Highlights
-
•
Extraction-assisted isomerization strategy was applied to produce d-tagatose.
-
•
The effect of the molar ratio of boron to sugar was systematically investigated.
-
•
The relationship between isomerization and absorption equilibrium was revealed.
-
•
The conversion yield of d-tagatose was significantly improved.
-
•
Excellent operational stability was conducive to sustainable application.
Keywords: d-tagatose, Extraction-assisted isomerization, High purity, Isomerization equilibrium, Components dynamical changes
Abstract
A one-pot extraction-assisted d-galactose-to-d-tagatose isomerization strategy was proposed based on the selective extraction of d-tagatose by phenylborate anions. 4-Vinylphenylboronic acid was selected with high extraction efficiency and selectivity towards d-tagatose. The extracted sugars could be desorbed through a two-staged stripping process with the purity of d-tagatose significantly increased. In-situ extraction-assisted d-galactose-to-d-tagatose isomerization was implemented for the first time ever reported, and the effect of boron-to-sugar ratio (boron: sugar) was investigated. The conversion yield of d-tagatose at 60 °C increased from ∼ 39 % (boron: sugar = 0.5) to ∼ 56 % (boron: sugar = 1) but then decreased to ∼ 44 % (boron: sugar = 1.5). With temperature increased to 70 °C, the conversion yield of d-tagatose was further improved to ∼ 61 % (boron: sugar = 1.5), with the minimized formation of byproducts. Moreover, high purity (∼83 %) and concentrated d-tagatose solution (∼40 g/L) was obtained after sequential desorption. The proposed extraction-assisted isomerization strategy achieved improving the yield and purity of d-tagatose, proving its feasibility in industrial applications.
1. Introduction
As a kind of rare ketose, d-tagatose has attracted much attention due to its unique properties and functions. d-tagatose has 92 % sweetness of sucrose with a lower caloric value, which can help to effectively control diabetes and promote loss of excess weight by reducing calorific intake (Kim, 2004, Lu et al., 2008, Rhimi et al., 2015). However, d-tagatose exists in very small quantities in nature (Beerens et al., 2012, Roy et al., 2018), which is unfavorable for its broad application. Currently, the production of d-tagatose is mainly achieved by chemical-based isomerization and enzymatic-based isomerization using d-galactose as a substrate (Wang et al., 2022). Production of d-tagatose by chemical catalysis is convenient and low-cost, which attracted high attention in the past decades. However, the unfavorable aldose-ketose equilibrium and the unwanted formation of byproducts confined the yield and purity of d-tagatose, obstructing its application in industrial production. Besides, the complicated and environment-unfriendly downstream treatment ultimately hampers its sustainable development (Roy et al., 2018). In recent years, the biotechnological production of d-tagatose by enzymatic isomerization has attracted high attention (Patel et al., 2016). The yield of d-tagatose varies from ∼ 30 % to ∼ 60 % with fewer byproducts using enzymes as biocatalysts, which is much higher than chemo-catalysis (Baptista et al., 2021, Rai et al., 2021, Xu et al., 2018, Yuan et al., 2021). However, due to the low enzyme activity, poor stability, and high cost, enzymatic production of d-tagatose is also hard to apply to industry (Liu et al., 2022, Zhang et al., 2020, Zhang et al., 2017).
Boron affinity chromatography (BAC) is a unique technology to selectively capture and enrich cis-diol-containing compounds, such as saccharides, nucleosides, catechols, and glycoproteins (Li, Chen, & Liu, 2015). A variety of applications based on BAC, such as molecularly imprinted polymers (Wang et al., 2022, Zhu et al., 2021), monolithic columns (Li et al., 2015, Li et al., 2011), silica particles (Li et al., 2016), and magnetic nanoparticles (Li et al., 2013b, Wang et al., 2013) have been widely used in capturing different kinds of cis-diol-containing molecules, which shows excellent absorption capacity and selectivity. Recently, an efficient extraction-assisted isomerization strategy using phenylboronic acids (PBAs) as extractants has been implemented to improve the yield and purity of ketose from aldose isomerization based on the higher affinity of PBAs towards ketose than aldose (Delidovich et al., 2018, Delidovich and Palkovits, 2016, Li et al., 2013a). Most PBAs have a pKa value varying from 8.0 to 9.0 (Pan et al., 2017, Vancoillie and Hoogenboom, 2016), and it can transform into sp3 hybridization and form the PBAs-sugar complexes at a high pH (Chen et al., 2019, Gunasekara and Zhao, 2017). Besides, the production of d-tagatose by chemical catalysis requires a high pH environment as well. Therefore, it is possible and applicable to utilize PBAs as extractants for d-galactose isomerization.
In this study, the extraction properties of PBAs on d-tagatose were first investigated in terms of extraction efficiency and selectivity. Then, the back-extraction process was conducted under acidic conditions along with a two-staged stripping strategy to purify and enrich the d-tagatose in the stripping solution. The extraction system was successfully applied to the isomerization reaction, which facilitated the conversion yield of d-tagatose and achieved synchronous purification. Meanwhile, pH changes were simultaneously monitored to analyze the dynamic changes in sugar composition during the isomerization. More importantly, the molar ratio of boron to sugar and the reaction temperature were optimized to further promote the equilibrium during the extraction-assisted isomerization process. Based on the dynamical detection of saccharide component changes during the isomerization, the potential mechanism was finally proposed to further understand the interrelationship of isomerization and extraction equilibrium.
2. Materials and methods
2.1. Chemicals
3-Acrylamidophenylboronic acid (AAPBA, purity 97 %), 2-(Hydroxymethyl) phenylboronic acid cyclic monoester (HMPBA, purity 97 %), and Aliquat 336 were purchased from Energy Chemical (Shanghai, China). 4-Vinylphenylboronic acid (VPBA, purity 97 %) and capryl alcohol were purchased from Innochem (Beijing, China). HPLC grade d-tagatose, d-galactose, and d-sorbose were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Other analytical grade chemicals and reagents were provided by Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).
