Abstract
Prized for their ability to generate chemical complexity rapidly, catalytic carbon–hydrogen (C–H) activation and functionalization reactions have enabled a paradigm shift in the standard logic of synthetic chemistry. Directing group strategies have been used extensively in C–H activation reactions to control regio- and enantioselectivity with transition metal catalysts. However, current methods rely heavily on coordination with nitrogen and/or oxygen atoms in molecules and have therefore been found to exhibit limited generality in asymmetric syntheses. Here, we report enantioselective C–H activation with unsaturated hydrocarbons directed by phosphorus centres to rapidly construct libraries of axially chiral phosphines through dynamic kinetic resolution. High reactivity and enantioselectivity are derived from modular assembly of an iridium catalyst with an endogenous phosphorus atom and an exogenous chiral phosphorus ligand, as confirmed by detailed experimental and computational studies. This reaction mode significantly expands the pool of enantiomerically enriched functional phosphines, some of which have shown excellent efficiency for asymmetric catalysis.
Subject terms: Asymmetric catalysis, Diversity-oriented synthesis, Reaction mechanisms
Directing group strategies have been used extensively in C–H activation reactions but current methods rely heavily on coordination with nitrogen or oxygen atoms in molecules and have therefore been found to exhibit limited generality in asymmetric syntheses. Here, the authors report enantioselective C–H activation with unsaturated hydrocarbons directed by phosphorus centres to rapidly construct libraries of axially chiral phosphines through dynamic kinetic resolution.
Introduction
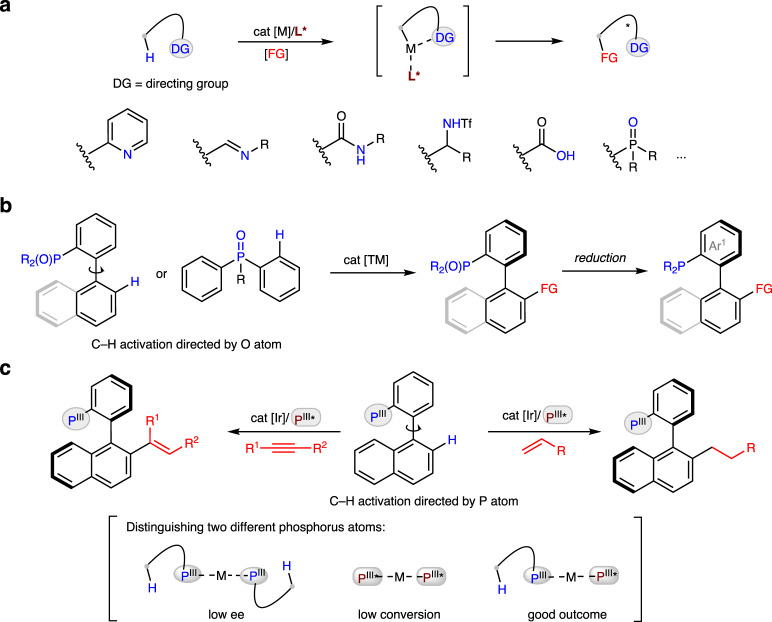
Generation of enantiopure molecules that operate efficiently with ideal atom and step economy is a long-standing challenge in organic synthesis1–3. Catalytic asymmetric C–H activation4–14 provides a reliable solution to this challenging task. Since a complex molecule typically contains multiple C–H bonds with comparable strengths and steric environments, the most successful method for this transformation is the use of a directing group15,16, either inherent or preinstalled in organic molecules, to position the metal catalyst at a particular C–H bond in a chiral environment (Fig. 1a)17–20. In this context, treatment of chiral ligands with metal catalysts has proven to be extremely effective when directed by aromatic nitrogen heterocycles21,22, carbonyl groups23–25, and amino derivatives26,27. Compared to oxygen and nitrogen atoms, phosphorus coordinates strongly with transition metals and is therefore challenging to use as a director in catalytic C−H activation28. Substantial progress has been made in ligand modification through phosphorus-directed C−H activation29–41. We have also demonstrated the viability of using phosphorus directing groups for the site-selective C−H functionalization of indoles at the benzene core42,43. Despite these advances, asymmetric C−H activation directed by a phosphorus center has not yet been overcome.
Fig. 1. Background and discovery.
a Pioneering examples of catalytic enantioselective C–H activation assisted by various directing groups. b State-of-the-art methods for catalytic asymmetric synthesis of chiral biaryl phosphines by O-directed C−H activation. c Enantioselective P(III)-directed C−H activation enabled by chiral phosphorus ligands.
Chiral biaryl phosphines are a class of promising ligands and organocatalysts and have become tremendously important in modern organic chemistry44–47. Preparations of these molecules typically require multistep syntheses, in which chiral auxiliaries and kinetic resolution are used most often in practice48,49. Palladium-catalyzed asymmetric C–C50–52 and C–P53,54 coupling of aryl (pseudo)halides have been disclosed for the synthesis of axially chiral phosphines. More recently, a series of catalytic asymmetric C–H activation strategies have also been developed to build the biaryl phosphines (Fig. 1b)55–59. However, the directing group in these methods is limited to O atom, and additional step for reduction of the formed phosphine oxides is needed. We reasoned that the development of a general strategy to enantioenriched phosphorus ligands through one-step syntheses would likely have a broad impact on asymmetric catalysis. Herein, we report that libraries of chiral biaryl phosphines can be generated by C–H activation with high regio-, stereo- and enantioselectivity (Fig. 1c). Chirality transfer occurs by combining a metal catalyst with a phosphorus directing group and a chiral phosphorus ligand. The main challenges for this process include strong background reactions with stoichiometric phosphines leading to racemization and formation of a thermodynamically stable metal-ligand complex resulting in low conversion. Therefore, the metal catalyst must distinguish between these two different phosphorus atoms and form a high equilibrium population of the required intermediates to achieve good outcome.
