Graphical abstract
Keywords: Electrospinning, Proteins, Electrospun fibers, Encapsulation, Bioactive compounds, Protein-based carriers
Highlights
-
•
Proteins serve as potential polymer matrices for engineering electrospun fibers.
-
•
The end attributes of protein electrospun fibers (EFs) are tunable by adjusting different production parameters.
-
•
Protein EFs can preserve (bio)stability and bioactivity of may functional ingredients.
-
•
In vivo behavior of EFs is highly challenging.
-
•
Further studies on their food applications via animal models could answer various queries.
Abstract
Electrospun fibers (EFs) have emerged as promising one-dimensional materials for a myriad of research/commercial applications due to their outstanding structural and physicochemical features. Polymers of either synthetic or natural precursors are applied to design EFs as carriers for bioactive compounds. For engineering food systems, it is crucial to exploit polymers characterized by non-toxicity, non-immunogenicity, biocompatibility, slow/controllable biodegradability, and structural integrity. The unique attributes of protein-based biomaterials endow a wide diversity of desirable features to EFs for meeting the requirements of advanced food/biomedical applications. In this review paper, after an overview on electrospinning, different protein materials (plant- and animal-based) as biodegradable/biocompatible building blocks for designing EFs will be highlighted. The potential application of protein-based EFs in loading bioactive compounds with the intention to inspire interests in both academia and industry will be summarized. This review concludes with a discussion of prevailing challenges in using protein EFs for the bioactive vehicle development.
Introduction
The literature survey clearly reveals that the overall therapeutic effects of bioactive compounds (bioactives) are not merely relative to their in vitro potency. Under physiological conditions, bioactives confront biological barricades, e.g., insolubility, aggregation, and degradation, threatening their therapeutic properties. Moreover, a bioactive's potency can severely decrease by various stresses (like pressure, heat, humidity, and pH), which can occur in vitro and in vivo (during storage, administration, or systemic circulation) (Falsafi et al., 2022, Fani et al., 2022).
To tackle these bottlenecks, a vast variety of carrier systems have been engineered. Efficient carriers can promote the stability, potency, bioaccesability, bioavailability, tolerability, and safety of different bioactives. As such, they can offer outstanding advantages over many naked bioactives and may even facilitate the application of bioactives, which would otherwise be unfeasible, owing to low (bio)stability/bioavailability. The proper design and formulation of a carrier system is, thus, usually as functionally important as the bioactive itself, and must be attentively considered (Liu et al., 2021).
Unique amongst the diverse strategies utilized for designing carrier systems is electrospinning (abbreviated as “E-spin”), which is the approach of applying an electrostatic force to a polymer solution to engineer fibrous structures of nanometer to micrometer diameter size fibers (Topuz & Uyar, 2017). The physical features of electrospun fibers (EFs) are very tunable, rendering them superior capability for loading a diverse array of bioactives. The density, diameter, and alignment of EFs can be adjusted by modifying different aspects of the E-spin process, e.g., design or rotation speed of the collecting plate, electrical field strength, type of solvent(s) or/and polymer(s), the flow rate of the solution from the spinneret, and the spinneret-to-collector distance (Drosou et al., 2022).
One of the keys to progressing in this field lies in the engineering of functional EFs, which can interact with or within biological environments. Such formulations can be emanated straight from nature, or designed in the lab using man-made synthetic polymers. Nevertheless, notwithstanding the diversity and great potential of synthetic polymers, their applications have been restricted by issues, e.g., bioresorbability and biocompatibility/ biodegradability.
Taking inspiration from nature’s toolkit, natural polymers have enjoyed a long history and emerged as viable substitutes for such applications in food/biomedical engineering (Falsafi, Wang, et al., 2023). Within the plethora of nature-inspired polymers, proteins, which comprise of tandem repeats of short peptide motifs, stand out as an unbeatable class of biomaterials. With inherent biochemical and biophysical properties at the molecular level (like excellent biocompatibility, absent/minimal immunogenicity, and ease of functionalization), protein matrices have been found to be appropriate architectures for designing EFs for bioactive delivery. Such properties can adjust the function of the protein matrix to create various EF behaviors, e.g., bioactive loading, release properties, and rate of degradation. Protein-based carriers are biodegradable into amino acids without toxic/acidic degradation products. Additionally, the degradation rate, in some cases, can be controlled to match the delivery application (Najafi et al., 2022).
This review is structured as follows: First, we overview the E-spin technique including processing parameters, various approaches for bioactive loading and release kinetics, and E-spin polymers. Next, we introduce proteins as biodegradable/biocompatible building blocks for E-spin and summarize the attributes of protein EFs, which are specifically critical in bioactive delivery. We also highlight the properties of protein EFs that either demonstrate or are anticipated to demonstrate potential in loading and controlled release of various bioactives. We conclude by offering our perspective on the current limitations and future directions in this realm. It is hoped that the comprehensive review offered here will acquaint the readers with protein EFs loaded by bioactives, and will inspire and interest the researchers to further explore more effective and novel active fibers for food and biomedical applications over a broad horizon.
E-spin background
E-spin is a fabrication technique used for the production of fibers with diameters from tens of nanometers to microns (Tebyetekerwa & Ramakrishna, 2020). The technique relies on electrostatic forces to draw charged threads from polymeric (Huang et al., 2003) and non-polymeric solutions (Dodero et al., 2021). During the E-spin process, a viscous solution is subjected to an electric potential to generate nonwoven materials in scalable quantities. This causes electrostatic repulsion forces between charges in the liquid and attraction forces between oppositely charged liquid and collector (Sill & von Recum, 2008). The electric forces at the liquid interface are balanced by the surface tension, which resists deformation of the liquid (Singh and Subramanian, 2020). When these forces dominate the surface tension, the stretching of the droplet occurs: under the high electric field, the droplet adhering to the nozzle exit first takes the shape of a cone (i.e., Taylor cone), and then, an electrified jet emerges from the apex of the Taylor cone (Jeong et al., 2022). As the jet travels in the air, it gets thinner and reaches a grounded metal plate as solid continuous EFs.
The E-spin technique has been applied to various polymeric and non-polymeric systems to produce EFs for a wide spectrum of applications, ranging from food to biomedical applications (Agarwal et al., 2013, Chen et al., 2020, Costoya et al., 2017, Topuz and Uyar, 2020a). Accordingly, the choice of polymer type, additives, and solvents vary from toxic to biocompatible ones. In the development of fibrous adsorbents/membranes from synthetic or natural polymers, polar aprotic (e.g., dimethylformamide, acetonitrile, tetrahydrofuran) and halogenated (e.g., trifluoethanol, chloroform) solvents have been used commonly. Whereas for EFs intended to be employed in biomedical applications, nontoxic polar aprotic solvents, like water, ethanol, and acetic acid, are desired to minimize the toxicity that can arise from the solvent residues remained in EFs. In the E-spin process, volatile solvents are preferred owing to their high evaporation rates; solvent molecules must vaporize before the jet hits the collector (Robb & Lennox, 2011). The stability of the resultant EFs in the respective solvent is another significant parameter, which might require physical or chemical cross-linking depending on the intended use of EFs.
E-spin has been implemented to generate fibrous materials for environmental (Dou et al., 2020), catalysis (Vempati et al., 2020), fuel cells (Waldrop et al., 2020), tissue engineering (Ghosal et al., 2019), drug/bioactive delivery (Rostamabadi et al., 2022, İnanç Horuz and Belibağlı, 2018), food packaging (Topuz & Uyar, 2020b), sensors (Zhang et al., 2019), agriculture (Noruzi, 2016), cosmetics (Zanin et al., 2011), and filtration (Lyu et al., 2021) applications. The ultrasmall size with a very high surface-to-volume ratio, tunable morphology, easy functionalization, flexibility, high porosity, interconnected pores, and many other benefits enabled the use of EFs for various purposes. Most applications of EFs focused on their use in (i) bioactive/drug delivery to benefit from tunable release by the structural control of EFs, (ii) environmental remediation to remove toxic pollutants from water and air, (iii) tissue engineering because of structural similarity to extracellular matrix (ECM), and (iv) catalysis owing to easy functionalization with catalytic nanoparticles (NPs). Unlike other EF fabrication techniques (e.g., molecular self-assembly, drawing, template synthesis, and temperature-induced phase separation), E-spin is a handy, low-cost, high speed, and versatile technique that produces handy fibrous bulk materials with desired structural features (Kumbar et al., 2008). The produced materials are highly flexible and can be used for applications that demand high workloads.
A typical E-spin system is composed of a high-voltage power supply, a syringe, a feeding pump, and a grounded metal collector (Fig. 1a). Depending on the need, various needles and collectors can be used (Fig. 1b, c). For hollow or co-axial EFs, a core–shell (coaxial) needle should be employed. To boost E-spin throughput, multiple needles can be used in the E-spin setup. Rotating collectors (i.e., rotating drum, mandrel, or wire drum) can be utilized to generate aligned EFs. By varying the E-spin parameters, smooth to porous EFs could be produced (Fig. 1d, e). Likewise, ribbon-like EFs could be obtained with the choice of appropriate parameters and polymer system (Topuz & Uyar, 2017). Each morphology offers its own pros and cons: for instance, porous EFs show better adsorption performance than smooth nonporous EFs because of their larger surface area and higher diffusion rate. However, the presence of porosity will reduce the mechanical properties of EFs.
Fig. 1.
Schematic illustration of a typical E-spin setup (a). Common needle types (b) and collectors (c) used in the E-spin system. A list of the E-spin parameters (d) and typical fiber morphology obtained (e).
Processing parameters
The E-spin process can be affected by process parameters, which include applied voltage, tip-to-collector distance, needle diameter, and feed rate (Fig. 1). Each of these parameters can affect the morphology and diameter of the resultant EFs. The influence of each parameter on EF morphology, thickness, and electrospinnability are summarized in Table 1. Since the E-spin process relies on an electric field, increasing the voltage can cause faster dragging of the jet from the Taylor cone and an increase in the electrostatic stress on the jet (Gu & Ren, 2005). Thus, thinner EFs is observed during the E-spin of many polymers with a rise in the applied voltage (Demir et al., 2002, Wang and Kumar, 2006). An opposite trend (i.e., diameter increase with the applied voltage) is also observed for some polymers as a result of higher mass flow (Tan et al., 2005, Zhang et al., 2005). The tip-to-collector distance (TCD) might affect the travelling time and path of the jet before the jet reaches to the collector. Since the electrostatic field is inversely proportional to the TCD, a critical distance is required for the solvent evaporation from the jet. Some works were reported a decrease in EF diameter with increasing TCD because of higher stretching of the jet (Akturk et al., 2020). However, an opposite trend was also reported for some EFs, which was attributed to the decrease in the electric field strength (Cai et al., 2014). For many polymers, variations in the inner needle diameter typically has no effect on the EF thickness. However, some papers reported an increase in EF diameter by the use of large-diameter needles (Heikkilä & Harlin, 2008). Unlike other process parameters, variations in the flow rate obviously change the EF morphology. Generally, increasing the flow rate is accompanied by the formation of thicker EFs as a result of higher mass flow (Topuz & Uyar, 2020b). However, the application of very high flow rates can also lead to the formation of non-uniform EFs (e.g., beaded EFs) (Topuz et al., 2021).
Table 1.
