Highlights
-
•
The 437 volatile organic compounds, of which 414 were shared metabolites in the three Triticeae crops, with 3 metabolites unique to oats.
-
•
The 307 differentially accumulated metabolites from all the comparison groups.
-
•
Terpenoids and esters are the key metabolites determining the differences in flavor.
-
•
The alpha-linolenic acid and phenylalanine pathways are the most significant metabolic pathways.
-
•
The 42 differentially accumulated metabolites may be leading to the flavor differences.
Keywords: Triticeae crops, Oats, Flavor quality, Metabolomics, Volatile organic compounds
Abstract
Oats is a cereal well known for its high nutritional value and unique flavor. This study investigated the metabolomics data from oats, wheat, and barley using broadly targeted GC–MS metabonomic techniques. A total of 437 volatile organic compounds (VOCs) were identified, of which 414 were shared metabolites, with three metabolites unique to oats. Three hundred and seven differentially accumulated metabolites (DAMs) were screened from all the comparison groups, of which 27 metabolites were shared by oats and barley, and 121 shared by oats and wheat. Terpenoids and esters were the key metabolites determining the differences in flavor. A KEGG analysis indicated that the alpha-linolenic acid and phenylalanine pathways were the most significant metabolic pathways. The 42 DAMs found may be the main substances leading to the flavor differences between the different varieties. Overall, this study reveals the main reasons for the unique flavor of oats through metabolomic evidence.
1. Introduction
Oats, wheat, and barley of the Gramineae family are the most widely cultivated Triticeae crops in the world (Khakimov et al., 2014), providing half of the calories that humans consume and containing compounds important for health, such as vitamins (Loskutov and Khlestkina, 2021, Kaur et al., 2014). Clinical studies have revealed that the increased consumption of whole grains is highly correlated with the reduced incidence of chronic diseases (Zhang et al., 2010). In the global food market, several grain derivatives have appeared, most of which are designed for nutritional purposes. The composition of the chemical components of these crops also determines their use in food and consequently its price. Compared with other grains, oats are rich in dietary fiber, and contain unique proteins and vitamins. These nutritional benefits have the potential to raise consumers' awareness of healthy eating habits (Kamal et al., 2022, Fu et al., 2020). As one of the most promising functional foods in the future, the demand for oats is increasing, which is promoting the development of oat-based foods, such as oatmeal, oat milk, oat rice, and oat flour (Rasane et al., 2015).
Most plant breeders have focused on improving producer-oriented traits, such as yield (Zhang et al., 2023, Zhang et al., 2023), and resistance against plant diseases (Nazareno et al., 2022), and abiotic stress (Kutasy et al., 2023). However, consumer-oriented traits, such as flavor, have often been overlooked because the relative concentrations of flavor substances are low, despite a large flavor increase achievable with a minimal loss in yield (Tieman et al., 2017). The chemical composition and differences in content determine the basis of flavor (Zheng et al., 2016). For example, hundreds of VOCs can be detected in most plants: 113 VOCs in strawberry (Fan et al., 2021), 148 VOCs during the ripening process of passion fruit (Li et al., 2021), 184 VOCs in green tea processing (Wang et al., 2021), and 170 VOCs in grapefruit pulp (Zheng et al., 2016). In general, the number and quantities of metabolites vary greatly between different plants and between different varieties of the same plant (Li et al., 2022). Recently, research has focused not only on flavor-related chemicals, but also on significantly related loci, such as Lin5 in tomato (Tieman et al., 2017). Flavor is one of the characteristic quality indices of oats, which are rich in many non-VOCs and VOCs (Zhao et al., 2022), and affect its consumer acceptability. Therefore, a comprehensive comparison of the metabolites present in different plants or varieties will help plant breeders to cultivate and develop varieties with better flavor characteristics.
With the recent advances in metabolomics technologies, a systems biology approach, commonly applied for the in-depth monitoring of the plant metabolome, can achieve a higher analytical sensitivity (Suo et al., 2023). Metabolomics is the quantitative and qualitative analysis of secondary and primary metabolites by high-throughput and rapid methods, which may open up a new approach for targeted plant breeding (Fernie and Schauer, 2009, Colantonio et al., 2022). Predicting flavor attributes through the use of metabolomics is of great significance in food science and genetics (Qi et al., 2021), because revealing changes in metabolic profiles and identifying changes in key components will help to assess their impact on food quality characteristics (Shi et al., 2022).
Consumer liking is highly associated with flavor intensity but analyzing the flavor metabolite profiles of oats, wheat, and barley by GC–MS has not yet been reported. The present study aims to determine the metabolic accumulation profiles of three Triticeae crops and three oat varieties by qualitative and quantitative analysis. Multivariate statistical analysis methods such as principal component analysis (PCA), orthogonal projections to latent structures-discriminant analysis (OPLS-DA), hierarchical cluster analysis (HCA), and KEGG pathway analysis will be used to explain differences between metabolites and differentially accumulated metabolites (DAMs). This study will help to better reveal and understand the differences in the characteristics of the unique flavoring substances in oats.
