Highlights
-
•
The difference volatile flavors between old and young ducks are firstly reported.
-
•
Characteristic aroma biomarkers for old ducks are explored.
-
•
Free fatty acids are closely associated with the characteristic old duck flavor.
-
•
Provide potential biomarkers for old duck identification.
Keywords: Sheldrake duck, Age, Volatile flavor compound, Free fatty acid
Abstract
In order to explore the characteristic aroma flavor and its formation mechanism of old ducks, two ages (30 days and 60 days) of young ducks and three ages of old ducks (300 days, 900 days, and 1500 days) were selected and studied. An electronic nose was applied to evaluate the overall aroma flavor, and the result showed significant differences between the five duck samples. By gas chromatography-mass spectrometry (GC–MS), forty-eight volatile flavor compounds were detected, including seven aldehydes, six esters, five alcohols, five nitrogen compounds, twenty-one hydrocarbons, and four others. Among these compounds, twelve components, such as hexanal and dimethyl anthranilate, were considered as the characteristic flavor compounds along with duck aging. Furthermore, correlation analysis indicated that meat's unsaturated free fatty acids, especially linoleic acid (C18:2), were responsible for the duck's characteristic flavor formation. These data contribute to the flavor research and identification of old ducks.
1. Introduction
Duck is widely prevalent among consumers for its abundant nutrients and unique flavor, especially in Asia, and the consumption of high-quality duck meat products is continuously increasing (Jo et al., 2018). Ducks for meat use are usually slaughtered and processed at seven to eight weeks of age (Liu et al., 2013). However, it is believed in China, the world’s largest duck meat producer and consumer country, that old ducks are more valuable with high quality and therapeutic efficacy (Xu et al., 2023). Generally, old ducks are spent laying sheldrake ducks aged over one year, and their preciousness is considered in line with the growing age. Old ducks are usually processed for their meat and soup, and the price of old ducks can be several times or even a dozen times higher than that of young ducks. Whereas, it is difficult to distinguish old ducks from other adult ducks in appearance, and the quality of old duck meat has not been well characterized yet, including the flavor.
The volatile flavor is one main part of meat flavor, and is also a crucial factor in consumer acceptance and preference for duck products (Xie et al., 2022, Zhang et al., 2022). The main precursors of meat aroma flavor include water-soluble amino acids, reducing sugars, lipids, and other small molecules, by which aroma flavor compounds are generated after proper cooking through the Maillard reaction, thermal degradation, oxidative decomposition, and other pathways (Mottram, 1998, Kosowska et al., 2017). During processing, the water-soluble precursors generate the basic meat aroma flavor (Khan et al., 2015), while lipids (meat fats), especially free fatty acids (FFAs), are considered as the most important source that contribute to the species-specific flavor of meat (Wood et al., 2008). Duck meat is characterized by its abundance of unsaturated fatty acids, e.g., linoleic and linolenic acid, and their oxidation produces various aromatic components such as aldehydes, esters, alcohols, ketones, and hydrocarbons (Qiao et al., 2017). Also, the effect of age on lipid metabolism has been carefully studied, and the activity of lipid biosynthesis in duck (sheldrake) is found to increase along with aging (Poureslami et al., 2010, He et al., 2018). Thus, it can be presumed that lipid probably plays a crucial role in the characteristic volatile flavor formation of old duck meat.
We hypothesize that the active lipid metabolism while aging leads to the differential lipid composition and contributes to the characteristic aroma flavor of old duck meat. Therefore, the objective of this study was to investigate the compositions of FFAs and volatile flavor in young and old duck meat with different ages (30 days, 60 days, 300 days, 900 days, and 1500 days), and to explore the potential responsible FFAs for the characteristic volatile flavor compounds of old duck meat. This work should provide valuable data for our knowledge of old ducks and may also contribute to their identification against meat adulteration.
2. Materials and methods
2.1. Animals and tissue collection
The sheldrake duck carcasses were purchased from a local experienced enterprise. The ages were chosen as follows: two young ducks, 30 days (D30, 0.67 ± 0.05 kg) and 60 days (D60, 1.12 ± 0.12 kg); three old ducks, 300 days (D300, 1.29 ± 0.07 kg), 900 days (D900, 1.46 ± 0.13 kg), and 1500 days (D1500, 1.46 ± 0.06 kg). Five replicates were set for each age. After being transported to the laboratory on ice, the leg muscle was carefully separated, trimmed to remove visible connective tissues, and cleaned, followed by cooking for 15 min in boiling water. Then, the duck meat was scooped out, cooled down to room temperature, vacuum-packed, and stored at 4 °C until use.
2.2. Electronic nose
As described by Li et al. (2022), the odors of the duck samples were evaluated by an electronic nose (PEN3 system; Airsense, Schwerin, Germany). Each sample (5.0 g) was accurately weighed in a 15 mL headspace vial (Merck KGaA, Darmstadt, Germany) and incubated at 40 °C for 30 min, then the electronic nose probe was inserted into the vial for detection. The detection parameters of the electronic nose were set as follows: the injection flow rate was 200 mL/min, the injection time was 3 s; the detection interval was 1 s, and the flushing time was 120 s. Samples from 56 to 60 s signal, using software WinMuster (Airsense, Schwerin, Germany) on the principal component analysis (PCA).
2.3. Determination of volatile flavor compounds
A headspace solid-phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC–MS, 7890B-7000C, Agilent, Santa Clara, CA) equipped with an HP-5MS column (30 m × 0.25 mm × 0.25 μm film thickness, Merck KGaA, Darmstadt, Germany) was used for the determination of volatile compounds in duck meat at different ages, based on the procedure described by Xia et al. (2021) with some modifications. Each sample (5.0 g) was placed into a 15 mL headspace vial (Merck KGaA, Darmstadt, Germany), which was sealed immediately with PTFE-silicone septum, then equilibrated at 60 °C for 10 min. The volatile flavor substances were extracted using a 75 μm CAR/SPME fiber (Merck KGaA, Darmstadt, Germany) at 60 °C for 40 min, which was preconditioned at 250 °C for 1 h and then used. The injection needle was inserted into the GC injection port, and desorption was performed at 280 °C in a non-shunt mode for 10 min. The temperature process of the column box is as follows: the initial temperature was maintained at 35 °C for 3 min, raised to 40 °C at a rate of 3 °C/min, then increases by 5 °C/min to 210 °C, and remains constant for 15 min. The carrier gas was helium. The transfer line to the MS was maintained at 280 °C, the ion source temperature was 230 °C, electron-impact mass spectra were produced at 70 eV, and an m/z scan range from 45 amu to 550 amu. The detected volatile compounds were identified by comparing the spectra with the database of NIST 14. L (NIST, Gaithersburg, MD) and by comparing their retention indices (RI) against the reported RI from literature and the selected pure standards (decyl aldehyde, hexanal, nonanal, heptaldehyde, 1-octen-3-ol, dodecane, tridecane, n-nonane and styrene) (>97%–99%, Sinopharm Group Co. Ltd., Shanghai, China). The contents of the detected volatile compounds were normalized by their peak areas as percentages of the total peak area.