Influence of E-spin parameters on the properties of produced fibers.
| Category | Parameter | Effect on EFs morphology, thickness, and electrospinnability | References |
|---|---|---|---|
| Process | Voltage |
|
|
| Tip-to-collector distance |
|
Akturk et al., 2020, Cai et al., 2014 | |
| Nozzle inner diameter |
|
||
| Flow rate |
|
||
| Collector/needle type |
|
||
| Formulation (feed) | Concentration |
|
|
| Viscosity |
|
||
| Mw |
|
||
| Conductivity |
|
||
| Solvent volatility |
|
||
| Surface tension |
|
||
| Environmental | Humidity |
|
|
| Temperature |
|
Formulation parameters
Unlike the process parameters, the impact of formulation parameters on the EF morphology can be apparent. Even variations in such parameters can lead to electrospraying process rather than E-spin. Formulation parameters include polymer concentration, molecular weight (Mw), viscosity, conductivity, solvent type/volatility, and surface tension (Table 1). For electrospinnability, a critical polymer concentration is required to produce continuous EFs. Otherwise, the electrospraying process can occur as a consequence of very low polymer concentrations. The formation of beads could be observed due to the electrospraying of low viscous solutions (Topuz et al., 2021). Further increasing the polymer concentration causes the production of thicker EFs (Zhang et al., 2005). Similar to polymer concentration, increasing the viscosity can lead to the generation of thicker EFs (Heikkilä & Harlin, 2008). The impact of Mw on the EF morphology is related to the solution viscosity. Usually, the application of polymers of higher Mw leads to the formation of thicker EFs (Jacobs et al., 2010).
The solvent used for dissolving polymer should be volatile enough to easily evaporate from the E-spin jet. However, the use of low boiling point solvents could cause the formation of porous EFs as a result of their rapid evaporation from the EF matrix. Solvents with a boiling point < 170 °C could be the proper candidates in this line. As the electrified jet is formed at higher electrostatic energy than the surface energy of the solution drop, lower surface tension is desired to produce continuous jets. Higher surface tension can cause the breaking of the jet, eventually leading to the formation of discontinuous EFs on the collecting plate. The surface tension of the solution could be lowered by changing the solvent or adding other ingredients, such as surfactants (Lin et al., 2004). Likewise, the solution conductivity played a critical role in the E-spin process. Several studies revealed that increasing solution conductivity improved electrospinnability and lowered the critical polymer concentration for the formation of bead-free EFs (Topuz et al., 2019).
A comprehensive review of the effects of E-spin parameters was compiled by Inn-Kyu Kang and colleagues (Haider et al., 2018). The authors explained the impact of each parameter on the E-spin process and the resultant EF morphology. Likewise, several papers have been reported to explore E-spin parameters for polymeric (Topuz et al., 2019, Topuz et al., 2021) and non-polymeric (Topuz & Uyar, 2020a) systems.
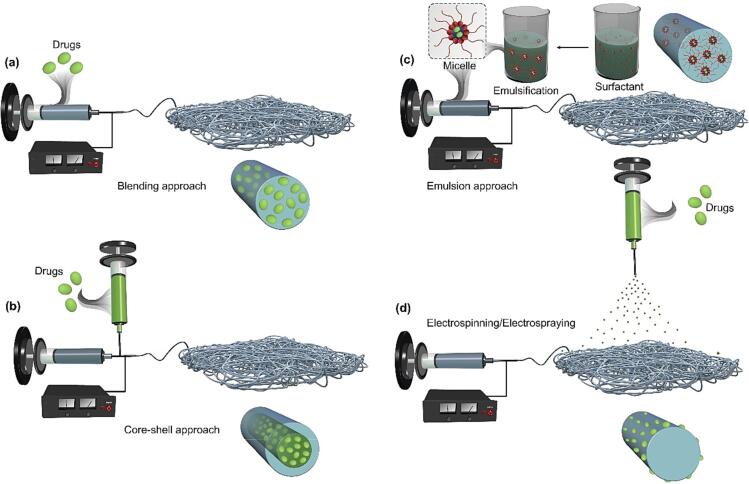
E-spin approaches for bioactive loading and release kinetics
The loading of bioactives into EFs can be done following different routes (Buzgo et al., 2018, Rychter et al., 2017), as depicted in Fig. 2. The most common route is the blending of polymer and bioactives, which leads to the amorphous dispersion of the compounds into the polymer (Fig. 2a). Eventually, this will lead to the homogenous distribution of the loadings within polymeric EFs. The approach is versatile and applicable to many polymers. In this regard, depending on the solvent system, excipients can be employed to enhance the loading capacity, especially for the waterborne E-spin of polymers. Most bioactives are poorly soluble in water and therefore, organic solvent might be required for their loading into EFs. The solubility of such molecules could be enhanced using excipients (Kazsoki et al., 2018). The release kinetics of the encapsulated bioactives could be tuned by changing the polymer type and porosity in the produced EFs. Furthermore, in the blending method, the surface of EFs could be modified through chemical or plasma techniques to control the release profile of the encapsulated bioactives (e.g., burst release) and improve EF compatibility for the targeted application. In the blending approach, bioactive-loaded NPs can be mixed with polymer solution and electrospun into EFs. In this regard, the presence of two polymer layers prolongs the release profile, while reducing the burst release.
Fig. 2.
Common approaches used for the loading of bioactive agents into EFs. Blending (a), coaxial (b), emulsion (c), and a combination of electrospraying/E-spin (d) approaches.
Bioactives can also be loaded into EFs through coaxial E-spin (Fig. 2b) (Yarin, 2011, Zhang et al., 2006). In this approach, bioactives are localized in the core of EFs without exposing them to organic solvents during the processing while the shell layer is used as a barrier and to sustain their release from the EF matrix. This will minimize the burst release of bioactives from EFs. Likewise, a tri-axial nozzle system can be employed for the better control in the release profile of the encapsulated bioactives. The emulsion approach can also be employed as an alternative to coaxial route to load bioactives into EFs (Fig. 2c) (Li et al., 2010). This method is mostly used in the case of the low soluble bioactives in the respective E-spin solvent and to prevent their contact with organic solvents. In this regard, an aqueous solution of bioactives could be prepared using a surfactant and then emulsified within an organic solvent, which is followed by the E-spin of the solution into EFs. With that approach, the aqueous phase of bioactives is encapsulated into polymeric EFs through a conventional E-spin system, as shown in Fig. 2c. This approach may arise the toxicity issue of the surfactant used, particularly in vivo applications.
Bioactives can be loaded into EFs through electrospraying/E-spin system (Fig. 2d). In this regard, a combination of electrospraying and E-spin techniques are applied in parallel: during the E-spin, the formed EFs are deposited by the electrospraying of loading solutions. This eventually will lead to the functionalization of EFs with electrosprayed particles of bioactives. The last method may suffer the stability issue, where the burst release of bioactives can be observed in case particles are decorated onto EF surface. It is noteworthy that each of these routes has its own pros and cons. Depending on the targeted applications, bioactives could be loaded based on the material choice and simultaneous/post-modification routes. Sometimes both approaches can be used in parallel to generate multi-bioactive-loaded EFs.
The release of bioactives from EFs can follow different release kinetics, which are dependent on the physicochemical properties of polymers and bioactives, loading approach, as well as polymer-bioactive interactions. Depending on those parameters, the release can happen as a burst, diffusion, erosion, or as a combination of them. In some applications, burst release might be desired but it is undesired for the most application, and therefore, the choice of polymer and method becomes important to prevent that. In this context, the use of a hydrophobic polymer matrix for hydrophobic bioactives can slow down the release profile as a result of hydrophobic interactions and the low wettability of the hydrophobic polymer matrix in aqueous environments. Biodegradation of the polymer also affects the release profiles of EFs. Biodegradable EFs can degrade through bulk degradation or surface erosion routes. If EFs degrade through bulk erosion, the release of the encapsulated bioactives mostly follows a diffusion mechanism. In such a scenario, burst release can be observed. On the other hand, during the surface erosion of rapidly degrading EFs, release and degradation take place in parallel, eventually resulting in a linear release profile.
In the case of core–shell EFs, the release profile of bioactives encapsulated in the axial core is mainly affected by the diffusion of the loadings through the polymer shell barrier. In this regard, some parameters can affect the release kinetics, including (i) the initial concentration difference between the shell and core layers, (ii) the diffusion rate of bioactives through the shell (i.e., barrier) layer, and (iii) texture and thickness of the shell layer. In addition to those, the physicochemical properties of bioactives and both polymer layers and their interactions with the encapsulated bioactives should be considered too. As observed for core–shell EFs, a delayed release will occur owing to the shell barrier for the emulsion EFs. The release of bioactives from the particle decorated EFs greatly affected by the used particle type and their deposition or incorporation onto/into EFs. If the particles are incorporated within EFs, the release kinetics will be directed by the axial core and shell polymer layer, as in the case of core–shell EFs. However, if the particles are decorated onto EFs, the release of the encapsulated bioactives will be solely affected by the NPs matrix and form. In this case, an initial burst release can be observed. Furthermore, the form of NPs has a profound effect on release kinetics. The faster release will take place for the capsule-like particles than the conventional particles as a result of short diffusion routes. For more details on the release of the encapsulated bioactives from EFs, a comprehensive review is available (Puppi & Chiellini, 2018).
E-spin of polymers
From the first EFs, which were reported by Cooley and Morton in 1902 (Cooley, 1902), the E-spin technique has sparked a tremendous interest to generate versatile materials to be used in various applications. The technique has been applied to a wide spectrum of synthetic polymers and composites to produce fibrous materials: numerous synthetic polymers could be electrospun into EFs using volatile organic compounds (VOCs). Likewise, many EFs were produced using natural polymers such as polysaccharides and proteins because of their accessibility, affordable price, biocompatibility, biodegradability, and many other intrinsic benefits (Falsafi, Maghsoudlou, et al., 2022). However, the electrospinnability of most biopolymers is limited by their poor solubility in nontoxic solvents and in conventional toxic VOCs. Recent studies showed that ionic liquids can be used as a solvent replacement for VOCs (Zavgorodnya et al., 2017). However, because of their high boiling points, they are not good enough to produce fibrous materials.
Currently, VOCs and acids have been employed for the E-spin of many biopolymers, such as proteins (e.g., gelatin, zein, collagen and silk). Since most proteins do not dissolve in water, mostly binary solvents or VOCs have been used to generate EFs. For instance, zein does not dissolve in water but in ethanol. In this case, the E-spin of zein has been reported from pure ethanol or ethanol/water mixtures (Yao et al., 2007). Zein could also be electrospun using acidic solutions (Selling et al., 2007). Gelatin also does not dissolve in cold water. It could be electrospun either using a hot-water circulation system to keep the injector warm or using nonaqueous systems, such as acids (acetic acid, trifluoroacetic acid, formic acid, (Topuz & Uyar, 2017). Other proteins like elastin, silk, soy, collagen, wheat, whey, and casein) could be electrospun into EFs using various solvents. Regarding the E-spin of biopolymers, Chronakis and colleagues reported a comprehensive review of the E-spin of food-derived proteins (Mendes et al., 2017).
Proteins as biodegradable/biocompatible building blocks for E-spin
Nowadays, awareness is raised concerning the need for safe, sustainable, green, and eco-friendly technologies. The current environmental conditions necessitate the modification and redesigning the entire material life cycle in terms of raw materials, processes, production, and even technologies to diminish the environmental impact (Rostamabadi, Chaudhary, et al. 2023). The inherent attributes of the protein-based biomaterials e.g., bioactivity, emulsibility, amphiphilicity, gel-forming capacity, biocompatibility/biodegradability, non-carcinogenicity, and nontoxicity can be preserved in the final product (Wang et al., 2022). For diverse food and biomedical applications, it is the physical/chemical features of the protein, which are exploited to develop electrospun architectures of specialized attributes. Despite their biological function in living organisms, proteins can improve the organoleptic properties of food formulations in terms of taste, flavor, and textural properties. The precise selection of the protein material and appropriate control over processing conditions are critical to attain EFs with specific and optimized attributes (Zhang et al., 2010).
