Abstract
We report here the first quantitative study of the branched-chain amino acid biosynthetic pathway in Salmonella typhimurium LT2. The intracellular levels of the enzymes of the pathway and of the 2-keto acid intermediates were determined under various physiological conditions and used for estimation of several of the fluxes in the cells. The results led to a revision of previous ideas concerning the way in which multiple acetohydroxy acid synthase (AHAS) isozymes contribute to the fitness of enterobacteria. In wild-type LT2, AHAS isozyme I provides most of the flux to valine, leucine, and pantothenate, while isozyme II provides most of the flux to isoleucine. With acetate as a carbon source, a strain expressing AHAS II only is limited in growth because of the low enzyme activity in the presence of elevated levels of the inhibitor glyoxylate. A strain with AHAS I only is limited during growth on glucose by the low tendency of this enzyme to utilize 2-ketobutyrate as a substrate; isoleucine limitation then leads to elevated threonine deaminase activity and an increased 2-ketobutyrate/2-ketoisovalerate ratio, which in turn interferes with the synthesis of coenzyme A and methionine. The regulation of threonine deaminase is also crucial in this regard. It is conceivable that, because of fundamental limitations on the specificity of enzymes, no single AHAS could possibly be adequate for the varied conditions that enterobacteria successfully encounter.
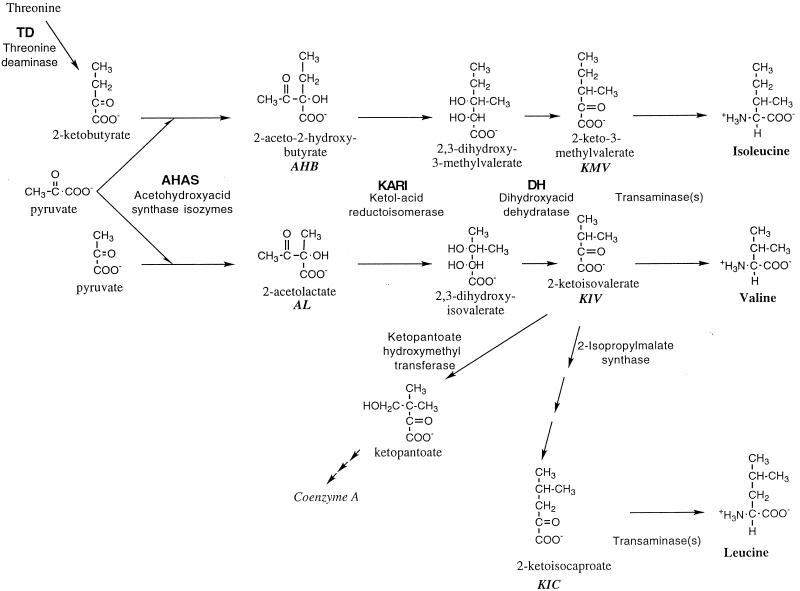
The branched-chain amino acid (BCAA) and pantothenate biosynthetic network in bacteria (Fig. 1) has many complex features which make an analysis of its regulation and control in whole cells interesting and challenging. These features include branching, homologous reactions catalyzed by single enzymes, multivalent regulation of both gene expression and allosteric enzymes, and the production of both amino acid building blocks and the critical cofactor molecule coenzyme A (67). Three enzymes, each with dual specificities, are common to the parallel pathways leading to the keto acid precursors of valine and isoleucine, 2-ketoisovalerate and 2-keto-3-methylvalerate, respectively (67). The first of these enzymes, acetohydroxy acid synthase (AHAS; EC 4.1.3.18; also called acetolactate synthase), can catalyze the decarboxylation of pyruvate and its condensation either with a second molecule of pyruvate, to produce acetolactate (a precursor of valine, leucine, and coenzyme A), or with 2-ketobutyrate, to produce acetohydroxybutyrate (a precursor of isoleucine). Any given AHAS catalyzes the formation of acetohydroxybutyrate and acetolactate at relative rates VAHB and VAL, respectively, which are proportional to the relative concentrations of 2-ketobutyrate and pyruvate, with a fixed specificity R characteristic of the enzyme (2, 28): VAHB/VAL = R × ([2-ketobutyrate]/[pyruvate]) (1)
FIG. 1.
Pathway for the biosynthesis of the BCAAs.
The reaction catalyzed by AHAS is an essentially irreversible branch point in the pathway and thus plays a key role in determining the relative fluxes to the two sets of end products.
The genomes of Escherichia coli and Salmonella typhimurium each encode three AHAS isozymes, which differ in their expression patterns, substrate specificity (R), sensitivity to feedback inhibition by valine, and other kinetic parameters (3, 18, 19, 67, 68). S. typhimurium LT2 expresses only two of these, AHAS I, encoded by ilvBN, and AHAS II, encoded by ilvGM. AHAS II is sufficient by itself for prototrophic growth on glucose (15). The marked preference of this enzyme for 2-ketobutyrate over pyruvate suggests that AHAS II will produce comparable amounts of acetolactate and acetohydroxybutyrate in vivo despite the large difference in the concentrations of these substrates (3); while pyruvate is an abundant, widely used central metabolite, 2-ketobutyrate is present in cells at low concentrations. Normal growth on acetate or oleate, on the other hand, requires AHAS I (15). Such carbon sources were reported to lead to much lower intracellular pyruvate concentrations (46) and are expected to lead to increased levels of glyoxylate (52), which has been reported to inhibit AHAS (14, 28). The cyclic AMP-catabolite gene activator protein-mediated activation of ilvBN expression (27) leads to sufficient AHAS I for growth, even in the presence of an elevated glyoxylate titer (15). It has been suggested that AHAS I, with its low selectivity for 2-ketobutyrate, is particularly appropriate for low pyruvate concentrations (2, 14, 15, 29). AHAS I is thus assumed to provide enterobacteria with the ability to adapt to a wider range of carbon sources and metabolic challenges. Despite this reasonable rationalization for the existence of multiple AHAS isozymes, the roles played by each isozyme in the partitioning of flux in the two parallel pathways have not been quantitatively assessed.
We now describe a quantitative study of the BCAA pathway in S. typhimurium LT2 and several mutants derived from it. We determined the intracellular levels of a number of enzymes, including threonine deaminase, AHAS isozymes I and II, ketol acid reductoisomerase, and dihydroxy acid dehydratase, under different physiological conditions. We also determined the intracellular levels of the 2-keto acids, since changes in their levels inside cells seem to be crucial for an understanding of the regulation and control of the pathway (25, 44, 57).
MATERIALS AND METHODS
Materials.
Racemic acetolactate was prepared by the method of Krampitz (38) as described previously (25). Dihydroxyisovalerate was a gift of Dennis Flint, E. I. du Pont de Nemours & Company, Wilmington, Del. Sulfometuron methyl was a gift from the same company.
Bacterial strains.
Bacterial strains used in this work, their genotypes, and their relevant phenotypes are listed in Table 1. P22-generalized transduction was used to construct the otherwise isogenic strains differing in ilv alleles. P22HT int-4 was grown on donor strains and subsequently used at a multiplicity of infection of 0.8 as previously described (17). Several S. typhimurium strains used here, TV105, TV108, TV493, TV496, TV497, TV503, and TV506, also harbor an F′ episome, F′ pro-lac zzf-1836::Tn10 Cm, which confers chloramphenicol resistance and an inducible β-galactosidase activity. This episome was useful for parallel experiments on responses to inhibitors (25) but is not relevant for the work presented here. For clarity and ease of reading, we do not include the notation F′ pro-lac zzf-1836::Tn10 Cm in the names of the strains in Table 1 or in the text.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Relevant phenotype | Source or reference |
|---|---|---|---|
| Salmonella typhimurium | |||
| DU503 | ilvG236 | 73 | |
| DU2616 | ilvB1 ilvG236 pan-187 ara-9 gal | 73 | |
| LT2 | Ilv+ | LaRossa laboratory collection | |
| MS1286 | ilvBN::MudI1734 | 15 | |
| TT66 | ilvG1007::Tn10 | 4, 40 | |
| TT2010 | zid-64::Tn10 metE338 hisD2421 ara-9 | 61 | |
| TV088 | ilvA219 | 43 | |
| TV105 | ilvBN::MudJ | Kmr Ilv+ Sms | P22 (MS1286) × LT2 → Kmr (Sms) |
| TV108 | ilvG1007::Tn10 | Tcr Ile− | P22 (TT66) × LT2 → Tcr (Ile−) |
| TV484 | zid-64::Tn10 ilvG236 | P22 (TT2010) × DU503 → Tcr (Ile−) | |
| TV486 | zid-64::Tn10 ilvB1 ilvG236 pan-187 ara-9 gal | P22 (TT2010) × DU2616 → Tcr (Ile− + Val−) | |
| TV493 | zid-64::Tn10 ilvG236 ilvBN::MudJ | Tcr Kmr Ilv− | P22 (TV484) × TV105 → Tcr (Ile− + Val−) (Kmr) |
| TV496 | zid-64::Tn10 ilvG236 | Tcr | P22 (TV486) × LT2 → Tcr (Ile− or Met− or Pan−) |
| TV497 | zid-64::Tn10 ilvG236 ilvA219 | Tcr Pan− | P22 (TV484) × TV088 → Tcr (Met− or Pan−) |
| TV503 | zid-64::Tn10 ilvG236 ilvBN::MudJ ilvA219 | Tcr Kmr Ilv− | P22 (MS1286) × TV497 → Kmr (Ile− + Val−) (Tcr) |
| TV506 | ilvBN::MudJ ilvA219 | Kmr Ilv+ Sms | P22 (MS1286) × TV088 → Kmr |
| Escherichia coli MM294 | F− endA1 hsdR17 (rK− mK+) supE44 thi-1 relA1 rfbD1? spo1 | Ilv+ |
Bacterial growth and media.