2.4. Analysis of FFAs
The FFA content was determined using the reported method (Wang et al., 2016) with slight modification. A minced sample of 3.0 g was mixed with 3.8 mL of chloroform–methanol in water (1:2:0.8, v/v/v, Sinopharm Group Co. Ltd., Shanghai, China), and the fat was extracted with saturated salt water. Following this, the fat was saponified with methanol solution at 60 °C for 2 h. After this process, 0.5 mL of boron fluoride-methanol solution was added to the cooled sample and methyl esterification at 60 °C for 1 h. Afterward, 1 g Na2SO4 (Anhui Haihua Chemical Technology Co., Ltd) was added to remove water and dissolved in 0.5 mL of hexane.
The GC–MS system (7890B-7000C; Agilent, Santa Clara, CA USA) equipped with a capillary column (CD-2560, 100 m × 250 μm × 0.20 μm; CNW, Duesseldorf, Germany) was applied. The column temperature was raised from 140 °C to 250 °C at 4 °C/min. Helium was used as carrier gas at 0.7 mL/min, and the injection split ratio was 20:1. The injector temperature was fixed at 250 °C. The mass spectrometer scanning range was 40–450 m/z, and the detector voltage was set at 70 eV. The ion source temperature was 230 °C. The free fatty acids were identified using the NIST 14.L database (NIST, Gaithersburg, MD). Only those compounds with a similarity of over 80 % were reported here.
2.5. Statistical analysis
Using SPSS 23.0 software (Version 23, SPSS Inc., Chicago, IL), experimental data are analyzed by one-way analysis of variance (ANOVA) and expressed as mean ± standard deviation. Duncan’s Multiple Range test (DMRT) is a post hoc test to measure specific differences between pairs of means. The correlation between free fatty acids and volatile flavor compounds was analyzed by Spearman correlation analysis in Origin 2021 software (OriginLab Corporation, Northampton, MA) and plotted into a heat map. The significance level is set to p < 0.05. Principal component analysis (PCA) and partial least squares discriminant (PLS-DA) were applied to multivariate analysis using SIMCA 14.0 (Malmo Metris, Sweden).
3. Results and discussion
3.1. Overall aroma evaluation of duck at different ages
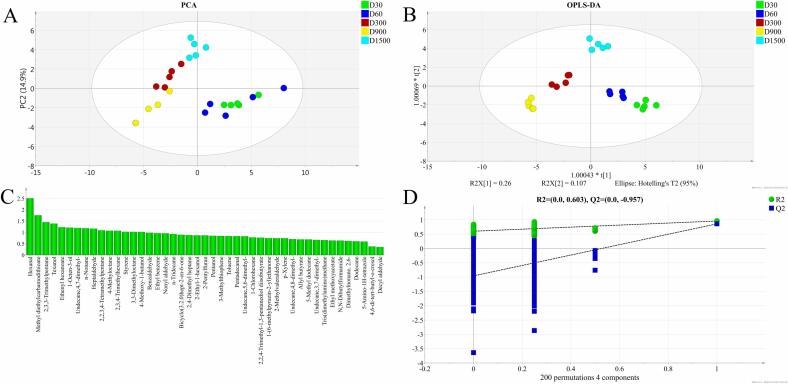
An electronic nose was used to evaluate the overall aroma of the collected duck meat at different ages in the present study. As illustrated in Fig. 1, the first and second principal components (PC1 and PC2) are 78.23 % and 15.67 %, respectively, which explains 93.9 % of the total variance of the five groups. It shows that the data points of each group cluster together, indicating good performance in the repeatability and stability of the determination. Meanwhile, the different groups of duck meat on the map are well separated. It suggests that the volatile flavors of the chosen young ducks and old ducks of various ages were significantly different.
Fig. 1.
Odor evaluation based on the electronic nose. D30, D60, D300, D900, and D1500 stand for 30 days, 60 days, 300 days, 900 days, and 1500 days ducks, respectively.
3.2. Volatile flavor compounds of young and old ducks
By GC–MS, a total of 48 volatile flavor substances were detected in all meat samples and shown in Table 1, including 21 hydrocarbons, 7 aldehydes, 6 esters, 5 alcohols, 5 nitrogen compounds, and 4 other compounds. The representative gas chromatograms of different duck samples are shown in Supplementary Fig. S1.
Table 1.
Volatile compounds in the leg muscles of Shaoxing ducks from different days.