2.2. Extraction of d-tagatose from the aqueous phase into the organic phase
Except specifically mentioned, all the extraction and back-extraction processes used the same volume of organic and aqueous phases. The phosphate buffer (PBS, 0.5 M, conditioned by NaOH to the target pH) was prepared as the aqueous phase to dissolve the sugars and activate the phenylboronic acid into sp3 hybridization. During the extraction and back-extraction process, the aqueous phase and organic phase were homogeneously mixed in an Erlenmeyer flask equipped with a screw cap along with magnetic stirring at 500 rpm. After that, the mixture was separated by centrifugation at 8000 rpm for 2 min. After dilution and desalination, the aqueous phase was then used for HPLC analysis.
The extraction properties of PBAs to sugars were systematically investigated following a previous study with some modifications (Wang et al., 2020). Three different monomers (HMPBA, pKa 7.2; AAPBA pKa 8.2; and VPBA pKa 8.8) with discrepant hydrophobicity were selected to determine the most efficient monomer for the extraction and the molar ratio of VPBA (111 mM) to Aliquat 336 (1:0.5, 1:1, 1:2, 1:4) were tested to investigate the influence of Aliquat 336 in the extraction process. The effect of the initial pH of the aqueous phase was investigated in 5 g/L d-tagatose-d-galactose binary solution with different pH values (8.5–10.5) in terms of extraction efficiency to d-tagatose and d-galactose. Effects of the molar ratio of VPBA to d-tagatose (0.5–6), d-tagatose to d-galactose (5:95–5:1), and the initial concentration of d-tagatose (1 g/L-50 g/L) were systematically investigated in terms of extraction efficiency and selectivity to d-tagatose. Extraction kinetics curves of VPBA towards d-tagatose were drawn by taking samples at predetermined time intervals.
The sugar extraction efficiency (Ee-s, %) and d-tagatose extraction selectivity (Es-Tag, %) were calculated as follows:
| (1) |
| (2) |
where C0-s (g/L) represents the initial concentration of sugars in the aqueous phase, and Ce-s (g/L) represents the equilibrium concentration of sugars in the aqueous phase.
2.3. Back-extraction of d-tagatose into aqueous phase under acidic conditions
After extraction, the d-tagatose-laden organic phase was mixed with HCl solution to investigate the back-extraction performance. Typically, 20 mL organic phase was mixed with HCl solution to desorb the sugar at room temperature with magnetic stirring at 500 rpm for 30 min. Then, the mixture was centrifuged at 8000 rpm for 2 min to separate the aqueous phase and organic phase. The aqueous phase was later used for the HPLC analysis.
The stripping efficiency (Se-s, %) and the purity of stripping aqueous (PuTag, %) were calculated as follows:
| (3) |
| (4) |
where Cs-s (g/L) represents the sugar concentration in the stripping aqueous phase, N devotes the volume ratio of the organic phase to the aqueous phase, and Cs-Tag (g/L) and Cs-Gal (g/L) represent the concentration of d-tagatose and d-galactose in the stripping aqueous phase, respectively.
2.4. Two-staged stripping strategy and operational stability of phenylboronic acid extraction system
Previous study has proved the feasibility to further increase the purity of the target molecule by performing a staged stripping process (Li et al., 2012). Herein, we conducted the two-staged stripping strategy with some adjustments to purify and enrich d-tagatose simultaneously. For the first step, the organic phase was mixed with a lower concentration HCl solution to rinse the most d-galactose. During the second step, the organic phase separated by centrifugation was mixed with a higher concentration HCl solution to rinse the remanent monosaccharide, which could improve the purity of d-tagatose in the stripping solution.
The operational stability of the VPBA extraction system was investigated by conducting the extraction and back-extraction process for six consecutive cycles using d-tagatose (5 g/L) as the substrate at 1:1 and 5:1 ratio of VO: VS (volume ratio of the organic phase to the stripping solution), respectively.
2.5. Catalytic isomerization of d-galactose and one-pot extraction-assisted isomerization
The substrate solution containing 25 g/L d-tagatose for isomerization was prepared by dissolving d-galactose in PBS buffer (pH 12, 0.5 M). The catalytic reaction was performed at different temperatures (50 °C, 60 °C, 70 °C) heated by an enzyme reactor. Sugar distribution in the reaction solution was investigated by drawing the sample at consecutive time intervals for HPLC analysis. The yield of d-tagatose (YTag, %), d-sorbose (YSorb, %), and byproduct (YByproduct, %) during the chemical-catalyzed isomerization process were calculated as the following equations:
| (5) |
| (6) |
| (7) |
where the Ct-Tag and Ct-Sorb represent the concentration of d-tagatose and d-sorbose in the isomerization solution. C0-Gal demonstrates the initial concentration of d-tagatose. CR-Gal represents the concentration of remnant d-tagatose in the isomerization solution.
The extraction-assisted isomerization system was formulated as previously study illustrated with some adjustments (Wang et al., 2020). Briefly, the organic phase (containing 69 ∼ 276 mM VPBA and 69 ∼ 276 mM Aliquat 336) was mixed with the d-galactose solution (25 g/L). The isomerization temperature and molar ratio of boron to sugar (boron: sugar) were investigated to optimize the isomerization conditions. At the same time, pH changes were monitored to further explain the dynamic changes of each component during the isomerization reaction. The yield of d-tagatose (YTag, %) and byproduct (YByproduct, %) during the extraction-assisted isomerization process were calculated as the following equations:
| (8) |
| (9) |
where the Ci-Tag and CR-Gal represent the concentration of d-tagatose and remnant d-galactose during the isomerization process, which consist of the sugars in both the aqueous phase after extraction and the stripping solution.
2.6. Determination and analysis method
All the HPLC analyses were performed on a Waters Alliance e2695 HPLC system (Waters Co., USA) equipped with an RI detector (Waters 2414; Waters Co., USA). An Asahipak chromatographic column NH2P-50 4E (4.6 mm × 250 mm; Showa Denko K.K, Japan) was chosen for the separation of sugars. The operation temperature was 35 °C and the flow rate was 1 mL/min using 75 % acetonitrile-25 % water (v/v) as the mobile phase (Wang et al., 2022).