Results
Reaction design
We started our project by identifying a class of biaryl phosphines for asymmetric C–H activation with unsaturated hydrocarbons, which would enable access to atropisomers through dynamic kinetic resolution60,61. Phosphine 1a fulfils this criterion and exhibits a barrier of 22.0 kcal/mol for atropisomer interconversion and a sufficiently high rotational barrier for formation of olefination product 3aa (~45 kcal/mol) from alkyne 2a (Table 1). When the reaction was conducted with [Ir(cod)Cl]2 (5.0 mol%) as a catalyst and chiral diene L162 as a ligand in toluene at 70 °C under a N2 atmosphere, the desired product 3aa was formed in racemic with 33% yield (entry 1). The reaction was then investigated by using the iridium catalyst with a variety of chiral ligands to obtain the enantioselectivity. The use of Carreira ligand L2 led to product 3aa in 79% yield with 51% ee (entry 3). Changing the ligand to the chiral spiro phosphoramidite L3 provided superior results with alkyne 2a, delivering product 3aa in 82% yield and 97% ee (entry 3). Here the absolute stereochemistry of product 3aa was determined by X-ray crystallographic analysis. Loading the BINOL-derived phosphoramidite L3 bearing an NMe2 motif decreased the enantioselectivity to 66% (entry 4). However, treatment of a TADDOL-derived ligand L5 in the system became very sluggish, leading to product 3aa only in trace amounts (entry 5). Other solvents, such as THF and DCM gave much lower enantioselectivities and yields (entries 6-7). Conducting the reaction at 90 °C further improved the yield of 3aa to 88% yield but with a reduced enantioselectivity (entry 8). Low efficiency was observed by conducting the reaction at room temperature (entry 9). It is noteworthy that other iridium sources like [Ir(coe)2Cl]2 also maintained an acceptable enantioselectivity (entry 10), but other transition metals such as [Rh(cod)Cl]2 gave a remarkably reduced ee value (entry 11) and Pd(OAc)2 completely failed (entry 12). Decreasing the [Ir(cod)Cl]2 loading to 2.5 mol% led to a substantial decrease in the yield (entry 13), and a control experiment revealed that the reaction did not proceed without the metal catalyst (entry 14).
Table 1.
Optimization of the reaction conditions.a
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Cat [M] (mol%) | L* (mol%) | Solvent | T (°C) | Ee of 3aa (%)b | Yield of 3aa (%)c |
| 1 | [Ir(cod)Cl]2 (5) | L1 (11) | Toluene | 70 | 0 | 33 |
| 2 | [Ir(cod)Cl]2 (5) | L2 (11) | Toluene | 70 | 51 | 79 |
| 3 | [Ir(cod)Cl]2 (5) | L3 (11) | Toluene | 70 | 97 | 82 |
| 4 | [Ir(cod)Cl]2 (5) | L4 (11) | Toluene | 70 | −66 | 76 |
| 5 | [Ir(cod)Cl]2 (5) | L5 (11) | Toluene | 70 | – | <5 |
| 6 | [Ir(cod)Cl]2 (5) | L3 (11) | THF | 70 | 71 | 44 |
| 7 | [Ir(cod)Cl]2 (5) | L3 (11) | DCM | 70 | 83 | 23 |
| 8 | [Ir(cod)Cl]2 (5) | L3 (11) | Toluene | 90 | 93 | 88 |
| 9 | [Ir(cod)Cl]2 (5) | L3 (11) | Toluene | rt | 99 | 11 |
| 10 | [Ir(coe)2Cl]2 (5) | L3 (11) | Toluene | 70 | 77 | 77 |
| 11 | [Rh(cod)Cl]2 (5) | L3 (11) | Toluene | 70 | 12 | 70 |
| 12 | Pd(OAc)2 (5) | L3 (11) | Toluene | 70 | – | 0 |
| 13 | [Ir(cod)Cl]2 (2.5) | L3 (5.5) | Toluene | 70 | 98 | 15 |
| 14 | – | L3 (11) | Toluene | 70 | – | 0 |
THF tetrahydrofuran, DCM dichloromethane.
aReaction conditions: cat [M] (2.5–5 mol%), L* (5.5–11 mol%), 1a (0.2 mmol), 2a (1.0 mmol) in 2 mL of dry solvent at 70 °C for 72 h under argon.
bEnantiomeric excess (ee) was determined by chiral HPLC analysis.
cIsolated yield.
Scope of the methodology
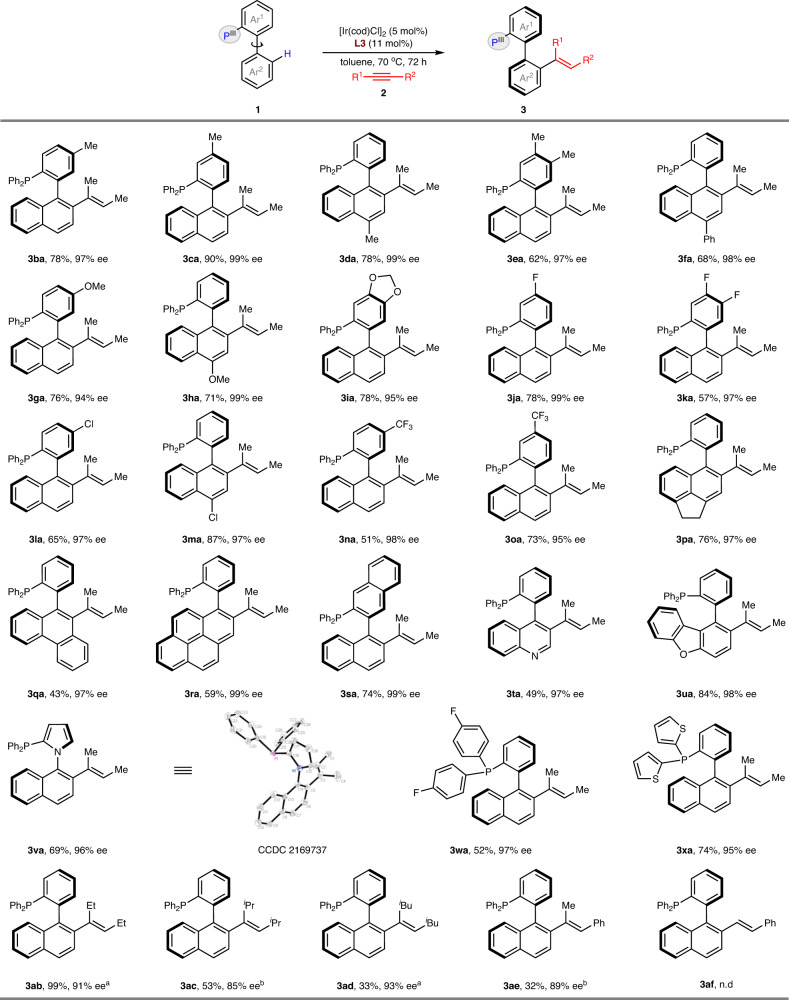
