Abstract
Background
Consumption of both caffeinated and decaffeinated coffee has been reported to attenuate long-term weight gain. Whether the association between coffee consumption and weight gain depends on the addition of sugar, cream, or coffee whitener remains unclear.
Objective
We aimed to study the associations between changes in coffee consumption, caffeine intake, and weight changes by considering the addition of sugar, cream, or a nondairy coffee whitener.
Methods
We used 3 large prospective cohorts – the Nurses’ Health Study (1986 – 2010), Nurses’ Health Study II (1991 – 2015) and Health Professional Follow-up Study (1991 – 2014). We applied multivariable linear regression models with robust variance estimators to assess the association of changes in coffee habits within each 4-y interval with concurrent weight changes. Results across the 3 cohorts were pooled using inverse-variance weights.
Results
After multivariable adjustment, each 1 cup per day increment in unsweetened caffeinated coffee was associated with a reduction in 4-y weight gain of -0.12 kg (95 % CI: -0.18, -0.05 kg) and of -0.12 kg (95 % CI: -0.16, -0.08 kg) for unsweetened decaffeinated coffee. The habits of adding cream or nondairy coffee whitener were not significantly linked to weight changes. Adding a teaspoon of sugar was associated with a 4-y weight gain of +0.09 kg (0.07, 0.12 kg). Stratified analyses suggested stronger magnitude of the observed associations with younger age and higher baseline BMI. Neither caffeine nor coffee modified the association of adding sugar to any food or beverage with weight changes.
Conclusions
An increase in intake of unsweetened caffeinated and decaffeinated coffee was inversely associated with weight gain. The addition of sugar to coffee counteracted coffee’s benefit for possible weight management. To the contrary, adding cream or coffee whitener was not associated with greater weight gain.
Keywords: coffee, caffeine, sugar, sugar-sweetening, weight gain, weight, diet, Nurses’ Health Study, Health Professional Follow-up Study, beverage consumption
Introduction
Caffeinated beverages are widely consumed globally. In the United States, about 85% of adults consume caffeine daily, with coffee as the predominant source [1]. Coffee consumption can be part of a healthy dietary pattern as a daily intake of 3 to 5 standard cups has been inversely associated with several chronic diseases, including type 2 diabetes, cardiovascular disease, some cancers, disease-specific mortality, and all-cause mortality [[2], [3], [4]]. Several of these health benefits may be mediated by the effect of coffee on energy metabolism, suggesting an antiobesity effect, only in part due to direct metabolic actions of caffeine [[5], [6], [7]]. An increase in sympathetic nervous system and energy expenditure are among the main proposed mechanisms [8].
Drinking coffee is often accompanied by adding sugar, artificial sweetener, cream, or coffee whitener. The evidence for sugar-sweetened and artificially sweetened beverages with obesity, cardiometabolic disease, and other chronic diseases raises concerns over adding sugar or artificial sweeteners to coffee due to their potential detrimental health effects [9,10]. A prospective cohort study in the UK Biobank found different dose-response relationships between coffee consumption and mortality depending on whether the coffee was consumed unsweetened, sugar-sweetened, or artificially sweetened with noncaloric sweeteners. The coffee habit of adding cream or a nondairy coffee whitener has not been studied. In comparison to unsweetened coffee, the mortality reduction for sugar-sweetened coffee was attenuated and U-shaped, insignificant at higher intakes, and the association became generally inconsistent for artificially sweetened coffee [11]. The authors concluded that adding sugar to coffee did not alter the relationship between coffee consumption and mortality despite the observed attenuation of an inverse association. It remains unknown whether sugar-sweetening of coffee affects metabolic health through changes in body weight due to its caloric contents. Its potential relevance as an effective weight management strategy, especially for people who are overweight, is reflected in the updated 17-item energy restricted Mediterranean Diet Adherence Screener (MEDAS), used in the ongoing Prevención con Dieta Mediterránea (PREDIMED)-Plus trial to assess calorie restriction within the pattern of the Mediterranean diet. The 17-item MEDAS includes a question on habitual adding of sugar to coffee or other beverages [12]. Besides sweetened coffee, cream, and coffee whiteners may affect body weight and metabolic health depending on their saturated fat and sugar content [12, [13], [14], [15], [16]].
The major goal of this study was to examine the association between changes in coffee consumption, caffeine intake, and weight changes by considering the addition of sugar, cream, or a nondairy coffee whitener. We also assessed the relationship between adding sugar to beverages and foods as a condiment in general and weight changes. Furthermore, we investigated whether weight change due to an increase in added sugar to beverages was modified by the concomitant increase in caffeine or coffee intake.
Methods
Study population
Our study used the data from 3 prospective cohorts. In 1976, the Nurses’ Health Study (NHS) started with 121,700 female registered nurses from 11 US states. In 1989, the Nurses’ Health Study II (NHS II) was initiated with 116,429 younger female registered nurses from 14 states. For the Health Professional Follow-up Study (HPFS), 51,529 US male health professionals from 50 states were recruited in 1986. Retention rate of participants in each 2-year interval was >90 % in all 3 cohorts. The first year for which detailed information on diet, physical activity, and tobacco habits was provided—1986 in the NHS and HPFS and 1991 in the NHS II—marked the baseline of our study. For the current analyses, follow-up of the NHS ended in 2010, in 2014 for the HPFS, and in 2015 for the NHS II.
We excluded participants with >70 missing responses in the baseline food frequency questionnaire (FFQ) or implausible energy intakes (females: <600 kcal/d or >3500 kcal/d; <800 or >4200 kcal/d for males). To minimize confounding by age and disease-related weight change, age≥65 years, self-reported baseline diagnosis of cardiovascular disease (heart disease, angina, coronary artery bypass graft, stroke, pulmonary embolism), diabetes, cancer, respiratory diseases (emphysema, active tuberculosis, chronic bronchitis), chronic kidney disease (chronic kidney failure), neurodegenerative disorders (Amyotrophic Lateral Sclerosis, Parkinson, Multiple Sclerosis, Alzheimer), gastric conditions (ulcerative colitis, gastric or duodenal ulcer, gastric surgery/intestinal bypass) and systemic lupus erythematosus were exclusion criteria. Participants with missing information on BMI and body weight, as well as with extreme values (BMI<15 or BMI>50) at baseline were also excluded. After excluding participants with missing information on baseline BMI, weight change, or change in exposure variables (caffeinated coffee, decaffeinated coffee, teaspoons of added sugar, coffee whitener, cream), 48,891 females in the NHS, 83,464 females in the NHSII, and 22,863 males in the HPFS were included in our study. During follow-up, pregnant females, deceased participants, those lost to follow-up, reaching the age of 65, or diagnosed with diabetes were censored at the time period of the reported diagnosis, and the following 4-year cycles were not included in the analysis. Participants with the other above-mentioned diseases included as baseline exclusions were censored 6 years before diagnosis. Four-year cycles with missing information on baseline BMI, weight change, or change in exposure variables (caffeinated coffee, decaffeinated coffee, teaspoons of added sugar, coffee whitener, cream) were not considered. All participants provided informed consent. The institutional review boards of Brigham and Women’s Hospital and Harvard School of Public Health approved the study protocol for all 3 cohorts.
