Highlights
-
•
Conjugated linoleic acid (CLA) is recently considered as a health-promoting compound.
-
•
CLA has anti-cancer, antidiabetic, antioxidant, anti-obesity, and anti-inflammatory effects.
-
•
CLA is naturally present in dairy and meat products of ruminants.
-
•
CLA can be produced by various chemical, microbial and enzymatic methods.
-
•
The microbial strategy is the most important one because of its many advantages.
Keywords: Conjugated linoleic acid (CLA), Biological effects, Linoleic acid, Microbial production, Vaccenic acid, Ricinoleic acid
Chemical compounds studied in this article: Conjugated linoleic acid (PubChem CID: 74607), Vaccenic acid (PubChem CID: 122325), Ricinoleic acid (PubChem CID: 643684)
Abstract
Conjugated linoleic acid (CLA) has recently attracted significant attention as a health-promoting compound. CLA is a group of positional isomers of linoleic acid (LA) with a conjugated double bond naturally occurring in dairy and ruminant meat products. Microbial biosynthesis of CLA is a practical approach for commercial production due to its high safety and purity. There are some factors for the microbial CLA production such as strain type, microbial growth phase, pH, temperature and incubation time, based on which the amount and type of CLA can be controlled. Understanding the interplay of these factors is essential in optimizing the quantity and composition of microbial CLA, as discussed in the current study. Further exploration of CLA and its influences on human health remains a dynamic and evolving area of study.
1. Introduction
Conjugated linoleic acid (CLA) is a general term for a mixture of positional and geometric isomers of linoleic acid (LA) (18: 2), in which two double bonds are conjugated (Wang, Li, Meng, Tong, & Liu, 2022). Cis-9, trans-11 CLA, and trans-10, cis-12 CLA are the most abundant CLA isomers, accounting for approximately 95 % of all isomers (Den Hartigh, 2019, Suksatan et al., 2022). Double-conjugated fatty acids (FAs) have long been identified in different amounts in dairy products and other foods derived from ruminants. Conjugated FAs are common in nature, and are usually part of the products of fat metabolism by bacteria that form in the rumen of cattle, sheep, and other ruminants (Zongo et al., 2021). In recent years, consumers have shifted to healthier foods, and it is expected that the trend will increase in the future (Arjeh, Akhavan, Barzegar, & Carbonell-Barrachina, 2020). Vegetable fats and oils are a source of energy in the diet of many people around the world. A lack of fats in the diet causes a feeling of fat hunger. Fats prevent premature hungry after a meal by delaying the digestion of food. Some unsaturated FAs are essential components of the diet, and their complete elimination causes fat-related diseases that appear as flaking of the skin, weight loss, kidney ulcers and eventually death. In humans, infants need essential fatty acids (EFAs), and a diet without fats causes itchy skin and eczema that goes away with the consumption of fats (Olejnik et al., 2023). EFAs are also involved in normal pregnancy and lactation. These compounds act as radiation resistance agents and prevent capillary rupture.
Today, researchers have found that foods, in addition to providing essential nutrients, contain components that may be helpful in maintaining health and preventing chronic diseases (Caballero et al., 2021(Arjeh, et al., 2022). The components in animal products are each associated with a specific effect on human health, which is why the interest in conjugated FAs has increased significantly. Studies showed that CLA has anti-cancer properties (Dachev et al., 2021). Further research has shown that anti-cancer isolates are composed of conjugated octadecadienoic acid isomers in which double bonds are separated by a methylene (–CH2–) group, instead of a single carbon-to-carbon bond. These isomers were generally referred to as CLA (Yurawecz, 2003). It has also been shown that CLA can have a variety of health effects such as inhibiting atherosclerosis, increasing immune function, liver metabolism and blood sugar (Chen & Park, 2019). But the most credible findings about the health effects of CLA include its anti-cancer and reduction in body fat effects. Some reported data suggest that these effects may be isomer-dependent; the c9, t11, and t10, c12 isomers are more important in terms of health (Amiri et al., 2020, Koba and Yanagita, 2014). The immune system is the central defense against many diseases, especially cancer. The anticancer activity of CLA could be the result of enhanced immune function, which has been associated with promoting T-cell (Lymphocyte) proliferation and increased IgA secretion and macrophage function against certain cancers (Kumar et al., 2009, Marín et al., 2018). In this regard, Trans-10, cis-12-CLA (t10c12-CLA) has been shown to alter immune function through modulating the activity of monocytes and macrophages (Kang, Lee, Jeung, & Yang, 2007).
Dairy products and meat are among the foods that naturally have the highest levels of CLA. The content of CLA in milk and meat is influenced by various factors including animal breed, age, diet and dietary supplements affecting the diet (Dhiman, Nam, & Ure, 2005). The amount of CLA in food can be increased by using various enzymatic, chemical and microbial methods. Microbial CLA production is recognized as an environmentally friendly method (Salamon et al., 2015). The naturally synthesized form of CLA has better properties than its chemically synthesized form, because most of the natural isomers are of c9, t11 type, which has a high biological activity; while in the chemical synthesis of CLA, four types of isomers are produced (Das et al., 2005, Wang et al., 2022). The recommended dose of CLA is approximately 3 g/day, and through its consumption, many health effects are manifested in humans (Gangidi & Proctor, 2004). Therefore, considering the importance of CLA in the diet and its effects on human health, it is necessary to study the safe production of CLA and the factors affecting the process.
2. Conjugated linoleic acid; an overview
2.1. Chemical structure
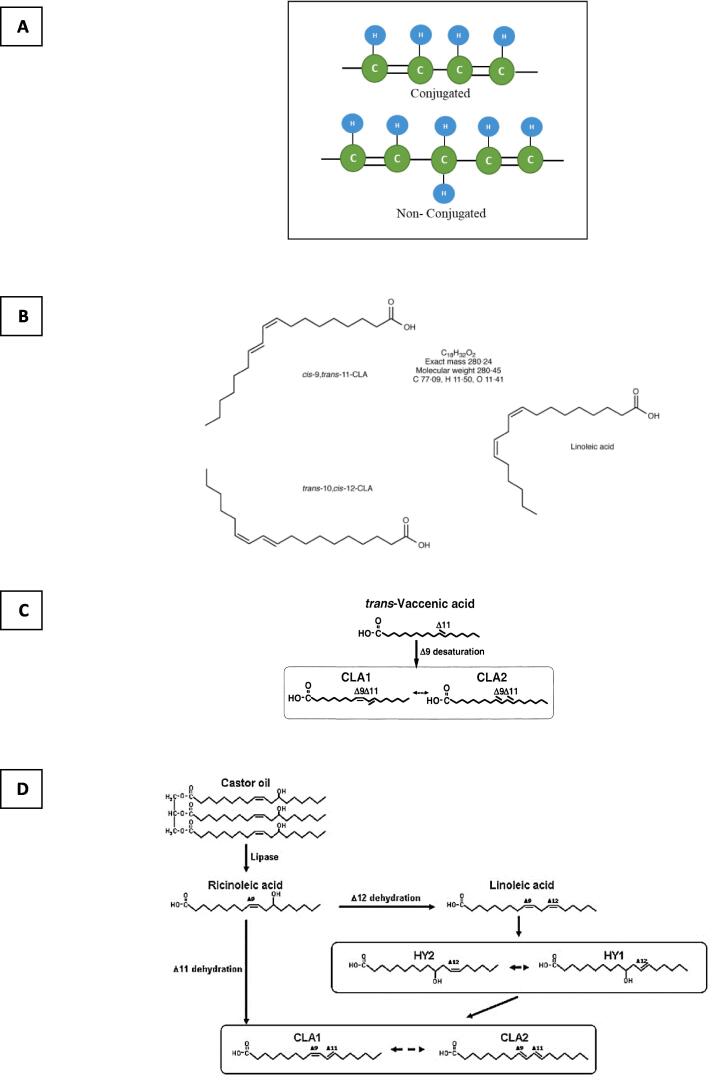
CLA is an 18-carbon fatty acid with two double bonds. Fatty acids with two double bonds have four fewer hydrogen atoms than similar saturated fatty acids and their general formula is CnH2n-4O2, of which LA (c9, c12 octadecadienoic acid) with the chemical formula (C18H32O2) is the most important fatty acid. As shown in Fig. 1A, CLA is the geometric (cis and trans) and positional (8 to 13) isomer of LA, in which the double bonds are conjugated (Khanal et al., 2005). More than 12 CLA isomers are found naturally in dairy and ruminant meat products, but c9, t11 and t10, c12 are two isomers (Fig. 1B) of high physiological importance (Banni et al., 2001, Kadamne et al., 2011; Lee, Paek, Lee, Park, & Lee, 2007).
Fig 1.
(A) General structure of conjugated and unconjugated bonds; (B) Linoleic acid and its important conjugated isomers; reproduced with permission from (Roche, Noone, & Gibney, 2001); (C) Isomerization of vaccenic acid to CLA isomers (Ogawa, Kishino, Ando, Sugimoto, Mihara, & Shimizu, 2005); (D) Conversion of ricinoleic acid in castor oil triglycerides to CLA from two different pathways (Kishino, Ogawa, Yokozeki, & Shimizu, 2009).
2.2. CLA production precursors
2.2.1. Vaccenic acid 18: trans-11
Vaccenic acid is a FA with the chemical formula C18H34O2 and the IUPAC name “Octadec-11-enoic acid- (E)” which is one of the precursors of CLA production. Vaccenic acid is found in milk fat and is considered a functional ingredient because it is a precursor to the endogenous synthesis (Fig. 1C) of rumenic acid (c9, t11 CLA) in cattle and humans (Caballero et al., 2021).