2. Materials and methods
2.1. Plant materials
To study the unique flavor substances in oats, three Triticeae crops, oats (3 varieties: Neiyou-5, RV; Neiyou-6, MV; and Huazao-2, FV), wheat (Chinese Spring, WV), and barley (Mengpimai, BV), were selected for metabolomics analysis (Fig. 1A). Mature grain samples were collected from the Key Laboratory of Germplasm Innovation and Utilization of Triticeae crops, Inner Mongolia Agricultural University, Hohhot, China. The samples were stored in the dark until needed and had a moisture content of less than 13 %. For each sample, six biological replicates were independently analyzed (1.5 g/replicate). The husks of the barley samples were removed before analysis.
Fig. 1.
Metabolite profiles of three Triticeae crops. (A), Photographs of the three Triticeae crops used in this study (scale bar = 1 cm); (B), Classification of the 437 metabolites of three Triticeae crops; (C), Principal component analysis of metabolic profiles of three Triticeae crops; and (D), Hierarchical cluster analysis of three Triticeae crops. Each Triticeae sample is represented by a column, and each metabolite is displayed in a single row. Red indicates a relatively high metabolite abundance, while green indicates a relatively low abundance. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Sample preparation and extraction
The samples were prepared and extracted by Wuhan Metville Biotechnology Co., Ltd. In brief, the oat, wheat, and barley seeds were frozen at −80 °C until further analysis. A portion of the samples was ground to a powder in liquid nitrogen, then 500 mg (1 mL) of the powder was weighed and transferred immediately to a 20-mL head-space vial (Agilent, Palo Alto, CA, USA), containing NaCl saturated solution to inhibit any enzymic reaction. The vials were then sealed using crimp-top caps with PTFE-silicone headspace septa (Agilent). For the solid phase microextraction (SPME) analysis, each vial was heated to 60 °C for 5 min then a 120-µm Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) fiber (Agilent) was exposed to the headspace of the sample for 15 min at 60 °C.
2.3. GC–MS conditions
After sampling, the VOCs were desorbed from the fiber coating in the injection port of the GC apparatus (Model 8890; Agilent) at 250 °C for 5 min in the splitless mode. The VOCs were identified and quantified using an Agilent Model 8890 GC and a 7000D mass spectrometer equipped with a 30 m × 0.25 mm × 0.25 μm DB-5MS (5 % phenyl-polymethylsiloxane) capillary column. Helium was used as the carrier gas at a flow rate of 1.2 mL/min. The injector temperature was maintained at 250 °C and the detector at 280 °C. The oven temperature was programmed from 40 °C (3.5 min), increasing at 10 °C/min to 100 °C, at 7 °C/min to 180 °C, at 25 °C/min to 280 °C, then held at 280 °C for 5 min. The mass spectra were recorded in the electron impact (EI) ionization mode at 70 eV. The quadrupole mass detector, ion source and transfer line temperatures were set at 150, 230, and 280 °C, respectively. The MS was used in the ion monitoring (SIM) mode to identify and quantify the analytes.
2.4. Screening of the differentially accumulated metabolites
To analyze the differences between two groups, the criteria for significantly differential metabolites were a VIP (variable importance in projection) value of ≥ 1, and an absolute Log2FC (fold change) of ≥ 1 calculated using the R package, MetaboAnalystR. The VIP values were extracted from the OPLS-DA results, which also provided score and permutation plots. The data were log-transformed (log2) and mean centered before OPLS-DA. To avoid overfitting, a permutation test using 200 permutations was performed.
2.5. Statistical analysis
To study the accumulation of the metabolites, PCA, HCA, Venn diagram, upset plot, volcano plot and K-means clustering analysis were performed using the R package (https://www.r-project.org). The identified metabolites were annotated using the KEGG Compound database (https://www.kegg.jp/kegg/compound/). The annotated metabolites were then mapped to the KEGG Pathway database (https://www.kegg.jp/kegg/pathway.html). Pathways with significantly regulated metabolites mapped were then fed into MSEA (metabolite set enrichment analysis), with P ≤ 0.05 considered as the threshold (Li et al., 2022).