Dietary exposure assessment
Participants filled out an FFQ providing information on food and beverage intake of more than 130 items over the previous year at baseline and every 4 years during follow-up. Participants indicated the quantity of consumed food using standard portion size and 9 possible frequencies, ranging from “never or less than once per month” to “6 or more times per day.” The FFQ captured coffee intake (“decaffeinated coffee” and “coffee with caffeine,” standard measure = 8 ounces [1 cup]), its associated habit of adding cream (standard measure = 1 tablespoon, excluding fat free) or nondairy coffee whitener (standard measure = 1 tablespoon, excluding fat free). Participant responses also included how many teaspoons of sugar they added to their beverages and food each day. Adding milk to coffee was not specifically captured in the FFQs and, therefore, was not examined.
Total caffeine intake was estimated by calculating the net intake per day from the main sources: caffeinated coffee, decaffeinated coffee, tea, and soda drinks. Reference values were derived from food composition tables provided by the US Department of Agriculture [17]. Change in caffeine intake within each 4-year period was calculated and transformed into a variable using 100 mg/d as the unit.
Reproducibility and validity of the FFQ used in the NHS and HPFS were shown previously [[18], [19], [20]]. Coffee and tea habits counted among the most reproducible items with correlation coefficients of 0.78 for coffee and 0.93 for tea over the 1-year period [21,22].
Body weight assessment
Baseline questionnaire and biennial questionnaires during follow-up captured the trajectory of height and weight among the participants. Self-reported measures could be generally confirmed in a validation subsample with staff-measured weight (Spearman’s correlation coefficient r = 0.96) in which self-reported weights were, on average, 1.5 kg lower than the measurements from staff [23]. A 4-year weight change was described using metric unit system (kg). Anthropometric measures and weight changes were strong predictors of disease risk and mortality in these cohorts [[24], [25], [26], [27]].
Covariate assessment
Other dietary factors with relevance for weight change were assessed in the FFQs every 4 years, including fruits, starchy and nonstarchy vegetables, legumes, whole grains, refined grains, cooked and fried potatoes, fresh and processed red meat, poultry, fish, eggs, butter, other dairy products, sweets and desserts, nuts, fried food consumed at home and away, sugar-sweetened beverages, fruit juice, and water. Intake of trans-fat, total fiber, and the ratio of unsaturated to saturated fats, which have been related to weight gain, were also included. Alcohol intake was estimated using information on consumption of beer, wine, and liquor. Overall diet quality was described with the Alternate Healthy Eating Index (AHEI)-2010 score [28]. Its changes have already been shown to powerfully predict weight change. It captures directly or indirectly the main food groups promoting weight gain except for sweets and desserts, cooked and fried potatoes, as well as fried food in general [[28], [29], [30]].
Information on medical conditions, medication and supplement use, body weight, screenings, and lifestyle habits (physical activity, smoking habits) were collected every 2 years with validated questionnaires. Sleep duration was assessed in 1986, 2000, and 2002 in NHS; in 2001 in the NHS II; and in 1987 and 2000 in the HPFS. Therefore, analyses were adjusted for sleep duration at baseline using categorical variables: missing data, <6, 6–7, 7–8, and >8 h per day. Sedentary behavior was approximated by hours per week of watching television at home: in 1992 and 2004 in the NHS; in 1991, 1997, 2001, and 2005 in the NHS II; and in 1988 and every 2 years thereafter in the HPFS. Hence, a baseline categorical variable for television watching was used in NHS and NHS II (missing data, 0–1, 2–5, 6–20, 21–40, and >40 h/wk), and absolute change (continuous variables) was used in the HPFS. Medications were not included as covariates because they can be a consequence of an unhealthy diet and its associated weight gain and, therefore, would mask the relationship between changes in diet and 4-year weight change by being their mediators or direct correlates [29].
Statistical analyses
We analyzed the relationship between changes in coffee intake, cream, coffee whitener, and added sugar and concurrent weight change in each 4-year period during 24 years of follow-up in the NHS (1986–2010) and NHS II (1991–2015) and 28 years in the HPFS (1986–2014). Dietary changes were expressed as changes in servings/d and used as continuous variables. Extreme values were truncated at the 0.5th and 99.5th percentile to avoid undue influence by outliers. Indicator variables for categorical behaviors (change in smoking status, sleep duration, time watching television) were made. For all variables describing a change in lifestyle habits, “no change” was the reference category. Missing lifestyle data during follow-up were imputed using the last observation forward for continuous variables or were coded as missing indicator if a categorical variable.
We used multivariate linear regression models (‘PROC GENMOD’ in SAS) with unstructured correlation matrix to factor in within-person repeated measures. Three main models were constructed to assess the relationship between change in intake of unsweetened coffee (caffeinated coffee and decaffeinated coffee, in cup/d), added sugar (teaspoon/d), coffee whitener, cream (both in tablespoon/d), and 4-year weight change (in kg). The first model was adjusted for the intercept of the 4-year time period and age only. The second multivariable adjusted model was additionally adjusted for BMI, sleep duration and time watching television (only NHS and NHS II) at baseline of the 4-year period, and 4-year change in time watching television (in h/wk, only HPFS), change in socioeconomic status, sugar-sweetened beverages, fruit juice, tea (tea was excluded for the analyses with caffeine intake as the exposure), water (all variables in servings/d), change in alcohol intake (g/d), change in adherence to AHEI-2010 excluding alcohol as a proxy for weight change triggered by changes in general dietary pattern, and the with weight strongly associated food groups, cooked and fried potatoes, other fried food and sweets and desserts (all variables in servings/d) which are not captured in the AHEI-2010. A third model was furthermore adjusted for change in total energy intake, allowing first to assess whether associations of coffee condiments with weight change are partially mediated through their calories and to indirectly include changes in food groups which account for changes in calories but have not been captured in the covariables of the second model yet.