3. Results and discussion
3.1. Extraction of d-tagatose into organic phase
The structure-determined hydrophobicity of PBAs plays an important role in the extraction of cis-diol-containing compounds (Wang et al., 2020). To investigate the effect of the hydrophobicity of PBAs on d-tagatose extraction, HMPBA, AAPBA, and VPBA were chosen as target monomers. As is shown in Fig. S1a, VPBA with the strongest hydrophobicity showed the highest extraction efficiency (∼90.30 % for d-tagatose and ∼ 68.52 % for d-galactose) compared with HMPBA (∼72.75 % for d-tagatose and ∼ 68.06 % for d-galactose) and AAPBA (∼77.96 % for d-tagatose and ∼ 54.39 % for d-galactose). The extraction efficiency of PBAs in the d-tagatose-d-galactose binary solution is shown in Fig. S1b. All the applied PBAs showed a higher Ee-Tag than Ee-Gal. Among them, VPBA showed the highest extraction efficiency for d-tagatose (∼75 %) and the lowest efficiency for d-galactose (∼29 %), which indicated that VPBA has the best selectivity in the mixed solution. Therefore, VPBA was selected for further study due to the best absorption selectivity.
Fig. 1.
Absorption characterization of VPBA extraction system. (a) Effect of pH values (8.5–10.5) of the aqueous phase (5 g/L d-tagatose and 5 g/L d-galactose) on extraction efficiency. (b) Effect of the molar ratio of VPBA to d-tagatose (0.5–6) on extraction efficiency and d-tagatose selectivity. (c) Effect of the molar ratio of d-tagatose to d-galactose (5:95–5:1) on extraction efficiency and d-tagatose selectivity. (d) Effect of the initial d-tagatose concentration (1 g/L-50 g/L) on extraction efficiency and d-tagatose selectivity. (e) Extraction kinetics of VPBA extraction system toward d-tagatose at pH 9.5, and pH 10.5, respectively.
Aliquat 336 could improve the solubility of PBAs and provide power to transfer the PBAs-cis-diols complex from the interfacial layer to the organic phase (Bérubé et al., 2008, Dowlut and Hall, 2006). As is shown in Fig. S1c, Aliquat 336 played a positive effect on the extraction of target sugar. With the content increase of Aliquat 336, Ee-Tag exhibited a significant increase. At a fixed Aliquat 336 concentration, VPBA showed higher extraction efficiency towards d-tagatose and d-galactose than HMPBA and AAPBA. This is mainly caused by the strongest hydrophobicity of VPBA, which enables it to realize phase transition with less assistance with Aliquat 336. Notably, the improvement in extraction efficiency caused by strong hydrophobicity was much lower than that of lactulose (Wang et al., 2020). This phenomenon could be attributed to the less “driving force” for d-tagatose (monosaccharide) to be transferred into the organic phase than lactulose (disaccharide).
The effect of the pH of the aqueous phase on the sugar extraction efficiency phase is shown in Fig. 1a. Ee-Tag significantly increased from 39.92 % (pH 8.5) to 80.05 % (pH 10.5), while Ee-Gal only increased from 6.28 % (pH 8.5) to 31.06 % (pH 10.5). Although the binding of d-galactose with VPBA showed the same pH-dependent behaviors, its extraction efficiency was much lower than d-tagatose due to the lower affinity to VPBA. Fig. 1b shows the effect of the molar ratio of VPBA to d-tagatose on Ee-Tag and Es-Tag, as can be seen, with VPBA concentration increasing, Ee-Tag significantly increased and stabilized at ∼ 55 % (pH 9.5) and ∼ 75 % (pH10.5). However, Es-Tag gradually decreased with the increase of the concentration of VPBA, which was ascribed to the more d-galactose absorption caused by the increase of absorption sites. In detail, the increase of VPBA concentration could provide more absorption sites, as d-tagatose was continuously absorbed, the concentration gradient of d-galactose between the two phases was much higher than d-tagatose, which caused the adverse absorption of d-galactose.
Fig. 1c shows the effect of the molar ratio of d-tagatose to d-galactose on Ee-Tag and Es-Tag. With the increase of d-tagatose concentration in the initial aqueous phase, Ee-Tag increased to ∼ 80 % (pH 10.5) and ∼ 65 % (pH 9.5), along with the Es-Tag increasing from 31.23 % to 88.26 % (pH 9.5), and 36.21 % to 94.79 % (pH 10.5).
As is shown in Fig. 1d, the extraction efficiency rapidly declined when the d-tagatose concentration increased in the initial aqueous phase, and the d-tagatose concentration in the organic phase gradually stabilized at ∼ 15 g/L (pH 10.5) and ∼ 7 g/L (pH 9.5). This phenomenon could be explained from two aspects: (1) the proportion of sp3 hybridization of VPBA is limited at determined pH, which greatly confined the number of absorption sites; (2) the cyclic ester structure formed by VPBA and d-tagatose has limited solubility in the organic phase, which has a side effect on d-tagatose absorption as well (Brennan et al., 2010, Li et al., 2012).
Fig. 1e demonstrates the extraction kinetics character at pH 9.5 and 10.5. The extraction efficiency rapidly reached equilibrium and stabilized at ∼ 55 % (pH 9.5) and ∼ 90 % (pH 10.5) in 2 min. The fast absorption rate of VPBA to d-tagatose can be ascribed to the large contact area caused by the sp3 hybridization structure of phenylborate anions (tetrahedral forms),which is a great advantage for further applications (Hasegawa et al., 2015, Wang et al., 2020).
3.2. Back-extraction of d-tagatose from organic phase under acidic conditions
The target compounds captured by PBAs through reversible covalent interaction could be released under acidic conditions. As is shown in Fig. S2a, the absorbed d-tagatose could be completely dissociated in a 0.1 M HCl solution (VO: VS = 1:1). However, due to the low affinity to PBAs, the d-galactose could be completely desorbed using 0.04 M HCl solution (Fig. S2b). The different requirements of H+ for desorption make it applicable to separate d-tagatose and d-galactose using a two-staged stripping strategy.