2.2.2. Ricinoleic acid 18: 1n 9
Ricinoleic acid is another FA with the chemical formula C19H25NO4 and the systematic name “12-hydroxy c9-18: 1”, which is found in abundance in castor oil. According to Fig. 1D, there are two possible pathways for the production of CLA from ricinoleic acid by lactic acid bacteria (LAB): (i) direct conversion of ricinoleic acid to CLA by dehydration at position 11; (ii) three-step conversion through LA; through dehydration at position 12 and sequential isomerization of LA.
2.3. Health benefits of CLA
CLA was first discovered as an anti-cancer compound (Yurawecz, 2003), but subsequent studies have shown several health effects for it, including reducing body fat, inhibiting atherosclerosis, increasing the immune system function, improving liver metabolism, hypoglycemia and bone health (Den Hartigh, 2019, Koba and Yanagita, 2014, Watkins et al., 2004). Isomers of CLA have several properties, and the isomers of rumenic acid (c9, t11 CLA) and t10, c12 CLA are the most biologically active isomers which can lower blood cholesterol, prevent atherosclerosis and carcinogenesis (den Hartigh, 2019). A summary of the health benefits of CLA are listed in Table 1.
Table 1.
Some health effects reported for CLA.
| Health effect | Description | Reference |
|---|---|---|
| Anti-cancer properties | c9, t11 isomer is an effective isomer in creating this effect. A diet containing 0.1, 1 and 5.5 % of the mixture of CLA isomers can reduce the number of tumors by 32, 56 and 60 %, respectively. | (Caballero et al., 2021, Gnadig et al., 2003, Huang et al., 1994, Knekt et al., 1996, Zeng et al., 2020) |
| Reducing body fat | CLA, while increasing body mass (protein), reduces fat. The action mechanism is probably to increase fat lipolysis, and to reduce the accumulation of FAs in adipose tissue. | (DeLany et al., 1999, Ibrahim and El-Sayed, 2021, Koba and Yanagita, 2014, Lehnen et al., 2015; Park & Pariza, 2007) |
| Improving immune system function | Consumption of diets containing CLA can increase the body's immune factors (antibodies, white blood cells and the enzyme lysozyme). The c9, t11 and t10, c12 CLA isomers have different effects on immune function and the population of immunoglobulin subtypes. | (Aydin, 2005, O'Shea et al., 2004, Viladomiu et al., 2016, Zhang et al., 2005) |
| Inhibition of atherosclerosis | Increasing CLA in the diet could inhibit atherosclerosis and, in addition, severely regenerate clogged arteries. | (Kritchevsky, et al., 2004; S Toomey, Roche, Fitzgerald, & Belton, 2003; Toomey, Harhen, Roche, Fitzgerald, & Belton, 2006) |
| Bone health | The effective isomers are c9, t11 and t10, c12. CLA, along with dietary calcium, can prevent osteoporosis by affecting the hormonal and enzymatic systems associated with bone. | (Dilzer & Park, 2012; Park et al., 2013, Watkins et al., 2004) |
| Reduction of inflammation | CLA can reduce inflammation by affecting cells involved in the inflammatory process (such as T cells, macrophages, and monocytes). | (Ávila et al., 2020, Bassaganya-Riera and Hontecillas, 2010, Butz et al., 2007, Gebauer et al., 2015) |
| Decrease of LDL cholesterol and increase of blood HDL | Many studies have confirmed the effect of CLA on lowering LDL and increasing HDL, but some studies have reported the opposite, so the effect of CLA on blood cholesterol still needs to be studied. | (Brouwer et al., 2010, Derakhshande-Rishehri et al., 2015, Gebauer et al., 2015, LeDoux et al., 2007) |
| Increasing energy | CLA probably increases the energy available to the body by increasing oxidation in fats. | (Choi, Jung, Park, & Song, 2004) |
| Reducing blood platelet clotting | Although lab studies confirm the effect of CLA on reducing platelet clotting, human studies suggest that short-term use of CLA in humans is unlikely to produce this effect. | (Benito et al., 2001, Kung and Yang, 2006, Uniacke-Lowe and Fox, 2012) |
As explained, there is a growing interest in incorporating CLA into food products. Despite its increasing demand in the food industry, CLA is very sensitive to light, heat, and oxygen, which can produce unpleasant odors and flavors during processing and storage, limiting its use in the food industry. Therefore, CLA should be provided in protected forms such as oil-in-water emulsions (Li, et al., 2022). In terms of industrial application, the utilization of CLA-producing LAB improves the functional potential of dairy products, e.g., fermented milks. Such innovative dairy products are considered as newer business growth drivers for the food industry (Dahiya & Puniya, 2018). Besides, the structure of CLA creates special conditions for its wider applications in various industries. For example, CLA is highly reactive towards polymerization, which makes it suitable for various industrial uses such as conjugated drying oils that have faster drying rates, better resistance to water, and improved toughness (Chen, Zhang, Zheng, & Zheng, 2017).
Although CLA consumption has health benefits, there are also potential risks associated with CLA consumption. The effects of CLA on weight loss and other health parameters have shown variability across studies, and not all individuals may respond in the same way. For example, high doses of CLA supplements may lead to side effects such as digestive issues, hepatic steatosis and induction of colon carcinogenesis in humans, and potential liver problems (Asbaghi et al., 2022, Jaudszus et al., 2010, Putera et al., 2023). Therefore, long-term safety of CLA has not been extensively studied. Before using CLA supplements, it is important to consulted with a healthcare professional or registered dietitian to determine the appropriate dosage and assess potential risks and benefits tailored to specific health goals and needs.
2.4. CLA natural resources
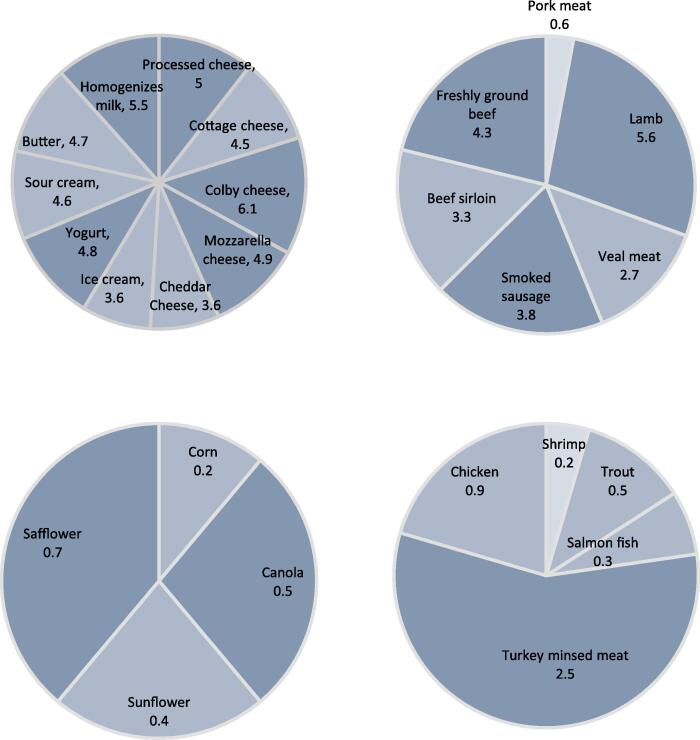
CLA isomers are found naturally in a variety of foods. Meat products, tissue fat, and dairy products have higher levels of CLA due to the production of this FA during bio-hydrogenation in ruminants (Jaglan et al., 2019, Paszczyk and Czarnowska-Kujawska, 2022). The CLA content in beef and dairy products varies from 3 to 10 mg/g fat (Dhiman, Nam, & Ure, 2005). The extent of CLA in human milk (1.7–36.4 mg/g fat), sheep (10.8–29.7 mg/g fat), goat (6.1–10.35 mg/g fat), buffalo (4.4–7.0 mg/g fat) and cattle (0.7–10.1 mg/g fat) are different from each other (Uniacke-Lowe & Fox, 2012). The content of CLA in different foods is presented in Fig. 2.
Fig 2.
The extent of CLA reported in different foods; adapted from (Chinnadurai and Tyagi, 2011, Guo, 2013; Kumar et al., 2018, Shaikh et al., 2018).
The optimal dosage of CLA for health benefits is not definitively established, and it can vary depending on individual factors. In general, CLA is considered to be a safe and effective supplement for most people when used at the directed dose. Studies have used doses ranging from 1 to 10 g/day (Asbaghi et al., 2022, Putera et al., 2023). Dietary sources of CLA, such as meat and dairy products, provide only low amounts of CLA, making it challenging to obtain therapeutic doses from food. Hence, supplementation is often considered to achieve higher CLA consumption.
3. CLA production methods
CLA production methods can be divided into three groups: chemical, microbial and enzymatic methods, as described in the following sections.
3.1. Chemical production of CLA
In general, chemical methods of CLA production can be divided into three classes, including ricinoleic acid dehydration (Berdeaux et al., 1997), alkaline isomerization (Reaney et al., 1999) and process catalysis with heterogeneous metals (Gnadig et al., 2003). Chemical methods require temperatures > 100 °C, use more chemicals, and have the potential to form by-products, such as cyclic, oxidized, and polymerized compounds. In addition, due to the lower selectivity of chemical catalysts, the product obtained is usually a mixture of CLA isomers with a purity < 40 % for each of the isomers (Saebo, 2003).
3.2. Enzymatic production of CLA
Linoleate isomerase is an enzyme that converts LA into CLA. By extracting and purifying the enzyme and using it in the production of CLA, more selectivity in the reaction and production of desirable isomers can be achieved. However, since some types of these enzymes are stabilized on the membrane and resistant to solubilization, they are very difficult to separate and purify. For this reason, many attempts in this field have not been very successful (Irmak et al., 2006).