Nonlinearity was assessed with additional polynomial regression models of degree 2.
Effect modification through age and BMI
Additionally, we examined the association between change in caffeine intake and 4-year weight change by adding change of caffeine to the age and multivariable adjusted models. As coffee was the major source of caffeine, we also constructed a multivariable model in which change in caffeinated coffee was left out for change in caffeine. Stratified analyses of the main models were performed by age group (>50 and ≤50 years) and BMI categories (≤ 25.0, 25.0 – 29.9, and ≥ 30.0 kg/m2) for the above-mentioned models.
Sensitivity analyses
The robustness of results was tested in several sensitivity analyses. First, change in intake of fruits, starchy and nonstarchy vegetables, legumes, whole grains, refined grains, fresh and processed red meat, poultry, fish, eggs, butter, other dairy products, nuts, calories from protein and fat sources, trans-fat, total fiber and the ratio of unsaturated to saturated fats were added to the third model. Next, a model excluding 4-year cycles in which participants added > 5 teaspoons of sugar per day were excluded to reduce distortion of the analysis through excessive added sugar, likely reflecting an overall unhealthy dietary pattern. Furthermore, 4-year weight changes in relation to changes in coffee and sugar habits were assessed among coffee drinkers who had a minimum intake of 1 cup/d both at the beginning and the end of each 4-y cycle.
Sugar-sweetened coffee and effect modification of the association between adding sugar and weight change through coffee or caffeine
Effect modification between adding sugar to coffee and weight was examined by adding 2 binary product terms for the age and multivariable adjusted models: the binary product term considered if a change in added sugar occurred with a concurrent change in caffeinated coffee or decaffeinated coffee, respectively. Cross-product terms to describe a concurrent change in coffee consumption, either caffeinated or decaffeinated, and added sugar up to 1 cup, between 1 and 2 cups, or > 2 cups/d were used in another separate model. In an alternative way to study effect modification using 3 categories, it was considered whether adding sugar was accompanied by an increase in caffeine intake up to 100 mg, between 100 and 200 mg, or > 200 mg. Effect modification was confirmed if coefficients were statistically significant (P<0.05).
Results across cohorts were pooled by means of inverse-variance–weighted, random-effects meta-analyses. Analyses were carried out with the use of SAS software, version 9.4 (SAS Institute Inc.), at a 2-tailed alpha level of 0.05.
Results
Cohort characteristics
Supplemental Figure 1 provides details on the prevalence of the exclusion criteria. After their application, the NHS counted 48,891 participants, NHS II and HPFS had 83,464 and 22,863, respectively. Table 1 summarizes the characteristics of the NHS, NHS II, and the HPFS, both at the average baseline of all 4-year periods and their changes. The average 4-year weight gain over all cycles was 1.2 kg (5th to 95th percentile: -6.8 to 9.1 kg) in the NHS, 1.7 kg (5th to 95th percentile: -7.7 to 11.3 kg) in the NHS II and 0.8 kg in the HPFS (5th to 95th percentile: -5.0 to 6.8 kg). Differences across cohorts may be explained in part by differences in sex and age: the mean age was 55.0 (5th to 95th percentile: 43.0 to 64.0), 45.0 (5th to 95th percentile: 32.0 to 59.0) and 53.7 (5th to 95th percentile: 41.0 to 64.0), in the NHS, NHS II, and HPFS, respectively.
TABLE 1.
Baseline characteristics and average 4-y changes in 3 US cohorts
| Variables | NHS∗ (48,891 females) |
NHS II∗ (83,464 females) |
HPFS∗ (22,863 males) |
|||
|---|---|---|---|---|---|---|
| Baseline | Changes within each 4-y period (5% – 95%) | Baseline | Changes within each 4-y period (5% – 95%) | Baseline | Changes within each 4-y period (5% – 95%) | |
| n | 48,891 | 83,464 | 22,863 | |||
| Number of 4-y cycles | 117,707 | 221,559 | 40,373 | |||
| Age (y) | 55.0 | 45.0 | 53.7 | |||
| BMI (kg/m2) | 25.6 | 0.4 (-2.4 to 3.3) | 25.6 | 0.7 (-2.5 to 4.1) | 25.7 | 0.3 (-1.6 to 2.2) |
| Overweight (%) | 30.5 | 25.7 | 45.9 | |||
| Obesity (%) | 15.9 | 18.1 | 9.2 | |||
| Body weight (kg) | 69.1 | 1.2 (-6.8 to 9.1) | 69.7 | 1.7 (-7.7 to 11.3) | 82.3 | 0.8 (-5.0 to 6.8) |
| Physical Activity (MET-h/wk)∗ | 18.1 | 8.1 (-27.5 to 30.3) | 21.0 | 1.4 (-30.9 to 36.2) | 29.3 | 10.9 (-38.0 to 52.8) |
| Sleep duration (h/d) | 7.0 | 7.0 | 7.1 | |||
| Sedentary time (h/wk) | 2.8 | 2.5 | 10.3 | -0.2 (-13.0 to 10.5) | ||
| Current smoker (in %) | 14.2 % | 8.1 % | 6.7 % | |||
| Adherence to AHEI-2010∗ | 48.1 | 1.1 (-13.2 to 15.7) | 49.4 | 2.8 (-11.5 to 18.0) | 47.4 | 2.1 (-10.9 – 15.8) |
