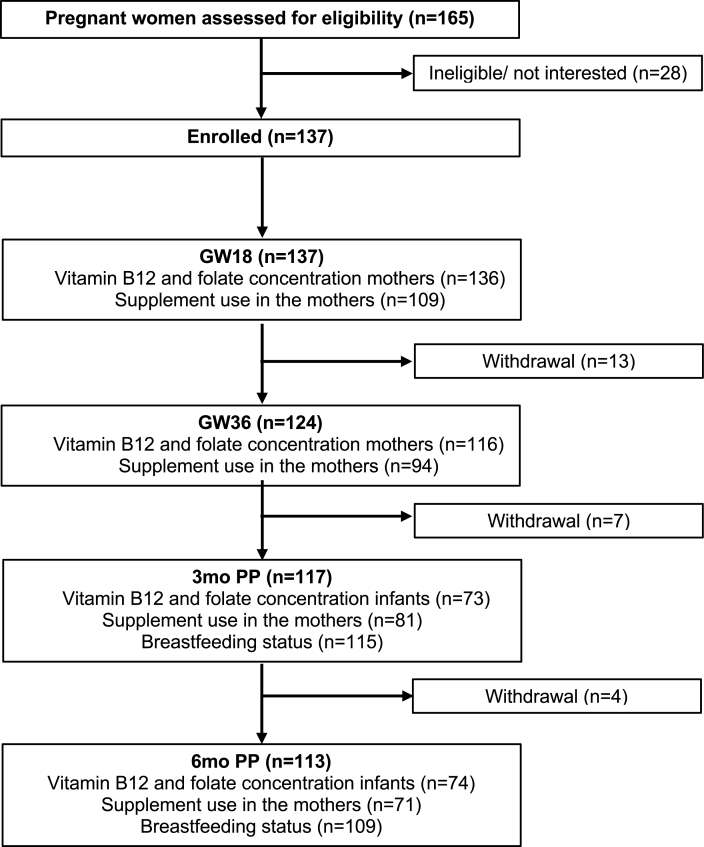
The present study is a secondary analysis from the “Mommy’s Food” study (NCT02610959), which was conducted in Bergen, Norway (2016–2018). The study was a randomized controlled trial, where a total of 137 pregnant females were assigned to either receive Atlantic cod (Gadus morhua) twice weekly or to continue with their habitual diet from gestational week (GW) 20 to 36. The main outcome of the study was to investigate the difference in urinary iodine concentrations between groups before and after the intervention. In this secondary analysis, the pregnant females and infants from both study arms were included in an observational cohort design. Further information about the study design and protocol is described in detail elsewhere [22,23]. Pregnant females were recruited from January 2016 until February 2017 prior to their routine ultrasound at GW 18 at the Women’s Health Clinic at Haukeland University Hospital, Bergen, Norway. Recruitment through online broadcasts was also used to achieve a higher participation rate. The pregnant females were followed-up, and data were collected at GW 18 and 36 and further with their infants at 3 and 6 mo of age. A flow chart of the study population, the number of participants, and the available data collected is given in Figure 1.
FIGURE 1.
Flowchart of the study population. Abbreviations: GW18, gestation week 18; GW36, gestation week 36; PP, postpartum.
The inclusion criteria included pregnant females with primiparous singleton pregnancies in GW ≤19 who spoke and/or understood Norwegian writing. Given the intervention with Atlantic cod, females who were allergic to fish or had chronic diseases affecting iodine status were excluded. All study participants provided written informed consent. The trial was conducted in accordance with the Declaration of Helsinki and was approved by the Regional Ethical Committee for Medical and Health Research Ethics West (2015/897).
Blood sampling
Blood samples were collected from the pregnant females at GW 18 and 36 and from the infants at ages 3 and 6 mo. Venous blood for plasma preparation was collected in BD Vacutainer K2EDTA 5.4 mg (adults) and 3.6 mg (infants) vials and centrifuged (1000–1300 × g, 20°C, 10 min) within 30 min prior to plasma separation. Capillary blood samples from the infants’ heel or fingertip were collected if venipuncture was not possible. The fingertip or heel was warmed with a hot water balloon prior to the capillary blood sampling to ensure sufficient blood flow. For the heel pricks, a Tenderfoot ITC heel-incision device (Accriva Diagnostics) was used. For the finger pricks, ACCU-Check Safe-T-Pro Plus lancets (Roche Diagnostics) was used. After collection, the capillary blood was placed in a BD Microtainer Blood Collection Tube K2EDTA (Becton, Dickinson and Co.) and centrifuged (1000–1300 × g, 20°C, 10 min) within 30 min prior to plasma separation. The infants were given 1 to 2 mL of 25% sucrose-water solution for pain relief prior to the sampling.
Analyses of vitamin B12 and folate status
Plasma samples were analyzed at Bevital Laboratory, Bergen, Norway (www.bevital.no). Concentrations of plasma cobalamin and folate were analyzed by microbiological assays based on colistin sulfate-resistant strain of Lactobacillus leichmannii [24] and a chloramphenicol-resistant strain of Lactobacillus casei [25], respectively. The within-day coefficient of variation (CV) for cobalamin and folate was 4%, while the between-day variation was 5% for both biomarkers [26]. Gas chromatography-tandem mass spectrometry based on methyl chloroformate derivatization was used to determine plasma t-Hcy and MMA concentrations [26]. The within-day CV ranged from 1% to 5%, whereas the between-day CV ranged from 1% to 3%.
Definition of vitamin B12 and folate status
Plasma cobalamin, t-Hcy, and MMA concentrations were used to describe vitamin B12 status. cB12 status was calculated as the log of (cobalamin/t-Hcy×MMA) multiplied by a constant while adjusting for folate concentration and age [19]. Higher cB12 indicates better vitamin B12 status. Plasma folate and t-Hcy concentrations were used to assess folate status. An overview of the cutoffs used to define low concentrations of vitamin B12 and folate is provided in Table 1 [27, 28].
TABLE 1.
Cutoffs used to assess vitamin B12 and folate status
| Indicator | Cutoff | Definition |
|---|---|---|
| Vitamin B12 status | ||
| Cobalamin | <148 pmol/L | Low B12-status [20,27] |
| t-Hcy | >13 μmol/L (mothers) | Elevated t-Hcy [16] |
| >6.5 μmol/L (infants) | Subclinical B12 deficiency [16] | |
| MMA | >0.26 | |
| cB12 | < −0.5 | Low B12 status [19] |
| Folate status | ||
| Folate | <10 nmol/L | Folate deficiency [28] |
| t-Hcy | >13 μmol/L (mothers) | Elevated t-Hcy [16] |
Abbreviations: cB12, combined indicator for vitamin B12 status based on the concentrations of t-Hcy, MMA, and cobalamin; MMA, methylmalonic acid; t-Hcy, total homocysteine.
