Highlights
-
•
The quenching mechanism of whey protein with epigallocatechin gallate was analyzed.
-
•
Whey protein was provided a binding site for epigallocatechin gallate to form complexes.
-
•
Epigallocatechin gallate and whey protein were combined by spontaneous reaction.
-
•
Binding modes after heat treatments were hydrophobic and electrostatic interactions.
Keywords: Epigallocatechin gallate, Whey protein, Interaction, Quenching mechanism
Abstract
This study aimed to examine the interaction mechanism of polyphenol protein in a heat-treated aqueous solution system using epigallocatechin gallate (EGCG) and whey protein (WP) as raw materials. Further, we hypothesized the binding characteristics of these two compounds. The results were as follows: The quenching mechanism between WP and EGCG was characterized as static quenching. As the temperature increased, the binding constant and the binding force between EGCG and WP both increased. The number of binding sites (denoted as n) between WP and EGCG was approximately 1. Hence, WP provided a single site to bind to EGCG to form a complex. The main binding modes between WP and EGCG were hydrophobic and electrostatic interactions, and they were spontaneously combined into complexes (ΔG < 0). This study provided a basis for the interaction between WP and EGCG under different heating conditions and their combination mode.
1. Introduction
The main component of tea polyphenols is epigallocatechin gallate (EGCG), which is easily soluble in water. The polyhydroxyl structure of EGCG enables it to exhibit numerous biological functions (Song & Yoo, 2017). Further, EGCG can also act as an antioxidant and antibacterial agent to inhibit lipid oxidation (Zhao, Lin, Gao, Gong, & Mao, 2022). Whey protein (WP) has rich nutritional value and good emulsification and water retention properties; it has been widely used in food production and processing (Abd El-Salam and El-Shibiny, 2018, Sanmartin et al., 2013).
However, the interactions between polyphenols and proteins have been found to affect their functional properties and nutritional effects (Bandyopadhyay et al., 2012, Liu et al., 2015, Tian et al., 2021). Therefore, people began to explore the possible mechanism underlying the interaction between polyphenols and proteins. Heat treatment is a basic step in the processing of milk and dairy products, and some chemical changes usually occur in the heat-treated milk (Ahmadi, Nasirpour, Goli, & Riahi, 2018). Additionally, the effects of EGCG and different structural proteins are extremely different at different temperatures and pH values, which might have a certain impact on the bioaccessibility of tea polyphenols and on the human body (Bayraktar et al., 2019, Ma et al., 2023). Xue, Tan, Zhang, Feng and Xia (2014) found that after casein was modified by glucan glycosylation, steric hindrance provided by glucan groups inhibited the interaction between EGCG and casein. Chang, Niu, Su, Qiu, Gu and Yang (2016) found that the emulsification ability of egg whey protein was enhanced after acid or acid–heat treatment, and the number of surface-exposed hydrophobic amino acids and charged groups increased, but the secondary structure changed little. Gao, Zhang, Wang, Sun, Wang and Wang (2019) reported that the changes in the physical and chemical properties of WP during heating were dependent on the type of heating (dry heat at 60 °C, wet heat at 80 °C, and the dry and wet combined heating) and the presence of inulin. Inulin did not affect the surface hydrophobicity of heated WP under dry heat conditions but decreased the surface hydrophobicity under hot and humid conditions. The surface hydrophobicity of WP with inulin was significantly increased, indicating that inulin had a significant effect on the conformation of WP. However, the surface hydrophobicity was reduced because of the presence of inulin under the combined heating condition. Pessato et al. (2018) found that whey protein isolate interacted with caffeic acid and EGCG at room temperature (25 °C), suggesting that EGCG complexation was primarily driven by noncovalent interactions, which might be a promising method to produce hypoallergenic dairy products. Radibratovic et al. (2019) examined the binding of EGCG to apo-alpha-lactalbumin (apo-ALA) and its stabilizing effect on protein structure. They found that the thermal stability of apo-ALA induced by EGCG was based on the increase in the conformational rigidity of apo-ALA. Cao, Xiong, Cao and True (2018) explored the effects of preheating (80 ℃, 9 min) and phenolic treatment on the adsorption behavior of whey protein isolate at the gas–water interface and subsequent foaming performance. They found that the enhanced foam stability caused by heat-induced protein aggregation improved the interface characteristics and led to the formation of proteins with higher foaming ability.
