Abstract
As part of a search for transcriptional regulatory genes, sequence analysis of several previously unsequenced gaps in the cephamycin biosynthetic cluster has revealed the presence in Streptomyces clavuligerus of seven genes not previously described. These include genes encoding an apparent penicillin binding protein and a transport or efflux protein, as well as the CmcI and CmcJ proteins, which catalyze late reactions in the cephamycin biosynthetic pathway. In addition, we discovered a gene, designated pcd, which displays significant homology to genes encoding semialdehyde dehydrogenases and may represent the gene encoding the long-sought-after dehydrogenase involved in the conversion of lysine to α-aminoadipate. Finally, two genes, sclU and rhsA, with no obvious function in cephamycin biosynthesis may define the end of the cluster. The previously described CcaR protein displays homology to a number of Streptomyces pathway-specific transcriptional activators. The ccaR gene was shown to be essential for the biosynthesis of cephamycin, clavulanic acid, and non-clavulanic acid clavams. Complementation of a deletion mutant lacking ccaR and the adjacent orf11 and blp genes showed that only ccaR was essential for the biosynthesis of cephamycin, clavulanic acid, and clavams and that mutations in orf11 or blp had no discernible effects. The lack of cephamycin production in ccaR mutants was directly attributable to the absence of biosynthetic enzymes responsible for the early and middle steps of the cephamycin biosynthetic pathway. Complementation of the ccaR deletion mutant resulted in the return of these biosynthetic enzymes and the restoration of cephamycin production.
Streptomyces species are well known for their possession of gene clusters which orchestrate antibiotic biosynthesis. These clusters consist of resistance, transport, and regulatory genes physically linked and coordinately regulated with genes encoding biosynthetic enzymes (11). Streptomyces clavuligerus produces a number of β-lactam compounds, including cephamycin C, clavulanic acid, and several structurally related clavams which differ from clavulanic acid in the stereochemistry of the clavam nucleus and nature of the substituent groups. The genes responsible for cephamycin biosynthesis in S. clavuligerus are clustered and may be flanked by the genes encoding the Bla (43) and PcbR (40) resistance proteins. The genes encoding three of the earliest enzymes in the biosynthetic pathway, lysine ɛ-aminotransferase (LAT), δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine synthetase (ACVS), and isopenicillin N synthase (IPNS), designated lat, pcbAB, and pcbC (42), are ordered sequentially in the gene cluster, and their transcriptional organization has been determined (45, 46). The lat promoter is thought to direct the synthesis of a polycistronic transcript of ∼14 kb responsible for lat, pcbAB, and pcbC expression. As well, a promoter located within the 3′ end of the pcbAB coding region was shown to be responsible for the production of a monocistronic pcbC transcript.
The LAT protein catalyzes the first of a two-step reaction converting lysine to α-aminoadipate (29), while the second step has only recently been characterized. The product of LAT activity, 1-piperideine-6-carboxylate, requires the activity of a piperideine-6-carboxylate dehydrogenase enzyme to be converted into α-aminoadipate (14), and yet no candidate genes have been discovered in any bacterial cephamycin clusters studied to date (42). The ACVS enzyme catalyzes the condensation of the three precursor amino acids, valine, cysteine, and α-aminoadipate, into δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine (ACV), which in turn undergoes an oxidative cyclization by the IPNS enzyme (42).
The cefD and cefE genes, located adjacent to one another about 10 kb upstream of the lat pcbAB pcbC operon, encode the enzymes isopenicillin N epimerase (IPNE) and desacetoxycephalosporin C synthase (DAOCS), which function in the middle part of the pathway (42). IPNE converts isopenicillin N to penicillin N, and DAOCS then catalyzes a further conversion to desacetoxycephalosporin C (42). The cefD and cefE genes are also organized into an operon which gives rise to a polycistronic transcript together with other, as yet uncharacterized genes (31). Genes encoding enzymes which function later in the cephamycin pathway (cefF [30]) and (cmcH [13]) have also been located within the cephamycin cluster.
The genes responsible for clavulanic acid biosynthesis are located directly adjacent to the cephamycin biosynthetic cluster in S. clavuligerus (55), and a large portion of the cluster has been sequenced (25). It is unclear whether the cas1 gene, encoding one of a pair of isozymes of clavaminate synthase (36), is part of a third group of biosynthetic genes responsible for the biosynthesis of non-clavulanic acid clavam compounds or is just the result of an apparent gene duplication event. Sequence analysis, both upstream and downstream of cas1, failed to demonstrate any additional homologs to genes within the cephamycin-clavulanic acid supercluster (56), and cas1 is apparently not linked to the supercluster since it is separated from cas2 by more than 40 kb (37).
Recently, Walters and coworkers (54) described the sequence analysis of a complementing fragment of DNA which restored clavulanic acid and cephamycin C production to nonproducing mutants. The gene was designated dclX (for decreased clavulanic acid) and was believed to encode a transcriptional activator because of its similarity to a number of pathway-specific transcriptional activators from various Streptomyces spp. The existence of a species-specific transcriptional activator affecting cephamycin production would be consistent with previous results which showed that the lat promoter displayed very strong activity in S. clavuligerus but 155-fold-weaker activity in S. lividans (45). Pérez-Llarena et al. (44) subsequently provided conclusive evidence that this gene was essential for the biosynthesis of both cephamycin and clavulanic acid and renamed it ccaR, for cephamycin and clavulanic acid regulator. In addition to ccaR, biosynthesis of clavulanic acid is also specifically regulated by claR, encoding a LysR-type transcriptional activator, located within the clavulanic acid gene cluster (39). A functional claR is essential for expression of the biosynthetic late genes required for the conversion of the intermediate clavaminic acid to clavulanic acid.
When the deduced amino acid sequence of CcaR was aligned with the homologous transcriptional activators (RedD, ActIIORF4 (open reading frame 4), AfsR, and DnrI), many regions of similarity were found to be present throughout the primary sequence. The ccaR gene also contains a rare leucine-encoding TTA codon often found in the N-terminal end of many of these activators (11) and demonstrated to be involved in the hierarchy of regulation for antibiotic biosynthesis and sporulation in S. coelicolor (33). Since the ccaR designation more accurately describes the involvement of this gene in the regulation of both cephamycin and clavulanic acid biosynthesis, we have chosen to adopt this terminology for subsequent studies. The sequence analysis of two genes (designated orf11 and blp) located downstream of ccaR has also been described (44). The orf11 gene did not display any significant similarity to known sequences, while orf12 was renamed blp, for β-lactamase-inhibitory protein (BLIP)-like protein because of its similarity to the bli gene previously described for S. clavuligerus (16). This terminology will also be used in the present study.
Insertional inactivation of ccaR has been shown to eliminate cephamycin and clavulanic acid biosynthesis in S. clavuligerus (44). We now show that biosynthesis of the clavams is also eliminated, and in the case of cephamycin biosynthesis, the ccaR mutation results in a failure to produce biosynthetic pathway enzymes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. S. clavuligerus was maintained on MYM agar (51) or ISP Medium 3 (Difco, Detroit, Mich.). Plasmid-containing cultures were supplemented with thiostrepton (5 μg/ml; Sigma Chemical Corp., St. Louis, Mo.), apramycin (25 μg/ml; Provel Inc., Scarborough, Ontario, Canada) or hygromycin (200 μg/ml; Boehringer Mannheim Biochemicals, Laval, Quebec, Canada) as appropriate. S. clavuligerus strains were stored as spore stocks in 20% glycerol at −70°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant features | Source or referencea |
|---|---|---|
| Bacterial strains | ||
| S. clavuligerus | ||
| NRRL 3585 | Wild type | NRRL |
| Δcob::tsrA/B | ccaR orf11 blp deletion mutant, Tsrr | This study |
| ΔccaR::tsrA/B | ccaR deletion mutant, Tsrr | This study |
| ccaR::aprA/B | ccaR disruption mutant, Aprr | This study |
| E. coli HB101 | General cloning host strain | 48 |
| Plasmid vectors | ||
| Cloning vectors | ||
| pSL1180 | E. coli general cloning vector, Ampr | Pharmacia |
| pBluescript KS/SK | E. coli general cloning vector, Ampr | Stratagene |
| pTZ18R | E. coli general cloning vector, Ampr | U.S. Biochemicals |
| pUC119 | E. coli general cloning vector, Ampr | 48 |
| pJOE829 | Streptomyces pIJ101 replicon, Hygr | J. Altenbuchner |
| pSET152 | E. coli replicon, Streptomyces ØC31 attachment site, Aprr | NRRL |
| pIJ486 | Streptomyces pIJ101 replicon, Tsrr | D. Hopwood |
| pMAL-c2 | E. coli MBP fusion expression vector, Ampr | New England Biolabs |
| Cephamycin cluster-containing cosmids | ||
| pOW309 | Cosmid containing some of the cephamycin cluster, Aprr | P. Skatrud |
| pLAFR/2-49 | Cosmid containing some of the cephamycin cluster, Tetr | 27 |
| Gene-targeting vectors | ||
| pDA517 | apr cassette cloned into pBluescript KS | This study |
| pDA551/552 | Shuttle vector carrying ccaR::apr construct | This study |
| pDA557/558 | Shuttle vector carrying ΔccaR::tsr construct | This study |
| pDA559/560 | Shuttle vector carrying Δcob::tsr construct | This study |
| E. coli high-level-expression constructs | ||
| pMAL-CcaR | ccaR ORF inserted into pMAL-c2 | This study |
| pMAL-LAT | lat ORF inserted into pMAL-c2 | This study |
| Complementation constructs | ||
| pDA1000 | tsr marker inserted into pSET152 | This study |
| pDA1001 | Wild-type cob fragment inserted into pSET152 | This study |
| pDA1002 | ccaR mutant cob fragment inserted into pSET152 | This study |
| pDA1003 | orf11 mutant cob fragment inserted into pSET152 | This study |
| pDA1004 | blp mutant cob fragment inserted into pSET152 | This study |
| pDA1006 | Wild-type ccaR fragment inserted into pSET152 | This study |
| pDA1007 | Mutant (Eco47III) ccaR fragment inserted into pSET152 | This study |
| pDA1008 | Mutant (EcoICRI) ccaR fragment inserted into pSET152 | This study |
NRRL, National Regional Research Laboratory. Some plasmids were generously provided by J. Altenbuchner, University of Stuttgart, Germany; D. Hopwood, John Innes Institute, Norwich, United Kingdom; and P. Skatrud, Eli Lilly, Indianapolis, Ind.