| Alcohol intake (g/d) | 5.9 | -0.2 (-8.6 to 8.0) | 4.8 | 0.7 (-5.7 to 8.9) | 11.4 | 0.2 (-13.4 to 14.3) |
| Energy intake (kcal/d) | 1768 | -6.4 (-728 to 709) | 1786 | 2.7 (-787 to 792) | 2025 | -11.4 (-832 to 806) |
| Fat intake (in % of total energy intake) | 31.3 | -0.8 (-10.8 to 9.1) | 31.5 | 0.4 (-10.2 to 11.2) | 31.2 | -0.4 (-9.8 to 9.0) |
| Fat ratio (saturated/ polyunsaturated) | 0.6 | 0.0 (-0.3 to 0.4) | 0.6 | 0.0 (-0.3 to 0.4) | 0.6 | 0.0 (-0.3 to 0.4) |
| Trans-fatty acids (g/d) | 2.6 | -0.2 (-2.2 to 1.8) | 2.4 | -0.4 (-2.2 to 1.2) | 3.0 | -0.1 (-2.5 to 2.3) |
| Fiber intake (g/d) | 19.8 | 0.7 (-9.9 to 11.7) | 20.1 | 0.9 (-10.7 to 12.9) | 21.7 | 0.9 (-10.6 to 12.7) |
| Dietary intake—servings/d | ||||||
| Decaffeinated coffee | 0.7 | -0.1 (-2.0 to 1.7) | 0.3 | 0.0 (-1.0 to 0.9) | 0.5 | 0.0 (-1.5 to 1.5) |
| Caffeinated coffee | 1.5 | -0.1 (-2.1 to 2.0) | 1.3 | 0.0 (-2.0 to 2.0) | 1.4 | 0.0 (-2.0 to 2.0) |
| Total coffee | 2.2 | -0.4 (-2.4 to 2.0) | 1.6 | 0.0 (-2.0 – 1.9) | 1.9 | 0.0 (-2.0 – 2.0) |
| Teaspoons of sugar | 0.8 | 0.0 (-2.0 to 2.0) | 0.7 | 0.0 (-2.0 – 2.0) | 1.0 | -0.1 (-2.0 – 1.0) |
| Adding sugar | 33.8 % | 82.5 % no change in habit | 31.1 % | 81.8 % no change in habit | 39.0 % | 83.3 % no change in habit |
| Caffeine [mg/d] | 259 | -30 (-320 to 240) | 210 | -11 (-261 to 215) | 228 | -14 (-288 to 253) |
| Coffee whitener1 [Tablespoon] | 0.2 | 0.0 (-0.8 to 0.6) | 0.3 | 0.0 (-0.8 to 1.0) | 0.2 | -0.1 (-1.0 to 0.4) |
| Cream2 [Tablespoon] | 0.2 | 0.0 (-0.4 to 0.4) | 0.3 | 0.0 (-0.8 to 1.0) | 0.2 | 0.0 (-0.4 to 0.4) |
| Water | 2.9 | -0.1 (-2.5 to 3.5) | 2.8 | 0.1 (-3.5 to 3.5) | 2.4 | -0.1 (-2.4 to 2.1) |
| Fruit juice | 0.7 | 0.0 (-1.0 to 1.0) | 0.5 | -0.1 (-1.0 to 0.9) | 0.8 | 0.0 (-1.1 to 1.0) |
| Fruits | 1.6 | -0.2 (-1.6 to 1.5) | 1.3 | 0.1 (-1.4 to 1.7) | 1.6 | 0.1 (-1.4 to 1.6) |
| Vegetables | 4.1 | 0.0 (-2.8 to 2.8) | 4.0 | 0.1 (-2.8 to 3.1) | 3.9 | 0.1 (-2.6 to 2.9) |
| Tea | 0.6 | 0.0 (-1.5 to 1.5) | 0.8 | 0.0 (-1.7 to 1.9) | 0.5 | 0.0 (-1.1 to 1.0) |
NHS = Nurses’ Health Study; NHSII = Nurses’ Health Study 2; HPFS = Health Professional Follow-up Study; MET = metabolic equivalent of task; AHEI-2010 = Alternate Healthy Eating Index-2010
nondairy coffee whitener (excluding fat free)
excluding fat free cream
In the NHS, participants consumed on average 1.5 (5th to 95th percentile: 0.0 – 4.5) cups of caffeinated coffee per day and 0.7 (5th to 95th percentile: 0.0 – 2.5) cups/d decaffeinated coffee; 1.3 (5th to 95th percentile: 0.0 – 4.5) cups of caffeinated coffee per day and 0.3 (5th to 95th percentile: 0.0 – 2.5) cups/d decaffeinated coffee in the NHS II, and 1.4 (5th to 95th percentile: 0.0 – 4.5) cups/d and 0.5 (5th to 95th percentile: 0.0 – 2.5) in the HPFS, at baseline, respectively. The average change in coffee consumption over all 4-year periods on the cohort level was minimal, but the 5th and 95th percentiles reported a change of about 2 cups/d in either direction. The average baseline level of adding sugar (0.8 [5th to 95th percentile: 0.0 – 4.0], 0.7 [5th to 95th percentile: 0.0 – 3.0], or 1.0 [5th to 95th percentile: 0.0 – 4.0] teaspoons of sugar per day in the NHS, NHS II, and HPFS, respectively), did not change on average over the 4-year periods. At baseline, about two-thirds of all participants reported not adding any sugar to beverages or food at all, and >80 % of all participants did not change their habit of adding sugar over the 4-year cycles.
Changes in coffee habits and weight changes
Table 2 presents the average 4-year weight changes associated with changes in coffee habits. An increase of one cup of unsweetened caffeinated coffee per day was associated with a moderate reduction in weight gain (multivariable adjusted model: -0.12 kg, 95% CI: -0.18, -0.05), as was unsweetened decaffeinated coffee (-0.12 kg; 95% CI: -0.16, -0.08). Increasing added sugar to any food or beverage by 1 teaspoon per day was associated with a weight gain of +0.09 kg (95% CI: 0.07, 0.12) over the 4-year cycles. Heterogeneity between studies was statistically significant, indicating a stronger inverse association between coffee intake and weight gain in the NHS II. Weight gain associated with adding sugar had a larger magnitude in NHS and NHS II than in the HPFS. The 4-year weight changes associated with changes in use of coffee whitener were marginally associated in the NHS only. Adding cream to coffee did not show any statistically significant association with weight change after adjustment for multiple covariates. Intermediate and multivariable adjusted model, which only differed from each other by adjustment for total energy intake, yielded the same results. A nonlinear relationship for changes in coffee habits was not observed when quadratic terms for all of them were added to the models (P>0.05).
TABLE 2.