Many scholars have recently investigated the mechanism underlying polyphenol–protein interaction. However, the nature of the polyphenol–protein complex under different temperature treatment conditions was less studied. Therefore, this study analyzed the fluorescence quenching effect, binding constant, interaction force, and thermodynamic significance of different concentrations of EGCG on WP by examining the interaction of WP–EGCG after heat treatments at different temperatures. It explored the influence of temperature on the polyphenol–protein interaction. This study provided data support for the interaction mechanism between tea polyphenols and WP during heating.
2. Materials and methods
2.1. Materials
EGCG (≥99 %) was purchased from Shanghai Pico Pharmaceutical Technology Co., Ltd. (Shanghai, China). WP was preparation from Desmodium intortum (Mill.) Urb. Tris (hydroxymethyl) aminomethane (Tris, ≥99 %) was procured from Yuanye Biotechnology Co., Ltd. (Shanghai, China). A water purification system produced ultrapure water.
2.2. Heat treatment
Tris (hydroxymethyl) aminomethane (Tris) and HCl (5 mol/L) were used as raw materials to prepare Tris–HCl buffer with pH 6.4. This buffer was used to prepare 20 μmol/L WP and 1.0 mmol/L EGCG solution for the solvent (Huang, Li, Ma, Feng, & He, 2010). WP and the corresponding concentration of EGCG were mixed and heated in a water bath at 60 ℃ for 10 min, 80 ℃ for 10 min, and 100 ℃ for 5 min in a boiling water bath. Then, the mixture was immediately cooled in an ice bath. WP or EGCG without mixing underwent the same heat treatment as blank controls (Ming, Chen, Khan, Wang, & Wang, 2020).
2.3. Fluorescence quenching spectroscopy
The concentration of WP was diluted to 10 μmol/L after heat treatment, and the concentration gradients of EGCG were 0, 50, 100, 150, 200, and 250 μmol/L. Each group of samples was placed in a water bath at 24 ℃, 31 ℃, and 38 ℃ for 1 h. Then, the excitation wavelength of 280 nm and the slit wavelength of 5 nm were set, and the fluorescence quenching spectra of the sample solution at the corresponding temperature were measured in the range of 300–450 nm. The corresponding concentration of EGCG buffer solution was used as a blank deduction to obtain the fluorescence intensity of each group of samples (Zhang, Wang, Qi, Zhao, Wang, & Zhang, 2022).
2.4. Fluorescence quenching constant
The Stern–Volmer quenching constant Ksv was obtained using the slope of the regression curve of F0/F against [Q] in the linear range, and Kq was calculated using the data measured by fluorescence spectra. These reflected the efficiency of quenching or the accessibility of fluorophore to the quencher, and also indicated the strength of the interaction between WP and EGCG (He, Chen, & Moser, 2015). The quenching of WP by EGCG at different temperatures (24 ℃, 31 ℃, and 38 ℃) was examined, and the Stern–Volmer equation was used for data analysis:
| (1) |
where F0 represents the maximum fluorescence intensity of the protein without EGCG; F represents the maximum fluorescence intensity of the protein with EGCG; Kq (L mol−1 s−1) and [Q] (μmol/L) are bimolecular quenching rate constant and EGCG concentration, respectively. τ0 (s) represents the average fluorescence lifetime of fluorescent molecules without a quencher, and τ0 of ordinary biomolecules is equal to 10−8 s; Ksv (L mol−1) is the Stern–Volmer quenching constant.
2.5. Binding constants and bits
The log[Q] by lg(F0/F – 1) was plotted to get a linear curve. According to its slope and intercept, the binding constant (Ka) and the number of binding sites (n) of the WP–EGCG complex was calculated as (Chen et al., 2020):
| (2) |
where Ka (L mol−1) is the binding constant, n is the number of binding sites, and [Q] is the concentration of EGCG.
2.6. Thermodynamic parameters
Using lnKa to plot 1/T, the enthalpy change ΔH and the entropy change ΔS were calculated from the slope and intercept, respectively. Then, the change in Gibbs free energy ΔG was calculated by combining the constants and the data obtained using the following formula. These thermodynamic parameters could be used to judge the direction of the spontaneous thermodynamic process and the type of binding force (Arroyo-Maya, Campos-Teran, Hernandez-Arana, & McClements, 2016).
| (3) |
| (4) |
| (5) |
where Ka represents the binding constant at 24 ℃, 31 ℃, and 38 ℃; and R is the molar gas constant of 8.314 J/(mol K).