It is worth noting that the isolate forms of proteins, e.g., whey, soy, and potato protein isolates, which comprise a blend of proteins, carbohydrates, and lipids, are mostly more cost-effective than single purified proteins. However, their application in E-spin may be more challenging, in some cases, due to their specific physicochemical attributes, biological properties, and shelf life, which can vary considerably from one to another. Isolate types of proteins mostly possess varying content of the individual proteins/peptides, purity, and overall composition as a function of factors like purification technique or origin. The sequence of amino acid residues in a protein molecule alongside the conditions of the surrounding medium (e.g., solvent, pH, or ionic strength) dictates the inter- and intra-molecular interactions and, therefore, the resulting 3-D tertiary and quaternary configuration of proteins (Kutzli et al., 2018). Some proteins possess high aqueous solubility owing to the presence of hydrophilic amino acids on their superficial parts. In the contrary, some fibrous proteins like silk, collagen, or elastin are arranged by repetitive amino acid groups and mostly covered by non-polar hydrophobic amino acids, rendering them aqueous insoluble.
Proteins are generally divided into two main categories based on their source; plant and animal proteins. Proteins with plant or animal origin are biodegradable and abundant natural materials, which can be tuned into eco-friendly architectures, typically under mild conditions. Due to their renewable sources and low emissions of greenhouse gases, plant-derived proteins count as cost-effective and scalable biomaterials, which offer a sustainable and green polymer origin. Moreover, proteins of plant origins are not generally associated with animal-borne diseases, providing a solution for individuals, who avoid consuming animal-based food, because of religious, personal preferences, and even ethical beliefs (Chen, Chuanbao, Xilan, Yin, & Hesun, 2006). Albeit typically costlier than plant-derived proteins, proteins of animal origin are obtainable from alternative sources or as side streams of the food industry or agriculture that render animal-based proteins cost-effective and ecologically friendly materials for biomedical applications. Generally, the majority of plant-based proteins possess a limited range of compatible solvents, rendering the design of EFs with plant origin more complicated. Strategies to improve the applicability/versatility of plant- and animal-based proteins in various applications include selecting the proper solvent, cross-linking, and blending with natural/synthetic polymers e.g., polysaccharides or other protein materials. Moreover, the fabrication of protein-based EFs by E-spin is relatively challenging because of their globular structure (in some cases), low viscosity, and the lack of intermolecular entanglements. Hence, proteins are often mixed with other synthetic or bio-based components to acquire mutually compatible hybrid polymeric systems, which can then be strongly electrospun to obtain fibrous structures (Wang et al., 2022).
Plant proteins
Zein
Zein, as a naturally friendly material with a Mw = 25–45 kDa, is a prolamin protein that can be manufactured from corn grains via wet milling (Zhang et al., 2022). Zein, as a Generally Recognized as Safe (GRAS) and low-price material, is rich in many sulfur-containing amino acids but demonstrates poor nutritional value owing to the lack of essential amino acids such as tryptophan and lysine. The existence of many uncharged amino acid residues in the zein structure makes it insoluble in aqueous environments, but soluble in certain concentrations of ethanol, anionic surfactants, or alkaline solutions (pH > 11) (Giteru, Ali, & Oey, 2021). Despite these attributes, it has been extensively exploited in various food and pharmaceutical formulations due to its good biocompatibility, biodegradability, self-assembly ability, film-forming capacity, and amphiphilicity. Zein, as a macromolecular carrier, can efficiently promote the stability and bioavailability of bioactives in vivo/in vitro alongside providing a bioactive sustained release in the intended locus. Additionally, zein is resistant to heat, oxidation, and moisture, which strengthens its potential to encapsulate/shield bioactives through E-spin (Wang et al., 2022).
Soy proteins
Soy protein, as the largest commercially available plant protein, is mainly comprised of two types of globular storage protein fractions; β-conglycinin (7S) and glycinin (11S). It is extensively utilized in food industry by virtue of its high gel-forming ability, water-holding capacity, accessibility, biodegradability, and biocompatibility. The changes in health concepts of consumers alongside the progress in the food industry have induced the wide application of soy protein isolate (SPI) as a processing raw material to enhance the nutritional value and promote the quality of EFs for various applications. Unlike fibrous proteins like collagen and gelatin that can be easily electrospun into fibril structures, the globular soy protein should be unfolded via denaturation to be enabled to produce fibrous bodies (Vega-Lugo & Lim, 2008). To solve such limitations, other synthetic/natural polymers can be added to the protein solution, facilitating the E-spin process. Another common solution could be the disruption of quaternary structure SPI by using alkaline and thermal treatments. This improves the solubility and unfolding of the protein lattice via exposing hydrophobic and sulfhydryl functional groups (Momen, Alavi, & Aider, 2021).
Gluten
Wheat gluten is one of the biocompatible, biodegradable, and eco-friendly storage proteins, which possesses a Mw = 30–90 kDa. As a promising biopolymer, gluten can be processed into EFs to be applied for a wide range of applications. This protein is essentially comprised of high Mw glutenins and low Mw gliadins (Pourmohammadi & Abedi, 2021). Glutenins with both inter- and intra-molecular SS-bond (Mw = ∼106 kDa and isoelectric point of 4.5 ∼ 4.6) are insoluble in aqueous alcohols, while gliadins (Mw = 25 ∼ 100 kDa and isoelectric point of 6.41 ∼ 7.1) as alcohol-soluble materials contain predominantly intra-molecular SS-bonds. Therefore, such discrepancies play a pivotal role in configuration and features of fibrous networks engineered by what gluten proteins, and, thus, the quality of final products (Pietsch, Bühler, Karbstein, & Emin, 2019).
Animal proteins
Casein
Caseins (approximately 80 % of the total protein in cow milk) demonstrate numerous advantages in E-spin, i.e., simple production, low price, and potential of encapsulating both hydrophobic and hydrophilic bioactives. Caseins with isoelectric point of ∼ 4.6 are typically spherical in shape and highly stable against high temperatures (their coagulation occurs at 140 °C for 15–20 min at the normal pH of milk). Caseins are generally classified as four main fractions including αs1, αs2, β, and κ-caseins at a ratio of 4:1:3.5:1.5, which possess exclusive structural and functional properties (Razzak & Cho, 2022). Distinct flexible structure and high amount of proline in caseins make them more resistant to thermal treatments than globular proteins like whey proteins (β-lactoglobulin or α-lactoalbumin). Caseins have good film-forming capacity thanks to the random coil structure of large amount of polar units. Casein molecules can be combined with calcium and assemble into a distinctive spherical micellar configuration, named casein micelles with a diameter ∼ 50–500 nm. The micellar casein is well-known to be a vehicle of calcium and an outstanding bioactive carrier. Besides the micellar architectures, caseins or casein salts (sodium/calcium caseinates) are extensively utilized as emulsifiers or delivery vehicles in the pharmaceutical and food industry (Wang & Zhao, 2022).
Whey proteins
Whey proteins, as an important co-product of dairy industry, are divided into four main components; β-lactoglobulin, α-lactalbumin, bovine serum albumin, and lactoferrin. These types of proteins are cost-effective with multiple functional attributes e.g., thickening, stabilizing, emulsifying, gelling, film-forming, fat replacing, whipping, coating, and water-holding properties. Whey proteins (with an isoelectric point around pH = 5) display superior nutritional values and manifold functionalities, rendering them interesting biomaterials for food and biomedical applications. Whey protein-based biomaterials also possesses amphiphilic configuration of excellent solubility over a wide pH range, but low stability above 70 °C because of denaturation, coagulation, and precipitation phenomenon. Functional features of this biopolymer greatly hinge upon their structural characteristics. Thanks to their natural source, whey protein-based materials reckon as biocompatible, biodegradable, sustainable, and safe building blocks for bioactive/drug delivery applications using E-spin.
Collagen and gelatin
Collagen counts as the most plentiful protein in the human body. It is a key element of the extracellular matrix (ECM) and confers tensile strength and structural integrity to tissues. Collagen, as an ideal and effective polymer for E-spin, has attained broad clinical/consumer acceptance due to its safety, high water-affinity, low antigenicity, excellent cell compatibility, non-immunogenicity, and available isolation approaches from different sources. This protein is characterized by the presence of a triple-helix architecture, which is supercoiled from 3 polyproline-II-like helices. Different types of collagens exhibit the triple helical molecular configuration. Collagens typically involved in creating fibrillar configurations are types I, II, III, V, and XI, with the fibril-forming collagens type I, II, and III being the most plentiful. The steric constraints for this structure lead to the presence of glycine as every third amino acid in the sequence to allow for close packing of the three polypeptide chains, while a high content of the amino acids proline and hydroxyproline promote the polyproline-II-like conformation of individual chains and provide stability. The denaturation of native collagen leads to the formation of gelatin and the individual chains in gelatin do not readily reform into the original aligned structure. However, shorter segments of triple-helix lacking the native alignment can assemble, offering junctional domains, which enable the gel network of gelatin to form. Gelatin produced by fractional hydrolysis of the collagen protein is widely used in the food/pharma disciplines due to its capability to enhance viscosity and stability, as well as high loading capacity for drug and bioactives. Gelatin hydrates and swells in cold water, while above 40 °C, a colloidal solution is formed (sol).
Elastin
As an insoluble protein, elastin is produced by the cross-linking of its soluble precursor tropoelastin (a protein of 750–800 amino acid residues). The hydrophobic sequences of the elastin are highly repetitive and rich in aliphatic residues, e.g., proline, alanine, valine, leucine, isoleucine, and glycine. Elastin is one of the structural components of ECM, which offers the necessary resilient and elastic attributes to tissues/organs (Rodríguez-Cabello, de Torre, Ibañez-Fonseca, & Alonso, 2018). This protein has now been successfully co-spun with synthetic polymers to offer bioactivity and elasticity (Ciarfaglia et al., 2020, Li et al., 2005, Sánchez-Cid et al., 2021, Wang et al., 2023).
Silk fibroin
Silk, as an intriguing medical-grade material, is exploited for centuries by virtue of its unique blend of material attributes and bioactivity. It is naturally produced by a vast diversity of insects/spiders and comprised of two distinct proteins: a glue-like sericin protein (holding fibers together) and a 325 kDa fibroin filament (the mechanical backbone). Silk has also been exhibited to decompose via proteolytic hydrolysis and be slowly absorbed in the body as biocompatible amino acids (notwithstanding it is considered a non-degradable material owing to the length of time over, which it breaks down). Furthermore, silk displays outstanding mechanical features not seen in other nature-derived proteins (Chen et al., 2018, Wang et al., 2021). The thermostability of silk material systems facilitate processing over a wide range of temperatures (<250 °C), as evidenced by the capability to autoclave them without loss of functional integrity (Zhang et al., 2009). Integrating these protein polymer advantages with the E-spin technique can results in scaffolds of combined mechanical, biochemical, and topographical cues with unique versatility for a range of biomaterial, cell, and tissue studies/applications.