Cultures for measurement of cephamycin production and/or production of cell extracts for Western analysis were prepared by inoculating spores of S. clavuligerus into Trypticase soy broth (Becton Dickinson and Company, Cockeysville, Md.) supplemented with 1% (wt/vol) soluble starch (TSBS) and incubating them on a rotary shaker for 30 to 36 h. Production cultures were prepared by subculturing seed cultures into fresh TSBS and incubating them for 48 to 72 h. All cultures of S. clavuligerus were incubated at 28°C and 250 or 280 rpm.
Production cultures for measurement of clavulanic acid or clavams were prepared as described above except that seed cultures were washed twice with sterile water before being subcultured into fresh TSBS, starch-asparagine (SA) medium (1), or soy medium (48) and incubated for 72 h.
Escherichia coli cultures were maintained on 2YT agar and grown in 2YT or Terrific Broth (49) at 37°C. Plasmid containing cultures were supplemented with ampicillin (100 μg/ml; Sigma), tetracycline (12.5 μg/ml; Sigma), apramycin (100 μg/ml), or hygromycin (50 μg/ml) as appropriate.
Transformation of S. clavuligerus.
Protoplast formation, transformation, and selection of transformants were carried out as described by Hopwood et al. (26) and Bailey and Winstanley (6).
Recombinant DNA techniques.
Restriction enzyme digestion was carried out according to the manufacturers’ recommendations. Purification of DNA fragments from agarose gel blocks was done with the GlassMax DNA isolation system (GIBCO BRL, Burlington, Ontario, Canada). Ligation reactions, generation of blunt ends on DNA fragments by using Klenow or T4 DNA polymerase, plasmid isolation, PCR, dephosphorylation of DNA with alkaline phosphatase, random primer labeling, and E. coli transformations were all done as described by Sambrook et al. (49). Plasmid cloning vectors, oligonucleotide primers, and plasmid constructs used for sequence analysis, gene disruption and replacement, complementation, or high-level expression studies are listed in Tables 1 and 2. More detailed explanations of plasmid constructions or intermediate plasmids are available upon request.
TABLE 2.
Oligonucleotide primers used in this study
| Oligonucleotide primer | Sequencea | Function |
|---|---|---|
| Dyl9 | GCCGGATCCATGGGCGAAGCAGCACGCCACCCC | 5′ lat primer |
| Dyl10 | GCCGAATTCGCGTCAGACGCTCTCGGCGACCGG | 3′ lat primer |
| Dyl12 | GCCGAATTCGCGGTTTCAGGCCGGGGTACGAC | 3′ ccaR primer |
| Dyl13 | GCCGGATCCATGAACACCTGGAATGATGTGACG | 5′ ccaR primer |
| Dyl19 | CTAGACTAGTCTAGCATG | Creation of stop codons |
Restriction enzyme recognition sites or the positions of stop codons within each oligonucleotide primer are underlined.
Sequence analysis of S. clavuligerus cephamycin cluster.
DNA fragments from the cephamycin cluster-containing cosmids, pOW309 and pLAFR/2-49, were subcloned into pBluescript KS(+), pBluescript SK(+), or pTZ18R, and exonuclease III-mediated deletions were generated with an Erase-a-Base kit (Promega Corp., Madison, Wis.). The resulting deletion clones were sequenced on both strands via ABI PRISM dye terminator cycle sequencing by the University of Alberta Department of Biological Sciences DNA synthesis lab, using a combination of universal and custom primers. No regions of the cluster which had already been sequenced by other groups were intentionally resequenced. Duplicated sequence was generated only when it was necessary to cross junctions of known and unknown regions or where sequences were published after completion of this sequencing project.
High-level production of CcaR and LAT in E. coli.
To obtain large amounts of CcaR and LAT for preparation of polyclonal antibodies, each protein was overproduced in E. coli. Large amounts of purified IPNS were available from a previous study (18). Overproduction of both LAT and CcaR was achieved by fusion of the coding regions of each gene in frame with the gene encoding the maltose binding protein (MBP) in the vector pMAL-c2. These fusion clones were created by using PCR to introduce artificial EcoRI and BamHI sites at the 3′ and 5′ ends, respectively, of each gene. For ccaR, the primers Dyl12 and Dyl13 and Vent DNA polymerase (New England Biolabs, Mississauga, Ontario, Canada) were used. For lat, the primers Dyl9 and Dyl10 and Taq DNA polymerase (Boehringer Mannheim) were used.
The amplified PCR fragments were inserted into pBluescript KS(+) and then subjected to sequence analysis. The lat clone contained a number of Taq-induced mutations; therefore, the entire ORF was replaced with wild-type DNA, leaving only a small amount of PCR-generated upstream sequence containing the desired BamHI site. Amplification of the ccaR clone occurred without the introduction of any unintended mutations. The ccaR and lat genes were then excised as BamHI/HindIII fragments and inserted into pMAL-c2 to create pMAL-CcaR and pMAL-LAT.
Cultures of E. coli HB101 carrying pMAL-CcaR and pMAL-LAT were grown in 2YT at 37°C to an optical density at 600 nm of about 0.5, induced by the addition of isopropyl-β-d-thiogalactopyranoside (0.5 mM, final concentration), and then incubated at 30°C for 4 h. The MBP fusion proteins were purified from induced cultures as directed by the manufacturer.
Preparation of polyclonal antibodies to CcaR, LAT, and IPNS.
Purified MBP fusion proteins and purified IPNS protein, approximately 150 μg of each, were run on separate sodium dodecyl sulfate (SDS)–7.5 or 10% polyacrylamide gels (9), stained for 30 min with 0.1% (wt/vol) Coomassie blue, and then destained in water for 10 min. Protein bands were excised from the gels and homogenized in phosphate-buffered saline (49), and the gel slurries were used to immunize rabbits by standard procedures (23).
Creation of ccaR and cob mutants. (i) ccaR::apr mutant construct.
A 3.7-kb EcoRI/BamHI fragment carrying the ccaR, orf11, and blp (cob) group of genes was inserted into pTZ18R. The blunt-ended apramycin resistance (apr) cassette (see below) was inserted at the unique EcoICRI site within the ccaR gene.
(ii) ΔccaR::tsr mutant construct.
The thiostrepton resistance (tsr) marker from pIJ486 was inserted as a 1.1-kb BclI fragment into pSL1180. A 2.8-kb NcoI/BamHI fragment of S. clavuligerus DNA containing the cefF-cmcH pair of genes was inserted upstream of tsr. A 2.3-kb NruI/EcoRI fragment containing the orf11-blp pair of genes was inserted downstream of tsr. The resulting construct carried a contiguous stretch of S. clavuligerus DNA extending from cefF to blp except that the 1.4-kb BamHI/NruI fragment containing ccaR had been replaced with tsr.
(iii) Δcob::tsr mutant construct.
By using the same strategy, a 2.8-kb NcoI/BamHI fragment containing the cefF-cmcH pair of genes was inserted upstream of tsr and a 1.7-kb EcoRI/ApaI fragment containing the lat gene was inserted downstream of tsr, all within pSL1180. The resulting construct carried a contiguous stretch of S. clavuligerus DNA extending from cefF to lat except that the 3.7-kb BamHI/EcoRI fragment containing the cob group of genes had been replaced with tsr.
All of the targeting vectors described above were prepared as parallel sets of constructs in which the antibiotic resistance marker was inserted in both orientations relative to the gene(s) being disrupted or deleted. In all cases where fragments with incompatible ends were to be ligated, the fragments were first made blunt by using Klenow or T4 DNA polymerase. The various disruption and deletion constructs are shown in Fig. 1. All of the targeting vectors described above were finally converted into E. coli-Streptomyces shuttle vectors by fusion with the Streptomyces replicon, pJOE829, at the unique HindIII site. The resulting gene targeting vectors were then introduced into S. clavuligerus by transformation. Disruption or deletion mutants defective in ccaR or the cob group of genes resulting from gene conversion by homologous recombination were isolated as described by Aidoo et al. (2). Disruption mutants defective in ccaR displayed an Aprr hygromycin-sensitive (Hygs) phenotype, while deletion mutants defective in ccaR or the cob group of genes displayed a Tsrr Hygs phenotype.