Breastfeeding status
Information regarding breastfeeding status was collected using 24-h dietary recalls conducted during the study visits at 3 and 6 mo postpartum. In the statistical analyses, the breastfeeding status was categorized as: 1) exclusively and partly breastfed infants or 2) nonbreastfed infants. Exclusively breastfed was defined using WHO’s definition of exclusively breastfeeding: “breastfeeding with no other food or drink, not even water.” With this definition, prescribed medication, vitamins, and minerals are not counted as fluids or foods [29].
Maternal vitamin B12 supplement use
Information regarding maternal supplement use was collected from a food frequency questionnaire (FFQ) completed at GW 18 and 36 and 3 and 6 mo postpartum. The FFQ was electronic and semiquantitative and aimed to capture participants’ habitual diet and dietary supplement use [30]. In the FFQ completed in GW 18, participants were asked to report an estimate of their diet since they became pregnant, whereas the FFQ in GW 36 covered the intake during the last 16 wk (approximately since the last completed FFQ). The FFQs completed at 3 and 6 mo postpartum were intended to cover participants’ diet during the past 3 mo. The questions regarding dietary supplement use included type and intake frequency. In the statistical analyses, participants were categorized into 2 categories: 1) B-vitamin supplement users: females that took a multivitamin and/or a supplement with B vitamins or 2) non-B vitamin supplement users: those only taking other supplements (not B vitamins or multivitamins) or no supplements.
Statistical analysis
For the baseline characteristics, categorical variables are reported as frequencies and percentages, while continuous variables are reported as mean values (SD) for normally distributed variables, and medians and interquartile ranges (IQRs) for variables with a skewed distribution. The correlations between the different biomarkers and between different time points were estimated using the Spearman rank order correlation. The associations were considered weak, moderate, or strong if the correlation coefficient (rho) was >0.10, >0.40, or >0.70, respectively [31]. Dose-response curves displaying the associations between maternal (GW 18 and 36) and infant (3 and 6 mo) status cB12 and folate status were also made using the “twoway fpfitci” command in STATA. Generalized linear models with an identity link function and of the Gaussian distribution family were used to investigate the association between maternal dietary supplement use and breastfeeding with infant biomarker-concentrations at ages 3 and 6 mo. The outcome variables (cobalamin, t-Hcy, MMA, and folate) were log-transformed to approximate normal distribution. Statistical analyses were performed using STATA version 17 (StataCorp). P values < 0.05 were considered statistically significant.
Results
A total of 137 of the 165 eligible pregnant females agreed to participate in the study (Figure 1). The pregnant females who were included in the study had an average age of 29 y. Most were either cohabiting or married and had a medium to high household income. At 3 mo of age, 79% of the infants were exclusively breastfed, while at 6 mo of age, only 3% was exclusively breastfed, with the majority (90%) of infants being partially breastfed. Data on supplement use in the infants were collected from 115 participants at 3 mo and 109 participants at 6 mo of age. Among these infants, 3 were found to be using supplements containing folate and other B vitamins at 3 mo, while only 1 infant was using such supplement at 6 mo of age. None of the infants reported the use of supplements containing vitamin B12 at either 3 or 6 mo of age. Demographic information and baseline characteristics of the mothers and infants included in the study are summarized in Table 2.
TABLE 2.
Baseline characteristics of mothers and infants in the Mommy’s Food Study
| Characteristics | n | mean (SD) or % |
|---|---|---|
| Mothers | ||
| Age, y | 135 | 29.3 (3.8) |
| BMI before pregnancy1, kg/m2 | 132 | 23.1 (4.0) |
| BMI during pregnancy2, kg/m2 | 118 | 24.5 (4.0) |
| Education, y | ||
| 13 | 19 | 14.3 |
| 14–17 | 33 | 24.8 |
| >17 | 81 | 60.9 |
| Household income, NOK | ||
| Low (<549,999) | 39 | 29.3 |
| Medium (550,000–1,249,999) | 77 | 57.9 |
| High (>1,250,000) | 17 | 12.8 |
| Marital status | ||
| Single or divorced | 2 | 1.5 |
| Cohabitant | 85 | 63.9 |
| Married | 43 | 32.3 |
| Other | 3 | 2.3 |
| Supplement users3 | ||
| GW18 | ||
| Users | 79 | 72.5 |
| Nonusers | 30 | 27.5 |
| GW36 | ||
| Users | 53 | 43.6 |
| Nonusers | 41 | 56.4 |
| 3 mo postpartum | ||
| Users | 39 | 48.2 |
| Nonusers | 42 | 51.9 |
| 6 mo postpartum | ||
| Users | 29 | 40.9 |
| Nonusers | 42 | 59.2 |
| Infants | ||
| Sex (female) | 58 | 50.4 |
| Birth weight, g | 93 | 3491 (536) |
| GA at time of delivery, wk | 122 | 40.2 (2.1) |
| Preterm delivery4 | 6 | 4.9 |
| Breastfeeding status, 3 mo | ||
| Not breastfed | 5 | 4.4 |
| Partially breastfed | 19 | 16.5 |
| Exclusively breastfed | 91 | 79.1 |
| Breastfeeding status, 6 mo | ||
| Not breastfed | 8 | 7.3 |
| Partially breastfed | 98 | 89.9 |
| Exclusively breastfed | 3 | 2.8 |
Abbreviations: BMI, body mass index; GA, gestational age; GW18, gestation week 18; GW36, gestation week 36; NOK, Norwegian Krone; SD, standard deviation.
Values are presented as means (SDs) or percentages.
Calculated using self-reported height and previous weight.
Calculated using self-reported current weight at gestation week 18 of the pregnancy.
Users defined as those taking B-vitamins and/or multivitamins, nonusers defined as those only taking other types of supplements or no supplement.
Preterm delivery defined if child was born before gestation week 37.
Direct and functional measures of vitamin B12 and folate status for the pregnant females and infants are presented in Table 3, together with the distribution of the participants according to the different biomarker cutoffs. At GW 18 and 36, 4.4% and 2.6% of the females had cobalamin concentrations <148 pmol/L, while 0.7% and 6.9% of the females had MMA concentrations >0.26 μmol/L, respectively. None of the mothers had t-Hcy >13 μmol/L or cB12 < −0.5 during pregnancy, indicating adequate vitamin B12 status. At GW 18, 3.7% of the pregnant females had folate concentrations <10 nmol/L, increasing to 30.2% at GW 36, indicating decreasing folate status throughout gestation. A total of 4.1% and 5.4% of the infants had cobalamin concentrations <148 pmol/L at 3 and 6 mo of age, respectively. At 3 mo of age, 63.0%, 58.9%, and 46.6% of the infants had t-Hcy >6.5 μmol/L, MMA >0.26 μmol/L and cB12 < −0.5, respectively, suggesting subclinical vitamin B12 deficiency. At 6 mo of age, the proportions increased to 74.3%, 66.2%, and 59.5%, indicating decreasing vitamin B12 status among the infants during the first 6 mo of life. None of the infants had folate concentrations <10 nmol/L at either 3 or 6 mo of age, indicating sufficient folate status.