Keratin
Keratin is a naturally occurring protein (found in hair, wool, feather, and fingernails), which has attracted the attention of many researchers due to its unique biocompatibility/biodegradability and hydrophilic properties. It is comprised of a series of amino acids, which are covalently bound by peptide linkages with complex structures (Ye et al., 2022). The diversity of amino acids in the protein structure render it an excellent carrier for hydrophilic, hydrophobic, and charged bioactive ingredients. Compositionally/structurally, keratin is much more complex compared to fiber-forming materials and is difficult to spin into electrospun fibril bodies. Besides, the engineered keratin-based EFs typically have poor stability and low mechanical strength in aqueous solutions (Zhao et al., 2022). In addition to this, due to its low molecular weight (around 60 to 25 kDa for mammal proteins and ∼ 10 kDa for feather proteins), keratin solution is generally characterized by extremely low viscosity, thus resulting in unstable E-spin, yielding droplets/beaded fibers. Reinforcing with fillers or blending with other polymers are main strategies utilized to tackle the aforementioned processing limitations (Schifino et al., 2022).
Protein EFs for loading and controlled release of bioactive compounds
Protein EFs are food-grade delivery materials with the capability to encapsulate a vast diversity of bioactives. A myriad of functions as well as the potential of changing the structure as a response to various triggers is the most well-known strengths of the protein-based EFs, as carriers of labile bioactives. As above-mentioned, many proteins possess specific features from which the designed EFs may benefit for bioactive delivery purposes. For instance, whey protein-based vehicles have been utilized for enhancing the stability and (bio)functionality of bioactives and biopharmaceuticals. Additionally, biomaterials, e.g., bioactive-loaded protein-based EFs intended for food packaging and wound healing, have been typically engineered from elastin or silk to benefit of proper mechanical features. Furthermore, protein-based EFs can be added to various biosystems like films or hydrogels to promote their bioactive and gelation properties. Several studies have revealed that the inherent features of the specific protein are typically conserved in the final EFs. Some proteins have been demonstrated to show antibacterial, anticancer, antiviral attributes, etc. Other proteins, such as collagen or elastin, possess the capability to promote cell proliferation and have demonstrated improved tissue regeneration ability. In what follows, application of protein EFs for loading different bioactives are discussed.
Polyphenols
Plant polyphenols are valuable bioactives that show beneficial health effects including antioxidant, anti-inflammatory, anticancer, and antimicrobial properties (Zhang et al., 2022). However, many polyphenols display limited bioaccessibility and stability against environmental conditions such as temperature, pH, light, and oxygen, which limit their application in a wide range of food and nutraceutical products. In this context, encapsulation is used as an effective strategy to overcome the challenges associated with the use of polyphenols. Among many different encapsulation systems available, EFs exhibit high porosity, high surface-to-volume ratio, and ultrafine architectures. E-spin, as a non-mechanical/thermal process, is well suited with sensitive phenolic compounds, improving their stability and bioavailability (Hosseini & Gómez-Guillén, 2018). Besides, the sensory quality of food products may be improved by masking the bitterness of polyphenols entrapped in EFs (Anu Bhushani & Anandharamakrishnan, 2014). Various animal and plant-based proteins are widely used as bio-based polymers in fabrication of EFs for polyphenol delivery. Findings of some recent studies focusing on the application of protein EFs for loading and controlled release of polyphenols and other bioactives are summarized in Table 2. It has been evident that polyphenol type/concentration and protein type/concentration are significant factors affecting the physicochemical properties, EE, release characteristics, and stability of protein EFs carrying polyphenols.
Table 2.
Some recent studies focusing on the application of protein EFs for loading and controlled release of bioactive molecules.
| Bioactive(s) | Protein type |
E-spin parameters |
Key findings | Reference | ||
|---|---|---|---|---|---|---|
| Voltage (kV) | Flow rate (mL/h) | Collector distance (cm) | ||||
| Eggplant peel extract rich in anthocyanins and phenolic compounds | Gelatin | 15 kV voltage, 1 mL h−1 flow rate, and 10 cm collector distance |
|
Estrella-Osuna et al., 2022 | ||
| Sour cherry concentrate rich in polyphenols | Gelatin or gelatin-lactalbumin | 25–28 kV voltage, 0.4–0.5 mL h−1 flow rate, and 10 cm collector distance |
|
Isik et al., 2018 | ||
| Polyphenols (kaempferol, tannic acid, and phlorotannin) | Zein | 12 kV voltage, 1.2 mL h−1 flow rate, and 15 cm collector distance |
|
Yingying et al., 2022 | ||
| Raspberry extract rich in anthocyanins | Soy protein | 20–30 kV voltage, 3.6–5.4 mL h−1 flow rate, and 20 cm collector distance |
|
Wang et al., 2013 | ||
| Moringa oleifera kernel oil rich in monounsaturated fatty acids | Zein | 20 kV voltage, 0.5–0.9 mL h−1 flow rate, and 10 cm collector distance |
|
Afolabi-owolabi et al., 2020 | ||
| European fish eel oil | European fish eel gelatin | 10–17 kV voltage, 0.04–0.5 mL h−1 flow rate, and 10 cm collector distance |
|
Taktak et al., 2021 | ||
| Echium oil | Pea protein | 11 kV voltage, 0.8 mL h−1 flow rate, and 15 cm collector distance |
|
Najafi et al., 2022 | ||
| Perillaldehyde and thymol | Gelatin-zein | 22 kV voltage, 1.8 mL h−1 flow rate, and 10 cm collector distance |
|
Wang et al., 2022 | ||
| Oliveria decumbens EO | Glutelin-hordein | 12–15 kV voltage, 0.5–1 mL h−1 flow rate, and 10–15 cm collector distance |
|
Zahabi et al., 2021 | ||
| Green tea EO | Casein in combination with polycaprolactone | 11 kV voltage, 1 mL h−1 flow rate, and 15 cm collector distance |
|
Yavari Maroufi et al., 2022 | ||
| Cumin EO | Gelatin combination with polycaprolactone | 0.25 mL h−1 flow rate, and 15 cm collector distance |
|
Shanbehzadeh et al., 2022 | ||
| Thyme EO | Zein | 20 kV voltage, 1 mL h−1 flow rate, and 15 cm collector distance |
|
Ansarifar & Moradinezhad, 2022 | ||
| Vitamin B12 | Zein | 0–20 kV voltage, 0.2–0.3 mL h−1 flow rate, and 7–15 cm collector distance |
|
Coelho et al., 2021 | ||
| Vitamin B12 | Potato protein | 25–30 kV voltage, 1.2–3.6 mL h−1 flow rate, and 15 cm collector distance |
|
Mendes et al., 2020 | ||
| Folic acid | Zein | 11 kV voltage, 1 mL h−1 flow rate, and 16 cm collector distance |
|
do Evangelho et al., 2019 | ||
| Zinc oxide and curcumin | Whey protein in combination with basil seed gum | 15 kV voltage, 5 mL h−1 flow rate, and 8 cm collector distance |
|
Larki et al., 2022 | ||
|
Lactobacillus plantarum |
Silk fibroin in combination with polyvinyl alcohol | 24 kV voltage, 2 mL h−1 flow rate, and 18 cm collector distance |
|
Wei et al., 2021 | ||
| Bifidobacterium | Whey protein in combination with pullulan | 12–14 kV voltage, 0.3 mL h−1 flow rate, and 7 cm collector distance |
|
López-Rubio et al., 2012 | ||
| Carotenoids extracted from tomato peels | Gelatin | 18 kV voltage, 0.9 mL h−1 flow rate, and 12.5 cm collector distance |
|
İnanç Horuz & Belibağlı, 2018 | ||
| β-carotene | Soy protein isolate in combination with polyvinyl alcohol | 18 kV voltage, 0.15 mL h−1 flow rate, and 17 cm collector distance |
|
Pinheiro Bruni et al., 2020 | ||
| β-carotene | Whey protein in combination with pullulan | 19–23 kV voltage, 2.0–2.4 mL h−1 flow rate, and 17–19 cm collector distance |
|
Drosou et al., 2022 | ||
Additionally, the encapsulation of bioactive ingredients can improve the water solubility and bioavailability of bioactive molecules during human digestion, i.e., protect them against unfavorable conditions in certain parts of the digestive tract (e.g., the stomach) and release them in targeted areas (e.g., the intestine). Moreover, the food safety and sensory quality may be improved by inhibiting microbial growth and masking unpleasant flavors (e.g., the bitterness of polyphenols), respectively.
In a recent study by Estrella-Osuna and coworkers (2022), gelatin EFs were loaded with anthocyanins and phenolic compounds extracted from eggplant peel by-product. Scanning electron microscopy (SEM) and transmission electronic microscopy (TEM) images indicated that the addition of antioxidant extract resulted in increased diameter and improved contour, which allowed the formation of smoother EFs (Fig. 3). The authors observed differences in the infrared spectrum of commercial gelatin powder and electrospun gelatin, indicating conformational rearrangement of protein during E-spin as a result of increased H-bonding interactions. Besides, gelatin EFs were observed to interact with the extract via intermolecular H-bonding. High EE (92–94 %) was reported independent of the extract concentration. Moreover, the bioactive concentration and pH had significant effects on the in vitro release profile of the bioactive-loaded gelatin EFs, where the maximum release was observed at 6 h (Estrella-Osuna et al., 2022).
Fig. 3.
SEM and TEM images of eggplant peel extract-loaded gelatin EFs. (a, d) empty electrospun gelatin fibers, (b, e) fibers loaded with 33.3% eggplant peel extract, and (c, f) fibers loaded with 20% eggplant peel extract (Estrella-Osuna et al., 2022).
Within the plant polyphenols, curcumin is among the most frequently studied bioactive for improvement of solubility, bioavailability, and stability via encapsulation (Jiang et al., 2020). In an attempt, Alehosseini and coworkers (2019) followed an interesting approach and incorporated a green tea extract (GTE) into the formulation as an antioxidant during encapsulating curcumin in gelatin and zein EFs. The authors reported high EE for both gelatin and zein; while zein provided enhanced protection and slower release of curcumin in hydrophobic media. Addition of GTE was reported to result in strong interactions with proteins, in particular for gelatin, improving the protective effect and modifying the release behavior from the EFs. It was suggested that the protein matrix should be selected depending on the final application in encapsulation of curcumin in protein EFs (Alehosseini et al., 2019).
Essential oils and essential fatty acids
Although oils rich in essential fatty acids are reported to show many health-beneficial effects; their widespread use in food systems is limited, since they are very sensitive to oxidative deterioration, which can result in rancidity and related quality defects. Therefore, several encapsulation techniques are applied to them to preserve their quality and prolong shelf life. Recently, protein EFs are efficiently used as carriers for various oils rich in essential fatty acids including fish oils, moringa kernel oil and echium oil (Table 2). Both animal-based proteins such as gelatin and plant-based proteins including zein, gliadin and pea proteins are shown to be effective polymers in fabrication of EFs for loading oils rich in essential fatty acids. Besides, among several carbohydrate and protein-based biomaterials tested for E-spin encapsulation of echium oil, pea protein was reported to provide the highest protection against oxidation (Najafi et al., 2022). Furthermore, natural antioxidants can be incorporated into the formulation for further improvement of oxidative stability. In a recent study, phenolic compounds were extracted from pecan nut shell, a by-product of the nut industry, and used as an antioxidant for encapsulation of sardine oil using gliadin-based carriers (Dórame-Miranda et al., 2021).