| Volatile flavor compounds | RI | Identification | Relative percentage content (%) |
||||
|---|---|---|---|---|---|---|---|
| D30 | D60 | D300 | D900 | D1500 | |||
| 2-Methylvaleraldehyde | 751.90 | MS, RI | 0.66 ± 0.15b | 0.48 ± 0.45b | 0.74 ± 0.10b | 1.14 ± 0.27a | – |
| Hexanal | 797.23 | MS, RI, PS | 17.34 ± 4.22c | 20.93 ± 10.05c | 29.88 ± 2.99ab | 36.53 ± 5.84a | 21.95 ± 6.46bc |
| Heptaldehyde | 898.78 | MS, RI, PS | 1.38 ± 0.24b | – | 2.22 ± 0.35a | 2.10 ± 0.46a | 1.23 ± 0.76b |
| Benzaldehyde | 958.77 | MS, RI | 3.96 ± 0.70a | 2.60 ± 0.89b | 3.51 ± 0.81ab | 2.43 ± 1.05b | 3.85 ± 0.32a |
| Nonanal | 1104.29 | MS, RI, PS | 16.9 ± 4.04 | 14.71 ± 4.26 | 15.53 ± 3.52 | 12.55 ± 2.67 | 15.69 ± 5.62 |
| Decyl aldehyde | 1205.34 | MS, RI, PS | 0.64 ± 0.16 | 0.66 ± 0.16 | 0.70 ± 0.08 | 0.54 ± 0.18 | 0.89 ± 0.52 |
| Pentadecanal | 1704.51 | MS, RI | 3.67 ± 3.34 | 1.74 ± 2.11 | 1.29 ± 0.93 | 1.09 ± 1.23 | 1.27 ± 0.95 |
| Aldehydes | 44.55 ± 6.26bc | 41.13 ± 13.72c | 53.86 ± 3.81ab | 56.38 ± 4.00a | 44.87 ± 9.01bc | ||
| Ethyl methoxy acetate | 758.82 | MS | 0.74 ± 0.57 | – | – | – | – |
| Ethenyl hexanoate | 985.38 | MS, RI | 2.95 ± 0.75b | 5.23 ± 3.11ab | 6.25 ± 1.50ab | 7.94 ± 3.86a | 3.92 ± 1.55b |
| Allyl butyrate | 1341.12 | MS, RI | – | – | 0.88 ± 0.87 | 0.91 ± 0.32 | – |
| Isobutyl isobutyrate | 1353.25 | MS, RI | 2.78 ± 2.20ab | 1.51 ± 0.77b | 1.64 ± 0.64b | 1.05 ± 0.41b | 4.66 ± 2.18a |
| Dimethyl anthranilate | 1363.02 | MS, RI | 3.46 ± 2.61b | 6.26 ± 3.77ab | 7.71 ± 2.50ab | 6.06 ± 4.30ab | 12.17 ± 8.10a |
| 2,2,4-Trimethyl-1,3-pentanediol di-isobutyrate | 1552.59 | MS, RI | 0.39 ± 0.62 | 1.01 ± 0.86 | – | 0.76 ± 0.48 | – |
| Esters | 10.32 ± 2.74b | 14.01 ± 3.17ab | 16.47 ± 2.46ab | 16.72 ± 4.58ab | 20.74 ± 8.27a | ||
| 4-Methoxy-1-butanol | 723.01 | MS, RI | 1.3 ± 0.96 | 6.03 ± 10.03 | 4.80 ± 0.87 | 4.99 ± 4.53 | 5.61 ± 6.73 |
| 1-Octen-3-ol | 980.70 | MS, RI, PS | 5.22 ± 0.97 | 3.99 ± 1.78 | 4.00 ± 0.80 | 5.64 ± 1.54 | 4.26 ± 0.57 |
| 2-Ethyl-1-hexanol | 1029.78 | MS, RI | 3.02 ± 0.80a | 2.19 ± 0.90ab | 1.42 ± 0.23bc | 1.16 ± 0.58c | 1.20 ± 0.45c |
| 2-Methyl-1-pentanol | 1032.29 | MS, RI | – | – | – | – | 0.07 ± 0.15 |
| Pentanol | 1071.47 | MS, RI | – | – | – | 0.97 ± 0.20 | – |
| Alcohol | 9.54 ± 1.96 | 12.21 ± 9.30 | 10.22 ± 1.02 | 12.76 ± 4.59 | 11.14 ± 6.87 | ||
| Toluene | 778.03 | MS, RI | 1.26 ± 0.36 | 1.05 ± 0.59 | – | – | – |
| Bicyclo(3.2.0)hept-2-en-6-one | 779.07 | MS, RI | – | 1.17 ± 0.71a | 0.87 ± 0.53b | 0.73 ± 0.42b | 1.58 ± 0.32ab |
| Ethylbenzene | 863.00 | MS, RI | 3.34 ± 1.15a | 2.73 ± 0.78ab | 1.03 ± 0.58c | 0.88 ± 0.56c | 1.83 ± 0.50bc |
| Styrene | 885.32 | MS, RI, PS | 1.08 ± 0.31 | 1.47 ± 0.31 | – | 1.15 ± 0.75 | 0.88 ± 0.83 |
| p-Xylene | 886.85 | MS, RI | 2.55 ± 0.53ab | 2.39 ± 0.43ab | 2.45 ± 0.83ab | 1.66 ± 0.64b | 3.32 ± 1.02a |
| n-Nonane | 897.55 | MS, RI, PS | 0.88 ± 0.44b | 2.30 ± 1.22a | – | – | 1.44 ± 1.50ab |
| 4-Methyloctane | 1013.79 | MS, RI | 0.67 ± 0.20b | – | 0.93 ± 0.16a | – | – |
| 3,3-Dimethyloctane | 1022.26 | MS, RI | 0.37 ± 0.35b | 0.56 ± 0.24ab | 0.72 ± 0.15a | – | – |
| 4,7-Dimethyl-Undecane | 1058.93 | MS, RI | 1.77 ± 0.59a | 2.19 ± 0.90a | 2.30 ± 0.42a | 0.86 ± 0.12b | 1.80 ± 0.23a |
| 2,6- Dimethylnonane | 1063.32 | MS, RI | 0.61 ± 0.11 | – | – | – | – |
| 2,2,3,4-Tetramethylpentane | 820.24 | MS, RI | 1.87 ± 0.46a | – | 1.02 ± 0.26b | – | 0.96 ± 0.58b |
| 2,3,4-Trimethylhexane | 850.00 | MS, RI | 0.67 ± 0.06b | 1.71 ± 0.28a | – | – | – |
| 2,3,3-Trimethylpentane | 750.85 | MS, RI | 3.93 ± 0.96a | 1.76 ± 0.78b | – | – | – |
| 2,4-Dimethyl heptane | 820.85 | MS, RI | 1.23 ± 0.34a | 1.19 ± 0.45a | 0.13 ± 0.30b | – | – |
| 5,6-Dimethyl-undecane | 1117.82 | MS, RI | 0.88 ± 0.23ab | 0.77 ± 0.20b | – | – | 1.07 ± 0.20a |
| 5-Methyl dodecane | 1127.06 | MS, RI | 0.62 ± 0.08a | 0.77 ± 0.35a | 0.13 ± 0.30b | – | – |
| Dodecane | 1200.00 | MS, RI, PS | 0.50 ± 0.06 | 0.58 ± 0.29 | – | – | – |
| 3,7-Dimethyl-undecane | 1280.78 | MS, RI | 0.66 ± 0.16bc | 0.89 ± 0.22b | 0.99 ± 0.27ab | 0.53 ± 0.18c | 1.27 ± 0.37a |
| Tridecane | 1300.00 | MS, RI, PS | 0.86 ± 0.35 | 0.54 ± 0.31 | 1.21 ± 1.17 | 0.63 ± 0.58 | – |
| 4,8-Dimethyl-undecane | 1463.08 | MS, RI | – | 0.73 ± 0.67 | – | – | – |
| 4,6-Di-tert-butyl-o-cresol | 1904.60 | MS, RI | 1.10 ± 0.53 | 0.88 ± 0.30 | 0.90 ± 0.23 | 0.76 ± 0.48 | 1.22 ± 0.15 |
| Hydrocarbon | 24.85 ± 4.20a | 23.68 ± 4.34a | 12.69 ± 2.67b | 7.20 ± 2.92c | 15.37 ± 3.95b | ||
| 3-Mercapto-4-methyl-1,2,4-triazole | 971.64 | MS | – | 0.42 ± 0.94 | – | – | – |
| 1-(6-Methylpyrazin-2-yl)ethanone | 1026.33 | MS | 0.80 ± 0.34ab | 1.19 ± 1.33a | 0.20 ± 0.44b | – | – |
| Tris(dimethylamino)methane | 1171.62 | MS | 0.80 ± 0.29a | 0.59 ± 0.25ab | – | 0.28 ± 0.29b | 0.62 ± 0.61ab |