TABLE 3.
Vitamin B12 and folate status in the mothers and infants
| Mothers |
Infants |
|||||||
|---|---|---|---|---|---|---|---|---|
| GW18 (n = 136)1 |
GW36 (n = 116)2 |
3 mo (n = 73)3 |
6 mo (n = 74) |
|||||
| Median (IQR) | Def, n (%) | Median (IQR) | Def, n (%) | Median (IQR) | Def, n (%) | Median (IQR) | Def, n (%) | |
| Cob | 220.0 (184.5, 301.9) | 6 (4.4) | 224.4 (187.9, 291.1) | 3 (2.6) | 244.9 (199.0, 321.8) | 3 (4.1) | 221.6, (194.2, 324.8) | 4 (5.4) |
| t-Hcy | 4.7 (4.3, 5.3) | 0 (0.0) | 5.7 (4.9, 6.5) | 0 (0.0) | 7.3 (6.2, 8.8) | 46 (63.0) | 7.5 (6.5, 8.8) | 55 (74.3) |
| MMA | 0.1 (0.1, 0.2) | 1 (0.7) | 0.2 (0.1, 0.2) | 8 (6.9) | 0.4 (0.2, 0.9) | 43 (58.9) | 0.5 (0.2, 1.0) | 49 (66.2) |
| Folate | 26.4 (16.4, 34.9) | 5 (3.7) | 14.2 (9.4, 27.3) | 35 (30.2) | 42.0 (34.7, 58.7) | 0 (0.0) | 45.7 (34.2, 63.4) | 0 (0.0) |
| cB12 | 0.7 (0.5, 0.9) | 0 (0.0) | 0.4 (0.2, 0.7) | 0 (0.0) | −0.4 (−1.0, 0.2) | 34 (46.6) | −0.7 (−1.0, 0.0) | 44(59.5) |
Abbreviations: cB12, combined indicator for vitamin B12 status based on the concentrations of t-Hcy, MMA, and cobalamin; Cob, cobalamin, Def, deficiency; GW18, gestation week 18; GW36, gestation week 36; IQR, interquartile range; MMA, methylmalonic acid; t-Hcy, total homocysteine.
Values are presented at medians and IQRs. The following cutoff values were used for calculating the percentage with low status of the biomarkers: Cob: <148 pmol/L; t-Hcy: >13 μmol/L for the mothers and >6.5 μmol/L for the infants; MMA: >0.26 μmol/L; folate: <10 nmol/L; and cB12: <−0.5.
n = 134 for cB12 at GW18.
n = 114 for cB12 and cobalamin at GW36.
n = 72 for folate concentrations in infants 3 mo.
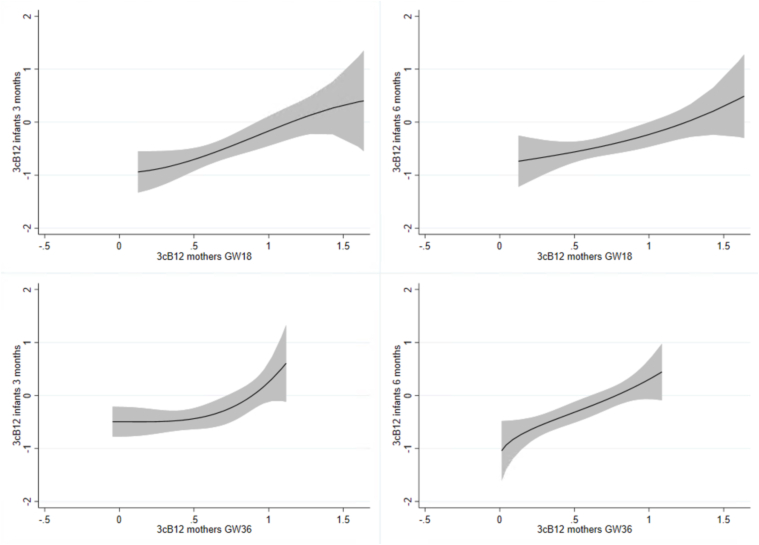
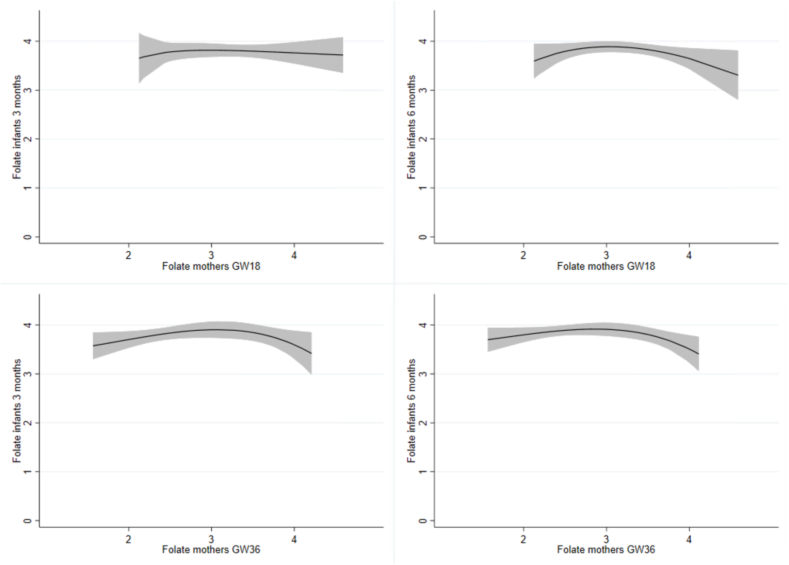
Several of the indicators for vitamin B12 status indicators in the infants (cobalamin, t-Hcy, and cB12) were associated with indicators of vitamin B12 status in the mothers (cobalamin, MMA, t-Hcy, and cB12) during pregnancy (Table 4). As depicted in Figure 2, maternal cB12 status during pregnancy at GW18 and GW36 was moderately associated with infant cB12 status at age 3 and 6 mo (P < 0.001). Maternal folate status had a weak association with infants’ t-Hcy status at 3 mo and infant cobalamin status at 6 mo but not with infant folate status at any of the time points (Table 4 and Figure 3).
TABLE 4.