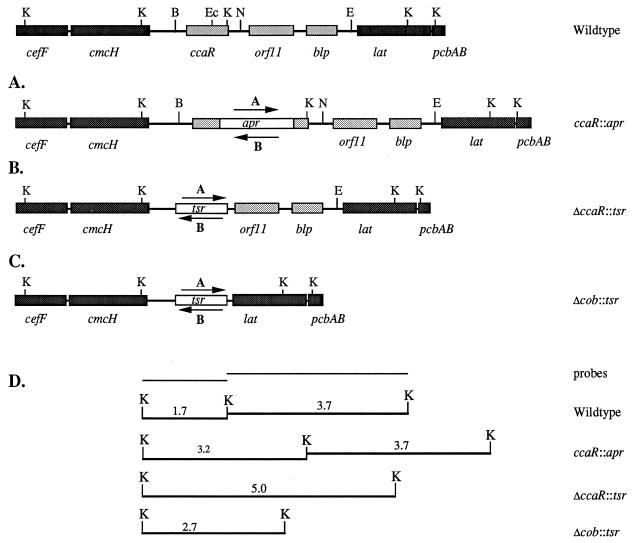
FIG. 1.
Strategy for disruption or deletion of the ccaR gene or deletion of the cob group of genes by gene replacement. The lightly shaded boxes represent the target gene(s), the darker boxes represent other ORFs within the cephamycin cluster, and the clear box represents the antibiotic resistance marker. The antibiotic resistance markers were introduced in both orientations (A and B). (A) Insertion of the apr cassette into the EcoICRI site of the ccaR gene; (B) deletion of the internal BamHI/NruI fragment containing the ccaR gene and replacement with the tsr marker; (C) deletion of the internal BamHI/EcoRI fragment containing the ccaR, orf11, and blp genes and replacement with the tsr marker; (D) Southern hybridization pattern of KpnI-digested genomic DNA from the wild type and ccaR::apr, ΔccaR::tsr, and Δcob::tsr mutants, using the 1.7- and 3.7-kb KpnI fragments as hybridization probes. Abbreviations for restriction sites: B, BamHI; E, EcoRI; Ec, EcoICRI; K, KpnI; N, NruI.
Creation of complementation constructs. (i) The apr cassette.
The apr marker from pOW309 was introduced into pBluescript KS(+), and then the translational stop fragment adapter oligonucleotide Dyl19 was inserted immediately upstream of apr to give pDA517. The apr marker together with adapter oligonucleotide (apr cassette) could then be released from pDA517 as a unit by HindIII/SmaI digestion, made blunt by treatment with Klenow DNA polymerase, and inserted into any cloned gene where a nonsense mutation was desired. Once the apr cassette had been introduced into a gene with selection for Aprr, the apr marker could be removed as a PstI fragment and the remaining plasmid could be self-ligated to leave only the 37-bp translational stop fragment (TSF) which contains stop codons in all three reading frames.
(ii) Mutation of the ccaR, orf11, or blp gene.
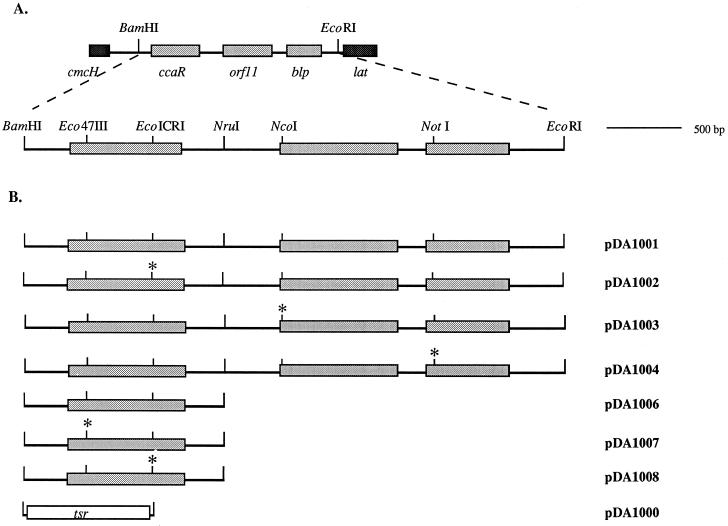
A plasmid construct carrying the cob group of genes was digested individually with each of the following enzymes which recognizes a unique site: Eco47III (near the 5′ end of ccaR), EcoICRI (near the 3′ end of ccaR), NcoI (near the 5′ end of orf11), and NotI (near the 5′ end of blp). The blunt-ended apr cassette was inserted into these sites, which were also made blunt ended if necessary. After confirmation that the desired orientation of the apr cassette had been obtained, the apr marker was removed by PstI digestion and religation, leaving behind the 37-bp TSF.
(iii) pSET152 constructs.
The 3.7-kb EcoRI/BamHI fragment of wild-type S. clavuligerus DNA which carries the cob group of genes (pDA1001) and the corresponding fragments from the mutant constructs described above—ccaR mutant (TSF in EcoICRI; pDA1002), orf11 mutant (TSF in NcoI; pDA1003), and blp mutant (TSF in NotI; pDA1004)—were inserted into the integrating vector, pSET152 (see Fig. 5). As well, a 1.4-kb BamHI/NruI fragment of wild-type DNA which carries only ccaR (pDA1006) and the corresponding fragments from the mutant constructs described above—ccaR mutant (TSF in Eco47III near the 5′ end; pDA1007) and ccaR mutant (TSF in EcoICRI near the 3′ end; pDA1008)—were inserted into pSET152. The tsr marker was also inserted into pSET152 to act as a negative control (pDA1000).
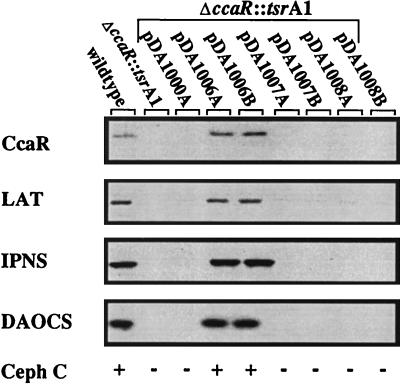
FIG. 5.
Complementation of the ΔccaR::tsrA1 mutant with pSET152 constructs. Western blot analysis and bioassay for production of cephamycin C were used to assess complementation. The designations A and B represent two independent clones generated during the same transformation. Five micrograms of cell extract protein from each strain, harvested after 48 h of growth, was separated by SDS-PAGE (10% gel) and transferred to PVDF membranes. The resulting Western blots were developed with polyclonal antibodies specific for the CcaR, LAT, IPNS, and DAOCS proteins. Each strain’s ability to produce cephamycin (Ceph) C was determined by bioassay, and the results were scored as + (production) and − (lack of production).
Southern hybridization analysis.
Genomic DNA for Southern analysis was purified as described by Hopwood et al. (26). Confirmation of gene conversion by homologous recombination was obtained by Southern analysis of KpnI-digested genomic DNA, using standard methods.
Heterologous probing of genomic DNA from a variety of β-lactam-producing and nonproducing actinomycetes was done with NcoI-digested genomic DNA separated by agarose gel electrophoresis, transferred to nylon membranes, and probed by standard techniques (49). Random primer-labeled probes were hybridized for 16 h followed by washing with 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])–0.1% SDS for 20 min and 1× SSPE–0.1% SDS for 20 min, all at 55°C.
Antibiotic quantitation and cell extract preparation.
Cultures of S. clavuligerus growing in SA, soy, or TSBS medium were sampled at 48 or 72 h. Culture filtrates from each sample were stored at −20°C and assayed within 1 week for cephamycin production (by bioassay [28]) and for clavulanic acid and clavam production (by high-pressure liquid chromatography [41]).
When cell extracts were to be analyzed, the cultures were harvested from TSBS cultures by centrifugation, washed with 0.85% (wt/vol) NaCl, and then resuspended in 1/5 original volume of TDE buffer (50 mM Tris-HCl [pH 7.0], 0.1 mM dithiothreitol, 0.01 mM EDTA). Washed cells were disrupted by sonication (two 20-s pulses; microprobe, power setting 2; Branson Sonifier 450; Branson Ultrasonic Corp., Danbury, Conn.) and then centrifuged for 5 min. Cell extracts were assayed for protein content (10) and stored at −20°C.
Western blot analysis.
Five- or ten-microgram amounts of cell extract protein were separated on duplicate SDS–10% polyacrylamide gels. The gels were transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore, Bedford, Mass.), using a Bio-Rad Transblot apparatus (Richmond, Calif.). The membranes were blocked in 5% (wt/vol) skim milk in Tris-buffered saline containing 0.1% (vol/vol) Tween 20 (TBST) for 16 h at 4°C. Proteins were detected by using the ECL (enhanced chemiluminescence) Western system reagents and protocols (Amersham, Chicago, Ill.). The primary antibodies were used at the following dilutions: CcaR, 1:5,000; LAT, 1:7,500; IPNS, 1:10,000; and DAOCS, 1:10,000. DAOCS antibodies were generously provided by C. Reeves, Panlabs Inc. Goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Amersham) was used as the secondary antibody.
Nucleotide sequence accession number.
The sequence information obtained for gaps B, C, and D has been deposited under GenBank accession no. AF073895, AF073896, and AF073897, respectively; sequence information for flanking region A has been deposited under accession no. AF073894. Each deposited sequence includes six nucleotides of sequence overlapping with the previously published sequence.
RESULTS
Sequence analysis of gaps in the cephamycin biosynthetic cluster.