Essential oils (EOs) obtained from various plant sources have also the potential to be used as effective antimicrobial agents due to their chemical constituents. However, the use of EOs as natural antimicrobials in food systems is limited as a result of their volatile nature, strong aroma, and sensitivity against environmental factors (Mukurumbira et al., 2022). Therefore, encapsulation is used as an effective strategy to overcome these challenges and improve the biological activity and controlled release of EOs and aromatic compounds. Literature survey demonstrated that EOs-incorporated protein EFs are mainly developed for use in active packaging applications (Table 2). Combination of two different types of proteins (Wang et al., 2022, Zahabi et al., 2021) or proteins with other biocompatible polymers such as polycaprolactone (Shanbehzadeh et al., 2022, Yavari Maroufi et al., 2022) are recently applied approaches in fabrication of EFs for loading and controlled release of EOs and aromatic compounds. In a recent research, Wang and coworkers (2022) fabricated gelatin/zein EFs loaded with perillaldehyde, thymol, or ɛ-polylysine for preservation of chilled chicken breast. The addition of perillaldehyde, thymol, and ɛ-polylysine was reported to influence the morphology and diameter of EFs. Furthermore, the type of antimicrobial agent added had a significant effect on the mechanical properties, water vapor and oxygen permeability and thermal stability of EF films. Gelatin/zein EF films loaded with perillaldehyde were observed to prolong the shelf life of chilled chicken breasts by 6 days (Wang et al., 2022). In another recent attempt, Shanbehzadeh and coworkers (2022) fabricated EF mats using a combination of gelatin-polycaprolactone and loaded with cumin EO and zinc oxide NPs for prolonging the shelf life of white cheese. Addition of cumin EO and zinc oxide NPs was observed to affect the thermal stability, surface and mechanical properties of EF mats. Fabricated mats were reported to slow the growth of Staphylococcus aureus in white cheese samples during 12 days of cold storage (Shanbehzadeh et al., 2022).
Vitamins, flavors, and minerals
Encapsulation technologies are widely applied to vitamins and minerals to improve their stability during processing and storage. Recent studies are focusing on encapsulation of vitamins by EFs and NPs. Zein EFs were fabricated for encapsulation of folic acid for improvement of stability against thermal treatment and irradiation. Folic acid-loaded EFs were reported to show increased stability against thermal treatment at 180 °C and exposure to 24 h of ultraviolet A light irradiation compared to unencapsulated folic acid (do Evangelho et al., 2019). In another recent study, potato protein EFs were used as carriers for vitamin B12. E-spin process was indicated to result in conformational changes in potato protein whereas addition of vitamin resulted in a slight increase in average diameter of EFs. The release characteristics of vitamin B12 from potato protein EFs indicated that the developed system can be potentially used as a delivery vehicle for hydrophilic bioactives (Mendes, Saldarini, & Chronakis, 2020). Liu and coworkers (2021) developed an interesting strategy and fabricated EF membranes of gelatin for co-encapsulation of hydrophobic fish oil and hydrophilic vitamin C. Addition of vitamin C was reported to result in some adverse effects including decreasing the loading capacity of fish oil and increased fragility of fibrous membranes. On the other hand, vitamin C was reported to prevent lipid oxidation and convert lipid hydroperoxides during the E-spin process, emphasizing the role of antioxidants in encapsulation of hydrophobic bioactives (Liu et al., 2021).
Probiotics
Probiotics are shown to have many beneficial health effects; however, they are highly sensitive towards harsh environmental conditions during processing and storage including temperature, oxygen, and relative humidity. Moreover, harsh conditions in the digestive system such as gastric acid and action of enzymes result in a significant decrease in the number of viable probiotics, limiting their beneficial role. Traditional encapsulation technologies have some limitations when they are applied to probiotics including introduction of high temperatures, dehydration stress and difficulty in controlling microcapsule particle size (Xu et al., 2022). Therefore, carriers such as EFs have emerged as an alternative to traditional techniques for encapsulation of probiotics. While fructooligosaccharides, polyvinyl alcohol and alginate are used as EFs for encapsulation of probiotics, there are limited reports on the use of proteins for this purpose. For instance, López-Rubio and coworkers (2012) used a combination of whey protein and pullulan for encapsulation of living Bifidobacterium strains with E-spin. The authors reported that encapsulation resulted in a significant increase in the viability of bifidobacterial strain at 20 °C. Moreover, whey protein was found to be more effective in protecting bacteria compared to pullulan, prolonging the survival at high relative humidity (López-Rubio et al., 2012). More recently, Wei and coworkers (2021) fabricated polyvinyl alcohol/silk fibroin EFs for encapsulating Lactobacillus plantarum. The authors reported that the mixing ratio of polyvinyl alcohol and silk fibroin affected EF morphology in such a way that polyvinyl alcohol allowed for formation of a continuous and uniform EF structure whereas silk fibroin increased the yield and diameter of EFs and had a positive effect on probiotics packaging. Encapsulation in polyvinyl alcohol/silk fibroin EFs was indicated to result in a significant improvement in the viability of L. plantarum under simulated gastric conditions (Wei et al., 2021).
Carotenoids
Carotenoids, which are potent antioxidants need protection during processing and storage since they are highly susceptible against environmental conditions. Encapsulation is applied to carotenoids not only for protection and prolonging shelf life, but also in some cases for improvement of solubility and bioavailability. Proteins can be used alone or in combination with other biocompatible polymers in EFs for encapsulation of carotenoids. Gelatin EFs were used for encapsulation of carotenoids extracted from tomato peels. Stability of the encapsulated carotenoid extract was reported to improve significantly during storage at varying temperatures of −20, 4, and 25 °C for 14 days, showing better retention of lycopene and its antioxidant activity. Moreover, encapsulation in gelatin EFs resulted in a remarkable increase in the solubility of carotenoid extract in water (İnanç Horuz & Belibağlı, 2018). In another recent attempt, β-carotene dissolved in corn oil was encapsulated in EFsEFs fabricated using pure pullulan or a combination of whey protein and pullulan. Both pullulan and whey protein:pullulan EFs were observed to improve the stability of β-carotene at low humidity environments. However, whey protein:pullulan (30:70) EFs provided higher stability against oxidation of β-carotene compared to pure pullulan EFs when tested under varying storage temperatures, water activity conditions and exposure to UV–Vis irradiation. Better protective effect observed for whey protein:pullulan EFs was attributed to the stronger interactions between protein and oil-β-carotene core mixture, reducing the availability of carotenoid molecules to oxidation by molecular oxygen (Drosou et al., 2022). On the other hand, Pinheiro Bruni and coworkers (2020) encapsulated β-carotene dissolved in soybean oil in a mixture of soy protein:polyvinyl alcohol electrospun onto a polymer film. Furthermore, a thermal process was applied to improve adhesion onto the packaging film. The annealing treatment observed to result in slower and more sustained release of encapsulated β-carotene in oil-based release medium (Pinheiro Bruni et al., 2020).
Challenges to be met and future perspective
The loading of bioactives into protein-based EFs has sparked a great interest for their use in diverse applications. Various bioactives have been loaded into different protein-based EFs through diversifying the E-spin process and loading mechanism, such as coaxial, emulsion, blending, or a simultaneous application of E-spin/electrospraying. Optimization of these methods allows effective loading of bioactives into EFs and their controlled release from EFs. Choosing the best loading route for bioactives is also related with the solubility and stability of bioactives of interest. Even though coaxial and emulsion E-spin routes are superior for loading bioactives than the other methods in terms of maintaining the bioactivity and tuning release profiles. In this regards, there are still major research challenges yet to be solved; these are (i) efficient bioactive loading without need of any toxic ingredients (e.g., surfactants) reduce the potential for adverse complications, (ii) preserving the bioactivity after the encapsulation for a long term, (iii) the use of nontoxic solvent alternatives, (v) a precise release profile, and (vi) industrialization of the concept for large scale productions.
Future studies should focus on high loading and improving therapeutic potential of bioactives. Since most bioactives are hydrophobic and poorly water soluble, the main challenge is their stability and bioavailability. Furthermore, the loading of volatile bioactives and flavoring agents into protein-based EFs and maintaining their presence can be problematic. In this regard, excipients, such as cyclodextrins (CDs), can be used as solubilization enhancers without the need of any toxic solvents and surfactants: since they form complexes with hydrophobic compounds that can fit into the CD cavity they enable the high loading of bioactives into EFs without the need for any toxic solvent. Also, they can stabilize and maintain the activity of bioactives hosted while boosting their bioavailability for in vivo applications.
Another major issue is the solubility of proteins in water or other conventional solvents to be electrospun into EFs. Many studies used toxic halogenated and polar aprotic solvents to able dissolve proteins. However, these solvents are not environmentally benign and highly toxic. Solvent residues in the produced EFs can cause toxicity, thereby limiting their bio-related use. In this regard, recent studies focused on the use of green solvent alternatives to produce protein EFs (Castilla-Casadiego et al., 2016, Lv et al., 2018, Miele et al., 2020, Mosher et al., 2021, Souzandeh et al., 2016). For the interested readers, a comprehensive review on the E-spin based on benign solvents is available (Avossa et al., 2022). Future studies should also focus on the engineering of protein-based EFs with better control on the release profiles of the encapsulated bioactives. In this regard, optimization of controlled release EF formulation can be done using artificial neural networks or response surface methodology, as also already reported for drug delivery NPs (Li et al., 2015, Reis et al., 2004).
Some bioactives are highly sensitive and can lose bioactivity during the storage of EFs. Recently, E-spin has been employed in the development of hydrogel structures (Li et al., 2020). Such an approach might improve the stability of the encapsulated bioactives because of the watery matrix of hydrogels while altering the release kinetics. The bioactives will be released from EFs first, followed by the release from the hydrogel matrix. This will increase the diffusion time and eventually prolong the release profile while enhancing the stability of bioactives. In future, particular interest should be given to alternative but effective routes for the loading of bioactive into the protein-based EFs. So far, the core–shell and emulsion approaches offer the best control on the release profile. As an alternative, polymers can be functionalized with complexation groups to encapsulate and slowly release the payloads from the EF matrix. Also, industrialization of loading approaches is another critical challenge, particularly for coaxial and emulsion E-spin, which would be problematic because of jet instability. Therefore, straightforward methods would be ideal routes to load bioactives into protein-based EFs at larger scales. Notwithstanding the great progress in the field, merely a few in vivo studies and clinical trials were reported in the literature over the years, and the biological features of protein-based EFs, in many cases, are restricted to in vitro assessments with cell lines.
Besides the aforementioned, although a huge progress in E-spin has been made at the laboratory scale in recent years, there are still some restrictions, which limit its commercial application in the food industry. One of the major limitations of protein EFs is the comparatively low productivity, which can be solved by modifying the structural aspects of the E-spin process (like the application of multi-needle setup). In addition, as described previously, the hydrophilicity of these structures may cause potent plasticization and deteriorates the barrier properties under increasing relative humidity, hindering their application in food industry. Hence, it is of great importance to improve the barrier characteristics of protein EFs to make them applicable in food sector. The most widely utilized strategies to enhance the performance of protein materials are the application of blends with biocompatible polymers and the use of cross-linkers (e.g., glutaraldehyde, genipin, transglutaminase, terephthalaldehyde, etc.).
