Abstract
Background:
Prior study of patients with urgency urinary incontinence by functional magnetic resonance imaging showed altered function in areas of the brain associated with interoception and salience and with attention. Our randomized controlled trial of hypnotherapy for urgency urinary incontinence demonstrated marked improvement in urgency urinary incontinence symptoms at two months. A sub-sample of these urgency urinary incontinent women underwent functional magnetic resonance imaging before and after treatment.
Objective:
To determine if hypnotherapy treatment of urgency urinary incontinence compared to pharmacotherapy was associated with altered brain activation or resting connectivity on functional Magnetic Resonance Imaging.
Study Design:
A sub-sample of women participating in a randomized control trial comparing hypnotherapy versus pharmacotherapy for treatment of urgency urinary incontinence was evaluated with functional magnetic resonance imaging. Scans were obtained pretreatment and 8–12 weeks after treatment initiation. Brain activation during bladder-filling and resting functional connectivity with an empty and partially filled bladder were assessed. Brain regions of interest were derived from those previously showing differences between healthy controls and untreated urgency urinary incontinence participants in our prior work, and included regions in the interoceptive and salience, ventral attentional and dorsal attentional networks.
Results:
Following treatment, participants in both groups demonstrated marked improvement in incontinence episodes (p < .001). Bladder filling task functional magnetic resonance imaging data from the combined groups (n=64, 30 Hypnotherapy, 34 Pharmacotherapy) demonstrated decreased activation of the left temporoparietal junction, a component of the ventral attentional network (p<.01) compared to baseline. Resting functional connectivity differed only with the bladder partially filled (n = 54). Compared to pharmacotherapy, hypnotherapy participants manifested increased functional connectivity between the anterior cingulate cortex and the left dorsolateral prefrontal cortex, a component of the dorsal attentional network (p<.001).
Conclusions:
Successful treatment of urgency urinary incontinence with both pharmacotherapy and hypnotherapy was associated with decreased activation of the ventral (bottom up) attentional network during bladder filling. This may be attributable to decreased afferent stimuli arising from the bladder in the pharmacotherapy group. In contrast, decreased ventral attentional network activation associated with hypnotherapy may be mediated by the counterbalancing effects of the dorsal (top down) attentional network.
Keywords: brain activation and connectivity, functional MRI (fMRI), hypnotherapy, mind/body therapy, overactive bladder, urgency urinary incontinence, women
Condensation:
Pharmacotherapy and hypnotherapy improve urgency urinary incontinence to a similar extent, the improvement following hypnotherapy possibly mediated though its effect on the “top down” attentional network
Introduction
Urgency urinary incontinence (UUI), involuntary urine loss associated with urgency, affects millions of women, significantly impairs quality of life and is commonly treated with medications that have side effects limiting adherence.1,2 Altered perceptual awareness may play a role in UUI and suggests an alternative treatment approach by addressing abnormalities in brain function. Evaluation of patients with UUI using functional Magnetic Resonance Imaging (fMRI) has identified specific brain sites that may function abnormally, potentially serving as targets for “brain-centered” therapies.3
Women with UUI manifest abnormal activation in brain regions that govern interoception, the perception and interpretation of physiologic stimuli. Output from interoceptive regions is modulated by interaction with brain regions responsible for executive control, including those responsible for direction of attention.4 These brain regions likely modify urgency perception in UUI and may be important in its genesis or persistence.3
Activity in brain regions associated with interoceptive and attentional control can be evaluated using fMRI by measuring the effects of localized changes in blood flow occurring in response to recurrent prompted tasks using blood oxygen level dependent (BOLD) imaging. Changes in brain blood flow measured over time with BOLD imaging can also assess which brain regions are integrated or connected.5 Connectivity between regions is most commonly assessed in the absence of a task and is inferred when areas of the brain show temporally coherent neural activation and deactivation constituting “functional networks.” Each functional network interconnects spatially distinct areas of the brain that together implement a unique component of cognition.7 Some anatomically defined areas of the brain can be components or “nodes” of more than one network; for example, the dorsolateral prefrontal cortex (DLPFC) is a component of both the fronto-parietal and dorsal attentional networks.8 Anatomy and function of the interoceptive network and the salience network, which is responsible for detecting and filtering stimuli, overlap considerably. Relevant networks and their nodes are noted in Table 1.
Table 1 -.