3. Results and discussion
3.1. Fluorescence quenching spectrum analysis
Fluorescence spectroscopy is widely used to study the interaction mechanism between ligands and proteins. Some important information about protein structure can be obtained by detecting the change in fluorescence spectral peak. The fluorophores in proteins include tryptophan, tyrosine, and phenylalanine, among which tryptophan is extremely sensitive to the change in molecular structure, and can be used as an endogenous probe to explore the characteristics of the interaction between WP and EGCG (Xie et al., 2022). The conformation of a protein can be determined indirectly by the change in its maximum internal fluorescence intensity (Fmax) and the corresponding maximum fluorescence emission wavelength (λmax) (Arroyo-Maya et al., 2016). Fig. 1 shows the fluorescence quenching diagrams of EGCG and WP at 24 ℃, 31 ℃, and 38 ℃ at concentrations of 0, 50, 100, 150, 200, and 250 μmol/L under heat treatment conditions.
Fig. 1.
Fluorescence quenching of EGCG (0 μmol/L, 50 μmol/L, 100 μmol/L, 150 μmol/L, 200 μmol/L, 250 μmol/L) and WP at 24 ℃, 31 ℃, 38 ℃ under heat treatment (C: room temperature). In the figure, from left to right are the room temperature group, 60 ℃ group, 80 ℃ group and 100 ℃ group respectively.
Fluorescence quenching refers to the process in which the fluorescence of a fluorophore is weakened due to the interaction between molecules. This figure shows that EGCG had a certain quenching effect on WP, and the change in heating conditions also affected the fluorescence intensity. The fluorescence intensity of WP was enhanced to a certain extent during heating, which might be because the complex was denatured and disintegrated with the increase in temperature (Arroyo-Maya et al., 2016, Pessato et al., 2018). The quenching rates in different groups changed after the addition of 200 μmol/L EGCG to WP; the quenching rate in the room temperature, 60 ℃, 80 ℃, and 100 ℃ groups after immersion in the water bath at 24 ℃ was 66.70 %, 67.56 %, 72.39 %, and 73.10 %, respectively. The quenching ability of EGCG increased after heating. This might be because the WP structure expanded with the increase in temperature, and the exposed amino acid residues exhibited a quenching effect with EGCG (Zhang, Sheng, Feng, Diao, Wang, & Zhang, 2022). Moreover, McCarthy, Kelly, O’Mahony and Fenelon (2014) found that when the temperature increased, monomer β-casein self-associated to form aggregates with hydrophilic surfaces and hydrophobic nuclei.
The maximum emission wavelength of WP at room temperature was 330 nm. Further, λmax value of <5 nm showed different degrees of redshift with the increase in the heating temperature to 80 ℃. λmax value also showed a redshift depending on EGCG concentration and water bath temperature. When the water bath temperature was 31 ℃, the redshift occurred at the EGCG concentration of 100 μmol/L; when the heating temperature increased to 80 ℃, the low-concentration EGCG also led to a redshift. It might be affected by temperature and led to the migration of fluorophores from hydrophobic to hydrophilic microenvironment, and the polarity of the microenvironment increased (Loveday, 2016).
The results showed that different concentrations of EGCG had a quenching effect on WP fluorescence, and the higher the concentration of EGCG, the stronger the quenching effect. It might be because the microenvironment changed after the addition of EGCG, facilitating the gradual unfolding of the molecular conformation of WP after being affected. Also, the amino acid residues were exposed to a more hydrophilic environment, forming more complexes. The fluorescence intensity also decreased with an increase in heat treatment temperature. This could be due to protein aggregation induced by heat treatment, resulting in the partial masking of tryptophan and tyrosine residues. After heating, EGCG might interact with stretched WP, bind to its exposed amino acids, reduce hydrophobicity, and form a ground-state complex that did not emit fluorescence. It was also possible that the fluorescence quenching of EGCG was enhanced under heating conditions.