Growth media used were the rich medium LB (48) and the minimal medium MOPS (51). Either glucose or acetate was added to the minimal medium to a final concentration of 0.4 or 0.8% (wt/vol), respectively. Chloramphenicol, ampicillin, kanamycin, and tetracycline were added when appropriate at 30, 100, 50, and 10 mg/liter, respectively. Bacteria were grown and growth was monitored as described previously (25).
Preparation of extracts.
The preparation of extracts for the determination of enzyme activity and analysis of keto acid content in bacterial extracts were performed as described previously (25). Intracellular metabolite concentrations were calculated from the cellular contents measured assuming that 1011 cells contain 0.06 ml of cytoplasmic volume (excluding the periplasmic volume) (30, 50).
Extracts for amino acid analysis were prepared essentially as described previously (54, 58). Exponentially growing cultures (4 × 108 cells ml−1; 40 Klett units) were filtered (four 30-ml portions) through nitrocellulose filters (47 mm; 0.45-μm-pore size). Cells on the filters were extracted by vigorous mixing (30 s) in boiling water (1 ml) containing 50 nmol of phenylalanine as an internal standard and 50 μl of toluene, heating at 100°C for 15 min, and cooling on ice. The suspensions were centrifuged at 5,000 × g for 15 min, and the combined supernatants were vacuum dried in a SpeedVac apparatus overnight. The resulting powder was resuspended in 400 μl of distilled water and filtered by centrifugation at 4°C through Ultrafree-MC filters (Millipore). The filtrate was lyophilized overnight.
Analysis of intracellular amino acids and keto acids.
Automated analysis of amino acids was performed by Aminolab (Rehovot, Israel) with a slow gradient (physiological amino acid analysis). The recovery of a standard amino acid mixture prepared in the same way was between 60 and 70%. Protein in the cell precipitates was determined by the method of Lowry et al. (47). The analysis of intracellular keto acids was performed as previously described (25).
Total amino acid contents of bacteria.
An exponentially growing culture (120 ml) of S. typhimurium (40 Klett units) was centrifuged at 5,000 × g at 4°C. The cell pellet was resuspended in 0.5 ml of medium and vacuum dried in a SpeedVac apparatus overnight to yield 17 mg of dry cells. Acid hydrolysis was carried out for 22 or 44 h, and the hydrolysate was analyzed by Aminolab with a standard automated procedure (64).
Determination of enzyme activities in bacterial extracts.
The enzyme activities of threonine deaminase, AHAS, and ketol acid reductoisomerase were determined at 37°C and pH 7.6 as previously described (25). The activity of diol dehydratase in 50 to 200 μl of bacterial extract was determined by the method of Kiritani and Wagner (35), except for use of a buffer at pH 7.6.
For the differential determination of AHAS isozymes I and II, parallel reactions were carried out with 100 mM phosphate buffer, 0.1 mM thiamine pyrophosphate, 40 mM pyruvate, 10 mM MgCl2, and 0.025 mM flavin adenine dinucleotide (FAD), with or without the addition of 100 μM sulfometuron methyl or 2 mM valine. Under the conditions of our assays, the AHAS activities of TV105 (AHAS II only) and TV496 (AHAS I only) were 98 and 4% inhibited, respectively, by 100 μM herbicide sulfometuron methyl and 1 and 96% inhibited, respectively, by 2 mM valine. For the differential determination of AHAS isozymes I and III in E. coli, extracts were prepared without FAD in the disruption buffer. The activities were then determined in four parallel reactions with a reaction buffer as described above, except that the FAD concentration was 0 or 0.25 mM and 0 or 116 μg of purified AHAS III small (regulatory) subunits, prepared as described previously (72), was added. In this assay, extracts of bacteria expressing only AHAS I had little or no activity in the absence of added FAD, while extracts of strains expressing only AHAS III had essentially the same activity in the absence or presence of added FAD. The activity in an extract of E. coli K-12 prepared without FAD was thus the contribution of AHAS III, while the difference in activities with and without added FAD was due to AHAS I. As AHAS III dissociates into its large and small subunits at high dilutions (62), we included in the assays of AHAS in E. coli K-12 an excess of purified AHAS III small subunits (72), which have no activity by themselves but which ensure that all the large subunits are in the active holoenzyme form.
The protein content in all extracts was determined by the procedure of Bradford (9). Bovine serum albumin (BSA) served as a standard.
For the calculation of estimated fluxes, the measured enzyme activities (in nanomoles minute−1 mg of protein−1; see Table 3) were converted to units of flux (e.g., millimolar minute−1) by multiplication by the extracted protein yield in each sample of cells and division by the internal cell volume represented by that sample.
TABLE 3.
Specific activities of enzymes of the BCAA pathway
| Strain | Mediuma | Sp act (nmol min−1 mg of protein−1) ofb:
|
|||||
|---|---|---|---|---|---|---|---|
| TDc | AHAS Id,e | AHAS IId | AHAS IIIe | KARI | DH | ||
| S. typhimurium | |||||||
| LT2 | Glc | 289 ± 40 | 8.9 ± 0.1 | 14.0 ± 0.3 | 7.8 ± 2.6 | 57 | |
| Glc + i.l.v. | 42 | 5.6 | 4.6 | 4.1 | NM | ||
| TV105 | Glc | 528 ± 20 | 0 | 30.4 ± 7.0 | 11.0 ± 0.7 | 97 | |
| Glc + i.l.v. | 35 | 0 | 3.7 | 4.5 | NM | ||
| TV506 | Glc | 659 ± 20 | 0 | 32.6 ± 2.2 | 8.3 ± 1.4 | 56 | |
| TV496 | Glc + Pan | 512 ± 68 | 31.3 ± 0.3 | 0 | 7.6 ± 1.2 | 101 | |
| Glc + Ile | 449 ± 70 | 31.3 ± 0.5 | 0 | 10.1 ± 1.2 | 115 | ||
| Glc + i.l.v. | 168 | 3.9 | 0 | 4.6 | NM | ||
| TV497 | Glc + Pan | 548 ± 98 | 34.7 ± 0.7 | 0 | 5.9 ± 1.7 | 156 | |
| TV108 | Glc + Ile | 21 | 61.0 | 0 | 9.3 | 1.4 | |
| TV493 | Glc + Ile, Val | 795 ± 46 | 0 | 0 | 4.5 ± 1.5 | NM | |
| TV503 | Glc + Ile, Val | 416 ± 23 | 0 | 0 | 4.7 ± 0.8 | NM | |
| LT2 | Acetate | 282 ± 14 | 27.0 ± 2.5 | 11.5 ± 0.9 | 9.6 ± 0.9 | 71 | |
| TV105 | Acetate | 510 ± 90 | 0 | 34.0 ± 5.0 | 13.3 ± 3.1 | 126 | |
| TV496 | Acetate | 334 ± 50 | 62.0 ± 6.0 | 0 | 6.4 ± 1.0 | 57 | |
| TV497 | Acetate + Pan | NM | 170.0 | 0 | 9.2 | NM | |
| TV108 | Acetate | 35 | 148.0 | 0 | 9.1 | NM | |
| E. coli K-12 MM294 | Glc | 104 ± 10 | 18.7 ± 3.0 | 15.4 ± 4.0 | 25.0 ± 3.2 | 25 | |
| Acetate | 145 ± 35 | 58.4 ± 6.6 | 37.7 ± 7.5 | 17.0 ± 4.0 | 8.4 | ||
The cells were grown in glucose (Glc) or acetate as described in Table 2, footnote a. Additions to the media were as follows: i.l.v., 0.38 mM each isoleucine, valine, and leucine (repressing conditions); Val, 0.85 mM valine; Ile, 0.38 mM isoleucine; and Pan, 0.34 mM pantothenate.
The enzyme activities were determined by standard methods as described in Materials and Methods. TD, threonine deaminase (EC 4.2.1.16); KARI, ketol acid reductoisomerase (EC 1.1.1.86); DH, dihydroxy acid dehydratase (EC 4.2.1.19). NM, not measured.
The determination of TD also included the addition of 0.1 to 1.4 mM Ile to assay mixtures to verify the presence of the normal ilvA gene product or the described ilvA219 mutation leading to feedback insensitivity.
AHAS isozymes I and II of S. typhimurium were determined differentially on the basis of their differential inhibition by Val and sulfometuron methyl as described in Materials and Methods. The levels of an isozyme were assumed to be zero in a strain deficient in the gene for that enzyme.
AHAS isozymes I and III of E. coli were determined differentially on the basis of their differential dependence on FAD (see Materials and Methods). Excess small subunits of AHAS III were also added to the assay mixtures (see Results).
RESULTS
Nutritional requirements and growth properties of the S. typhimurium strains.
The experiments reported here were carried out with isogenic strains derived from S. typhimurium LT2 (Table 1). The behavior of the strains (Table 2) was generally similar to that reported for other strains with mutations in the same genes (15, 57, 63).
TABLE 2.