Definitions
| Terminology | Abbreviation | Description Anatomic Areas and Function/Networks |
|---|---|---|
| Anterior Cingulate Cortex, dorsal Anterior Cingulate Cortex | ACC, dACC | Anterior portion of the cingulate gyrus located in the medial cerebral hemisphere, important in emotion and coordinating responses to internal and external events3 (See Figure 6) |
| Brain Oxygen Level Dependent | BOLD | fMRI sequence which detects local changes in blood oxygen levels caused by increased cerebral blood flow (hemodynamic response) at sites of increased neural activity. |
| Corona Radiata | Continuation of white matter tracts above level of the internal capsule, terminating in the cerebral cortex | |
| Default Mode Network | DMN | Brain regions with increased activity when mind is not engaged in cognitive tasks and deactivated during cognitive tasks nodes include the Ventromedial PFC and the posterior cingulate cortex.6 |
| Dorsal Attentional Network | DAN | Brain regions associated with focused attention on external stimuli. |
| Dorsolateral Prefrontal Cortex | DLPFC | Region in prefrontal cortex (PFC). A component of both the fronto-parietal control network and the dorsal attention network. 7 (See Figure 6) |
| Fronto-Parietal Control Network | Network typically activated during cognitively demanding tasks; nodes include the lateral prefrontal cortex including DLPFC, Intraparietal Sulcus (IPS), Superior Parietal Lobule (SPL).8 | |
| Mid-Cingulate Cortex | MCC | Middle portion of cingulate cortex including anterior MCC (sometimes referred to as “dorsal ACC” and posterior MCC); activated in emotion and pain processing as well as intentional motor control |
| Posterior Medial Cortex | PMC | Brain region including the precuneus and posterior cingulate; functions as the posterior node of the DMN |
| Interoceptive/Salience Network | Brain regions that integrate visceral, autonomic, emotional sensory data to assess salience. The ACC is a component of this network | |
| Temporal Parietal Junction | TPJ | Brain region at the junction of the temporal and parietal lobes; a component of the ventral attention network (See Figure 6) |
| Ventral Attentional Network | VAN | Brain regions activated in response to unexpected behavioral stimuli, or a “bottom-up” network; includes the temporoparietal junction and ventral frontal/prefrontal cortex. 11 |
| Ventrolateral Prefrontal Cortex | VLPFC | Brain region located in prefrontal cortex (PFC); a region which is part of the ventral attention network (See Figure 6) |
Pioneering fMRI evaluation of UUI identified increased activation of the interoceptive/salience network compared to controls, and suggested that this activation could be modulated by components of the fronto-parietal control network. 3,9 Our prior UUI fMRI study demonstrated increased activation in regions in the interoceptive/salience network among UUI patients, as well as differences between UUI patients and controls with respect to activation and connectivity of attentional networks.10 Abnormal function of these brain networks may contribute to UUI symptoms. Since mind-body therapies have the potential to alter function of interoceptive/salience, fronto-parietal control and attentional networks, they warrant exploration as a means of treating UUI.
Mind body therapies may alter attentional network function by their effect on the DLPFC. This anatomic site is a component of the dorsal attentional network, the network responsible for top down direction of attention.11, 12,13 Hypnosis, a mind-body therapy, is defined as: ”A state of consciousness involving focused attention and reduced peripheral awareness characterized by an enhanced capacity for response to suggestion.”14 Functional MRI of subjects under hypnosis has suggested augmented connectivity of the dorsal attentional network and interoceptive networks.15,16 Hypotherapy’s efficacy in treating UUI in our recent randomized controlled trial (RCT) provides the opportunity to identify neural correlates of therapeutic success.17
The objective of this study was to determine if improvement in UUI symptoms following hypnotherapy, a mind-body therapy, is associated with amelioration of abnormal brain function on fMRI. We hypothesized that hypnotherapy treatment of UUI would modulate neurologic interactions between the brain and bladder and that hypnotherapy participants would manifest different changes in brain activation and/or functional connectivity than participants using UUI medications. Based on our prior work evaluating pre-intervention fMRI characteristics in a subset of the participants of this trial,10 we hypothesized that post-intervention differences in activation and connectivity would involve the interoceptive/salience and attentional networks.
Materials and Methods
Study Design.
The current study represents a sub-sample of participants from our RCT comparing hypnotherapy versus pharmacotherapy for treatment of UUI (ClinicalTrials.gov. #NCT01829425) in women. The sub-sample consists of those participants who consented to undergo pre and post treatment fMRI scanning. 17 The study was approved by the University of New Mexico Institutional Review Board (HRRC #09–314, 1/24/2012 initial approval with yearly re-approval) and all participants gave written consent. Methodology for the Hypnotherapy Or Pharmacotherapy Trial has been described previously.17 Briefly, participants were recruited from an academic urogynecology clinic and the community at large between March 2013-April 2016. Pilot study results comparing overactive bladder and controls suggested effect sizes >1 for between group differences in interoceptive and salience network activation and functional connectivity. Power calculations made with the assumption of 40–60% improvement abnormal activation and connectivity following hypnotherapy suggested that 60–75 participants could achieve a power of 0.8.18
UUI participants were non-pregnant woman ≥ 18 years old who had ≥3 UUI episodes (UUIEs)/week for ≥3 months, were free of significant neurologic illness and pelvic organ prolapse beyond the hymen.19 Women who participated in this optional fMRI sub-study were identified prior to treatment randomization. Randomization was stratified by UUI severity (≤3 or ≥4 UUIEs on three-day diary). Baseline data collection included validated questionnaire results and UUIEs on diaries (Table 2). Participants underwent cystometrics to determine their individual “strong desire to void” volumes, bladder volumes eliciting a “persistent desire to pass urine without fear of leakage.”20
Table 2 –
Clinical Parameters and Between Group Comparisons
| Clinical & Questionnaire Characteristics | Hypnotherapy N= 30 | Medication N=34 | P value of Difference |
|---|---|---|---|
| Age Mean (SD) | 54(13) | 57(10) | P = 0.30 |
| UUIE1 at baseline Mean (SD) | 8 (8) | 7 (12) | P = 0.50 |
| UUIE1 at 2 month follow-up Mean (SD) | 1 (4) | 1 (3) | P = 1.00 |
| Change in UUIE1 Median (Q1, Q3) | −5 (−2,−10) | −4.5 (−3,−7) | P = 0.60 |
Results reported as means (SD), except change UUIE. UUIE change reported as median (Q1,Q3) due to skewness of data
Urgency Urinary Incontinence Episodes on 3 day diary
RCT Interventions.