3. Results
3.1. Overview of metabolic profiles
3.1.1. Gc–ms-based quantitative metabolomic analysis
To examine the diversity of their metabolites, the GC–MS metabolic profiling analyses of oat, wheat, and barley provided details of the overall metabolic differences (Fig. 1B and Table S1): 437 metabolites were identified and classified into 15 types in detail according to their properties. These consisted of esters (16.93 %), terpenoids (16.7 %), heterocyclic compounds (13.96 %), hydrocarbons (12.59 %), alcohols (9.61 %), ketones (7.78 %), aldehydes (7.55 %), aromatics (7.09 %), phenols (1.83 %), acids (1.6 %), nitrogen compounds (1.14 %), amines (0.92 %), halogenated hydrocarbons (0.92 %), sulfur compounds (0.46 %), and others (0.92 %). This showed that esters and terpenoids were the most abundant metabolites in the three Triticeae crops. These VOCs also play a crucial role in fruit aroma (Urrutia et al., 2017).
Of these metabolites, 427, 431, 431, 427 and 433 VOCs were detected in the RV, MV, FV, WV, and BV samples, respectively: 414 VOCs were shared metabolites in the three Triticeae crops. Three VOCs, characteristic metabolites of oats, the aromatics (1,2,3-trimethoxy-5- (1-propenyl)-, and (E)- benzene), and the phenol (4- (3-hydroxy-1-propenyl)-phenol), were present in RV and MV, and the terpenoid, (E)-4,8-dimethylnona-1,3,7-triene, was present in MV and FV (Figure S1 and Table S1). These may be the VOCs that influence the flavor of different oat varieties. These results indicated that the different Triticeae crops contained a wide variety of metabolites.
3.1.2. Multivariate statistical analysis
PCA revealed the overall metabolic differences between each group and the variability within a particular group (Zhang et al., 2023). To illustrate the flavor substances present in different Triticeae crops, we used data on the 437 metabolites identified to analyze their metabolic profiles using PCA. Two principal components accounted for 57.84 % and 14.31 % of the metabolic variances among the three Triticeae crops. PCA revealed a lower variability among the biological replicates. The three Triticeae crops were obviously separated, a result consistent with those of previous studies (Khakimov et al., 2014). In terms of the metabolome, based on PC1, the three oat varieties were well clustered together, and more closely related to barley, with wheat far from the other groups.
The clustering heat profiles for the three Triticeae crops were constructed based on the metabolomics data (Fig. 1D). The metabolite accumulation pattern of WV was quite different from the others. Although BV and FV, RV, MV belong to the same category, their content of metabolites was also obviously different. This species-dependent accumulation pattern was further supported by HCA. The adjacent tree reflects the same affinities. Previous studies have shown that metabolomics data reflected genetic relationships (Zhao et al., 2022, Qi et al., 2021).
Overall, these results indicated good homogeneity and high reliability of data between the biological replicates. The metabolites were significantly different among the genotypes with distinct metabolic profiles.
3.2. Overview of DAMs
OPLS-DA was found to be an effective method for identifying DAMs and maximizing the differences between groups. For the paired comparison of the three Triticeae crops, the values of R2Y and Q2 were both greater than 0.9, indicating that the model had a high fitting accuracy (Wang et al., 2019) (Figure S2). Depending on the method, we filtered the results to get 307 DAMs that were assigned to 8 different profiles (Fig. 2). From profiles 6 and 8, 91 DAMs showed a higher accumulation pattern in oats than in wheat, with only 67 DAMs in oats being greater or equal to those in barley, and more than 60 % of metabolites being terpenoids, hydrocarbons or esters. Profiles 5 and 7 included 167 DAMs, mainly terpenoids, heterocyclic compounds and esters that were lower in oats than in wheat and barley. From the metabonomic analysis, the changes in the relative abundance of the metabolites indicated that the terpenoid- and ester-related pathways may explain the reason for the flavor differences between oats, wheat and barley.
Fig. 2.
K-means clustering analysis of differentially accumulated metabolites of three Triticeae crops. The y-axis shows the standardized amount of each metabolite, and the x-axis shows the different samples.
3.3. KEGG pathway analysis of oat DAMs
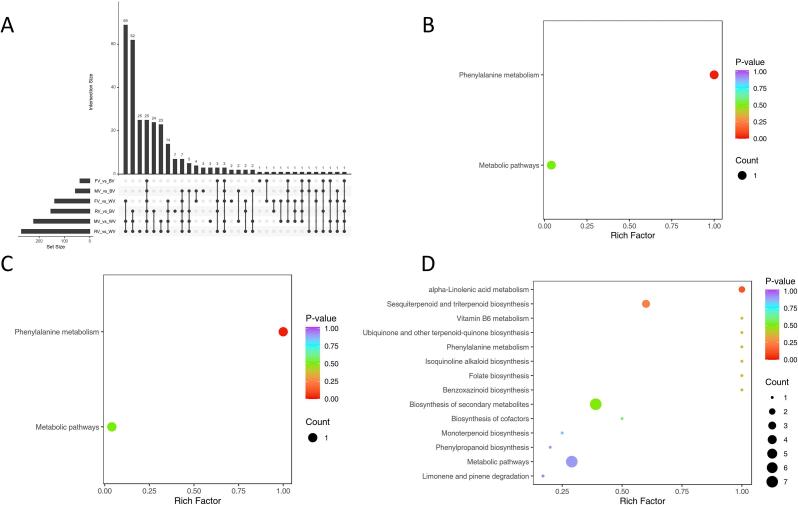
To show the dependence of the metabolites in the three Triticeae crops of the species, an upset plot of DAMs was constructed (Fig. 3A). This showed that 25 metabolites were shared by six comparable groups and that 27 metabolites were different between oats and barley (RV/FV/MV vs BV), and 121 metabolites different between oats and wheat (RV/FV/MV vs WV).