Fig. 2.
(a)-(b) Two-staged stripping strategy to improve the purity of d-tagatose in the stripping solution. The initial aqueous phase contains 5 g/L d-tagatose and 5 g/L d-galactose. After extraction, the back-extraction was performed at (a) VO: VS = 1:1, and (b) VO: VS = 5:1. (c)-(d) Operational stability of VPBA extraction system in terms of d-tagatose extraction efficiency and stripping efficiency. The organic phase was continuously used for 6 cycles to investigate the stability at (c) VO: VS = 1:1, and (d) VO: VS = 5:1, respectively.
Fig. S2c shows the back-extraction kinetics character of VPBA. Similar to the extraction process, d-tagatose was completely desorbed in 2 min. The fast extraction and back-extraction process enabled the VPBA extraction system applicable for selectively capturing and enriching d-tagatose during extraction-assisted isomerization.
3.3. Two-staged stripping strategy and operational stability
The different requirement of H+ to completely desorb d-galactose and d-tagatose was mainly caused by the discrepant combination affinity between phenylborate anions and sugar molecules. Compared with d-galactose, the stronger combination of VPBA and d-tagatose is more resistant to H+, leading to the dissociation at a lower pH. Therefore, the sequential desorption of the weakly bound d-galactose-VPBA complex followed by the strongly bound d-tagatose-VPBA complex with increasing HCl concentration in the stripping solution is an effective method to further improve the purity of d-tagatose. Fig. 2a-b illustrates the two-staged stripping strategy to further improve the purity of d-tagatose. The initial aqueous phase containing 5 g/L d-tagatose and 5 g/L d-galactose was used for extraction and back-extraction. After extraction, PuTag was greatly improved from ∼ 50 % to ∼ 67 %. An HCl solution (0.02 M) was used for the first step back-extraction at VO: VS = 1:1 (Fig. 2a and S3a). In the first step, most of the d-galactose (∼73 %) and only a small part of d-tagatose (∼15 %) were rinsed. In the second step, an HCl solution in a higher concentration (0.08 M) was used for desorbing the residual sugars, and PuTag was significantly improved to 85.74 %. Besides, the same trend of the two-staged stripping strategy is also observed at VO: VS = 5:1, which improved PuTag to 83.16 % with the d-tagatose concentration increased to ∼ 18 g/L (Fig. 2b and S3b). The two-staged stripping strategy achieved efficient purification and enrichment of d-tagatose simultaneously, proving the feasibility of being applied to extraction-assisted isomerization.
Operational stability is an important factor in industrial practice. Fig. 2c-d shows the excellent operational stability of the VPBA extraction system at VO: VS = 1:1 and VO: VS = 5:1, respectively. After six consecutive cycles, no significant decrease in extraction and stripping efficiency was observed, which maintained ∼ 95 % extraction efficiency and ∼ 100 % stripping efficiency during the six cycles. The economical and sustainable separation method exhibits great application prospects for d-tagatose production.
3.4. Optimization of extraction-assisted isomerization and dynamic analysis of monosaccharide composition during the isomerization
3.4.1. Traditional chemical-based isomerization of d-galactose to d-tagatose
d-galactose can be catalyzed into d-tagatose in the presence of bases. However, d-tagatose can also be catalyzed into d-sorbose, which hampers obtaining high-purity tagatose (Drabo & Delidovich, 2018). The d-galactose-d-tagatose and d-tagatose-d-sorbose equilibrium is significantly influenced by the isomerization conditions. Thus, the effect of pH, temperature, and isomerization time on YTag was investigated. As is shown in Table S1, with the increase of pH and temperature, the reaction took less time to reach isomerization equilibrium. However, d-tagatose in increasing concentrations was catalyzed into d-sorbose and other colored products, which confined the conversion yield. YTag was confined to ∼ 25 %, while YByproduct could even reach over 50 %. To further increase the conversion yield of d-tagatose, it is of significant importance to remove the d-tagatose in the reaction mixture to eliminate the product inhibition effect. As discussed above, the VPBA extraction system had excellent affinity and absorption capacity to d-tagatose. Therefore, it is applicable to transfer the d-tagatose into the organic phase to promote the isomerization forward assisted by the VPBA extraction system.
3.4.2. Extraction-assisted isomerization of d-galactose to d-tagatose
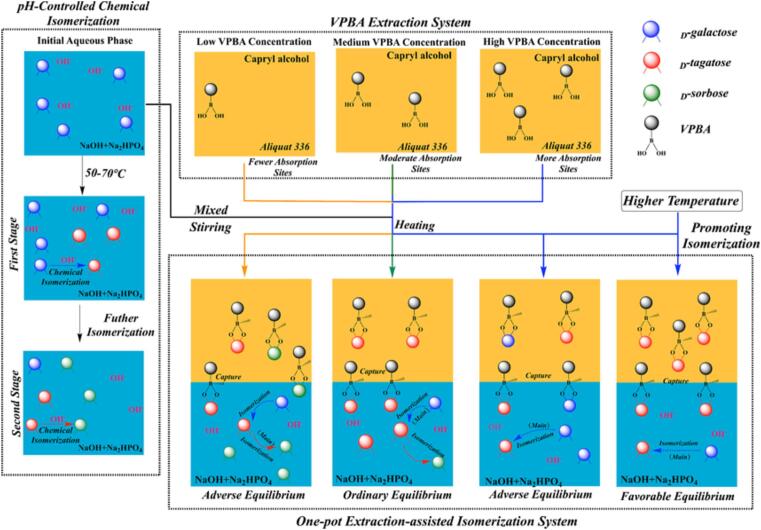
Fig. 3 shows the proposed mechanism of the one-pot extraction-assisted isomerization strategy. The aqueous phase containing 25 g/L d-galactose was mixed with the organic phase and the pH of the aqueous phase was adjusted to 12. Then, the substrate solution was mixed with the VPBA-laden organic phase and adjusted to the target reaction temperature to initiate isomerization. At the beginning of the isomerization, d-galactose which took up the largest proportion of the aqueous phase was firstly absorbed into the organic phase, while the remnant d-galactose in the aqueous phase was catalyzed into d-tagatose continually. Then, due to the higher affinity, the d-tagatose would reversibly substitute the d-galactose, which guarantees a low concentration of d-tagatose in the aqueous phase throughout to avoid unnecessary side effects. When the whole reaction system reached isomerization equilibrium, the reaction was stopped by cooling to room temperature and separated by centrifugation. After isomerization, the d-tagatose-laden organic phase was desorbed using the two-staged stripping strategy (VO: VS = 5:1) to further improve the concentration and purity of d-tagatose. The organic was recycled for the next extraction-assisted isomerization.