3.3. Microbial production of CLA
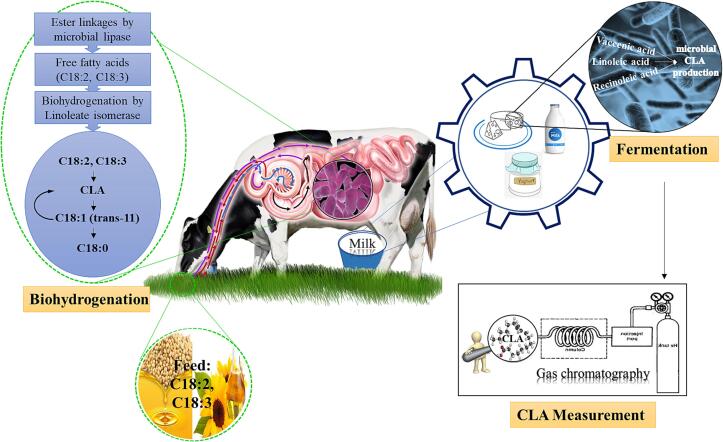
Since the discovery of B. fibrisolvens in the rumen of ruminants as a CLA-producing bacterium, there have been many reports of CLA production by various bacteria. In addition to rumen bacteria, some other bacteria, such as Propionibacterium, Lactobacillus, Lactococcus, Bifidobacterium, Enterococcus, Closteridium, Pediococcus and Megasphaera are also capable of producing CLA (Adamczak et al., 2008). In this method, bacteria are used as biocatalysts to convert LA (or ricinoleic acid) into CLA. After production, pure CLA or a mixture of FAs containing CLA is obtained. CLA can be used as a capsule or dietary supplement or in the production of activator feed (Ogawa et al., 2005). Fig. 3 summarizes the production of CLA in cow rumen and cow's milk fermented products. It should be noted that lipolytic and proteolytic reactions are favorable for the formation of CLA. Lipolysis produces free fatty acids, as the precursors of CLA, which are formed via the microbiological hydrogenation pathway. Proteolysis breaks down proteins into low molecular weight compounds that act as hydrogen donors (Lin, Boylston, Luedecke, & Shultz, 1999). In addition, proteolysis is a pre-requisite for the growth of LAB and the subsequent degradation of proteins, leading to the release of peptides and free amino acids (Olivo, et al., 2021). In this regard, Moslemi, Moayedi, Khomeiri, and Maghsoudlou (2023) observed a positive correlation between the amount of proteolysis and CLA synthesis.
Fig 3.
Production of CLA in cow rumen and cow's milk fermented products.
The CLA content in milk and meat is affected by several factors, such as animal's breed, age, diet, and animal keeping conditions (Dhiman, Nam, & Ure, 2005). Ruminant diet supplementation with sunflower oil, fish oil and soybean oil (with a high percentage of C18:2, C18:3) has been reported as a good strategy for enhancing CLA in dairy cows (Dohme-Meier and Bee, 2012, Lounglawan and Suksombat, 2011). In the animal rumen, ester linkages are first catalyzed by microbial lipase and then bio-hydrogenation of unsaturated FAs by linoleate isomerase occurs. Linoleate isomerase is an enzyme involved in the formation of conjugated double bonds of LA as well as α and γ-linolenic acids (Weiss, Martz, & Lorenzen, 2004). During fermentation in dairy products, CLA precursor FAs (such as vaccenic, LA and ricinoleic acid) or oils containing a high level of these FAs turn into CLA. The microbial mechanism of CLA production is unclear, but a number of theories have been proposed. It was illustrated that the presence of high levels of free LA inhibits bacterial growth because the production of CLA is considered as a detoxification mechanism for the bacterial cell (Yang et al., 2017).
In general, bio-isomerization of LA into CLA is carried out in three ways: (i) using CLA-producing bacteria with the aim of producing functional fermented foods; (ii) production of CLA by microbes using pure substrate such as LA or ricinoleic acid; (iii) extraction of enzymes for the conversion of LA to CLA from bacteria with potential CLA production, such as Lactobacillus, Lactococcus, Propionibacterium, Bifidobacterium, Megasphaera elsdenii and Butyrivibrio fibrisolvence.
In terms of efficiency, different CLA production methods are comparable as follows (Boyaval et al., 1995, Farmani et al., 2010, Kabara et al., 1972):
-
•
Cost-effectiveness: chemical > microbial > enzymatic method
-
•
Product purity: chemical < microbial < enzymatic method
Table 2 presents some advantages and disadvantages of CLA production methods.
Table 2.
Advantages and disadvantages of CLA production methods.
| Methods | Advantages | Disadvantages |
|---|---|---|
| Chemical | Better control Larger scale More profit margins |
Low selectivity Less isomeric purity producing more by-products Consuming more chemic |
| Microbial | Production of biologically active isomers No need for a cofactor Production of functional foods using microbial fermentation |
Bacterial sensitivity to CLA precursors Low solubility of CLA precursors Lower efficiency Production of by-products Cost of production |
| Enzymatic | More purity Less by-product |
More cost Low enzyme stability Requires cofactor |
As it turns out, each of the chemical, microbial, and enzymatic methods has several advantages and disadvantages. However, the use of food as a functional product to deliver CLA into the body is still preferred because food is one of the most essential human needs. On the other hand, the consumption of micronutrients in the form of drugs is less popular. Therefore, the best way to deliver micronutrients is to enrich/fortify food products. In this regard, various formulated products such as iron-enriched flour or vitamin d-containing oils have been successfully produced in the past. Therefore, the use of microbial and fermentation methods to produce functional food products can be a good way to deliver CLA to individuals in the form of a daily diet (Blasi & Cossignani, 2019).
Today, various strategies have been proposed to increase the efficiency of microbial production of CLA and eliminate its disadvantages. LA, as one of the main precursors of CLA production, causes toxicity in bacteria (Kim, 2003). This compound stimulates the production of CLA in bacteria. The conversion of LA into a less toxic compound is the most important strategy of bacteria to reduce its toxicity; hence, by converting it to CLA, bacteria reduce its toxic effects. One way to reduce the toxicity of LA is to use washed or resting (immobile) cells; bacterial-washed cells are alive but do not grow. Bacterial cells at the end of the logarithmic phase or at the beginning of the stationary phase are considered washed cells; therefore, in this phase, they are less sensitive to the toxicity of LA (Ogawaet al., 2005). The precursors of CLA production, such as oils and FAs (LA and ricinoleic acid), must be dissolved in the medium to be consumed by bacteria. The use of supplementary compounds, such as bovine serum albumin (BSA), Tween 80, starch and lipid compounds including cholesterol, lecithin and surfactants of FAs, can act as dispersing agents, which concurrently reduce the antimicrobial effects of FAs (Kabara et al., 1972).
3.3.1. CLA-producing bacteria
CLA production by bacterial strains is influenced by various factors. Studies have shown that most strains with the potential to produce CLA are from LAB (lactic acid bacteria) (Wu & Li, 2018). The term of LAB is used for bacteria that cause fermentation and coagulation of milk, and produce lactic acid from lactose. LAB are one of the most important microbial groups used in food fermentation; they help to create the texture of fermented products (Blasi & Cossignani, 2019). Interestingly, probiotic bacteria have been used for centuries in the form of dairy foods containing LAB (Song et al., 2021). Among LAB, Lactobacilli are more commonly known CLA-producing strains (Song et al., 2021, Tapia et al., 2019, Tyagi et al., 2020). In the meantime, many studies have been performed on L. plantarum strains; it was revealed that using L. plantarum cells as a catalyst, the production of CLA from LA in optimal conditions reached to 40 mg/L. This was the highest amount of CLA produced among Lactobacillus bacteria (Ogawa et al., 2005, Tapia et al., 2019).
Table 3 reports the amount of CLA production in LAB, ranging from 1 up to 40,000 mg/L. In addition to lactobacilli, other LABs can also produce CLA. Coakley, Ross, Nordgren, Fitzgerald, Devery, and Stanton (2003) examined the different strains of Lactobacillus, Lactococcus, Pediococcus, and Bifidobacterium in CLA-free LA medium and found that B. breve and B. dentium were the most efficient CLA producers among the strains used, and 65 % of LA was converted to c9, t11 CLA (Coakleyet al., 2003). Alonso et al. (2003) studied two bacterial strains of Lactobacillus acidophilus and two bacterial strains of Lactobacillus casei for CLA production in culture medium containing free LA. The results showed that all strains were able to produce CLA, and the highest amount of CLA was between 130 and 180 μg/mL after 24 h in medium containing 0.02 % LA. In addition, an increase in CLA in skim milk was also observed. Therefore, they found that the use of CLA-producing LAB in fermented dairy products may have potential health benefits. Therefore, if CLA-producing bacteria are included in starter cultures, the produced CLA could be readily available for absorption through the gastrointestinal tract (Alonso et al., 2003).
Table 3.