With the optimal reaction conditions in hand, we evaluated the scope of two components in this reaction (Fig. 2). The scope of phosphines 1 was first examined with alkyne 2a. Phosphines with electron-donating groups, including methyl (1b-e), phenyl (1f) and ether (1g-i) substituents at the P-phenyl ring or naphthalene ring, all delivered excellent enantioselectivities for both hydroarylation reactions. Electron-withdrawing substituents, such as F (1j-k), Cl (1l-m) and CF3 (1n-o), also worked well. Given the steric requirements of ligands, we further surveyed reactants with in creasingly bulky backbones. Phosphines bearing 1,2-dihydroacenaphthyl (1p), phenanthrenyl (1q), pyrenyl (1r) and a 1,2’-binaphthalenyl (1s) motif did not noticeably affect the reaction efficiency and enantioselectivity for either set of reaction conditions. We were pleased to find that heteroaryl-based phosphines, such as quinolone (1t) and dibenzo[b,d]furan (1u) were very well tolerated in enantioselective olefination. The reaction of alkyne 2a with N-aryl pyrrole-based phosphine 1v delivered the desired product 3va in 69% yield and 96% ee, showing the same absolute configuration as the biaryl substrates. In addition, variations in the aryl substituents (1w) at the phosphorus atom with different electronic properties, were well tolerated under reaction conditions. Furthermore, a thiophenl-containing ligand 1x also proved to be highly efficient. We then explored the scope for alkynes 2 with the developed conditions. Increasing the steric hindrance in the internal alkynes (2b-d) with ethyl to isobutyl groups maintained the high enantioselectivities but resulted in a gradual decrease in the reaction yield. Nonsymmetric alkynes, such as prop-1-yn-1-ylbenzene (2e), led to enantioenriched product 3ae in a highly regioselective manner, albeit with diminished yield. In addition, the employment of a terminal alkyne 2f as a substrate completely failed to undergo C–H activation in the current system.
Fig. 2. Atroposelective P(III)-directed C–H olefination of biaryl phosphines with alkynes.
Reaction conditions: [Ir(cod)Cl]2 (5 mol%), L3 (11 mol%), 1 (0.2 mmol), 2 (1.0 mmol) in 2 mL of dry toluene at 70 °C for 72 h under argon; ee was determined by chiral HPLC analysis; Isolated yield. aAt 75 °C. bAt 90 °C.
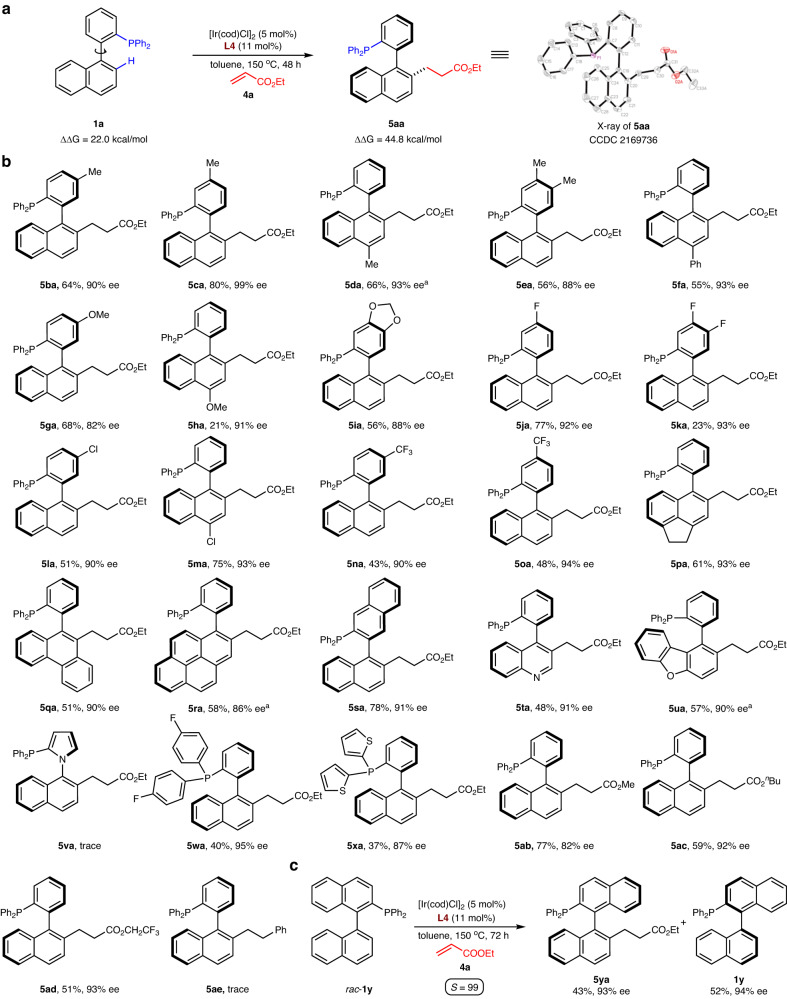
Hydroarylation of biaryl phosphines with olefins was then studied in the transformation (Fig. 3). Initially, the different ligands and reaction parameters were evaluated with phosphine 1a and olefin 4a (Fig. 3a). We found that the selection of the BINOL-derived phosphoramidite L4 under 150 °C for 48 h could give the desired product 5aa (with ~44.8 kcal/mol rotational barrier) in 77% yield and 91% ee. The absolute configuration of this product was confirmed by X-ray diffraction, and the stereochemistry of other products was assigned by analogy to this crystal. Using the same substrates as in Fig. 2, we next evaluated the reaction efficiency with alkene 4a (Fig. 3b). A wide range of biaryl phosphines that incorporate electron-neutral (1b-e), electron-donating (1f-i) and electron-withdrawing (1j-o) substituents, were readily tolerated with alkene 4a to produce the related products 5ba-oa in modest to good yields and with excellent levels of enantioselectivities. Moreover, the use of polycyclic phosphine 1p-s and heterocycle-containing phosphines 1t-u did not interfere with productive atroposelective hydroarylation. However, the pyrrole-based phosphine 1v only delivered the desired product 5va in trace amounts at the current reaction conditions. Phosphines 1w-x with different substituents at the P atom were also compatible with this reaction. In addition to alkene 4a, we found that steric hindrance of the acrylate (4b-d) had a minimal impact on the reaction outcome, but styrene (4e) was not compatible with this C–H activation process. Finally, one example highlights the kinetic resolution of racemic 1,1’-binaphthalenyl phosphine 1y by asymmetric hydroarylation of alkene 4a (Fig. 3c). Chiral product 5ya was formed in 93% ee, and the remaining starting material 1y exhibited an excellent selectivity factor (S = 99).