Correlation matrix of vitamin B12 and folate status in the mothers and infants
| Mothers GW18 (n = 136) |
Mothers GW36 (n = 116) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cobalamin | t-Hcy | MMA | Folate | cB12 | Cobalamin | t-Hcy | MMA | Folate | cB12 | ||
| Infants 3 mo (n = 73) | Cobalamin | 0.36 (0.002) | −0.29 (0.013) | −0.15 (0.218) | 0.21 (0.075) | 0.37 (0.001) | 0.39 (0.001) | −0.25 (0.037) | −0.27 (0.022) | 0.25 (0.036) | 0.44 (<0.001) |
| t-Hcy | −0.28 (0.017) | 0.18 (0.124) | 0.21 (0.081) | −0.27 (0.023) | −0.33 (0.004) | −0.39 (0.001) | 0.40 (<0.001) | 0.34 (0.004) | −0.32 (0.006) | −0.54 (<0.001) | |
| MMA | −0.15 (0.196) | 0.06 (0.598) | 0.32 (0.006) | 0.04 (0.730) | −0.29 (0.013) | −0.22 (0.077) | −0.02 (0.884) | 0.31 (0.010) | 0.08 (0.521) | −0.26 (0.031) | |
| Folate | 0.14 (0.265) | 0.01 (0.909) | −0.01 (0.965) | −0.06 (0.627) | 0.03 (0.831) | 0.06 (0.655) | −0.10 (0.405) | −0.05 (0.685) | 0.01 (0.904) | 0.08 (0.534) | |
| cB12 | 0.26 (0.026) | −0.19 (0.113) | −0.34 (0.004) | 0.04 (0.735) | 0.41 (<0.001) | 0.33 (0.006) | −0.14 (0.243) | −0.38 (0.001) | 0.04 (0.732) | 0.43 (<0.001) | |
| Infants 6 mo (n = 74) | Cobalamin | 0.22 (0.060) | −0.15 (0.217) | −0.07 (0.578) | 0.24 (0.043) | 0.22 (0.059) | 0.29 (0.014) | −0.19 (0.119) | −0.17 (0.142) | 0.29 (0.016) | 0.31 (0.009) |
| t-Hcy | −0.13 (0.287) | 0.16 (0.184) | 0.25 (0.031) | −0.18 (0.126) | −0.24 (0.036) | −0.21 (0.076) | 0.27 (0.021) | 0.36 (0.002) | −0.13 (0.287) | −0.42 (<0.001) | |
| MMA | −0.17 (0.146) | 0.07 (0.528) | 0.43 (<0.001) | 0.05 (0.700) | −0.40 (<0.001) | −0.27 (0.025) | 0.19 (0.106) | 0.48 (<0.001) | 0.01 (0.923) | −0.49 (<0.001) | |
| Folate | 0.18 (0.130) | −0.04 (0.726) | −0.04 (0.737) | −0.06 (0.612) | 0.10 (0.397) | 0.07 (0.580) | 0.06 (0.618) | −0.09 (0.469) | −0.07 (0.575) | 0.04 (0.767) | |
| cB12 | 0.23 (0.053) | −0.09 (0.442) | −0.42 (<0.001) | 0.06 (0.612) | 0.43 (<0.001) | 0.36 (0.002) | −0.26 (0.031) | −0.52 (<0.001) | 0.11 (0.357) | 0.59 (<0.001) | |
Abbreviations: cB12, combined indicator for vitamin B12 status based on the concentrations of t-Hcy, MMA, and cobalamin; GW18, gestation week 18; GW36, gestation week 36; MMA, methylmalonic acid; t-Hcy, total homocysteine.
Pairwise Spearman correlation was used investigate the relationship between the infants and mothers’ biomarkers for folate and vitamin B12 status. The results are presented as Spearman’s rho (P value). Significant values in bold.
FIGURE 2.
Dose-response curves of cB12 concentrations in the mothers and infants. The figure displays the relationship between mothers’ cB12 status during pregnancy and infants’ cB12 status at 3 and 6 mo of age with 95% confidence intervals. Abbreviations: cB12, combined indicator for vitamin B12 status based on the concentrations of total homocysteine, methylmalonic acid, and cobalamin; GW18, gestation week 18, GW36, gestation week 36.
FIGURE 3.
Dose-response curves of log-transformed folate concentrations in the mothers and infants. The figure displays the relationship between mothers’ folate status during pregnancy and infants’ folate status at 3 and 6 mo of age with 95% confidence intervals. Abbreviations: GW18, gestation week 18; GW36, gestation week 36.
Breastfed infants had lower vitamin B12 status, as indicated by plasma cobalamin and t-Hcy concentrations, and cB12, compared with nonbreastfed infants at 3 and 6 mo of age (Table 5 and Table 6).
TABLE 5.
| Log cob |
Log t-Hcy |
Log MMA |
Log folate |
cB12 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp (95%CI) | P | Exp (95%CI) | P | Exp (95%CI) | P | Exp (95%CI) | P | Coef. (95%CI) | P | |
| Infant status 3 mo | ||||||||||
| BF status 3 mo | 0.041 | |||||||||
| Non-BF (n = 3) | Ref | Ref | Ref | Ref | Ref | |||||
| BF (n = 70) | 0.64 (0.43, 0.94) | 0.023 | 1.44(1.04, 2.00) | 0.030 | 2.04(0.60, 6.94) | 0.251 | 0.63(0.38, 1.06) | 0.080 | −0.94(−1.83, −0.04) | 0.041 |
| Infant status 6 mo | ||||||||||
| BF status 6 mo | ||||||||||
| Non-BF (n = 7) | Ref | Ref | Ref | Ref | Ref | |||||
| BF (n = 66) | 0.50 (0.40, 0.64) | <0.001 | 1.55 (1.30, 1.85) | <0.001 | 3.15 (1.58, 6.29) | 0.001 | 0.70 (0.52, 0.93) | 0.015 | −1.40 (−1.89, −0.90) | <0.001 |
Abbreviations: BF, breastfed; cB12, combined indicator for vitamin B12 status based on the concentrations of t-Hcy, MMA, and cobalamin; CI, confidence interval; cob, cobalamin; GW18, gestation week 18, GW36, gestation week 36; MMA, methylmalonic acid; PP, postpartum; Ref, reference; t-Hcy, total homocysteine.
Generalized linear models were used to investigate the infant’s status at 3 and 6 mo depending on the breastfeeding status at 3 and 6 mo. Significant values in bold.
n = 72 for Log folate 3 mo.
The results are presented as Exp (exponentiated coefficients and corresponding 95% confidence intervals) or Coef (coefficient estimates and corresponding 95% confidence intervals).
TABLE 6.