Fig. 3.
Upset map and KEGG bubble maps of differentially accumulated metabolites in oats, wheat, and barley. (A), An upset map of differential metabolites in oats, wheat, and barley; (B), KEGG pathway impact analysis showing altered metabolism in six comparable groups; (C) KEGG pathway impact analysis showing altered metabolism in oats vs. barley; and (D) KEGG pathway impact analysis showing altered metabolism in oats vs. wheat. The x-axis indicates the enrichment factor, and the y-axis the pathways. The larger red dots represent the main pathway enrichment and higher pathway impact values, individually. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The KEGG database is a major public pathway database, which can be used to study metabolite accumulation in general networks (Kanehisa & Goto, 2000). In the present study, the results of the KEGG pathway analysis of the significant DAMs in oats, wheat, and barley are shown using a bubble diagram in Fig. 3B–D. Fourteen metabolic pathways with higher levels of alpha-linolenic acid metabolism in oats than in wheat were observed. For oats and barley, the DAMs enriched two pathways, mainly phenylalanine metabolism. Similarly, the level of phenylalanine metabolism was higher in oats than in barley and wheat. The great majority of metabolites were mapped to the related pathways of amino acid metabolism, terpenoid metabolism and fatty acid metabolism, especially alpha-linolenic acid and phenylalanine, a precursor of esters, which was expected. A comprehensive analysis of the metabolomics data has indicated that fatty acid and amino acid metabolism were involved in the synthesis of important VOCs in passion fruit (Xia et al., 2021).
3.4. Odor activity value analysis
Oats are widely consumed because of their unique flavor and nutritive value. Flavor components can objectively reflect the flavor characteristics of different samples and are important indices to evaluate flavor quality. The sensory analysis of DAMs can be used to further understand their contributions to the overall flavor profile. Using these databases of odor sensory information (https://www.odour.org.uk; https://www.flavornet.org; https://www.femaflavor.org/flavor-library), we analyzed the influence of DAMs on flavor. Compared with barley, oats contained a greater number of chemical, floral, rose, honey, gasoline, fruity, cauliflower, fatty, terpenic (provides the characteristic flavor of many herbs and spices), and winey flavor combinations (Fig. 4A). Compared with wheat, oats exhibited more attributes related to fatty, spice, balsamic, musty, honey, rose, terpenic, winey, chemical, and cauliflower flavors (Fig. 4B). From this analysis, the overlap of the chemical, rose, honey, cauliflower, fatty, terpenic, and winey flavors probably explains the unique flavor of oats.
Fig. 4.
Flavor wheel of differentially accumulated metabolites. (A), Flavor wheel of DAMs common to oats and barley; and (B), Flavor wheel of DAMs common to oats and wheat. Inner circle, number of common differential metabolites; Middle circle, related odor description; and Outer circle, characteristic component KEGG annotation.
3.5. Dams in different oat varieties
To further understand the differences in metabolites, the three oat varieties were compared in pairs. The OPLS-DA plots showed notable differences in metabolic phenotypes between the three genotypes (Fig. S3A–C), and a volcano plot was used to compare the expression of the DAMs (Fig. S3D–F). A total of 31 DAMs were detected in RV and FV (29 up-regulated, 2 down-regulated). Similarly, a total of 12 DAMs were detected in MV and FV (10 up-regulated, 2 down-regulated), and a total of 24 DAMs in RV and MV (19 up-regulated, 5 down-regulated).