Conclusion
The high versatility of proteins in nature is outstanding. Due to the great tunability of different protein architectures for specific application, it is not surprising that EFs designed by proteins can be applicable in a myriad of disciplines as food packaging, wound dressings, and more importantly bioactive delivery. Utilizing protein-based biopolymers alongside promising encapsulation approaches like E-spin open the way for innovation in the field of food science and technology. Protein EFs are very proper candidates for loading and encapsulating bioactives, thanks to their superior attributes over various synthetic polymers by being biodegradable and biocompatible and a sustainable origin. E-spin, as one of the most adaptable strategies for engineering a range of protein-based EFs can be applied in diverse encapsulation techniques to combine the inherent attributes of proteins for encapsulation and loading of bioactives. Many studies revealed that protein-based EFs promote (bio)stability, physicochemical characteristics, and release mechanism of bioactives. Considering such properties, protein-based EFs are potent to offer a range of functions required for food/biomedical applications in the near future. By changing/controlling some important parameters of E-spin, e.g., the protein/bioactive concentration, viscosity of the feeding polymer solution, electrostatic field, and ambient humidity, a prompt growth of bioactive architectures could be achieved. Nonetheless, further in vivo studies are needed to assess previously engineered protein-based EFs in detail. Furthermore, as outlined in this work, some bioactive-loaded protein EFs are investigated broadly in vitro, and we believe that it is time to focus on their behavior in vivo. Aside from the food and biomedical applications of bioactive-loaded protein-based EFs, researchers are about to introduce further applications of these structures by strictly modifying system attributes to match the intended tissues/cells. To promote the features of such architectures, a combination of bio-based and synthetic materials together with surface decoration of EFs might constitute another challenge. All in all, what is important is that the protein-based EFs as potential carriers of bioactives will be key players in the future of food science and engineering.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Seid Mahdi Jafari, Email: smjafari@gau.ac.ir.
Hadis Rostamabadi, Email: rostamabadi@nutr.mui.ac.ir.
Data availability
Data will be made available on request.
References
- Afolabi-owolabi O.T., Abidin S.Z., Ariffin F. Electrospun Polymer Nanofiber from Moringa Oleifera Kernel Oil with Coaxial Electrospinning Method. Current Nutrition & Food Science. 2020;16(1):90–97. [Google Scholar]
- Agarwal S., Greiner A., Wendorff J.H. Functional materials by electrospinning of polymers. Progress in Polymer Science. 2013;38(6):963–991. doi: 10.1016/j.progpolymsci.2013.02.001. [DOI] [Google Scholar]
- Akturk A., Erol Taygun M., Goller G. Optimization of the electrospinning process variables for gelatin/silver nanoparticles/bioactive glass nanocomposites for bone tissue engineering. Polymer Composites. 2020;41(6):2411–2425. doi: 10.1002/pc.25545. [DOI] [Google Scholar]
- Alehosseini A., Gómez-Mascaraque L.G., Martínez-Sanz M., López-Rubio A. Electrospun curcumin-loaded protein nanofiber mats as active/bioactive coatings for food packaging applications. Food Hydrocolloids. 2019;87:758–771. doi: 10.1016/j.foodhyd.2018.08.056. [DOI] [Google Scholar]
- Ansarifar E., Moradinezhad F. Encapsulation of thyme essential oil using electrospun zein fiber for strawberry preservation. Chemical and Biological Technologies in Agriculture. 2022;9(1):2. doi: 10.1186/s40538-021-00267-y. [DOI] [Google Scholar]
- Anu Bhushani J., Anandharamakrishnan C. Electrospinning and electrospraying techniques: Potential food based applications. Trends in Food Science & Technology. 2014;38(1):21–33. doi: 10.1016/j.tifs.2014.03.004. [DOI] [Google Scholar]
- Avossa J., Herwig G., Toncelli C., Itel F., Michel Rossi R. Electrospinning based on benign solvents: Current definitions, implications and strategies. Green Chemistry. 2022;24(6):2347–2375. doi: 10.1039/D1GC04252A. [DOI] [Google Scholar]
- Buzgo M., Mickova A., Rampichova M., Doupnik M. In: Core-Shell Nanostructures for Drug Delivery and Theranostics. Focarete M.L., Tampieri A., editors. Woodhead Publishing; 2018. 11—Blend electrospinning, coaxial electrospinning, and emulsion electrospinning techniques; pp. 325–347. [DOI] [Google Scholar]
- Cai X.L., Jiang T.T., Qiao C.M., Cheng B.W., Kang W.M. Effect of Electrospinning Process on Electrospun Chlorinated Polyvinyl Chloride (CPVC) Nanofibers. Applied Mechanics and Materials. 2014;633–634:11–14. doi: 10.4028/www.scientific.net/AMM.633-634.11. [DOI] [Google Scholar]
- Casper C.L., Stephens J.S., Tassi N.G., Chase D.B., Rabolt J.F. Controlling Surface Morphology of Electrospun Polystyrene Fibers: Effect of Humidity and Molecular Weight in the Electrospinning Process. Macromolecules. 2004;37(2):573–578. doi: 10.1021/ma0351975. [DOI] [Google Scholar]
- Castilla-Casadiego D.A., Maldonado M., Sundaram P., Almodovar J. “Green” electrospinning of a collagen/hydroxyapatite composite nanofibrous scaffold. MRS Communications. 2016;6(4):402–407. doi: 10.1557/mrc.2016.43. [DOI] [Google Scholar]
- Chen C., Chuanbao C., Xilan M., Yin T., Hesun Z. Preparation of non-woven mats from all-aqueous silk fibroin solution with electrospinning method. Polymer. 2006;47(18):6322–6327. [Google Scholar]
- Chen S., John J.V., McCarthy A., Xie J. New forms of electrospun nanofiber materials for biomedical applications. Journal of Materials Chemistry B. 2020;8(17):3733–3746. doi: 10.1039/D0TB00271B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Li R., Li X., Xie J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Advanced drug delivery reviews. 2018;132:188–213. doi: 10.1016/j.addr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Chuangchote S., Sagawa T., Yoshikawa S. Electrospinning of poly(vinyl pyrrolidone): Effects of solvents on electrospinnability for the fabrication of poly(p-phenylene vinylene) and TiO2 nanofibers. Journal of Applied Polymer Science. 2009;114(5):2777–2791. doi: 10.1002/app.30637. [DOI] [Google Scholar]
- Ciarfaglia N., Pepe A., Piccirillo G., Laezza A., Daum R., Schenke-Layland K., Bochicchio B. Nanocellulose and elastin act as plasticizers of electrospun bioinspired scaffolds. ACS Applied Polymer Materials. 2020;2(11):4836–4847. [Google Scholar]
- Coelho S.C., Laget S., Benaut P., Rocha F., Estevinho B.N. A new approach to the production of zein microstructures with vitamin B12, by electrospinning and spray drying techniques. Powder Technology. 2021;392:47–57. doi: 10.1016/j.powtec.2021.06.056. [DOI] [Google Scholar]
- Cooley J.F. Google Patents; 1902. Apparatus for electrically dispersing fluids. [Google Scholar]
- Costoya A., Concheiro A., Alvarez-Lorenzo C. Electrospun Fibers of Cyclodextrins and Poly(cyclodextrins) Molecules. 2017;22(2):Article 2. doi: 10.3390/molecules22020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vrieze S., Van Camp T., Nelvig A., Hagström B., Westbroek P., De Clerck K. The effect of temperature and humidity on electrospinning. Journal of Materials Science. 2009;44(5):1357–1362. doi: 10.1007/s10853-008-3010-6. [DOI] [Google Scholar]
- Demir M.M., Yilgor I., Yilgor E., Erman B. Electrospinning of polyurethane fibers. Polymer. 2002;43(11):3303–3309. doi: 10.1016/S0032-3861(02)00136-2. [DOI] [Google Scholar]
- do Evangelho J.A., Crizel R.L., Chaves F.C., Prietto L., Pinto V.Z., de Miranda M.Z., Dias A.R.G., Zavareze E. da R. Thermal and irradiation resistance of folic acid encapsulated in zein ultrafine fibers or nanocapsules produced by electrospinning and electrospraying. Food Research International. 2019;124:137–146. doi: 10.1016/j.foodres.2018.08.019. [DOI] [PubMed] [Google Scholar]
- Dodero A., Schlatter G., Hébraud A., Vicini S., Castellano M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydrate Polymers. 2021;264 doi: 10.1016/j.carbpol.2021.118042. [DOI] [PubMed] [Google Scholar]
- Dórame-Miranda R.F., Gámez-Meza N., Ovando-Martínez M., Medina-Juárez L.A., Cárdenas-López J.L., Ramírez-Bon R., Santos-Sauceda I., Castro-Enríquez D.D., Burruel-Ibarra S.E. Encapsulation of Sardine Oil by Electrospraying with Gliadins and Pecan Nutshell Extracts for its Stabilization. Food and Bioprocess Technology. 2021;14(3):457–470. doi: 10.1007/s11947-020-02567-x. [DOI] [Google Scholar]
- Dou Y., Zhang W., Kaiser A. Electrospinning of Metal-Organic Frameworks for Energy and Environmental Applications. Advanced Science. 2020;7(3):1902590. doi: 10.1002/advs.201902590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosou C., Krokida M., Biliaderis C.G. Encapsulation of β-carotene into food-grade nanofibers via coaxial electrospinning of hydrocolloids: Enhancement of oxidative stability and photoprotection. Food Hydrocolloids. 2022;133 doi: 10.1016/j.foodhyd.2022.107949. [DOI] [Google Scholar]
- Estrella-Osuna D.E., Tapia-Hernández J.A., Ruíz-Cruz S., Márquez-Ríos E., de Ornelas-Paz J., Del-Toro-Sánchez C.L., Ocaño-Higuera V.M., Rodríguez-Félix F., Estrada-Alvarado M.I., Cira-Chávez L.A. Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release. Nanomaterials. 2022;12(13):Article 13. doi: 10.3390/nano12132303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsafi S.R., Bangar S.P., Chaudhary V., Hosseini E., Mokhtari Z., Karaca A.C.…Rostamabadi H. Recent advances in oral delivery of bioactive molecules: Focus on prebiotic carbohydrates as vehicle matrices. Carbohydrate Polymers. 2022;120074 doi: 10.1016/j.carbpol.2022.120074. [DOI] [PubMed] [Google Scholar]
- Falsafi S.R., Maghsoudlou Y., Aalami M., Jafari S.M., Raeisi M., Nishinari K., Rostamabadi H. Application of multi-criteria decision-making for optimizing the formulation of functional cookies containing different types of resistant starches: A physicochemical, organoleptic, in-vitro and in-vivo study. Food Chemistry. 2022;393 doi: 10.1016/j.foodchem.2022.133376. [DOI] [PubMed] [Google Scholar]
- Falsafi S.R., Wang Y., Ashaolu T.J., Sharma M., Rawal S., Patel K.…Rostamabadi H. Biopolymer Nanovehicles for Oral Delivery of Natural Anticancer Agents. Advanced Functional Materials. 2023;33(4):2209419. [Google Scholar]
- Fani N., Enayati M.H., Rostamabadi H., Falsafi S.R. Encapsulation of bioactives within electrosprayed κ-carrageenan nanoparticles. Carbohydrate Polymers. 2022;294 doi: 10.1016/j.carbpol.2022.119761. [DOI] [PubMed] [Google Scholar]