Hypnotherapy:
Participants randomized to hypnotherapy received eight weekly, one-hour, one-on-one bladder-directed hypnotherapy sessions delivered by a board-certified hypnotherapist using a standardized format, also described previously.17,18 Pharmacotherapy: Participants randomized to medications received extended release oxybutynin 10 mg/day initially or extended release tolterodine 4 mg/day. Participants also received eight weekly, one-on-one medication counseling sessions delivered using a standardized format.17,19
fMRI Task and Resting Connectivity:
fMRI Scans were obtained pretreatment and 8–12 weeks after treatment initiation. In order to activate specific sites within the brain during MRI, “tasks” are performed. In this case, the fMRI task consisted of low, medium and high volume bladder infusions as previously described (See Supplement A).10 Our prior study comparing UUI participants to controls demonstrated differences between groups largely confined to high bladder fill volumes, and therefore, comparisons for the current study were confined to the high volume task.10
Resting state connectivity data collection was performed over 5 minutes, initially only with an empty bladder. After study initiation, newly published literature reported UUI vs control differences in resting functional connectivity acquired with a partially filled bladder.21 Accordingly, the study protocol was amended to include a second resting data collection performed with the bladder filled to half the previously determined “strong desire to void volume” for that particular patient.
The imaging acquisition for our fMRI data has been described previously.10 Multiple Regions of Interest (ROIs) were derived from our prior work, identified as showing significant activation differences between control and UUI participants. These included the dorsal Anterior Cingulate Cortex (dACC), Mid-cingulate Cortex (MCC), bilateral Ventrolateral Prefrontal Cortex (VLPFC), bilateral Temporoparietal Junction (TPJ) and Posteromedial Cortex (PMC) (See Table 1).
Statistical Analysis.
During the bladder filling task functional activation values within the above ROIs were acquired for participants pre- and post-treatment and Analysis of Variance (ANOVAs) were performed to evaluate differences in functional activation across groups (hypnotherapy vs pharmacotherapy) and time (pre-treatment vs. post-treatment). Treatment Group, Time and the Treatment Group × Time interactions were the effects of interest in this statistical framework.
The same ROIs were used as seed points for resting state functional connectivity analysis examining pre- to post-treatment group differences. Correlations were calculated between the average time-course of activation in these regions and voxel-wise time-course of activation throughout the brain. ROIs and brain sites showing increased connectivity were assigned to corresponding brain networks based on previously reported Talairach spatial coordinates or Brodmann areas. Whole brain resting state functional connectivity results were corrected for false positives at p < 0.05 based on 10,000 Monte Carlo simulations (p < 0.001; minimum cluster size = 1,024 microliters).
Between group differences in baseline values for ROI activation or ROI functional connectivity were evaluated by t-tests. If paired t-tests suggested significant baseline differences between groups, the pre-post treatment differences were further analyzed with ANCOVA utilizing baseline values as a co-variate.
Clinical differences between groups were also assessed. Between group differences in questionnaire and baseline UUIEs were compared using t-tests. Because our prior work showed that change in UUIEs two months post-treatment was not normally distributed, pre-post treatment changes in UUIEs difference between groups were compared using Mann Whitney U.