The extent of CLA production in lactic acid bacteria.
| Lactic acid bacteria | CLA (mg/L) | Reference |
|---|---|---|
| Bifidobacterium | ||
| B. breve | 99–600 | (Chung et al., 2008, Coakley et al., 2003, Lee et al., 2003, Ogawa et al., 2005, Song et al., 2005, Van Nieuwenhove et al., 2007) |
| B. adolescentis | 3–4 | (Coakley, et al., 2003) |
| B. bifidum | 1–207 | |
| B. angulatum | 1 | |
| B. infantis | 4–25 | |
| B. lactis | 170 | |
| B. pseudolongum | 214 | (Gorissen, et al., 2010) |
| B. animalis | 7–48 | (Rodríguez-Alcalá, Braga, Malcata, Gomes, & Fontecha, 2011) |
| B. pseudocatenulatum | 23–135 | (Coakley, et al., 2003) |
| Lactobacillus | ||
| L. plantarum | 7–40000 | (Ando et al., 2004, Hou et al., 2011, Kishino et al., 2002, Lee et al., 2007) |
| L. casei | 100–175 | (Van Nieuwenhove, et al., 2007) |
| L. acidophilus | 4–4900 | (Alonso et al., 2003, Li et al., 2011, Ogawa et al., 2001, Rodríguez-Alcalá et al., 2011) |
| L. delbrueckii | 78 | (Lin, 2000) |
| L. rhamnosus | 1–190 | (Van Nieuwenhove et al., 2007, Xu et al., 2004) |
| L. reuteri | 119–300 | (Lee et al., 2003, Roman-Nunez et al., 2007) |
| L. casei | 11–80 | (Alonso, et al., 2003) |
| L. curvatus | 8 | (Gorissen, et al., 2011) |
3.3.2. Other CLA-producing bacteria
In addition to LAB, some butyric acid and propionic acid bacteria are also known as CLA-producing strains. Propionibacterium freundenreichii, P. shermanii, P. thoenii and Butyrivibrio fibrisolvens A38 are bacteria whose ability to produce CLA has been proven. (Jiang et al., 1998, Kim, 2003, Rainio et al., 2002). As mentioned earlier, CLA production varies from species to species and is not limited to a specific class of microbes; some strains of Lactobacillus, Propionibacterium, Bifidobacterium and Enterococcus were studied for CLA production by Deng et al. (2007); they found that propionibacteria had the highest CLA production among these species (Deng et al., 2007). Butyrivibrio fibrisolvens A38 is one of the most active rumen bacteria in the production of CLA. It has been shown that LA has an inhibitory effect not only on bacterial growth, but also on hydrogenation of LA, and this effect is more pronounced at higher concentrations of LA. CLA levels increase significantly when LA is added to the medium with a inhibitory agent such as glycolytic and iodoacetate (Kim, 2003, Mei et al., 2021).
4. Factors affecting CLA production by bacteria
There are numerus factors that can affect CLA production. The pH of the culture medium, the type of bacterial strain, the type and concentration of the substrate, the temperature and time of fermentation, and the physical conditions of the culture medium all can affect the extent and type of isomers produced (Dahiya and Puniya, 2018, Özer and Kılıç, 2021). In the following, we will discuss some of these important factors.
4.1. Microbial strains
Microbial strains do not have the same potential for substrate conversion and CLA production (Gao et al., 2021, Palachum et al., 2018, Srivastava et al., 2021, Zahed et al., 2021). Kishinoet al., (2009) compared over 250 strains of LAB from 14 genera in terms of CLA production. They found that strains of the Enterococcus, Pediococcus, Propionibacterium and Lactobacillus produce significant amounts of CLA from LA. All strains produced two specific isomers called c9, t11-18: 2 (CLA1) and t9, t11-18: 2 (CLA2). Among the evaluated bacterial strains, L. plantarum strain AKU 1009a displayed the highest potential for CLA production (Kishinoet al., 2009). Ando et al., (2003) also reviewed 250 strains of LAB, belonging to the genera Lactobacillus, Streptococcus, Pediococcus, Leuconostoc, Propionibacterium, Bifidobacterium, Aquaspirillum, Enterococcus, Tetragenococcus, Aerococcus, Butyrivibrio, Lactococcus and weissella, for CLA production. They found that CLA producing strains belonged to Lactobacillus, Propionibacterium, Streptococcus, Leuconostoc and Pediococcus, and L. plantarum had the highest CLA production among all strains. Overall CLA production, 21 % was CLA1 and 79 % was CLA2 (Ando et al., 2003).
4.2. Bacterial growth phase
It has been discovered that CLA production depends on the bacterial growth phases; in some of them, CLA is produced in the logarithmic phase and in others in the stationary phase. The production potential of CLA by L. lactis in culture medium and milk containing sunflower oil was studied. The results showed that at all concentrations of sunflower oil, when the oil was added at the beginning of incubation (the cells are in the growth stage), bacteria produced more CLA than when the cells were in a stationary phase (Kim & Liu, 2002). In another study, production of CLA in probiotic yogurt and the optimization of the production process by mixing the starter and sunflower oil as a source of LA have been investigated. Temperature of 37 °C, sunflower oil at 0.5 mL/L milk and pH = 5 were estimated as the optimal conditions for CLA production in yogurt. Increasing the temperature and oil content had a decreasing effect on the production of CLA, while adding oil at the end of the growth phase increased the production rate. Under these conditions, 10.07 mg CLA/g milk fat was produced, which indicates a 161 % increase compared to regular yogurt (Khorasani, 2005).
4.3. Substrate
Studies have shown that the type of carbon source used for bacterial growth affects the extent and type of CLA produced. For example, when different sources of carbon (e.g., glucose, lactose or a combination of glucose-lactose) were used for CLA production by L. plantarum in biofilm reactors, the extent of biomass and CLA was changed. The combination of lactose-glucose in the ratio of 1:1 resulted in the highest amount of biomass (3.66 g/L) and the highest concentration of CLA (37.08 μg/mL) (Razmjooei, et al., 2020).
4.4. Triglyceride esters
LA is one of the most important substrates for the production of CLA in bacterial cultures. The form of LA in the substrate can be free or esterified in the triglyceride structure. According to studies, LAB use only the free form of LA to produce CLA and cannot use LA in the ester structure or triacylglycerol form (Kishino et al., 2009). In addition, ricinoleic acid is another substrate used by LABs to produce CLA. It has been revealed that bacteria use its free form to produce CLA, and they cannot use the esterified form and triacylglycerol of ricinoleic acid (Kishino et al., 2009). But, some studies have shown that the free or esterification forms of LA did not influence the CLA production. In a study, three strains of Propionibacterium freundenreichii ssp. shermanii, which converts free LA to CLA in vitro, were used as auxiliary strains along with Geotrichum candidum and Yarrowia lipolytica to make cheese. It was observed that total CLA levels (esterified and free) were similar in control and experimental cheeses and remained unchanged during the four months of ripening. The addition of LA-rich safflower oil increased the concentration of free LA produced in the cheese, but the CLA content did not change (Das et al., 2005).
4.5. Type and quantity of oil
Vegetable oils could be a more suitable substrate for CLA production depending on the content of CLA precursor FAs (Turek & Wszołek, 2021). Accordingly, castor, sunflower, safflower, and sesame oils are the most common vegetable oils used as a microbial substrate for CLA production. Ogawa et al., (2005) used castor oil as a substrate to produce CLA by LAB. Castor oil is rich in triacylglycerol ricinoleic acid, which was used as a substrate for CLA production using lipase enzyme and oil hydrolysis (to release ricinoleic acid as a substrate for CLA production). This study showed that L. plantarum AKU 1009a had the highest CLA production among the studied strains. L. plantarum AKU 1009a firstly produced α- and γ-linolenic acids from conjugated trienoic FAs. Trienoic FAs produced from α-linolenic acid include c9, t11, c15-octadecatrienoic acid (18:3) and t9, t11, c15-18: 3 and those from γ-linolenic include c6, c9, t11-18:3 and c6, t9, t11-18:3. Conjugated trienoic FAs produced from α- and γ-linolenic acids were converted to t10, c15-18:2 and c6, t10-18:2 by L. plantarum AKU 1009a, respectively (Ogawa et al., 2005).
Van Nieuwenhove et al., (2007) investigated the effect of adding sunflower oil on CLA production in buffalo cheese. The results showed that the level of CLA in cheese was higher than raw milk, especially after ripening period. In addition, they found that sunflower oil increased the CLA content of fresh cheese. CLA can also be formed under optimal conditions in milk containing sunflower oil during fermentation by LAB. In another study, Kim and Liu (2002) evaluated the factors and methods that increase the amount of CLA in fermented milk. They studied 14 LAB strains to produce CLA using sunflower oil (containing 70 % LA) as a substrate. Based on the obtained data, it was found that among the screened strains, Lactococcus lactis I-01 had the highest ability to produce CLA. The optimum concentration of sunflower oil for CLA production was estimated as 0.1 g/L milk, which was 0.25 % of total milk fat. The results showed that the formation of CLA in fermented milk can be affected by many factors, such as bacterial strain, cell number, optimal substrate concentration and incubation time at neutral pH (Kim & Liu, 2002).
4.6. Lipase enzyme
Researchers have found that oil, either intact or lipolyzed, could have a significant effect on CLA content. Oil lipolysis could be due to endogenous oil lipase or external lipase added to the product. Studies performed by Vahvaselkä and Laakso (2010) showed that by introducing oat meal (water activity = 0.7), lipolytic activity started after three weeks and LA was released; then, in the lipolyzed form of oats, Propionibacterium freundenreichii was cultured and the CLA content was studied. It was revealed that the content of CLA produced by bacteria in the fermented substrate was significantly higher than the non-fermented substrate. Therefore, fermentation is mediated by the activity of endogenous enzymes and the production of LA, which is a precursor for CLA production; it can be another effective factor in CLA production.