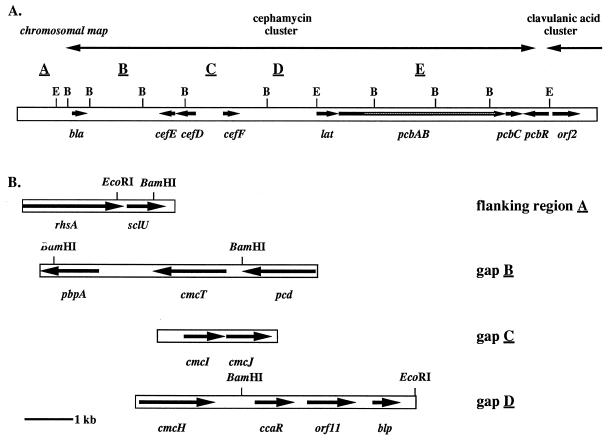
Many of the structural genes encoding biosynthetic enzymes from the early and middle parts of the cephamycin C pathway of S. clavuligerus have been sequenced; however, several unsequenced gaps in the gene cluster remain. A rough restriction map of the cluster (Fig. 2A), indicating locations of the BamHI and EcoRI restriction sites and positions of the genes for which sequence information was available at the onset of this study, was created by compilation of the restriction maps generated in other studies (35, 50, 54). Four of these unsequenced gaps (B through E) exist between regions of known sequence, while the other (flanking region A) lies adjacent to the previously sequenced bla gene and may represent a region beyond the end of the cephamycin cluster. The sizes of gaps B, C, and D were estimated to be 5.9, 2.3, and 6.0 kb, respectively. The gap E region represents about 8 kb from the middle of the pcbAB gene. In an attempt to identify a gene encoding a transcriptional activator which could be responsible for species-specific activation of the lat promoter (45), the remaining gaps of the cephamycin biosynthetic cluster were sequenced. Flanking region A was also sequenced to determine whether the genes present in this region appeared to be involved in cephamycin biosynthesis or whether bla represented the end of the cephamycin cluster.
FIG. 2.
Physical map of the cephamycin biosynthetic gene cluster in S. clavuligerus. (A) The chromosomal restriction map was created by compiling the restriction maps created in other studies (35, 50, 54). E and B, EcoRI and BamHI restriction sites; solid arrows, genes previously sequenced; shaded bar, region of the pcbAB gene which has not been sequenced. Gaps within or regions flanking the known sequence of the cephamycin cluster are noted with underlined letters. (B) Diagrammatic representation of the genes present within the gaps or region flanking the cephamycin biosynthetic gene cluster as determined by sequence analysis. Arrows indicate the direction of transcription for each gene, positions of the EcoRI and BamHI restriction sites within each fragment are shown.
Cosmid pOW309 (47) (generously provided by P. Skatrud, Eli Lilly Ltd.) was isolated from an S. clavuligerus DNA library constructed in the vector pKC462a when screened with a pcbAB-specific probe. Cosmid pLAFR/2-49 was isolated from an S. clavuligerus pLAFR3 cosmid library when screened with a cefE-specific probe (27). Cosmid pOW309 was mapped by using EcoRI and BamHI, and the insert contained within this cosmid provided the template to complete the sequence of gaps located within the cephamycin cluster.
The BamHI fragments were subcloned from pOW309 into pBluescript KS(+) or SK(+) vector and used to generate exonuclease III deletion subclones for sequencing. The EcoRI/BamHI fragment was sequenced to obtain information on flanking region A. To obtain more DNA sequence information from this flanking region, digests of cosmid pLAFR/2-49 were hybridized with the EcoRI/BamHI fragment, and a KpnI/BamHI fragment which provided a contiguous stretch of DNA extending downstream from the end of the pOW309 cosmid DNA was identified.
A combination of exonuclease III deletion subclones, custom primers, and additional deletion subclones created by using unique restriction enzymes was used to sequence the cluster fragments. Sequence across the junctions between subclones was confirmed by using custom primers or by sequencing overlapping subclones. All templates were sequenced on both strands by using cycle sequencing methodologies.
The sequence of the pcbAB gene from S. clavuligerus has been only partially determined at the 5′ (53, 57) and 3′ (17) ends, but since the unsequenced internal region of the gene (gap E) was judged to be unlikely to contain regulatory genes, it was left unsequenced. Regions of the cephamycin cluster for which published sequence information was available at the outset of this project were not sequenced again except to provide overlap with newly sequenced regions. Shortly after this project had been initiated, unpublished nucleotide sequence information for ccaR (dclX [54]) was generously provided by B. Barton, SmithKline Beecham, and so this region was not resequenced. Despite the fact that ccaR clearly resembled genes encoding transcriptional regulators, we persisted with the sequence analysis of unsequenced regions to ensure that all regulatory genes had been identified.
After the sequences of gaps B, C, and D and flanking region A had been completed, the sequences of the cob, group of genes (nucleotide accession no. Z81324) were reported by Pérez-Llarena et al. (44), and so these regions of sequence within gap D were inadvertently duplicated. All duplicated sequence information was checked for agreement with the corresponding published sequence. Differences noted between the newly determined sequence and the published sequences are summarized in Table 3. It is not clear whether these discrepancies are due to allelic differences or sequencing errors, but careful reexamination of our raw sequence data and those of Walters et al. (54) failed to show any regions of ambiguity (7). Therefore, these changes may represent genuine differences between the two separate wild-type strains (NRRL 3585 and ATCC 27064) despite their recent common origin. Interestingly, all of these differences are located in the intergenic regions around the cob group of genes. Sequence information for a very small segment of the cmcH gene from S. clavuligerus was published previously by Coque et al. (13) but was not deposited in the databases, and so the entire region was resequenced.
TABLE 3.
Differences between newly determined sequence and published sequence
| Sequence location in gap Da | Gene | Sequence comparison
|
|
|---|---|---|---|
| Publishedb | This work | ||
| 2278–2280 | ccaR upstream | cc | ccc |
| 2356–2358 | ccaR upstream | cc | ccc |
| 2450–2451 | ccaR upstream | a | aa |
| 3502–3503 | orf11 upstream | c | cc |
| 3513 | orf11 upstream | gg | g |
| 3643–3645 | orf11 upstream | ccg | cgc |
| 3729–3730 | orf11 upstream | gg | gc |
| 5754–5756 | blp downstream | cc | ccc |
| 5761–5764 | blp downstream | ggg | gggg |
| 5790–5791 | blp downstream | g | gg |
| 5803–5804 | blp downstream | c | cc |
A diagrammatic representation of the genes present within the gaps of the cephamycin cluster was generated from the compiled sequence containing both previously published and newly sequenced regions (Fig. 2B). The total amount of compiled sequence spanning from the rhsA gene to the 5′ end of the pcbAB gene was 26,035 nucleotides; when the unsequenced region of pcbAB and the sequences of the pcbC and pcbR genes were included, it was estimated that the entire cephamycin cluster would be approximately 38 kb. Nucleotide sequence accession numbers for previously sequenced genes used in this study are as follows: bla, Z54190 (43); cefD, M32324 (31); cefE, M24140 (31); cefF, M63809 (30); lat and the 5′ end of pcbAB, M64834 (53, 57). Each new gene was given a designation based on homology of its putative protein to known proteins from other species (see below). The organization of the genes was determined by using a combination of the CODONPREFERENCE program (15) from the Genetics Computer Group Wisconsin Package, which identifies ORFs on the basis of the characteristic third-position G+C bias typical of Streptomyces, as well as DNA Strider 1.2 and BLASTX and BLASTP searches of the databases at the National Center for Biotechnology Information (4, 5).
Analysis of ORFs in the newly sequenced regions.
As a result of the sequence analysis and database homology searches of gaps B and C and flanking region A, we identified seven ORFs which have not been described previously for S. clavuligerus.
The PbpA protein shows weak homology to a number of low-molecular-weight penicillin binding proteins (PBPs). Although the Nocardia lactamdurans cephamycin gene cluster (12) also contains a pbp gene, that sequence was not identified as homologous in the database search. The Nocardia Pbp shows greater homology to d-Ala-d-Ala carboxypeptidases and β-lactamases. The PbpA protein in S. clavuligerus may function as an autoresistance mechanism with a role similar to that already demonstrated for PcbR, another PBP-type protein involved in resistance of S. clavuligerus to β-lactam antibiotics (40).
The CmcT protein shows homology to efflux pump proteins and therefore may function as a transporter for export of cephamycin from the cell.
The PCD protein shows strong homology to semialdehyde dehydrogenases. The location of pcd within the cephamycin cluster suggests that the PCD protein may function as a semialdehyde dehydrogenase in cephamycin biosynthesis. This dehydrogenase activity may be required to convert 1-piperideine-6-carboxylate to α-aminoadipate, the second of two steps required for the conversion of lysine into α-aminoadipate.
The CmcI and CmcJ proteins show significant homology only to the corresponding proteins from N. lactamdurans and are presumed to represent the cephalosporin-7-α-hydroxylase and methyltransferase activities responsible for the conversion of O-carbamoyl-desacetylcephalosporin C (OCDAC) to cephamycin C via 7-α-hydroxy-OCDAC (21).
Sequence information from flanking region A indicated the presence of two ORFs. The SclU (for S. clavuligerus unknown) protein, encoded by the first of these genes, does not shown significant homology to any known proteins, and therefore no presumed function can be ascribed. The sequence of the second ORF is apparently incomplete, but when analyzed by gapped BLASTP databases searches, a number of rhs genes from E. coli were identified as similar. The function of the putative Rhs proteins from E. coli is not completely understood, but the proteins contain a core ORF resembling a cell surface ligand binding protein and also contain a peptide motif that is repeated 28 times (24). The hybrid genes are also known to be recombination hot spots, for which they are named.