Cohort-specific and pooled results for the relationships between changes in coffee habits (1 serving per day) and absolute weight change (kg) within each 4-y period in 3 prospective cohorts.
| Increase dietary intake (servings/day) | Weight change (kg) within each 4-y period (95 % confidence interval) |
|||||
|---|---|---|---|---|---|---|
| NHS | NHS II | HPFS | Pooled results | P value for heterogeneity | ||
| UNSWEETENED | Caffeinated coffee | |||||
| Minimal adjustment | -0.10 (-0.13, -0.07) | -0.18 (-0.20, -0.15) | -0.06 (-0.10, -0.02) | -0.11 (-0,18, -0,05) | < 0.001 | |
| Intermediate adjustment | -0.10 (-0.13, -0.07) | -0.18 (-0.21, -0.15) | -0.07 (-0.10, -0.03) | -0.11 (-0,18, -0,05) | < 0.001 | |
| Multivariable adjustment | -0.10 (-0.13, -0.07) | -0.18 (-0.21, -0.15) | -0.06 (-0.10, -0.02) | -0.12 (-0.18, -0.05) | < 0.001 | |
| Decaffeinated coffee | ||||||
| Minimal adjustment | -0.12 (-0.15, -0.09) | -0.19 (-0.24, -0.15) | -0.13 (-0.18, -0.08) | -0.14 (-0.19, -0.10) | 0.02 | |
| Intermediate adjustment | -0.10 (-0.13, -0.07) | -0.16 (-0.20, -0.12) | -0.12 (-0.17, -0.07) | -0.12 (-0.16, -0.08) | 0.03 | |
| Multivariable adjustment | -0.10 (-0.13, -0.07) | -0.16 (-0.20, -0.12) | -0.11 (-0.16, -0.06) | -0.12 (-0.16, -0.08) | 0.04 | |
| ADDING TO COFFEE | Teaspoon of sugar | |||||
| Minimal adjustment | 0.14 (0.12, 0.17) | 0.16 (0.14, 0.19) | 0.09 (0.05, 0.12) | 0.13 (0.09, 0.18) | < 0.001 | |
| Intermediate adjustment | 0.11 (0.09, 0.14) | 0.10 (0.07, 0.12) | 0.06 (0.03, 0.09) | 0.09 (0.07, 0.12) | 0.05 | |
| Multivariable adjustment | 0.11 (0.09, 0.13) | 0.10 (0.07, 0.12) | 0.07 (0.04, 0.10) | 0.09 (0.07, 0.12) | 0.06 | |
| Adding cream | ||||||
| Minimal adjustment | 0.06 (0.00, 0.12) | 0.06 (0.02, 0.10) | 0.10 (0.01, 0.18) | 0.07 (0.04, 0.10) | 0.72 | |
| Intermediate adjustment | 0.03 (-0.03, 0.09) | 0.02 (-0.02, 0.06) | 0.07 (-0.01, 0.16) | 0.03 (0.00, 0.06) | 0.45 | |
| Multivariable adjustment | 0.03 (-0.03, 0.08) | 0.02 (-0.02, 0.06) | 0.05 (-0.03, 0.13) | 0.03 (0.00, 0.06) | 0.45 | |
| Coffee whitener | ||||||
| Minimal adjustment | 0.05 (0.02, 0.09) | 0.04 (0.00, 0.07) | -0.01 (-0.06, 0.05) | 0.03 (0.00, 0.06) | 0.37 | |
| Intermediate adjustment | 0.04 (-0.01, 0.08) | 0.01 (-0.04, 0.05) | -0.01 (-0.06, 0.05) | 0.01 (-0.01, 0.04) | 0.38 | |
| Multivariable adjustment | 0.04 (-0.01, 0.08) | 0.01 (-0.03, 0.05) | -0.02 (-0.07, 0.03) | 0.02 (-0.01, 0.04) | 0.40 | |
NHS = Nurses’ Health Study; NHS II = Nurses’ Health Study 2; HPFS = Health Professional Follow-up Study. Minimally adjusted model: adjustment for intercept of 4-y time period and age. Intermediate adjustment: additional adjustment for BMI, daily hours of sleep (≤6 h/d, >6 ∼ ≤7 h/d, >7 ∼ ≤8 h/d, >8 h/d), change in physical activity (MET-hour per week), change in smoking status (never-never, current-past, past-current, never-current, past-past, current-current), changes in sugar-sweetened beverages, fruit juice, water, tea (all variables in servings/d), socioeconomic status, change in alcohol intake (g/d), adherence to AHEI-2010 excluding alcohol, cooked and fried potatoes, other fried food and sweets and desserts. In NHS and NHS II: sedentary time (≤1 h/wk, 2–5 h/wk, 6–20 h/wk, 21–40 h/wk, 41+ h/wk); in HPFS change in sedentary time (>4hr less, 1.5h ∼ 4h less, 0 ∼ 1.5h less, no change, up to 1.5h more, 1.5 ∼ 4h more, > 4h). Multivariable adjustment: additional adjustment for change in total energy intake. Results were pooled by an inverse-variance-weighted random-effects meta-analysis across the 3 cohorts.
Subgroup analyses
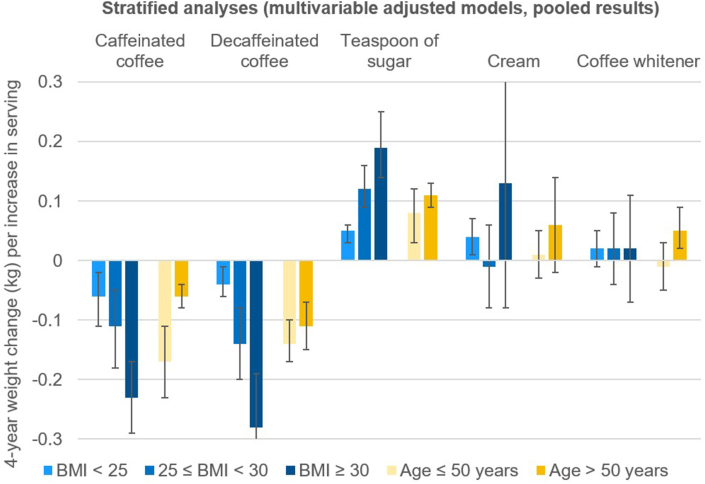
Stratification across BMI categories showed stronger associations of changes in coffee or adding sugar with 4-year weight change among people with overweight and obesity, compared with healthy weight (Figure 1 and Supplemental Table 1). Analyses stratified by age group (≤ or >50 years of age) indicated stronger weight reduction among the younger subgroup with increasing coffee consumption. Multivariate adjusted models including all strata using product terms confirmed a statistically significant effect modification of the relationship between changes in caffeinated, decaffeinated coffee, sugar, and weight change through overweight and obesity. Among the product terms to test for effect modification through age group, only the one for caffeinated coffee reached statistical significance (Supplemental Table 2).
FIGURE 1.