Doubling times of S. typhimurium strains in various mediaa
| Strain | AHAS(s) expressed | Addition(s)b | Doubling time (h) inc:
|
|
|---|---|---|---|---|
| Glucose | Acetate | |||
| LT2 | I + II | None | 1.3 | 2.5 |
| Val | 1.3 | 3.0 | ||
| Val + Ile | 1.3 | 2.5 | ||
| TV105 | II | None | 1.3 | 7–10 |
| Val | 1.3 | 5.0 | ||
| Ile | 1.3 | 5.0 | ||
| Val + Ile | 1.3 | 2.5 | ||
| Pan | 1.3 | 3.8 | ||
| TV506d | II | None | 1.5 | ND |
| Val | 1.3 | 2.5 | ||
| Ile | 1.5 | ND | ||
| Val + Ile | 1.3 | 2.5 | ||
| Pan | 1.3 | ND | ||
| Met | 1.4 | ND | ||
| TV496 | I | None | >5e | 3.5 |
| Thr | 2.3f | NM | ||
| KB | 2.7f | NM | ||
| Ile | 1.4 | 3.5 | ||
| Val | ND | NM | ||
| Val + Ile | 1.3 | 2.4 | ||
| Pan | 1.5 | 2.9 | ||
| Met | 1.8 | 2.5 | ||
| TV497d | I | None | ND | ND |
| Ile | ND | ND | ||
| Val + Ile | 1.4 | 3.4 | ||
| Pan | 1.5 | 5.2 | ||
| Met | 1.6 | 4.5 | ||
| Leu + Pan | 1.5 | NM | ||
| TV108 | I | None | 10–15 | 3.0 |
| Thr | 2.2 | NM | ||
| KB | 2.3 | NM | ||
| Ile | 1.3 | 3.0 | ||
| Val + Ile | 1.3 | 2.5 | ||
| Pan | 10–15 | 3.1 | ||
| Met | 10–15 | 3.1 | ||
Cells were grown in glucose or acetate in the presence of limiting amounts (10 μg/ml) of valine and isoleucine as described in Materials and Methods, washed twice with buffer by centrifugation and resuspension, and transferred to the appropriate medium.
Additions to the media were at the following concentrations (millimolar): valine (Val), 0.85; isoleucine (Ile), 0.38; leucine (Leu), 0.45; threonine (Thr), 1.0; methionine (Met), 0.34; pantothenate (Pan), 0.34; and ketobutyrate (KB), 1.0.
ND, no visible growth detected during 30 h; NM, not measured.
AHAS mutants TV506 and TV497 carry an additional mutation in the ilvA gene. This ilvA219 mutation codes for a threonine deaminase that is not sensitive to feedback inhibition by Ile (11).
TV496 begins growing in glucose with a generation time of 2.2 h, which increases to >5 h after 10-fold dilution.
TV496 grows in glucose with the addition of Thr or KB only after a lag of approximately 5 h.
S. typhimurium TV105, which expresses AHAS II only, was able to grow in glucose as well as wild-type LT2 but grew very slowly in acetate (Table 2). The slow growth of TV105 continued for more than 15 generations, ruling out the possibility that it was an artifact of nutrient carryover. In contrast, the S. typhimurium ilvBN mutant reported by Dailey et al. (15) did not grow at all in acetate as a carbon source. Pantothenate most dramatically improved the growth rate, although it was incapable of restoring it to wild-type levels, while valine or isoleucine individually slightly improved it.
TV496, which expresses AHAS I only, grew with difficulty in glucose (63) (Table 2). The isoleucine precursors 2-ketobutyrate and threonine provided partial relief of the apparent isoleucine limitation only after a lag of approximately 5 h. Pantothenate or methionine strongly stimulated the growth of this strain. In acetate, the growth of TV496 was slower than that of the wild type, and the addition of either pantothenate, methionine, or isoleucine plus valine stimulated growth. In contrast to TV496, which contains a point mutation in the ilvGM gene, strain TV108, which contains a Tn10 transposon insertion in ilvGM, was unable to grow well in glucose with the addition of pantothenate or methionine.
Both strain TV497 and strain TV506 also contain the ilvA219 mutation, which codes for a threonine deaminase which is insensitive to feedback inhibition by isoleucine (11). However, rather than making up for the low 2-ketobutyrate specificity of AHAS I (the single AHAS isozyme expressed in TV497), this absence of control in the production of 2-ketobutyrate interfered with growth in both glucose and acetate media so that TV497 grew far more slowly than isogenic strain TV496 expressing wild-type threonine deaminase. The effect of the ilvA219 mutation was also observed in a comparison of TV105 and TV506, which express AHAS II only.
Cellular enzyme levels.
The five enzyme activities determined with freshly prepared extracts from S. typhimurium LT2 (Table 3) are similar to those determined by Primerano and Burns for this strain under slightly different conditions (57). The differential determination of AHAS I and AHAS II in S. typhimurium LT2 was based on the facts that AHAS I is almost completely inhibited by 2 mM valine, while AHAS II is valine resistant (19, 20), and that AHAS II is almost completely inhibited by 100 μM sulfometuron methyl, while AHAS I is barely affected (40). It should be noted that the enzyme activities reported in Table 3 were determined in vitro under standard, near-optimal conditions and thus reflect the relative amounts of the proteins present. They are informative as indirect indicators of the induction or repression of synthesis of the enzymes. These activities were also used, together with other data, to calculate estimated fluxes in the cells (see below). In wild-type LT2 grown in minimal medium with glucose, the levels of expression of the enzymes encoded by the ilvGMEDA, ilvBN, and ilvYC operons (with the exception of threonine deaminase) were elevated by only two- to threefold compared to those measured in the presence of high concentrations of all three BCAAs. Most of the mutant S. typhimurium strains examined here showed high levels of the enzymes encoded by the ilvGMEDA and/or ilvBN operons in minimal medium, compared to LT2, under similar conditions (Table 3).
Mutants expressing only AHAS I were too limited in growth in minimal medium MOPS with glucose (Table 2) for meaningful measurements of steady-state enzyme levels. However, in the presence of 0.34 mM pantothenate, TV496 and TV497 had doubling times of 1.5 h. We expected the enzyme levels observed under these conditions (Table 3) to be relevant to the availability of the amino acids, as pantothenate is not a known effector of the BCAA pathway (67). The fourfold derepression of the ilvBN operon in these mutants relative to the wild type reflected the lack of AHAS II, which must play the major role in isoleucine synthesis in wild-type bacteria (see below). The levels of AHAS I were elevated during growth in acetate compared to growth in glucose, in accord with the well-known activation of ilvBN expression by the catabolite gene activator protein-cyclic AMP catabolite repression system (14, 66).
The very low levels of threonine deaminase and diol dehydratase, two of the other enzymes encoded by ilvGMEDA, observed in strain TV108 were a result of the Tn10 insertion in ilvGM, which clearly has a polar effect on the expression of the downstream genes (37, 45, 53). The behavior of TV496, which expresses these genes at levels much closer to those observed in wild-type LT2 (Table 3), is thus more directly relevant to the role of AHAS isozymes in the pathway than is that of TV108.
Ketol acid reductoisomerase activity is expected to reflect intracellular levels of the acetohydroxy acids acetolactate and acetohydroxybutyrate, since the expression of ilvC is induced by these compounds (6, 55, 60, 74). However, strains TV493 and TV503, which are completely devoid of AHAS activity and hence are unable to synthesize the inducing acetohydroxy acids, had levels of ketol acid reductoisomerase only twofold lower than the highest levels observed with other strains. This finding suggests that under most conditions, the other strains do not exhibit strong induction of ilvYC.
Table 3 also includes the levels of enzymes expressed in an E. coli K-12 strain, MM294, expressing both AHAS I and AHAS III. The differential determination of these two isozymes was based on the tight binding of FAD by AHAS III (1). This analysis showed that 55% ± 5% or 61% ± 5% of the specific activity of AHAS in E. coli MM294 was contributed by AHAS I when the bacteria were grown in glucose or acetate, respectively, as the carbon source (Table 3).
Intracellular keto acid levels.
Keto acids were determined, as previously described (25), in amounts equivalent to intracellular concentrations of 0.5 μM to 1 mM (Table 4). Pyruvate was also determined independently by the lactate dehydrogenase assay (25). The pyruvate level in S. typhimurium ranged from 0.8 to 1.5 mM when glucose was the carbon source and decreased to 0.5 to 0.8 mM when the cells were grown in acetate. As expected, because of the importance of the glyoxylate shunt to the utilization of acetate or fatty acids as sole carbon sources (52, 59), glyoxylate levels were 5- to 10-fold higher in acetate than in glucose. The resolution of the peak for the glyoxylate derivative was not always possible without special modification of the high-pressure liquid chromatography gradient, so the data presented for this keto acid are limited.
TABLE 4.