Results
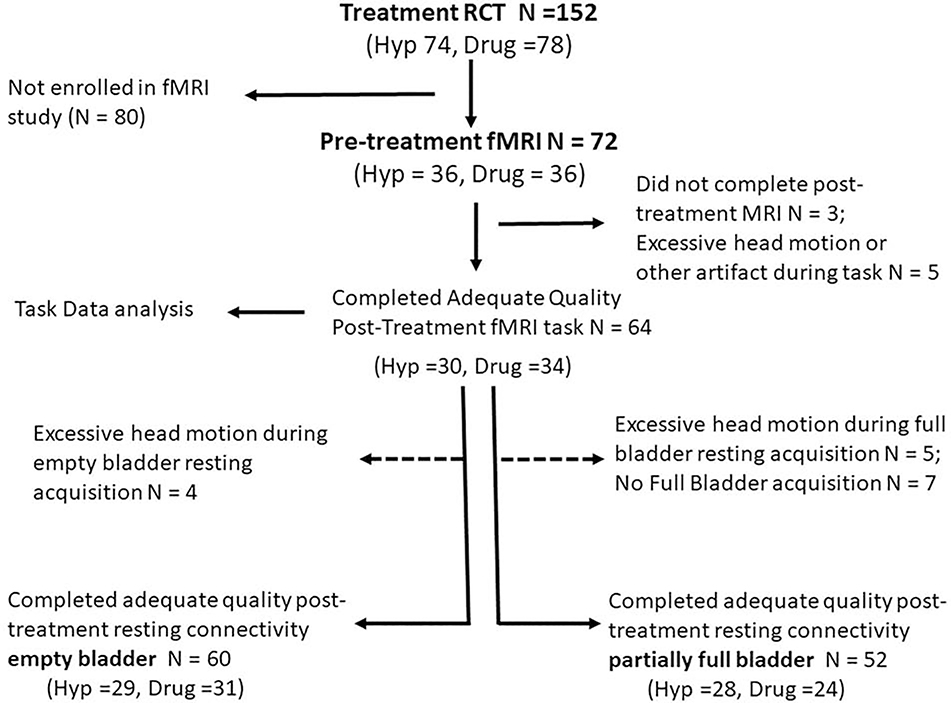
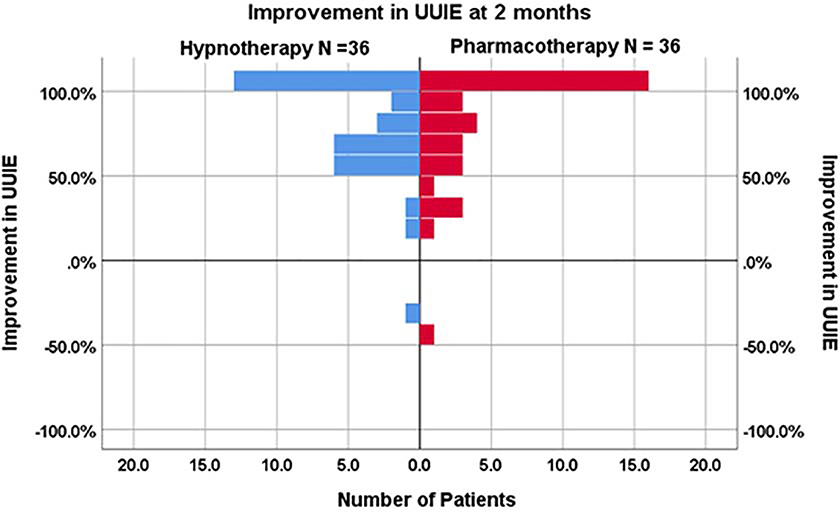
Seventy-two participants, 36 treated with hypnotherapy and 36 with pharmacotherapy, enrolled in this imaging study; three did not complete post treatment scanning and five were excluded secondary to technical problems with fMRI acquisition or due to excessive head motion (defined as 3 times the inter-quartile range relative to their cohort based on frame-wise displacement, during the bladder filling task).24 Figure 1 displays fMRI data inclusion/exclusion. Task data from 64 participants, empty bladder resting connectivity data from 60 participants, and partially filled bladder resting connectivity data from 52 participants were analyzed. Groups did not differ in participant baseline questionnaire scores or in UUIEs (Table 2). Following treatment, both hypnotherapy and the medication groups demonstrated marked improvement in UUIEs (both p <.001) and the degree of improvement did not differ between groups (p=0.60; Figure 2).
Figure 1 – Consort Diagram.
Diagram displaying inclusion/exclusion of Hypnotherapy and Medication treated participants in activation and connectivity analysis
Figure 2 – Improvement in urgency urinary incontinence.