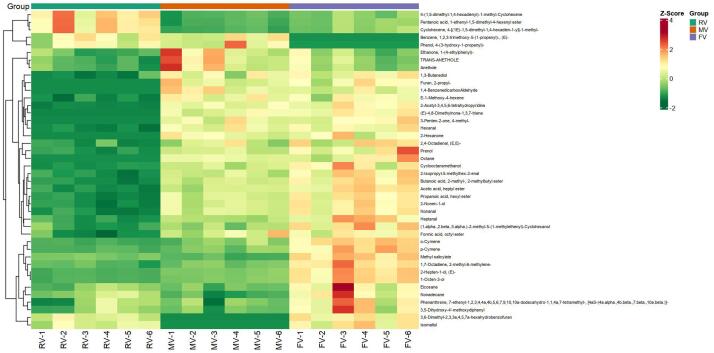
HCA was used to summarize the variation in the levels of the differential metabolites in the three oat varieties (Fig. 5). The differences in the pairwise comparison of the 42 VOCs could be better observed by HCA than from the OPLS-DA plots and could be divided into three main clusters. Most of the DAMs accumulated in the FV samples and mainly consisted of alcohols, aldehydes, and hydrocarbons. FV is usually used for processing oat flour, possibly because it has more flavor metabolites, which guarantees oats its unique flavor. Compared with FV and MV, only three substances in RV were significantly up-regulated. From this map, MV was intermediate between FV and RV, but each group had its own distinct metabolites. Overall, the results showed that the differences in the composition and relative abundance may be the main reason for the differences in flavor between different varieties. Previous studies of metabolites have demonstrated that each green tea has its own unique flavor characteristics due to its VOCs (Shi et al., 2022), and that metabolomics is an effective and reliable method to identify DAMs among different varieties of buckwheat (Li et al., 2022).
Fig. 5.
Heatmap of the pairwise comparisons of the 42 differentially accumulated metabolites in the FV, RV, and MV samples. Red indicates a relatively high metabolite abundance, and green indicates a relatively low abundance. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Metabonomics has developed into an effective tool for revealing metabolite accumulation patterns and has been widely used in many cereal analyses (Zhao et al., 2022). Examples include investigating the metabolite composition of black, red, glutinous, and white rice using widely targeted metabolomics (Zhang et al., 2023), and analyzing the metabolomics of phenols and organic acids in wheat, barley, oats, and rye by GC–MS (Khakimov et al., 2014). Another study identified a total of 201 flavonoid metabolites and 29 carotenoid metabolites from 17 wheat, maize, rice, sorghum, millet, and broomcorn samples by metabolome analysis (Tang et al., 2022). In the present study, a total of 437 metabolites was identified in three Triticeae crops by GC–MS analysis. The PCA and HCA results showed that different species and varieties have different metabolite characteristics. An excellent OPLS-DA model was established with high R2Y and Q2 values. The conditions for screening the DAMs in this method allowed comparisons between the different groups (Table 1), and their analysis included using K-means clustering, and the KEGG database. The flavor metabolome profiles of the three oat varieties were also determined. This study provides metabolomic evidence for understanding flavor differences in oats.
Table 1.
This is a table. Tables should be placed in the main text near to the first time they are cited.
| Group name | All sig diff | Down regulated | Up regulated |
|---|---|---|---|
| FV_vs_BV | 39 | 6 | 33 |
| FV_vs_WV | 139 | 80 | 59 |
| MV_vs_BV | 57 | 7 | 50 |
| MV_vs_FV | 12 | 2 | 10 |
| MV_vs_WV | 222 | 85 | 137 |
| RV_vs_BV | 154 | 5 | 149 |
| RV_vs_FV | 31 | 2 | 29 |
| RV_vs_MV | 24 | 5 | 19 |
| RV_vs_WV | 270 | 81 | 189 |
| WV_vs_BV | 110 | 21 | 89 |
Due to their richness in various valuable nutrients, Triticeae crops are playing an increasingly important role in the human diet and will gain more scientific attention. Previous studies have mainly focused on nutrient content (Poonia et al., 2022), yield (Zhang et al., 2023), while only relatively few have reported on flavor, which is one of the most relevant for consumers evaluating quality and their purchasing preference. Most secondary metabolites are associated with flavor formation (Suo et al., 2023), and are widely distributed in plants (Urrutia et al., 2017). For example, the characteristic aromatic substance of prickly ash is terpenoids (Fei et al., 2021), Suo et al. revealed changes in flavor quality by metabolomics, with terpenoids showing a gradual accumulation pattern (Suo et al., 2023), and similarly, the main aromatic components of postharvest Torreya grandis nuts are terpenoids (63.0 %–90.8 %) (Hu et al., 2022). Another study on strawberry flavor showed that esters were the most common VOC and highly correlated with liking (Fan et al., 2021). Oats have a unique flavor, with the present study revealing that terpenoids and esters were the predominant flavor compounds and DAMs in oats compared with wheat and barley. The DAMs are mainly enriched in the alpha-linolenic acid and phenylalanine metabolic pathways that provide the precursors of VOCs, such as esters (Aragüez and Valpuesta, 2013). The odor activity value analysis showed that the greater number of chemical, rose, honey, cauliflower, fatty, terpenic, and winey combinations may explain the unique flavor of oats. As important future step where one can pair volatile profiling of oat varieties with consumer preference surveys to determine the preferred flavours in oats destined for use in different products. As the willingness of consumers to pay a premium for higher quality products has increased, a demand has been created for high-yielding varieties of oats with special flavors.
