Highlights
-
•
Light-wave and conventional fixation methods for green tea were compared.
-
•
Light-wave-dried tea leaves (LWL) exhibited jade green leaves, fresh and mellow taste.
-
•
Fixation produces more amino acids, peptides, flavonoids and trans-catechins.
-
•
The fresh and mellow taste of LWL is related to its high level of γ-glutamyl peptides.
-
•
Pinocembrin, phloretin and bitter peptides caused the bitterness of roller-dried tea.
Keywords: Kokumi peptides, Bitter peptides, Flavonoids, Amino acids, Trans-catechins
Abstract
Fresh leaves of Echa 1 were fixed by roller, steam/hot air and light-wave, and the effects of the three fixation methods on the chemical characteristics of straight-shaped green teas (GTs) were studied by widely targeted metabolomic analysis. 1001 non-volatile substances was identified, from which 97 differential metabolites were selected by the criteria of variable importance in projection (VIP) > 1, p < 0.05, and |log2(fold change)| > 1. Correlation analysis indicated that 14 taste-active metabolites were the major contributors to the taste differences between differently processed GTs. High-temperature fixation induces protein oxidation or degradation, γ-glutamyl peptide transpeptidation, degradation of flavonoid glycosides and epimerization of cis-catechins, resulting in the accumulation of amino acids, peptides, flavonoids and trans-catechins, which have flavor characteristics such as umami, sweetness, kokumi, bitterness and astringency, thereby affecting the overall taste of GTs. These findings provided a scientific basis for the directional processing technology of high-quality green tea.
1. Introduction
Tea is the second most popular beverage worldwide after water, because of its unique taste and health benefits. China is the world’s largest tea producer and the only country that produces all six tea categories (green, black, oolong, etc.), with more than 2000 tea products. The traditional tea processing techniques and associated social practices in China were added to the intangible cultural heritage list of UNESCO in November 2022. Green tea has the largest production and consumption in China (Zhang, Ho, Zhou, Santos, Armstrong & Granato, 2019), with green leaves and a clear infusion, a fresh and mellow taste, and a strong and lasting aroma (Wang et al., 2021b). Meanwhile, the shapes of green tea are also diverse, including flat, single bud, straight, curly, spiral and round. Among them, straight-shaped green tea is favored by many consumers due to its tight, thin, round and straight appearance, but its heavy bitter and astringent taste is usually unacceptable. The processing of green tea includes fixation, rolling and drying, of which fixation is the key process that determines its quality (Wang et al., 2023). Fresh leaves are fixed at high temperatures, and the endogenous enzymes polyphenol oxidase and peroxidase are rapidly inactivated, which prevents the enzymatic oxidation of polyphenols from turning the leaves dark brown, as occurs in black tea (Zhang et al., 2019). The non-volatile components in fresh leaves, including primary metabolites such as protein, amino acids, sugars and lipids, as well as secondary metabolites such as flavonoids and alkaloids, undergo extensive transformation during the fixation process.
Depending on the heat transfer medium (air, metal, or electromagnetic radiation), the fixation methods of green tea can be classified as thermal convection, thermal conduction and thermal radiation. The main methods currently used industrially to fix green tea are heated metal rollers, steam and hot air (Wang et al., 2020, Song et al., 2023); new methods, such as light-wave fixation are also being developed. Roller fixation is suitable for large-scale production, because of its low cost, and it is currently the most widely used fixation process for green tea production in China (Wang et al., 2020). The high temperature of roller fixation achieves high conversion of volatile substance precursors and the green tea produced has a strong and lasting aroma, but the taste is heavy bitter and astringent (Wang et al., 2021a). Steam/hot air fixation is a combination of steam and hot air, which combines the advantages of steam fixation to maintain the “three green” color characteristics, namely, green dry tea leaves, green tea infusion and green infused leaves, while using hot air to promote the conversion of volatile substance precursors. The hot air produces an intense and lasting green tea aroma, combined with the stable green color of the leaves, produces a high-quality product, so the steam/hot air process has been adopted rapidly by the tea processing industry (Ye et al., 2019). Tea dried with light-wave contains high levels of free amino acids and has a sweet and mellow taste (Zhu et al., 2022), but the application of light-wave to the fixation of green tea has received little research attention. Light-wave heat has high radiation intensity and energy efficiency, which can fix tea leaves completely and evenly in a short time. However, light-wave fixation has not been widely adopted for industrial tea production, mainly because its influence on the sensory quality of green tea is still unclear, compared with traditional fixation methods, such as roller and steam.
The content of non-volatile components in tea is influenced by various factors such as the environment, tea plant variety, and processing method (Li et al., 2018, Ma et al., 2021, Shi et al., 2022). Therefore, by comparing the differences in sensory quality and taste compound composition between green teas, it is possible to clarify the impact of different fixation methods on their quality and explore the underlying mechanisms, by using different methods to fix the same fresh leaf variety (Echa 1).
Common taste-active compounds in green tea include catechins, caffeine, amino acids and flavonoids (Zhang, Cao, Granato, Xu & Ho, 2020). Taste-active peptides are also present in tea, exhibiting sweetness (Val-Gly-Val), umami (Val-Phe, Glu-Glu), kokumi (γ-Glu-Glu), bitterness (Pro-Ala, Ile-Ile, Leu-Leu-Leu), and sourness (Glu-Asp) (Xue et al., 2022, Zhao et al., 2022). Understanding how the composition of these various taste compounds changes during fixing by light-wave is vital information for development and industrial application of this novel process.
Widely targeted metabolomics analysis combines the advantages of non-targeted (comprehensive) metabolomics and targeted (analysis of specific compound classes) metabolomics, has a short analysis time, high throughput, high sensitivity and wide coverage, and can obtain a comprehensive qualitative and quantitative metabolic profile of a wide range of sample types (Chen et al., 2013). Widely targeted metabolomics is used extensively to study tea processing and the chemical mechanisms that determine tea quality (Wang et al., 2021b, Shi et al., 2022, Zhou et al., 2022). The non-volatile differential compounds in roasted, baked, steamed and sun-dried green teas are mainly flavonoids, amino acids, lipids and phenolic acids (Shi et al., 2022). The probiotic fungus, Eurotium cristatum makes a major contribution to the transformation of flavonoids and phenolic acids during the pile fermentation of dark tea (Xiao et al., 2022). Previous widely targeted metabolomics studies mainly focused on the differential components between samples, but the influence of these differential components on the taste of tea remains unclear.
Therefore, in this study, widely targeted metabolomics based on ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) was used to analyze the non-volatile metabolites of fresh tea leaves (Echa 1) and three straight-shaped green teas, produced by different fixation methods (roller, steam/hot air and light-wave). This approach revealed the impact of the different fixation methods on the sensory quality and chemical composition of green tea. The findings will help to elucidate the formation mechanism of green tea flavor quality, inform the development of key processing technology regulations to improve the quality and stability of green tea, and promote technology development in the tea industry.