Hosseini et al., (2015) used sunflower and castor oils as cost-effective substrates (compared to LA) for CLA production. In this study, bacterial lipase was used to produce free FAs from sunflower and castor oils. In addition, they examined the effect of some significant parameters on CLA production, such as substrate type and concentration, incubation time, and the effect of probiotic lipase on sunflower and castor oils. Among the 5 probiotic strains studied, L. plantarum (ATCC 8014) cells produced the highest concentration of CLA isomers. Their results also showed that the produced CLA was a mixture of two bioactive isomers, including c9, t11-CLA (0.38 mg/mL) and t10, c12-CLA (0.42 mg/mL), which were obtained from 8 mg/mL of sunflower oil in the presence of bacterial lipase. Therefore, they discovered the important capability of L. plantarum (ATCC 8014) to produce active CLA isomers from cost-effective substrates for the production of probiotic supplements such as CLA and other bioactive compounds (Hosseini et al., 2015).
4.7. pH
The pH of medium plays an important role in the fermentation process, as it can alter the surface charge of microorganisms, thereby affecting their growth and the internal metabolic pathways responsible for nutrient uptake. It is now well understood that lactobacilli convert LA to CLA through the activity of LA isomerase, and this enzyme is sensitive to pH. Studies have shown that at pH = 5.0, CLA isomers gradually increased for 48 h after incubation and then decreased. The highest quantity of CLA isomers produced at this pH included the c9, t11: 16.0; t10, c12: 4.36 g/mL and t9, t11: 5.51 g/mL. Similarly, at pH = 6 and 7, an increasing trend in CLA biosynthesis was observed for the first 48 h; the isomers produced at pH = 6 and 7 were included, (c9, t11: 31.97 and t10, c12: 5.94 and t9, t11, 9.99 g/mL) and (c9, t11: 24.21 and t10, c12: 4.04 and t9, t11: 6.31 g/mL), respectively (Dahiya & Puniya, 2018). Dahiya and Puniya (2018) showed that the highest quantity of CLA was produced at pH = 6 (compared to pH = 5 and 7). This may be due to the slower growth of strains at this pH, which in turn affects linoleate isomerase.
Researchers have shown that the maximum CLA biosynthesis from LAB will be attained in the optimal pH range, which is mainly related to better enzyme performance. For instance, Ando et al., (2003) found that the maximum CLA synthesis from L. plantarum JCM 1551 cells was at pH = 6.5. In another study, Li et al. (2013) demonstrated that efficient production of CLA with L. acidophilus F0221 from LA substrate was possible when the initial pH of the medium was in the range of 6.0–7.0. However, data showed that lactobacilli have the ability to produce CLA at a wide pH range; lower (pH = 5.0) and higher (pH = 7.0). These results indicate that lactobacilli have the potential to produce CLA in different conditions and this varies from one strain to another (Dahiya & Puniya, 2018).
4.8. Incubation time
Some works have revealed that CLA production could increase over time, but gradually decreases over long periods of time. As an example, Dahiya and Puniya (2018) observed that CLA levels increased up to 48 h after incubation, but after this time its production decreased. Incubation for > 48 h reduces the density of living cells, which could be due to excessive accumulation of c9, t11-CLA, since it inhibits bacterial activity. In another study, at higher incubation time from 24 to 72 h, a gradual increase in c9, t11-CLA biosynthesis was observed, but in any case, long-term fermentation seems to reduce the production of c9, t11-CLA (Wang et al., 2022). The ability of L. plantarum Ip15 to synthesize CLA in MRS liquid culture medium was evaluated by adding 0.2 mg/mL LA; the results showed that CLA increased to 48.7 μg/mL within 48 h and then, it dropped quickly. In addition, data showed that with increasing incubation time up to 48 h, CLA production increased and then, a decreasing trend. They found that in the medium inoculated with L. acidophilus and Streptococcus thermophilus, the maximum synthesis of CLA was 120.37 μg/mL. Therefore, according to the different stages of bacterial growth, LA concentration and isomeric activity of linoleate, the highest amount of CLA can be obtained at a specific time (Wang et al., 2022).
5. Conclusions and perspectives
In recent years, CLA has attracted a lot of attention due to its health-promoting properties, such as anti-cancer, anti-inflammatory, anti-obesity, immune-boosting function, and anti-diabetic activity. The present study was a critical review of different CLA production strategies in which microorganisms with high potential in CLA production, factors affecting microbial production and CLA-rich sources were compared and studied. Due to many health-promoting effects of CLA for humans, it is recommended to use it in the diet. However, the testing of CLA in clinical trials is still limited, and further research is needed in the future. It is also necessary to provide reliable information considering the functional activities of CLA in dairy products in future research so that the consumer can choose the product with more knowledge and confidence. In terms of production costs, chemical methods are likely to be economical than fermentation methods. However, the quality and purity of microbial CLA are far higher than the product of chemical processes. Therefore, future studies should focus more on optimizing the microbial production process and screening species with a high production capacity and selectivity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Last author acknowledges the Chinese Ministry of Science and Technology “Belt and Road” Innovative Talent Exchange Foreign Expert Project (Grant Number DL2023003001L).
Data availability
No data was used for the research described in the article.
References
- Adamczak M., Bornscheuer U.T., Bednarski W. Properties and biotechnological methods to produce lipids containing conjugated linoleic acid. European Journal of Lipid Science and Technology. 2008;110(6):491–504. [Google Scholar]
- Alonso L., Cuesta E., Gilliland S. Production of free conjugated linoleic acid by Lactobacillus acidophilus and Lactobacillus casei of human intestinal origin. Journal of Dairy Science. 2003;86(6):1941–1946. doi: 10.3168/jds.S0022-0302(03)73781-3. [DOI] [PubMed] [Google Scholar]
- Amiri S., Mokarram R.R., Khiabani M.S., Bari M.R., Khaledabad M.A. In situ production of conjugated linoleic acid by Bifidobacterium lactis BB12 and Lactobacillus acidophilus LA5 in milk model medium. LWT. 2020;132 [Google Scholar]
- Asbaghi O., Shimi G., Naseri K., Saadati S., Kelishadi M.R., Doaei S., Haghighat N. The effects of conjugated linoleic acid supplementation on blood pressure and endothelial function in adults: A systematic review and dose-response meta-analysis. European Journal of Pharmacology. 2022;175162 doi: 10.1016/j.ejphar.2022.175162. [DOI] [PubMed] [Google Scholar]
- Asbaghi O., Shimi G., Shiraseb F., Karbasi A., Nadery M., Ashtary-Larky D.…Haghighat N. The effects of conjugated linoleic acid supplementation on liver function enzymes and malondialdehyde in adults: A GRADE-assessed systematic review and dose-response meta-analysis. Pharmacological Research. 2022;106518 doi: 10.1016/j.phrs.2022.106518. [DOI] [PubMed] [Google Scholar]
- Ando A., Ogawa J., Kishino S., Shimizu S. CLA production from ricinoleic acid by lactic acid bacteria. Journal of the American Oil Chemists' Society. 2003;80(9):889–894. [Google Scholar]
- Ando A., Ogawa J., Kishino S., Shimizu S. Conjugated linoleic acid production from castor oil by Lactobacillus plantarum JCM 1551. Enzyme and Microbial Technology. 2004;35(1):40–45. [Google Scholar]
- Arjeh E., Akhavan H.-R., Barzegar M., Carbonell-Barrachina Á.A. Bio-active compounds and functional properties of pistachio hull: A review. Trends in Food Science & Technology. 2020;97:55–64. [Google Scholar]
- Arjeh E., Khodaei S.M., Barzegar M., Pirsa S., Karimi Sani I., Rahati S., Mohammadi F. Phenolic compounds of sugar beet (Beta vulgaris L.): Separation method, chemical characterization, and biological properties. Food Science & Nutrition. 2022;10:4238–4246. doi: 10.1002/fsn3.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila G., Catozzi C., Pravettoni D., Sala G., Martino P., Meroni G.…Ceciliani F. In vitro effects of conjugated linoleic acid (CLA) on inflammatory functions of bovine monocytes. Journal of Dairy Science. 2020;103(9):8554–8563. doi: 10.3168/jds.2020-18659. [DOI] [PubMed] [Google Scholar]
- Aydin R. Conjugated linoleic acid: Chemical structure, sources and biological properties. Turkish Journal of Veterinary and Animal Sciences. 2005;29(2):189–195. [Google Scholar]
- Banni S., Carta G., Angioni E., Murru E., Scanu P., Melis M.P.…Ip C. Distribution of conjugated linoleic acid and metabolites in different lipid fractions in the rat liver. Journal of Lipid Research. 2001;42(7):1056–1061. [PubMed] [Google Scholar]