3.2. Fluorescence quenching mechanism
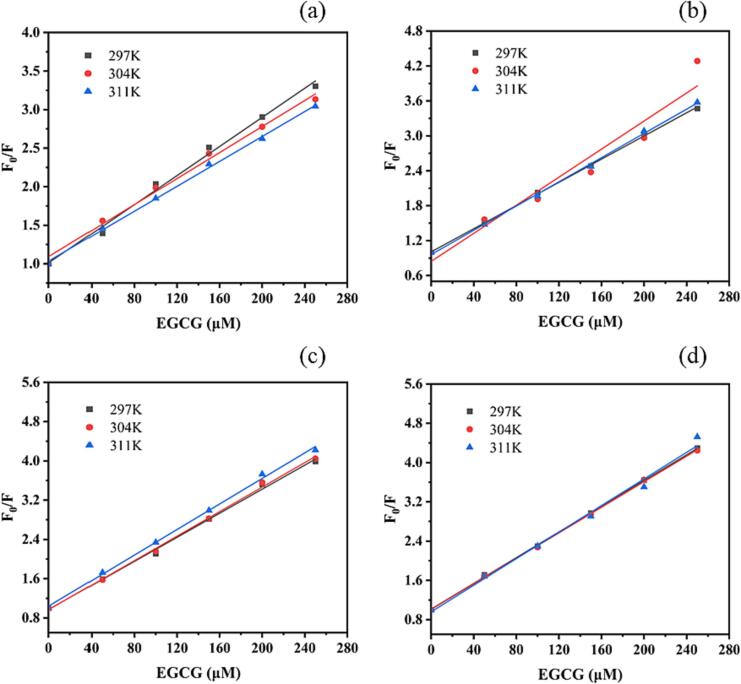
The quenching constant Ksv was calculated using the Stern–Volmer Eq. (1), and then the mechanism of the quenching of WP fluorescence by EGCG was analyzed (He, Chen, & Moser, 2015). Fig. 2 shows the Stern–Volmer curves of WP–EGCG at 297 K, 304 K, and 311 K after heat treatment, where (a), (b), (c), and (d) correspond to the room temperature, 60 ℃, 80 ℃, and 100 ℃ groups.
Fig. 2.
Stern-Volmer curves of WP-EGCG at 297 K, 304 K and 311 K after heat treatment. (a): Room temperature group; (b): 60 ℃ group; (c): 80 ℃ group; (d): 100 ℃ group.
EGCG could quench the fluorescence of WP based on the fluorescence quenching spectrum, and the common causes of fluorescence quenching were dynamic and static quenching. Static quenching referred to the intermolecular force between the quencher and the fluorescer, forming a nonfluorescing substance. The degree of turbulence in the system increased with the increase in temperature, leading to a decrease in the quenching constant. Dynamic quenching occurred when the fluorescence intensity decreased due to collisions between the quencher and the fluorescence molecule in the excited state. Therefore, the collision rate intensified and the quenching constant increased with the increase in temperature and turbulence of the system (He et al., 2020, Liu et al., 2021). The quenching rate constant was calculated to determine the quenching type using the Stern–Volmer equation. The slope Ksv was determined, and the Kq values (Table 1) were obtained at different temperatures (297 K, 304 K, and 311 K). This figure and table clearly depict that in the quenching curve of the interaction between WP and EGCG at room temperature, Ksv decreased with the increase in heating temperature and was 9.69 × 103, 8.79 × 103, and 8.06 × 103 L mol−1, which conformed to the static quenching mechanism. After heat treatment at different temperatures, the Ksv of WP–EGCG did not decrease with the increase in temperature. After heat treatment at 60 ℃, Ksv increased first and then decreased. After heat treatment at 80 ℃, Ksv increased with the increase in temperature. After heat treatment at 100 ℃, Kq decreased first and then increased, but the Kq after heat treatment was greater than the maximum dynamic quenching value (2 × 1010 L mol−1 s−1). It was speculated that the fluorescence quenching mechanism of EGCG and WP after heat treatment might result from complex static and dynamic quenching. In summary, in the linear range, it was believed that the fluorescence quenching of WP by EGCG was mainly caused by the formation of new complexes, that is, the quenching mechanism was considered to be static quenching.
Table 1.
Fluorescence quenching constants of EGCG complex with whey protein under different temperature treatment conditions.
| Groups | T/K | Ksv/(104 L mol−1) | Kq/(1012 L mol−1 s−1) |
|---|---|---|---|
| Room temperature | 297 | 0. 969 ± 0.023de | 0. 969 ± 0.023de |
| 304 | 0. 879 ± 0.031ef | 0. 879 ± 0.031ef | |
| 311 | 0. 806 ± 0.027f | 0. 806 ± 0.027f | |
| 60 ℃ | 297 | 1.004 ± 0.027d | 1.004 ± 0.027d |
| 304 | 1.198 ± 0.046c | 1.198 ± 0.046c | |
| 311 | 1.056 ± 0.019d | 1.056 ± 0.019d | |
| 80 ℃ | 297 | 1.252 ± 0.034bc | 1.252 ± 0.034bc |
| 304 | 1.339 ± 0.079ab | 1.339 ± 0.079ab | |
| 311 | 1.351 ± 0.107a | 1.351 ± 0.107a | |
| 100 ℃ | 297 | 1.327 ± 0.025ab | 1.327 ± 0.025ab |
| 304 | 1.310 ± 0.027ab | 1.310 ± 0.027ab | |
| 311 | 1.366 ± 0.017a | 1.366 ± 0.017a | |
Note: Different letters indicate significant differences in the same column of data (p < 0.05).