| 5-Amino-1H-tetrazole | 1378.40 | MS | 0.41 ± 0.24ab | 0.56 ± 0.19a | – | 0.32 ± 0.23b | – |
| N, N-Dibutylformamide | 1302.07 | MS, RI | 3.46 ± 1.44a | 2.49 ± 0.87ab | 2.48 ± 1.06ab | 1.46 ± 1.20b | 2.83 ± 1.24ab |
| Nitrogen compounds | 5.47 ± 2.12a | 5.26 ± 3.24a | 2.68 ± 1.26ab | 2.06 ± 1.62b | 3.46 ± 0.78ab | ||
| 3-Methylthiophene | 780.97 | MS, RI | 0.96 ± 0.42 | – | 1.07 ± 0.51 | 0.67 ± 0.10 | 0.98 ± 0.25 |
| 1-Chlorohexane | 867.89 | MS, RI | 1.17 ± 0.21a | 1.13 ± 0.38a | 0.15 ± 0.34b | – | 0.96 ± 0.61a |
| 2-Pentylfuran | 990.35 | MS, RI | 3.13 ± 1.14ab | 2.59 ± 1.14b | 2.86 ± 0.32ab | 4.03 ± 1.42a | 2.48 ± 0.35b |
| 2-Methyl-3-octane | 1086.83 | MS, RI | – | – | – | 0.19 ± 0.27 | – |
| Others | 5.26 ± 1.49 | 3.72 ± 0.80 | 4.08 ± 0.58 | 4.89 ± 1.70 | 4.43 ± 0.86 | ||
The contents of the volatile compounds were expressed as mean ± standard deviation (n = 5). For each component, data with different superscript letters differ significantly at p < 0.05. RI, retention index; PS, pure standard; MS, mass spectrum; D30, D60, D300, D900, and D1500 stand for 30 days, 60 days, 300 days, 900 days, and 1500 days ducks, respectively. Hyphens in content columns mean not detected.
Based on the relative percentage content, aldehydes accounted for the most part (about 41%∼56%) of the duck meat aroma, which was consistent with our previous study (He et al. 2020). Aldehydes are the main products of fatty acid degradation and usually have a great influence on the flavor of meat products due to their high content and low odor threshold (Ba et al., 2019, Guo et al., 2019, Chen et al., 2021, Chen et al., 2021). Then it could be confirmed in the present study that aldehydes contributed greatly to the aroma flavor of duck meat. The data in Table 1 show that the total aldehydes contents of old ducks (D300, D900) were almost significantly higher than those of young ducks (D30 and D60). It implies that aldehydes were probably responsible for the characteristic volatile flavor of old ducks, although the D1500 old ducks presented a decreasing trend. Among the aldehydes, hexanal was the most abundant one, which was reported with the odor of apple, grass, and leaves (Duan et al., 2020). Similar to the total aldehydes, the hexanal content was significantly higher in D300 and D900 old ducks than in young ducks (p < 0.05), with a reducing trend in D1500 ducks. It indicates that hexanal is potentially an important candidate substance for the characteristic aroma flavor of old ducks. The second abundant one was nonanal, which was derived from oleic acid degradation and gave duck meat pleasant citrus and rose aroma (Chen, Li, et al., 2021), but the content did not significantly vary with the ages. Moreover, 2-methylvaleraldehyde (vegetable or fruit odor) was found with a significantly higher content in D900 duck meat (p < 0.05) (Mason, Johnson, & Hamming, 1967), while the benzaldehyde bitter almond flavor content was relatively lower in D60 and D900 duck meat.
Esters also have a lower odor threshold with fruit flavor and are synthesized from esterification reactions between alcohols and carboxylic acids in meat products (Hu et al., 2020, Lorenzo et al., 2014). A total of six esters were detected, and their total content tended to increase along with aging. However, a significant difference (p < 0.05) was only detected between D30 and D1500 duck meat. Some esters were found age-specific, e.g., ethyl methoxy acetate for D30, allyl butyrate for D300 and D900. These volatiles might be used as potential biomarkers for some specific ages, as well as two alcohols, 2-methyl-1-pentanol, and pentanol, which were only observed in D1500 and D900 old ducks, respectively.
Alcohols, especially fatty alcohols, contribute significantly to the formation of flavor in meat products and are important flavor components in duck meat (He et al., 2020, Cai et al., 2020). Whereas, the total alcohol content did not significantly change among the five ages of ducks, except that 2-ethyl-1-hexanol (fruity grass aroma) (Vera et al., 2020) presented a decreasing trend. Intriguingly, there was no significant difference found on 1-octen-3-ol, which is a common oxidation product of lipids, specifically linoleic acid (Zhang, Cao, et al., 2019) and arachidonic acid (Wang et al., 2021), with a strong mushroom aroma and a low odor threshold (Garcia-Gonzalez et al., 2008). Our previous study showed that 1-octen-3-ol was a key aroma compound of sauced ducks processed using various duck sources (Xia et al., 2021). It indicates that 1-octen-3-ol contributes to the characteristic flavor of ducks from different breeds, rather than different ages.