2. Materials and methods
2.1. Chemicals and reagents
The deionized water used for experiment was prepared by Milli-Q Water Purification System (Millipore, Billerica, MA, USA). The methanol, formic acid , acetic acid and acetonitrile used for MS analysis were LC/MS-grade and purchased from Thermo Fisher (Thermo Scientific, Waltham, MA, USA). The standard solution, namely, (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), (−)-epigallocatechin gallate (EGCG), (+)-catechin (C), (−)-catechin gallate (CG), (−)-gallocatechin (GC), (−)-gallocatechin gallate (GCG), l-aspartic acid (Asp), l-glutamic acid (Glu), l-glutamine (Gln), l-theanine (Thea), l-serine (Ser), l-glycine (Gly), l-threonine (Thr), l-alanine (Ala), l-proline (Pro), l-cystine (Cys), l-arginine (Arg), l-tyrosine (Tyr), l-valine (Val), l-lysine (Lys), l-isoleucine (Ile), l-leucine (Leu), l-phenylalanine (Phe), l-ornithine (Orn), gallic acid and caffeine, all with a purity of ≥ 98%, were purchased from the Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
2.2. Tea processing and sample collection
Clonal tea leaves of the Echa 1 variety (single bud or one bud with one leaf initially displayed) were harvested from the tea garden of Gongyide Tea Industry Co., Ltd., Jiaoyuan Town, Xuanen County, Enshi Prefecture, Hubei Province, China. Fresh leaves (FL) were spread on a bamboo sieve for 8–10 h (with a moisture content of approximately 70% (w.b.)), and fixed using different methods: (1) roller fixation: the leaves were heated at 280–300 °C for 2 min in a 6CST-60 roller fixation machine (Sichuan Dengyao Machinery Equipment Co., Ltd, Leshan, China); (2) steam/hot air fixation: the leaves were fixed in steam/hot air at 580 °C for 10 s, then heated in hot air at 135 °C for 3 min in a 6CZQC-100 steam/hot air coupling fixation machine (Chuangyu Machinery Co., Ltd, Yaan, China); (3) light-wave fixation: the leaves were heated in a GB-80 light-wave fixation machine at 85 °C for 2 min, and the parameters of phase A, phase B and phase C were set at 150 A, 160 A and 155 A, respectively. The fixed leaves were cooled for 1 h under room temperature, then rolled using a 6CR-35 roller (Zhejiang Shangyang Machinery Co. Ltd., Quzhou, China) for 50 min. The rolling process was as follows: without pressure for 15 min, with light pressure for 10 min, with high pressure for 10 min, with light pressure for 10 min, and without pressure again for 5 min. Next, the leaves were shaped in a tea striping machine (Sichuan Dengyao Machinery Equipment Co., Ltd, Leshan, China), with a temperature of 80–100 °C for 8 min. Afterward, the leaves were parched at 100 °C for 5 min, followed by drying at 80 °C for 60 min in a 6CHZ-3 hot air-drying machine (Anxi County Shengong Agricultural and Forestry Machinery Co., Ltd, Quanzhou, China). Finally, three straight-shaped green teas (GTs) under different fixation methods with moisture content less than 6% were obtained, namely, roller-dried tea leaves (RL), light-wave-dried tea leaves (LWL) and steam/hot air-dried tea leaves (SHL). In addition, FL were freeze-dried using a lyophilizer (Scientz-100F). The experimental samples (FL, RL, LWL, SHL) were collected and stored in the refrigerator at −80 °C until analysis.
2.3. Targeted absolute quantification of catechins and free amino acids by high-performance liquid chromatography (HPLC)
The detection method of catechins was as follows: the samples were ground to powder using a ball mill (MM400, Retsch company, Haan, Germany) with zirconia beads for 1.5 min at 30 Hz. The powder (0.2 g) was weighed into 10.0 mL tubes and mixed with 5 mL of 70% methanol, vortexed for 30 s and extracted in 70 °C water-bath for 10 min, centrifuged for 10 min (1307 × g, 4 °C). Transfer the supernatant into a 10 mL volumetric flask and repeat the extraction procedure. Two parts of supernatants were merged and adjusted to 10 mL, then filtered through a 0.22 μm microporous membrane for HPLC analysis using external standard method. The measurement of catechins was measured by HPLC system (Waters 2695, Milford, America) coupled with an Agilent ZORBAX SB-Aq C18 column (4.6 mm × 250 mm, 5 μm). The mobile phase comprised phase A (2% acetic acid in water) and phase B (100% acetonitrile). The linear gradient elution program was set as follows: 0–16 min, 6.5%–85% B; 16–25 min, 85%–75% B; 25–30 min, 75%–6.5% B; 30–40 min, 6.5% B. The column oven was 40 °C, the sample injection volume was 10 μL, and the flow rate was 1.0 mL/min (Feng et al., 2020). Each tea sample was performed in triplicates.
The detection method of free amino acids was as follows: the powder (0.1 g) was weighed into 15.0 mL tubes and added 10 mL water in 100 °C water-bath for 30 min, and then centrifuged for 10 min (1307 × g, 4 °C). Transfer the supernatant into a volumetric flask and adjust to 10 mL, filter through a 0.22 μm microporous membrane for HPLC analysis using external standard method. The measurement of amino acids was performed on a Waters 2695 HPLC system coupled with a Waters AccQ.Tag C18 column (3.9 mm × 150 mm, 4 μm) according to the methods in the literature (Feng et al., 2020). Each tea sample was performed in triplicates.
The content of proteinaceous amino acids (PAAs) is the sum of Asp, Glu, Gln, Ser, Gly, Thr, Ala, Pro, Cys, Arg, Tyr, Val, Lys, Ile, Leu and Phe. The content of non-proteinaceous amino acids (NPAAs) is the sum of Thea and Orn. The content of total amino acids (TAAs) is the sum of PAAs and NPAAs. The content of total catechins is the sum of EC, ECG, EGC, EGCG, C, CG, GC and GCG.
2.4. Widely targeted metabolomics analysis by UHPLC-MS/MS
The powder (100 mg) was weighted into 2.0-mL tubes and added 1.2 mL of 70% methanol. The homogenate was vortexed for 30 s with an interval of 30 min, vortexed for 6 times totally, and then kept in a refrigerator at 4 °C overnight for extraction. Following centrifugation at 12,000 × g for 10 min at 4 °C, the supernatant was filtrated through a 0.22 μm microporous membrane and transferred into a glass vial for UHPLC–MS/MS analysis. Each tea sample was performed in triplicates.
The metabolites measurement were measured by UHPLC system coupled to a tandem mass spectrometry(MS/MS, Applied Biosystems 4500 QTRAP)equipped with an Agilent SB-C18 column (2.1 mm × 100 mm, 1.8 μm). The mobile phase consisted of phase A (0.1% formic acid in water) and phase B (0.1% formic acid in acetonitrile). The linear gradient elution program was performed as follows: 0.00 min, 5% B; 0.00–9.00 min, 5%−95% B, and held for 1.00 min; 10.00–11.10 min, 95%−5% B; 11.10–14.00 min, 5% B. The flow rate was 0.35 mL/min. The column oven was kept at 40 °C, and the sample injection volume was set at 4 μL.
Linear ion trap and triple quadrupole (QQQ) scans were acquired on a triple quadrupole-linear ion trap mass spectrometer (QTRAP, API 4500 QTRAP UPLC–MS/MS system) equipped with an electrospray ionization (ESI) Turbo Ion-Spray interface. The positive and negative ion modes were controlled by Analyst 1.6.3 software (AB Sciex, Framingham, MA). The ESI source operation parameters were as follows: the ion source was turbine spray, and the source temperature was 550 °C; the ion spray voltage was set at 5.5 kV in positive ionization mode and −4.5 kV in negative ionization mode; the ion source gas I, gas II, and curtain gas were set at 50, 60, and 30 psi, respectively; and the collision gas was high. Multiple reaction monitoring (MRM) mode was used for QQQ scanning, and the pressure of collision gas (nitrogen) was 5 psi. By further optimizing the clustering potential (DP) and collision energy (CE), the DP and CE for a single MRM transformation were determined. A specific set of MRM transitions was monitored for each period according to the metabolites eluted within this period. Qualitative metabolite analysis was performed by matching the retention times, fragmentation patterns, and accurate m/z values with standards in the self-constructed metabolite database (MetWare, Wuhan, China). Quantitative analysis was carried out according to the signal intensity of metabolites obtained from characteristic ions. MultiaQuant software was used to integrate and calibrate the chromatographic peaks. The area of each chromatographic peak represented the relative content of the corresponding substance.
2.5. Sensory evaluation
The sensory evaluation of three straight-shaped green teas was performed by five expert panelists (2 females and 3 males, aged 25–40 years) in accordance with national standards ‘methodology for sensory evaluation of tea’ (GB/T 23376, 2018) and ‘tea vocabulary for sensory evaluation’ (GB/T 14487, 2017). 100 g tea samples were placed in white evaluation trays, and their appearance was evaluated and scored. 3.0 g of tea samples were infused with 150 mL 100 °C water for 4 min in a teapot, then tea infusion was poured into a tea bowl. Evaluated the color, aroma, taste, and infused leaves of tea in turn, and scored based on a percentage system. The total score of tea samples is calculated according to the following formula: Total scores = appearance score × 25% + infusion color score × 10% + aroma score × 25% + taste score × 30% + infused leaves score × 10%.