3.3. Combination of constant and thermodynamic analysis
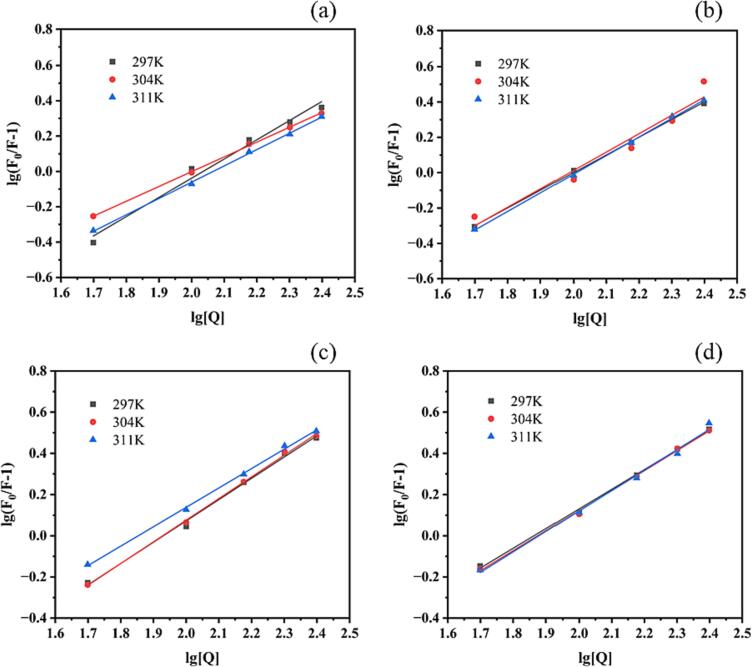
The combination of WP and EGCG was a static quenching process. Fig. 3 shows the double-log curves of WP–EGCG at 297 K, 304 K, and 311 K after heat treatment, where (a), (b), (c), and (d) correspond to the room temperature, 60 ℃, 80 ℃, and 100 ℃ groups. The binding constant Ka and the number of binding sites n were calculated based on the static quenching mechanism by substituting Eq. (2). Then, the thermodynamic parameters were calculated using Eqs. (3), (4), (5), and the binding types were predicted. The calculation results are listed in Table 2.
Fig. 3.
Double-log curves of WP-EGCG at 297 K, 304 K and 311 K after heat treatment. (a): Room temperature group; (b): 60 ℃ group; (c): 80 ℃ group; (d): 100 ℃ group.
Table 2.
Binding constants Ka, number of binding sites n and thermodynamic parameters of EGCG and whey protein under heat treatment.
| Groups | T/K | Ka/(104 L mol−1) | n | ΔH/(kJ mol−1) | ΔG/(kJ mol−1) | ΔS/(J mol−1 K−1) |
|---|---|---|---|---|---|---|
| Room temperature | 297 | 0.628 ± 0.046f | 1.089 ± 0.011 | 54.008 | −21.594 | 254.550 |
| 304 | 2.060 ± 0.054a | 0.849 ± 0.010 | −25.105 | 260.239 | ||
| 311 | 1.610 ± 0.379b | 0.877 ± 0.050 | −25.053 | 254.213 | ||
| 60 ℃ | 297 | 1.023 ± 0.037de | 0.998 ± 0.011 | −10.010 | −22.799 | 43.064 |
| 304 | 0.866 ± 0.084ef | 1.038 ± 0.026 | −22.916 | 42.454 | ||
| 311 | 0.857 ± 0.097ef | 1.035 ± 0.022 | −23.416 | 43.105 | ||
| 80 ℃ | 297 | 1.019 ± 0.082de | 1.038 ± 0.014 | 27.744 | −22.788 | 170.142 |
| 304 | 0.879 ± 0.133ef | 1.077 ± 0.037 | −22.953 | 166.765 | ||
| 311 | 1.712 ± 0.159b | 0.957 ± 0.030 | −25.205 | 170.255 | ||
| 100 ℃ | 297 | 1.631 ± 0.017b | 0.961 ± 0.006 | −14.076 | −23.950 | 33.249 |
| 304 | 1.511 ± 0.022bc | 0.971 ± 0.007 | −24.321 | 33.703 | ||
| 311 | 1.262 ± 0.138 cd | 1.013 ± 0.024 | −24.416 | 33.248 | ||
Note: Different letters indicate significant differences in the same column of data (p < 0.05).