Neither the sclU nor the rhsA gene of S. clavuligerus has any apparent relationship to cephamycin biosynthesis, but since the function of each is unknown, neither gene can be eliminated as part of the cluster without further investigation.
Presence of homologous sequences in other β-lactam-producing actinomycetes.
Genomic DNA preparations from a number of actinomycetes were subjected to Southern analysis using moderate-stringency conditions to look for evidence of cross-hybridization with various fragments of S. clavuligerus DNA from the cephamycin gene cluster. The ccaR, orf11, blp, and pcd genes were of particular interest because they had no apparent counterparts in the cephamycin gene cluster from N. lactamdurans. The ccaR probe was the same fragment as that generated by PCR for use in high-level expression, the orf11 probe comprised two consecutive SmaI fragments extending from 130 bp downstream of the start codon to 80 bp upstream of the stop codon, and the blp probe was a NotI/PvuI fragment extending from 40 bp downstream of the start codon to 170 bp downstream of the stop codon. The pcd probe was a KpnI/BamHI fragment extending from 48 bp downstream of the start codon to 27 bp downstream of the stop codon. The β-lactam-producing actinomycetes examined for evidence of hybridization were S. clavuligerus, S. cattleya, S. griseus, S. jumonjinensis, S. lipmanii, and N. lactamdurans. A variety of non-β-lactam-producing actinomycetes were also included: S. antibioticus, S. fradiae, S. griseofuscus, S. lividans, and S. venezuelae. Both the ccaR probe and the pcd probe hybridized to all of the β-lactam producers tested except N. lactamdurans (Table 4) and did not hybridize to any of the non-β-lactam producers. The orf11 probe hybridized only to S. clavuligerus, S. jumonjinensis, and S. lipmanii among the β-lactam producers and to none of the nonproducers, while the blp probe hybridized only to S. clavuligerus DNA. A lat probe (NcoI/BamHI fragment extending from the lat start codon to 166 bp downstream of the lat stop codon), acting as a positive control for β-lactam producers, showed hybridization with all β-lactam-producing species, including N. lactamdurans, and was absent from all nonproducers.
TABLE 4.
Presence of cross-hybridizing DNA in other β-lactam-producing actinomycetes
| Actinomycetea | β-Lactam antibiotic(s) | Extent of hybridizationb
|
||||
|---|---|---|---|---|---|---|
| lat | ccaR | orf11 | blp | pcd | ||
| S. clavuligerus | Cephamycin C, clavams, clavulanic acid | +++ | +++ | +++ | +++ | +++ |
| S. cattleya | Cephamycin C | ++ | + | − | − | ++ |
| S. griseus | Cephamycins A and B | ++ | + | − | − | ++ |
| S. jumonjinesis | Cephamycin C, clavulanic acid | + | + | + | − | + |
| S. lipmanii | 7-Methoxycephalosporin C, clavaminic acid | ++ | + | ++ | − | +++ |
| N. lactamdurans | Cephamycin C | ++ | − | − | − | − |
A combination of β-lactam-producing actinomycetes (S. clavuligerus NRRL 3585, S. cattleya NRRL 3841, S. griseus NRRL 3851, S. jumonjinensis NRRL 5741, S. lipmanii NRRL 3584, and N. lactamdurans NRRL 3802) was used.
Qualitative score relative to the hybridization to the S. clavuligerus DNA, indicated as strong hybridization (+++), moderate hybridization (++), weak or very weak hybridization (+), and no hybridization (−).
The presence of ccaR homologs in all β-lactam producers except N. lactamdurans suggests that a common mechanism for the regulation of β-lactam biosynthesis exists in the β-lactam-producing Streptomyces spp. and that it proceeds through a CcaR-like regulator. The fact that N. lactamdurans is excluded from this group suggests that it must rely on a different regulator.
Disruption or deletion of the ccaR gene eliminates antibiotic production.
The presence of a pathway-specific regulatory gene, ccaR (also named dclX), controlling antibiotic production in S. clavuligerus has recently been reported by two separate groups (44, 54). We have also investigated this regulatory gene in order to better understand its mechanism of regulation and the role, if any, of the two uncharacterized ORFs (orf11 and blp, downstream of ccaR) in the regulation of β-lactam metabolite production in S. clavuligerus.
To investigate the roles of ccaR, orf11, and blp in antibiotic production in S. clavuligerus, we prepared a series of targeting vectors and used them to create mutants with disruptions or deletions in these genes. Plasmids pDA559 and pDA560 carry a stretch of S. clavuligerus DNA from the cephamycin gene cluster in which a 3.7-kb BamHI/EcoRI fragment containing the cob group of genes has been replaced with the tsr marker in both orientations. The plasmid constructs were introduced into protoplasts of wild-type S. clavuligerus by transformation, and then transformants were allowed to sporulate in the absence of antibiotic selection to promote the loss of free plasmid. Mutants in which the cob genes had been deleted due to homologous recombination between the plasmid construct and the corresponding region of the chromosome (Δcob::tsr) were detected initially by their Tsrr Hygs phenotype and then confirmed by Southern analysis. Genomic DNA from wild-type S. clavuligerus, when digested with KpnI, gave (i) a 1.7-kb fragment containing most of ccaR and part of cmcH and (ii) a 3.7-kb fragment containing orf11, blp, and part of lat (Fig. 1D). When these DNA fragments were labeled and used as probes, the KpnI digests of genomic DNA from presumptive Δcob::tsr mutants showed a single 2.7-kb hybridizing KpnI fragment, consistent with the replacement of the cob genes with a tsr marker.
Four independently created Δcob::tsr mutants (two mutants for each orientation of tsr) were grown on TSBS for 48 and 72 h, and then culture supernatants were assayed for cephamycin production by bioassay. All of the Δcob::tsr mutants showed no detectable production of cephamycin, whereas the wild-type culture gave large zones of inhibition. The cephamycin-negative phenotype occurred regardless of the orientation of the tsr marker. Previous studies (3) had indicated that transcription can proceed through the tsr marker when it is oriented in the same direction as transcription. Therefore, the antibiotic-negative phenotype was presumably due to the loss of the cob genes and not to a polar effect on downstream genes.
In a related series of experiments, disruption mutants were prepared in which only the ccaR gene was affected. Using plasmids pDA551 and pDA552, we created disruption mutants (ccaR::apr) in which the wild-type ccaR gene was replaced with a mutant copy disrupted by insertion of the apr cassette into the unique EcoICRI site. Similarly, using plasmids pDA557 and pDA558, we created deletion mutants (ΔccaR::tsr) in which the wild-type ccaR gene was replaced with a tsr marker. As described earlier, each of these mutants was isolated based on its antibiotic resistance phenotype and then confirmed by Southern hybridization. The ccaR::apr mutants gave 3.2- and 3.7-kb hybridizing KpnI fragments, while the ΔccaR::tsr mutants gave a single 5.0-kb KpnI fragment. Both of these results were consistent with the insertion of the apr cassette and deletion of the ccaR gene, respectively.
When these various ccaR and cob mutants were subsequently tested for cephamycin, clavulanic acid, and clavam production, all were found to be negative for all of these metabolites regardless of the orientation of the antibiotic resistance marker.
Complementation with ccaR restores β-lactam metabolite production.
Since complete loss of β-lactam metabolite production was seen in all ΔccaR::tsr and ccaR::apr mutants as well as in Δcob::tsr mutants, any possible effects of mutations in orf11 or blp on metabolite production were obscured by the overriding effect of ccaR. To test the function of each gene independently, we prepared three separate pSET152-based plasmid constructs carrying the cob group of genes in which one of the three genes was mutated by insertion of the TSF carrying stop codons in all three reading frames. Plasmid pSET152 integrates site specifically into the Streptomyces chromosome, using the attP site and integrase present on the plasmid (8), and therefore is present at single copy.
The Δcob::tsr deletion mutant was transformed with the various pSET152 plasmid constructs containing wild-type or mutant ccaR, orf11, or blp genes (Fig. 3). Genomic DNA from the transformants was analyzed to confirm that the complementing plasmid had integrated into the chromosome; then transformants were grown in TSBS, soy, and SA media, and culture supernatants were analyzed for cephamycin, clavulanic acid, and clavam metabolites. The pDA1001 construct carrying the three cob genes in wild-type form was capable of complementing Δcob::tsr to restore production of cephamycin, clavulanic acid, and clavams. Mutation to either orf11 or blp (pDA1003 or pDA1004, respectively) had no discernible effect on production of any of the metabolites, providing that an intact ccaR gene was present. Metabolite production was also restored to ΔccaR::tsr mutants when a functional ccaR gene (pDA1006) alone was added. Since the mutations were designed to interrupt translation without affecting transcription, the effects observed could be unambiguously attributed to the mutated genes.
FIG. 3.
The cob group of genes and pSET152 complementation constructs created with these genes. (A) Restriction map of the region of DNA containing the cob genes and unique restriction sites present within this region; (B) diagram of wild-type and mutant constructs created in pSET152 for the complementation experiments using the ΔccaR::tsr or Δcob::tsr deletion mutant strains. Asterisks represent locations of stop codon-containing TSFs.
CcaR is essential for synthesis of the cephamycin C biosynthetic enzymes.