2-Keto acid contents of S. typhimurium and E. coli K-12 strains
| Strain | Mediuma | Intracellular concn (μM) ofb:
|
KIV/KB ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pyr + PEP (HPLC) | Pyr (LDH) | KB | KIV | KIC | KMV | Glyoxylate | |||
| LT2 | Glc | 1,670 ± 170 | 1,370 ± 130 | 17 ± 4 | 66 ± 16 | 7 ± 1 | 27 ± 4 | 11 ± 1 | 4.0 |
| TV105 | Glc | 1,190 ± 90 | 930 ± 1 | 9 ± 2 | 20 ± 3 | 7 ± 2 | 19 ± 2 | 11 | 2.2 |
| TV105 | Glc + Ile, Val | 1,720 | NM | 22 | 150 | 10 | 113 | NC | 6.8 |
| TV506 | Glc | 1,780 ± 30 | 1,570 ± 130 | 36 ± 9 | 10 ± 4 | 6 ± 1 | 180 ± 60 | NC | 0.3 |
| TV496 | Glc + Pan | 1,215 ± 90 | 890 ± 40 | 62 ± 6 | 17 ± 1 | 1.7 ± 1 | ND | NC | 0.28 |
| TV496 | Glc + Ile | 1,550 ± 300 | 1,040 ± 30 | 17 ± 2 | 8 ± 2 | 2 ± 1 | 93 ± 11 | NC | 1.1 |
| TV497 | Glc + Pan | 1,250 ± 120 | 990 ± 70 | 140 ± 7 | 23 ± 2 | ND | 64 ± 17 | NC | 0.17 |
| TV108 | Glc | 1,095 | 952 | 132 | 64 | 10 | 45 | NC | 0.48 |
| TV108 | Glc + Ile | 2,150 | NM | 6 | 290 | 82 | 340 | NC | 48 |
| MM294 | Glc | 1,370 ± 140 | 820 ± 70 | 28 ± 2 | 58 ± 3 | 8 ± 2 | 8 ± 1 | NC | 2.1 |
| LT2 | Acetate | 1,200 ± 80 | 660 ± 10 | 17 ± 2 | 10 ± 1 | 7 ± 1 | 29 ± 4 | 120 ± 20 | 0.6 |
| TV105 | Acetate | 730 | 720 | 3 | 1.7 | ND | 1.8 | 47 | 0.5 |
| TV496 | Acetate | 880 ± 70 | 770 ± 20 | 23 ± 2 | 6 ± 1 | ND | 11 ± 2 | 100 ± 10 | 0.26 |
| MM294 | Acetate | 650 | 400 | 16 | 7 | ND | 18 | NC | 0.44 |
Bacterial cultures were grown in glucose (Glc) or acetate. Additions to the media were as follows: Pan, 0.34 mM pantothenate; Ile, 0.38 mM isoleucine; and Val, 0.76 mM valine. Extracts were prepared as described in Materials and Methods.
Except for pyruvate (Pyr), all keto acids were determined by high-pressure liquid chromatography (HPLC) with precolumn derivatization with 1,2-diamino-4,5-methylenedioxybenzene as described in Materials and Methods. Pyr was determined by a spectrophotometric method with lactate dehydrogenase (LDH) as described previously (25). Concentrations were calculated assuming the intracellular volume as described in the text. PEP, phosphoenolpyruvate; KB, 2-ketobutyrate; KIV, 2-ketoisovalerate; KIC, 2-keto-4-methylvalerate (ketoisocaproate); KMV, 2-keto-3-methylvalerate. NM, not measured (the enzyme-coupled assay was not performed); ND, not detected (≤1 μM); NC, not calculated (the determination of glyoxylate required collection and analysis of early data from the HPLC run, which was not always carried out).
The 2-keto acids specifically related to the BCAA biosynthetic pathway, including 2-ketobutyrate, were minor metabolites in wild-type S. typhimurium LT2 grown in either glucose or acetate (Table 4). Mutants expressing AHAS I only (TV496, TV497, and TV108) showed elevated levels of 2-ketobutyrate (60 to 140 μM, compared to 17 μM in LT2). The addition of isoleucine to the growth medium of TV496 and TV108 decreased 2-ketobutyrate levels presumably by effective feedback inhibition of threonine deaminase by isoleucine and increased 2-keto-3-methylvalerate levels by transamination of the isoleucine. The addition of isoleucine and valine together to the growth medium of TV105, expressing AHAS II only, also caused the expected increase in the levels of the keto acids derived from them.
The ilvA219 mutation caused defective feedback inhibition of threonine deaminase; this led to higher intracellular 2-ketobutyrate levels, as expected; compare TV506 to TV105 or TV497 to TV496 (Table 4). In TV506, expressing AHAS II only, this mutation led to an increased doubling time which could be relieved by valine or pantothenate (Table 2).
Levels of 2-ketobutyrate in S. typhimurium LT2 grown in acetate were not very different from those in cells grown in glucose (Table 4), but ketoisovalerate levels were significantly lower in cells grown in acetate. Despite this finding, the BCAAs did not seem to be limiting under these conditions (Table 2). Very low levels of 2-ketobutyrate, ketoisovalerate, and 2-keto-3-methylvalerate were found during growth in acetate in strain TV105, expressing AHAS II only (Table 4). Under these conditions, TV105 did indeed seem to be limited in pantothenate, valine, and isoleucine syntheses (Table 2).
Intracellular free amino acid contents.
When two independent analyses of the free amino acid contents were carried out (for strains LT2 and TV497), they showed 20 to 30% deviation between the analyses as a result of the complexity of the bacterial extracts. Recovery for all of the amino acids of interest was about 60 to 70%, as judged by analysis of a control containing known amounts of the amino acids. Despite this uncertainty in the analysis, the data (Table 5) can provide a semiquantitative picture of free amino acid levels in the cells. The concentrations of amino acids relevant to the pathway were in general quite similar to those reported for E. coli grown in glucose (58); comparable data for S. typhimurium are not available.
TABLE 5.
Free amino acids in bacterial strainsa
| Strain | Medium | Intracellular concn (mM) ofb:
|
|||||
|---|---|---|---|---|---|---|---|
| Thr | Val | Leu | Ile | Met | 2-Aminobutyrate | ||
| LT2 | Glucose | 0.08 ± 0.003 | 1.14 ± 0.01 | 0.72 ± 0.27 | 0.17 ± 0.04 | 0.070 ± 0.006 | ND |
| TV105 | Glucose | — | 1.56 | 0.65 | 0.46 | 0.11 | ND |
| TV496 | Glucose + pantothenate | ND | 1.79 | 1.42 | 0.37 | 0.08 | 0.7 |
| TV496 | Glucosec | ND | 2.37 | 0.52 | 0.11 | 0.07 | 0.3 |
| TV497 | Glucose + pantothenate | 0.05 ± 0.012 | 1.08 ± 0.32 | 1.12 ± 0.05 | 5.90 ± 0.43 | 0.054 ± 0.010 | 0.50 ± 0.03 |
| MM294 | Glucose | 0.29 | 3.7 | 1.08 | 0.31 | 0.59 | 0.1 |
| LT2 | Acetate | 0.03 | 3.76 | 0.26 | 0.19 | 0.11 | ND |
The amino acid analysis was performed as described in Materials and Methods.
Bacteria were grown to the steady state, rapidly filtered, and extracted with boiling water as described in the text. Intracellular concentrations were calculated from the results of automated amino acid analysis with a slow gradient (see Results). Data shown for LT2 and TV497 in glucose medium are the averages of two independent analyses. ND, not detected (the limits of detection were apparently ≤0.02 mM). —, peaks of threonine and serine were too close for a reliable estimate of the threonine concentration in this sample.
TV496 does not attain a constant growth rate in glucose minimal medium without additions. An aliquot of a culture grown to the steady state with the addition of pantothenate was diluted 10-fold to an absorbance of 4 Klett units in glucose minimal medium and allowed to double three times (40 Klett units) before being harvested for amino acid analysis.
The apparent free threonine concentration was surprisingly low in all S. typhimurium strains examined; in TV496 it was too low for adequate determination (Table 5). Methionine levels were also very low in the S. typhimurium strains.
The relative levels of the BCAAs in different strains were in general parallel to the levels of their cognate 2-keto acids (Tables 4 and 5). Strain TV497, which lacks AHAS II and harbors a feedback-insensitive form of threonine deaminase, showed relatively high levels of isoleucine, as expected from the high levels of 2-keto-3-methylvalerate observed (Table 4) and from the fact that isoleucine addition to the medium did not improve the growth of this strain (Table 2). The transamination product of 2-ketobutyrate, 2-aminobutyrate (AB), was detected in strains which showed relatively high 2-ketobutyrate levels (Tables 4 and 5).
In order to be able to estimate the required flux of valine plus leucine and isoleucine into protein in growing cells, the total amino acid contents (after acid hydrolysis) of S. typhimurium LT2 were measured. The amino acid contents found were similar to those reported for E. coli (50).
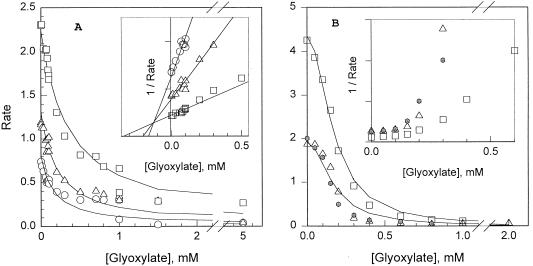
Inhibition of AHAS isozymes by glyoxylate.
The inhibition of AHAS by glyoxylate has been reported previously (14), but details are available in the literature only for AHAS III (28). We examined the kinetics of the other isozymes under the standard conditions that we assume are relevant to their intracellular function, pH 7.6. The Km of isozyme I for pyruvate was 1.6 mM under these conditions (data not shown). Glyoxylate behaved as a competitive inhibitor of AHAS I, with a Ki of 0.14 mM (Fig. 2A). The behavior of isozyme II, with a Km for pyruvate of 5.3 mM under our standard conditions, was more complex (Fig. 2B); the nonlinear Dixon plots for glyoxylate inhibition suggested that there is more than one mode of interaction of glyoxylate with the enzyme, perhaps involving competition with each of the two substrate pyruvate molecules (28). On the other hand, the inhibition observed in the presence of 1 mM pyruvate plus 0.1 mM 2-ketobutyrate was quantitatively similar to that observed with pyruvate alone. The total rates of these reactions in the absence of glyoxylate were expected to be the same, even though in the former case most of the product was expected to be acetohydroxybutyrate (28).