Fig. 3.
Mechanism of one-pot extraction-assisted isomerization.
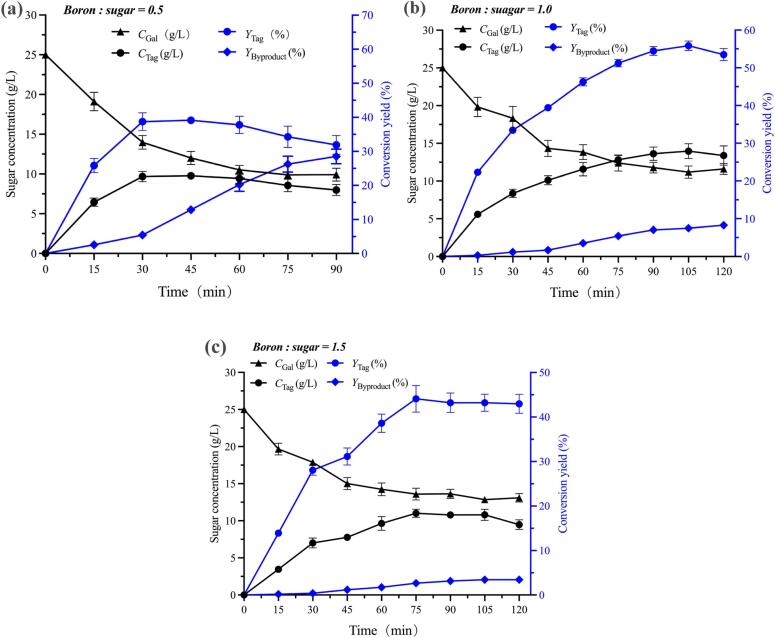
As mentioned above, the main mechanism of extraction-assisted isomerization to improve d-tagatose conversion yield is to eliminate the product inhibition effect by transferring d-tagatose into the organic phase. Therefore, the absorption capacity which relies on the concentration of VPBA in the organic phase performs a decisive role in the isomerization (Wang et al., 2023, Wang et al., 2022). Fig. 4 demonstrates the effect of the molar ratio of boron to sugar on YTag. As is shown in Fig. 4a (boron: sugar = 0.5), YTag reached the maximum (39.09 %) at 30 mins, while the byproduct conversion yield was 12.82 %. However, as the reaction proceeded, YByproduct rapidly increased to 28.47 %, while YTag slowly decreased to 31.89 % at 90 mins. According to the HPLC profile (Fig. S5a), the organic phase was laden with d-tagatose and d-sorbose, and almost no d-galactose was detected, which demonstrated that d-tagatose had reached the maximum capacity. With concentration increasing, d-tagatose which was not absorbed into the organic phase was constantly catalyzed into d-sorbose and other byproducts, leading to a decrease in conversion yield. Therefore, a higher molar ratio of VPBA to sugar (1:1) was utilized to further enhance YTag. As is shown in Fig. 4b, the reaction system reached the equilibrium stage at 105 mins when YTag reached 55.89 % and YByproduct was only 7.50 %. Besides, benefiting from the two-stage stripping strategy, a high concentration (32.42 g/L) and high purity (85.23 %) d-tagatose solution was obtained, which avoided the complicated downstream purification process. Nevertheless, an over-high concentration of VPBA also has adverse effects (Wang et al., 2022). Theoretically, more absorption sites in the organic phase could enhance the absorption capacity toward d-tagatose, which was favorable for isomerization. However, as is shown in Fig. 4c, YTag and YByproduct respectively decreased to 44.07 % and 2.65 % at 75 mins during the isomerization. According to the HPLC profile (Fig. S5c), more d-galactose appeared in the stripping solution, which simultaneously affected the purity of d-tagatose. This phenomenon can be explained in terms of reaction equilibrium. Associated with Fig. 3, it can be inferred that the whole isomerization system has three reaction equilibriums: (i) d-galactose-to-d-tagatose isomerization equilibrium, (ii) d-tagatose-to-d-sorbose isomerization equilibrium, and (iii) competitive adsorption equilibrium between d-galactose and d-tagatose. At the beginning of isomerization, a large part of d-galactose in the initial aqueous phase is transferred into the organic phase, leading to the low concentration of the isomerization substrate, which has an adverse effect on the production of d-tagatose. Besides, insufficient production of d-tagatose was unable to efficiently substitute the d-galactose in the organic phase, leading to the inadequate substrate for the isomerization. This unfavorable isomerization-absorption equilibrium eventually confined the yield of d-tagatose. As is shown in Fig. 4c and Fig. S5c, when the reaction time exceeded 75 mins, the stable YTag and the amount of d-galactose absorbed in the organic phase indicated that the whole isomerization system had reached equilibrium.
Fig. 4.
Effect of the molar ratio of boron to sugar (a) 0.5:1, (b) 1:1, and (c) 1.5:1 in terms of sugar concentration and conversion yield during the extraction-assisted isomerization process. The initial aqueous phase contains 25 g/L d-galactose as the reaction substrate at pH 12. The sugar concentration, conversion yield of d-tagatose, and byproducts were dynamically monitored by drawing the aqueous phase and the stripping solution for HPLC analysis at consecutive time intervals.