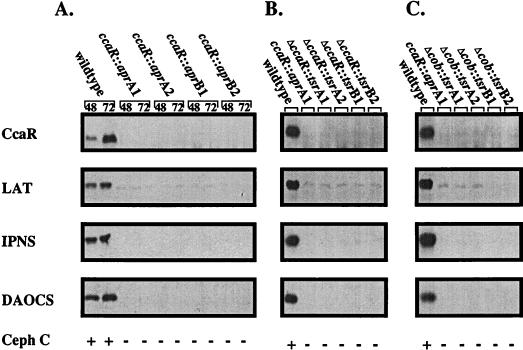
Further studies into the CcaR-dependent regulation of β-lactam metabolite production concentrated on cephamycin biosynthesis since the biochemistry and genetics of this pathway are quite well understood (42), whereas the clavulanic acid and clavam biosynthetic pathways are only beginning to be elucidated (19, 25, 36, 41). Western analyses of cell extracts prepared from ccaR mutants were carried out to determine if expression of any of the genes encoding biosynthetic enzymes was affected in these mutants. Equivalent amounts of cell extract protein prepared from the various mutants were separated by SDS-PAGE, blotted onto PVDF membranes, and then probed with antisera specific for CcaR, LAT, IPNS, and DAOCS. As shown in Fig. 4, all four of these proteins were absent in all of the mutant strains, in contrast to the parental wild-type S. clavuligerus strain. The ccaR mutants also do not produce the ACVS protein, as determined by inspection of SDS-polyacrylamide gels, nor do they have detectable activities for the ACVS, IPNS, IPNE, and DAOCS biosynthetic enzymes (data not shown).
FIG. 4.
Presence of the CcaR protein and cephamycin C biosynthetic enzymes in ccaR mutant strains. (A) Wild-type and ccaR::apr insertion strains; (B) wild-type and ΔccaR::tsr deletion strains; (C) wild-type and Δcob::tsr deletion strains. Ten micrograms of cell extract protein from each strain, harvested after 72 h of growth unless otherwise noted, was separated by SDS-PAGE (10% gel) and transferred to PVDF membranes. The resulting Western blots were developed with polyclonal antibodies specific for the CcaR, LAT, IPNS, and DAOCS proteins. Each strain’s ability to produce cephamycin (Ceph) C was determined by bioassay, and the results were scored as + (production) and − (lack of production).
Complementation by ccaR restores production of cephamycin C biosynthetic enzymes.
Introduction of plasmid pDA1006 into either the Δcob::tsr or the ΔccaR::tsr mutants resulted in integration of the ccaR gene in trans and at single copy into the chromosome, and this was sufficient to restore antibiotic production. Figure 5 shows that complementation resulted in restoration of the production not only of CcaR but also of three biosynthetic enzymes required for cephamycin production. Cephamycin production was, however, restored only to 89% of wild-type levels. Insertion of the TSF into the Eco47III or the EcoICRI site of the ccaR gene used in complementation studies (Fig. 3) would be expected to create truncated CcaR proteins, 38 and 191 amino acids in length, respectively, instead of forming a full-length CcaR protein of 256 amino acids. Since both of these constructs (pDA1007 and pDA1008, respectively) were unable to complement a ccaR mutant, it was concluded that full-length CcaR gene products were needed before any complementation could occur. Western analysis did not show any evidence of these truncated forms of CcaR (Fig. 5, lanes 7 and 8), and therefore instability of the truncated products may prevent complementation.
DISCUSSION
Nucleotide sequence information has been obtained for several previously unsequenced regions of the cephamycin C biosynthetic gene cluster of S. clavuligerus; with this new information, the sequence of the cluster is complete except for an internal region of the pcbAB gene. Some uncertainty also remains as to whether sclU and/or rhsA represent one end of the cluster, but database searches do not suggest any identifiable roles for their putative proteins in cephamycin biosynthesis. New genes identified as a result of these sequence analyses appear to encode proteins responsible for resistance, transport, biosynthesis, and regulation functions in cephamycin biosynthesis. The pbpA gene likely acts as a resistance gene which along with bla (43) and pcbR (40) serves to protect the cell from the potentially lethal effects of the metabolites that it produces. Resistance genes also flank the cephamycin cluster in N. lactamdurans (12), but in S. clavuligerus the gene organization differs because pcbR has no counterpart in N. lactamdurans.
The cmcT gene could also be considered a resistance mechanism since genes encoding efflux proteins can confer resistance when cloned at high copy number, but its primary function more likely involves transporting cephamycin from the cell. The cmcT gene along with pcd and possibly pbpA may be transcribed as a polycistronic message together with the cefD and cefE genes, from a promoter located upstream of cefD. Kovacevic et al. (31) reported a cefD/cefE hybridizing mRNA of approximately 10 kb, which would be large enough to included all five of the genes within one transcript. Both the bla gene downstream of pbpA and the cmcI gene upstream of cefD are oriented in the opposite direction to the group of five genes and so cannot be part of the multicistronic transcript.
The pcd gene represents one of the more interesting genes present in the newly described sequences. The production of α-aminoadipate for cephamycin biosynthesis is a two-step process. The first step is catalyzed by LAT, which removes the ɛ amino group from lysine to create the intermediate 1-piperideine-6-carboxylate (cyclized form of aminoadipic semialdehyde). The second step, converting 1-piperideine-6-carboxylate to α-aminoadipate, is catalyzed by a PCD which has recently been purified from S. clavuligerus and characterized (14). However, a number of gene clusters from β-lactam-producing species have now been partially or fully sequenced and no candidate dehydrogenase-type genes have been identified (42), which led to speculation that the reaction might be catalyzed by a primary metabolic enzyme. pcd may represent that missing gene, a speculation consistent with the location of pcd within the cephamycin cluster and with the estimated size of the purified enzyme, 52.6 kDa, which corresponds well with the deduced molecular mass of the pcd gene product, 54.0 kDa. However, it seems unusual that since LAT and PCD are proposed to catalyze sequential steps in the pathway, their corresponding genes would not be more closely grouped, especially in view of the multicistronic transcriptional relationship that exists between lat and the pcbAB and pcbC genes, encoding enzymes for the next two steps in the biosynthetic pathway. Instead, pcd is grouped together with genes encoding enzymes from the middle part of the pathway and is likely cotranscribed with them as a polycistronic message. A second area of concern is that no pcd gene has been identified in N. lactamdurans cephamycin cluster (consistent with the lack of cross-hybridization between pcd and N. lactamdurans genomic DNA), but PCD activity has been detected in N. lactamdurans (14). The fact that N. lactamdurans can form α-aminoadipate despite the absence of the pcd gene implies that another route is available. If that alternative route also exists in S. clavuligerus, then it follows that pcd may not actually be involved in α-aminoadipate formation but rather have some other unrecognized function which has no equivalent in N. lactamdurans. However, pcd was shown by Southern hybridization to have an equivalent in all other β-lactam species examined, and so whatever its role, it seems closely associated with the production of cephamycin.
cmcI and cmcJ are other newly identified biosynthetic genes from S. clavuligerus which act together with lat, pcbAB, pcbC, cefD, cefE, cefF, and cmcH (and possibly pcd) to make cephamycin. The cmcI and cmcJ genes were expected to be located within the unsequenced gaps in the S. clavuligerus cephamycin cluster, since similar genes had previously been located in N. lactamdurans. However, their organization within the S. clavuligerus cephamycin biosynthetic cluster differs markedly from that of N. lactamdurans. cmcI and cmcJ along with cefF and cmcH appear to have moved as a late gene cassette from their location between cefD and ccaR in S. clavuligerus to reside between pcbC and bla in N. lactamdurans. This variable organization could suggest that the different cephamycin-producing species have acquired component parts of the cephamycin biosynthetic gene cluster in separate stages rather than obtaining the entire cluster in a single horizontal gene transfer event. Alternatively, the different organization of the cephamycin gene cluster in S. clavuligerus may be related to constraints imposed by the presence of the adjacent clavulanic acid biosynthetic gene cluster, which is apparently coregulated with cephamycin production.
Like pcd, the ccaR orf11 blp group of genes is also absent from the N. lactamdurans cluster. No roles for the orf11 and blp genes in β-lactam biosynthesis were evident from homology searches. However, orf11 cross-hybridizing DNA was found in S. jumonjinensis and S. lipmanii, which produce clavulanic acid and clavulanic acid precursors like clavaminic acid, respectively, in addition to cephamycin (7, 20), but was absent in the other β-lactam producers. blp, which shows some sequence similarity to bli, the gene encoding the β-lactamase inhibitor protein from S. clavuligerus, hybridized only with genomic DNA from S. clavuligerus, and then only to itself and not to the bli gene.
Since this sequencing project was originally undertaken to search for potential transcriptional activators, the ccaR gene became the focus of subsequent studies. Involvement of CcaR in transcriptional regulation of the cephamycin biosynthetic pathway was predicted from its homology to other pathway-specific transcriptional activators, and an essential role in the production of both cephamycin and clavulanic acid has been demonstrated (44). We have confirmed this essential role for the CcaR protein in cephamycin and clavulanic acid production and have also demonstrated that CcaR is required for clavam production.