Vitamin B12 status in the infants according to breastfeeding status
| 3 mo |
6 mo |
|||||||
|---|---|---|---|---|---|---|---|---|
| BF(n = 70) |
Non-BF (n = 3) |
BF (n = 66) |
Non-BF (n = 7) |
|||||
| Median (IQR) | Def, n (%) | Median (IQR) | Def, n (%) | Median (IQR) | Def, n (%) | Median (IQR) | Def, n (%) | |
| Cob | 239.1 (194.9, 313.0) | 3 (4.3) | 390.8 (364,0, 446.8) | 0 (0.0) | 212.8 (188.2, 277.1) | 4 (6.1) | 461.0, 374,5, 562,4) | 0 (0.0) |
| t-Hcy | 7.5 (6.3, 9.0) | 46 (65.7) | 4.7 (4.7, 6.1) | 0 (0.0) | 7.8 (6.7, 9.0) | 54 (81.8) | 5.6 (4.3, 5.6) | 0 (0.0) |
| MMA | 0.4 (0.2, 1.0) | 42 (60.0) | 0.2 (0.2, 0.3) | 1 (33.3) | 0.6 (0.3, 1.1) | 49 (74.2) | 0.2 (0.2, 0.3) | 0 (0.0) |
| cB12 | −0.5 (−1.0, 1.4) | 34 (48.6) | 0.5 (0.3, 0.5) | 0 (0.0) | −0.8 (−1.1, −0.2) | 44 (66.7) | 0.6 (0.6, 1.1) | 0 (0.0) |
Abbreviations: cB12, combined indicator for vitamin B12 status based on the concentrations of t-Hcy, MMA, and cobalamin; Cob, cobalamin, Def, deficiency; IQR, interquartile range; MMA, methylmalonic acid; t-Hcy, total homocysteine.
Values are presented at medians and IQRs. The following cutoff values were used for calculating the percentage with low status of the biomarkers: Cob: <148 pmol/L, t-Hcy: >6.5 μmol/L, MMA: >0.26 μmol/L, and cB12: <-0.5.
Infant plasma cobalamin concentrations at 3 mo of age were estimated to be 33% (9%–63%) higher if the mother used B-vitamin supplements at GW 18 (P = 0.005), and 20%(1%–44%) higher at 6 mo of age if the mother used B-vitamin supplements at GW 36. Further, infant t-Hcy concentrations at 6 mo of age were estimated to be 15% (1%–27%) lower if the mother used B-vitamin supplements at GW 18. No associations between maternal B-vitamin supplement use and infant folate status were observed (Table 7).
TABLE 7.
Infants vitamin B12 and folate status according to maternal supplement use3
| Log cob |
Log t-Hcy |
Log MMA |
Log folate |
cB12 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp (95%CI) | P | Exp (95%CI) | P | Exp (95%CI) | P | Exp (95%CI) | P | Coef. (95%CI) | P | |
| Infants’ status 3 mo | ||||||||||
| Supplement use GW18, (n = 60)1 | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 1.33 (1.09, 1.63) | 0.005 | 0.89 (0.76, 1.05) | 0.165 | 0.95 (0.50, 1.79) | 0.863 | 1.07 (0.81, 1.41) | 0.641 | 0.28 (−0.20, 0.77) | 0.249 |
| Supplement use GW36 (n = 57)2 | ||||||||||
| No | ||||||||||
| Yes | 1.10 (0.91, 1.32) | 0.323 | 0.98 (0.84, 1.14) | 0.777 | 1.11 (0.64, 1.91) | 0.717 | 1.00 (0.80, 1.26) | 0.985 | 0.01 (−0.39, 0.42) | 0.954 |
| Supplement use 3 mo PP (n = 48) | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 1.09 (0.91, 1.32) | 0.351 | 0.86 (0.74, 1.00) | 0.056 | 1.00 (0.55, 1.81) | 0.995 | 1.08 (0.84, 1.38) | 0.553 | 0.15 (−0.30, 0.60) | 0.512 |
| Infants’ status 6 mo | ||||||||||
| Supplement use GW18 (n = 59) | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 1.21 (0.95, 1.52) | 0.118 | 0.85 (0.73, 0.99) | 0.042 | 0.74 (0.42, 1.28) | 0.276 | 0.88 (0.69, 1.12) | 0.295 | 0.41 (−0.05, 0.86) | 0.084 |
| Supplement use GW36 (n = 60) | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 1.20 (1.01, 1.44) | 0.043 | 0.89 (0.78, 1.02) | 0.104 | 0.91 (0.56, 1.49) | 0.707 | 0.87 (0.71, 1.06) | 0.161 | 0.24 (−0.15, 0.63) | 0.223 |
| Supplement use 3 mo PP (n = 51) | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 1.10 (0.91, 1.33) | 0.334 | 0.92 (0.81, 1.05) | 0.212 | 1.39 (0.85, 2.25) | 0.188 | 1.01 (0.83, 1.24) | 0.900 | −0.09 (−0.48, 0.29) | 0.641 |
| Supplement use 6 mo PP (n = 48) | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 1.02 (0.82, 1.27) | 0.875 | 0.88 (0.77, 1.02) | 0.089 | 1.26 (0.73, 2.18) | 0.398 | 0.95 (0.77, 1.18) | 0.670 | −0.06 (−0.48, 0.36) | 0.785 |
Abbreviations: cB12, combined indicator for vitamin B12 status based on the concentrations of t-Hcy, MMA, and cobalamin; CI, confidence interval, cob, cobalamin; GW18, gestation week 18, GW36, gestation week 36; MMA, methylmalonic acid; PP, postpartum; Ref, reference; t-Hcy, total homocysteine.
Generalized linear models were used to investigate the infant’s status at 3 and 6 mo depending on the mothers supplement status at 4 time points (GW18, GW36, and 3 and 6 mo postpartum). Mothers who used multivitamins and/or B-vitamins were considered supplement users while those taking only other supplements or no supplements were considered nonsupplement users. Significant values in bold.
n = 59 for folate.
n = 56 for folate.
The results are presented as Exp (exponentiated coefficients and corresponding 95% confidence intervals) or Coef (coefficient estimates and corresponding 95% confidence intervals).
Discussion
In this study of healthy pregnant females and their infants from Norway, we found that a considerable number of infants had low vitamin B12 status at 3 and 6 mo of age, but sufficient folate status. The pregnant females had sufficient vitamin B12 status during pregnancy, but a high proportion had folate deficiency at the end of pregnancy. We also found an association between maternal vitamin B12 status during pregnancy and infant vitamin B12 status at 3 and 6 mo of age. Additionally, the study indicated that breastfed infants had lower vitamin B12 status compared with nonbreastfed infants, and B-vitamin supplement use during pregnancy was associated with better vitamin B12 status among infants during the first 6 mo of life.
In the current study, more than half of the infants had elevated plasma t-Hcy concentrations at 3 and 6 mo. t-Hcy is the preferred marker of vitamin B12 status in the first 2 y of life, but it increases with both vitamin B12 and folate deficiency [16]. In the current study, none of the infants had folate deficiency (defined as plasma folate concentrations <10 nmol/L); thus, the elevated plasma t-Hcy concentrations most likely reflect subclinical vitamin B12 deficiency in this group. Previous studies in Norway have reported a prevalence of approximately 45% to 70% of infants with elevated t-Hcy concentrations [[5], [6], [7]]; thus, our study confirms and adds to these previous findings.