The genetic background is a key factor influencing the production of primary and secondary metabolites. Various studies have reported differences in both the quality and quantity of VOCs between different varieties (Yu et al., 2020), resulting in variations in flavor (Ulrich et al., 2018). Because oats can self-breed, counterfeit seeds are difficult to identify, therefore any variety is controversial. From our results, RV, FV and MV could be clearly separated. Therefore, analysis of metabolites based on the GC–MS platform may be an effective method to distinguish between varieties caused by a different synthesis and decomposition of metabolites in different genotypes (Jiang et al., 2022).
Flavor quality is a complex characteristic (Klee & Tieman, 2018). The flavor metabolites of oats, barley, and wheat were analyzed in the present study, but how the flavor formation is related to the biosynthetic genes and molecular regulation mechanisms is yet to be determined. Identifying genes that regulate the synthesis of flavor chemicals, especially alleles of genes that provide more favorable chemical compositions, will help improvement by use of molecular tools. Studies have shown that green tea has its own unique flavor quality due to different processing techniques, which is closely related to its metabolite composition (Shi et al., 2022). The greatest influences during processing, such as heat treatment and milling, can trigger the unique flavor of oats (Rasane et al., 2015), therefore the significant changes in metabolites during oat processing need further study. Volatile substances can be converted to non-volatile substances thereby reducing flavor formation (Tikunov et al., 2013), but this phenomenon in oats is unclear, with a lack of targeted and comprehensive identification. Therefore, improving the flavor of oats will be a challenging task.
5. Conclusions
This study compared the differences in the metabolic profiles of oats, wheat and barley using a widely targeted GC–MS metabonomic analysis and found 437 metabolites. Terpenoids and esters were found to be potentially associated with the flavor of oats. By screening DAMs in different comparison groups, further KEGG enrichment revealed that alpha-linolenic acid and phenylalanine pathways were the key metabolic pathways. A flavor wheel analysis showed that, compared with wheat and barley, oats contained more flavor combinations of rose, honey, and cauliflower. Analysis of the DAMs of the three oat cultivars showed that the differences in the composition and concentration of metabolites may be the main cause of the flavor differences. The results of this study will enrich knowledge of the metabolomics of Triticeae crops and provide evidence on the flavor of Triticeae crops and a basis for the targeted use of different varieties of oats.
CRediT authorship contribution statement
Ting Wang: Methodology, Software, Formal analysis, Investigation, Data curation, Writing – original draft. Jinghong An: Conceptualization, Methodology. Mingna Chai: Formal analysis. Zhiqiang zhu: Software. Yulian Jiang: Investigation. Xuejie Huang: Data curation. Bing Han: Conceptualization, Validation, Resources, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Key Research and Development Program (Inter-governmental Special Project for International Scientific and Technological Innovation Cooperation) and the Collection, Evaluation and Innovative Utilization of Germplasm Resources of Oats in China and Mongolia (2022YFE0119800); Germplasm Innovation and Molecular Breeding of Forage Crops and Useful Microbiology (TD202103).
Author contributions
Conceptualization, H.B. and J.A.; methodology, H.B, J.A. and T.W.; software, T.W. and Z.Z.; validation, B.H.; formal analysis, T.W. and M.C.; investigation, T.W. and Y.J.; resources, B.H; data curation, T.W. and X.H.; writing—original draft preparation, T.W.; writing—review and editing, B.H.; supervision, B.H.; project administration, B.H. All authors have read and agreed to the published version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.101000.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Aragüez I., Valpuesta V. Metabolic engineering of aroma components in fruits. Biotechnology Journal. 2013;8(10):1144–1158. doi: 10.1002/biot.201300113. [DOI] [PubMed] [Google Scholar]