The biosynthetic gene clusters responsible for daunorubicin, actinorhodin, and undecylprodigiosin production in other Streptomyces spp. are known to be regulated by proteins homologous to CcaR (DnrI [52], ActII-ORF4 [22], and RedD [38], respectively). Mutants lacking the DnrI or RedD transcriptional activators displayed an antibiotic-nonproducing phenotype and lacked the transcripts responsible for the biosynthetic enzymes (34, 38). Disruption of the ccaR gene by insertion of the apr cassette or deletion of the ccaR gene, either by itself or together with orf11 and blp, resulted in loss of biosynthetic ability for cephamycin, clavulanic acid, and the clavams. This makes the CcaR protein unique in that it simultaneously regulates the activity of three distinct biosynthetic pathways. Typically, multiple pathways within a single organism are each regulated by a pathway-specific transcriptional regulator, as in the case of S. coelicolor, where RedD and ActII-ORF4 regulate undecylprodigiosin biosynthesis and actinorhodin biosynthesis, respectively (22, 38).
Mutant strains defective in ccaR, orf11, and blp used in these studies demonstrated that only ccaR has a clear cut role in the regulation of β-lactam metabolite production. Plasmid constructs used to give rise to the mutants were designed to ensure that polar mutations could be ruled out, but this precaution may have been unnecessary since a previous study demonstrated that ccaR and blp were transcribed as monocistronic messages and no transcript was detected for orf11 (44). Complementation of the Δcob::tsr strain with the wild-type cob fragment rescued production of the metabolites. When complemented with cob constructs carrying mutations in any one of the three genes, only the ccaR mutated construct was unable to complement production of the metabolites. This eliminates an essential role for orf11 or blp in the production of any of the metabolites. The lack of orf11 involvement in metabolite production was further confirmed by demonstrating metabolite production in orf11 gene disruption mutants resulting from replacement of the wild-type gene with a mutant version with the apr cassette inserted into the NcoI site of orf11 (data not shown).
Despite this inability to demonstrate a role for orf11 in production of any of the three classes of metabolites examined, it is still tempting to suggest that it may play a role in either clavulanic acid or clavam biosynthesis since orf11 cross-hybridizing DNA was found in S. jumonjinensis and S. lipmanii but was absent in the other β-lactam producers. Perhaps orf11 functions under environmental conditions which are difficult to reproduce in the laboratory.
The presence of ccaR cross-hybridizing DNA in Streptomyces β-lactam antibiotic-producing species but not in N. lactamdurans suggests that β-lactam production in Streptomyces spp. proceeds through a method of transcriptional regulation which is conserved throughout the genus but not in N. lactamdurans. As an alternative, transcriptional regulation of cephamycin production in N. lactamdurans has been suggested to involve the orf12 gene product (12) or the bla gene product (32) rather than ccaR.
Since pathway-specific activators similar to ccaR, such as dnrI, actII-ORF4, and redD, typically control antibiotic biosynthesis by regulating the transcription of biosynthetic genes, ccaR mutants were characterized for the synthesis of selected enzymes required for cephamycin biosynthesis. As expected, mutants were unable to make the CcaR protein, but they were also shown to be unable to make the LAT, ACVS, IPNS, and DAOCS proteins, thus explaining the defect in cephamycin production. Therefore, the CcaR protein was essential to cephamycin production by activating production of at least these pathway enzymes. Presumably similar situations occur in the clavulanic acid and clavam biosynthetic pathways to prevent their biosyntheses.
The ability to complement a ccaR disruption mutant was demonstrated previously when the gene was cloned at a high copy number (44). The present study shows that a ccaR deletion mutant can be complemented by a single copy of the ccaR gene delivered by the integrating vector pSET152 and that successful complementation rescues cephamycin biosynthesis by restoring the production of the cephamycin biosynthetic enzymes. The levels of cephamycin production were lower in complemented mutants than in the wild type, but this may be due to positional effects since pSET152 constructs integrate at the attB site. Alternatively, some upstream regulating elements needed for optimum expression of ccaR may have been unknowingly omitted from the complementing fragment used. The placement of stop codons within the ccaR gene created mutant alleles that were unable to complement, showing that ccaR does not contain alternate start codons capable of creating truncated but still functional versions of the CcaR protein.
In most instances, transcriptional activators like CcaR regulate a number of biosynthetic genes present within the cluster (11). Since LAT, ACVS, and IPNS arise from a tricistronic operon, CcaR could potentially regulate cephamycin production simply by controlling expression from the lat promoter. However, pcbC can also be transcribed as a monocistronic message from an independent promoter within the operon (46), yet no IPNS protein is seen in ccaR mutants. Similarly, DAOCS, an enzyme from the middle of the cephamycin pathway, arises from a separate polycistronic message which includes cefD, cefE (31), and possibly pcd, cmcT, and pbpA. The fact that DAOCS is also not produced in ccaR mutants indicates that CcaR must regulate expression from a number of separate promoters.
ACKNOWLEDGMENTS
We thank B. Barton, SmithKline Beecham Inc., for providing the dclX (ccaR) DNA sequence and many helpful ideas. We also thank C. Reeves, Panlabs Inc., for DAOCS antiserum, P. Skatrud, Eli Lilly Ltd., for providing cosmid pOW309, and L. Lee for excellent technical assistance.
This research was supported by Natural Sciences and Engineering Research Council of Canada and Alberta Heritage Foundation for Medical Research graduate fellowships to D.C.A. and a Natural Sciences and Engineering Research Council of Canada operating grant to S.E.J.
REFERENCES
- 1.Aharonowitz Y, Demain A L. Carbon catabolite regulation of cephalosporin production in Streptomyces clavuligerus. Antimicrob Agents Chemother. 1978;14:159–164. doi: 10.1128/aac.14.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aidoo K A, Wong A, Alexander D C, Rittammer R A R, Jensen S E. Cloning, sequencing and disruption of a gene from Streptomyces clavuligerus involved in clavulanic acid biosynthesis. Gene. 1994;147:41–46. doi: 10.1016/0378-1119(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 3.Aidoo, K. A., and S. E. Jensen. Unpublished data.
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey C R, Winstanley D J. Inhibition of restriction in Streptomyces clavuligerus by heat treatment. J Gen Microbiol. 1986;132:2945–2947. doi: 10.1099/00221287-132-10-2945. [DOI] [PubMed] [Google Scholar]
- 7.Barton, B. Personal communication.
- 8.Bierman M, Logan R, O’Brien K, Seno E T, Rao R N, Schoner B E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 9.Blackshear P J. Systems for polyacrylamide gel electrophoresis. Methods Enzymol. 1984;104:327–355. doi: 10.1016/s0076-6879(84)04093-3. [DOI] [PubMed] [Google Scholar]
- 10.Bradford M M. A rapid, sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Chater K F, Bibb M J. Regulation of bacterial antibiotic production. In: Kleinkauf H, von Döhren H, editors. Biotechnology. 6. Products of secondary metabolism. Weinheim, Germany: VCH; 1997. pp. 57–105. [Google Scholar]
- 12.Coque J J R, Liras P, Martín J F. Genes for a β-lactamase, a penicillin-binding protein and a transmembrane protein are clustered with the cephamycin biosynthetic genes in Nocardia lactamdurans. EMBO J. 1993;12:631–639. doi: 10.1002/j.1460-2075.1993.tb05696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coque J J R, Pérez-Llarena F J, Enguita F J, de la Fuente J L, Martín J F, Liras P. Characterization of the cmcH genes of Nocardia lactamdurans and Streptomyces clavuligerus encoding a functional 3′-hydroxymethylcephem O-carbamoyltransferase from cephamycin biosynthesis. Gene. 1995;162:21–27. doi: 10.1016/0378-1119(95)00308-s. [DOI] [PubMed] [Google Scholar]
- 14.de la Fuente J L, Rumbero A, Martín J F, Liras P. Δ-1-Piperideine-6-carboxylate dehydrogenase, a new enzyme that forms α-aminoadipate in Streptomyces clavuligerus and other cephamycin C-producing actinomycetes. Biochem J. 1997;327:59–64. [PMC free article] [PubMed] [Google Scholar]
- 15.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doran J L, Leskiw B K, Aippersbach S, Jensen S E. Isolation and characterization of a β-lactamase inhibitory protein from Streptomyces clavuligerus and cloning and analysis of the corresponding gene. J Bacteriol. 1990;172:4909–4918. doi: 10.1128/jb.172.9.4909-4918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doran J L, Leskiw B K, Petrich A K, Westlake D W S, Jensen S E. Production of Streptomyces clavuligerus isopenicillin N synthase in Escherichia coli using two-cistron expression systems. J Indust Microbiol. 1990;5:197–206. doi: 10.1007/BF01569677. [DOI] [PubMed] [Google Scholar]
- 18.Durairaj M, Doran J L, Jensen S E. High-level expression of the Streptomyces clavuligerus isopenicillin N synthase gene in Escherichia coli. Appl Environ Microbiol. 1992;58:4038–4041. doi: 10.1128/aem.58.12.4038-4041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan L A, Busby R W, Iwata-Reuyl D, Townsend C A. Probable role of clavaminic acid as the terminal intermediate in the common pathway to clavulanic acid and the antipodal clavam metabolites. J Am Chem Soc. 1997;119:2348–2355. [Google Scholar]
- 20.Elson, S. W., K. H. Baggaley, J. Gillett, S. Holland, N. H. Nicholson, J. T. Sime, and S. R. Woroniecki. 1987. Isolation of two novel intracellular β-lactams and a novel dioxygenase cyclising enzyme from Streptomyces clavuligerus. J. Chem. Soc. Chem. Commun. 1736–1738.