Notably, total nitrogenous compounds were found with a decreasing trend along with aging, especially for D900 ducks with a significantly lower content (p < 0.05), compared to young ducks (D30 and D60). It suggests that old duck meat generally has a relatively weaker ammonia flavor. Hydrocarbons are considered insufficient fragrance contributors due to their high odor thresholds (Ansorena et al., 2001). However, the hydrocarbon content was significantly higher in young ducks (D30 and D60, p < 0.05), implying the heavier aroma flavor of old ducks. Some other compounds were also detected, including 3-methyl thiophene, 1-chlorohexidine, 2-pentyl furan, and 2-methyl-3-octanone, which might not contribute much to the flavor of duck meat due to their relatively low contents and moderate thresholds. However, 2-methyl-3-octanone could also be used as a flavor marker of D900 duck meat.
GC–MS analysis revealed the differential volatile flavor compositions of duck meat with various ages. Generally, compared to the volatile compounds of young ducks, the old ducks' aroma contained fewer hydrocarbons, but more odor-active aldehydes or esters. Moreover, there were also odor differences among old ducks, i.e., fewer aldehydes and inclined more esters for D1500 duck meat, compared to D300 and D900 duck meat. It could be concluded that these diversities contributed to the characteristic duck volatile flavor.
3.3. Duck FFA composition changes during aging
FFAs are mainly produced by the hydrolysis of triglycerides and phospholipids. FFAs are oxidized to form large amounts of hydroperoxides, which are involved in multiple decomposition pathways to generate abundant volatile compounds (Huang et al., 2014, Wan et al., 2023). Here, a total of eleven FFAs were detected in all meat samples, including three saturated fatty acids (SFAs), two monounsaturated fatty acids (MUFAs), and six polyunsaturated fatty acids (PUFAs). It can be seen from Table 2 that in duck meat of various days, the relative content of SFAs of D30 and D60 was significantly higher than that of D300 (p < 0.05), while the reduction in D900 or D1500 was not significant, compared to young ducks. However, stearic acid (C18:0), the only significantly changed SFA, presented with lower proportions in all old duck meat (D300, D900, and D1500) (p < 0.05), indicating the decreasing tendency along with aging. It is noteworthy that reducing the intake of stearic acid is beneficial to the prevention of cardiovascular disease (Hunter, Zhang & Kris Etherton, 2010), suggesting that old duck is fitter for human health than young duck. The old duck meat (D300 and D900) contained higher UFAs than young duck meat (p < 0.05), except that D1500 did not reach a significant level. Among UFAs, C18:2 (linoleic acid), a key FFA for meat aroma formation (Wu et al., 2021), was found significantly accumulated in D300 and D900 meat, compared to young ducks (p < 0.05). Although the linoleic acid accumulation was not remarkable, D1500 old duck meat contained higher C18:3 (linolenic acid), another crucial FFA for meat volatile flavor (Soro et al., 2022), than other old and young ducks (p < 0.05), except D60 duck. It also indicates the differential aroma flavor of old duck with aging. There was also some significance found on C20:4 (arachidonic acid) and C22:6 (docosahexaenoic acid, DHA), while C17:0 (margaric acid), C20:2 (eicosadienoic acid), and C20:3 (eicosatrienoic acid) were only observed in D300 duck, which was worthy of further investigation.
Table 2.
Free fatty acids compositions of Shaoxing ducks from various ages.
| FFA (%) | D30 | D60 | D300 | D900 | D1500 |
|---|---|---|---|---|---|
| C16:0 | 18.92 ± 1.69 | 19.65 ± 1.28 | 17.43 ± 8.05 | 21.86 ± 1.48 | 20.69 ± 1.52 |
| C16:1 | 0.58 ± 0.22 | 0.68 ± 0.27 | 0.6 ± 0.6 | 0.96 ± 0.07 | 0.85 ± 0.17 |
| C17:0 | – | – | 0.1 ± 0.23 | – | – |
| C18:0 | 22.06 ± 1.54a | 21.52 ± 1.72a | 17.68 ± 0.52b | 17.9 ± 2.1b | 17.88 ± 1.45b |
| C18:1 | 22.09 ± 4.15 | 23.63 ± 3.26 | 24.98 ± 1.58 | 26.00 ± 2.53 | 25.06 ± 2.96 |
| C18:2 | 12.58 ± 0.8 cd | 10.71 ± 1.49d | 17.6 ± 1.06a | 16.14 ± 2.95ab | 14.36 ± 1.93bc |
| C18:3 | 0.43 ± 0.27b | 0.52 ± 0.14ab | 0.24 ± 0.53b | 0.15 ± 0.33b | 0.92 ± 0.27a |
| C20:2 | – | – | 0.17 ± 0.24 | – | – |
| C20:3 | – | – | 0.46 ± 1.03 | – | – |
| C20:4 | 18.77 ± 2.36a | 18.7 ± 2.56a | 14.15 ± 1.17b | 13.73 ± 0.72b | 16.58 ± 1.52a |
| C22:6 | 4.57 ± 0.81a | 4.60 ± 0.84a | 2.96 ± 0.45b | 3.26 ± 0.35b | 3.66 ± 0.88ab |
| SFA | 40.97 ± 2.05a | 41.16 ± 1.45a | 35.22 ± 8.04b | 39.76 ± 1.05ab | 38.57 ± 1.23ab |
| UFA | 59.03 ± 2.05b | 58.84 ± 1.45b | 61.17 ± 1.00a | 60.24 ± 1.05ab | 61.44 ± 1.23a |
| MUFA | 22.68 ± 4.36 | 24.32 ± 3.48 | 25.58 ± 1.47 | 26.96 ± 2.53 | 25.91 ± 3.08 |
| PUFA | 36.35 ± 2.78 | 34.52 ± 2.14 | 35.59 ± 0.88 | 33.28 ± 2.19 | 35.53 ± 2.92 |
FFA, free fatty acid; SFA, saturated fatty acid; UFA, unsaturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; D30, D60, D300, D900 and D1500 stand for 30 days, 60 days, 300 days, 900 days, and 1500 days ducks, respectively; hyphens in content columns mean not detected. Data are shown as mean ± standard deviation (n = 5), and the contents with different superscript letters of each FFA item mean significant differences at p < 0.05.