FIG. 2.
Inhibition of AHAS activity of glyoxylate. (A) Effect of glyoxylate on AHAS I. The activity in the presence of various glyoxylate concentrations was monitored by a standard method at pyruvate concentrations of 0.5 mM (○), 1 mM (▵), or 3 mM (□). The data could be fit (curves) by assuming competitive inhibition, with a Km (pyruvate) of 1.6 mM and a Ki (glyoxylate) of 0.14 mM. The insert is a Dixon plot of the data. (B) Effect of glyoxylate on AHAS II. In addition to experiments at 1 mM (▵) or 3 mM (□) pyruvate, measurements were made in the presence of 1 mM pyruvate plus 0.1 mM 2-ketobutyrate ( ), conditions under which >85% of the product was expected to be acetohydroxybutyrate (24). The data could not be fit by any simple inhibition mechanism; the curves are a fit of the data (pyruvate only) to competition dependent on the square of the glyoxylate concentration: V(inhibited) = V0/{1 + ([glyoxylate]/K)2}, where K is 0.169 mM. The insert is a Dixon plot of the data.
), conditions under which >85% of the product was expected to be acetohydroxybutyrate (24). The data could not be fit by any simple inhibition mechanism; the curves are a fit of the data (pyruvate only) to competition dependent on the square of the glyoxylate concentration: V(inhibited) = V0/{1 + ([glyoxylate]/K)2}, where K is 0.169 mM. The insert is a Dixon plot of the data.
DISCUSSION
Despite the great interest in the BCAA pathway, reinforced in recent years by the economic importance of herbicides which inhibit it, there has not yet been a comprehensive attempt to analyze the function of its components as they act together. There have in fact been only a few such quantitative studies of the biosynthesis of any amino acid (10, 75, 76). In our present study of BCAA synthesis in S. typhimurium, we made simultaneous measurements in growing cells of the levels of several enzymes, substrates, and intermediates using a number of isogenic mutants and different carbon sources. This investigation provided unique data which allow for the first time a quantitative assessment of the roles of the AHAS isozymes. The measured levels of the intermediates require a revision of our suggestions (2, 3, 29) concerning the way in which AHAS isozymes contribute to the fitness of enterobacteria.
Calculated fluxes.
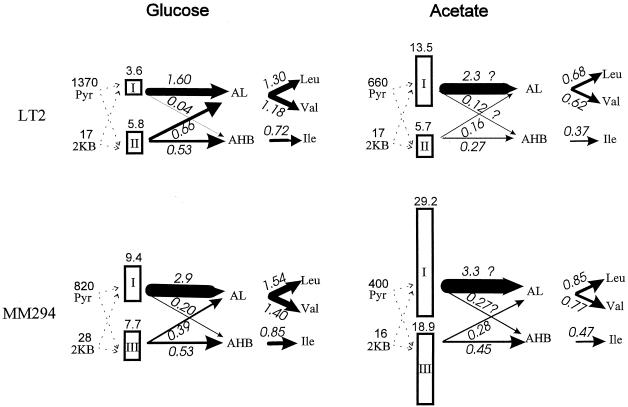
The measurement of intracellular pyruvate and ketobutyrate concentrations (Table 4) and of the specific activities of the AHAS isozymes (Table 3) under a single set of conditions allowed us to calculate predicted fluxes through each isozyme to each of the acetohydroxy acids (Fig. 3). The kinetic parameters used were obtained under conditions close to those expected for the intracellular environment in which the isozymes function. The minimal required flux to each of the BCAAs, calculated from the doubling times for the strains and media in question (Table 2) and the amino acid compositions of the cells, are given in Fig. 3. Because of the uncertainties in the data, we estimate that the calculated fluxes in Fig. 3 have an uncertainty of about 30%.
FIG. 3.
Estimated fluxes in the BCAA biosynthetic pathway. Fluxes estimated for S. typhimurium LT2 and E. coli K-12 MM294 in glucose and in acetate minimal media are given in units of millimolar minute−1 over the flux arrows, which are drawn with thickness proportional to flux. Fluxes through the AHAS isozymes to the two acetohydroxy acids (acetolactate [AL] and acetohydroxybutyrate [AHB]) were calculated from the intracellular pyruvate and 2-ketobutyrate (2KB) micromolar concentrations (at left in each portion of the figure [from Table 4]) and from the measured activity of each isozyme (Vmax in units of flux of millimolar minute−1, calculated from the specific activities shown in Table 3 as described in Materials and Methods). Fluxes were calculated from V = Vmax[Pyr]/(Km + [Pyr]), where the Km values are 1.64, 5.3, and 6 mM for AHAS I, II, and III, respectively. Fluxes in acetate medium were approximated for AHAS I or III by assuming that Km(app) = Km(1 + 100 μM/Ki), where the values of Ki, the inhibition constant for glyoxylate, are 140 and 150 μM, respectively, for isozymes I and III. For isozyme II, we used the phenomenological relationship shown in Fig. 2B. The partitioning of flux to the two products, AL and AHB, was calculated from VAHB/VAL = R([KB]/[Pyr]), where the R values are 2, 65, and 40 for AHAS I, II, and III, respectively (29). The required (minimal) flux, J, to each amino acid was calculated from the doubling time of the cells, τ (Table 2), the total amino acid content, A (320, 350, and 190 μmol of Val, Leu, and Ile g [dry weight]−1, respectively), and the assumption that 1 g (dry weight) of cells contains 2.4 ml of intracellular water (50, 58): J = (ln2/τ) × (A/2.4).
There is a reassuring agreement between the calculated fluxes to acetolactate and acetohydroxybutyrate, on the one hand, and the required fluxes to leucine plus valine and to isoleucine, on the other hand, in LT2 grown in glucose (Fig. 3). The flux to pantothenate can be disregarded in this comparison since it is estimated to be about 1/20 the combined flux to leucine and valine (13). Although the apparent specific activity of AHAS II is higher than that of AHAS I under these conditions, AHAS I provides about two thirds of the combined flux to leucine and valine because isozyme I has a lower Km for pyruvate than isozyme II and because almost all of the flux through it is to acetolactate. More than 90% of the flux to isoleucine is provided by AHAS II (Fig. 3), even though this isozyme is calculated to produce at least as much acetolactate as acetohydroxybutyrate at the intracellular pyruvate/ketobutyrate concentration ratio observed (Table 4).
When strain LT2 grows in acetate, a number of changes affect the fluxes in the pathway. Because the doubling time for cells in acetate is nearly twice as long as that in glucose (Table 2), the minimal required fluxes to the amino acids for growth in acetate are only about half those calculated for growth in glucose (Fig. 3). The change in carbon source leads to a more than twofold decrease in the intracellular pyruvate concentration (Table 4), but it is important to note that we did not observe the drastic decrease in pyruvate levels previously reported for E. coli (16, 46). More important, it appears that the intracellular levels of glyoxylate, a potent inhibitor of the AHAS isozymes of enterobacteria (14, 28), increase from about 10 μM in glucose-grown cells to approximately 100 μM when acetate is the sole carbon source (Table 4). A calculation based on the parameters used in Fig. 2 indicates that the effective rates of the AHAS reactions will be decreased by 1.5- to 3-fold by 100 μM glyoxylate. S. typhimurium is able to continue to synthesize the BCAAs under these circumstances because growth in acetate leads to a nearly fourfold induction of AHAS I expression (Table 3). We have included an estimation of the effects of glyoxylate in all of the calculated fluxes given here for growth in acetate as a carbon source. Figure 3 shows that over 90% of the calculated flux to acetolactate in S. typhimurium LT2 grown in acetate is due to AHAS I, although AHAS II remains the major source of flux (60 to 70%) to acetohydroxybutyrate.
We also estimated the relative contributions of AHAS isozymes I and III to the fluxes in the pathway. The calculated fluxes to the acetohydroxy acids in E. coli K-12 strain MM294 (which is wild type with regard to the BCAA pathway) grown in glucose minimal medium are in reasonable agreement with the minimal required fluxes to the amino acid end products. AHAS I seems to play a more major role in this case than it does in S. typhimurium LT2, perhaps because AHAS III has a lower specificity for 2-ketobutyrate than does AHAS II (R values, 40 and 65, respectively). The slightly higher growth rate (Table 2) and lower intracellular pyruvate concentrations (Table 4) in the E. coli strain also may be significant in this regard. Surprisingly, the expression of both isozymes is significantly higher when this E. coli strain is grown in acetate (Table 3), so that AHAS III plays almost as significant a calculated role in this medium as it does during growth in glucose, e.g., providing 65% of the flux to acetohydroxybutyrate and ∼10% of the flux to acetolactate.
The calculations of predicted fluxes in Fig. 3 (see Fig. 4) do not take into consideration the regulatory effect of valine as an allosteric feedback inhibitor of AHAS I. The measured free valine concentrations reported in Table 5 would lower the activity of AHAS I at the observed pyruvate levels by a factor of 3 to 10 or more (calculations not shown). The resulting overestimation of the flux through AHAS I might be more serious for growth in acetate, since the valine level measured in S. typhimurium LT2 grown in acetate is significantly higher than that for cells grown in glucose.