Pooled results for the relationships between changes in coffee habits (1 serving per day) and absolute weight change (kg) within each 4-y period: stratified by age or BMI. Pooled results are derived from the results of NHS, NHS II, and HPFS using inverse-variance-weighted random-effects meta-analysis. All models were multivariable adjusted: intercept of 4-y time period, age, BMI, daily hours of sleep (≤6 h/d, >6 ∼ ≤7 h/d, >7 ∼ ≤8 h/d, >8 h/d), change in physical activity (MET-h per week), change in smoking status (never-never, current-past, past-current, never-current, past-past, current-current), changes in sugar-sweetened beverages, fruit juice, water, tea (all variables in servings/d), socioeconomic status, change in alcohol intake (g/d), adherence to AHEI-2010 ex alcohol, cooked and fried potatoes, other fried food, sweets and desserts and change in total energy intake. In NHS and NHS II: sedentary time (≤1 h/wk, 2–5 h/wk, 6–20 h/wk, 21–40 h/wk, 41+ h/wk); in HPFS change in sedentary time (> 4h less, 1.5h ∼ 4h less, 0 ∼ 1.5h less, no change, up to 1.5h more, 1.5 ∼ 4h more, > 4h).
The error bars indicate 95% CIs.
Effect modification of the relationship between adding sugar and weight gain through coffee
The absence of effect modification in the relationship between increasing sugar and weight gain through a concomitant increase in caffeinated or decaffeinated coffee was further supported in pooled analyses including cross-product terms (Supplemental Figure 2, Supplemental Table 3). The nonsignificant cross-product terms did not support the hypothesis that coffee modifies the weight gain promoted by added sugar. In analyses using 3 semiquantitative variables, no effect modification through caffeinated or decaffeinated coffee was observed either (Supplemental Table 4). Stratification across BMI and age groups did not yield significant results for effect modification through coffee in any subgroup (results not shown).
Caffeine consumption and weight changes
Supplemental Table 5 shows the results of analyses using changes of 100 mg/d of caffeine as the exposure variable. An increase in caffeine intake by 100 mg/d was inversely associated with weight gain (-0.08 kg, 95% CI: -0.13, -0.03). The results were similar to caffeinated coffee (-0.12 kg, 95% CI: -0.18, -0.05). Effect modification of the relationship between increase in sugar intake and weight gain through caffeine was likewise not observed. Caffeine intake from other sources than coffee could not be studied due to the high correlation between caffeine and coffee intake in our study (correlation coefficients >0.9).
Sensitivity analyses
The robustness of the main results was confirmed in sensitivity analyses (shown in Supplemental Table 6), where the multivariable models were further adjusted for additional dietary variables, including change in intake of fruits, starchy and nonstarchy vegetables, legumes, whole grains, refined grains, fresh and processed red meat, poultry, fish, eggs, butter, other dairy products, nuts, fiber intake, ratio of the intake of polyunsaturated and saturated acids, percentage of calories from fats, protein, total energy intake and trans-fatty acids. Results also remained consistent after excluding participants who add more than 5 teaspoons of sugar per day to food and beverages and when limiting the analyses to coffee drinkers with at least 1 cup of coffee at the beginning and the end of each 4-year cycle.
Discussion
In 3 separate large prospective US cohorts, we found that increasing consumption of coffee was associated with lower weight gain over 4-year cycles. However, this association was attenuated by adding sugar to beverages and foods. Increasing the use of coffee whitener or cream did not show any statistically significant association with weight change. Neither caffeine nor coffee modified the association between increasing use of sugar and weight gain.
To the best of our knowledge, our study is the first to report that sweetening coffee with sugar counteracts the long-term reduction in weight gain associated with increasing coffee intake. The results emphasize the critical role of sugar added to beverages: it has long been recognized that sugar-sweetened beverages promote weight gain, chronic diseases, and increase mortality risk [9,31]. One serving of sugar-sweetened beverage (355 ml) contains 35.0 to 37.5 g of sugar and ∼140 to 150 calories [9]. Our findings are notable as even one teaspoon of sugar, equal to 4 to 5 g, contributed to small amounts of long-term weight gain. Given the stronger association among participants with overweight and obesity and high amounts of added sugar in popular coffee drinks [32], our study has important public health relevance.
To study the above-mentioned association between change in adding sugar to coffee and weight change, we relied on the variable providing the number of teaspoons of sugar to any beverage and food in general. Our study focused on changes in added sugar, coffee whitener, and cream in the context of coffee consumption. A previous publication using the same cohorts showed that an increase in tea consumption did not attenuate long-term weight gain [7]. However, the observed association between adding sugar and weight gain is likely to apply to other beverages, including tea.
The use of coffee whitener and cream was not associated with weight gain in our study. The metabolic effects of these products are likely to depend on the specific composition, which often includes glucose-based sweetener and fat as ingredients [33]. Because our FFQ did not capture the exact coffee whitener products used by the participants, our findings cannot provide more conclusive data for specific products.
Adding milk to coffee was not specifically captured in the FFQs and, therefore, was not examined. However, its impact on weight management is likely less relevant given that the findings from the same cohorts showed that increased milk intake was not associated with long-term weight gain [7].
A principal bioactive compound of coffee is caffeine, an adenosine-receptor antagonist, shown to increase activity of the sympathetic nervous system and energy expenditure [8]. In both caffeinated and decaffeinated coffee, chlorogenic acid is considered to exert an antiobesity effect [34], providing reasonable biological mechanisms for the inverse association between increases in both caffeinated and decaffeinated coffee consumption and weight change in our current study.
The second part of our study investigated the possible effect of the modification of sugar on weight through concomitant coffee or caffeine intake with added sugar. Our research was driven by the hypothesis that the caffeine caused stimulation of the sympathetic nervous system, resulting in an increase in energy expenditure, which could make the body burn the extra calories provided by the teaspoon of sugar faster when both intakes occur simultaneously [35]. However, our overall results did not support this hypothesis as decaffeinated coffee did not modify the association between adding sugar and weight gain either. These results imply that increasing added sugar and coffee consumption influence weight change independently from each other.
As the main caffeine source was coffee in our cohorts, we could not study caffeine independently from coffee. The absence of an effect modification by caffeine or coffee on the relationship between adding sugar and weight change calls into question the overemphasized calorie burning effect in advertisements for energy drinks. More research is needed to investigate the effects of energy drinks on weight gain and health in general.