- Colantonio V., Ferrão L.F.V., Tieman D.M., Bliznyuk N., Sims C., Klee H.J., Munoz P., Resende M.F.R., Jr Metabolomic selection for enhanced fruit flavor. Proceedings of the National Academy of Sciences of the United States of America. 2022;119(7) doi: 10.1073/pnas.2115865119. e2115865119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Hasing T., Johnson T.S., Garner D.M., Schwieterman M.L., Barbey C.R., Colquhoun T.A., Sims C.A., Resende M.F.R., Whitaker V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Horticulture Research. 2021;8(1):66. doi: 10.1038/s41438-021-00502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei X., Qi Y., Lei Y., Wang S., Hu H., Wei A. Transcriptome and metabolome dynamics explain aroma differences between green and red prickly ash fruit. Foods. 2021;10(2):391. doi: 10.3390/foods10020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie A.R., Schauer N. Metabolomics-assisted breeding: A viable option for crop improvement? Trends in Genetics: TIG. 2009;25(1):39–48. doi: 10.1016/j.tig.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Fu J., Zhang Y., Hu Y., Zhao G., Tang Y., Zou L. Concise review: Coarse cereals exert multiple beneficial effects on human health. Food Chemistry. 2020;325 doi: 10.1016/j.foodchem.2020.126761. 126761. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang Z., Hua B., Tao L., Chen W., Gao Y., Suo J., Yu W., Wu J., Song L. The interaction of temperature and relative humidity affects the main aromatic components in postharvest Torreya grandis nuts. Food Chemistry. 2022;368 doi: 10.1016/j.foodchem.2021.130836. [DOI] [PubMed] [Google Scholar]
- Jiang L., Lu M., Rao T., Liu Z., Wu X., An H. Comparative analysis of fruit metabolome using widely targeted metabolomics reveals nutritional characteristics of different Rosa roxburghii genotypes. Foods. 2022;11(6):850. doi: 10.3390/foods11060850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal N., Tsardakas Renhuldt N., Bentzer J., Gundlach H., Haberer G., Juhász A.…Sirijovski N. The mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature. 2022;606(7912):113–119. doi: 10.1038/s41586-022-04732-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K.D., Jha A., Sabikhi L., Singh A.K. Significance of coarse cereals in health and nutrition: A review. Journal of Food Science And Technology. 2014;51(8):1429–1441. doi: 10.1007/s13197-011-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakimov B., Jespersen B.M., Engelsen S.B. Comprehensive and comparative metabolomic profiling of wheat, barley, oat and rye using gas chromatography-mass spectrometry and advanced chemometrics. Foods. 2014;3(4):569–585. doi: 10.3390/foods3040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H.J., Tieman D.M. The genetics of fruit flavour preferences. Nature Reviews. Genetics. 2018;19(6):347–356. doi: 10.1038/s41576-018-0002-5. [DOI] [PubMed] [Google Scholar]
- Kutasy E., Diósi G., Buday-Bódi E., Nagy P.T., Melash A.A., Forgács F.Z., Virág I.C., Vad A.M., Bytyqi B., Buday T., Csajbók J. Changes in PLANT AND GRAIN QUALITY OF WINTER OAT (Avena sativa L.) varieties in response to silicon and sulphur foliar fertilisation under abiotic stress conditions. Plants. 2023;12(4):969. doi: 10.3390/plants12040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Xin M., Li L., He X., Yi P., Tang Y., Li J., Zheng F., Liu G., Sheng J., Li Z., Sun J. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chemistry. 2021;355 doi: 10.1016/j.foodchem.2021.129685. [DOI] [PubMed] [Google Scholar]
- Li H., Lv Q., Liu A., Wang J., Sun X., Deng J., Chen Q., Wu Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131125. [DOI] [PubMed] [Google Scholar]
- Loskutov I.G., Khlestkina E.K. Wheat, barley, and oat breeding for health benefit components in grain. Plants. 2021;10(1):86. doi: 10.3390/plants10010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareno E.S., Fiedler J., Miller M.E., Figueroa M., Kianian S.F. A reference-anchored oat linkage map reveals quantitative trait loci conferring adult plant resistance to crown rust (Puccinia coronata f. sp. avenae) TAG. Theoretical AND Applied Genetics. Theoretische und angewandte Genetik. 2022;135(10):3307–3321. doi: 10.1007/s00122-022-04128-6. [DOI] [PubMed] [Google Scholar]
- Poonia A., Phogat D.S., Versha N.S., Sharma P., Kumar V. Biochemical assessment of oat genotypes revealed variability in grain quality with nutrition and crop improvement implications. Food chemistry. 2022;377 doi: 10.1016/j.foodchem.2021.131982. [DOI] [PubMed] [Google Scholar]
- Qi J., Li K., Shi Y., Li Y., Dong L., Liu L., Li M., Ren H., Liu X., Fang C., Luo J. Cross-species comparison of metabolomics to decipher the metabolic diversity in ten fruits. Metabolites. 2021;11(3):164. doi: 10.3390/metabo11030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasane P., Jha A., Sabikhi L., Kumar A., Unnikrishnan V.S. Nutritional advantages of oats and opportunities for its processing as value added foods - a review. Journal of Food Science And Technology. 2015;52(2):662–675. doi: 10.1007/s13197-013-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhu Y., Ma W., Shi J., Peng Q., Lin Z., Lv H. Comprehensive investigation on non-volatile and volatile metabolites in four types of green teas obtained from the same tea cultivar of Longjing 43 (Camellia sinensis var. sinensis) using the widely targeted metabolomics. Food chemistry. 2022;394 doi: 10.1016/j.foodchem.2022.133501. [DOI] [PubMed] [Google Scholar]