3.4. Correlation between characteristic volatile flavor and FFAs during duck aging
The PCA and OPLS-DA (orthogonal partial least-squares discrimination analysis) were used to characterize the population of all meat samples based on the data obtained from GC–MS. In the PCA model, PC1 and PC2 accounted for 29.8% and 14.9% of the data variance, respectively (Fig. 2A). It was observed that the old duck samples were completely separated from young ducks, and the three ages of old ducks were also generally isolated from each other. But the two young ducks, D30 and D60, could not be discriminated. Afterward, OPLS-DA analysis was performed to further understand the characteristic distribution of the volatile flavor of various duck samples (Fig. 2B). The results of the 200 permutation tests showed that the Y-intercept standards of R2 (cum) and Q2 (cum) are 0.603 and −0.957, respectively, indicating that the established model was reliable (Fig. 2D). Then, as showed in Fig. 2B, all five groups of ducks with different ages are well separated from each other. It is consistent with the result of electronic nose analysis and indicates that OPLS-DA is more efficient to analyze the characteristic aroma flavor of differentially aged old ducks and young ducks. The volatile flavor compounds were analyzed by load maps corresponding to the model, and the projection score (VIP) values were established based on the peak intensity of each compound to describe the contribution extent of the variables (Lou et al., 2018). In the present study, a volatile compound with VIP > 1 and p < 0.05 was considered as a potential characteristic aroma biomarker, and the higher VIP value represented the greater contribution to duck aroma flavor (Zhu et al., 2022). As a result, twelve volatile compounds were found as the potential aroma biomarkers of old and young ducks (Fig. 2C and Table 3), including two aldehydes, three esters, and seven hydrocarbons. It was predictable that the aldehydes and esters turned out to be characteristic aroma biomarkers of duck meat. Hydrocarbons, despite less contribution to aroma flavor, might also be applied in the discrimination of old and young ducks.
Fig. 2.
Volatile flavor compounds of young and old ducks. A, Principal Component Analysis (PCA); B, Orthogonal partial least squares discriminant analysis (OPLS-DA); C, OPLS-DA model Variable Importance for Projection (VIP) scores plot; D, Permutation test plot of the OPLS-DA model. D30, D60, D300, D900, and D1500 stand for 30 days, 60 days, 300 days, 900 days, and 1500 days ducks, respectively.
Table 3.
Potential volatile flavor biomarkers of ducks.
| No. | Compounds | VIP score |
|---|---|---|
| 1 | Hexanal | 2.50844 |
| 2 | Dimethyl anthranilate | 1.75816 |
| 3 | 2,3,3-Trimethylpentane | 1.45542 |
| 4 | Isobutyl isobutyrate | 1.39109 |
| 5 | Ethenyl hexanoate | 1.22914 |
| 6 | Undecane,4,7-dimethyl- | 1.19563 |
| 7 | n-Nonane | 1.18402 |
| 8 | Heptaldehyde | 1.16758 |
| 9 | 2,2,3,4-Tetramethylpentane | 1.09281 |
| 10 | 4-Methyloctane | 1.07522 |
| 11 | 2,3,4-Trimethylhexane | 1.07406 |
| 12 | 3,3-Dimethyloctane | 1.02270 |
VIP score is the variable weight value of OPLS-DA model variables, which can be used to measure the influence strength and explanatory ability of accumulation difference of various compounds on the classification and discrimination of samples of each group. VIP > 1 is a common screening criterion for differential compounds.
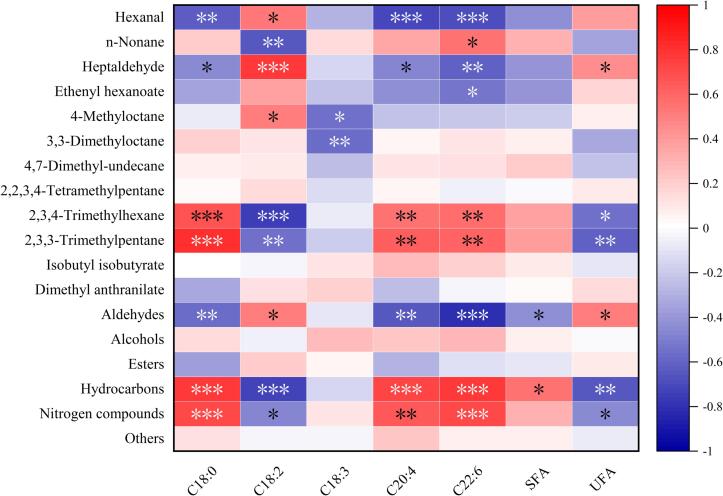
FFAs are the main precursors of meat's volatile flavor. Here, Pearson correlation analysis was performed (Fig. 3) to explore the relationship between the characteristic aroma compounds by OPLS-DA and the significant FFAs (p < 0.05 by ANOVA), which should be conducive to explaining the formation mechanism of characteristic old duck flavor. Aldehydes were found significantly correlated with C18:2 and total UFAs, among which hexanal was significantly associated with C18:2. Previous reports have shown that aldehydes are the main products of UFAs, and linoleic acid (C18:2) is regarded as a key fatty acid associated with the formation of saturated aldehydes such as heptaldehyde and hexanal (Wang et al., 2018, Xie et al., 2022). It is consistent with the result in this study that C18:2 was also found positively associated with aldehydes, especially hexanal, which was the most abundant aroma component of all ducks with the five selected ages and also with the highest VIP value. It suggested that aldehydes and C18:2 contributed greatly to the formation of the characteristic aroma flavor of old and young ducks. Meanwhile, there were also negative associations observed between aldehydes and several fatty acids, e.g., C18:0, C20:4, and C22:6. These negative associations might not be direct, but probably due to the percentage relationship with other fatty acids. Since esters are generated from the esterification reactions between alcohols and carboxylic acids in meat products (Hu et al., 2020, Lorenzo et al., 2014), it was understandable that almost no significant association was observed between esters and FFAs, except a negative correlation for ethenyl hexanoate and C22:6 which might be caused by a similar reason of the negative association between aldehydes and fatty acids as mentioned above. Volatile nitrogen compounds are mainly derived from the catabolism of proteins, free amino acids, and nucleic acids (Chen, Song, & Ma, 2009). Alkanes are generally generated by the homolysis of fatty acid alkoxy radicals (Yao et al., 2022). Intriguingly, a similar correlation with C18:0, C20:4, and C22:6 was both observed for nitrogen compounds and hydrocarbons in the present study, it could be deduced that C18:0, C20:4, and C22:6 contributed little to the formation of duck flavor, which was also indicated by the discussion above. However, the effects of these FFAs on the formation of nitrogen compounds and hydrocarbons are still unknown, the mechanism of which should be indirect and worth further investigating.
Fig. 3.