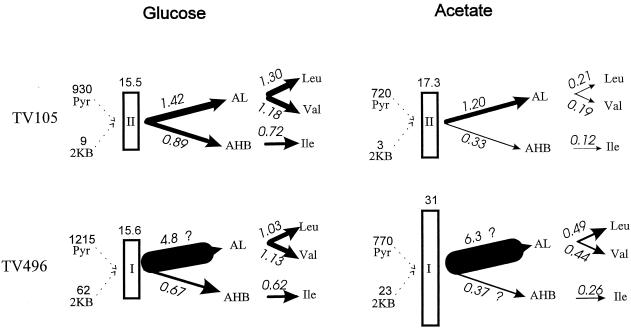
FIG. 4.
Fluxes estimated for two mutant strains of S. typhimurium containing a single AHAS isozyme in glucose or acetate minimal medium. Calculated fluxes to the acetohydroxy acids were estimated from the data shown, and required fluxes to the three BCAAs were calculated from the doubling times as described in the legend to Fig. 3. Note that the data for TV496 were for growth in the presence of added pantothenate.
Behavior of isogenic mutants.
The quantitative analysis of the pathway in isogenic mutants of S. typhimurium LT2 containing a single AHAS isozyme further illuminates the roles of multiple isozymes in enterobacteria (Fig. 4). TV105, which expresses only AHAS II, is able to grow in glucose at a normal rate (Table 2) because of two regulatory adaptations: (i) the level of AHAS II expressed is two times that in the wild type (Table 3), and (ii) the steady-state concentration of 2-ketobutyrate is reduced by about twofold (Table 4). These adaptations are the result of known regulatory mechanisms; the expression of the ilvGMEDA operon is attenuated by multivalent action of the three BCAAs, and the activity of threonine deaminase is regulated allosterically by the relative levels of valine and isoleucine (12, 23, 32, 65).
On the other hand, TV496, which expresses only isozyme I of AHAS, grows very slowly in glucose (Table 2). The addition of pantothenate allows this strain to grow nearly as rapidly as the wild type. The adaptation of this strain to growth in glucose in the presence of pantothenate involves a fourfold increase in the expression of AHAS I relative to that in LT2 (Table 3), as well as the accumulation of 2-ketobutyrate to a level about four times higher than the steady-state level observed in the wild type (Table 4). These adaptations seem to satisfy the requirements for BCAA synthesis (Fig. 4), and we must look elsewhere to understand why pantothenate or methionine is required for the rapid growth of TV496 in glucose (see below).
In acetate, TV105 expressing only AHAS II grows very slowly; the addition of both valine and isoleucine to the medium is required to raise the growth rate to that of the wild type (Table 2). Figure 4 shows that AHAS II, at a level which apparently represents maximum derepression of the ilvGMEDA operon, is able to provide a barely sufficient flux through each of the branches of the pathway to sustain the observed slow growth. The flux through this enzyme is calculated to be low chiefly because of the inhibitory effect of the high glyoxylate levels in cells grown in acetate (Table 4). TV496, containing AHAS I only, is also somewhat limited in growth in acetate (Table 2), despite a very high level of expression of this isozyme (Table 3). The calculated fluxes shown in Fig. 4 suggest that isoleucine production should be the limiting factor in this strain; it is not clear why the addition of isoleucine has so little effect on growth under these conditions (Table 2).
Regulation of AHAS I by valine.
In some cases, e.g., the growth of TV496 in glucose with added pantothenate, the inclusion of the inhibitory effect of valine in the estimation of flux through AHAS I (Fig. 4) would be inconsistent with the observed growth rate (Table 2). It is possible that the measured values for free amino acids represent in part sequestered labile intermediates or some other experimental error of unknown origin. However, it is also conceivable that some form of metabolic channeling among the enzymes of the pathway is responsible for the apparent paradox. Ratzkin and coworkers suggested some years ago, on the basis of indirect genetic evidence, the existence of a complex of the BCAA pathway enzymes in E. coli which synthesizes valine from pyruvate (60). The isolation of such a complex from Neurospora crassa by gradient centrifugation has also been reported (5).
Imbalance among 2-keto acids.
Due to the close structural relationship among the 2-keto acids involved in the BCAA pathway, enzymes cannot always completely differentiate among them (42, 56). In the reaction catalyzed by ketopantoate hydroxymethyl transferase (EC 2.1.2.11), competition between 2-ketoisovalerate and 2-ketobutyrate can lead to the formation of a desmethyl analog of ketopantoate (56) which might be an antimetabolite for coenzyme A synthesis. The synthesis of norvaline from 2-ketobutyrate by the enzymes of the leucine branch of the pathway is a further possible result of an unusually low 2-ketoisovalerate/2-ketobutyrate ratio (7, 36, 69). Together, these effects are expected to have ramifications which lead to a methionine deficiency (41, 44, 57, 70).
TV496, which expresses only AHAS I, shows the characteristic behavior ascribed to such problems (57); it grows very slowly in glucose, but pantothenate, methionine, or isoleucine supplementation can each support its growth at nearly wild-type rates (Table 2). Similar reversals by these nutrients were obtained (69) when sulfometuron methyl, a specific inhibitor of AHAS II (39), was added to cells of the parent LT2 strain in glucose medium supplemented with adenine. This result suggests that the conditional nature of the defect in TV496 is not a consequence of the specific ilvG mutation in the strain. In TV496 grown in glucose, the ratio of 2-ketoisovalerate to 2-ketobutyrate is less than 0.3, about 1 order of magnitude lower than that observed in S. typhimurium strains which are prototrophic in glucose, such as LT2 or TV105 (Table 4). This observation demonstrates that an imbalance in 2-keto acid concentrations could be the cause of the auxotrophy of this strain, which we could not explain on the basis of the calculated fluxes.
Isoleucine can support the growth of the strain that expresses only AHAS I, TV496, with glucose as the sole carbon source because it depresses the 2-ketobutyrate concentration (Table 4). The fact that the growth of TV497, harboring a feedback-insensitive form of threonine deaminase (43), cannot be supported by isoleucine in glucose minimal medium (Table 2) indicates that an elevated concentration of 2-ketobutyrate can be detrimental. The ratio between 2-ketoisovalerate and 2-ketobutyrate is lower upon growth in acetate than upon growth in glucose for LT2 and is particularly low for TV496. This finding may explain why this strain grows more slowly than the wild type in acetate medium (Table 2), despite expressing AHAS I, the isozyme presumed optimal for growth in a carbon source such as acetate.
Threonine deaminase function.
The physiological importance of threonine deaminase in the regulation of flux to valine as well as isoleucine has long been recognized (65). The data presented here show that the enzyme operates in S. typhimurium at a threonine concentration (≤0.1 M) (Table 5) at which its activity is far below its maximal velocity and is inversely proportional to the isoleucine concentration (21–23). The minimal required flux through threonine deaminase in strain LT2 (i.e., the calculated flux to isoleucine, 0.72 mM min−1) is less than 1% the measured activity of the enzyme in this strain under optimal conditions (equivalent to 140 mM min−1). These observations demonstrate the quantitative importance of this feedback regulation in S. typhimurium.
Further evidence for the physiological importance of this regulation is seen in the properties of S. typhimurium strains carrying the ilvA219 mutation, leading to a threonine deaminase defective in feedback inhibition. These strains grow poorly under many conditions compared to isogenic strains which have a single AHAS isozyme and which express wild-type threonine deaminase (Table 2). As expected, they generally have elevated intracellular levels of 2-ketobutyrate and 2-keto-3-methylvalerate and reduced levels of ketoisovalerate compared to those with feedback-sensitive threonine deaminase (Table 4). A milder version of this phenotype was previously ascribed to a strain carrying the ilvA219 allele in conjunction with a full complement of AHAS I and AHAS II encoded by ilvBN+ and ilvGM+; this strain accumulated 2-keto-3-methylvalerate, although it did not show a growth defect (43, 69).
Conclusions.
Although the enterobacteria have adopted multiple isozymes, each regulated in a different manner, as a common strategy for controlling flux to different end products in branching pathways (31), the multiple isozymes of AHAS clearly do not represent a simple example of such a strategy. In addition to maintaining the availability of each of the amino acids for protein synthesis, the machinery for the biosynthesis of the three BCAAs must allow close regulation of concentrations of intermediates which may potentially interact with other pathways. These problems arise because of fundamental limitations in the specificities of enzymes.
The role of multiple AHAS isozymes in the enterobacteria in meeting these challenges is complex. In wild-type LT2, isozyme I provides most of the flux to valine, leucine, and pantothenate, while isozyme II provides most of the flux to isoleucine. Either of these isozymes by itself is able to provide for adequate flux to both sets of products under the physiological conditions appropriate for the isozyme. The limitations on strains with single isozymes are different. A strain with isozyme I only is limited during growth in glucose by the low specificity of this enzyme for 2-ketobutyrate. The elevated threonine deaminase activity engendered by isoleucine limitation then leads to increased 2-ketobutyrate and decreased 2-ketoisovalerate levels, which ultimately interfere with the synthesis of coenzyme A and methionine. AHAS isozyme II (or III) is optimized to cope with these problems by preferring 2-ketobutyrate over pyruvate with a specificity close to the physicochemical limits for the selective recognition of a methyl group by a protein (28). On the other hand, a strain with isozyme II only is limited in growth in, e.g., acetate, because of its high Km for pyruvate and its sensitivity to inhibition by glyoxylate. AHAS I, with its low Km for pyruvate, is better designed for dealing with competition by glyoxylate for the pyruvate binding site on AHAS. It is conceivable that no single enzyme could possibly be adequate for the varied conditions that the enterobacteria successfully encounter.