Percentage improvement in UUI episodes at two months for the subset of RCT participants undergoing fMRI, comparing Hypnotherapy (N=36) and Pharmacotherapy (N =36) groups. Between group difference was not significant
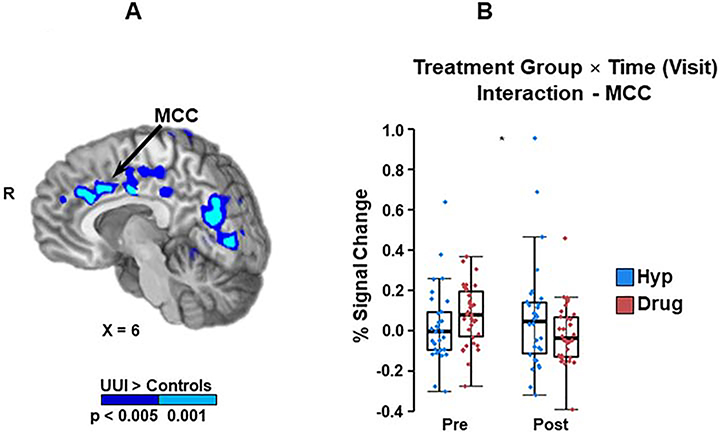
Mixed-measures ANOVA of task-based activation demonstrated that a single Treatment Group × Time interaction approached significance; the MCC trended towards increased activation in hypnotherapy relative to pharmacotherapy (p = 0.05; Figure 3). In contrast, results showed a definitive main effect of time (initial visit vs. follow up visit) for the left TPJ, with activation diminishing in the combined hypnotherapy and pharmacotherapy groups following treatment (p = 0.01; Figure 4). The left VLPFC and the right TPJ showed a similar trend, but did not reach statistical significance (p = 0.06 and 0.07, respectively).
Figure 3-. Middle Cingulate Cortex (MCC) region of interest (ROI) and Treatment Group x Time Interaction.
A: Midline cortical sites showing increased task activation in UUI participants compared to controls in a previous publication (10). Midline cortical sites showing increased task activation in UUI participants compared to controls at the baseline on pre-intervention fMRIs. Of these potential areas, only the MCC (at arrow tip) demonstrated a trend separating intervention groups over time, pre-intervention compared to post-intervention. (Treatment Group x Time interaction)
B: Treatment Group × Time interaction: Post treatment the MCC demonstrated a trend towards increased activation in the hypnotherapy compared to the pharmacotherapy group (p = .05). This trend was not present after controlling for baseline activation in the two treatment groups.
Figure 4 -. Ventral attentional network regions of interest (ROIs) and demonstration of post treatment deactivation.
A:) Cortical sites showing increased task activation in UUI participants compared to controls at the baseline, pre-intervention fMRIs (10). The ventral attentional network sites (TPJ and VLPFC) are denoted by the arrows.
B: Main Effect of Time (Visit): The left TPJ demonstrated a statistically significant decrease in activation in all participants (i.e. combined hypnotherapy and medication participants)(p=0.01). Other ventral attentional network sites, right TPJ and left VLPFC demonstrated a similar trend, but change did not reach statistical significance.
Pre to post-treatment functional connectivity from the left VLPFC to the right superior and posterior corona radiata (1565 μl) decreased in both groups, but no change in VLPFC connectivity to cortical sites was observed. Pre-treatment to post-treatment connectivity between the dACC and DLPFC increased in the hypnotherapy group compared to pharmacotherapy (p < .001) (Figure 5). Baseline connectivity between the dACC and the DLPFC was lower in the hypnotherapy group (p=.02), but when baseline connectivity was entered as a covariate (using Analysis of Covariance), the between group difference in connectivity remained significant (p < .01)
Figure 5-. Effect of therapy on functional connectivity to the dorsal attentional network.
A: Resting functional connectivity analysis performed with the bladder filled to half “strong desire to void volume”. Pre-post treatment change in connectivity between the ACC and the dorsal attentional network (DLPFC) differed between groups (p < .001).
B: Treatment Group × Time interaction. Connectivity increased in participants in the Hypnotherapy treatment group compared to the pharmacotherapy group following treatment (p < .001. This difference persisted when results were controlled for differences in baseline connectivity (p <.01).
Comment
Principal Findings:
Participants treated with hypnotherapy and those treated with pharmacotherapy did not differ in post-treatment change in brain activation related to a bladder-filling task. Treatment with either therapy resulted in decreased activation of the left TPJ during bladder filling. Other components of the ventral attentional network also showed similar trends. In contrast, post-treatment resting connectivity performed with a partially filled bladder demonstrated increased connectivity between the dACC (interoceptive/salience network) and the DLPC (dorsal attentional network) only in participants receiving hypnotherapy.
Results:
This study demonstrated changes in attentional network activation and connectivity in response to UUI treatment. Specifically, among women treated with either hypnotherapy or medication, decreased activation of the TPJ was observed. The ventral attention network has been described as “bottom up” attentional network that responds to unexpected or infrequent behaviorally relevant stimuli.”22 This network demonstrated abnormal activation in UUI participants relative to controls in our prior work.10 The observed decrease in ventral attention activation following treatment represents amelioration of this abnormality. Decrease in ventral network activation with bladder filling was associated with marked decrease in UUIEs in both groups post-treatment.