Fig. 3. Atroposelective P(III)-directed C–H alkylation of biaryl phosphines with alkenes.
a Atroposelective hydroarylation of phosphine 1a with olefin 4a. b Reaction substrate scope. c, Kinetic resolution of racemic phosphine 1y by hydroarylation of olefin 4a. Reaction conditions: [Ir(cod)Cl]2 (5 mol%), L4 (11 mol%), 1 (0.2 mmol), 4 (1.0 mmol) in 2 mL of dry toluene at 150 °C for 48 h under argon; ee was determined by chiral HPLC analysis; Isolated yield. aUsing 10.0 equiv. of 4.
Synthetic applications
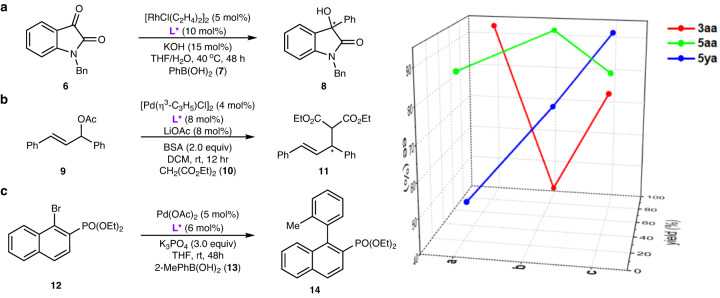
To emphasize the practicality of our chemistry, we chose some chiral products, including 3aa, 5aa and 5ya, with different steric and electronic properties, to generate a ligand library and tested them for asymmetric catalysis. In the first example (Fig. 4a), use of 3aa as a ligand in the rhodium-catalyzed arylation of isatin 6 with boronic acid 7 afforded alcohol 8 with good yield and high ee (91%, 93% ee); these were much higher than those obtained using the original MeO-MOP ligand (72%, 75% ee)63. In the second case (Fig. 4b), compound 5aa showed high reactivity for palladium-catalyzed asymmetric allylic alkylation64 with substrate 9 and dimethyl malonate (10), which afforded the desired product 11 in 93% yield and with 92% ee. In the third example (Fig. 4c), phosphine 5ya was used as a ligand for palladium-catalyzed Suzuki-Miyaura cross-coupling of aryl halide 12 with boronic acid 13 to form atropisomeric biaryl 14 in 80% yield and with 93% ee50,51. These in situ-modified chiral ligands have proven valuable in accelerating the optimization of asymmetric catalysis.
Fig. 4. Applications of the developed chiral phosphines in asymmetric catalysis.
a Asymmetric aryl-addition reaction. b Asymmetric allylic alkylation reaction. c Asymmetric Suzuki-Miyaura cross-coupling reaction.
Discussion
We next performed several experiments to investigate this asymmetric C–H activation. Reacting 1.0 equiv. of L3 with a stoichiometric quantity of [Ir(cod)Cl]2 yielded complex 15, as confirmed by X-ray analysis (Fig. 5a). Using 15 as the catalyst for hydroarylation of alkyne 2a with phosphine 1a yielded compound 3aa with good efficiency, and addition of a catalytic quantity of 3aa to the system as a ligand led to low enantioselectivity (Fig. 5b). These results showed that the formed products did not serve as ligands. Furthermore, a clear linear effect for the reaction of 1a and 2b indicated that only one chiral ligand could coordinate to the Ir center (see the Supplementary Information for details). A series of deuterium labeling experiments were then performed (Fig. 5c). The reaction of phosphine d-1a with alkyne 2a resulted in 47% deuterium incorporation at the olefinic position of product 3aa. Further addition of 10 equiv. of D2O to the system dramatically improved the level of deuterium incorporation (85% D), suggesting considerable H/D exchange occurred between the trace water in the solvent and the reaction intermediates. In addition, KIE experiments (kH/kD = 2.03) indicated that C−H bond cleavage was the rate-determining step in the reaction (Fig. 5d).65
Fig. 5. Mechanistic experiments.
a Isolation of an iridium complex 15. b Test of the reactivity of 15 and 3aa for C–H activation of 1a. c Observation of H/D exchange during the reaction of d-1a with alkyne 2a. d Kinetic isotope effect (KIE) experiments.