Quantitative descriptive analysis (QDA) was conducted by five experts, and the scores of five taste attributes (umami, sweetness, kokumi, bitterness, and astringency) were assessed on a ten-point scale, in which 0–2 represented “extremely weak”, 2–4 represented “weak”, 4–6 represented “neutral”, 6–8 represented “strong”, and 8–10 represented “extremely strong” (Zeng et al., 2023).
2.6. Statistical analysis
Student's t-test (for two groups) and analysis of variance (ANOVA) followed by Duncan's multiple-range (for ≥ three groups) test were performed using SPSS software (version 26.0, SPSS Inc., Chicago, USA). Principal component analysis (PCA) and orthogonal partial least squares-discrimination analysis (OPLS-DA) were conducted using Simca 14.1 software (Umetrics, Umea, Sweden). HCA analysis was performed using an integrative toolkit-TBtools. The bar charts, flavor wheel, radar map, and volcano maps were drawn using the Origin 2022 (OriginLab, Northampton, MA).
3. Results and discussion
3.1. Effects of fixation methods on sensory quality of GTs
LWL had the best sensory quality (total score = 92.35, Fig. 1A), with jade green leaves, light green infusion, fresh and mellow taste, and bright infused leaves (Fig. 1B). LWL was superior to RL and SHL in terms of leaf appearance, infusion color, taste and infused leaf, but its aroma score was the lowest at 89.6, with a clean and slightly grassy odour. The most prominent quality characteristic of RL was its excellent aroma (aroma score = 93.8), with a tender chestnut-like aroma, but a slightly bitter and astringent taste. The high temperature and long duration of roller fixation facilitate the rapid conversion or volatilization of low-boiling volatile compounds with grassy odour, while promoting the formation of higher-boiling volatile compounds with chestnut-like aromas (Wang et al., 2020). There was no difference in total sensory score between SHL and RL, and the evaluation results were similar. Clearly, the different fixation methods had significantly different effects on the quality of green tea, which is consistent with a previous report (Wang et al., 2020).
Fig. 1.
Sensory and quantitative descriptive analysis of straight-shaped green teas (GTs) under different fixation methods. (A) The total score of straight-shaped green teas. (B) The vocabulary and scores of appearance, soup colour, aroma, taste and infused leaves of straight-shaped green teas. (C) Quantitative descriptive analysis of taste attributes of straight-shaped green teas. Different lowercase letters in bar chart indicate significant differences between samples (p < 0.05, Duncan's test). RL, roller-dried tea leaves; LWL, light-wave-dried tea leaves; SHL, steam/hot air-dried tea leaves. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
QDA was used to quantify the taste attributes of GTs, to more objectively characterize their intensity in each taste dimension. The intensity of umami, sweetness, and kokumi for the GTs were all in the order LWL > SHL > RL, whereas bitterness and astringency showed the opposite trend. LWL had high taste intensity scores for umami, sweetness and kokumi (7.07, 5.00 and 5.23, respectively), as well as being slightly bitter and astringent (Fig. 1C). Overall, therefore, it presented a sweet, mellow and strong umami taste, which is popular with consumers. RL had the most intense bitterness and astringency, with scores of 3.47 and 4.47, respectively, mainly related to its high content of bitter or astringent flavonoid glycosides, EC and GC. The taste properties of tea are often inter-related, for example, tea with a strong umami taste usually also has strong sweetness and kokumi, and strongly bitter tea is usually also strongly astringent, which may be related to synergistic interactions between taste attributes. Research has shown that there is a synergistic enhancement between umami, sweetness and kokumi (Kuroda et al., 2012, Yin et al., 2014, Yang et al., 2021), and bitter and astringent substances often enhance each other’s taste intensity (Yin et al., 2014).
3.2. Effects of fixation methods on changes in amino acids and catechins content of GTs
Amino acids are major taste components of green tea, contributing umami, sweet and mellow tastes (Wang et al., 2021b, Zhang et al., 2020). There were 18 different amino acids present, detected by absolute quantitative methods, of which four had umami taste, six sweet, seven bitter and one was tasteless (Table 1). LWL had the highest content of umami amino acids (25.77 mg/g), followed by SHL and RL. Thea was the most abundant umami amino acid, followed by Glu and Asp. The GTs contained 3.09–3.45 mg/g of sweet amino acids, mainly Ser, Thr and Pro. Bitter amino acids were the most numerous, but their total content was low (2.88–2.93 mg/g), with Arg the most abundant. The TAAs of SHL was the highest at 31.14 mg/g, and that of RL was the lowest at 24.40 mg/g, but all GTs were significantly higher in TAAs than FL. The ratio of PAAs/NPAAs in FL was 0.81, and that in GTs was > 1. The significant increase in TAAs of GTs was mainly accounted for by increased PAAs (such as Asp, Glu and Ser). The main reason is the thermal degradation of protein during fixation, which releases a large amount of proteinaceous amino acids (Yu & Yang, 2020).
Table 1.
Content of amino acids and total catechins in tea samples under different fixation methods.
| Name | Taste | Contents (mg/g) |
|||
|---|---|---|---|---|---|
| FL | RL | SHL | LWL | ||
| Asp | Umami | 1.39 ± 0.13c | 2.76 ± 0.18b | 3.66 ± 0.17a | 3.37 ± 0.09a |
| Glu | 2.16 ± 0.25d | 3.58 ± 0.22c | 4.21 ± 0.29b | 6.01 ± 0.13a | |
| Gln | 0.99 ± 0.01c | 1.32 ± 0.12b | 1.82 ± 0.13a | 1.35 ± 0.01b | |
| Thea | 10.41 ± 0.35d | 11.98 ± 0.52c | 13.87 ± 0.53b | 15.02 ± 0.32a | |
| Total | 14.96 ± 0.74d | 19.66 ± 1.02c | 23.58 ± 1.10b | 25.77 ± 0.45a | |
| Ser | Sweetness | 0.50 ± 0.05c | 1.36 ± 0.09a | 1.38 ± 0.09a | 1.12 ± 0.01b |
| Gly | 0.74 ± 0.50a | 0.04 ± 0.00b | 0.06 ± 0.00b | 0.05 ± 0.00b | |
| Thr | 0.38 ± 0.03c | 0.56 ± 0.03b | 0.61 ± 0.01b | 1.03 ± 0.06a | |
| Ala | 0.19 ± 0.01c | 0.34 ± 0.02a | 0.27 ± 0.01b | 0.33 ± 0.00a | |
| Pro | 0.74 ± 0.08b | 0.69 ± 0.09b | 1.01 ± 0.04a | 0.82 ± 0.05b | |
| Cys | 0.01 ± 0.00b | 0.08 ± 0.00a | 0.08 ± 0.00a | 0.08 ± 0.00a | |
| Total | 2.6 ± 0.68b | 3.09 ± 0.15ab | 3.43 ± 0.10a | 3.45 ± 0.11a | |
| Arg | Bitterness | 1.19 ± 0.09bc | 1.32 ± 0.12b | 1.52 ± 0.02a | 1.04 ± 0.03c |
| Tyr | 0.05 ± 0.00d | 0.15 ± 0.01b | 0.11 ± 0.00c | 0.23 ± 0.02a | |
| Val | 0.06 ± 0.00c | 0.27 ± 0.01b | 0.26 ± 0.00b | 0.33 ± 0.01a | |
| Lys | 0.07 ± 0.00c | 0.31 ± 0.01a | 0.29 ± 0.01a | 0.23 ± 0.00b | |
| Ile | 0.03 ± 0.00c | 0.16 ± 0.01b | 0.14 ± 0.00b | 0.2 ± 0.00a | |
| Leu | 0.1 ± 0.01b | 0.38 ± 0.02a | 0.39 ± 0.01a | 0.39 ± 0.01a | |
| Phe | 0.08 ± 0.00c | 0.31 ± 0.00b | 0.33 ± 0.01b | 0.43 ± 0.04a | |
| Total | 1.61 ± 0.09b | 2.93 ± 0.11a | 3.07 ± 0.08a | 2.88 ± 0.11a | |
| Orn | Tasteless | 0.03 ± 0.00c | 0.07 ± 0.00a | 0.06 ± 0.00ab | 0.05 ± 0.00b |
| PAAs | 8.44 ± 0.54c | 12.34 ± 0.84b | 14.98 ± 0.85a | 16.05 ± 0.31a | |
| NPAAs | 10.44 ± 0.36d | 12.05 ± 0.51c | 13.94 ± 0.53b | 15.08 ± 0.32a | |
| TAAs | 18.88 ± 0.89c | 24.4 ± 1.31b | 28.93 ± 1.35a | 31.14 ± 0.60a | |
| Total catechins | 109.18 ± 7.18b | 126.64 ± 4.43a | 116.41 ± 1.67ab | 129.36 ± 8.27a | |
Note: The data were expressed as mean value ± standard deviation (mean ± SD). Different lowercase letters in a row indicate significant differences between samples (p < 0.05, Duncan's test). PAAs, proteinaceous amino acids; NPAAs, non-proteinaceous amino acids; TAAs, total amino acids; FL, fresh leaves; RL, roller-dried tea leaves; LWL, light-wave-dried tea leaves; SHL, steam/hot air-dried tea leaves.