The mechanisms by which pharmacotherapy and hypnotherapy affect “bottom up” attention likely differ. In the pharmacotherapy group, decreased ventral attentional activation may be mediated by suppression of afferent stimuli due to anti-muscarinic effects.23 In contrast, we speculate that the decreased activation of ventral attention network sites following hypnotherapy more likely results from opposing input from the “top down” dorsal attentional network connectivity. The dorsal attentional network could modulate input received by the ventral attentional network via its increased connectivity with the interoceptive/salience network (Figure 6). This kind of interaction between the ventral and dorsal attentional networks via connectivity to an intermediary brain network has been previously demonstrated. For instance, the cognitive ability to suppress distraction may depend upon the connectivity between the ventral and dorsal attention networks and the default mode network (see Table 1) rather than on connectivity to each other.24 In addition the ventral and dorsal networks may interact with each other more directly. Prior work has demonstrated that the dorsal attentional network (DLPFC) may filter information available to the ventral attentional network via its connections to the TPJ.11,25
Figure 6 – Components of attentional and the interoceptive and salience networks.
Diagram of anatomic relationships of selected components of the ventral attentional (VLPFC), (TPJ), dorsal attentional (DLPFC) and interoceptive/salience networks (ACC). Locations on the surface of the brain are denoted by solid blue. Midline structures (e.g. ACC) appear in red cross-hatched pattern. Dotted blue line represents the augmented connectivity between the dorsal attentional and interoceptive/salience network associated with hypnotherapy. VLPFC – ventral lateral prefrontal cortex, TPJ -temporoparietal junction, DLPFC – dorsal lateral prefrontal cortex, ACC- anterior cingulate cortex
In our prior study of baseline (pre-treatment) differences between UUI and controls, women with UUI demonstrated increased connectivity between the MCC (interoceptive/salience network) and DLPFC.10 We speculated that this connectivity could represent an (ineffective) attempt by “top-down” dorsal attentional control to repress UUI symptoms. Our current finding suggests that hypnotherapy may further augment “top down” attentional control such that it becomes sufficient to suppress UUI symptoms. Recently the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network investigators suggested that similar changes in attention allocation are important to the clinical response to urologic chronic pelvic pain syndrome. Investigators observed an association between increased resting connectivity of the left frontal parietal cortex (which includes the left DLPFC) and remission of symptoms. 26
Substantial evidence supports DLPFC function as an important component of hypnotherapy. Interactions between the DLPFC and the interoceptive/salience network (particularly the ACC), underlie the enhanced somatic and emotional control seen in the hypnotic state.27 Connectivity between the interoceptive/salience network and the DLPFC appears to be important in pain modulation during hypnosis, and pre-hypnosis connectivity may predict the strength of analgesic response to hypnotic suggestion.28
Clinical Implications:
The data presented here suggest that hypnotherapy, a mind-body therapy, can augment DLPFC to interoception/salience network connectivity that persists outside of the hypnotic state. Similar changes have been documented with meditation, a related mind body therapy. Meditation is characterized as “full attention to internal and external experiences as they occur in the present moment”.29 Training in meditation is associated with durable augmentation of connectivity within several regions including the interoceptive/salience network (ACC) and components of the ventral and dorsal attentional networks.29,30
This changed connectivity in hypnotherapy treated participants is to our knowledge, the first demonstration of durable effects of hypnotherapy on cognition. Most studies of neural function related to hypnotherapy have focused on changes in brain activation during the hypnotic state. In contrast, this study reports a therapeutic alteration of brain connectivity associated with hypnosis that persists long after cessation of the hypnotic state. These findings suggest that further evaluation of resting connectivity is warranted in patients with UUIE treated with mind body therapies.
Research implications:
Our findings suggest that evaluation of UUI treatment with mind body therapies might focus on changes in functional connectivity, particularly those affecting the attentional networks. Results from our parent clinical trial demonstrated that the efficacy of hypnotherapy in treating UUI compared to pharmacotherapy increased over time, improving at 6 and 12 months.17 It is possible that continued brain remodeling could yield further changes in connectivity in participants re-imaged at later time-points. Alternatively, changes in connectivity among hypnotherapy participants could remain stable despite further decrease in UUIE, suggesting that brain remodeling presages clinical improvement. Future evaluations of mind body related changes resting connectivity among UUIE might be designed with longer-term follow up MRI evaluations.
Potential interactions between pharmacotherapy and hypnotherapy also remains unexplored. Since the mechanism by which hypnotherapy and pharmacotherapy affect the ventral attentional network differ, the therapies may be synergistic. Alternatively, once anticholinergic treatment has diminished bladder afferent stimulation that activates the ventral attentional network, the “top down” attentional network could be ineffective in further suppressing activation.
Limitations and Strengths:
Despite marked improvement in UUI, our study did not demonstrate the decreased bladder filling task- related activation of the interoceptive/salience network reported in other studies following treatment with pelvic floor muscle training and sacral neuromodulation.31 This could be attributable to modest differences in the bladder filling technique used in our study, as we used a manual fluid infusion in our study versus the use of an infusion pumps in other protocols. Recent work has also demonstrated limited test-retest reproducibility observed with the bladder-filling tasks, and this may have degraded our ability to demonstrate pre-post treatment differences in brain activation in response to this stimulus.32
With regards to connectivity, our findings are limited to demonstrating that among hypnotherapy patients, increased dorsal attentional to interoceptive/salience network connectivity is associated with decreased ventral network activation and associated improvement in UUI. The findings do not prove this relationship is causal. As noted above, neither does this study explore the alteration of attentional network activation and connectivity in affecting longer term (> 2 month) improvement in UUI symptoms.