- Bassaganya-Riera J., Hontecillas R. Dietary CLA and n-3 PUFA in inflammatory bowel disease. Current Opinion in Clinical Nutrition and Metabolic Care. 2010;13(5):569. doi: 10.1097/MCO.0b013e32833b648e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito P., Nelson G., Kelley D., Bartolini G., Schmidt P., Simon V. The effect of conjugated linoleic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids. 2001;36(3):221–227. doi: 10.1007/s11745-001-0711-y. [DOI] [PubMed] [Google Scholar]
- Berdeaux O., Christie W., Gunstone F., Sebedio J.-L. Large-scale synthesis of methyl cis-9, trans-11-octadecadienoate from methyl ricinoleate. Journal of the American Oil Chemists' Society. 1997;74(8):1011–1015. [Google Scholar]
- Blasi F., Cossignani L. Fermentation as a strategy to increase conjugated linoleic acid in dairy products. Large Animal Review. 2019;25(3):101–104. [Google Scholar]
- Boyaval P., Corre C., Dupuis C., Roussel E. Effects of free fatty acids on propionic acid bacteria. Le Lait. 1995;75(1):17–29. [Google Scholar]
- Brouwer I.A., Wanders A.J., Katan M.B. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans–a quantitative review. PLoS One1. 2010;5(3):e9434. doi: 10.1371/journal.pone.0009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz D.E., Li G., Huebner S.M., Cook M.E. A mechanistic approach to understanding conjugated linoleic acid's role in inflammation using murine models of rheumatoid arthritis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;293(2):R669–R676. doi: 10.1152/ajpregu.00005.2007. [DOI] [PubMed] [Google Scholar]
- Caballero D., Ríos-Reina R., Amigo J. Reference Module in Food Science. Encyclopedia of Food and Health. 2021:132–138. [Google Scholar]
- Chen J., Zhang L., Zheng X., Zheng Y. Revealing ruthenium and basicity synergetic effects in Ru–MgAl catalysts for isomerization of linoleic acid to conjugated linoleic acid. RSC advances. 2017;7:54747–54755. [Google Scholar]
- Chen P.B., Park Y. Conjugated linoleic acid in human health: Effects on weight control. Nutrition in the Prevention and Treatment of Abdominal Obesity. 2019:355–382. [Google Scholar]
- Chinnadurai K., Tyagi A. Conjugated linoleic acid: A milk fatty acid with unique health benefit properties. Soybean and Health. 2011;111 [Google Scholar]
- Choi J.S., Jung M.H., Park H.S., Song J. Effect of conjugated linoleic acid isomers on insulin resistance and mRNA levels of genes regulating energy metabolism in high-fat–fed rats. Nutrition. 2004;20(11–12):1008–1017. doi: 10.1016/j.nut.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Chung S.H., Kim I.H., Park H.G., Kang H.S., Yoon C.S., Jeong H.Y.…Kim Y.J. Synthesis of conjugated linoleic acid by human-derived Bifidobacterium breve LMC 017: Utilization as a functional starter culture for milk fermentation. Journal of Agricultural and Food Chemistry. 2008;56(9):3311–3316. doi: 10.1021/jf0730789. [DOI] [PubMed] [Google Scholar]
- Coakley M., Ross R., Nordgren M., Fitzgerald G., Devery R., Stanton C. Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. Journal of Applied Microbiology. 2003;94(1):138–145. doi: 10.1046/j.1365-2672.2003.01814.x. [DOI] [PubMed] [Google Scholar]
- Dachev M., Bryndová J., Jakubek M., Moučka Z., Urban M. The effects of conjugated linoleic acids on cancer. Processes. 2021;9(3):454. [Google Scholar]
- Dahiya D.K., Puniya A.K. Optimisation of fermentation variables for conjugated linoleic acid bioconversion by Lactobacillus fermentum DDHI 27 in modified skim milk. International Journal of Dairy Technology. 2018;71(1):46–55. [Google Scholar]
- Das S., Holland R., Crow V., Bennett R., Manderson G. Effect of yeast and bacterial adjuncts on the CLA content and flavour of a washed-curd, dry-salted cheese. International Dairy Journal. 2005;15(6–9):807–815. [Google Scholar]
- DeLany J.P., Blohm F., Truett A.A., Scimeca J.A., West D.B. Conjugated linoleic acid rapidly reduces body fat content in mice without affecting energy intake. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1999;276(4):R1172–R1179. doi: 10.1152/ajpregu.1999.276.4.R1172. [DOI] [PubMed] [Google Scholar]
- Deng M.-D., Grund A.D., Schneider K.J., Langley K.M., Wassink S.L., Peng S.S., Rosson R.A. Linoleic acid isomerase from Propionibacterium acnes: Purification, characterization, molecular cloning, and heterologous expression. Applied Biochemistry and Biotechnology. 2007;143(3):199–211. doi: 10.1007/s12010-007-8075-1. [DOI] [PubMed] [Google Scholar]
- Den Hartigh L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients. 2019;11(2):370. doi: 10.3390/nu11020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshande-Rishehri S.-M., Mansourian M., Kelishadi R., Heidari-Beni M. Association of foods enriched in conjugated linoleic acid (CLA) and CLA supplements with lipid profile in human studies: A systematic review and meta-analysis. Public Health Nutrition. 2015;18(11):2041–2054. doi: 10.1017/S1368980014002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman T.R., Nam S.-H., Ure A.L. Factors affecting conjugated linoleic acid content in milk and meat. Critical Reviews in Food Science and Nutrition. 2005;45(6):463–482. doi: 10.1080/10408390591034463. [DOI] [PubMed] [Google Scholar]
- Dilzer A., Park Y. Implication of conjugated linoleic acid (CLA) in human health. Critical reviews in food science and nutrition. 2012;52(6):488–513. doi: 10.1080/10408398.2010.501409. [DOI] [PubMed] [Google Scholar]
- Dohme-Meier F., Bee G. Feeding unprotected CLA methyl esters compared to sunflower seeds increased milk CLA level but inhibited milk fat synthesis in cows. Asian-Australasian Journal of Animal Sciences. 2012;25(1):75. doi: 10.5713/ajas.2011.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmani J., Safari M., Roohvand F., Razavi S.H., Aghasadeghi M.R., Noorbazargan H. Conjugated linoleic acid-producing enzymes: A bioinformatics study. European Journal of Lipid Science and Technology. 2010;112(10):1088–1100. [Google Scholar]
- Gangidi R., Proctor A. Photochemical production of conjugated linoleic acid from soybean oil. Lipids. 2004;39(6):577–582. doi: 10.1007/s11745-004-1266-7. [DOI] [PubMed] [Google Scholar]
- Gao H., Yang B., Stanton C., Ross R.P., Zhang H., Chen H., Chen W. Linoleic acid induces different metabolic modes in two Bifidobacterium breve strains with different conjugated linoleic acid-producing abilities. LWT. 2021;142 [Google Scholar]
- Gebauer S.K., Destaillats F., Dionisi F., Krauss R.M., Baer D.J. Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect LDL cholesterol: A double-blind, randomized controlled trial. The American Journal of Clinical Nutrition. 2015;102(6):1339–1346. doi: 10.3945/ajcn.115.116129. [DOI] [PubMed] [Google Scholar]
- Gnadig S., Xue Y., Berdeaux O., Chardigny J., Sebedio J. Conjugated linoleic acid (CLA) as a functional ingredient. Functional Dairy Products. 2003:263–297. [Google Scholar]
- Gorissen L., Raes K., Weckx S., Dannenberger D., Leroy F., De Vuyst L., De Smet S. Production of conjugated linoleic acid and conjugated linolenic acid isomers by Bifidobacterium species. Applied Microbiology and Biotechnology. 2010;87(6):2257–2266. doi: 10.1007/s00253-010-2713-1. [DOI] [PubMed] [Google Scholar]
- Gorissen L., Weckx S., Vlaeminck B., Raes K., De Vuyst L., De Smet S., Leroy F. Linoleate isomerase activity occurs in lactic acid bacteria strains and is affected by pH and temperature. Journal of Applied Microbiology. 2011;111(3):593–606. doi: 10.1111/j.1365-2672.2011.05087.x. [DOI] [PubMed] [Google Scholar]
- Guo M. Elsevier; 2013. Functional foods: Principles and technology. [Google Scholar]
- Hosseini E.S., Kermanshahi R.K., Hosseinkhani S., Shojaosadati S.A., Nazari M. Conjugated linoleic acid production from various substrates by probiotic Lactobacillus plantarum. Annals of Microbiology. 2015;65(1):27–32. [Google Scholar]
- Hou J., Liu Y., Wang Y., Xiao Z., Liu F., Yu W.…Xu J. Promoting the production of conjugated linoleic acid by optimizing the fermentation parameters of Lactobacillus sp. Milchwissenschaft. 2011;66(4):368–371. [Google Scholar]
- Huang Y.-C., Luedecke L.O., Shultz T.D. Effect of cheddar cheese consumption on plasma conjugated linoleic acid concentrations in men. Nutrition Research. 1994;14(3):373–386. [Google Scholar]
- Ibrahim K.S., El-Sayed E.M. Dietary conjugated linoleic acid and medium-chain triglycerides for obesity management. Journal of Biosciences. 2021;46(1):1–14. [PubMed] [Google Scholar]
- Irmak S., Dunford N.T., Gilliland S.E., Banskalieva V., Eisenmenger M. Biocatalysis of linoleic acid to conjugated linoleic acid. Lipids. 2006;41(8):771–776. doi: 10.1007/s11745-006-5030-9. [DOI] [PubMed] [Google Scholar]