- 21.Enguita F J, Liras P, Leitão A L, Martín J F. Interaction of the two proteins of the methoxylation system involved in cephamycin C biosynthesis. J Biol Chem. 1996;271:33225–33230. doi: 10.1074/jbc.271.52.33225. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Moreno M A, Caballero J L, Hopwood D A, Malpartida F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell. 1991;66:769–780. doi: 10.1016/0092-8674(91)90120-n. [DOI] [PubMed] [Google Scholar]
- 23.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 24.Hill C W, Sandt C H, Vlazny D A. Rhs elements of Escherichia coli: a family of genetic composites each encoding a large mosaic protein. Mol Microbiol. 1994;12:865–871. doi: 10.1111/j.1365-2958.1994.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 25.Hodgson J E, Fosberry A P, Rawlinson N S, Ross H N M, Neal R J, Arnell J C, Earl A J, Lawlor E J. Clavulanic acid biosynthesis in Streptomyces clavuligerus: gene cloning and characterization. Gene. 1995;166:49–55. doi: 10.1016/0378-1119(95)00560-9. [DOI] [PubMed] [Google Scholar]
- 26.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: John Innes Foundation; 1985. [Google Scholar]
- 27.Jensen, S. E. Unpublished data.
- 28.Jensen S E, Westlake D W S, Wolfe S. Cyclization of δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine to penicillins by cell-free extracts of Streptomyces clavuligerus. J Antibiot. 1982;35:483–490. doi: 10.7164/antibiotics.35.483. [DOI] [PubMed] [Google Scholar]
- 29.Kern B A, Hendlin D, Inamine E. l-Lysine ɛ-aminotransferase involved in cephamycin C synthesis in Streptomyces lactamdurans. Antimicrob Agents Chemother. 1980;17:676–685. doi: 10.1128/aac.17.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacevic S, Miller J R. Cloning and sequencing of the β-lactam hydroxylase gene (cefF) from Streptomyces clavuligerus: gene duplication may have led to separate hydroxylase and expandase activities in the actinomycetes. J Bacteriol. 1991;173:398–400. doi: 10.1128/jb.173.1.398-400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovacevic S, Towbin M B, Miller J R. The β-lactam biosynthesis genes for isopenicillin N epimerase and desacetoxycephalosporin C synthetase are expressed from a single transcript in Streptomyces clavuligerus. J Bacteriol. 1990;172:3952–3958. doi: 10.1128/jb.172.7.3952-3958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar V, de la Fuente J L, Leitão A L, Liras P, Martín J F. Effect of amplification or targeted disruption of the β-lactamase gene of Nocardia lactamdurans on cephamycin biosynthesis. Appl Microbiol Biotechnol. 1996;45:621–628. doi: 10.1007/s002530050739. [DOI] [PubMed] [Google Scholar]
- 33.Leskiw B K, Bibb M J, Chater K F. The use of a rare codon specifically during development. Mol Microbiol. 1991;5:2861–2867. doi: 10.1111/j.1365-2958.1991.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 34.Madduri K, Hutchinson C R. Functional characterization and transcriptional analysis of the dnrR1 locus, which controls daunorubicin biosynthesis in Streptomyces peucetius. J Bacteriol. 1995;177:1208–1215. doi: 10.1128/jb.177.5.1208-1215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madduri K, Stuttard C, Vining L C. Cloning and location of a gene governing lysine ɛ-aminotransferase, an enzyme initiating β-lactam biosynthesis in Streptomyces spp. J Bacteriol. 1991;173:985–988. doi: 10.1128/jb.173.3.985-988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh E N, Chang M D T, Townsend C A. Two isozymes of clavaminate synthase central to clavulanic acid formation: cloning and sequencing of both genes from Streptomyces clavuligerus. Biochemistry. 1992;31:12648–12657. doi: 10.1021/bi00165a015. [DOI] [PubMed] [Google Scholar]
- 37.Mosher, R. H., and S. E. Jensen. Unpublished data.
- 38.Narva K E, Feitelson J S. Nucleotide sequence and transcriptional analysis of the redD locus of Streptomyces coelicolor A3(2) J Bacteriol. 1990;172:326–333. doi: 10.1128/jb.172.1.326-333.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paradkar A S, Aidoo K A, Jensen S E. A pathway-specific transcriptional activator regulates late steps of clavulanic acid biosynthesis in Streptomyces clavuligerus. Mol Microbiol. 1998;27:831–843. doi: 10.1046/j.1365-2958.1998.00731.x. [DOI] [PubMed] [Google Scholar]
- 40.Paradkar A S, Aidoo K A, Wong A, Jensen S E. Molecular analysis of a β-lactam resistance gene encoded within the cephamycin cluster of Streptomyces clavuligerus. J Bacteriol. 1996;178:6266–6274. doi: 10.1128/jb.178.21.6266-6274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paradkar A S, Jensen S E. Functional analysis of the gene encoding the clavaminate synthase 2 isoenzyme involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. J Bacteriol. 1995;177:1307–1314. doi: 10.1128/jb.177.5.1307-1314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paradkar A S, Jensen S E, Mosher R H. Comparative genetics and molecular biology of β-lactam biosynthesis. In: Strohl W R, editor. Biotechnology of industrial antibiotics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 241–277. [Google Scholar]
- 43.Pérez-Llarena F, Martín J F, Galleni M, Coque J J R, de la Fuente J L, Frère J-M, Liras P. The bla gene of the cephamycin cluster of Streptomyces clavuligerus encodes a class A β-lactamase of low enzymatic activity. J Bacteriol. 1997;179:6035–6040. doi: 10.1128/jb.179.19.6035-6040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pérez-Llarena F, Liras P, Rodríguez-García A, Martín J F. A regulatory gene (ccaR) required for cephamycin and clavulanic acid production in Streptomyces clavuligerus: amplification results in overproduction of both β-lactam compounds. J Bacteriol. 1997;179:2053–2059. doi: 10.1128/jb.179.6.2053-2059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrich A K, Leskiw B K, Paradkar A S, Jensen S E. Transcriptional mapping of the genes encoding the early enzymes of the cephamycin biosynthetic pathway of Streptomyces clavuligerus. Gene. 1994;142:41–48. doi: 10.1016/0378-1119(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 46.Petrich A K, Wu X, Roy K L, Jensen S E. Transcriptional analysis of the isopenicillin N synthase-encoding gene of Streptomyces clavuligerus. Gene. 1992;111:77–84. doi: 10.1016/0378-1119(92)90605-o. [DOI] [PubMed] [Google Scholar]
- 47.Rao R N, Richardson M A, Kuhstoss S. Cosmid shuttle vectors for cloning and analysis of Streptomyces DNA. Methods Enzymol. 1987;153:166–198. doi: 10.1016/0076-6879(87)53053-1. [DOI] [PubMed] [Google Scholar]
- 48.Salowe S P, Marsh E N, Townsend C A. Purification and characterization of clavaminate synthase from Streptomyces clavuligerus: an unusual oxidative enzyme in natural product biosynthesis. Biochemistry. 1990;29:6499–6508. doi: 10.1021/bi00479a023. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Smith D J, Burnham M K R, Bull J H, Hodgson J E, Ward J M, Browne P, Brown J, Barton B, Earl A J, Turner G. β-Lactam antibiotic biosynthetic genes have been conserved in clusters in prokaryotes and eukaryotes. EMBO J. 1990;9:741–747. doi: 10.1002/j.1460-2075.1990.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuttard C. Temperate phages of Streptomyces venezuelae: lysogeny and host specificity shown by phages SV1 and SV2. J Gen Microbiol. 1982;128:115–121. [Google Scholar]
- 52.Stutzman-Engwall K J, Otten S L, Hutchinson C R. Regulation of secondary metabolism in Streptomyces spp. and overproduction of daunorubicin in Streptomyces peucetius. J Bacteriol. 1992;174:144–154. doi: 10.1128/jb.174.1.144-154.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tobin M B, Kovacevic S, Madduri K, Hoskins J A, Skatrud P L, Vining L C, Stuttard C, Miller J R. Localization of the lysine ɛ-aminotransferase (lat) and δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine synthetase (pcbAB) genes from Streptomyces clavuligerus and production of lysine ɛ-aminotransferase activity in Escherichia coli. J Bacteriol. 1991;173:6223–6229. doi: 10.1128/jb.173.19.6223-6229.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walters N J, Barton B, Earl A J. Novel compounds. 1994. International patent WO 94/18326-A 1. [Google Scholar]
- 55.Ward J M, Hodgson J E. The biosynthetic genes for clavulanic acid and cephamycin production occur as a ‘super-cluster‘ in three Streptomyces. FEMS Microbiol Lett. 1993;110:239–242. doi: 10.1111/j.1574-6968.1993.tb06326.x. [DOI] [PubMed] [Google Scholar]
- 56.Wu T-K, Busby R W, Houston T A, McIlwaine D B, Egan L A, Townsend C A. Identification, cloning, sequencing, and overexpression of the gene encoding proclavaminate amidino hydrolase and characterization of protein function in clavulanic acid biosynthesis. J Bacteriol. 1995;177:3714–3720. doi: 10.1128/jb.177.13.3714-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu H, Serpe E, Romero J, Coque J J R, Maeda K, Oelgeschläger M, Hintermann G, Liras P, Martín J F, Demain A L, Piret J. Possible involvement of the lysine ɛ-aminotransferase gene (lat) in the expression of the genes encoding ACV synthetase (pcbAB) and isopenicillin N synthase (pcbC) in Streptomyces clavuligerus. Microbiology. 1994;140:3367–3377. doi: 10.1099/13500872-140-12-3367. [DOI] [PubMed] [Google Scholar]