The study showed that the greater the binding constant Ka, the greater the binding force between the molecule and the protein (Chen et al., 2020). As shown in this table, the Ka after different heat treatments increased from 6.28 × 103 to 1.63 × 104 L mol−1 at the temperature of 297 K. The binding constant of EGCG increased with the increase in temperature, indicating that the binding force of the complex between EGCG and WP increased. The number of binding sites n of WP and EGCG was about 1, indicating that WP provided a site to bind to EGCG to form a complex. The Ka analysis of EGCG and WP indicated that the binding constant was the smallest at room temperature. The binding constant increased and the binding force became stronger after heat treatment. This might be because the molecular structure of WP was affected after heat treatment, and it was easier to interact with EGCG after WP was stretched out. This might also be the heat causing the protein structure to shrink rapidly, and the soluble or insoluble aggregation of polyphenol proteins occurred. Notably, the binding force of the two increased during the aggregation process.
The interaction forces between the protein and the ligand were mainly electrostatic interaction, hydrophobic interaction, hydrogen bond, and van der Waals force (Niccoli, Oliva, & Castronuovo, 2017). The thermodynamic parameters ΔG, ΔH, and ΔS were calculated. ΔG is the change in Gibbs free energy, which can be used to determine the direction of spontaneous thermodynamic processes. ΔH and ΔS can determine the type of binding force of the protein and ligand reaction system. The reaction is spontaneous at ΔG < 0, and the reaction is spontaneous in the reverse direction at ΔG > 0. Further, ΔH < 0 and ΔS < 0 indicate mainly hydrogen bonds or van der Waals forces; ΔH < 0 and ΔS > 0 indicate mainly electrostatic interaction; ΔH > 0 and ΔS > 0 indicate mainly hydrophobic interaction; and ΔH > 0 and ΔS < 0 indicate mainly electrostatic and hydrophobic interactions (Arroyo-Maya et al., 2016). It was observed based on the aforementioned rules and the calculation results that ΔG < 0 under different heat treatment conditions indicated that EGCG and WP were combined into a complex through a spontaneous reaction. After heat treatment at room temperature and 80 ℃, ΔH > 0 and ΔS > 0 indicated that the force between EGCG and WP in this system was mainly hydrophobic interaction. After heat treatment at 60 ℃ and 100 ℃, ΔH < 0 and ΔS > 0 indicated that the interaction force between EGCG and WP in this system was mainly electrostatic interaction. We found that heat treatment had some effect on the type of binding force between WP and EGCG.
4. Conclusions
Further exploration of the WP–EGCG system under various heat treatments revealed that heat treatment and different concentrations of EGCG induced a fluorescence quenching effect on WP fluorescence. The higher the concentration of EGCG, the more pronounced the quenching effect; furthermore, the quenching ability increased after heat treatment. In the quenching curve of the interaction between WP and EGCG at room temperature, Ksv decreased with the increase in heating temperature. The temperature increase caused the unfolding of the WP structure, the decrease in hydrophobicity, and the quenching effect between exposed amino acid residues and EGCG. Within the linear range, the fluorescence quenching of WP by EGCG was considered to be due to the formation of complexes. Therefore, the quenching mechanism involved static quenching. Moreover, the number of binding sites n of WP and EGCG under different heat treatments was about 1, indicating that WP provided a single site for EGCG binding to form a complex. The thermodynamic analysis revealed that EGCG and WP formed a complex through a spontaneous reaction. The main binding types of WP and EGCG following different heat treatments were found to be hydrophobic and electrostatic interactions. The main binding type might alternate between hydrophobic and electrostatic interactions with the change in heat treatment.
CRediT authorship contribution statement
Yu-qi Song: Conceptualization. Ying Zhao: Formal analysis. Guanglong Yao: Methodology. Rong-shu Dong: . Jian Chen: .
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by National Natural Science Foundation of Hainan Province (320RC471) and National Natural Science Foundation of China (32160529).
Contributor Information
Rong-shu Dong, Email: dongrongshu@126.com.
Jian Chen, Email: chenjian19850702@163.com.
Data availability
No data was used for the research described in the article.