Last, meaningful analysis of the relationship between the extent of clinical response and changes in brain activation and connectivity was limited by the widespread clinical improvement noted in both treatment groups, particularly at 2 months following treatment. In light of the marked clinical response of the majority of participants in both groups, analysis of “responders” vs. “non-responders” would result in non-responder sample sizes that were prohibitively small for fMRI sub-analysis.
This study does present additional data from a randomized controlled trial of UUI treatment methods with a much larger cohort than most previous reports. Additionally, between group differences in functional connectivity following UUI therapy have not been widely reported. Our findings suggest that that evaluation of treatment effects using resting functional connectivity with a partially filled bladder, may be an avenue for assessing UUI treatment effects on the brain. This could avoid both methodological problems of bladder filling task at high volumes and patient discomfort attendant with that procedure.
Conclusions:
UUI improvement is associated with decreased activation of a component of the ventral attentional network during bladder filling. Pharmacotherapy mediated improvement in UUI symptoms may diminish of “bottom up” attentional activation via its effect on the bladder. Conversely, following hypnotherapy, bottom up attention may suppressed by interactions with the dorsal (top down) attentional network. In that framework, pharmacotherapy mediated decrease in UUI symptoms would drive changes in attentional networks. Conversely, hypnotherapy mediated changes in attentional networks would drive the decrease in UUI symptoms
Supplementary Material
AJOG at a glance.
Why was the study conducted? The specific aim was to determine if treatment of urgency urinary incontinence with hypnotherapy compared to pharmacotherapy would be associated with changes in brain networks which direct attention.
What are the key findings? After three months of treatment both therapies markedly improved symptoms and were associated with decreased activation of the ventral “bottom up” attentional network during bladder filling. Only hypnotherapy was associated with increased connectivity of the dorsal “top down” attentional network.
What does this study add to what is already known? Hypnotherapy, a mind body therapy, may decrease “bottom up“ attentional activation associated with bladder filling without pharmacologic alteration of bladder function. This may be mediated by changes in function of “top down” attention.
Acknowledgments
Financial Support/Funding Source & Sponsor’s Role:
National Center for Complementary & Integrative Health, National Institutes of Health, Award Number R01AT007171
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors report the following potential conflicts of interest:
Drs. Ketai, Komesu, Rogers, Sapien, Schrader & Mayer received support from above NIH grant. The authors below have the additional disclosures:
Yuko Komesu MD: Other NIH grants. Site PI for CookMyosite® CELLEBRATE trial
Rebecca Rogers MD: Other NIH grants, UpToDate royalties, ABOG & ACOG travel & stipend, International Urogynecologic Association travel and stipend and editorship.
Robert Sapien MD: International Board of Hypnotherapy President, Global Hypnotherapy Advancement Foundation, Sapien Wellness LLC, It’s Mental LLC.
Andrew Mayer PhD: Other NIH grants
Footnotes
Parent Study registered with ClinicalTrials.gov; https://clinicaltrials.gov ID#: NCT01829425
Study Conducted in : Albuquerque New Mexico
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodynam. 2010;29:4–20. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in regional managed care plan. J Manag Care Pharm. 2008;14(3):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths D, Clarkson B, Tadic SD, Resnick NM. Brain Mechanisms Underlying Urge Incontinence and its Response to Pelvic Floor Muscle Training. J Urol. 2015; 194(3):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farb NA, Segal ZV, Anderson KA. Attentional Modulation of Primary Interoceptive and Exteroceptive Cortices. Cerebral Cortex January 2013;23:114–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, et al. Resting-State Functional MRI: Everything That Non-experts Have Always Wanted to Know. January 18, 2018. as 10.3174/ajnr.A5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raichle ME. The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc London B Biol Sci. 2015; 370:1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brosnan MB, Wiegand. The Dorsolateral Prefrontal Cortex, a Dynamic Cortical Area to Enhance Top-Down Attentional Control. J. Neurosci, March 29, 2017. • 37(13):3445–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marek S The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci. 2018. Jun; 20(2): 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urology. 2005; 174:1862–67. [DOI] [PubMed] [Google Scholar]