Fig. 5.
Effect of temperature (a) 50 °C, (b) 60 °C, and (c) 70 °C on sugar concentration and conversion yield. Extraction-assisted isomerization was performed at pH12, boron: sugar = 1.5 (Initial aqueous phase containing 25 g/L d-galactose).
As is shown in Fig. 4b, despite the improvement of YTag to ∼ 55 %, nearly half of d-galactose was not consumed, which was caused by the thermodynamic constraints. Therefore, the effect of isomerization temperature on YTag was further investigated aiming to promote the isomerization. The HPLC profile (Fig. S5b) exhibits that scarcely any d-galactose was absorbed into the organic phase, which indicated that d-tagatose had occupied most absorption sites. Consequently, a higher VPBA concentration (boron: sugar = 1.5) was selected for further study. The results of the extraction-assisted isomerization performed at 50 °C, 60 °C, and 70 °C are shown in Fig. 5 in terms of YTag and YByproduct. YTag stabilized at 30.36 % at 50 °C (Fig. 5a), 43.18 % at 60 °C (Fig. 5b), and 61.58 % at 70 °C (Fig. 5c). Besides, combined with the HPLC profile (Fig. S5c, S5d, and S5e), with the increase of the isomerization temperature, less d-galactose was discovered in the stripping solution, which manifested that the isomerization was constantly improving. Therefore, it is evident that a higher temperature is favorable for the improvement in YTag. However, it is not said that a higher temperature is a better reaction condition. The over-high temperature could simultaneously facilitate the production of byproducts, leading to the acceleration of the yield of d-sorbose (1.86 % at 50 °C, 3.44 % at 60 °C, and 11.68 % at 70 °C), which is hard to separate using PBAs due to the similarity in structure (Wang et al., 2022).
3.4.3. Proposed mechanism
As discussed above, the conversion yield and purity of d-tagatose were closely connected with the reaction and absorption equilibrium. To further explain the underlying mechanism, the interrelationship of the reaction and adsorption equilibrium was systematically described in Fig. 6. The d-galactose-d-tagatose and d-tagatose-d-sorbose isomerization occur in the aqueous phase under alkali conditions. d-Tagatose isomerized from d-galactose will participate in the isomerization to d-sorbose as substrate. Benefiting from the selective extraction by VPBA, d-tagatose can be maintained at a low concentration, which can promote d-galactose-d-tagatose isomerization by reducing the production inhibition effect. Meanwhile, low-concentration d-tagatose efficiently reduces the generation of d-sorbose, which simultaneously improves the purity. As the main extractant, the concentration of VPBA in the organic phase determines the number of accessible combination sites, which directly affects the maximum absorption capacity toward target molecules. At low VPBA concentration, due to inadequate absorption, remnant d-tagatose remaining in high concentration in the aqueous phase rapidly isomerize to d-sorbose. At the same time, d-sorbose also can be captured by VPBA and then decrease the purity of d-tagatose after stripping. At a medium VPBA concentration, more absorption sites efficiently decrease the concentration of residual d-tagatose in the aqueous phase and minimize the production of d-sorbose, simultaneously promoting the isomerization. However, at a high VPBA concentration, a large part of d-galactose is extracted to the organic phase at the beginning of isomerization, leading to its low concentration in the aqueous phase, which has an adverse effect on the production of d-tagatose and simultaneously affects the purity of d-tagatose in the stripping solution. After enhancing the reaction temperature, residual d-galactose in low concentration can further isomerize to d-tagatose and substitute d-galactose in the organic phase. The isomerization and extraction equilibrium are further promoted. Besides, due to the high concentration of VPBA, d-tagatose can maintain in low concentration in the reaction system, which improves the purity of d-tagatose after stripping. Remarkably, based on the proposed isomerization-absorption equilibrium mechanism, the conversion yield of d-tagatose was further enhanced to 61.58 % at a 1.5:1 molar ratio of boron to sugar, which is higher than our former study (52.92 % at a 1:1 molar ratio of boron to sugar) (Wang et al., 2022).
Fig. 6.
Mechanism of the interrelationship between isomerization and absorption equilibrium.
4. Conclusions
In this study, an efficient and facile one-pot extraction-assisted isomerization strategy was applied to promote the production of d-tagatose by chemical catalysis based on the different absorption properties of VPBA towards d-tagatose and d-galactose. Typically, the conversion yield of tagatose is confined to ∼ 22 % using chemical catalysts. However, by adopting the one-pot extraction-assisted isomerization strategy, the yield of d-tagatose can be significantly improved to 61.58 %, which is nearly three-fold to the chemical method, and similar to the efficiency of enzymatic catalysis but more convenient and stable than that, and the yield of byproducts was minimized to only 10.60 %, which overcomes the biggest drawback of chemical methods. Besides, a high concentration (40.49 g/L) and high purity (83.27 %) d-tagatose solution was simultaneously obtained through the two-staged stripping strategy, which greatly reduces the cost of the downstream purification and concentration. A potential mechanism involving the effect of temperature and molar ratio of boron to sugar on isomerization-absorption equilibrium was proposed to account for the improvement in the yield of d-tagatose. Overall, the proposed extraction-assisted isomerization strategy using VPBA as absorbents shows excellent efficiency, stability, and sustainability, which has great potential for industrial production.
CRediT authorship contribution statement
Guangzhen Wang: Data curation, Investigation, Methodology. Xiaomei Lyu: Data curation, Methodology. Lu Wang: Investigation, Writing – review & editing. Mingming Wang: Funding acquisition, Supervision, Writing – review & editing. Ruijin Yang: Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors gratefully acknowledge the financial support provided by The National Natural Science Foundation of China (32072150, and 32201932), the National Key Research and Development Program of China (2019YFC160580401), the priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the National First-Class Discipline Program of Food Science and Technology (JUFSTR20180202), and the China Postdoctoral Science Foundation (2022M712999).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100928.