References
- Abd El-Salam M.H., El-Shibiny S. Glycation of whey proteins: Technological and nutritional implications. International of Biological Macromolecules. 2018;112:83–92. doi: 10.1016/j.ijbiomac.2018.01.114. [DOI] [PubMed] [Google Scholar]
- Ahmadi S.F., Nasirpour A., Goli S.A.H., Riahi E. Effect of heat treatment and solution preparation procedure on colloidal stability of whey protein sour cherry beverage. International Journal of Dairy Technology. 2018;71(3):781–790. doi: 10.1111/1471-0307.12498. [DOI] [Google Scholar]
- Arroyo-Maya I.J., Campos-Teran J., Hernandez-Arana A., McClements D.J. Characterization of flavonoid-protein interactions using fluorescence spectroscopy: Binding of pelargonidin to dairy proteins. Food Chemistry. 2016;213:431–439. doi: 10.1016/j.foodchem.2016.06.105. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay P., Ghosh A.K., Ghosh C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food & Function. 2012;3(6):592–605. doi: 10.1039/c2fo00006g. [DOI] [PubMed] [Google Scholar]
- Bayraktar M.K., Harbourne N.B., Fagan C.C. Impact of heat treatment and acid gelation on polyphenol enriched milk samples. LWT-Food Science and Technology. 2019;113 doi: 10.1016/j.lwt.2019.108282. [DOI] [Google Scholar]
- Cao Y., Xiong Y.L., Cao Y., True A.D. Interfacial properties of whey protein foams as influenced by preheating and phenolic binding at neutral pH. Food Hydrocolloids. 2018;82:379–387. doi: 10.1016/j.foodhyd.2018.04.020. [DOI] [Google Scholar]
- Chang C., Niu F., Su Y., Qiu Y., Gu L., Yang Y. Characteristics and emulsifying properties of acid and acid-heat induced egg white protein. Food Hydrocolloids. 2016;54:342–350. doi: 10.1016/j.foodhyd.2015.09.026. [DOI] [Google Scholar]
- Chen W., Wang H., Wang W., Ma X., Guo M., Ding T.…Liu D. Binding affinity and antioxidant activity of the complex of (-)-epigallocatechin-3-gallate and whey protein isolate: Effect of ultrasound pretreatment. Journal of Food Process Engineering. 2020;43(5):e13081. [Google Scholar]
- Gao F., Zhang X., Wang H., Sun X., Wang J., Wang C. Comparison of dry- and wet-heat induced changes in physicochemical properties of whey protein in absence or presence of inulin. Food Science and Biotechnology. 2019;28(5):1367–1374. doi: 10.1007/s10068-019-00577-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A., Guan X., Song H., Li S., Huang K. Encapsulation of (-)-epigallocatechin-gallate (EGCG) in hordein nanoparticles. Food Bioscience. 2020;37 doi: 10.1016/j.fbio.2020.100727. [DOI] [Google Scholar]
- He Z., Chen J., Moser S.E. Interaction of beta-lactoglobulin with (-)-epigallocatechin-3-gallate under different processing conditions of pH and temperature by the fluorescence quenching method. European Food Research and Technology. 2015;241(3):357–366. doi: 10.1007/s00217-015-2466-2. [DOI] [Google Scholar]
- Huang H., Li L., Ma Q., Feng Y.-Q., He Z.-K. Determination of melamine in milk by fluorescent spectrophotometry with cetyltrimethylammonium bromide. Chinese Journal of Analytical Chemistry. 2010;38(2):249–252. doi: 10.3724/SP.J.1096.2010.00249. [DOI] [Google Scholar]
- Liu C., Lv N., Ren G., Wu R., Wang B., Cao Z., Xie H. Explore the interaction mechanism between zein and EGCG using multi-spectroscopy and molecular dynamics simulation methods. Food Hydrocolloids. 2021;120 doi: 10.1016/j.foodhyd.2021.106906. [DOI] [Google Scholar]
- Liu Y., Liu Y., Wang S., Dong S., Chang P., Jiang Z. Structural characteristics of (-)-epigallocatechin-3-gallate inhibiting amyloid A beta 42 aggregation and remodeling amyloid fibers. RSC Advances. 2015;5(77):62402–62413. doi: 10.1039/c5ra09608a. [DOI] [Google Scholar]
- Loveday S.M. Beta-lactoglobulin heat denaturation: A critical assessment of kinetic modelling. International Dairy Journal. 2016;52:92–100. doi: 10.1016/j.idairyj.2015.08.001. [DOI] [Google Scholar]