- 10.Ketai LH, Komesu YM, Dodd AB, Rogers R, Ling J, Mayer AR, Urgency urinary incontinence and the interoceptive network: a functional MRI study. Am J Obstet Gynecol. 2016. October; 215(4): 449.e1–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vossel S, Geng J, Fink G. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014; 20(2):150–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taren AT MD, Gianaros PJ, Greco CM, Lindsay EK, Fairgrieve A, Brown KW, Mindfulness Meditation Training and Executive Control Network Resting State Functional Connectivity: A Randomized Controlled Trial Center for Psychosom Med. 2017; 79(6): 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen NKF. Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond. Evidence-Based Complementary and Alternative Medicine. 2012. 2012:680407. doi: 10.1155/2012/680407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins GR, Barabasz AF, Council JR, Spiegel D. Advancing Research and Practice: The Revised APA Division 30 Definition of Hypnosis, International Journal of Clinical and Experimental Hypnosis, 2015. 63:1, 1–9 [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, White MP, Greicius MD, Waelde LC, Spiegel D. Brain Activity and Functional Connectivity Associated with Hypnosis. Cerebral Cortex, August 2017;27: 4083–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeft F, Gabrieli JD, ,Whitfield-Gabrieli S, Haas BW, Roland Bammer R, Menonn V, Spiegel, Functional Brain Basis of Hypnotizability. Arch Gen Psychiatry. 2012. October; 69(10): 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komesu YM, Schrader RM,2 Rebecca RG, Sapien RE, Mayer AR,4 Ketai LH. Hypnotherapy or Medications: A Randomized Non-Inferiority Trial in Urgency Urinary Incontinent Women. Am J Obstet Gynecol. 2020. Feb;222(2):159.e1–159.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komesu YM, Ketai LH, Mayer AR, Teshiba TM, Rogers RG. Functional MRI of the Brain in Women with Overactive Bladder: Brain Activation During Urinary Urgency. Female Pelvic Med Reconstr Surg. 2011;17(1):50–54. doi: 10.1097/SPV.0b013e3182065507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komesu YM, Rogers RG, Sapien RE, Schrader RM, Simmerman-Sierra T, Ketai LH. Methodology for a Trial of Brain-Centered versus Anti-cholinergic Therapy for Women with Urgency Urinary Incontinence. Int Urogynecol J. 2017;28(6):865874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: The OAB-q. Quality of Life Research. 2002;11(6):563–74. [DOI] [PubMed] [Google Scholar]
- 21.Nardos R, Karstens L, Carpenter S2, Aykes K, Krisky C, Stevens C, Gregory WT, Fair DA. Abnormal functional connectivity in women with urgency urinary incontinence: Can we predict disease presence and severity in individual women using Rs-fcMRI. Neurourol Urodyn. 2016. Jun;35(5):564–73. [DOI] [PubMed] [Google Scholar]
- 22.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008; 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel MC. Therapeutic Modulation of Urinary Bladder Function: Multiple Targets at Multiple Levels Rev. Pharmacol. Toxicol 2015. 55:269–87 [DOI] [PubMed] [Google Scholar]
- 24.Poole VN, Robinson ME, Singleton O, DeGutis J, Milberg WP, McGlinchey RE, et al. Intrinsic functional connectivity predicts individual differences in distractibility. Neuropsychologia. 2016. Jun;86:176–82 [DOI] [PubMed] [Google Scholar]
- 25.Igelström KM, Graziano MSA. The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia. 2017. Oct;105:70–83 [DOI] [PubMed] [Google Scholar]
- 26.Kutch JJ, Labus JS, nbHarris RE, MartuccI KT,Farmer MA, Fenske S et al. , Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: A MAPP Network Study. Pain. 2017. June; 158(6): 1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landry M, Lifshitz M, Raz A. Brain correlates of hypnosis: A systematic review and metaanalytic exploration. Neurosci Biobehav Rev. 2017. Oct;81(Pt A):75–98 [DOI] [PubMed] [Google Scholar]
- 28.Huber A, Lui F, Porro CA. Hypnotic susceptibility modulates brain activity related to experimental placebo analgesia. Pain. 154 (2013) 1509–1518 [DOI] [PubMed] [Google Scholar]
- 29.Gotink RA, Meijboom R, Vernooij MW, Smits M, Hunink MGM. 8-week Mindfulness Based Stress Reduction induces brain changes similar to traditional long-term meditation practice – A systematic review Brain and Cognition 108 (2016) 32–4132 [DOI] [PubMed] [Google Scholar]
- 30.Kwak S, Lee TY, Jung WH, Hur JW, Bae D1, Hwang WJ, Cho KIK et al.The Immediate and Sustained Positive Effects of Meditation on Resilience Are Mediated by Changes in the Resting Brain. Front Hum Neurosci. 2019. Mar 26;13:101. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissbart SJ, Bhavsar R, Rao H, Wein AJ, Detre JA, Arya LA, Smith AL. Specific Changes in Brain Activity during Urgency in Women with Overactive Bladder after Successful Sacral Neuromodulation: A Functional Magnetic Resonance Imaging Study..J Urol. 2018. Aug;200(2):382–388. [DOI] [PubMed] [Google Scholar]
- 32.Clarkson BD, Tyagi S, Griffiths DJ, Resnick NM, Test-retest repeatability of patterns of brain activation provoked by bladder filling Neurourol Urodyn. 2017. August; 36(6): 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.