Contributor Information
Mingming Wang, Email: wmm@ouc.edu.cn.
Ruijin Yang, Email: yrj@jiangnan.edu.cn.
Appendices
Optimization of the phenylboronic acid extraction system; desorption properties of VPBA extraction system; HPLC-profiles of two-staged stripping solution; results of the base-catalyzed isomerization; HPLC-profiles of the base-catalyzed isomerization; HPLC-profiles of the stripping solution during extraction-assisted isomerization; pH dynamic changes during extraction-assisted isomerization; brownish changes in the aqueous phase during the isomerization; extraction-assisted isomerization cooperated with adjusting pH.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Baptista S.L., Romaní A., Oliveira C., Ferreira S., Rocha C.M.R., Domingues L. Galactose to tagatose isomerization by the l-arabinose isomerase from Bacillus subtilis: A biorefinery approach for Gelidium sesquipedale valorisation. LWT. 2021;151 doi: 10.1016/j.lwt.2021.112199. [DOI] [Google Scholar]
- Beerens K., Desmet T., Soetaert W. Enzymes for the biocatalytic production of rare sugars. Journal of Industrial Microbiology & Biotechnology. 2012;39(6):823–834. doi: 10.1007/s10295-012-1089-x. [DOI] [PubMed] [Google Scholar]
- Bérubé M., Dowlut M., Hall D.G. Benzoboroxoles as Efficient Glycopyranoside-Binding Agents in Physiological Conditions: Structure and Selectivity of Complex Formation. The Journal of Organic Chemistry. 2008;73(17):6471–6479. doi: 10.1021/jo800788s. [DOI] [PubMed] [Google Scholar]
- Brennan T.C.R., Datta S., Blanch H.W., Simmons B.A., Holmes B.M. Recovery of Sugars from Ionic Liquid Biomass Liquor by Solvent Extraction. BioEnergy Research. 2010;3(2):123–133. doi: 10.1007/s12155-010-9091-5. [DOI] [Google Scholar]
- Chen Y., Huang A., Zhang Y., Bie Z. Recent advances of boronate affinity materials in sample preparation. Analytica Chimica Acta. 2019;1076:1–17. doi: 10.1016/j.aca.2019.04.050. [DOI] [PubMed] [Google Scholar]
- Delidovich I., Gyngazova M.S., Sánchez-Bastardo N., Wohland J.P., Hoppe C., Drabo P. Production of keto-pentosesviaisomerization of aldo-pentoses catalyzed by phosphates and recovery of products by anionic extraction. Green Chemistry. 2018;20(3):724–734. doi: 10.1039/c7gc03077k. [DOI] [Google Scholar]
- Delidovich I., Palkovits R. Fructose production via extraction-assisted isomerization of glucose catalyzed by phosphates. Green Chemistry. 2016;18(21):5822–5830. doi: 10.1039/c6gc01712f. [DOI] [Google Scholar]
- Dowlut M., Hall D.G. An Improved Class of Sugar-Binding Boronic Acids, Soluble and Capable of Complexing Glycosides in Neutral Water. Journal of the American Chemical Society. 2006;128(13):4226–4227. doi: 10.1021/ja057798c. [DOI] [PubMed] [Google Scholar]
- Drabo P., Delidovich I. Catalytic isomerization of galactose into tagatose in the presence of bases and Lewis acids. Catalysis Communications. 2018;107:24–28. doi: 10.1016/j.catcom.2018.01.011. [DOI] [Google Scholar]
- Gunasekara R.W., Zhao Y. A General Method for Selective Recognition of Monosaccharides and Oligosaccharides in Water. Journal of the American Chemical Society. 2017;139(2):829–835. doi: 10.1021/jacs.6b10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa U., Nishida T., van der Vlies A.J. Dual Stimuli-Responsive Phenylboronic Acid-Containing Framboidal Nanoparticles by One-Step Aqueous Dispersion Polymerization. Macromolecules. 2015;48(13):4388–4393. doi: 10.1021/acs.macromol.5b00574. [DOI] [Google Scholar]
- Kim P. Current studies on biological tagatose production using L-arabinose isomerase: A review and future perspective. Applied Microbiology and Biotechnology. 2004;65(3):243–249. doi: 10.1007/s00253-004-1665-8. [DOI] [PubMed] [Google Scholar]
- Li B., Relue P., Varanasi S. Simultaneous isomerization and reactive extraction of biomass sugars for high yield production of ketose sugars. Green Chemistry. 2012;14(9) doi: 10.1039/c2gc35533g. [DOI] [Google Scholar]
- Li B., Varanasi S., Relue P. High yield aldose–ketose transformation for isolation and facile conversion of biomass sugar to furan. Green Chemistry. 2013;15(8) doi: 10.1039/c3gc40795k. [DOI] [Google Scholar]
- Li D., Chen Y., Liu Z. Boronate affinity materials for separation and molecular recognition: Structure, properties and applications. Chemical Society Reviews. 2015;44(22):8097–8123. doi: 10.1039/c5cs00013k. [DOI] [PubMed] [Google Scholar]
- Li D., Li Y., Li X., Bie Z., Pan X., Zhang Q., Liu Z. A high boronate avidity monolithic capillary for the selective enrichment of trace glycoproteins. Journal of Chromatography. A. 2015;1384:88–96. doi: 10.1016/j.chroma.2015.01.050. [DOI] [PubMed] [Google Scholar]
- Li H., Liu Y., Liu J., Liu Z. A Wulff-type boronate for boronate affinity capture of cis-diol compounds at medium acidic pH condition. Chemical Communications (London) 2011;47(28):8169–8171. doi: 10.1039/c1cc11096a. [DOI] [PubMed] [Google Scholar]
- Li H., Shan Y., Qiao L., Dou A., Shi X., Xu G. Facile synthesis of boronate-decorated polyethyleneimine-grafted hybrid magnetic nanoparticles for the highly selective enrichment of modified nucleosides and ribosylated metabolites. Analytical Chemistry. 2013;85(23):11585–11592. doi: 10.1021/ac402979w. [DOI] [PubMed] [Google Scholar]