- Jaglan N., Kumar S., Choudhury P.K., Tyagi B., Tyagi A.K. Isolation, characterization and conjugated linoleic acid production potential of bifidobacterial isolates from ruminal fluid samples of Murrah buffaloes. Anaerobe. 2019;56:40–45. doi: 10.1016/j.anaerobe.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Jaudszus A., Moeckel P., Hamelmann E., Jahreis G. Trans-10, cis-12-CLA-caused lipodystrophy is associated with profound changes of fatty acid profiles of liver, white adipose tissue and erythrocytes in mice: Possible link to tissue-specific alterations of fatty acid desaturation. Annals of Nutrition and Metabolism. 2010;57(2):103–111. doi: 10.1159/000319877. [DOI] [PubMed] [Google Scholar]
- Jiang J., Björck L., Fonden R. Production of conjugated linoleic acid by dairy starter cultures. Journal of Applied Microbiology. 1998;85(1):95–102. doi: 10.1046/j.1365-2672.1998.00481.x. [DOI] [PubMed] [Google Scholar]
- Kabara J.J., Swieczkowski D.M., Conley A.J., Truant J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrobial Agents and Chemotherapy. 1972;2(1):23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadamne J.V., Castrodale C.L., Proctor A. Measurement of conjugated linoleic acid (CLA) in CLA-rich potato chips by ATR-FTIR spectroscopy. Journal of Agricultural and Food Chemistry. 2011;59(6):2190–2196. doi: 10.1021/jf104204e. [DOI] [PubMed] [Google Scholar]
- Kang J.-H., Lee G.-S., Jeung E.-B., Yang M.-P. Trans-10, cis-12-conjugated linoleic acid increases phagocytosis of porcine peripheral blood polymorphonuclear cells in vitro. British Journal of Nutrition. 2007;97(1):117–125. doi: 10.1017/S0007114507280584. [DOI] [PubMed] [Google Scholar]
- Khanal R., Dhiman T., Ure A., Brennand C., Boman R., McMahon D.J. Consumer acceptability of conjugated linoleic acid-enriched milk and cheddar cheese from cows grazing on pasture. Journal of Dairy Science. 2005;88(5):1837–1847. doi: 10.3168/jds.S0022-0302(05)72858-7. [DOI] [PubMed] [Google Scholar]
- Kim Y., Liu R. Increase of conjugated linoleic acid content in milk by fermentation with lactic acid bacteria. Journal of Food Science. 2002;67(5):1731–1737. [Google Scholar]
- Kim Y.J. Partial inhibition of biohydrogenation of linoleic acid can increase the conjugated linoleic acid production of Butyrivibrio fibrisolvens A38. Journal of Agricultural and Food Chemistry. 2003;51(15):4258–4262. doi: 10.1021/jf034057r. [DOI] [PubMed] [Google Scholar]
- Kishino S., Ogawa J., Omura Y., Matsumura K., Shimizu S. Conjugated linoleic acid production from linoleic acid by lactic acid bacteria. Journal of the American Oil Chemists' Society. 2002;79(2):159–163. [Google Scholar]
- Kishino S., Ogawa J., Yokozeki K., Shimizu S. Microbial production of conjugated fatty acids. Lipid Technology. 2009;21(8–9):177–181. [Google Scholar]
- Knekt P., Järvinen R., Seppänen R., Pukkala E., Aromaa A. Intake of dairy products and the risk of breast cancer. British Journal of Cancer. 1996;73(5):687–691. doi: 10.1038/bjc.1996.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba K., Yanagita T. Health benefits of conjugated linoleic acid (CLA) Obesity Research & Clinical Practice. 2014;8(6):e525–e532. doi: 10.1016/j.orcp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S.A., Wright S., Czarnecki S.K., Wilson T.A., Nicolosi R.J. Conjugated linoleic acid isomer effects in atherosclerosis: Growth and regression of lesions. Lipids. 2004;39(7):611–616. doi: 10.1007/s11745-004-1273-8. [DOI] [PubMed] [Google Scholar]
- Kumar R., Bhatia A., Arora D. Health benefits of conjugated linoleic acid: A review. J Clinical Diagnostic Res. 2009;3:1953–1967. [Google Scholar]
- Kumar S., Sharma B., Bhadwal P., Sharma P., Agnihotri N. Therapeutic foods. Elsevier; 2018. Lipids as nutraceuticals: A shift in paradigm; pp. 51–98. [Google Scholar]
- Kung F.-C., Yang M.-C. Effect of conjugated linoleic acid immobilization on the hemocompatibility of cellulose acetate membrane. Colloids and Surfaces B: Biointerfaces. 2006;47(1):36–42. doi: 10.1016/j.colsurfb.2005.11.019. [DOI] [PubMed] [Google Scholar]
- LeDoux M., Laloux L., Fontaine J.-J., Carpentier Y.A., Chardigny J.-M., Sébédio J.-L. Rumenic acid significantly reduces plasma levels of LDL and small dense LDL cholesterol in hamsters fed a cholesterol-and lipid-enriched semi-purified diet. Lipids. 2007;42(2):135–141. doi: 10.1007/s11745-007-3023-y. [DOI] [PubMed] [Google Scholar]
- Li M., Liu Y., Zhao J., Yu R., Hussain M.A., Qayum A.…Qu B. Glycosylated whey protein isolate enhances digestion behaviors and stabilities of conjugated linoleic acid oil in water emulsions. Food Chemistry. 2022;383 doi: 10.1016/j.foodchem.2022.132402. [DOI] [PubMed] [Google Scholar]
- Lee K., Paek K., Lee H., Park J.H., Lee Y. Antiobesity effect of trans-10, cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. Journal of Applied Microbiology. 2007;103(4):1140–1146. doi: 10.1111/j.1365-2672.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- Lee S.O., Hong G.W., Oh D.K. Bioconversion of linoleic acid into conjugated linoleic acid by immobilized Lactobacillus reuteri. Biotechnology Progress. 2003;19(3):1081–1084. doi: 10.1021/bp0257933. [DOI] [PubMed] [Google Scholar]
- Lehnen T.E., da Silva M.R., Camacho A., Marcadenti A., Lehnen A.M. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. Journal of the International Society of Sports Nutrition. 2015;12(1):1–11. doi: 10.1186/s12970-015-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang L., Han X., Yi H., Guo C., Zhang Y.…Shan Y. Effect of incubation conditions and possible intestinal nutrients on cis-9, trans-11 conjugated linoleic acid productions by Lactobacillus acidophilus F0221. International Dairy Journal. 2013;29(2):93–98. [Google Scholar]
- Li Y.-C., Chen Q., Wan X.-Z., Yang X.-L., Liu X., Zhong L. Effects of conjugated linoleic acid on cleavage of amyloid precursor protein via PPARγ. Neurological Sciences. 2011;32(6):1095–1101. doi: 10.1007/s10072-011-0711-4. [DOI] [PubMed] [Google Scholar]
- Lin H., Boylston T., Luedecke L., Shultz T. Conjugated linoleic acid content of Cheddar-type cheeses as affected by processing. Journal of Food Science. 1999;64(5):874–878. [Google Scholar]
- Lin T.Y. Conjugated linoleic acid concentration as affected by lactic cultures and additives. Food Chemistry. 2000;69(1):27–31. [Google Scholar]
- Lounglawan P., Suksombat W. Effect of soybean oil and lactic acid bacteria supplementation on performance and CLA accumulation in milk of dairy cows. Journal of Animal and Veterinary Advances. 2011;10(10):868–874. [Google Scholar]
- Marín M., Meléndez P., Aranda P., Ríos C. Conjugated linoleic acid content and fatty acids profile of milk from grazing dairy cows in southern Chile fed varying amounts of concentrate. Journal of Applied Animal Research. 2018;46(1):150–154. [Google Scholar]
- Mei Y., Chen H., Yang B., Zhao J., Zhang H., Chen W. Linoleic Acid Triggered a Metabolomic Stress Condition in Three Species of Bifidobacteria Characterized by Different Conjugated Linoleic Acid-Producing Abilities. Journal of Agricultural and Food Chemistry. 2021;69(38):11311–11321. doi: 10.1021/acs.jafc.1c03752. [DOI] [PubMed] [Google Scholar]
- Moslemi M., Moayedi A., Khomeiri M., Maghsoudlou Y. Development of a whey-based beverage with enhanced levels of conjugated linoleic acid (CLA) as facilitated by endogenous walnut lipase. Food Chemistry: X. 2023;17 doi: 10.1016/j.fochx.2022.100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnik A., Gornowicz-Porowska J., Jenerowicz D., Polańska A., Dobrzyńska M., Przysławski J.…Ferreri C. Fatty Acids Profile and the Relevance of Membranes as the Target of Nutrition-Based Strategies in Atopic Dermatitis: A Narrative Review. Nutrients. 2023;15(17):3857. doi: 10.3390/nu15173857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea M., Bassaganya-Riera J., Mohede I.C. Immunomodulatory properties of conjugated linoleic acid. The American Journal of Clinical Nutrition. 2004;79(6):1199S–S1206. doi: 10.1093/ajcn/79.6.1199S. [DOI] [PubMed] [Google Scholar]
- Ogawa J., Kishino S., Ando A., Sugimoto S., Mihara K., Shimizu S. Production of conjugated fatty acids by lactic acid bacteria. Journal of Bioscience and Bioengineering. 2005;100(4):355–364. doi: 10.1263/jbb.100.355. [DOI] [PubMed] [Google Scholar]
- Ogawa J., Matsumura K., Kishino S., Omura Y., Shimizu S. Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Applied and Environmental Microbiology. 2001;67(3):1246–1252. doi: 10.1128/AEM.67.3.1246-1252.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özer C.O., Kılıç B. Optimization of pH, time, temperature, variety and concentration of the added fatty acid and the initial count of added lactic acid Bacteria strains to improve microbial conjugated linoleic acid production in fermented ground beef. Meat Science. 2021;171 doi: 10.1016/j.meatsci.2020.108303. [DOI] [PubMed] [Google Scholar]
- Olivo P.M., Dos Santos G.T., Rodrigues B.M., Osmari M.P., Marchi F.E.D., Madrona G.S.…Pozza M.S. Starter bacteria as producers of CLA in ripened cheese. Anais da Academia Brasileira de Ciências. 2021;93:e20190677. doi: 10.1590/0001-3765202120190677. [DOI] [PubMed] [Google Scholar]
- Palachum W., Choorit W., Chisti Y. Accumulation of conjugated linoleic acid in Lactobacillus plantarum WU-P19 is enhanced by induction with linoleic acid and chitosan treatment. Annals of Microbiology. 2018;68(10):611–624. [Google Scholar]