- Ghosal K., Agatemor C., Špitálsky Z., Thomas S., Kny E. Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chemical Engineering Journal. 2019;358:1262–1278. doi: 10.1016/j.cej.2018.10.117. [DOI] [Google Scholar]
- Giteru S.G., Ali M.A., Oey I. Recent progress in understanding fundamental interactions and applications of zein. Food Hydrocolloids. 2021;120:106948. [Google Scholar]
- Gu S.-Y., Ren J. Process Optimization and Empirical Modeling for Electrospun Poly(D, L-lactide) Fibers using Response Surface Methodology. Macromolecular Materials and Engineering. 2005;290(11):1097–1105. doi: 10.1002/mame.200500215. [DOI] [Google Scholar]
- Haghi A.K., Akbari M. Trends in electrospinning of natural nanofibers. Physica Status Solidi (a) 2007;204(6):1830–1834. doi: 10.1002/pssa.200675301. [DOI] [Google Scholar]
- Haider A., Haider S., Kang I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arabian Journal of Chemistry. 2018;11(8):1165–1188. doi: 10.1016/j.arabjc.2015.11.015. [DOI] [Google Scholar]
- Han S.O., Son W.K., Youk J.H., Lee T.S., Park W.H. Ultrafine porous fibers electrospun from cellulose triacetate. Materials Letters. 2005;59(24):2998–3001. doi: 10.1016/j.matlet.2005.05.003. [DOI] [Google Scholar]
- Heikkilä P., Harlin A. Parameter study of electrospinning of polyamide-6. European Polymer Journal. 2008;44(10):3067–3079. doi: 10.1016/j.eurpolymj.2008.06.032. [DOI] [Google Scholar]
- Hosseini S.F., Gómez-Guillén M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends in Food Science & Technology. 2018;79:125–135. [Google Scholar]
- Huang Z.-M., Zhang Y.-Z., Kotaki M., Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology. 2003;63(15):2223–2253. doi: 10.1016/S0266-3538(03)00178-7. [DOI] [Google Scholar]
- İnanç Horuz T., Belibağlı K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chemistry. 2018;268:86–93. doi: 10.1016/j.foodchem.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Isik B.S., Altay F., Capanoglu E. The uniaxial and coaxial encapsulations of sour cherry (Prunus cerasus L.) concentrate by electrospinning and their in vitro bioaccessibility. Food Chemistry. 2018;265:260–273. doi: 10.1016/j.foodchem.2018.05.064. [DOI] [PubMed] [Google Scholar]
- Jacobs V., Anandjiwala R.D., Maaza M. The influence of electrospinning parameters on the structural morphology and diameter of electrospun nanofibers. Journal of Applied Polymer Science. 2010;115(5):3130–3136. doi: 10.1002/app.31396. [DOI] [Google Scholar]
- Jeong J., Park K., Kim H., Park I., Choi J., Lee S.S. Multiplexed electrospraying of water in cone-jet mode using a UV-embossed pyramidal micronozzle film. Microsystems & Nanoengineering. 2022;8(1):Article 1. doi: 10.1038/s41378-022-00391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Liao W., Charcosset C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Research International. 2020;132 doi: 10.1016/j.foodres.2020.109035. [DOI] [PubMed] [Google Scholar]
- Singh K.S., Subramanian A. Phase-field simulations of electrohydrodynamic jetting for printing nano-to-microscopic constructs. RSC Advances. 2020;10(42):25022–25028. doi: 10.1039/D0RA04214E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazsoki A., Szabó P., Domján A., Balázs A., Bozó T., Kellermayer M., Farkas A., Balogh-Weiser D., Pinke B., Darcsi A., Béni S., Madarász J., Szente L., Zelkó R. Microstructural Distinction of Electrospun Nanofibrous Drug Delivery Systems Formulated with Different Excipients. Molecular Pharmaceutics. 2018;15(9):4214–4225. doi: 10.1021/acs.molpharmaceut.8b00646. [DOI] [PubMed] [Google Scholar]
- Kumar A., Wei M., Barry C., Chen J., Mead J. Controlling Fiber Repulsion in Multijet Electrospinning for Higher Throughput. Macromolecular Materials and Engineering. 2010;295(8):701–708. doi: 10.1002/mame.200900425. [DOI] [Google Scholar]
- Kumbar S.G., James R., Nukavarapu S.P., Laurencin C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomedical Materials. 2008;3(3) doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- Kutzli I., Gibis M., Baier S.K., Weiss J. Formation of whey protein isolate (WPI)–maltodextrin conjugates in fibers produced by needleless electrospinning. Journal of Agricultural and Food Chemistry. 2018;66(39):10283–10291. doi: 10.1021/acs.jafc.8b02104. [DOI] [PubMed] [Google Scholar]
- Larki M., Enayati M.H., Rostamabadi H. Basil seed gum promotes the electrospinnability of WPI for co-encapsulation of ZnO nanoparticles and curcumin. Carbohydrate Polymers. 2022;296 doi: 10.1016/j.carbpol.2022.119966. [DOI] [PubMed] [Google Scholar]
- Li J., Pan K., Tian H., Yin L. The Potential of Electrospinning/Electrospraying Technology in the Rational Design of Hydrogel Structures. Macromolecular Materials and Engineering. 2020;305(8):2000285. doi: 10.1002/mame.202000285. [DOI] [Google Scholar]
- Li M., Mondrinos M.J., Gandhi M.R., Ko F.K., Weiss A.S., Lelkes P.I. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26(30):5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Li X., Su Y., Liu S., Tan L., Mo X., Ramakrishna S. Encapsulation of proteins in poly(l-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids and Surfaces B: Biointerfaces. 2010;75(2):418–424. doi: 10.1016/j.colsurfb.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Li Y., Abbaspour M.R., Grootendorst P.V., Rauth A.M., Wu X.Y. Optimization of controlled release nanoparticle formulation of verapamil hydrochloride using artificial neural networks with genetic algorithm and response surface methodology. European Journal of Pharmaceutics and Biopharmaceutics. 2015;94:170–179. doi: 10.1016/j.ejpb.2015.04.028. [DOI] [PubMed] [Google Scholar]
- Lin T., Wang H., Wang H., Wang X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology. 2004;15(9):1375–1381. doi: 10.1088/0957-4484/15/9/044. [DOI] [Google Scholar]
- Liu L., Tao L., Chen J., Zhang T., Xu J., Ding M., Wang X., Zhong J. Fish oil-gelatin core-shell electrospun nanofibrous membranes as promising edible films for the encapsulation of hydrophobic and hydrophilic nutrients. LWT. 2021;146 doi: 10.1016/j.lwt.2021.111500. [DOI] [Google Scholar]
- López-Rubio A., Sanchez E., Wilkanowicz S., Sanz Y., Lagaron J.M. Electrospinning as a useful technique for the encapsulation of living bifidobacteria in food hydrocolloids. Food Hydrocolloids. 2012;28(1):159–167. doi: 10.1016/j.foodhyd.2011.12.008. [DOI] [Google Scholar]
- Lv D., Zhu M., Jiang Z., Jiang S., Zhang Q., Xiong R., Huang C. Green Electrospun Nanofibers and Their Application in Air Filtration. Macromolecular Materials and Engineering. 2018;303(12):1800336. doi: 10.1002/mame.201800336. [DOI] [Google Scholar]
- Lyu C., Zhao P., Xie J., Dong S., Liu J., Rao C., Fu J. Electrospinning of Nanofibrous Membrane and Its Applications in Air Filtration. A Review. Nanomaterials. 2021;11(6), Article 6 doi: 10.3390/nano11061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A.C., Saldarini E., Chronakis I.S. Electrohydrodynamic processing of potato protein into particles and fibers. Molecules. 2020;25(24):5968. doi: 10.3390/molecules25245968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A.C., Stephansen K., Chronakis I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocolloids. 2017;68:53–68. doi: 10.1016/j.foodhyd.2016.10.022. [DOI] [Google Scholar]
- Miele, D., Catenacci, L., Rossi, S., Sandri, G., Sorrenti, M., Terzi, A., Giannini, C., Riva, F., Ferrari, F., Caramella, C., & Bonferoni, M. C. (2020). Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution. Materials, 13(21), Article 21. 10.3390/ma13214698. [DOI] [PMC free article] [PubMed]
- Momen S., Alavi F., Aider M. Alkali-mediated treatments for extraction and functional modification of proteins: Critical and application review. Trends in Food Science & Technology. 2021;110:778–797. [Google Scholar]
- Mosher C.Z., Brudnicki P.A.P., Gong Z., Childs H.R., Lee S.W., Antrobus R.M., Fang E.C., Schiros T.N., Lu H.H. Green electrospinning for biomaterials and biofabrication. Biofabrication. 2021;13(3) doi: 10.1088/1758-5090/ac0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukurumbira A.R., Shellie R.A., Keast R., Palombo E.A., Jadhav S.R. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control. 2022;136 doi: 10.1016/j.foodcont.2022.108883. [DOI] [Google Scholar]
- Najafi Z., Bildik F., Şahin-Yeşilçubuk N., Altay F. Enhancing oxidative stability of encapsulated echium oil by incorporation of saffron extract loaded nanoliposomes into electrospun pullulan-pea protein isolate-pectin. Food Hydrocolloids. 2022;129 doi: 10.1016/j.foodhyd.2022.107627. [DOI] [Google Scholar]
- Noruzi M. Electrospun nanofibres in agriculture and the food industry: A review. Journal of the Science of Food and Agriculture. 2016;96(14):4663–4678. doi: 10.1002/jsfa.7737. [DOI] [PubMed] [Google Scholar]
- Pietsch V.L., Bühler J.M., Karbstein H.P., Emin M.A. High moisture extrusion of soy protein concentrate: Influence of thermomechanical treatment on protein-protein interactions and rheological properties. Journal of Food Engineering. 2019;251:11–18. [Google Scholar]
- Pinheiro Bruni G., de Oliveira J.P., Gómez-Mascaraque L.G., Fabra M.J., Guimarães Martins V., da Zavareze E. da R., López-Rubio A. Electrospun β-carotene–loaded SPI:PVA fiber mats produced by emulsion-electrospinning as bioactive coatings for food packaging. Food Packaging and Shelf Life. 2020;23 doi: 10.1016/j.fpsl.2019.100426. [DOI] [Google Scholar]
- Pourmohammadi K., Abedi E. Enzymatic modifications of gluten protein: Oxidative enzymes. Food Chemistry. 2021;356:129679. doi: 10.1016/j.foodchem.2021.129679. [DOI] [PubMed] [Google Scholar]
- Puppi D., Chiellini F. In: Core-Shell Nanostructures for Drug Delivery and Theranostics. Focarete M.L., Tampieri A., editors. Woodhead Publishing; 2018. 12—Drug release kinetics of electrospun fibrous systems; pp. 349–374. [DOI] [Google Scholar]
- Razzak M.A., Cho S.J. Molecular characterization of capsaicin binding interactions with ovalbumin and casein. Food Hydrocolloids. 2022;133:107991. [Google Scholar]
- Reis M.A.A., Sinisterra R.D., Belchior J.C. An Alternative Approach Based on Artificial Neural Networks to Study Controlled Drug Release. Journal of Pharmaceutical Sciences. 2004;93(2):418–430. doi: 10.1002/jps.10569. [DOI] [PubMed] [Google Scholar]
- Robb B., Lennox B. In: Electrospinning for Tissue Regeneration. Bosworth L.A., Downes S., editors. Woodhead Publishing; 2011. 3—The electrospinning process, conditions and control; pp. 51–66. [DOI] [Google Scholar]