Our study had several strengths: First, we used 3 independent, large prospective cohorts with detailed information on diet, lifestyle habits, and health. The “changes on changes” analysis approach capitalizes on the repeated measures of diet and long-term follow-ups of cohorts. Second, our linear regression models were simultaneously adjusted for multiple diet and lifestyle factors. In previous studies, the changes in diet and lifestyle factors were largely independent from each other (correlation r<0.1) [29]. Third, we examined the association between adding sugar and weight change with coffee and from coffee independently, providing insight into a potential effect modification through coffee and caffeine, respectively. Fourth, despite the inevitable source of error from self-reported diet, coffee was one of the most accurately reported items on the validated FFQs and, therefore, provided reliable information for assessment of changes in beverage and diet intake [19,20,22].
Our study had several limitations. First, our study cohorts do not represent the general population because they were primarily white US health care professionals, and therefore, the findings might not be generalizable to other groups. Second, as coffee was the dominant source of caffeine in our cohorts, we could not study the role of caffeine in other caffeinated beverages, such as energy drinks. The absence of effect modification in the relationship between sugar-sweetening and weight gain through caffeine is considered a preliminary result and needs replication in other cohorts with higher consumption of caffeine from diverse sources. Third, our study did not have information on adding artificial sweeteners to coffee as an alternative to sugar. Fourth, our study did not capture commercial sugar-sweetened coffee drinks with higher caloric load. Fifth, body weight was self-reported by participants; however, self-reported weight was highly correlated with measured weight in our subcohorts [23]. Finally, because of the observational nature of our analysis, cautions are warranted to make any causal inferences.
Conclusions
In our study, an increase in both caffeinated and decaffeinated coffee was associated with a reduction in weight gain over 4 years in both males and females. Adding a teaspoon of sugar largely counteracted coffee’s benefit for weight management, as it was associated with additional weight gain. Our results may be particularly relevant as an effective weight management strategy among people with overweight and obesity, as the observed weight gain associated with adding sugar was higher in these participants.
The findings raise questions about a potentially even more detrimental impact on weight by commercial coffee drinks with higher amounts of added sugar. Despite the moderate magnitude of the associations between coffee habits and weight change, our findings have important public health relevance given the widespread consumption of coffee and the high prevalence of overweight and obesity globally.
Acknowledgments
We acknowledge the Channing Division of Network Medicine, Department of Biomedicine, Brigham and Women’s Hospital, and Harvard Medical School. We thank the participants in the Health Professionals Follow-up Study, Nurses’ Health Study, and Nurses’ Health Study II for their continued support and dedication.
The authors’ responsibilities were as follows–– MH, MAM-G, WCW, FBH: designed the study; WCW: has been instrumental in the data collection since study inception; MH: conducted the statistical analyses; MH: wrote the first manuscript draft; FBH had primary responsibility for final content. All authors contributed to the interpretation of the results and critical revision of the manuscript, and all authors are responsible for the final content, and have read and approved the final manuscript. The authors report no conflicts of interest.
Conflict of interest
All authors report no conflicts of interest.
Funding
Sources of support: National Institutes of Health (UM1 CA186107, U01 CA176726, U01 CA167552, R01 HL034594, R01 HL088521, R01 HL60712, R01 CA50385, P01 CA87969, R01 AR049880, R01 DK112940, R01 DK119268, R01 DK120870, P30 DK046200, R01 HL118264, and R01 DK127601).
Data availability
The data that support the findings of this study are available from the Channing Division of Network Medicine, but restrictions apply to the availability of these data, which were used under license for the current study and, therefore, are not publicly available.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.09.023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mitchell D.C., Knight C.A., Hockenberry J., Teplansky R., Hartman T.J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014;63:136–142. doi: 10.1016/j.fct.2013.10.042. https://doi:10.1016/J.FCT.2013.10.042 [DOI] [PubMed] [Google Scholar]
- 2.van Dam R.M., Hu F.B., Willett W.C. Coffee, caffeine, and health. N. Engl. J. Med. 2020;383:369–378. doi: 10.1056/NEJMra1816604. https://doi:10.1056/NEJMRA1816604 [DOI] [PubMed] [Google Scholar]
- 3.Grosso G., Godos J., Galvano F., Giovannucci E.L. Coffee, caffeine, and health outcomes: an umbrella review. Annu. Rev. Nutr. 2017;37:131–156. doi: 10.1146/annurev-nutr-071816-064941. https://doi:10.1146/annurev-nutr-071816-064941 [DOI] [PubMed] [Google Scholar]
- 4.Kim Y., Je Y., Giovannucci E. coffee consumption and all-cause and cause-specific mortality: a meta-analysis by potential modifiers. Eur. J. Epidemiol. 2019;34:731–752. doi: 10.1007/s10654-019-00524-3. https://doi:10.1007/s10654-019-00524-3 [DOI] [PubMed] [Google Scholar]
- 5.Tabrizi R., Saneei P., Lankarani K.B., Akbari M., Kolahdooz F., Esmaillzadeh A., et al. the effects of caffeine intake on weight loss: a systematic review and dose-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019;59:2688–2696. doi: 10.1080/10408398.2018.1507996. https://doi:10.1080/10408398.2018.1507996 [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Garcia E., Van Dam R.M., Rajpathak S., Willett W.C., Manson J.A.E., Hu F.B. Changes in caffeine intake and long-term weight change in men and women. Am. J. Clin. Nutr. 2006;83:674–680. doi: 10.1093/ajcn.83.3.674. https://doi:10.1093/ajcn.83.3.674 [DOI] [PubMed] [Google Scholar]