The existence of multiple isozymes of AHAS is not the rule among autotrophic organisms. A variety of organisms, including plants (49), algae (71), yeasts (26), gram-positive bacteria (33), cyanobacteria (34), and archaebacteria (8), appear to have a single AHAS regulated by a single amino acid. Some of these organisms no doubt manage well with only a single AHAS due to the compartmentalization of the BCAA pathway and glyoxylate shunt in separate organelles, while others may be more restricted in the range of external conditions that they cope with naturally.
Studies of the quantitative details of a complex metabolic pathway such as that involved in the biosynthesis of a group of amino acids do not deal with a completely defined system because of interactions with other pathways and possible effects of still-undefined protein-protein interactions. They are thus unlikely to lead rapidly to a comprehensive quantitative model (e.g., one that can be tested as a mathematical model). Nonetheless, such studies are likely to provide insight into the workings of pathways within the crowded confines of the cell and into the evolution of metabolic function. Such studies of metabolic complexity are crucial for an understanding of the biochemistry of living cells and may have relevance to systems as diverse as multicomponent regulatory cascades and inborn errors of human metabolism.
ACKNOWLEDGMENTS
This work was supported by grant 338/92 from the Israel Science Foundation.
We are grateful to Monika Einav and Neora Levy for excellent technical assistance.
REFERENCES
- 1.Barak Z, Calvo J M, Schloss J V. Acetolactate synthase isozyme III from Escherichia coli. Methods Enzymol. 1988;166:455–458. doi: 10.1016/s0076-6879(88)66059-9. [DOI] [PubMed] [Google Scholar]
- 2.Barak Z, Chipman D M, Gollop N. Physiological implications of the specificity of acetohydroxy acid synthase isozymes of enteric bacteria. J Bacteriol. 1987;169:3750–3756. doi: 10.1128/jb.169.8.3750-3756.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barak Z, Kogan N, Gollop N, Chipman D M. Importance of AHAS isozymes in branched chain amino acid biosynthesis. In: Barak Z, Chipman D M, Schloss J V, editors. Biosynthesis of branched chain amino acids. Weinheim, Germany: VCH; 1990. pp. 91–107. [Google Scholar]
- 4.Berg, C. M. Personal communication.
- 5.Berquist A, Eakin E A, Murali D K, Wagner R P. A pyruvate-valine enzyme complex that is dependent upon the metabolic state of the mitochondria. Proc Natl Acad Sci USA. 1974;71:4352–4355. doi: 10.1073/pnas.71.11.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blazey D L, Burns R O. Regulation of Salmonella typhimurium ilvYC genes. J Bacteriol. 1984;159:951–957. doi: 10.1128/jb.159.3.951-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogosian G, Violand B N, Doward-King E J, Workman W E, Jung P E, Kane J F. Biosynthesis and incorporation into protein of norleucine in Escherichia coli K-12. J Biol Chem. 1989;264:531–539. [PubMed] [Google Scholar]
- 8.Bowen T L, Union J, Tumbula D L, Whitman W B. Cloning and phylogenetic analysis of the genes encoding acetohydroxyacid synthase from the archaeon Methanococcus aeolicus. Gene. 1997;188:77–84. doi: 10.1016/s0378-1119(96)00779-2. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Brenner M, Ames B N. The histidine operon and its regulation. In: Vogel H J, editor. Metabolic regulation. New York, N.Y: Academic Press, Inc.; 1971. pp. 349–387. [Google Scholar]
- 11.Burns R O, Hofler J G, Luginbuhl G H. Threonine deaminase from Salmonella typhimurium. Substrate-specific patterns of inhibition in an activator-deficient form of the enzyme. J Biol Chem. 1979;254:1074–1079. [PubMed] [Google Scholar]
- 12.Burns R O, Zarlengo M H. Threonine deaminase from Salmonella typhimurium. J Biol Chem. 1968;243:178–185. [PubMed] [Google Scholar]
- 13.Cronan J E, Jr, Littel K J, Jakowski S. Genetic and biochemical analyses of pantothenate biosynthesis in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1982;149:916–922. doi: 10.1128/jb.149.3.916-922.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dailey F E, Cronan J E., Jr Acetohydroxy acid synthase I, a required enzyme for isoleucine and valine biosynthesis in Escherichia coli K-12 during growth on acetate as the sole carbon source. J Bacteriol. 1986;165:453–460. doi: 10.1128/jb.165.2.453-460.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dailey F E, Cronan J E, Jr, Maloy S R. Acetohydroxy acid synthase I is required for isoleucine and valine biosynthesis by Salmonella typhimurium LT2 during growth on acetate or long-chain fatty acids. J Bacteriol. 1987;169:917–919. doi: 10.1128/jb.169.2.917-919.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danchin A, Dondon L, Daniel J. Metabolic alterations mediated by 2-ketobutyrate in Escherichia coli K-12. Mol Gen Genet. 1984;193:473–478. doi: 10.1007/BF00382086. [DOI] [PubMed] [Google Scholar]
- 17.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 18.DeFelice M, Griffo G, Lago C T, Limauro D, Ricca E. Detection of the acetolactate synthase isozymes I and III of Escherichia coli K12. Methods Enzymol. 1988;166:241–244. doi: 10.1016/s0076-6879(88)66032-0. [DOI] [PubMed] [Google Scholar]
- 19.DeFelice M, Lago C T, Squires C H, Calvo J M. The acetohydroxy acid synthase isozymes of Escherichia coli and Salmonella typhiumurium. Ann Microbiol (Paris) 1982;133A:251–256. [PubMed] [Google Scholar]
- 20.DeFelice M, Squires C H, Levinthal M. A comparative study of the acetohydroxy acid synthase isozymes of Escherichia coli K-12. Biochim Biophys Acta. 1978;541:9–17. [Google Scholar]
- 21.Eisenstein E. Energetics of cooperative ligand binding to the active sites of threonine deaminase. J Biol Chem. 1994;269:29416–29422. [PubMed] [Google Scholar]
- 22.Eisenstein E. Allosteric regulation of biosynthetic threonine deaminase from Escherichia coli: effects of isoleucine and valine on active-site ligand binding and catalysis. Arch Biochem Biophys. 1995;316:311–318. doi: 10.1006/abbi.1995.1042. [DOI] [PubMed] [Google Scholar]
- 23.Eisenstein E, Yu H D, Schwarz F P. Cooperative binding of the feedback modifiers isoleucine and valine to threonine deaminase. J Biol Chem. 1994;269:29423–29429. [PubMed] [Google Scholar]
- 24.Epelbaum S, Chipman D M, Barak Z. Determination of products of acetohydroxy acid synthase by the colorimetric method, revisited. Anal Biochem. 1990;191:96–99. doi: 10.1016/0003-2697(90)90393-n. [DOI] [PubMed] [Google Scholar]
- 25.Epelbaum S, Chipman D M, Barak Z. Metabolic effects of inhibitors of two enzymes of the branched-chain amino acid pathway in Salmonella typhimurium. J Bacteriol. 1996;178:1187–1196. doi: 10.1128/jb.178.4.1187-1196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falco S C, Dumas K S. Genetic analysis of mutants of Saccharomyces cerevisiae resistant to the herbicide sulfometuron methyl. Genetics. 1985;109:21–35. doi: 10.1093/genetics/109.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freundlich M, Burns R O, Umbarger H E. Control of isoleucine, valine and leucine biosynthesis. I. Multi-valent repression. Proc Natl Acad Sci USA. 1962;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gollop N, Damri B, Barak Z, Chipman D M. Kinetics and mechanism of acetohydroxy acid synthase isozyme III from Escherichia coli. Biochemistry. 1989;28:6310–6317. doi: 10.1021/bi00441a024. [DOI] [PubMed] [Google Scholar]
- 29.Gollop N, Damri B, Chipman D M, Barak Z. Physiological implications of the substrate specificities of acetohydroxy acid synthases from varied organisms. J Bacteriol. 1990;172:3444–3449. doi: 10.1128/jb.172.6.3444-3449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodsell D S. Inside a living cell. Trends Biochem Sci. 1991;16:203–206. doi: 10.1016/0968-0004(91)90083-8. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann K M, Somerville R L. Amino acids: biosynthesis and genetic regulation. Reading, Mass: Addison-Wesley Publishing Co.; 1983. [Google Scholar]
- 32.Hoffler J G, Burns R O. Threonine deaminase from Salmonella typhimurium. J Biol Chem. 1978;253:1245–1251. [PubMed] [Google Scholar]
- 33.Inui M, Vertes A A, Kobayashi M, Kurusu Y, Yukawa H. Cloning and sequence determination of the acetohydroxy acid synthase genes from Brevibacterium flavum MJ233 by using the polymerase chain reaction. DNA Sequence. 1993;3:303–310. doi: 10.3109/10425179309020828. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko T, Sato S, Kotani H, et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 35.Kiritani K, Wagner R P. α,β-Dihydroxy acid dehydratase. Methods Enzymol. 1970;17A:755–764. [Google Scholar]