- Park Y., Kim J., Scrimgeour A.G., Condlin M.L., Kim D., Park Y. Conjugated linoleic acid and calcium co-supplementation improves bone health in ovariectomised mice. Food Chemistry. 2013;140(1–2):280–288. doi: 10.1016/j.foodchem.2012.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Pariza M.W. Mechanisms of body fat modulation by conjugated linoleic acid (CLA) Food Research International. 2007;40(3):311–323. [Google Scholar]
- Paszczyk B., Czarnowska-Kujawska M. Fatty Acid Profile, Conjugated Linoleic Acid Content, and Lipid Quality Indices in Selected Yogurts Available on the Polish Market. Animals. 2022;12(1):96. doi: 10.3390/ani12010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putera H.D., Doewes R.I., Shalaby M.N., Ramírez-Coronel A.A., Clayton Z.S., Abdelbasset W.K.…Nattagh-Eshtivani E. The effect of conjugated linoleic acids on inflammation, oxidative stress, body composition and physical performance: A comprehensive review of putative molecular mechanisms. Nutrition & Metabolism. 2023;20(1):1–16. doi: 10.1186/s12986-023-00758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainio A., Vahvaselkä M., Suomalainen T., Laakso S. Production of conjugated linoleic acid by Propionibacterium freudenreichii ssp. shermanii. Le Lait. 2002;82(1):91–101. [PubMed] [Google Scholar]
- Razmjooei M., Shad E., Nejadmansouri M., Safdarianghomsheh R., Delvigne F., Khalesi M. Effect of metal support and different carbon sources on CLA production using Lactobacillus plantarum. Biochemical Engineering Journal. 2020;162 [Google Scholar]
- Reaney M.J., Liu Y.-D., Westcott N.D. Commercial production of conjugated linoleic acid. Advances in Conjugated Linoleic Acid Research. 1999;1:39–54. [Google Scholar]
- Roche H.M., Noone E., Gibney A.N.M.J. Conjugated linoleic acid: A novel therapeutic nutrient? Nutrition Research Reviews. 2001;14(1):173–188. doi: 10.1079/NRR200122. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Alcalá L.M., Braga T., Malcata F.X., Gomes A., Fontecha J. Quantitative and qualitative determination of CLA produced by Bifidobacterium and lactic acid bacteria by combining spectrophotometric and Ag+-HPLC techniques. Food Chemistry. 2011;125(4):1373–1378. [Google Scholar]
- Roman-Nunez M., Cuesta-Alonso E., Gilliland S. Influence of sodium glycocholate on production of conjugated linoleic acid by cells of Lactobacillus reuteri ATCC 55739. Journal of Food Science. 2007;72(4):M140–M143. doi: 10.1111/j.1750-3841.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Saebo A. Commercial synthesis of conjugated linoleate. Advances in Conjugated Linoleic Acid Research. 2003;2:71–81. [Google Scholar]
- Salamon R., Vargáné-Visi É., András C.D., Csapóné Kiss Z., Csapó J. Synthetic methods to obtain conjugated linoleic acids (CLAs) by catalysis–A review. Acta Alimentaria. 2015;44(2):229–234. [Google Scholar]
- Shaikh A., Pawar A., Parmar H., Vadgama R., Lali A., Odaneth A. Conjugated Linoleic Acid production by Lactic Acid Bacteria: A Bio-transformation study in media with oil hydrolysates. Journal of Applied Biotechnology & Bioengineering. 2018;5:321–327. [Google Scholar]
- Song N.-E., Kim N.-J., Kim Y.-H., Baik S.-H. Probiotic Properties of Lactic Acid Bacteria with High Conjugated Linoleic Acid Converting Activity Isolated from Jeot-Gal High-Salt Fermented Seafood. Microorganisms. 2021;9(11):2247. doi: 10.3390/microorganisms9112247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.-S., Kang S.-W., Oh D.-K., Rho Y.-T., Hong S.-I., Kim S.-W. Bioconversion of linoleic acid to conjugated linoleic acid byBifidobacterium breve. Biotechnology and Bioprocess Engineering. 2005;10(4):357–361. [Google Scholar]
- Srivastava A., Kumar S., Tyagi A., Shrivastava N., Varma A., Tyagi A.K. Butyrivibrio fibrisolvens F7 dietary supplementation increases levels of cis 9-trans 11 conjugated linoleic acid in gut and adipose tissue in mice. Current Research in Biotechnology. 2021;3:300–307. [Google Scholar]
- Suksatan W., Putera H.D., Abdulkadhim A.H., Hammid A.T., Ismailov J.A., Jannat B.…Izadi F. The effect of conjugated linoleic acid supplementation on oxidative stress markers: A systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition ESPEN. 2022;49:121–128. doi: 10.1016/j.clnesp.2022.04.004. [DOI] [PubMed] [Google Scholar]
- Tapia A.M., Bautista J.A.N., Mendoza B.C., Pham L.J., Sarmago I.G., Oliveros M.C.R. Production of conjugated linoleic acid by lactic acid bacteria: Screening and optimization. Philippine Journal of Science. 2019;148(3):457–464. [Google Scholar]
- Toomey S., Harhen B., Roche H.M., Fitzgerald D., Belton O. Profound resolution of early atherosclerosis with conjugated linoleic acid. Atherosclerosis. 2006;187(1):40–49. doi: 10.1016/j.atherosclerosis.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Toomey S., Roche H., Fitzgerald D., Belton O. Regression of pre-established atherosclerosis in the apoE−/− mouse by conjugated linoleic acid. Biochemical Society Transactions. 2003;31(5):1075–1079. doi: 10.1042/bst0311075. [DOI] [PubMed] [Google Scholar]
- Turek K., Wszołek M. Comparative study of walnut and Camelina sativa oil as a functional component for the unsaturated fatty acids and conjugated linoleic acid enrichment of kefir. LWT. 2021;147 [Google Scholar]
- Tyagi A.K., Kumar S., Choudhury P.K., Tyagi B., Tyagi N. Conjugated linoleic acid producing potential of lactobacilli isolated from goat (AXB) rumen fluid samples. Asian-Australasian Journal of Animal Sciences. 2020;33(8):1233. doi: 10.5713/ajas.19.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniacke-Lowe T., Fox P.F. Equid milk: Chemistry, biochemistry and processing. Food Biochemistry and Food Processing. 2012:491–530. [Google Scholar]
- Vahvaselkä M., Laakso S. Production of cis-9, trans-11-conjugated linoleic acid in camelina meal and okara by an oat-assisted microbial process. Journal of Agricultural and Food Chemistry. 2010;58(4):2479–2482. doi: 10.1021/jf903383x. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhove C.P., Oliszewski R., González S.N., Chaia A.B.P. Influence of bacteria used as adjunct culture and sunflower oil addition on conjugated linoleic acid content in buffalo cheese. Food Research International. 2007;40(5):559–564. [Google Scholar]
- Viladomiu M., Hontecillas R., Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. European Journal of Pharmacology. 2016;785:87–95. doi: 10.1016/j.ejphar.2015.03.095. [DOI] [PubMed] [Google Scholar]
- Wang J., Li H., Meng X., Tong P., Liu X. Biosynthesis of c9, t11-conjugated linoleic acid and the effect on characteristics in fermented soy milk. Food Chemistry. 2022;368 doi: 10.1016/j.foodchem.2021.130866. [DOI] [PubMed] [Google Scholar]
- Watkins B.A., Li Y., Lippman H.E., Reinwald S., Seifert M.F. A test of Ockham's razor: Implications of conjugated linoleic acid in bone biology. The American Journal of Clinical Nutrition. 2004;79(6):1175S–S1185. doi: 10.1093/ajcn/79.6.1175S. [DOI] [PubMed] [Google Scholar]
- Weiss M., Martz F., Lorenzen C. REVIEWS: Conjugated Linoleic Acid: Historical Context and Implications. The Professional Animal Scientist. 2004;20(2):127–135. [Google Scholar]
- Wu W., Li H. Springer; 2018. Metabolites of lactic acid bacteria. In Lactic acid bacteria in foodborne hazards reduction; pp. 87–113. [Google Scholar]
- Xu S., Boylston T.D., Glatz B.A. Effect of lipid source on probiotic bacteria and conjugated linoleic acid formation in milk model systems. Journal of the American Oil Chemists' Society. 2004;81(6):589–595. [Google Scholar]
- Yang B., Gao H., Stanton C., Ross R.P., Zhang H., Chen Y.Q.…Chen W. Bacterial conjugated linoleic acid production and their applications. Progress in Lipid Research. 2017;68:26–36. doi: 10.1016/j.plipres.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Yurawecz M.P. vol. 2. The American Oil Chemists Society; 2003. (Advances in conjugated linoleic acid research). [Google Scholar]
- Zahed O., Khosravi-Darani K., Mortazavian A.M., Mohammadi A. Bacterial conjugated linoleic acid bio-fortification of synbiotic yogurts using Propionibacterium freudenreichii as adjunct culture. Italian Journal of Food Science. 2021;33(SP1):1–11. [Google Scholar]
- Zeng Y., Liu P., Yang X., Li H., Li H., Guo Y.…Liu X. The dietary c9, t11-conjugated linoleic acid enriched from butter reduces breast cancer progression in vivo. Journal of Food Biochemistry. 2020;44(4):e13163. doi: 10.1111/jfbc.13163. [DOI] [PubMed] [Google Scholar]
- Zhang H., Guo Y., Yuan J. Conjugated linoleic acid enhanced the immune function in broiler chicks. British Journal of Nutrition. 2005;94(5):746–752. doi: 10.1079/bjn20051482. [DOI] [PubMed] [Google Scholar]
- Zongo K., Krishnamoorthy S., Moses J.A., Yazici F., Çon A.H., Anandharamakrishnan C. Total conjugated linoleic acid content of ruminant milk: The world status insights. Food Chemistry. 2021;334 doi: 10.1016/j.foodchem.2020.127555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.