Similar to the high prevalence of subclinical vitamin B12 deficiency, defined as t-Hcy concentrations >6.5 μmol/L in our study, our results suggest the infants also had low vitamin B12 status according to the combined indicator of vitamin B12 status, cB12, where a total of 47% of infants had a low vitamin B12 status at 3 mo, which further increased to 60% at 6 mo. Despite the utility of cB12, taking more than one biomarker of vitamin B12 into account when assessing vitamin B12 status, it is important to note that the use of cB12 as a marker of vitamin B12 status has not yet been validated for use in infancy. Therefore, further studies evaluating the use of the combined indicator in infant populations are necessary. Nonetheless, taking our infant vitamin B12 status observations together with the findings of previous studies suggests that a high proportion of Norwegian infants have biochemical signs of subclinical vitamin B12 deficiency.
Few of the mothers had poor vitamin B12 status during pregnancy, but maternal vitamin B12 status at GW 18 and 36 were moderately associated with infants’ vitamin B12 status at 3 and 6 mo of age. These results are in line with previous studies showing that maternal vitamin B12 status during pregnancy is associated with infant vitamin B12 status from birth until 2 y of age [[32], [33], [34], [35], [36], [37], [38]]. cB12 was the index with the strongest association between the mothers’ and infants’ statuses at all time points. Our results add to the existing knowledge that mothers’ vitamin B12 status during pregnancy is important for infants’ status during the first months of life.
Maternal folate status during pregnancy was not associated with infants’ folate status at 3 and 6 mo of age. Similarly, Hay et al. [33] did not find an association between maternal folate status during pregnancy and the infants’ folate status at 6 mo of age. Our findings indirectly support the view that folate concentration in human milk is relatively stable and independent of the mothers’ folate status [39] and that infants are protected against low folate status even when the mothers’ folate status is low [40].
We found that the t-Hcy in breastfed infants was 44% (4%–100%) higher compared with that of the nonbreastfed infants at age 3 mo, increasing to 55% (30%–85%) higher at age 6 mo. Previous studies among Norwegian and Danish infants also concluded that breastfed infants had lower vitamin B12 status compared with nonbreastfed infants at 6 mo to 2 y of age [41,42]. Also, human milk from well-nourished females contains lower concentrations of vitamin B12 compared with infant formula [43]. In our study, only 3 and 7 infants included in the analysis were not breastfed at 3 and 6 mo, respectively, limiting the strength of our results. However, our results and the abovementioned studies lend further support to the suggestion that breastfed infants may not receive adequate amounts of vitamin B12 throughout the period of recommended breastfeeding.
Overall, maternal B-vitamin supplement use during pregnancy was not clearly associated with vitamin B12 status in infancy. Exceptions were maternal supplement use at GW 18, which was associated with a 33% (9%–63%) increase in infant cobalamin concentrations at 3 mo of age and an 15% (1%–27%) decrease in t-Hcy concentrations at 6 mo of age. Further, supplement use in GW 36 was associated with an 20% (1%–44%) increase in infant cobalamin concentrations at 6 mo of age. Previous studies have shown conflicting results regarding maternal supplement use [33,[44], [45], [46]], where interventions with high-dose vitamin B12 supplements to mothers (50 μg/d) have shown the strongest impact on infants’ status [45,46]. Importantly, we did not consider other dietary sources of vitamin B12 and folate, and we grouped all supplement use (multivitamins and/ or B-vitamins) together in our analysis. Thus, more studies are needed to investigate the effect of maternal supplement use on infants’ vitamin B12 and folate statuses.
A strength of our study is the use of both direct and functional biomarkers when assessing vitamin B12 status, as is recommended [18]. We also measured the status in the mothers and infants at 2 time points. However, there was a considerable number of missing data on supplement use among the mothers and biochemical measures of vitamin B12 and folate status in the infants at 3 and 6 mo of age. Furthermore, the mothers in our study had relatively low BMI and high education, which limits the generalizability of our study. However, the participants were recruited through routine ultrasound at the hospital and through online broadcasts, and few infants were born prematurely or with low birth weight. Therefore, our infants are likely a representative sample of healthy Norwegian infants. In our study, we used capillary blood from the fingertip or heel to sample blood when venipuncture was not possible. Sampling capillary blood may result in different concentrations of certain biomarkers, which could have influenced our results.
A major limitation of our study is the cutoff used to define low vitamin B12 status in our participants. There is a lack of consensus regarding the reference values to define deficiency and subclinical deficiency for vitamin B12 and folate, and most of the indicators do not have age-specific cutoffs or cutoffs for pregnant females. Some randomized controlled trials have investigated if supplementing infants with vitamin B12 improves cognitive development [7,10,[47], [48], [49]]. However, the results from these trials are not conclusive, and more studies are needed. Therefore, whether the observed high prevalence of subclinical vitamin B12 deficiency in Norwegian infants reflects a clinical deficiency or if these lower values are normal fluctuations during early life without further consequences is not certain.
In conclusion, we found a high prevalence of subclinical vitamin B12 deficiency in the infants in our study but adequate folate status. Infant’s vitamin B12 status was associated with mother’s status during pregnancy and breastfeeding. Further studies are needed to investigate if this observed subclinical vitamin B12 deficiency has clinical implications for the infants, or if this reflects the need to develop specific cutoffs used to define overt and subclinical vitamin B12 deficiency during infancy.
Author contributions
The authors’ responsibilities were as follows—MK, LD, MWM: designed and conducted the research; SMGB: analyzed the data, performed the statistical analysis, and wrote the first draft of the paper; CK, TAS, IK: supervised; PMU, AM: analyzed the blood samples; CK, IK, AM, PMU, SNS, LD, MK, TAS, MWM: contributed to the critical revision of the manuscript; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
The Mommy’s Food study was financially supported by The Norwegian Seafood Research Fund (http://www.fhf.no/) (grant no. 901038) (to MWM). This work was supported by a grant from Innlandet Hospital Trust (grant no. 150455) (to TAS) The funder had no role in the design of the study, the reporting of the results, or the decision to publish this article. The cod used in the trial was purchased, after tender, from Lerøy A/S, Bergen, Norway.
Data availability
Requests for data collected in the Mommy’s Food study (such as deidentified participant data) can be made to the corresponding author following publication, and requests will be considered on an individual basis. Any requests require completion and approval of the application for use of data from the Mommy's Food study. The trial project group will review and, if acceptable and approved by the Regional Committee for Medical and Health Research Ethics West, Norway, provide approval of the request. A signed data sharing access agreement will be required. To facilitate the data access process, please contact, data will be available after publication of the study results, Maria Wik Markhus at maria.wik.markhus@hi.no and mammasmat@hi.no.
Acknowledgments
We are thankful for all assistance from the staff at Institute of Marine Research.