Correlation between characteristic volatile flavor and FFAs during duck aging. SFA, saturated fatty acid; UFA, unsaturated fatty acid; the transverse axis is free fatty acids, and the longitudinal axis is volatile compounds. Red, positively correlated; blue, negatively correlated. Asterisks indicate significant differences: * p < 0.05, ** p < 0.01, *** p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
The volatile flavors of differentially-aged old and young ducks are significantly different. Their aroma is mainly composed of aldehydes, esters, alcohols, and hydrocarbons, among which several components, e.g., hexanal, heptaldehyde, and dimethyl anthranilate, are considered the characteristic flavor volatiles by OPLS-DA. Based on correlation analysis, the FFAs of duck meat are closely associated with the characteristic aroma flavor, especially UFAs, and C18:2. The results could verify the characteristic aroma flavor of old ducks and conduce to explain its formation mechanism, and may also provide potential biomarkers for old duck identification.
CRediT authorship contribution statement
Mingcai Duan: Investigation, Data curation, Writing – original draft. Ligen Xu: Methodology. Tiantian Gu: Data curation. Yangying Sun: Formal analysis. Qiang Xia: Software. Jun He: Conceptualization, Methodology, Writing – review & editing. Daodong Pan: Supervision. Lizhi Lu: Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Key R&D Program of China (2021YFD2100103).
China Agriculture Research System of MOF and MARA (CARS-42-6; CARS-42-25), and Zhejiang Science and Technology Major Program on Agricultural New Variety Breeding (2021C02068-10).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100899.
Contributor Information
Jun He, Email: junhe2019@hotmail.com.
Lizhi Lu, Email: lulizhibox@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Ansorena D., Gimeno O., Astiasaran I., Bello J. Analysis of volatile compounds by GC-MS of a dry fermented sausage: Chorizo de Pamplona. Food Research International. 2001;34(1):67–75. doi: 10.1016/s0963-9969(00)00133-2. [DOI] [Google Scholar]
- Ba H.V., Seo H.W., Seong P.N., Cho S.H., Kang S.M., Kim Y.S., Moon S.S., Choi Y.M., Kim J.H. Live weights at slaughter significantly affect the meat quality and flavor components of pork meat. Animal science journal. 2019;90(5):667–679. doi: 10.1111/asj.13187. [DOI] [PubMed] [Google Scholar]
- Chen G., Song H., Ma C. Aroma-active compounds of Beijing roast duck. Flavour and Fragrance Journal. 2009;24(4):186–191. doi: 10.1002/ffj.1932. [DOI] [Google Scholar]
- Cai Z., Ruan Y., He J., Dang Y., Cao J., Sun Y.…Tian H. Effects of microbial fermentation on the flavor of cured duck legs. Poultry Science. 2020;99(9):4642–4652. doi: 10.1016/j.psj.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Luo J., Lou A., Wang Y., Yang D., Shen Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.128138. [DOI] [PubMed] [Google Scholar]
- Chen Y., Li P., Liao L., Qin Y., Jiang L., Liu Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chemistry. 2021;361 doi: 10.1016/j.foodchem.2021.130055. [DOI] [PubMed] [Google Scholar]
- Duan Z., Dong S., Sun Y., Dong Y., Gao Q. Response of Atlantic salmon (Salmo salar) flavor to environmental salinity while culturing between freshwater and seawater. Aquaculture. 2020;530 doi: 10.1016/j.aquaculture.2020.735953. [DOI] [Google Scholar]
- Garcia-Gonzalez D.L., Tena N., Aparicio-Ruiz R., Morales M.T. Relationship between sensory attributes and volatile compounds qualifying dry-cured hams. Meat Science. 2008;80(2):315–325. doi: 10.1016/j.meatsci.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Guo Q., Kong X., Hu C., Zhou B., Wang C., Shen Q.W. Fatty acid content, flavor compounds, and sensory quality of pork loin as affected by dietary supplementation with l-arginine and glutamic acid. Journal of food science. 2019;84(12):3445–3453. doi: 10.1111/1750-3841.14959. [DOI] [PubMed] [Google Scholar]
- Hunter J.E., Zhang J., Kris-Etherton P.M. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. American Journal of Clinical Nutrition. 2010;91(1):46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- Huang Y., Li H., Huang T., Li F., Sun J. Lipolysis and lipid oxidation during processing of Chinese traditional smoke-cured bacon. Food Chemistry. 2014;149:31–39. doi: 10.1016/j.foodchem.2013.10.081. [DOI] [PubMed] [Google Scholar]
- He J., Zheng H., Pan D., Liu T., Sun Y., Cao J.…Zeng X. Effects of aging on fat deposition and meat quality in Sheldrake duck. Poultry Science. 2018;97(6):2005–2010. doi: 10.3382/ps/pey077. [DOI] [PubMed] [Google Scholar]
- He Y., Zhou M., Xia C., Xia Q., He J., Cao J.…Sun Y. Volatile flavor changes responding to heat stress-induced lipid oxidation in duck meat. Animal Science Journal. 2020;91(1) doi: 10.1111/asj.13461. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang L., Zhang H., Wang Y., Chen Q., Kong B. Physicochemical properties and flavour profile of fermented dry sausages with a reduction of sodium chloride. LWT-Food Science and Technology. 2020;124 doi: 10.1016/j.lwt.2020.109061. [DOI] [Google Scholar]
- Jo Y., An K.-A., Arshad M.S., Kwon J.-H. Effects of e-beam irradiation on amino acids, fatty acids, and volatiles of smoked duck meat during storage. Innovative Food Science & Emerging Technologies. 2018;47:101–109. doi: 10.1016/j.ifset.2017.12.008. [DOI] [Google Scholar]
- Khan M.I., Jo C., Tariq M.R. Meat flavor precursors and factors influencing flavor precursors-A systematic review. Meat Science. 2015;110:278–284. doi: 10.1016/j.meatsci.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Kosowska M., Majcher M.A., Fortuna T. Volatile compounds in meat and meat products. Food Science and Technology. 2017;37(1):1–7. doi: 10.1590/1678-457x.08416. [DOI] [Google Scholar]
- Liu C., Pan D., Ye Y., Cao J. H-1 NMR and multivariate data analysis of the relationship between the age and quality of duck meat. Food Chemistry. 2013;141(2):1281–1286. doi: 10.1016/j.foodchem.2013.03.102. [DOI] [PubMed] [Google Scholar]