Generally, an excessive catechins content results in tea with a bitter and astringent taste, but too little catechins results in a weak, bland taste, so catechins are considered the most characteristic secondary metabolites in green tea. The total catechins content in GTs was 116.41–129.36 mg/g (Table 1), of which EGCG was the most abundant, followed by EGC and ECG (Table S1). The contents of EGCG, CG and GC in RL were higher than those in LWL, but the contents of EC, GCG and ECG were higher in LWL. Combining the catechins content and sensory evaluation results, the bitterness and astringency of RL were significantly stronger than those of LWL, indicating that EGCG, CG and GC were the main contributors to the bitterness and astringency of tea infusion.
3.3. Full mass spectrometric analysis of non-volatile metabolites of GTs
To determine the impact of the different fixation methods on the metabolic profile of green tea, a widely targeted metabolomics analysis based on UHPLC-MS/MS was carried out to identify comprehensively and systematically the metabolites of FL, RL, LWL and SHL. Overlay analysis of the total ion chromatograms of the quality control samples (Fig. S1) and the multi-peak detection of tea samples (Fig. S2), indicated that the analysis had good repeatability and reliability. A total of 1001 non-volatile metabolites were identified (Table S2), namely, 230 flavonoids, 186 phenolic acids, 134 lipids, 86 amino acids and their derivatives, 76 organic acids, 70 saccharides and alcohols, 57 nucleotides and their derivatives, 48 alkaloids, 30 lignans and coumarins, 30 tannins, 20 terpenoids, 16 vitamins and 18 other metabolites. Flavonoids, phenolic acids, lipids and amino acids and their derivatives, accounted for 22.98%, 18.58%, 13.39% and 8.59% of the 1001 metabolites, respectively, i.e., these were the major classes of non-volatile metabolites in the tea samples, which is consistent with a previous report (Shi et al., 2022).
To visualize the overall differences in the non-volatile metabolic profiles of the four tea samples, an unsupervised PCA analysis was performed (Fig. S3). The first principal component explained 34.02% of the compositional variation, and RL, SHL and LWL were clearly separated from FL, indicating that GTs had major compositional differences from FL. The second principal component (PC2) explained 19.66% of the variation and there was a distinct separation on PC2 of the different fixation methods. LWL and SHL were close together, but they were well separated from RL. This separation was also reflected in the heatmap analysis of the metabolites. The content of Cluster I metabolites was lower in FL and higher in SHL, whereas those of Clusters II and III were higher in FL and lower in GTs (Fig. S4).
3.3.1. Screening for differential non-volatile metabolites in GTs
OPLS-DA is a supervised multivariate statistical analysis method, which needs preset classification information and quantifies the contribution of different compounds to classification differences through deep data mining. OPLS-DA provides information on the effects of the different processing methods on non-volatile differential metabolites. Therefore, to determine the influence of the three fixation methods on the non-volatile metabolic profiles of GTs, the key differential metabolites between each sample were explored using a supervised OPLS-DA model. The OPLS-DA models of RL vs. LWL, RL vs. SHL and SHL vs. LWL all exhibited good explained variance and high predictive capability (Fig. 2A). To assess the performance of these models, 200 permutation tests were used for cross validation, and the p-values of R2Y and Q2 were both < 0.005, indicating that there was no over-fitting in these models (Fig. S5).
Fig. 2.
Analysis of non-volatile differential metabolites in straight-shaped green teas under different fixation methods. (A) Score plot for the OPLS-DA model. (B) Volcano plot. (C) Venn diagram. Numbers in venn diagram represent the differential metabolites in groups; RL, roller-dried tea leaves; LWL, light-wave-dried tea leaves; SHL, steam/hot air-dried tea leaves. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The differential metabolites among GTs were determined by the criteria of variable importance in projection (VIP) > 1, p < 0.05, and |log2FC (fold change)| > 1 (Table S3). There were 67 differential metabolites between RL and LWL (Fig. 2-B1, 15 upregulated and 52 downregulated), 57 differential metabolites between RL and SHL (Fig. 2-B2, 10 upregulated and 47 downregulated) and 28 differential metabolites between SHL and LWL (Fig. 2-B3, 9 upregulate and 19 downregulated). The Venn diagram (Fig. 2C) showed that there were both common and unique differential metabolites in these comparison groups. LysoPE 14:0 (2n isomer), uracil and adenosine were shared in RL vs. LWL, RL vs. SHL and SHL vs. LWL, which were the common differential metabolites in GTs. LysoPE 14:0 (2n isomer) and adenosine were abundant in RL, whereas uracil was abundant in LWL. There were 15, 22 and 8 unique differential metabolites between RL vs. LWL, RL vs. SHL and SHL vs. LWL, respectively. These differential metabolites characterize the quality differences resulting from the three fixation methods.
3.3.2. Effects of different fixation methods on changes in significant differential metabolites of GTs
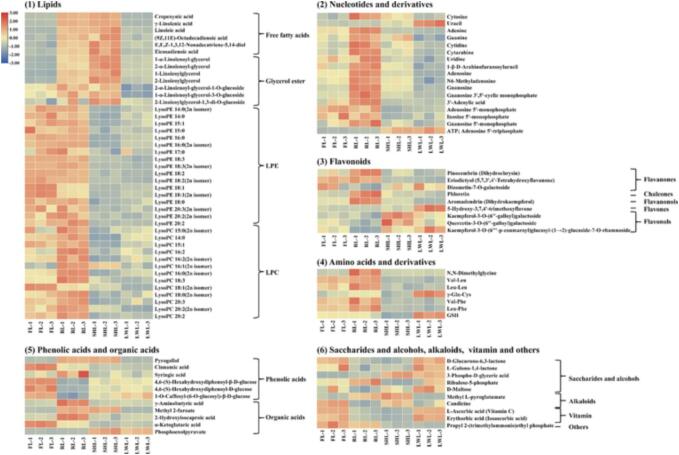
Fixation is a key process in the processing of green tea, which causes major changes in the content and composition of thermally unstable substances. A total of 97 differential metabolites in GTs were screened (Fig. 2C), namely, 43 lipids, 17 nucleotides and derivatives, nine flavonoids, seven amino acids and derivatives, six phenolic acids, five organic acids, five saccharides and alcohols, two alkaloids, two vitamins and one other. Lipids and nucleotides, and their derivatives, were the main differential non-volatile metabolites. Distribution heatmaps (Fig. 3) of differential non-volatile metabolites between FL and GTs were plotted to visualize the influence of fixation methods on these differential metabolites.
Fig. 3.