- Rodríguez-Cabello J.C., de Torre, Ibañez-Fonseca A., Alonso M. Bioactive scaffolds based on elastin-like materials for wound healing. Advanced Drug Delivery Reviews. 2018;129:118–133. doi: 10.1016/j.addr.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Rostamabadi H., Chaudhary V., Chhikara N., Sharma N., Nowacka M., Demirkesen I.…Falsafi S.R. Ovalbumin, an outstanding food hydrocolloid: Applications, technofunctional attributes, and nutritional facts, A systematic review. Food Hydrocolloids. 2023;108514 [Google Scholar]
- Rostamabadi M.M., Falsafi S.R., Nishinari K., Rostamabadi H. Seed gum-based delivery systems and their application in encapsulation of bioactive molecules. Critical Reviews in Food Science and Nutrition. 2022:1–24. doi: 10.1080/10408398.2022.2076065. [DOI] [PubMed] [Google Scholar]
- Rychter M., Baranowska-Korczyc A., Lulek J. Progress and perspectives in bioactive agent delivery via electrospun vascular grafts. RSC Advances. 2017;7(51):32164–32184. doi: 10.1039/C7RA04735E. [DOI] [Google Scholar]
- Sánchez-Cid P., Perez-Puyana V., Jiménez-Rosado M., Guerrero A., Romero A. Influence of elastin on the properties of hybrid PCL/elastin scaffolds for tissue engineering. Journal of Applied Polymer Science. 2021;138(35):50893. [Google Scholar]
- Schifino G., Gasparini C., Drudi S., Giannelli M., Sotgiu G., Posati T.…Aluigi A. Keratin/Polylactic acid/graphene oxide composite nanofibers for drug delivery. International Journal of Pharmaceutics. 2022;623 doi: 10.1016/j.ijpharm.2022.121888. [DOI] [PubMed] [Google Scholar]
- Selling G.W., Biswas A., Patel A., Walls D.J., Dunlap C., Wei Y. Impact of Solvent on Electrospinning of Zein and Analysis of Resulting Fibers. Macromolecular Chemistry and Physics. 2007;208(9):1002–1010. doi: 10.1002/macp.200700056. [DOI] [Google Scholar]
- Shanbehzadeh F., Saei-Dehkordi S.S., Semnani D. Fabrication and characterization of electrospun nanofibrous mats of polycaprolactone/gelatin containing ZnO nanoparticles and cumin essential oil and their anti-staphylococcal potency in white cheese. Food Bioscience. 2022;49 doi: 10.1016/j.fbio.2022.101904. [DOI] [Google Scholar]
- Sill T.J., von Recum H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Souzandeh H., Wang Y., Zhong W.-H. “Green” nano-filters: Fine nanofibers of natural protein for high efficiency filtration of particulate pollutants and toxic gases. RSC Advances. 2016;6(107):105948–105956. doi: 10.1039/C6RA24512A. [DOI] [Google Scholar]
- Taktak W., Nasri R., López-Rubio A., Chentir I., Gómez-Mascaraque L.G., Boughriba S., Nasri M., Karra-Chaâbouni M. Design and characterization of novel ecofriendly European fish eel gelatin-based electrospun microfibers applied for fish oil encapsulation. Process Biochemistry. 2021;106:10–19. doi: 10.1016/j.procbio.2021.03.031. [DOI] [Google Scholar]
- Tan S.-H., Inai R., Kotaki M., Ramakrishna S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer. 2005;46(16):6128–6134. doi: 10.1016/j.polymer.2005.05.068. [DOI] [Google Scholar]
- Tebyetekerwa M., Ramakrishna S. What Is Next for Electrospinning? Matter. 2020;2(2):279–283. doi: 10.1016/j.matt.2020.01.004. [DOI] [Google Scholar]
- Topuz F., Uyar T. Electrospinning of gelatin with tunable fiber morphology from round to flat/ribbon. Materials Science and Engineering: C. 2017;80:371–378. doi: 10.1016/j.msec.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Topuz F., Uyar T. Electrospinning of Cyclodextrin Nanofibers: The Effect of Process Parameters. Journal of Nanomaterials. 2020;2020:e7529306. [Google Scholar]
- Topuz F., Uyar T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Research International. 2020;130 doi: 10.1016/j.foodres.2019.108927. [DOI] [PubMed] [Google Scholar]
- Topuz F., Abdulhamid M.A., Szekely G. Superoleophilic oil-adsorbing membranes based on porous and nonporous fluorinated polyimides for the rapid remediation of oil spills. Chemical Engineering Journal. 2022;449 doi: 10.1016/j.cej.2022.137821. [DOI] [Google Scholar]
- Topuz F., Abdulhamid M.A., Holtzl T., Szekely G. Nanofiber engineering of microporous polyimides through electrospinning: Influence of electrospinning parameters and salt addition. Materials & Design. 2021;198 doi: 10.1016/j.matdes.2020.109280. [DOI] [Google Scholar]
- Topuz F., Satilmis B., Uyar T. Electrospinning of uniform nanofibers of Polymers of Intrinsic Microporosity (PIM-1): The influence of solution conductivity and relative humidity. Polymer. 2019;178 doi: 10.1016/j.polymer.2019.121610. [DOI] [Google Scholar]
- Vega-Lugo A.C., Lim L.T. Electrospinning of soy protein isolate nanofibers. Journal of Biobased Materials and Bioenergy. 2008;2(3):223–230. [Google Scholar]
- Vempati S., Ranjith K.S., Topuz F., Biyikli N., Uyar T. Electrospinning Combined with Atomic Layer Deposition to Generate Applied Nanomaterials: A Review. ACS Applied Nano Materials. 2020;3(7):6186–6209. doi: 10.1021/acsanm.0c01120. [DOI] [Google Scholar]
- Waldrop K., Wycisk R., Pintauro P.N. Application of electrospinning for the fabrication of proton-exchange membrane fuel cell electrodes. Current Opinion in Electrochemistry. 2020;21:257–264. doi: 10.1016/j.coelec.2020.03.007. [DOI] [Google Scholar]
- Wang D., Sun J., Li J., Sun Z., Liu F., Du L., Wang D. Preparation and characterization of gelatin/zein nanofiber films loaded with perillaldehyde, thymol, or ɛ-polylysine and evaluation of their effects on the preservation of chilled chicken breast. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131439. [DOI] [PubMed] [Google Scholar]
- Wang S., Marcone M.F., Barbut S., Lim L.-T. Electrospun soy protein isolate-based fiber fortified with anthocyanin-rich red raspberry (Rubus strigosus) extracts. Food Research International. 2013;52(2):467–472. doi: 10.1016/j.foodres.2012.12.036. [DOI] [Google Scholar]
- Wang T., Kumar S. Electrospinning of polyacrylonitrile nanofibers. Journal of Applied Polymer Science. 2006;102(2):1023–1029. doi: 10.1002/app.24123. [DOI] [Google Scholar]
- Wang X., Bai Z., Li K., Dong J., Zhang H., Liu X.…Li Q. Bioinspired PLCL/Elastin Nanofibrous Vascular Tissue Engineering Scaffold Enhances Endothelial Cells and Inhibits Smooth Muscle Cells. Biomacromolecules. 2023;2741–2754 doi: 10.1021/acs.biomac.3c00175. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhao Z. Improved encapsulation capacity of casein micelles with modified structure. Journal of Food Engineering. 2022;333:111138. [Google Scholar]
- Wang Z., Wang Y., Yan J., Zhang K., Lin F., Xiang L.…Zhang H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Advanced Drug Delivery Reviews. 2021;174:504–534. doi: 10.1016/j.addr.2021.05.007. [DOI] [PubMed] [Google Scholar]
- Wei L., Zhou D., Kang X. Electrospinning as a novel strategy for the encapsulation of living probiotics in polyvinyl alcohol/silk fibroin. Innovative Food Science & Emerging Technologies. 2021;71 doi: 10.1016/j.ifset.2021.102726. [DOI] [Google Scholar]
- Xu C., Ban Q., Wang W., Hou J., Jiang Z. Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems. Journal of Controlled Release. 2022;349:184–205. doi: 10.1016/j.jconrel.2022.06.061. [DOI] [PubMed] [Google Scholar]
- Yao C., Li X., Song T. Electrospinning and crosslinking of zein nanofiber mats. Journal of Applied Polymer Science. 2007;103(1):380–385. doi: 10.1002/app.24619. [DOI] [Google Scholar]
- Yarin A. Coaxial electrospinning and emulsion electrospinning of core–shell fibers. Polymers for Advanced Technologies. 2011;22(3):310–317. doi: 10.1002/pat.1781. [DOI] [Google Scholar]
- Yavari Maroufi L., PourvatanDoust S., Naeijian F., Ghorbani M. Fabrication of Electrospun Polycaprolactone/Casein Nanofibers Containing Green Tea Essential Oils: Applicable for Active Food Packaging. Food and Bioprocess Technology. 2022;15(11):2601–2615. doi: 10.1007/s11947-022-02905-1. [DOI] [Google Scholar]
- Ye W., Qin M., Qiu R., Li J. Keratin-based wound dressings: From waste to wealth. International Journal of Biological Macromolecules. 2022;211:183–197. doi: 10.1016/j.ijbiomac.2022.04.216. [DOI] [PubMed] [Google Scholar]
- Yingying M., Xiu-xia L., Luyun C., Jianrong L. PH-Sensitive ε-polylysine/polyaspartic acid/zein nanofiber membranes for the targeted release of polyphenols. Food & Function. 2022;13(12):6792–6801. doi: 10.1039/D1FO03051E. [DOI] [PubMed] [Google Scholar]
- Zahabi N., Golmakani M.-T., Fazaeli M., Ghiasi F., Khalesi M. Electrospinning of glutelin-hordein incorporated with Oliveria decumbens essential oil: Characterization of nanofibers. Colloids and Surfaces B: Biointerfaces. 2021;208 doi: 10.1016/j.colsurfb.2021.112058. [DOI] [PubMed] [Google Scholar]
- Zanin M.H.A., Cerize N.N.P., de Oliveira A.M. In: Nanocosmetics and Nanomedicines: New Approaches for Skin Care. Beck R., Guterres S., Pohlmann A., editors. Springer; 2011. Production of Nanofibers by Electrospinning Technology: Overview and Application in Cosmetics; pp. 311–332. [DOI] [Google Scholar]
- Zavgorodnya O., Shamshina J.L., Bonner J.R., Rogers R.D. Electrospinning Biopolymers from Ionic Liquids Requires Control of Different Solution Properties than Volatile Organic Solvents. ACS Sustainable Chemistry & Engineering. 2017;5(6):5512–5519. doi: 10.1021/acssuschemeng.7b00863. [DOI] [Google Scholar]
- Zhang C., Yuan X., Wu L., Han Y., Sheng J. Study on morphology of electrospun poly(vinyl alcohol) mats. European Polymer Journal. 2005;41(3):423–432. doi: 10.1016/j.eurpolymj.2004.10.027. [DOI] [Google Scholar]
- Zhang H., Zhao C., Zhao Y., Tang G., Yuan X. Electrospinning of ultrafine core/shell fibers for biomedical applications. Science China Chemistry. 2010;53(6):1246–1254. doi: 10.1007/s11426-010-3180-3. [DOI] [Google Scholar]
- Zhang, S., Jia, Z., Liu, T., Wei, G., & Su, Z. (2019). Electrospinning Nanoparticles-Based Materials Interfaces for Sensor Applications. Sensors, 19(18), Article 18. 10.3390/s19183977. [DOI] [PMC free article] [PubMed]
- Zhang X., Reagan M.R., Kaplan D.L. Electrospun silk biomaterial scaffolds for regenerative medicine. Advanced drug delivery reviews. 2009;61(12):988–1006. doi: 10.1016/j.addr.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.Z., Wang X., Feng Y., Li J., Lim C.T., Ramakrishna S. Coaxial Electrospinning of (Fluorescein Isothiocyanate-Conjugated Bovine Serum Albumin)-Encapsulated Poly(ε-caprolactone) Nanofibers for Sustained Release. Biomacromolecules. 2006;7(4):1049–1057. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Li X., Sang S., McClements D.J., Chen L., Long J., Jiao A., Jin Z., Qiu C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation. Nano-Delivery, and Application. Foods. 2022;11(15):Article 15. doi: 10.3390/foods11152189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Niu J., Xiao C., Qin Z., Jin X., Wang W., Zhu Z. Separator with high ionic conductivity and good stability prepared from keratin fibers for supercapacitor applications. Chemical Engineering Journal. 2022;444 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.