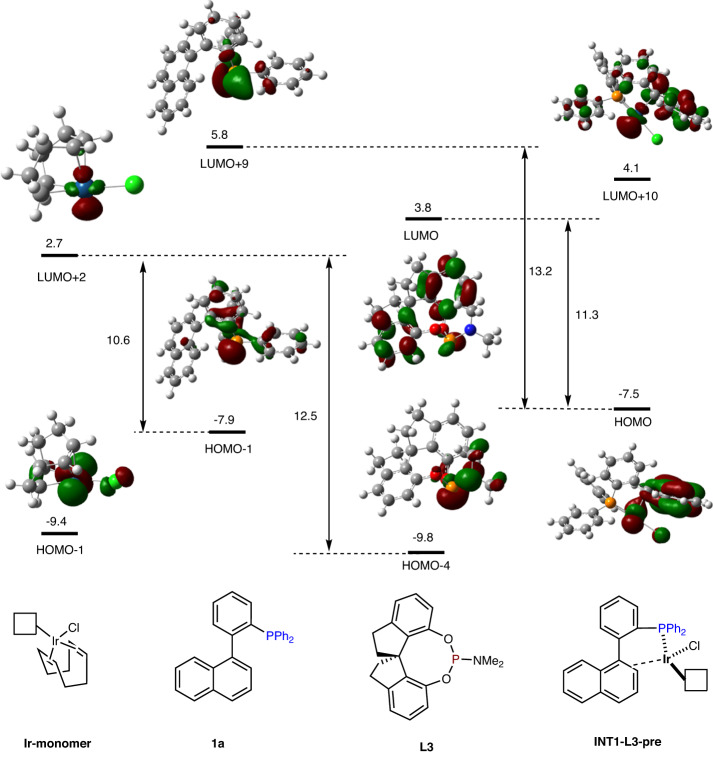
The asymmetric C–H activation involves a key intermediate INT1-L3 in which the Ir center coordinates with two phosphorus atoms in the ligand and substrate (Ir/L3/1a = 1/1/1). The high equilibrium population of this species can be illustrated by analysis of the frontier molecular orbitals (Fig. 6). The main contribution to the unoccupied molecular orbital (LUMO+2) of the intermediate Ir-monomer came from the Ir 5d orbital, which serves as an electronic acceptor for the phosphorus lone pair. The energy difference between Ir-monomer and the phosphorus-occupied molecular orbital (HOMO-1) of 1a was 1.9 eV less than that of the LUMO+2-HOMO-4 gap between Ir-monomer and the chiral ligand L3 (10.6 eV vs. 12.5 eV), suggesting that strong σ donation makes the electron-rich phosphine 1a more likely to coordinate with Ir-monomer. Then, the η4-cod ligand dissociates to provide vacancies around the Ir center (INT1-L3-pre) to facilitate the coordination of chiral ligand. The Ir 5d occupied molecular orbital (LUMO) of INT1-L3-pre is thermodynamically favorable to interact with the phosphine 3d unoccupied molecular orbital (HOMO) of L3 than with another phosphine ligand 1a (11.3 eV vs. 13.2 eV). This outcome indicates that the second coordination with L3 mainly occurs via π-back bonding, thus stabilizing the formation of intermediate INT1-L3.
Fig. 6. Analysis of frontier molecular orbitals.
HF/6-31G(d)-Lanl2DZ/CPCM.
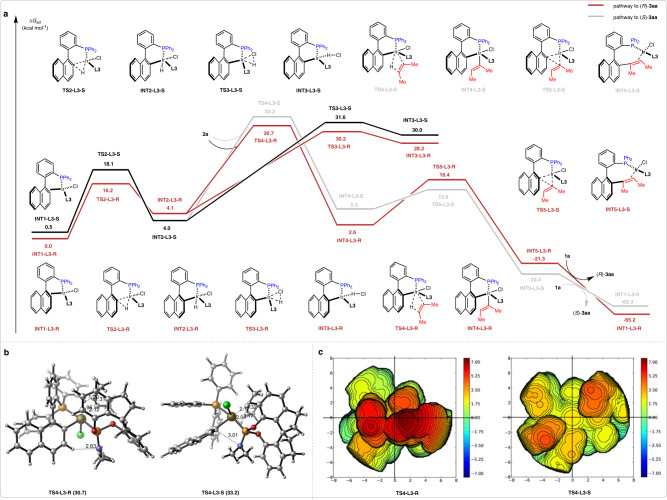
Based on the aforementioned results, the energy profile for the reaction between phosphine 1a and alkyne 2a is shown in Fig. 7a66–70. Biaryl atropisomers could easily undergo interconversion and the axial chirality of 1a leads to the formation of two enantiomers INT1-L3-R and INT1-L3-S, where INT1-L3-R was set as the relative zero point of Gibbs free energy. The two enantiomers INT1-L3 undergo C–H activation, generating the iridium complex INT2-L3 through a reversible oxidative addition. Subsequent reductive elimination of H–Cl via transition state TS3-L3 reversibly generates intermediate INT3-L3, and this process is predominantly a H/D exchange with trace water in the solvent. As a competing pathway, insertion of alkyne 2a into the Ir–H bond of INT2-L3-R leads to INT4-L3-R via TS4-L3-R with a relative free energy barrier of 30.7 kcal·mol−1, which is comparable in energy to the transition state TS3-L3, but 2.5 kcal·mol−1 lower than that for the formation of INT4-L3-S (30.7 vs 33.2 kcal·mol−1, Fig. 7b). The irreversible alkyne insertion has the highest activation energy in the catalytic cycle and is therefore proposed to be the rate-determining and enantio-determining step, in accordance with the results of KIE experiments. The stereochemical model can be further visualized by steric maps around the Ir catalyst using SambVca 2.1 tool (Fig. 7c). The geometries of TS4-L3-R and TS4-L3-S are octahedron, where Ir–Cl bond is defined as Z axis and chiral ligand L3 is located in SE quadrant of the steric map. In both transition state TS4-L3s, C–H bond adjacent to the Ir–C bond towards to the direction of Ir–Cl bond and interacts with nitrogen atom of L3 by weak hydrogen bond, resulting L3 appears vertically more extended in favored transition state TS4-L3-R and horizontally more extended in disfavored TS4-L3-S. The horizontal extension increases the repulsion interaction between methyl group of alkyne and phenyl ring of L3, resulting the energy barrier of TS4-L3-S is significantly higher than that of TS4-L3-R. The dominant intermediate INT4-L3-R further undergoes reductive elimination through transition state TS5-L3-R with an activation free energy of 13.8 kcal·mol−1 to form the INT5A-L3-R complex. Finally, ligand exchange between INT5A-L3-R and phosphine 1a releases product 3aa and regenerate INT1-L3 to complete the catalytic cycle.
Fig. 7. Computational investigations.
a DFT-computed reaction pathways (ωb97xd/6-311+G(d,p)-SDD(Ir)/CPCM//B3LYP-D3BJ/6-31G(d)-Lanl2DZ(Ir)/CPCM). b Stereochemical mode. c Steric maps of the Ir(III) transition states TS4-L3-R and TS4-L3-S.
In summary, we have demonstrated an enantioselective catalytic strategy for phosphorus-directed C–H activation enabled by chiral phosphorus ligands with iridium catalysts. This method represents an effective approach for modular syntheses of chiral phosphorus ligands via one-step transformations with widely available parent ligands, which considerably expands the toolbox of reactions available to synthetic chemists. Furthermore, the results of this study constitute a proof of principle for asymmetric C–H activations in more general molecules, which may furnish solutions for the remaining limitations in this field.