- 36.Kisumi N, Siguira M, Chibata I. Biosynthesis of norvaline, norleucine, and homoisoleucine in Serratia marcescens. J Biochem. 1976;80:333–339. doi: 10.1093/oxfordjournals.jbchem.a131281. [DOI] [PubMed] [Google Scholar]
- 37.Kleckner N J, Roth J, Botstein D. Genetic engineering in vivo using translocatable drug-resistant elements. New methods in bacterial genetics. J Mol Biol. 1977;116:125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- 38.Krampitz L O. Preparation and determination of 2-acetolactic acid. Arch Biochem. 1948;17:81. [PubMed] [Google Scholar]
- 39.LaRossa R A, Schloss J V. The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonella typhimurium. J Biol Chem. 1984;259:8753–8757. [PubMed] [Google Scholar]
- 40.LaRossa R A, Smulski D R. ilvB-Encoded acetolactate synthase is resistant to the herbicide sulfometuron methyl. J Bacteriol. 1984;160:391–394. doi: 10.1128/jb.160.1.391-394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaRossa R A, VanDyk T K. Metabolic mayhem caused by 2-ketoacid imbalances. Bioessays. 1987;7:125–130. doi: 10.1002/bies.950070308. [DOI] [PubMed] [Google Scholar]
- 42.LaRossa R A, VanDyk T K. Leaky pantothenate and thiamin mutations of Salmonella typhimurium conferring sulphometuron methyl sensitivity. J Gen Microbiol. 1989;135:2209–2222. doi: 10.1099/00221287-135-8-2209. [DOI] [PubMed] [Google Scholar]
- 43.LaRossa R A, VanDyk T K, Smulski D R. Toxic accumulation of α-ketobutyrate caused by inhibition of the branched-chain amino acid biosynthetic enzyme acetolactate synthase in Salmonella typhimurium. J Bacteriol. 1987;169:1372–1378. doi: 10.1128/jb.169.4.1372-1378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaRossa R A, VanDyk T K, Smulski D R. A need for metabolic insulation: lessons from sulfonylurea genetics. In: Barak Z, Chipman D M, Schloss J V, editors. Biosynthesis of branched chain amino acids. Weinheim, Germany: VCH; 1990. pp. 109–121. [Google Scholar]
- 45.Lopes J M, Soliman N, Smith P K, Lawther R P. Transcriptional polarity enhances the contribution of the internal promoter, ilv-Ep, in the expression of the ilvGMEDA operon in wild-type Escherichia coli K12.Mol. Microbiol. 1989;3:1039–1052. doi: 10.1111/j.1365-2958.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 46.Lowry O H, Carter J, Ward J B, Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971;246:6511–6521. [PubMed] [Google Scholar]
- 47.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 48.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 49.Mourad G, Haughn G, King J. Intragenic recombination in the CSR1 locus of Arabidopsis. Mol Gen Genet. 1994;243:178–184. doi: 10.1007/BF00280315. [DOI] [PubMed] [Google Scholar]
- 50.Neidhardt F C. Chemical composition of Escherichia coli. In: Neidhardt F C, Ingraham J L, Low K L, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 3–6. [Google Scholar]
- 51.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nunn W D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol Rev. 1986;50:179–192. doi: 10.1128/mr.50.2.179-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parekh B S, Hatfield G W. Growth rate-related regulation of the ilvGMEDA operon of Escherichia coli K-12 is a consequence of the polar frameshift mutation in the ilvG gene of this strain. J Bacteriol. 1997;179:2086–2088. doi: 10.1128/jb.179.6.2086-2088.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Payne S M, Ames B N. A procedure for rapid extraction and high-pressure liquid chromatographic separation of the nucleotides and other small molecules from bacterial cells. Anal Biochem. 1982;123:151–162. doi: 10.1016/0003-2697(82)90636-4. [DOI] [PubMed] [Google Scholar]
- 55.Pledger W J, Umbarger H E. Isoleucine and valine metabolism in Escherichia coli. XXII. A pleotropic mutation affecting induction of isomeroreductase activity. J Bacteriol. 1973;114:195–207. doi: 10.1128/jb.114.1.195-207.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powers S G, Snell E E. Ketopantoate hydroxymethyltransferase. 2. Physical catalytic and regulatory properties. J Biol Chem. 1976;251:3786–3793. [PubMed] [Google Scholar]
- 57.Primerano D A, Burns R O. Metabolic basis for the isoleucine, pantothenate, or methionine requirement of ilvG strains of Salmonella typhimurium. J Bacteriol. 1982;150:1202–1211. doi: 10.1128/jb.150.3.1202-1211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quay S C, Dick T E, Oxender D. Role of transport systems in amino acid metabolism: leucine toxicity and the branched-chain amino acid transport systems. J Bacteriol. 1977;129:1257–1265. doi: 10.1128/jb.129.3.1257-1265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramseier T M. Cra and the control of carbon flux via metabolic pathways. Res Microbiol. 1996;147:489–493. doi: 10.1016/0923-2508(96)84003-4. [DOI] [PubMed] [Google Scholar]
- 60.Ratzkin B, Arfin S, Umbarger H E. Isoleucine and valine metabolism in Escherichia coli. XVIII. Induction of acetohydroxy acid isomeroreductase. J Bacteriol. 1972;112:131–141. doi: 10.1128/jb.112.1.131-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanderson K E, Roth J R. Linkage map of Salmonella typhimurium, edition VI. Microbiol Rev. 1983;47:410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sella C, Weinstock O, Barak Z, Chipman D M. Subunit association in acetohydroxy acid synthase isozyme III. J Bacteriol. 1993;175:5339–5343. doi: 10.1128/jb.175.17.5339-5343.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw K J, Berg C M, Sobol T J. Salmonella typhimurium mutants defective in acetohydroxy acid synthases I and II. J Bacteriol. 1980;141:1258–1263. doi: 10.1128/jb.141.3.1258-1263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spackman D H, Stein W H, Moore S. Automatic recording apparatus for use in the chromatography of amino acids. Anal Chem. 1958;30:1190–1206. [PubMed] [Google Scholar]
- 65.Squires C H, Levinthal M, DeFelice M. A role for threonine deaminase in the regulation of α-acetolactate biosynthesis in Escherichia coli K12. J Gen Microbiol. 1981;127:19–25. doi: 10.1099/00221287-127-1-19. [DOI] [PubMed] [Google Scholar]
- 66.Sutton A, Freundlich M. Regulation by cyclic AMP of ilvB-biosynthetic acetohydroxy acid synthase in Escherichia coli K-12. Mol Gen Genet. 1980;178:179–183. doi: 10.1007/BF00267227. [DOI] [PubMed] [Google Scholar]
- 67.Umbarger H E. Biosynthesis of the branched chain amino acids. In: Neidhardt F C, Ingraham J L, Low K L, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 352–367. [Google Scholar]
- 68.Umbarger H E. The study of branched chain amino acid biosynthesis—its roots and its fruits. In: Barak Z, Chipman D M, Schloss J V, editors. Biosynthesis of branched chain amino acids. Weinheim, Germany: VCH; 1990. pp. 1–24. [Google Scholar]
- 69.VanDyk T K, LaRossa R A. Prevention of endogenous 2-ketobutyrate toxicity in Salmonella typhimurium. In: Barak Z, Chipman D M, Schloss J V, editors. Biosynthesis of branched chain amino acids. Weinheim, Germany: VCH; 1990. pp. 123–130. [Google Scholar]
- 70.VanDyk T K, Smulski D R, Chang Y Y. Pleiotropic effects of poxA regulatory mutations of Escherichia coli and Salmonella typhimurium, mutations conferring sulfometuron methyl and α-ketobutyrate hypersensitivity. J Bacteriol. 1987;169:4540–4546. doi: 10.1128/jb.169.10.4540-4546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van-Moppes D, Barak Z, Chipman D M, Gollop N, Arad S. An herbicide (sulfometuron-methyl) resistant mutant in Porphyridium (Rhodophyta) J Phycol. 1989;25:108–112. [Google Scholar]
- 72.Vyazmensky M, Sella C, Barak Z, Chipman D M. Isolation and characterization of subunits of acetohydroxy acid synthase isozyme III and reconstitution of the holoenzyme. Biochemistry. 1996;35:10339–10346. doi: 10.1021/bi9605604. [DOI] [PubMed] [Google Scholar]
- 73.Weinberg R, Burns R. Regulation of expression of the ilvB operon in Salmonella typhimurium. J Bacteriol. 1984;160:833–841. doi: 10.1128/jb.160.3.833-841.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wek R C, Hatfield G W. Transcriptional activation at adjacent operators in the divergent-overlapping ilvY and ilvC promoters of Escherichia coli. J Mol Biol. 1988;203:643–663. doi: 10.1016/0022-2836(88)90199-4. [DOI] [PubMed] [Google Scholar]
- 75.Yanofsky C, Horn V. Role of regulatory features of the trp operon of Escherichia coli in mediating a response to a nutritional shift. J Bacteriol. 1994;176:6245–6254. doi: 10.1128/jb.176.20.6245-6254.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanofsky C, Kelley R L, Horn V V. Repression is relieved before attenuation in the trp operon of Escherichia coli as tryptophan starvation becomes increasingly severe. J Bacteriol. 1984;158:1018–1024. doi: 10.1128/jb.158.3.1018-1024.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]