Heatmap of 97 differential metabolites in straight-shaped green teas under different fixation methods. FL, fresh leaves; RL, roller-dried tea leaves; LWL, light-wave-dried tea leaves; SHL, steam/hot air-dried tea leaves. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3.2.1. Lipids
Lipids help to maintain the structure of tea leaves and are the precursors of many tea aroma compounds. The lipid metabolites detected consisted of free fatty acids, glycerol esters, lysophosphatidylethanolamines (LPE) and lysophosphatidylcholines (LPC). Phospholipids, including phosphatidylcholines and phosphatidylethanolamines, are the main components of cell membrane structure and have important physiological functions in cells. Phospholipids undergo degradation reactions catalyzed by enzymes or heat (Li et al., 2017, Li et al., 2021), forming degradation products such as lysophospholipids (LPC, LPE) and free fatty acids (Siriwardane, Wang, Jiang & Mudalige, 2020), for example, the content of LPC increased during the drying of black tea (Li et al., 2017, Zhou et al., 2022). In this study, the content of most LPC species increased significantly after heat treatment by roller fixation, such as LysoPC 16:1 (2n isomer) and LysoPC 16:2, which were 3.15- and 1.81-fold higher than in FL. Compared with FL, most free fatty acids were significantly more abundant in GTs, with the highest content in SHL, followed by RL and LWL. For example, the content of linoleic acid in SHL, RL and LWL was 2.41-, 2.12- and 1.14-fold higher than that of FL, respectively. Clearly, the increased LPC and free fatty acids in GTs resulted from degradation of phospholipids by the high-temperature during fixation.
Unlike LPC, LPE did not consistently increase after fixation and had a higher content in RL but a lower content in LWL. The degradation of LPE liberates free unsaturated fatty acids (linoleic acid, linolenic acid). During the processing of green tea, linoleic acid, an important aroma precursor in tea, was degraded to form 1-octen-3-ol, 1-octen-3-one and trans-4,5-epoxy-(E)-2-decenal that contributed to the aroma characteristics of green tea (Ho et al., 2015, Baba et al., 2017).
3.3.2.2. Nucleotides and derivatives
Nucleotides and their derivatives are important secondary metabolites in tea plants, with an umami taste that can improve the taste of tea infusion. Seventeen nucleotides were significantly different among GTs. The contents of uracil and adenosine 5′-triphosphate were highest in LWT, and those of the other nucleotides were highest in RL. The umami taste intensity is in the order guanosine 5′-monophosphate (GMP) > inosine 5′-monophosphate (IMP) > adenosine 5′-monophosphate (AMP) (Manninen, Rotola-Pukkila, Aisala, Hopia & Laaksonen, 2018), and the contents of GMP, IMP and AMP in RL were 3.39-, 1.79- and 2.03-fold those in LWL, respectively. Therefore, it can be inferred that the contribution of GMP to the umami taste of RL tea infusion was stronger than those of IMP and AMP. Compared with FL, the content of GMP in RL significantly increased (1.92-fold), mainly by transformation of xanthosine 5′-monophosphate into GMP (Wang et al., 2022). Notably, AMP and IMP did not increase like GMP, although they have similar chemical structures, mainly because they are transformed into hypoxanthine, which exhibits a bitter taste, during fixation at high temperature (Zhang et al., 2018). However, the QDA results showed that the umami taste of LWL was significantly stronger than that of RL, indicating that nucleosides were not major contributors to the umami taste of LWL.
3.3.2.3. Flavonoids
Nine flavonoids presented significant differences in GTs, namely, three flavanones, three flavonols, one chalcone, one flavanonol, and one flavone. Since the structures of flavonoids contain hydroxyl and ketone groups, double bonds, and many are glycosylated, their thermal stability is low and they are extensively transformed by heat treatment (Chaaban et al., 2017). Compared with FL, the contents of most flavonoids in GTs markedly increased, consistent with previous reports (Chaaban et al., 2017, Shi et al., 2022). Notably, the content of 5-hydroxy-3,7,4′-trimethoxyflavone in LWL was significantly higher (7.45-fold) than that in the other GTs. The contents of flavonoid aglycones (pinocembrin, phloretin, eriodictyol and aromadendrin) were high in RL, whereas flavonoid glycosides such as kaempferol-3-O-(6′'-galloyl)galactoside, quercetin-3-O-(6′'-galloyl)galactoside and kaempferol-3-O-(6′''-p-coumaroyl)glucosyl-(1 → 2)-glucoside-7-O-rhamnoside were low. In LWL, flavonoid glycosides such as kaempferol-3-O - (6′'- galloyl) galactoside and kaempferol-3-O - (6′' - p-coumaroyl) glucosyl - (1 → 2) - glucoside-7-O-rhamnoside have more reservations. Studies have shown that the sensitivity of glycosylated flavonoids to heat treatment is higher than that of its aglycon flavonoids, and diglycosylated flavonoids are more sensitive to heat treatment and may be the precursor of monoglycosylated and aglycon flavonoids (Liu, Wang, Corke & Zhu, 2022). The fixation temperature of RL was 280–300 °C, much higher than that of LWL (85 °C), so thermal degradation of flavonoid glycosides during RL significantly increased the content of aglycon flavonoids. The different fixation temperatures resulted in significant differences in flavonoid structural modification.
3.3.2.4. Amino acids and derivatives
Amino acids and their derivatives have taste characteristics such as umami, sweetness, kokumi and bitterness, which contribute strongly to the taste of tea. Six peptides were significantly different in GTs. Notably, peptides composed of l-amino acid residues, such as Val-Leu, Leu-Leu, Val-Phe and Leu-Phe, were abundant in RL but low in LWL, and their ratios of RL/LWL were all over 2.0. Peptides (e,g., Val-Leu, Leu-Leu and Leu-Phe) with hydrophobic amino acid residues such as Val, Leu, and Phe, tend to be bitter (Asao, Iwamura, Akamatsu & Fujita, 1987). The sensory evaluation results indicate that the slight bitterness of RL may be related to its high content of bitter peptides. Amino acids and reducing sugars undergo a Maillard reaction at high temperatures, and free radicals (Maillard intermediates) attack proteins, resulting in free-radical-mediated oxidative fragmentation of the main chain (Ebner, Baum & Pischetsrieder, 2016). The fixation temperature of RL was much higher than that of LWL, so the Maillard reaction would have been more extensive, with a higher degree of protein oxidation fragmentation. Val-Phe, which has an umami taste, has been identified in black tea and its content increased gradually during the processing of black tea and reached a maximum during the drying stage (Xue et al., 2022). It appears that the enrichment of peptides composed of l-amino acid residues is induced by the thermal processing of tea, such as fixation and drying, and is mediated by the Maillard reaction.
In addition, non-proteinaceous peptides, such as γ-glutamyl peptides (γ-Glu-Cys and glutathione (GSH)) have been identified in tea. Kokumi peptides, when added to the five basic taste solutions, can enhance the intensity of their tastes (especially sweet and umami) even at concentrations below their taste thresholds (Kuroda et al., 2012). The content of γ-Glu-Cys and GSH in LWL was the highest, 1.73- and 1.65-fold that of RL, respectively, and the fresh, mellow taste of LWL may be related to its high content of these kokumi peptides. γ-Glutamyl peptides are formed by the transpeptidation activity of γ-glutamyl transferase (GGT), which hydrolyses γ-glutamyl linkages in γ-glutamyl peptides and transfers γ-glutamyl moieties to other amino acids or peptides to form new γ-glutamyl peptides. GGT is a heat-activated enzyme and the moderate fixation temperature of LWL (85 °C) would be expected to activate GGT, thereby inducing transpeptidation reactions and increasing the γ-glutamyl peptides content.
3.3.2.5. Phenolic acids and organic acids
Pyrogallol, γ-aminobutyric acid, and syringic acid were significantly more abundant after fixation, with the highest concentrations in RL, which were 6.0-, 2.5- and 1.8-fold higher than those in FL, respectively. Conversely, cinnamic acid was significantly less abundant, and its content in RL, SHL and LWL was 40%, 20% and 20% of that in FL, respectively. Moreover, the content of 4,6-(S)-hexahydroxydiphenoyl-β-d-glucose, 4,6-(S)-hexahydroxydiphenoyl-d-glucose and 1-O-caffeoyl-(6-O-glucosyl)-β-d-glucose in RL decreased markedly to 28%, 22% and 41% of those in FL, respectively. Obviously, phenolic acid glucosides were decreased remarkably during the fixation process, which was consistent with a previous report (Shi et al., 2022). Heat treatment facilitates the release of d-glucose and the corresponding phenolic acids, such as caffeic acid and hexahydroxidiphenic acids, from phenolic acid glycosides. Evidently, fixation of green tea promotes the hydrolysis of phenolic acid glycosides to produce phenolic acid aglycones.