- 7.Pan A., Malik V.S., Hao T., Willett W.C., Mozaffarian D., Hu F.B. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int. J. Obes. (Lond) 2013;37:1378–1385. doi: 10.1038/ijo.2012.225. https://doi:10.1038/ijo.2012.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glade M.J. Caffeine—not just a stimulant Nutrition. 2010;26:932–938. doi: 10.1016/j.nut.2010.08.004. https://doi:10.1016/j.nut.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 9.Malik V.S., Hu F.B. The Role of Sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022;18:205–218. doi: 10.1038/s41574-021-00627-6. https://doi:10.1038/s41574-021-00627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirahatake K.M., Jacobs D.R., Shikany J.M., Jiang L., Wong N.D., Steffen L.M., et al. Cumulative intake of artificially sweetened and sugar-sweetened beverages and risk of incident type 2 diabetes in young adults: the coronary artery risk development in young adults (CARDIA) study. Am. J. Clin. Nutr. 2019;110:733–741. doi: 10.1093/ajcn/nqz154. https://doi:10.1093/ajcn/nqz154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu D., Li Z.H., Shen D., Zhang P.D., Song W.Q., Zhang W.T., et al. Association of sugar-sweetened, artificially sweetened, and unsweetened coffee consumption with all-cause and cause-specific mortality: a large prospective cohort study. Ann. Intern. Med. 2022;175:909–917. doi: 10.7326/M21-2977. https://doi:10.7326/M21-2977 [DOI] [PubMed] [Google Scholar]
- 12.Schröder H., Zomeño M.D., Martínez-González M.A., Salas-Salvadó J., Corella D., Vioque J., et al. Validity of the energy-restricted Mediterranean diet adherence screener. Clin. Nutr. 2021;40:4971–4979. doi: 10.1016/j.clnu.2021.06.030. https://doi:j.clnu.2021.06.030 [DOI] [PubMed] [Google Scholar]
- 13.Lee B., Lee H.J., Cho E., Hwang K.T. Fatty acid compositions of fats in commercial coffee creamers and instant coffee mixes and their sensory characteristics. J. Korean Soc. Food Sci. Nutr. 2012;41:362–368. https://doi:10.3746/JKFN.2012.41.3.362 [Google Scholar]
- 14.Neelakantan N., Seah J.Y.H., van Dam R.M. The effect of coconut oil consumption on cardiovascular risk factors: a systematic review and meta-analysis of clinical trials. Circulation. 2020;141:803–814. doi: 10.1161/CIRCULATIONAHA.119.043052. https://doi:10.1161/CIRCULATIONAHA.119.043052 [DOI] [PubMed] [Google Scholar]
- 15.Field A.E., Willett W.C., Lissner L., Colditz G.A. Dietary fat and weight gain among women in the nurses’ health study. obesity (Silver Spring) 2007;15:967–976. doi: 10.1038/oby.2007.616. https://doi:10.1038/oby.2007.616 [DOI] [PubMed] [Google Scholar]
- 16.Coffee Mate Original Powdered Creamer Canister 12 x 11 Oz. https://www.nestleprofessional.us/coffee-mate/original-powder-creamer-12-x-11-oz Available online:
- 17.FoodData Central. https://fdc.nal.usda.gov/fdc-app.html#/ Available online:
- 18.Willett W.C., Sampson L., Browne M.L., Stampfer M.J., Rosner B., Hennekens C.H., et al. The use of a self-administered questionnaire to assess diet four years in the past. Am. J. Epidemiol. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. https://doi:10.1093/oxfordjournals.aje.a114780 [DOI] [PubMed] [Google Scholar]
- 19.Willett W.C., Sampson L., Stampfer M.J., Rosner B., Bain C., Witschi J., et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. https://doi:10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 20.Rimm E.B., Giovannucci E.L., Stampfer M.J., Colditz G.A., Litin L.B., Willett W.C. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. https://doi:10.1093/oxfordjournals.aje.a116211 [DOI] [PubMed] [Google Scholar]
- 21.Salvini S., Hunter D.J., Sampson L., Stampfer M.J., Colditz G.A., Rosner B., et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int. J. Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. https://doi:10.1093/ije/18.4.858 [DOI] [PubMed] [Google Scholar]
- 22.Feskanich D., Rimm E.B., Giovannucci E.L., Colditz G.A., Stampfer M.J., Litin L.B., et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J. Am. Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. https://doi:10.1016/0002-8223(93)91754-e [DOI] [PubMed] [Google Scholar]
- 23.Manson J.E., Willett W.C., Stampfer M.J., Colditz G.A., Hunter D.J., Hankinson S.E., et al. Body weight and mortality among women. N. Engl. J. Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. https://doi:10.1056/NEJM199509143331101 [DOI] [PubMed] [Google Scholar]
- 24.Thygesen L.C., Grønbsek M., Johansen C., Fuchs C.S., Willett W.C., Giovannucci E. Prospective weight change and colon cancer risk in male us health professionals. Int. J. Cancer. 2008;123:1160–1165. doi: 10.1002/ijc.23612. https://doi:10.1002/ijc.23612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eliassen A.H., Colditz G.A., Rosner B., Willett W.C., Hankinson S.E. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. https://doi:10.1001/jama.296.2.193 [DOI] [PubMed] [Google Scholar]
- 26.Colditz G.A., Willett W.C., Rotnitzky A., Manson J.E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. https://doi:10.7326/0003-4819-122-7-199504010-00001 [DOI] [PubMed] [Google Scholar]
- 27.Chan J.M., Rimm E.B., Colditz G.A., Stampfer M.J., Willett W.C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. https://doi:10.2337/diacare.17.9.961 [DOI] [PubMed] [Google Scholar]
- 28.Fung T.T., Pan A., Hou T., Chiuve S.E., Tobias D.K., Mozaffarian D., et al. Long-term change in diet quality is associated with body weight change in men and women. J. Nutr. 2015;145:1850. doi: 10.3945/jn.114.208785. https://doi:10.3945/JN.114.208785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozaffarian D., Hao T., Rimm E.B., Willett W.C., Hu F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. https://doi:10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertoia M.L., Mukamal K.J., Cahill L.E., Hou T., Ludwig D.S., Mozaffarian D., et al. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12(9) doi: 10.1371/journal.pmed.1001878. https://doi:10.1371/JOURNAL.PMED.1001878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Ruyter J.C., Olthof M.R., Seidell J.C., Katan M.B. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012;367:1397–1406. doi: 10.1056/NEJMoa1203034. https://doi:10.1056/NEJMoa1203034 [DOI] [PubMed] [Google Scholar]
- 32.Caramel Macchiato Nutrition: Starbucks Coffee Company. https://www.starbucks.com/menu/product/413/hot/nutrition Available online:
- 33.Coffee Mate Original Powdered Creamer Canister 12 x 11 Oz. https://www.nestleprofessional.us/coffee-mate/original-powder-creamer-12-x-11-oz Available online:
- 34.Watanabe T., Kobayashi S., Yamaguchi T., Hibi M., Fukuhara I., Osaki N. Coffee abundant in chlorogenic acids reduces abdominal fat in overweight adults: a randomized, double-blind, controlled trial. Nutrients. 2019;11(7):1617. doi: 10.3390/nu11071617. https://doi:10.3390/nu11071617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haraguchi A., Yamazaki T., Ryan C., Ito K., Sato S., Tamura K., et al. Caffeine suppresses high-fat diet-induced body weight gain in mice depending on feeding timing. J. Funct. Foods. 2022;99 https://doi:10.1016/J.JFF.2022.105307 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Channing Division of Network Medicine, but restrictions apply to the availability of these data, which were used under license for the current study and, therefore, are not publicly available.