Methods
Due to slight variations in the experimental protocols for the processes presented herein, we refer the reader to the Supplementary Information for experimental details.
Supplementary information
Source data
Acknowledgements
We would like to thank the National Natural Science Foundation of China (Grants 22025104, 22171134 and 21972064), Natural Science Foundation of Jiangsu Province (BK20220033), Natural Science Foundation for Excellent Youth of Anhui Education Department (Grant 2022AH030056), and the Fundamental Research Funds for the Central Universities (Grant 020514380254) for their financial support. We are also grateful to the High-Performance Computing Center of Nanjing University for performing the numerical calculations in this paper on its blade cluster system.
Author contributions
Z.S. conceived and designed the study and wrote the paper. Z.L. performed the experiments, mechanistic studies, and analyzed the data. Y.Y. made contributions during the revision. K.H and Y.L. supervised the DFT calculation and commented on the manuscript. M.W. and X.C. performed the density functional theory (DFT) calculations. Y.Z. performed the crystallographic studies.
Peer review
Peer review information
Nature Communications thanks Wei-Liang Duan, Bing-Feng Shi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Crystallographic data for the structures of 3aa, 3va, 5aa and 15 reported in this paper have been deposited at the Cambridge Crystallographic Data Center under deposition numbers CCDC 2169735, 2169736, 2169737 and 2169738. Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of the study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Information, or from the corresponding author upon request. Coordinates of the optimized structures are provided in the source data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zexian Li, Minyan Wang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-44202-1.
References
- 1.Mohr JT, Krout MR, Stoltz BM. Natural products as inspiration for the development of asymmetric catalysis. Nature. 2008;455:323–332. doi: 10.1038/nature07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt JR, Salerno F, Fuchter MJ. The added value of small-molecule chirality in technological applications. Nat. Rev. Chem. 2017;1:0045. doi: 10.1038/s41570-017-0045. [DOI] [Google Scholar]
- 3.Bhadra S, Yamamoto H. Substrate directed asymmetric reactions. Chem. Rev. 2018;118:3391–3446. doi: 10.1021/acs.chemrev.7b00514. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RG. C–H activation. Nature. 2007;446:391–393. doi: 10.1038/446391a. [DOI] [PubMed] [Google Scholar]
- 5.Wencel-Delord, Dröge JT, Liu F, Glorius F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 2011;40:4740–4761. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]
- 6.Wang C-S, Dixneuf PH, Soulé J-F. Photoredox catalysis for building C–C bonds from C(sp2)–H bonds. Chem. Rev. 2018;118:7532–7585. doi: 10.1021/acs.chemrev.8b00077. [DOI] [PubMed] [Google Scholar]
- 7.Davies HML, Liao K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 2019;3:347–360. doi: 10.1038/s41570-019-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta U, Maiti S, Bhattacharya T, Maiti D. Arene diversification through distal C(sp2)−H functionalization. Science. 2021;372:eabd5992. doi: 10.1126/science.abd5992. [DOI] [PubMed] [Google Scholar]
- 9.Guillemard L, Kaplaneris N, Ackermann L, Johansson MJ. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 2021;5:522–545. doi: 10.1038/s41570-021-00300-6. [DOI] [PubMed] [Google Scholar]
- 10.McNally A, Haffemayer B, Collins BSL, Gaunt MJ. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature. 2014;510:129–133. doi: 10.1038/nature13389. [DOI] [PubMed] [Google Scholar]
- 11.Shu C, Noble A, Aggarwal VK. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature. 2020;586:714–719. doi: 10.1038/s41586-020-2831-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, et al. Phosphorus-mediated sp2–sp3 couplings for C–H fluoroalkylation of azines. Nature. 2021;594:217–222. doi: 10.1038/s41586-021-03567-3. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, et al. A radical approach for the selective C–H borylation of azines. Nature. 2021;595:677–683. doi: 10.1038/s41586-021-03637-6. [DOI] [PubMed] [Google Scholar]
- 14.Sambiagio C, et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018;47:6603–6743. doi: 10.1039/C8CS00201K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, et al. Transition-metal-catalyzed, coordination-assisted functionalization of nonactivated C(sp3)–H bonds. Chem. Rev. 2021;121:14957–15074. doi: 10.1021/acs.chemrev.1c00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giri R, et al. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. Chem. Soc. Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. [DOI] [PubMed] [Google Scholar]
- 17.Newton CG, Wang S-G, Oliveira CC, Cramer N. Catalytic enantioselective transformations involving C–H bond cleavage by transition-metal complexes. Chem. Rev. 2017;117:8908–8976. doi: 10.1021/acs.chemrev.6b00692. [DOI] [PubMed] [Google Scholar]
- 18.Liu C-X, et al. Synthesis of atropisomers by transition-metal-catalyzed asymmetric C–H functionalization reactions. J. Am. Chem. Soc. 2021;143:14025–14040. doi: 10.1021/jacs.1c07635. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Wu L-S, Shi B-F. Forging C−heteroatom bonds by transition-metal-catalyzed enantioselective C–H functionalization. Chem. 2022;8:384–413. doi: 10.1016/j.chempr.2021.11.015. [DOI] [Google Scholar]