3.4. Effects of fixation methods on changes in main taste metabolites of GTs
3.4.1. The main taste difference metabolites of GTs
The taste of GTs is a comprehensive reflection of its taste attributes such as umami, sweetness, kokumi, bitterness and astringency, of which umami, sweetness and kokumi are favored by consumers, whereas bitterness and astringency are less appealing. The taste of tea is not only closely related to its chemical composition, but also influenced by synergistic or antagonistic interactions between taste-active compounds. Therefore, identifying the main taste-active compounds and their interactions is vital for understanding the factors influencing the taste quality of green tea.
Correlation analysis is a powerful tool for investigating the relationship between sensory attributes and metabolites. Pearson correlation coefficients were calculated to examine the associations between 41 different taste substances (14 identified by widely targeted metabolomics and 24 by absolute quantification) and five sensory attributes (umami, sweetness, kokumi, bitterness and astringency). Fourteen taste compounds correlated closely with sensory attributes (r > 0.6, p < 0.05), indicating that they are important factors underlying the taste properties of GTs. The kokumi, umami and sweetness taste attributes of GTs were positively correlated with GSH, γ-Glu-Cys, d-maltose, Thr, Glu, and Thea. Pinocembrin, phloretin, Val-Leu, Leu-Leu, Leu-Phe, GC, EGCG and CG positively correlated with the bitterness and astringency of GTs (Fig. 4A).
Fig. 4.
Correlation between sensory attributes of straight-shaped green teas and major taste-active components (A) and their changes (B) under different fixation methods. Different lowercase letters in bar chart indicate significant differences between samples (p < 0.05, Duncan's test). FL, fresh leaves; RL, roller-dried tea leaves; LWL, light-wave-dried tea leaves; SHL, steam/hot air-dried tea leaves. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
According to the clustering of the taste attributes, substances with bitter and astringent tastes were clustered into one group, and the others with kokumi, umami and sweet taste clustered into a separate group, indicating that there are significant interactions between taste-active compounds. There is usually a synergistic effect between umami and sweet substances. For example, Thea and sucrose interact to produce a taste enhancement (Yin et al., 2014). In this study, sweet substances such as d-maltose and Thr not only showed a significant positive correlation with the sweetness of GTs (r = 0.93, 0.92, respectively), but also enhanced the umami and kokumi tastes. Similarly, Glu and Thea (umami taste) contributed significantly to the umami taste of GTs (r = 0.96, 0.91, respectively), and strongly correlated with the sweet and kokumi tastes. γ-Glutamyl peptides (GSH and γ-Glu-Cys; kokumi taste) had a significant positive correlation with the umami taste of GTs (r = 0.68, 0.73, respectively), and with their sweetness (r = 0.65, 0.68, respectively).
γ-Glutamyl peptides are perceived by the human tongue through the calcium-sensing receptor (CaSR) (Ohsu et al., 2010). CaSR is a close relative of the G protein-coupled receptors T1R1, T1R2 and T1R3 (sweet and umami receptors) (Ohsu et al., 2010), and this explains why γ-glutamyl peptides can enhance umami and sweetness, even below their kokumi taste-thresholds (Kuroda et al., 2012). γ-Glutamyl peptides enhance the umami taste of MSG, mainly through a synergy with MSG in activating the T1R3 receptor. γ-Glu-Cys exerts an umami-enhancing effect by binding to Ala-302 and Gln-389 on receptor proteins, whereas GSH binds to Ser-276 and Asp-216 (Yang et al., 2021). Therefore, the kokumi taste substances that contributed significantly to the taste of GTs would have enhanced the sweetness and umami tastes by binding to the corresponding receptor proteins.
3.4.2. Fixation facilitates the accumulation of amino acids, peptides, flavonoids and catechins
Fixation methods have different influences on the variety, content and proportion of tea chemical components (especially taste-active substances), because of differences in temperature, heating rate and heat transfer medium. This means that the same fresh leaves can become green teas with different sensory characteristics when fixed by different methods.
A high content of free amino acids is considered to be essential for high-quality green tea (Yu and Yang, 2020). In this study, amino acids with umami (Thea, Glu) and sweet (Thr) tastes enhanced the freshness, sweetness and kokumi of LWL. Thea can also improve the taste quality of green tea by reducing the astringency of polyphenols and the bitterness of caffeine (Ye et al., 2023). The contents of Thea, Glu and Thr in GTs increased significantly compared with FL, with the highest content in LWL, followed by SHL and RL (Fig. 4B). The significant increase in proteinaceous amino acids such as Glu and Thr mainly results from the extensive degradation of precursor proteins during green tea processing (Yu and Yang, 2020).
Peptides with umami, sweet, kokumi and bitter tastes also have important effects on tea taste (Chen et al., 2020, Zhang et al., 2020, Xue et al., 2022). γ-Glutamyl peptides are typical kokumi peptides, widely occurring in fermented foods, such as cheese and soy sauce, and are the main contributors to kokumi taste (Yang, Bai, Zeng & Cui, 2019), for example, γ-Glu-Glu contributes to the taste of black tea (Xue et al., 2022). In this study, γ-glutamyl peptides, including γ-Glu-Cys and GSH, also made major contributions to the kokumi taste of GTs. The contents of GSH and γ-Glu-Cys were highest in LWL, followed by RL and SHL. LWL contains high levels of γ-glutamyl peptides, because of activation of the GGT enzyme in fresh leaves, which catalyzes transpeptidation reactions. Compared with FL, the contents of Val-Leu, Leu-Leu and Leu-Phe in RL were significantly higher, whereas those in SHL and LWL were markedly lower. The content of bitter peptides was significantly higher in RL, which appears to result from oxidative fragmentation of proteins at high temperatures (Ebner et al., 2016).
Pinocembrin and phloretin are important flavonoids in GTs and are major contributors to the bitter taste, because they can activate the human bitter receptors, hTAS2R14 and hTAS2R39 (Li et al., 2023). Flavonoid glycosides are unstable at high temperatures and easily undergo heat-induced degradation reactions (Zhu et al., 2021), for example, phloretin is the aglycone of phlorizin, which is easily hydrolyzed to phloretin by heat. The contents of pinocembrin and phloretin in RL were much higher than those in FL, indicating that flavonoid glycosides undergo hydrolysis reactions during the processing of green tea.
Compared with FL, the contents of CG, GC and EGCG in RL increased by 4.55-fold, 16.0% and 8.2%, respectively (p < 0.05). Cis-catechins (such as ECG and EGC) undergo epimerization at the C-2 position during high-temperature fixation to form trans-catechins (CG, GC), because the heat of formation of the cis-catechin is higher than that of trans-catechins, making them susceptible to epimerization reactions; the higher the temperature, the faster the epimerization reaction (Donlao & Ogawa, 2019). The fixation temperature of RL is much higher than that of LWL, so the epimerization of cis-catechins to trans-catechins is more extensive and results in the high content of CG and GC in RL.
A proposed mechanism of the effect of different fixation methods on the quality of GTs is shown in Fig. 5. By heating fresh leaves from the outside to the inside with light-wave, protein degradation and γ-glutamyl peptide transpeptidation reactions were promoted, resulting in enrichment of free amino acids (Glu, Thr) and γ-glutamyl peptides (GSH, γ-Glu-Cys). Therefore, LWL had a fresh, sweet and mellow taste. The high temperature of roller fixation accelerates chemical reactions in tea leaves, such as protein oxidation, flavonoid glycoside degradation and epimerization of cis-catechins, which contributes to the enrichment of peptides (Val-Leu, Leu-Leu, Leu-Phe), flavonoids (pinocembrin, phloretin), and trans-catechins (GC, CG) with a bitter or astringent taste. Therefore, RL has a more umami, mellow, and slightly bitter and astringent taste.
Fig. 5.