- Suo J., Ma Z., Zhao B., Ma S., Zhang Z., Hu Y., Yang B., Yu W., Wu J., Song L. Metabolomics reveal changes in flavor quality and bioactive components in post-ripening Torreya grandis nuts and the underlying mechanism. Food chemistry. 2023;406 doi: 10.1016/j.foodchem.2022.134987. [DOI] [PubMed] [Google Scholar]
- Tang J., Li X., Zhang Y., Yang Y., Sun R., Li Y., Gao J., Han Y. Differential Flavonoids and Carotenoids Profiles in Grains of Six Poaceae Crops. Foods. 2022;11(14):2068. doi: 10.3390/foods11142068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman D., Zhu G., Resende M.F., Jr, Lin T., Nguyen C., Bies D., Rambla J.L., Beltran K.S., Taylor M., Zhang B., Ikeda H., Liu Z., Fisher J., Zemach I., Monforte A., Zamir D., Granell A., Kirst M., Huang S., Klee H. A chemical genetic roadmap to improved tomato flavor. Science. 2017;355(6323):391–394. doi: 10.1126/science.aal1556. [DOI] [PubMed] [Google Scholar]
- Tikunov Y.M., Molthoff J., de Vos R.C., Beekwilder J., van Houwelingen A., van der Hooft J.J., Nijenhuis-de Vries M., Labrie C.W., Verkerke W., van de Geest H., Viquez Zamora M., Presa S., Rambla J.L., Granell A., Hall R.D., Bovy A.G. Non-smoky glycosyltransferase1 prevents the release of smoky aroma from tomato fruit. The Plant cell. 2013;25(8):3067–3078. doi: 10.1105/tpc.113.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D., Kecke S., Olbricht K. What do we know about the chemistry of strawberry aroma? Journal of Agricultural and Food Chemistry. 2018;66(13):3291–3301. doi: 10.1021/acs.jafc.8b01115. [DOI] [PubMed] [Google Scholar]
- Urrutia M., Rambla J.L., Alexiou K.G., Granell A., Monfort A. Genetic analysis of the wild strawberry (Fragaria vesca) volatile composition. Plant Physiology and Biochemistry: PPB. 2017;121:99–117. doi: 10.1016/j.plaphy.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Wang F., Chen L., Chen H., Chen S., Liu Y. Analysis of flavonoid metabolites in citrus peels (Citrus reticulata “Dahongpao”) using UPLC-ESI-MS/MS. Molecules (Basel, Switzerland) 2019;24(15):2680. doi: 10.3390/molecules24152680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Hua J., Yu Q., Li J., Wang J., Deng Y., Yuan H., Jiang Y. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food chemistry. 2021;363 doi: 10.1016/j.foodchem.2021.130131. [DOI] [PubMed] [Google Scholar]
- Xia Z., Huang D., Zhang S., Wang W., Ma F., Wu B., Xu Y., Xu B., Chen D., Zou M., Xu H., Zhou X., Zhan R., Song S. Chromosome-scale genome assembly provides insights into the evolution and flavor synthesis of passion fruit (Passiflora edulis Sims) Horticulture Research. 2021;8(1):14. doi: 10.1038/s41438-020-00455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Xiao J., Chen S., Yu Y., Ma J., Lin Y., Li R., Lin J., Fu Z., Zhou Q., Chao Q., Chen L., Yang Z., Liu R. Metabolite signatures of diverse Camellia sinensis tea populations. Nature Communications. 2020;11(1):5586. doi: 10.1038/s41467-020-19441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Liu C., Mao L., Li Y., Shen Y. Divergent response of hay and grain yield of oat: Effects of environmental factors and sowing rate. Journal of the Science of Food and Agriculture. 2023;103(1):233–242. doi: 10.1002/jsfa.12135. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cui D., Ma X., Han B., Han L. Comparative analysis of rice reveals insights into the mechanism of colored rice via widely targeted metabolomics. Food Chemistry. 2023;399 doi: 10.1016/j.foodchem.2022.133926. [DOI] [PubMed] [Google Scholar]
- Zhang M.W., Zhang R.F., Zhang F.X., Liu R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. Journal of Agricultural And Food Chemistry. 2010;58(13):7580–7587. doi: 10.1021/jf1007665. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhai G., Li X., Tao H., Li L., He Y., Zhang X., Wang F., Hong G., Zhu Y. Metabolomics reveals nutritional diversity among six coarse cereals and antioxidant activity analysis of grain sorghum and sweet sorghum. Antioxidants. 2022;11(10):1984. doi: 10.3390/antiox11101984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Zhang Q., Quan J., Zheng Q., Xi W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chemistry. 2016;205:112–121. doi: 10.1016/j.foodchem.2016.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.