References
- 1.Venkatramanan S., Armata I.E., Strupp B.J., Finkelstein J.L. Vitamin B-12 and cognition in children. Adv. Nutr. 2016;7(5):879–888. doi: 10.3945/an.115.012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Rest O., van Hooijdonk L.W., Doets E., Schiepers O.J., Eilander A., de Groot L.C. B vitamins and n-3 fatty acids for brain development and function: review of human studies. Ann. Nutr. Metab. 2012;60(4):272–292. doi: 10.1159/000337945. [DOI] [PubMed] [Google Scholar]
- 3.Rush E.C., Katre P., Yajnik C.S. Vitamin B12: one carbon metabolism, fetal growth and programming for chronic disease. Eur. J. Clin. Nutr. 2014;68(1):2–7. doi: 10.1038/ejcn.2013.232. [DOI] [PubMed] [Google Scholar]
- 4.Lipari Pinto P., Florindo C., Janeiro P., Santos R.L., Mexia S., Rocha H., et al. Acquired vitamin B12 deficiency in newborns: positive impact on newborn health through early detection. Nutrients. 2022;14(20):4397. doi: 10.3390/nu14204397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjørke-Monsen A.L., Torsvik I., Saetran H., Markestad T., Ueland P.M. Common metabolic profile in infants indicating impaired cobalamin status responds to cobalamin supplementation. Pediatrics. 2008;122(1):83–91. doi: 10.1542/peds.2007-2716. [DOI] [PubMed] [Google Scholar]
- 6.Ljungblad U.W., Paulsen H., Mørkrid L., Pettersen R.D., Hager H.B., Lindberg M., et al. The prevalence and clinical relevance of hyperhomocysteinemia suggesting vitamin B12 deficiency in presumed healthy infants. Eur. J. Paediatr. Neurol. 2021;35:137–146. doi: 10.1016/j.ejpn.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Torsvik I.K., Ueland P.M., Markestad T., Midttun Ø., Bjørke Monsen A.L. Motor development related to duration of exclusive breastfeeding, B vitamin status and B12 supplementation in infants with a birth weight between 2000-3000 g, results from a randomized intervention trial. BMC Pediatr. 2015;15:218. doi: 10.1186/s12887-015-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandyo R.K., Ulak M., Kvestad I., Hysing M., Shrestha M., Ranjitkar S., et al. Cobalamin and folate status among breastfed infants in Bhaktapur, Nepal. Nutrients. 2018;10(5):639. doi: 10.3390/nu10050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strand T.A., Ulak M., Kvestad I., Henjum S., Ulvik A., Shrestha M., et al. Maternal and infant vitamin B12 status during infancy predict linear growth at 5 years. Pediatr. Res. 2018;84(5):611–618. doi: 10.1038/s41390-018-0072-2. [DOI] [PubMed] [Google Scholar]
- 10.Kvestad I., Taneja S., Kumar T., Hysing M., Refsum H., Yajnik C.S., et al. Vitamin B12 and folic acid improve gross motor and problem-solving skills in young North Indian children: a randomized placebo-controlled trial. PLOS ONE. 2015;10(6) doi: 10.1371/journal.pone.0129915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukumar N., Rafnsson S.B., Kandala N.B., Bhopal R., Yajnik C.S., Saravanan P. Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2016;103(5):1232–1251. doi: 10.3945/ajcn.115.123083. [DOI] [PubMed] [Google Scholar]
- 12.Crider K.S., Bailey L.B., Berry R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3(3):370–384. doi: 10.3390/nu3030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson R.D., O’Connor D.L. Maternal folic acid and multivitamin supplementation: international clinical evidence with considerations for the prevention of folate-sensitive birth defects. Prev. Med. Rep. 2021;24 doi: 10.1016/j.pmedr.2021.101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Norwegian Directorate of Health . The Norwegian Directorate of Health; Oslo: 2018. Nasjonal faglig retningslinje for svangerskapsomsorgen.https://www.helsedirektoratet.no/retningslinjer/svangerskapsomsorgen [Internet] [updated 6 June, 2023]. Available from. [Google Scholar]
- 15.Dror D.K., Allen L.H. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr. Rev. 2008;66(5):250–255. doi: 10.1111/j.1753-4887.2008.00031.x. [DOI] [PubMed] [Google Scholar]
- 16.Hannibal L., Lysne V., Bjørke-Monsen A.L., Behringer S., Grünert S.C., Spiekerkoetter U., et al. Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front. Mol. Biosci. 2016;3:27. doi: 10.3389/fmolb.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoey L., Strain J.J., McNulty H. Studies of biomarker responses to intervention with vitamin B-12: a systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2009;89(6):1981S–1996S. doi: 10.3945/ajcn.2009.27230C. [DOI] [PubMed] [Google Scholar]
- 18.Sobczyńska-Malefora A., Delvin E., McCaddon A., Ahmadi K.R., Harrington D.J. Vitamin B12 status in health and disease: a critical review. Diagnosis of deficiency and insufficiency - clinical and laboratory pitfalls. Crit. Rev. Clin. Lab. Sci. 2021;58(6):399–429. doi: 10.1080/10408363.2021.1885339. [DOI] [PubMed] [Google Scholar]
- 19.Fedosov S.N., Brito A., Miller J.W., Green R., Allen L.H. Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin. Chem. Lab. Med. 2015;53(8):1215–1225. doi: 10.1515/cclm-2014-0818. [DOI] [PubMed] [Google Scholar]
- 20.Harrington D.J. Laboratory assessment of vitamin B12 status. J. Clin. Pathol. 2017;70(2):168–173. doi: 10.1136/jclinpath-2015-203502. [DOI] [PubMed] [Google Scholar]
- 21.Sobczyńska-Malefora A., Harrington D.J. Laboratory assessment of folate (vitamin B9) status. J. Clin. Pathol. 2018;71(11):949–956. doi: 10.1136/jclinpath-2018-205048. [DOI] [PubMed] [Google Scholar]
- 22.Markhus M.W., Kvestad I., Midtbø L.K., Nerhus I., Ødegaard E.R., Graff I.E., et al. Effects of cod intake in pregnancy on iodine nutrition and infant development: study protocol for Mommy’s Food - a randomized controlled trial. BMC Nutr. 2018;4:7. doi: 10.1186/s40795-018-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markhus M.W., Hysing M., Midtbø L.K., Nerhus I., Næss S., Aakre I., et al. Effects of two weekly servings of cod for 16 weeks in pregnancy on maternal iodine status and infant neurodevelopment: Mommy’s Food, a randomized-controlled trial. Thyroid. 2021;31(2):288–298. doi: 10.1089/thy.2020.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelleher B.P., Broin S.D. Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J. Clin. Pathol. 1991;44(7):592–595. doi: 10.1136/jcp.44.7.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molloy A.M., Scott J.M. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. doi: 10.1016/s0076-6879(97)81007-5. [DOI] [PubMed] [Google Scholar]
- 26.Midttun Ø., McCann A., Aarseth O., Krokeide M., Kvalheim G., Meyer K., et al. Combined measurement of 6 fat-soluble vitamins and 26 water-soluble functional vitamin markers and amino acids in 50 μL of serum or plasma by high-throughput mass spectrometry. Anal. Chem. 2016;88(21):10427–10436. doi: 10.1021/acs.analchem.6b02325. [DOI] [PubMed] [Google Scholar]