A proposed mechanism of the effect of different fixation methods on the quality of straight-shaped green teas. FL, fresh leaves; RL, roller-dried tea leaves; LWL, light-wave-dried tea leaves; SHL, steam/hot air-dried tea leaves. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
The effects of roller, steam/hot air, and light-wave fixation on the quality and metabolic profile of straight-shaped green teas were investigated by sensory evaluation and chemical analysis. LWL had the best overall sensory quality, with a clear aroma and a fresh, mellow taste, whereas RL had a tender chestnut-like aroma and a slightly bitter and astringent taste, with the quality of SHL intermediate between them. A total of 1001 non-volatile metabolites were detected in GTs, and 93 differential metabolites were identified, based on the criteria of VIP > 1, p < 0.05, and |log2FC| > 1. The main taste-active compounds, i.e., amino acids (Thea, Glu and Thr), peptides (GSH, γ-Glu-Cys, Val-Leu, Leu-Leu and Leu-Phe), catechins (GC, EGCG and CG), flavonoids (pinocembrin, phloretin) and d-maltose directly contributed to the taste quality differences between GTs. Roller fixation promoted protein oxidation, degradation of flavonoid glycosides, epimerization of cis-catechin, and the accumulation of bitter and astringent substances, such as peptides, flavonoids and trans-catechins, resulting in the bitter and astringent taste of RL. Light-wave fixation promoted protein degradation and γ-glutamyl peptides transpeptidation, accumulating substances with umami, sweet and kokumi tastes, giving LWL a fresh, sweet and mellow taste. These findings help to elucidate the mechanism of the impact of different fixation methods on the quality of green tea, and provide a theoretical basis for improved quality control of green tea, as well as strong support for the application and promotion of light-wave fixation in industrial green tea production.
CRediT authorship contribution statement
Jinjin Xue: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. Panpan Liu: Data curation, Formal analysis, Methodology, Software, Investigation, Writing – original draft. Lin Feng: Software, Visualization. Lin Zheng: Visualization, Investigation. Anhui Gui: Methodology, Formal analysis. Xueping Wang: Methodology, Software. Shengpeng Wang: Formal analysis, Investigation. Fei Ye: Formal analysis, Investigation. Jing Teng: Formal analysis, Software. Shiwei Gao: Conceptualization, Supervision, Project administration, Writing – review & editing. Pengcheng Zheng: Conceptualization, Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFD1601103, 2022YFD1600804), China Agriculture Research System of MOF and MARA (CARS-19), Hubei Rural Revitalization Strategy and Technology Support Project (2022BBA095), and the Cultivation Plan for Young Backbone Talents of the Fruit and Tea Research Institute of the Hubei Academy of Agricultural Sciences (2023).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100943.
Contributor Information
Shiwei Gao, Email: gsw0609@126.com.
Pengcheng Zheng, Email: zpct@hbaas.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Asao M., Iwamura H., Akamatsu M., Fujita T. Quantitative structure-activity relationships of the bitter thresholds of amino acids, peptides, and their derivatives. Journal of Medicinal Chemistry. 1987;30(10):1873–1879. doi: 10.1021/jm00393a031. [DOI] [PubMed] [Google Scholar]
- Baba R., Amano Y., Wada Y., Kumazawa K. Characterization of the potent odorants contributing to the characteristic aroma of matcha by gas chromatography–olfactometry techniques. Journal of Agricultural and Food Chemistry. 2017;65(14):2984–2989. doi: 10.1021/acs.jafc.7b00421. [DOI] [PubMed] [Google Scholar]
- Chaaban H., Ioannou I., Chebil L., Slimane M., Gérardin C., Paris C.…Ghoul M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. Journal of Food Processing and Preservation. 2017;41(5):e13203. [Google Scholar]
- Chen Q.C., Shi J., Mu B., Chen Z., Dai W.D., Lin Z. Metabolomics combined with proteomics provides a novel interpretation of the changes in nonvolatile compounds during white tea processing. Food Chemistry. 2020;332 doi: 10.1016/j.foodchem.2020.127412. [DOI] [PubMed] [Google Scholar]
- Chen W., Gong L., Guo Z.L., Wang W.S., Zhang H.Y., Liu X.Q.…Luo J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: application in the study of rice metabolomics. Molecular Plant. 2013;6(6):1769–1780. doi: 10.1093/mp/sst080. [DOI] [PubMed] [Google Scholar]
- Donlao N., Ogawa Y. The influence of processing conditions on catechin, caffeine and chlorophyll contents of green tea (Camelia sinensis) leaves and infusions. Lwt-Food Science and Technology. 2019;116 doi: 10.1016/j.lwt.2019.108567. [DOI] [Google Scholar]
- Ebner J., Baum F., Pischetsrieder M. Identification of sixteen peptides reflecting heat and/or storage induced processes by profiling of commercial milk samples. Journal of Proteomics. 2016;147:66–75. doi: 10.1016/j.jprot.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Feng L., Liu P.P., Zheng P.C., Zhang L., Zhou J., Gong Z.M.…Wan X.C. Chemical profile changes during pile fermentation of Qingzhuan tea affect inhibition of α-amylase and lipase. Scientific Reports. 2020;10(1):3489. doi: 10.1038/s41598-020-60265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.T., Zheng X., Li S.M. Tea aroma formation. Food Science and Human Wellness. 2015;4(1):9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- Kuroda M., Kato Y., Yamazaki J., Kai Y., Mizukoshi T., Miyano H., Eto Y. Determination and quantification of γ-glutamyl-valyl-glycine in commercial fish sauces. Journal of Agricultural and Food Chemistry. 2012;60(29):7291–7296. doi: 10.1021/jf3012932. [DOI] [PubMed] [Google Scholar]
- Li J., Hua J.J., Yuan H.B., Deng Y.L., Zhou Q.H., Yang Y.Q.…Jiang Y.W. Investigation on green tea lipids and their metabolic variations during manufacturing by nontargeted lipidomics. Food Chemistry. 2021;339 doi: 10.1016/j.foodchem.2020.128114. [DOI] [PubMed] [Google Scholar]
- Li J., Hua J.J., Zhou Q.H., Dong C.W., Wang J.J., Deng Y.L.…Jiang Y.W. Comprehensive lipidome-wide profiling reveals dynamic changes of tea lipids during manufacturing process of black tea. Journal of Agricultural and Food Chemistry. 2017;65(46):10131–10140. doi: 10.1021/acs.jafc.7b03875. [DOI] [PubMed] [Google Scholar]
- Li L.J., Yan X., Chen F.Y., Zheng L.Y., Hu Y., He F.…Li Q.B. A comprehensive review of the metabolism of citrus flavonoids and their binding to bitter taste receptors. Comprehensive Reviews in Food Science and Food Safety. 2023;22(3):1763–1793. doi: 10.1111/1541-4337.13129. [DOI] [PubMed] [Google Scholar]
- Li P.L., Dai W.D., Lu M.L., Xie D.C., Tan J.F., Yang C.…Lin Z. Metabolomic analysis reveals the composition differences in 13 Chinese tea cultivars of different manufacturing suitabilities. Journal of the Science of Food and Agriculture. 2018;98(3):1153–1161. doi: 10.1002/jsfa.8566. [DOI] [PubMed] [Google Scholar]
- Liu F., Wang Y., Corke H., Zhu H.K. Dynamic changes in flavonoids content during congou black tea processing. LWT-Food Science and Technology. 2022;170 doi: 10.1016/j.lwt.2022.114073. [DOI] [Google Scholar]
- Ma W.J., Zhu Y., Shi J., Wang J.T., Wang M.Q., Shao C.Y.…Lv H.P. Insight into the volatile profiles of four types of dark teas obtained from the same dark raw tea material. Food Chemistry. 2021;346 doi: 10.1016/j.foodchem.2020.128906. [DOI] [PubMed] [Google Scholar]