- 20.Saint-Denis TG, et al. Enantioselective C(sp3)‒H bond activation by chiral transition metal catalysts. Science. 2018;359:eaao4798. doi: 10.1126/science.aao4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi B-F, Maugel N, Zhang Y-H, Yu J-Q. PdII-catalyzed enantioselective activation of C(sp2)–H and C(sp3)–H bonds using monoprotected amino acids as chiral ligands. Angew. Chem. Int. Ed. 2008;47:4882–4886. doi: 10.1002/anie.200801030. [DOI] [PubMed] [Google Scholar]
- 22.Lou S-J, et al. Modular access to spiro-dihydroquinolines via scandium-catalyzed dearomative annulation of quinolines with alkynes. J. Am. Chem. Soc. 2021;143:20462–20471. doi: 10.1021/jacs.1c10743. [DOI] [PubMed] [Google Scholar]
- 23.Ye B, Cramer N. Chiral cyclopentadienyl ligands as stereocontrolling element in asymmetric C–H functionalization. Science. 2012;338:504–506. doi: 10.1126/science.1226938. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, et al. Ligand-accelerated enantioselective methylene C(sp3)–H bond activation. Science. 2016;353:1023–1027. doi: 10.1126/science.aaf4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q-F, et al. Formation of α-chiral centers by asymmetric β-C(sp3)–H arylation, alkenylation, and alkynylation. Science. 2017;355:499–3503. doi: 10.1126/science.aal5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu L, Xiao K-J, Yu J-Q. Room-temperature enantioselective C–H iodination via kinetic resolution. Science. 2014;346:451–455. doi: 10.1126/science.1258538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidal X, Mascareñas JL, Gulías M. Palladium-catalyzed, enantioselective formal cycloaddition between benzyltriflamides and allenes: straightforward access to enantioenriched isoquinolines. J. Am. Chem. Soc. 2019;141:1862–1866. doi: 10.1021/jacs.8b12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Dixneuf PH, Soulé J-F. Late stage modifications of P-containing ligands using transition-metal-catalysed C–H bond functionalisation. Chem. Commun. 2018;54:7265–7280. doi: 10.1039/C8CC02821D. [DOI] [PubMed] [Google Scholar]
- 29.Crawford KM, Ramseyer TR, Daley CJA, Clark TB. Phosphine-directed C–H borylation reactions: Facile and selective access to ambiphilic phosphine boronate esters. Angew. Chem. Int. Ed. 2014;53:7589–7593. doi: 10.1002/anie.201402868. [DOI] [PubMed] [Google Scholar]
- 30.Wright SE, et al. Accessing ambiphilic phosphine boronates through C–H borylation by an unforeseen cationic iridium complex. Angew. Chem. Int. Ed. 2019;58:2834–2838. doi: 10.1002/anie.201812857. [DOI] [PubMed] [Google Scholar]
- 31.Wen J, et al. Rhodium-catalyzed PIII-directed ortho-C–H borylation of arylphosphines. Angew. Chem. Int. Ed. 2019;58:2078–2082. doi: 10.1002/anie.201813452. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda K, Iwasawa N, Takaya J. Ruthenium-catalyzed ortho C−H borylation of arylphosphines. Angew. Chem. Int. Ed. 2019;58:2850–2853. doi: 10.1002/anie.201813278. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, et al. Rhodium(II)-catalyzed dehydrogenative silylation of biaryl-type monophosphines with hydrosilanes. Angew. Chem. Int. Ed. 2019;58:12529–12533. doi: 10.1002/anie.201906975. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, et al. Revealing silylation of C(sp2)/C(sp3)–H bonds in arylphosphines by ruthenium catalysis. Angew. Chem. Int. Ed. 2020;59:10909–10912. doi: 10.1002/anie.202003865. [DOI] [PubMed] [Google Scholar]
- 35.Shelby Q, Kataoka N, Mann G, Hartwig J. Unusual in situ ligand modification to generate a catalyst for room temperature aromatic C−O bond formation. J. Am. Chem. Soc. 2000;122:10718–10719. doi: 10.1021/ja002543e. [DOI] [Google Scholar]
- 36.Qiu X, Wang M, Zhao Y, Shi Z. Rhodium(I)-catalyzed tertiary phosphine directed C−H arylation: Rapid construction of ligand libraries. Angew. Chem. Int. Ed. 2017;56:7233–7237. doi: 10.1002/anie.201703354. [DOI] [PubMed] [Google Scholar]
- 37.Luo X, et al. Synthesis of peri-substituted (naphthalen−1-yl)phosphine ligands by rhodium(I)-catalyzed phosphine-directed C–H arylation. Org. Lett. 2018;20:1810–1814. doi: 10.1021/acs.orglett.8b00305. [DOI] [PubMed] [Google Scholar]
- 38.Li J-W, et al. Ruthenium-catalyzed gram-scale preferential C–H arylation of tertiary phosphine. Org. Lett. 2019;21:2885–2889. doi: 10.1021/acs.orglett.9b00888. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, et al. Rhodium-catalysed direct hydroarylation of alkenes and alkynes with phosphines through phosphorous-assisted C−H activation. Nat. Commun. 2019;10:3539. doi: 10.1038/s41467-019-11420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Cordier M, Dixneuf PH, Soulé J-F. Late-stage diversification of biarylphosphines through rhodium(I)-catalyzed C–H bond alkenylation with internal alkynes. Org. Lett. 2020;22:5936–5940. doi: 10.1021/acs.orglett.0c02023. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Roisnel T, Dixneuf PH, Soulé J-F. RhI-catalyzed PIII-directed C−H Bond alkylation: design of multifunctional phosphines for carboxylation of aryl bromides with carbon dioxide. Angew. Chem. Int. Ed. 2019;58:14110–14114. doi: 10.1002/anie.201906913. [DOI] [PubMed] [Google Scholar]
- 42.Borah AJ, Shi Z. Rhodium-catalyzed, remote terminal hydroarylation of activated olefins through a long-range deconjugative isomerization. J. Am. Chem. Soc. 2018;140:6062–6066. doi: 10.1021/jacs.8b03560. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, et al. Phosphorus(III)-assisted regioselective C–H silylation of heteroarenes. Nat. Commun. 2021;12:524. doi: 10.1038/s41467-020-20531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang W, Zhang X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 2003;103:3029–3070. doi: 10.1021/cr020049i. [DOI] [PubMed] [Google Scholar]
- 45.Fernández-Pérez H, Etayo P, Panossian A, Vidal-Ferran A. Phosphine−phosphinite and phosphine−phosphite ligands: Preparation and applications in asymmetric catalysis. Chem. Rev. 2011;111:2119–2176. doi: 10.1021/cr100244e. [DOI] [PubMed] [Google Scholar]