- Ma Y., Zhang S., Feng Y., Wang H., Liu Y., Wang C. Modification of the structural and functional characteristics of mung bean globin polyphenol complexes: Exploration under heat treatment conditions. Foods. 2023;12(11):2091. doi: 10.3390/foods12112091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy N.A., Kelly A.L., O’Mahony J.A., Fenelon M.A. Sensitivity of emulsions stabilised by bovine beta-casein and lactoferrin to heat and CaCl2. Food Hydrocolloids. 2014;35:420–428. doi: 10.1016/j.foodhyd.2013.06.021. [DOI] [Google Scholar]
- Ming Y., Chen L., Khan A., Wang H., Wang C. Effects of tea polyphenols on physicochemical and antioxidative properties of whey protein coating. Food Science and Biotechnology. 2020;29(12):1655–1663. doi: 10.1007/s10068-020-00824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli M., Oliva R., Castronuovo G. Cyclodextrin-protein interaction as inhibiting factor against aggregation. Journal of Thermal analysis and Calorimetry. 2017;127(2):1491–1499. doi: 10.1007/s10973-016-5736-8. [DOI] [Google Scholar]
- Pessato T.B., de Morais F.P.R., de Carvalho N.C., Figueira A.C.M., Fernandes L.G.R., Zollner R. de L., Netto F.M. Protein structure modification and allergenic properties of whey proteins upon interaction with tea and coffee phenolic compounds. Journal of Functional Foods. 2018;51:121–129. doi: 10.1016/j.jff.2018.10.019. [DOI] [Google Scholar]
- Radibratovic M., Al-Hanish A., Minic S., Radomirovic M., Milcic M., Stanic-Vucinic D., Cirkovic Velickovic T. Stabilization of apo α-lactalbumin by binding of epigallocatechin-3-gallate: Experimental and molecular dynamics study. Food Chemistry. 2019;278:388–395. doi: 10.1016/j.foodchem.2018.11.038. [DOI] [PubMed] [Google Scholar]
- Sanmartin B., Diaz O., Rodriguez-Turienzo L., Cobos A. Functional properties of caprine whey protein concentrates obtained from clarified cheese whey. Small Ruminant Research. 2013;110(1):52–56. doi: 10.1016/j.smallrumres.2012.11.029. [DOI] [Google Scholar]
- Song Y., Yoo S.-H. Quality improvement of a rice-substituted fried noodle by utilizing the protein-polyphenol interaction between a pea protein isolate and green tea (Camellia sinensis) extract. Food Chemistry. 2017;235:181–187. doi: 10.1016/j.foodchem.2017.05.052. [DOI] [PubMed] [Google Scholar]
- Tian L., Yang K., Zhang S., Yi J., Zhu Z., Decker E.A., McClements D.J. Impact of tea polyphenols on the stability of oil-in-water emulsions coated by whey proteins. Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128448. [DOI] [PubMed] [Google Scholar]
- Xie D., Deng F., Shu J., Zhu C., Hu X., Luo S., Liu C. Impact of the frying temperature on protein structures and physico-chemical characteristics of fried surimi. International Journal of Food Science and Technology. 2022;57(7):4211–4221. doi: 10.1111/ijfs.15741. [DOI] [Google Scholar]
- Xue J., Tan C., Zhang X., Feng B., Xia S. Fabrication of epigallocatechin-3-gallate nanocarrier based on glycosylated Casein: Stability and interaction mechanism. Journal of Agricultural and Food Chemistry. 2014;62(20):4677–4684. doi: 10.1021/jf405157x. [DOI] [PubMed] [Google Scholar]
- Zhang S., Sheng Y.-N., Feng Y.-C., Diao J.-J., Wang C.-Y., Zhang D.-J. Changes in structural and functional properties of globulin-polyphenol complexes in mung beans: Exploration under different interaction ratios and heat treatment conditions. International Journal of Food Science and Technology. 2022;57(4):1920–1935. doi: 10.1111/ijfs.15180. [DOI] [Google Scholar]
- Zhang X., Wang C., Qi Z., Zhao R., Wang C., Zhang T. Pea protein based nanocarriers for lipophilic polyphenols: Spectroscopic analysis, characterization, chemical stability, antioxidant and molecular docking. Food Research International. 2022;160 doi: 10.1016/j.foodres.2022.111713. [DOI] [PubMed] [Google Scholar]
- Zhao J., Lin W., Gao J., Gong H., Mao X. Limited hydrolysis as a strategy to improve the non-covalent interaction of epigallocatechin-3-gallate (EGCG) with whey protein isolate near the isoelectric point. Food Research International. 2022;161 doi: 10.1016/j.foodres.2022.111847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.