- Manninen H., Rotola-Pukkila M., Aisala H., Hopia A., Laaksonen T. Free amino acids and 5′-nucleotides in Finnish forest mushrooms. Food Chemistry. 2018;247:23–28. doi: 10.1016/j.foodchem.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Ohsu T., Amino Y., Nagasaki H., Yamanaka T., Takeshita S., Hatanaka T.…Eto Y. Involvement of the calcium-sensing receptor in human taste perception. Journal of Biological Chemistry. 2010;285(2):1016–1022. doi: 10.1074/jbc.M109.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.L., Zhu Y., Ma W.J., Shi J., Peng Q.H., Lin Z., Lv H.P. Comprehensive investigation on non-volatile and volatile metabolites in four types of green teas obtained from the same tea cultivar of Longjing 43 (Camellia sinensis var. sinensis) using the widely targeted metabolomics. Food Chemistry. 2022;394 doi: 10.1016/j.foodchem.2022.133501. [DOI] [PubMed] [Google Scholar]
- Siriwardane D.A., Wang C.G., Jiang W.L., Mudalige T. Quantification of phospholipid degradation products in liposomal pharmaceutical formulations by ultra performance liquid chromatography-mass spectrometry (UPLC-MS) International Journal of Pharmaceutics. 2020;578 doi: 10.1016/j.ijpharm.2020.119077. [DOI] [PubMed] [Google Scholar]
- Song F.H., Zheng Y., Li R.Y., Li Z.F., Liu B.Y., Wu X. Intelligent control of green tea fixation with microwave processing. Journal of Food Engineering. 2023;349 doi: 10.1016/j.jfoodeng.2023.111481. [DOI] [Google Scholar]
- Wang D., Shi L.J., Fan X.W., Lou H.Q., Li W.T., Li Y.L.…Yi L.Z. Development and validation of an efficient HILIC-QQQ-MS/MS method for quantitative and comparative profiling of 45 hydrophilic compounds in four types of tea (Camellia sentences) Food Chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131201. [DOI] [PubMed] [Google Scholar]
- Wang H.J., Hua J.J., Jiang Y.W., Yang Y.Q., Wang J.J., Yuan H.B. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Research International. 2020;136 doi: 10.1016/j.foodres.2020.109479. [DOI] [PubMed] [Google Scholar]
- Wang H.J., Hua J.J., Yu Q.Y., Jiang Y.W., Wang J.J., Yang Y.Q., Yuan H.B. Based on IRAE-HS-SPME / GC-MS analysis of the effect of fixation methods on the formation of chestnut-like aroma in green tea (in Chinese) Food Science. 2021;42(14):209–217. doi: 10.7506/spkx1002-6630-20200604-052. [DOI] [Google Scholar]
- Wang H.J., Hua J.J., Yu Q.Y., Li J., Wang J.J., Deng Y.L.…Jiang Y.W. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chemistry. 2021;363 doi: 10.1016/j.foodchem.2021.130131. [DOI] [PubMed] [Google Scholar]
- Wang Y.J., Ren Z.Y., Li M.Y., Lu C.Y., Deng W.W., Zhang Z.Z., Ning J.M. From lab to factory: A calibration transfer strategy from HSI to online NIR optimized for quality control of green tea fixation. Journal of Food Engineering. 2023;339 doi: 10.1016/j.jfoodeng.2022.111284. [DOI] [Google Scholar]
- Xiao Y., He C., Chen Y.L., Ho C.T., Wu X., Huang Y.X.…Liu Z.H. UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2021.131999. [DOI] [PubMed] [Google Scholar]
- Xue J.J., Liu P.P., Guo G.Y., Wang W.W., Zhang J.Y., Wang W.…Jiang H.Y. Profiling of dynamic changes in non-volatile metabolites of shaken black tea during the manufacturing process using targeted and non-targeted metabolomics analysis. LWT-Food Science and Technology. 2022;156 doi: 10.1016/j.lwt.2021.113010. [DOI] [Google Scholar]
- Yang J., Bai W.D., Zeng X.F., Cui C. Gamma glutamyl peptides: The food source, enzymatic synthesis, kokumi-active and the potential functional properties–A review. Trends in Food Science and Technology. 2019;91:339–346. doi: 10.1016/j.tifs.2019.07.022. [DOI] [Google Scholar]
- Yang J., Huang Y.R., Cui C., Dong H., Zeng X.F., Bai W.D. Umami-enhancing effect of typical kokumi-active γ-glutamyl peptides evaluated via sensory analysis and molecular modeling approaches. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.128018. [DOI] [PubMed] [Google Scholar]
- Ye F., Gui A.H., Gong Z.M., Gao S.W., Wang X.P., Zheng P.C., Liu P.P. Effects of microwave fixation process on preserving green color and reducing bitterness in autumn green tea (in Chinese) Chinese Journal of Tropical Crops. 2019;40(2):396–402. doi: 10.3969/j.issn.1000-2561.2019.02.026. [DOI] [Google Scholar]
- Ye Y.Y., Yan W., Peng L.J., Zhou J.J., He J.L., Zhang N.…Cai J. Insights into the key quality components in se-enriched green tea and their relationship with selenium. Food Research International. 2023;112460 doi: 10.1016/j.foodres.2023.112460. [DOI] [PubMed] [Google Scholar]
- Yin J.F., Zhang Y.N., Du Q.Z., Chen J.X., Yuan H.B., Xu Y.Q. Effect of Ca2+ concentration on the tastes from the main chemicals in green tea infusions. Food Research International. 2014;62:941–946. doi: 10.1016/j.foodres.2014.05.016. [DOI] [Google Scholar]
- Yu Z.M., Yang Z.Y. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Critical Reviews in Food Science and Nutrition. 2020;60(5):844–858. doi: 10.1080/10408398.2018.1552245. [DOI] [PubMed] [Google Scholar]
- Zeng L., Fu Y.Q., Liu Y.Y., Huang J.S., Chen J.X., Yin J.F.…Xu Y.Q. Comparative analysis of different grades of Tieguanyin oolong tea based on metabolomics and sensory evaluation. LWT-Food Science and Technology. 2023;174 doi: 10.1016/j.lwt.2023.114423. [DOI] [Google Scholar]
- Zhang L., Cao Q.Q., Granato D., Xu Y.Q., Ho C.T. Association between chemistry and taste of tea: A review. Trends in Food Science and Technology. 2020;101:139–149. doi: 10.1016/j.tifs.2020.05.015. [DOI] [Google Scholar]
- Zhang L., Ho C.T., Zhou J., Santos J.S., Armstrong L., Granato D. Chemistry and biological activities of processed Camellia sinensis teas: a comprehensive review. Comprehensive Reviews in Food Science and Food Safety. 2019;18(5):1474–1495. doi: 10.1111/1541-4337.12479. [DOI] [PubMed] [Google Scholar]
- Zhang R.J., Qiu W.Q., Zhang M.S., Row K.H., Cheng Y.D., Jin Y.Z. Effects of different heating methods on the contents of nucleotides and related compounds in minced Pacific white shrimp and Antarctic krill. Lwt-Food Science and Technology. 2018;87:142–150. doi: 10.1016/j.lwt.2017.08.078. [DOI] [Google Scholar]
- Zhao F., Qian J., Liu H., Wang C., Wang X.J., Wu W.X.…Lin Y. Quantification, identification and comparison of oligopeptides on five tea categories with different fermentation degree by Kjeldahl method and ultra-high performance liquid chromatography coupled with quadrupole-orbitrap ultra-high resolution mass spectrometry. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2022.132130. [DOI] [PubMed] [Google Scholar]
- Zhou J., Fang T.T., Li W., Jiang Z.D., Zhou T.S., Zhang L., Yu Y.B. Widely targeted metabolomics using UPLC-QTRAP-MS/MS reveals chemical changes during the processing of black tea from the cultivar Camellia sinensis (L.) O. Kuntze cv Huangjinya. Food Research International. 2022;162 doi: 10.1016/j.foodres.2022.112169. [DOI] [PubMed] [Google Scholar]
- Zhu J.Y., Yang X., Chen Y.Q., Yu Z., Ni D.J., Chen S.Z., Chen X.L. Effects of different drying methods on quality of dark tea (in Chinese) Journal of Food Safety and Quality. 2022;13(14):4423–4430. doi: 10.19812/j.cnki.jfsq11-5956/ts.2022.14.051. [DOI] [Google Scholar]
- Zhu Y.M., Dong J.J., Jin J., Liu J.H., Zheng X.Q., Lu J.L.…Ye J.H. Roasting process shaping the chemical profile of roasted green tea and the association with aroma features. Food Chemistry. 2021;353 doi: 10.1016/j.foodchem.2021.129428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.