- 46.Ni H, Chan W-L, Lu Y. Phosphine-catalyzed asymmetric organic reactions. Chem. Rev. 2018;118:9344–9411. doi: 10.1021/acs.chemrev.8b00261. [DOI] [PubMed] [Google Scholar]
- 47.Guo H, et al. Phosphine organocatalysis. Chem. Rev. 2018;118:10049–10293. doi: 10.1021/acs.chemrev.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pereira MM, et al. Synthesis of binaphthyl based phosphine and phosphite ligands. Chem. Soc. Rev. 2013;42:6990–7027. doi: 10.1039/c3cs60116a. [DOI] [PubMed] [Google Scholar]
- 49.Dutartre M, Bayardona J, Jugé S. Applications and stereoselective syntheses of P-chirogenic phosphorus compounds. Chem. Soc. Rev. 2016;45:5771–5794. doi: 10.1039/C6CS00031B. [DOI] [PubMed] [Google Scholar]
- 50.Yin J, Buchwald SL. A catalytic asymmetric Suzuki coupling for the synthesis of axially chiral biaryl compounds. J. Am. Chem. Soc. 2000;122:12051–12052. doi: 10.1021/ja005622z. [DOI] [Google Scholar]
- 51.Yamamoto T, Akai Y, Nagata Y, Suginome M. Highly enantioselective synthesis of axially chiral biarylphosphonates: Asymmetric suzuki–miyaura coupling using high-molecular-weight, helically chiral polyquinoxaline-based phosphines. Angew. Chem. Int. Ed. 2011;50:8844–8847. doi: 10.1002/anie.201103792. [DOI] [PubMed] [Google Scholar]
- 52.Ji W, Wu H-H, Zhang J. Axially chiral biaryl monophosphine oxides enabled by palladium/WJ-Phos-catalyzed asymmetric Suzuki–Miyaura cross-coupling. ACS Catal. 2020;10:1548–1554. doi: 10.1021/acscatal.9b04354. [DOI] [Google Scholar]
- 53.Bhat V, Wang S, Stoltz BM, Virgil SC. Asymmetric synthesis of QUINAP via dynamic kinetic resolution. J. Am. Chem. Soc. 2013;135:16829–16832. doi: 10.1021/ja409383f. [DOI] [PubMed] [Google Scholar]
- 54.Ramírez-López P, et al. A dynamic kinetic C–P cross–coupling for the asymmetric synthesis of axially chiral P,N ligands. ACS Catal. 2016;6:3955–3964. doi: 10.1021/acscatal.6b00784. [DOI] [Google Scholar]
- 55.Li S-X, Ma Y-N, Yang S-D. P(O)R2-Directed enantioselective C–H olefination toward chiral atropoisomeric phosphine–olefin compounds. Org. Lett. 2017;19:1842–1845. doi: 10.1021/acs.orglett.7b00608. [DOI] [PubMed] [Google Scholar]
- 56.Jang Y-S, Woźniak Ł, Pedroni J, Cramer N. Access to P- and axially chiral biaryl phosphine oxides by enantioselective CpxIrIII-catalyzed C−H arylations. Angew. Chem. Int. Ed. 2018;57:12901–12905. doi: 10.1002/anie.201807749. [DOI] [PubMed] [Google Scholar]
- 57.Duan L, et al. Enantioselective ring-opening/oxidative phosphorylation and P-transfer reaction of cyclic diaryliodoniums. ACS Catal. 2019;9:9852–9858. doi: 10.1021/acscatal.9b03454. [DOI] [Google Scholar]
- 58.Hu P, et al. Twofold C−H activation-based enantio- and diastereoselective C−H arylation using diarylacetylenes as rare arylating reagents. Angew. Chem. Int. Ed. 2021;60:20424–20429. doi: 10.1002/anie.202106871. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C-W, et al. Asymmetric C–H activation for the synthesis of P- and axially chiral biaryl phosphine oxides by an achiral Cp*Ir catalyst with chiral carboxylic amide. ACS Catal. 2022;12:193–199. doi: 10.1021/acscatal.1c05080. [DOI] [Google Scholar]
- 60.Cheng JK, et al. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 2021;121:4805–4902. doi: 10.1021/acs.chemrev.0c01306. [DOI] [PubMed] [Google Scholar]
- 61.Carmona JA, et al. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 2021;50:2968–2983. doi: 10.1039/D0CS00870B. [DOI] [PubMed] [Google Scholar]
- 62.Nagamoto M, Nishimura T. Asymmetric transformations under iridium/chiral diene catalysis. ACS Catal. 2017;7:833–847. doi: 10.1021/acscatal.6b02495. [DOI] [Google Scholar]
- 63.Lu S, Poh SB, Siau W-Y, Zhao Y. Kinetic resolution of tertiary alcohols: Highly enantioselective access to 3-hydroxy-3-substituted oxindoles. Angew. Chem. Int. Ed. 2013;52:1731–1734. doi: 10.1002/anie.201209043. [DOI] [PubMed] [Google Scholar]
- 64.Trost BM, Vranken DLV. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 1996;96:395–422. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]
- 65.Simmons EM, Hartwig JF. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- 66.Huang G, Liu P. Mechanism and origins of ligand-controlled linear versus branched selectivity of iridium-catalyzed hydroarylation of alkenes. ACS Catal. 2016;6:809–820. doi: 10.1021/acscatal.5b02201. [DOI] [Google Scholar]
- 67.Peng Q, Duarte F, Paton RS. Computing organic stereoselectivity from concepts to quantitative calculations and predictions. Chem. Soc. Rev. 2016;45:6093–6107. doi: 10.1039/C6CS00573J. [DOI] [PubMed] [Google Scholar]
- 68.Woźniak Ł, et al. Catalytic enantioselective functionalizations of C–H bonds by chiral iridium complexes. Chem. Rev. 2020;120:10516–10543. doi: 10.1021/acs.chemrev.0c00559. [DOI] [PubMed] [Google Scholar]
- 69.Jiang R, Ding L, Zheng C, You S-L. Iridium-catalyzed Z-retentive asymmetric allylic substitution reactions. Science. 2021;371:380–386. doi: 10.1126/science.abd6095. [DOI] [PubMed] [Google Scholar]
- 70.Li B, Xu H, Dang Y, Houk KN. Dispersion and steric effects on enantio-/diastereoselectivities in synergistic dual transition-metal catalysis. J. Am. Chem. Soc. 2022;144:1971–1985. doi: 10.1021/jacs.1c12664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Crystallographic data for the structures of 3aa, 3va, 5aa and 15 reported in this paper have been deposited at the Cambridge Crystallographic Data Center under deposition numbers CCDC 2169735, 2169736, 2169737 and 2169738. Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of the study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Information, or from the corresponding author upon request. Coordinates of the optimized structures are provided in the source data file. Source data are provided with this paper.