- 27.Aparicio-Ugarriza R., Palacios G., Alder M., González-Gross M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 2015;53(8):1149–1159. doi: 10.1515/cclm-2014-0784. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization . World Health Organization; Geneva: 2015. Serum and red blood cell folate concentrations for assessing folate status in populations. [Google Scholar]
- 29.World Health Organization, UNICEF . World Health Organization; Geneva: 2021. Indicators for assessing infant and young child feeding practices: definitions and measurement methods. [Google Scholar]
- 30.Næss S., Aakre I., Kjellevold M., Dahl L., Nerhus I., Midtbø L.K., et al. Validation and reproducibility of a new iodine specific food frequency questionnaire for assessing iodine intake in Norwegian pregnant women. Nutr. J. 2019;18(1):62. doi: 10.1186/s12937-019-0489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schober P., Boer C., Schwarte L.A. Correlation coefficients: appropriate use and interpretation. Anesth. Analg. 2018;126(5):1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 32.Varsi K., Ueland P.M., Torsvik I.K., Bjørke-Monsen A.L. Maternal serum cobalamin at 18 weeks of pregnancy predicts infant cobalamin status at 6 months-a prospective, observational study. J. Nutr. 2018;148(5):738–745. doi: 10.1093/jn/nxy028. [DOI] [PubMed] [Google Scholar]
- 33.Hay G., Clausen T., Whitelaw A., Trygg K., Johnston C., Henriksen T., et al. Maternal folate and cobalamin status predicts vitamin status in newborns and 6-month-old infants. J. Nutr. 2010;140(3):557–564. doi: 10.3945/jn.109.117424. [DOI] [PubMed] [Google Scholar]
- 34.Finkelstein J.L., Fothergill A., Krisher J.T., Thomas T., Kurpad A.V., Dwarkanath P. Maternal vitamin B12 deficiency and perinatal outcomes in southern India. PLOS ONE. 2021;16(4) doi: 10.1371/journal.pone.0248145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjørke Monsen A.L., Ueland P.M., Vollset S.E., Guttormsen A.B., Markestad T., Solheim E., et al. Determinants of cobalamin status in newborns. Pediatrics. 2001;108(3):624–630. doi: 10.1542/peds.108.3.624. [DOI] [PubMed] [Google Scholar]
- 36.Fréry N., Huel G., Leroy M., Moreau T., Savard R., Blot P., et al. Vitamin B12 among parturients and their newborns and its relationship with birthweight. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992;45(3):155–163. doi: 10.1016/0028-2243(92)90076-b. [DOI] [PubMed] [Google Scholar]
- 37.Muthayya S., Dwarkanath P., Mhaskar M., Mhaskar R., Thomas A., Duggan C., et al. The relationship of neonatal serum vitamin B12 status with birth weight, Asia Pac. J. Clin. Nutr. 2006;15(4):538–543. [PubMed] [Google Scholar]
- 38.Lubree H.G., Katre P.A., Joshi S.M., Bhat D.S., Deshmukh U.S., Memane N.S., et al. Child’s homocysteine concentration at 2 years is influenced by pregnancy vitamin B12 and folate status. J. Dev. Orig. Health Dis. 2012;3(1):32–38. doi: 10.1017/S2040174411000602. [DOI] [PubMed] [Google Scholar]
- 39.Allen L.H. B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Adv. Nutr. 2012;3(3):362–369. doi: 10.3945/an.111.001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen L.H. B vitamins: proposed fortification levels for complementary foods for young children. J. Nutr. 2003;133(9):3000S–3007S. doi: 10.1093/jn/133.9.3000S. [DOI] [PubMed] [Google Scholar]
- 41.Hay G., Johnston C., Whitelaw A., Trygg K., Refsum H. Folate and cobalamin status in relation to breastfeeding and weaning in healthy infants. Am. J. Clin. Nutr. 2008;88(1):105–114. doi: 10.1093/ajcn/88.1.105. [DOI] [PubMed] [Google Scholar]
- 42.Kvammen J.A., Thomassen R.A., Eskerud M.B., Rugtveit J., Henriksen C. Micronutrient status and nutritional intake in 0- to 2-year-old children consuming a cows’ milk exclusion diet. J. Pediatr. Gastroenterol. Nutr. 2018;66(5):831–837. doi: 10.1097/MPG.0000000000001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen L.H., Miller J.W., de Groot L., Rosenberg I.H., Smith A.D., Refsum H., et al. Biomarkers of Nutrition for Development (BOND): vitamin B-12 review. J. Nutr. 2018;148(suppl 4):1995S–2027S. doi: 10.1093/jn/nxy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mørkbak A.L., Ramlau-Hansen C.H., Møller U.K., Henriksen T.B., Møller J., Nexø E. A longitudinal study of serum cobalamins and its binding proteins in lactating women. Eur J Clin Nutr. 2007;61(2):184–189. doi: 10.1038/sj.ejcn.1602502. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqua T.J., Ahmad S.M., Ahsan K.B., Rashid M., Roy A., Rahman S.M., et al. Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: a randomized clinical trial in Bangladesh. Eur. J. Nutr. 2016;55(1):281–293. doi: 10.1007/s00394-015-0845-x. [DOI] [PubMed] [Google Scholar]
- 46.Duggan C., Srinivasan K., Thomas T., Samuel T., Rajendran R., Muthayya S., et al. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J. Nutr. 2014;144(5):758–764. doi: 10.3945/jn.113.187278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torsvik I., Ueland P.M., Markestad T., Bjørke-Monsen A.L. Cobalamin supplementation improves motor development and regurgitations in infants: results from a randomized intervention study. Am. J. Clin. Nutr. 2013;98(5):1233–1240. doi: 10.3945/ajcn.113.061549. [DOI] [PubMed] [Google Scholar]
- 48.Strand T.A., Ulak M., Hysing M., Ranjitkar S., Kvestad I., Shrestha M., et al. Effects of vitamin B12 supplementation on neurodevelopment and growth in Nepalese infants: a randomized controlled trial. PLOS Med. 2020;17(12) doi: 10.1371/journal.pmed.1003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulak M., Kvestad I., Chandyo R.K., Ranjitkar S., Hysing M., Schwinger C., et al. The effect of infant vitamin B12 supplementation on neurodevelopment: a follow-up of a randomized placebo-controlled trial in Nepal. Br. J. Nutr. 2023;129(1):41–48. doi: 10.1017/S0007114522000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for data collected in the Mommy’s Food study (such as deidentified participant data) can be made to the corresponding author following publication, and requests will be considered on an individual basis. Any requests require completion and approval of the application for use of data from the Mommy's Food study. The trial project group will review and, if acceptable and approved by the Regional Committee for Medical and Health Research Ethics West, Norway, provide approval of the request. A signed data sharing access agreement will be required. To facilitate the data access process, please contact, data will be available after publication of the study results, Maria Wik Markhus at maria.wik.markhus@hi.no and mammasmat@hi.no.