- Lorenzo J.M., Franco D., Carballo J. Effect of the inclusion of chestnut in the finishing diet on volatile compounds during the manufacture of dry-cured “Lacon” from Celta pig breed. Meat Science. 2014;96(1):211–223. doi: 10.1016/j.meatsci.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Lou X., Ye Y., Wang Y., Sun Y., Pan D., Cao J. Effect of high-pressure treatment on taste and metabolite profiles of ducks with two different vinasse-curing processes. Food Research International. 2018;105:703–712. doi: 10.1016/j.foodres.2017.11.084. [DOI] [PubMed] [Google Scholar]
- Li C., Al-Dalali S., Wang Z., Xu B., Zhou H. Investigation of volatile flavor compounds and characterization of aroma-active compounds of water-boiled salted duck using GC-MS-O, GC-IMS, and E-nose. Food Chemistry. 2022;386 doi: 10.1016/j.foodchem.2022.132728. [DOI] [PubMed] [Google Scholar]
- Mason M.E., Johnson B., Hamming M.C. Volatile components of roasted peanuts. Major monocarbonyls and some noncarbonyl components. Journal of Agriculture and Food Chemistry. 1967;15(1):66–73. doi: 10.1021/jf60149a029. [DOI] [Google Scholar]
- Mottram D.S. Flavour formation in meat and meat products: A review. Food Chemistry. 1998;62(4):415–424. doi: 10.1016/s0308-8146(98)00076-4. [DOI] [Google Scholar]
- Poureslami R., Raes K., Turchini G.M., Huyghebaert G., De Smet S. Effect of diet, sex and age on fatty acid metabolism in broiler chickens: N-3 and n-6 PUFA. British Journal of Nutrition. 2010;104(2):189–197. doi: 10.1017/s0007114510000395. [DOI] [PubMed] [Google Scholar]
- Qiao Y., Huang J., Chen Y., Chen H., Zhao L., Huang M., Zhou G. Meat quality, fatty acid composition and sensory evaluation of Cherry Valley, Spent Layer and Crossbred ducks. Animal Science Journal. 2017;88(1):156–165. doi: 10.1111/asj.12588. [DOI] [PubMed] [Google Scholar]
- Soro A.B., Harrison S.M., Whyte P., Bolton D.J., Tiwari B.K. Impact of ultraviolet light and cold plasma on fatty acid profile of raw chicken and pork meat. Journal of Food Composition and Analysis. 2022;114 doi: 10.1016/j.jfca.2022.104872. [DOI] [Google Scholar]
- Vera P., Canellas E., Nerin C. Compounds responsible for off-odors in several samples composed by polypropylene, polyethylene, paper and cardboard used as food packaging materials. Food Chemistry. 2020;309 doi: 10.1016/j.foodchem.2019.125792. [DOI] [PubMed] [Google Scholar]
- Wood J.D., Enser M., Fisher A.V., Nute G.R., Sheard P.R., Richardson R.I.…Whittington F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Science. 2008;78(4):343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang Y.-T., Cao J.-X., Chen Y.-J., Sun Y.-Y., Zeng X.-Q.…Gan N. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chemistry. 2016;190:33–40. doi: 10.1016/j.foodchem.2015.05.048. [DOI] [PubMed] [Google Scholar]
- Wang W., Feng X., Zhang D., Li B., Sun B., Tian H., Liu Y. Analysis of volatile compounds in Chinese dry-cured hams by comprehensive two-dimensional gas chromatography with high-resolution time-of-flight mass spectrometry. Meat Science. 2018;140:14–25. doi: 10.1016/j.meatsci.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Wang F., Gao Y., Wang H., Xi B., He X., Yang X., Li W. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS) Meat Science. 2021;175 doi: 10.1016/j.meatsci.2021.108449. [DOI] [PubMed] [Google Scholar]
- Wu S., Yang J., Dong H., Liu Q., Li X., Zeng X., Bai W. Key aroma compounds of Chinese dry-cured Spanish mackerel (Scomberomorus niphonius) and their potential metabolic mechanisms. Food chemistry. 2021;342 doi: 10.1016/j.foodchem.2020.128381. [DOI] [PubMed] [Google Scholar]
- Wan J., Liu Q., Ma C., Muhoza B., Huang Y., Sun M., Song S., Ho C.T. Characteristic flavor fingerprint disclosure of dzo beef in Tibet by applying SAFE-GC-O-MS and HS-GC-IMS technology. Food research international (Ottawa. 2023;Ont.), 166 doi: 10.1016/j.foodres.2023.112581. [DOI] [PubMed] [Google Scholar]
- Xia C., He Y., Cheng S., He J., Pan D., Cao J., Sun Y. Free fatty acids responsible for characteristic aroma in various sauced-ducks. Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128493. [DOI] [PubMed] [Google Scholar]
- Xie Q., Xu B., Xu Y., Yao Z., Zhu B., Li X., Sun Y. Effects of different thermal treatment temperatures on volatile flavour compounds of water-boiled salted duck after packaging. LWT-Food Science and Technology. 2022;154 doi: 10.1016/j.lwt.2021.112625. [DOI] [Google Scholar]
- Xu L., Duan M., Cai Z., Zeng T., Sun Y., Cheng S.…Pan D. Colloidal nanoparticles isolated from duck soup exhibit antioxidant effect on macrophages and enterocytes. Foods (Basel, Switzerland) 2023;12(5):981. doi: 10.3390/foods12050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W., Cai Y., Liu D., Chen Y., Li J., Zhang M.…Zhang H. Analysis of flavor formation during production of Dezhou braised chicken using headspace-gas chromatography-ion mobility spec-trometry (HS-GC-IMS) Food Chemistry. 2022;370 doi: 10.1016/j.foodchem.2021.130989. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cao J., Pei Z., Wei P., Xiang D., Cao X.…Li C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Research International. 2019;123:217–225. doi: 10.1016/j.foodres.2019.04.069. [DOI] [PubMed] [Google Scholar]
- Zhang M., Chen M., Fang F., Fu C., Xing S., Qian C.…Jin C. Effect of sous vide cooking treatment on the quality, structural properties and flavor profile of duck meat. International Journal of Gastronomy and Food Science. 2022;29 doi: 10.1016/j.ijgfs.2022.100565. [DOI] [Google Scholar]
- Zhu Z., Bassey A.P., Cao Y., Du X., Huang T., Cheng Y., Huang M. Meat quality and flavor evaluation of Nanjing water boiled salted duck (NWSD) produced by different Muscovy duck (Cairina moschata) ingredients. Food Chemistry. 2022;397 doi: 10.1016/j.foodchem.2022.133833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.