- Li H., Zhang X., Zhang L., Wang X., Kong F., Fan D.…Wang W. Preparation of a boronate affinity silica stationary phase with enhanced binding properties towards cis-diol compounds. Journal of Chromatography. A. 2016;1473:90–98. doi: 10.1016/j.chroma.2016.10.050. [DOI] [PubMed] [Google Scholar]
- Liu W., Jiang C., Zhang Y., Zhu L., Jiang L., Huang H. Self-assembling protein scaffold-mediated enzymes' immobilization enhances in vitro d -tagatose production from lactose. Food Bioengineering. 2022;1(1):47–57. doi: 10.1002/fbe2.12001. [DOI] [Google Scholar]
- Lu Y., Levin G.V., Donner T.W. Tagatose, a new antidiabetic and obesity control drug [https://doi.org/10.1111/j.1463-1326.2007.00799.x] Diabetes, Obesity and Metabolism. 2008;10(2):109–134. doi: 10.1111/j.1463-1326.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- Pan J., Liu J., Ma Y., Huang X., Niu X., Zhang T.…Qiu F. Wulff-type boronic acids suspended hierarchical porous polymeric monolith for the specific capture of cis -diol-containing flavone under neutral condition. Chemical Engineering Journal. 2017;317:317–330. doi: 10.1016/j.cej.2017.02.078. [DOI] [Google Scholar]
- Patel M.J., Patel A.T., Akhani R., Dedania S., Patel D.H. Bioproduction of D-Tagatose from D-Galactose Using Phosphoglucose Isomerase from Pseudomonas aeruginosa PAO1. Applied Biochemistry and Biotechnology. 2016;179(5):715–727. doi: 10.1007/s12010-016-2026-7. [DOI] [PubMed] [Google Scholar]
- Rai S.K., Kaur H., Singh A., Kamboj M., Jain G., Yadav S.K. Production of d-tagatose in packed bed reactor containing an immobilized l-arabinose isomerase on alginate support. Biocatalysis and Agricultural Biotechnology. 2021;38 doi: 10.1016/j.bcab.2021.102227. [DOI] [Google Scholar]
- Rhimi M., Bermudez-Humaran L.G., Huang Y., Boudebbouze S., Gaci N., Garnier A.…Maguin E. The secreted L-arabinose isomerase displays anti-hyperglycemic effects in mice. Microbial Cell Factories. 2015;14:204. doi: 10.1186/s12934-015-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Chikkerur J., Roy S.C., Dhali A., Kolte A.P., Sridhar M., Samanta A.K. Tagatose as a Potential Nutraceutical: Production, Properties, Biological Roles, and Applications. Journal of Food Science. 2018;83(11):2699–2709. doi: 10.1111/1750-3841.14358. [DOI] [PubMed] [Google Scholar]
- Vancoillie G., Hoogenboom R. Synthesis and polymerization of boronic acid containing monomers. Polymer Chemistry. 2016;7(35):5484–5495. doi: 10.1039/c6py00775a. [DOI] [Google Scholar]
- Wang H., Bie Z., Lü C., Liu Z. Magnetic nanoparticles with dendrimer-assisted boronate avidity for the selective enrichment of trace glycoproteins. Chemical Science. 2013;4(11) doi: 10.1039/c3sc51623g. [DOI] [Google Scholar]
- Wang M., Wang L., Hua X., Yang R. Production of high-purity lactulose via an integrated one-pot boronate affinity adsorbent based adsorption-assisted isomerization and simultaneous purification. Food Chemistry. 2023;429 doi: 10.1016/j.foodchem.2023.136935. [DOI] [PubMed] [Google Scholar]
- Wang M., Ye F., Wang H., Yang R., Hua X. Highly Efficient Production and Simultaneous Purification of Lactulose via Isomerization of Lactose through an Innovative Sustainable Anion-Extraction Process. ACS Sustainable Chemistry & Engineering. 2020;8(8):3465–3476. doi: 10.1021/acssuschemeng.9b07779. [DOI] [Google Scholar]
- Wang Z., Wang M., Lyu X., Wang C., Tong Y., Hua X., Yang R. Recycling preparation of high-purity tagatose from galactose using one-pot boronate affinity adsorbent-based adsorption-Assisted isomerization and simultaneous purification. Chemical Engineering Journal. 2022;446 doi: 10.1016/j.cej.2022.137089. [DOI] [Google Scholar]
- Xu W., Zhang W., Zhang T., Jiang B., Mu W. l -arabinose isomerases: Characteristics, modification, and application. Trends in Food Science & Technology. 2018;78:25–33. doi: 10.1016/j.tifs.2018.05.016. [DOI] [Google Scholar]
- Yuan J., Ravikumar Y., Zhang G., Yun J., Zhang Y., Zabed H.M., Qi X. L-arabinose isomerase from Lactobacillus parabuchneri and its whole cell biocatalytic application in D-tagatose biosynthesis from D-galactose. Food Bioscience. 2021;41 doi: 10.1016/j.fbio.2021.101034. [DOI] [Google Scholar]
- Zhang G., Zabed H.M., Yun J., Yuan J., Zhang Y., Wang Y., Qi X. Two-stage biosynthesis of D-tagatose from milk whey powder by an engineered Escherichia coli strain expressing L-arabinose isomerase from Lactobacillus plantarum. Bioresource Technology. 2020;305 doi: 10.1016/j.biortech.2020.123010. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhang T., Jiang B., Mu W. Enzymatic approaches to rare sugar production. Biotechnology Advances. 2017;35(2):267–274. doi: 10.1016/j.biotechadv.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang M., Hua X., Yao C., Yang R. An innovative and sustainable adsorption-assisted isomerization strategy for the production and simultaneous purification of high-purity lactulose from lactose isomerization. Chemical Engineering Journal. 2021;406 doi: 10.1016/j.cej.2020.126751. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.